Abstract
Mutations in photoreceptor cilium genes CEP290 and NPHP5 cause a form of Leber congenital amaurosis (LCA) which typically lacks rods but retains central cones. The current study evaluated the transient pupillary light reflex (TPLR) as an objective outcome measure to assess efficacy of ongoing and future therapies. Eleven eyes of six patients selected for retained cone function were tested with TPLR using full-field stimuli in the dark-adapted state. Stimuli were red or blue with 1 s duration and spanned a 6-log unit dynamic range. TPLR response amplitude was quantified at fixed times of 0.9 and 2 s after stimulus onset and TPLR latency was defined as the time to reach 0.3 mm constriction. Full-field stimulus testing (FST) and static perimetry were used to correlate subjective perception with objective TPLR parameters. TPLR and FST thresholds with both red and blue stimuli were abnormally elevated in patients to near -1.25 log phot-cd.m−2 consistent with the lack of rods. TPLR latencies were delayed on average but showed some differences among patients. Remnant extrafoveal vision was correlated with faster TPLR latencies. Our results support the use of a short TPLR protocol with full-field red stimuli of 0.7 log phot-cd.m−2 or brighter as an objective and convenient outcome measure of cone function in CEP290- and NPHP5-LCA. The latency parameter of the TPLR would be expected to show a detectable change when an intervention modifies cone sensitivity in the extrafoveal region.
Keywords: Ciliopathy, Gene Therapy, LCA10, IQCB1, NPHP6, Photoreceptor, Retina, Retinal Degeneration
1. Introduction
Ciliopathies are rare genetic disorders characterized by dysfunction of cilia – a nearly ubiquitous cellular organelle (Reiter & Leroux, 2017). Rod and cone photoreceptor cells have one of the longest cilia known, and retinal ciliopathies refer to monogenic diseases caused by genes expressed at the connecting cilium located between inner and outer segments (Estrada-Cuzcano et al., 2012). Retinal ciliopathies can be part of syndromic diseases with multiple organ manifestations such as Joubert syndrome or nephronophthisis, or only affect the retina. LCA-ciliopathies refer to the most severe forms of retinal ciliopathies with congenital blindness which is often diagnosed as Leber congenital amaurosis (LCA) or syndromic conditions where the retinal phenotype is consistent with LCA. Implicated in LCA-ciliopathies are mutations in at least 9 different genes, the most common of which is the centrosomal protein 290 (CEP290 or NPHP6) gene (den Hollander et al., 2006; Perrault et al., 2007). Mutations in a related gene, nephrocystin 5 (NPHP5 or IQCB1), can also cause an LCA-ciliopathy (Otto et al., 2005; Stone et al., 2011). Patients with CEP290- or NPHP5-LCA tend to retain macular cone photoreceptors that are either partially or completely dysfunctional (Cideciyan et al., 2007a, 2011; Cideciyan & Jacobson, 2019; Jacobson et al., 2017b) thus providing a potential to improve vision with the discovery of effective treatments (Sumaroka et al., 2019). Recent preliminary results with anti-sense oligonucleotides suggest that visual improvement can indeed be realized (Cideciyan et al., 2019).
Outcome measures for clinical trials evaluating experimental interventions in LCA have involved subjective measures of visual function such as visual acuity, perimetry, full-field stimulus testing (FST), or mobility courses (Cideciyan et al., 2019; Jacobson et al., 2012). Ideally, objective measures of function should confirm subjective measures. The pupillary light reflex (PLR) is an objective functional imaging method that can measure the fidelity of the retino-pretectal tract driven with calibrated light stimuli over a very wide dynamic range. PLR is potentially activated by rods, short-wavelength (S-), middle-wavelength (M-), and long-wavelength (L-) sensitive cones, and intrinsically photosensitive retinal ganglion cells (ipRGCs) depending on the previous adaptation of the eye, ambient conditions during the recording, and strength and color of the stimulus (Charng et al., 2017; Dacey et al., 2005; Kelbsch et al., 2019; McDougal & Gamlin, 2010).
Previously, PLR has been used to evaluate the functioning of the visual system in patients with retinal diseases. Such studies included patients with inherited (outer) retinal diseases but without a specific genetic diagnosis or a specific retinal phenotype (Aleman et al., 2004; Barricks et al., 1977; Kardon et al., 2011; Kelbsch et al., 2017; Leon et al., 2012; Park et al., 2011; Richter et al., 2016) or multifactorial inner and outer retinal diseases (Feigl et al., 2011; Feigl et al., 2012; Feigl and Zele, 2014). Directly relevant to the current work, there have also been studies of patients with specific genotypes and an associated understanding of the underlying molecular pathophysiology (Aguirre et al., 2007; Ba-Ali et al., 2017; Charng et al., 2017; Cideciyan et al., 2008; Collison et al., 2015, 2016, 2019; Jacobson et al., 2009a, 2011, 2013, 2017a, 2017b; Kawasaki et al., 2012; Lisowska et al., 2017; Lorenz et al., 2012; Schatz et al., 2019; Stingl et al., 2019). The current work was undertaken to better understand the PLR in the subset of LCA-ciliopathy patients with CEP290 or NPHP5 mutations retaining a central island of abnormal cone vision as a prerequisite for potentially using this method as an objective outcome in ongoing or planned clinical trials.
2. Methods
2.1. Human Subjects
Six subjects (ages: 12–33 years) with severe visual impairment due to LCA associated with biallelic mutations in CEP290 (6 eyes of 3 subjects) or NPHP5 (5 eyes of 3 subjects) genes, and four control subjects (ages: 22–26 years) participated in this study. All subjects underwent a complete eye examination. The tenets of the Declaration of Helsinki were followed, and informed consent, assent and parental permission were obtained. The research was approved by the institutional review board at the University of Pennsylvania.
2.2. Retinal Imaging
Cross-sectional images of the macula with line scans along the horizontal and vertical meridian crossing the fovea, were obtained using a spectral-domain optical coherence tomography (OCT, RTvue-100; Optovue Inc., Fremont, CA, USA). A confocal scanning laser ophthalmoscope (Spectralis HRA; Heidelberg Engineering, Heidelberg, Germany) was used for near infra-red reduced-illuminance autofluorescence imaging (NIR-RAFI). Details of imaging methods and analyses have been published previously (Cideciyan et al., 2007b; Jacobson et al., 2017b).
2.3. Visual fields
Kinetic (Goldmann) visual fields (VFs) were performed with the V-4e target light adapted. Static perimetry was performed using a modified HFA (Humphrey Field Analyzer; Zeiss Meditec Inc, Dublin, CA, USA). Visual sensitivity was determined in dark-adapted eyes using 31 loci (with 2° spacing) that sampled the horizontal meridian centered on fixation with achromatic white stimuli (200 ms, Goldmann size V, 1.7° diameter) (Jacobson et al., 2009b). All patients shared foveal peak vision within ±2° from fixation but differed in terms of extrafoveal remnant (3–4 log below peak sensitivity) vision. The extent and sensitivity of this extrafoveal remnant vision was quantified within ±20° from fixation, excluding foveal locations.
2.4. Full-field stimulus testing (FST)
Full-field stimulus testing (FST) was performed to estimate the perceptual light sensitivity as previously described (Roman et al., 2005; Roman et al., 2007). Briefly, red (637 nm) or blue (465 nm) full-field stimuli (200 ms) were presented to dark-adapted eyes, via a computer-driven stimulator (Colordome; Diagnosys LLC, Littleton, MA) and subjects responded when they perceived the lights presented. At least 10 threshold estimates for each color were obtained and averaged.
2.5. Transient pupillary light reflex (TPLR)
Recordings were performed with a commercial pupillometer (RETI-port; Roland Consult Stasche & Finger GmbH, Brandenburg a.d. Havel, Germany) after at least 40 minutes of binocular dark adaptation in a dark room. Direct pupil response in each eye was assessed with a sequence of full-field stimuli, with the contralateral eye patched. Brief (1 s) stimuli were red (peak 630 nm) or blue (peak 470 nm) color, increasing by 1 log unit steps. The photopic luminance of the maximal red stimulus was 2.7 log phot-cd.m−2 and the scotopic luminance of the maximal blue stimulus was 3.9 log scot-cd.m−2. The nominal luminance values provided by the manufacturer were experimentally confirmed for most stimuli. The dimmest red stimulus was at or below the noise level of the photometer (IL1700, International Light, Peabody, MA), and its value was taken to be equal to the value provided by the manufacturer. Spectral measurements provided further insight into relative effectiveness of the two colors stimulating different opsin systems (Lucas et al., 2014). Blue stimuli were 0.1, 2.0, 2.3 and 2.4 log units more effective in stimulating cone (Vλ), rod (V’λ), melanopsin and short-wavelength (S-) sensitive cone opsins, respectively, as compared to nominally paired red stimuli. Using simplifying assumptions (Charng et al., 2017), the radiant flux at the cornea for the maximal red and blue stimuli were estimated to be 10.8 and 11.3 log quanta.deg−2.s−1 corresponding to 13.8 and 14.3 log quanta.cm−2.s−1, respectively, at the retina before accounting for pre-retinal transmission losses. Most red stimuli could be paired with a blue stimulus that was a near match scotopically. Exceptions were the dimmest two red stimuli (which did not have scotopically-matched blue stimuli) and the brightest two blue stimuli (which did not have scotopically-matched red stimuli).
Pupil responses for all red stimuli were always recorded first and at least 2 recordings were obtained for each luminance. Interstimulus intervals increased with stimulus luminance for up to 2 minutes to allow recovery of the pupil diameter to fully dark-adapted values measured at the start of the recording session. An infra-red sensitive video camera (uEye, IDS, Obersulm, Germany) acquired the videos at 30 frames per second, starting from 1 s before the stimulus onset (pre-stimulus baseline) for a total duration of 15 s. Pupil magnification was fixed (cornea to camera distance 0.3 m, 0.05 mm/pixel). The pupil was detected and tracked automatically by the commercial software (Roland Consult Ver.1017.2.0.6).
Post-acquisition analyses included visual inspection of individual video frames for pupil detection errors (due to blinks or head movements). If possible, detection errors were corrected manually or sections of recording that were affected were excluded from the analysis. Data containing the stimulus parameters, timestamps, pupil centers and pupil diameter were exported for further analysis. The baseline pupil diameter was preliminarily defined as the average pupil diameter during the pre-stimulus baseline. This value was manually adjusted to account for small drifts occurring during the 1 s long pre-stimulus recording period. Three TPLR parameters were defined and measured. Response amplitude was defined as the difference between baseline pupil diameter and pupil diameter measured at one of two fixed times (0.9 or 2 s) after the stimulus onset. Response latency was defined as the time initial pupil constriction reached a criterion value of 0.3 mm – a value chosen based on previous estimates of hippus (Aleman et al., 2004). All three TPLR parameters were based on the absolute pupil diameter and constriction (in mm) consistent with our previous studies (Aguirre et al., 2007; Aleman et al., 2004; Charng et al., 2017; Cideciyan et al., 2008; Jacobson et al., 2009a, 2011, 2013, 2017a, 2017b); relative measures (such as percent constriction from baseline diameter) were not used. The two TPLR amplitudes and latency were plotted as a function of stimulus intensity to obtain intensity response functions. TPLR amplitude threshold was defined as the dimmest light that elicited a TPLR amplitude of 0.3 mm at 0.9 s: the thresholds were estimated for each eye and condition by interpolating the relevant intensity response functions. For some control subjects with small but detectable responses to the dimmest red stimulus, thresholds were extrapolated. For all control subjects, blue thresholds were assumed to be equal to red thresholds in scotopic units. A mixed-effects model with luminance as fixed-effect and patient and eye (nested) as random effects was used to estimate the within- and between-subject standard deviations of the PLR parameters in patients (R Core Team 2019; Bates et al. 2015).
3. Results
3.1. Stereotypical Retinal Structure and Visual Function
All patients selected for the current study (Table 1) had very similar retinal disease phenotype in both of their eyes as demonstrated by the results in the left eyes of S2 and S5 (Fig.1). In the CEP290-LCA patient S2, cross-sectional imaging with OCT at age 15 showed preserved outer nuclear layer (ONL) at the fovea supporting the existence of near normal numbers of cone photoreceptor nuclei (Fig.1A, left panel, arrow). Inner and outer segment structure distal to the ONL was abnormal, consistent with previous work (Cideciyan et al., 2011). Surrounding the fovea, there was increasingly greater loss of photoreceptors with eccentricity (Fig.1A, left panel, arrowheads). En face imaging with NIR-RAFI showed an elliptical macular region of relatively higher signal intensity signifying retained RPE melanization (Cideciyan et al., 2007) encircled by lower signal corresponding to degenerated and/or demelanized RPE (Fig.1A, middle panel). A very small VF of 2–4° diameter was detectable with kinetic perimetry (Fig.1A, right panel). Visual acuity in this eye was 20/100 (Table 1) and likely originated from within the small VF. NPHP5-LCA patient S5 at age 18 had similar results. The OCT showed preserved ONL at the fovea with abnormal inner and outer segments (Fig.1B, left panel, arrow), surrounded by greater degeneration (Fig.1B, left panel, arrowheads). NIR-RAFI showed an elliptical macular region of relatively higher signal (Fig.1B, middle panel), and a small VF was detectable by kinetic perimetry (Fig.1B, right panel). All patients had color perception (based on subjective reports of stimulus color during pupillometry) except for S4 who could not distinguish colors with certainty (Table 1). Retinal disease phenotype of S1, S4 and S6 were similar to S2 and S5. The exception was S3 who had severely reduced visual acuities bilaterally but retained foveal fixation, a small VF, and a retinal structure similar to other patients. All patients had a 2–4 deg diameter residual central VF but some patients had remnant sensitivity around that vision. Electroretinograms were not detectable in all patients.
Table 1.
Clinical characteristics of subjects with CEP290- and NPHP5-LCA
| Color Perception | BCVA | |||||
|---|---|---|---|---|---|---|
| ID* | Sex | Age | RE | LE | RE | LE |
| S1 | F | 14 | Yes | Yes | 20/80 | 20/80 |
| S2 | F | 15 | Yes | Yes | 20/125 | 20/100 |
| S3 | F | 16 | Yes | Yes | 20/5000 | 20/5000 |
| S4 | M | 12 | ns | ns | 20/125 | 20/100 |
| S5 | F | 18 | Yes | Yes | 20/125 | 20/100 |
| S6† | F | 33 | Yes | Yes | 20/63 | 20/125 |
Subjects S1–S3 are CEP290-LCA and S4–S6 are NPHP5-LCA.
PLR was assessed only in the right eye of S6.
Abbreviations: BCVA: Best corrected visual acuity in Snellen equivalent, RE: Right eye, LE: Left eye. ns: Not sure.
Fig.1.
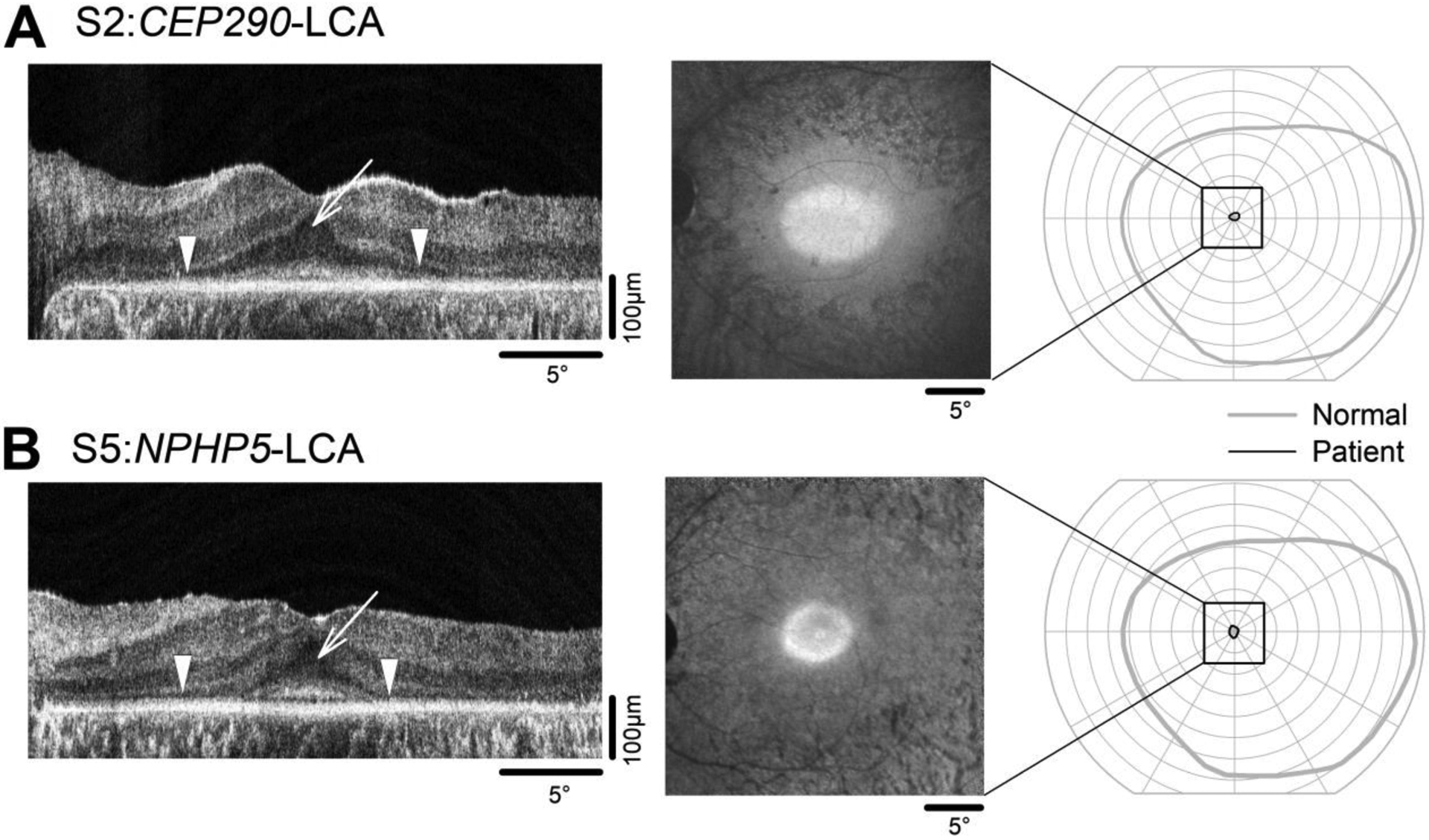
Retinal structure and visual function in representative CEP290- (A) and NPHP5-LCA (B) patients included in the current study. Cross-sectional imaging along the horizontal meridian with OCT imaging (left panels) demonstrates the relatively healthy outer nuclear layer in the foveal region (arrows) but severe photoreceptor loss more peripherally (arrowheads). Retinal en face imaging with NIR-RAFI (middle panels) shows elliptical areas of retained signal intensity in the foveal and parafoveal regions. Goldmann kinetic perimetry with V4e target (right panels) shows a small retained central visual field but no evidence of residual vision anywhere else in the retina. Also shown for comparison is the visual field extent of a control subject (gray trace). Black square depicts the extent of the macular visual field imaged with OCT and NIR-RAFI.
3.2. Transient Pupillary Light Reflex (TPLR) in Control Subjects
To better understand quantitative features of TPLR in selected LCA subjects, we first evaluated control subjects. Raw traces describing changes to pupil diameter as a function of time before and after the onset of red and blue stimuli over a range of luminances are shown for a representative subject (Fig.2A). The same sets of chromatic traces are shown twice: once by photopic matching (left), and once by scotopic matching (right). With the dimmest available red stimulus (-3.3 log phot-cd.m−2) there was a small TPLR, whereas with the dimmest available blue stimulus there was a robust response. With increasing luminance of the red and blue stimuli, the most obvious change was an increase in the delay of the redilation phase (Fig.2A). Time-courses of photopically-matched blue and red waveforms (Fig.2A, left) appeared dissimilar, whereas time-courses of scotopically-matched (Fig.2A, right) waveforms appeared similar. This observation was consistent with the expectation of rod photoreceptors dominating the rapid constriction phase of the TPLR in dark-adapted control eyes (McDougal and Gamlin, 2010). Also consistent with rod mediation was the exquisite sensitivity of the TPLR with a threshold near or just below the dimmest red stimulus corresponding to -4.1 log scot-cd.m−2
Fig. 2.
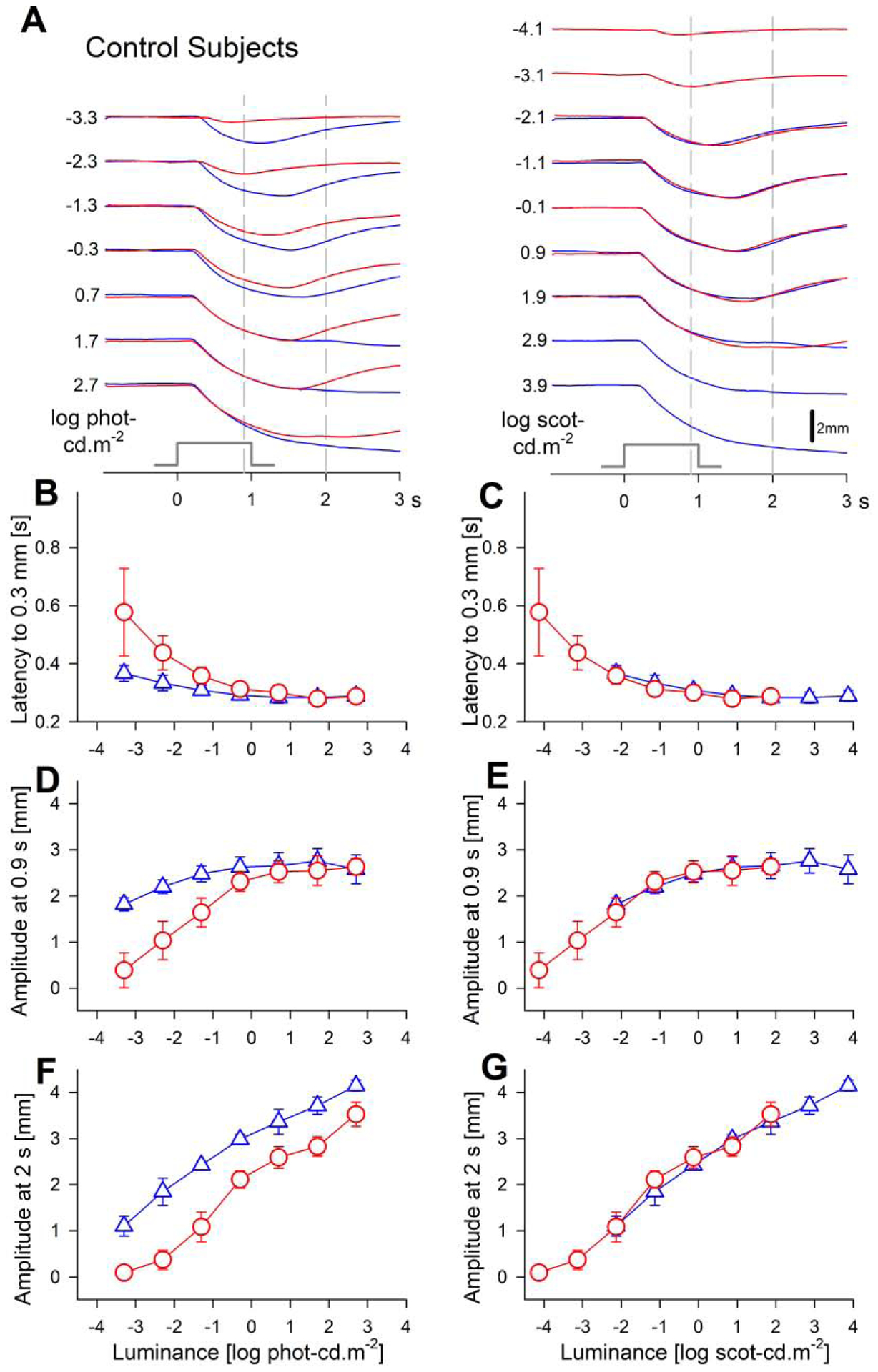
Transient pupillary light responses in dark-adapted control subjects evoked with red and blue stimuli over a 6-log unit dynamic range. (A) Traces of pupil diameter as a function of time for red and blue stimuli. Responses to photopically (left) and scotopically (right) paired chromatic stimuli are shown. Time zero corresponds to the onset of 1 second long stimuli (stimulus monitor shown). Vertical gray dashed lines depict the fixed times (0.9 and 2 s) when the amplitude of the pupillary constriction was measured. (B, C) The response latency to reach a criterion amplitude accelerates with increasing luminance to reach a plateau near 0.3 s. (D–G) Response amplitudes show saturation near 2.6 mm when measured at the early time of 0.9 s, but monotonic increase with luminance when measured at a later time of 2 s. Parameters associated with red stimuli are shown with red circles, and blue stimuli with blue triangles. Error bars represent ±1 sd.
Four key parameters were used to quantify chromatic TPLR families across 6 log units of dynamic range. The baseline pupil diameters in dark-adapted control eyes before the presentation of the first stimulus was on average 7.9 mm, and this value did not change substantially across the chromatic luminance series confirming full recovery of pupil diameter before presentation of each stimulus during the experimental protocol. The response latencies evoked by the dimmest stimuli were 0.58±0.15 s and 0.37±0.03 s, for red and blue, respectively. With increasing luminance until -0.3 log phot-cd.m−2, latencies quickly decreased to 0.31±0.03 s and 0.29±0.02 s for red and blue, respectively. With even higher luminances, acceleration of the rapid constriction phase tended to plateau reaching 0.29±0.03 s (Fig.2B,C). Red and blue latencies were mismatched when plotted on a photopic luminance axis (Fig.2B), but well matched when plotted on a scotopic luminance axis (Fig.2C) confirming rod domination of this parameter throughout most of the available dynamic range where scotopic matching of two colors was possible. Specifically, any additional contributions from cones and ipRGCs that would have been expected to be stimulated above 0 log phot-cd.m−2 were not detectable with the latency parameter as defined.
The amplitude of the rapid TPLR component was measured at the fixed time of 0.9 s after the onset and immediately before the offset of the 1-s-long stimulus (Fig.2A, left vertical dashed lines). Amplitudes at 0.9 s increased from 0.39±0.38 mm and 1.82±0.14 mm to 2.31±0.21 mm and 2.62±0.22 mm for red and blue TPLR families, respectively, until -0.3 log phot-cd.m−2 (Fig.2D). With higher red and blue luminances, TPLR amplitudes at 0.9 s appeared to plateau near 2.6 mm. Like the latency results, amplitudes at early times were mismatched on the photopic axis (Fig.2D) but well matched on the scotopic axis (Fig.2E). The TPLR amplitude near the onset of the redilation phase (at lower luminances) was measured at the fixed time of 2 s after the onset (and 1 s after the offset) of the stimulus (Fig.2A, right vertical dashed lines). Amplitudes at 2 s monotonically increased throughout the full range of red and blue stimuli and were also scotopically matched (Fig.2F, 2G), further confirming mediation by rod photoreceptors at least through 0.7 log phot-cd.m−2 blue and 2.7 log phot-cd.m−2 red stimuli. Possible contributions from other photoreceptors to the two highest blue stimuli (which did not have scotopically matched red stimuli) especially at later times could not be ruled out.
3.3. TPLR in LCA Subjects
One of the clinical characteristics listed for LCA, in the pre-molecular era, was an abnormal or absent pupillary light reflex. But we now know that LCA encompasses a variety of molecular defects and a wide range of disease stages. Here we evaluated the details of the TPLR in our cohort of CEP290- and NPHP5-LCA subjects selected not only for a common cilial defect but also for a specific disease stage of retained cone function and structure. Given the pathophysiological and phenotypical similarity of the two LCA cohorts, the results will be presented together when possible.
Early (up to 4 s) components of raw TPLR traces evoked by red and blue stimuli over a range of luminances are shown for the representative CEP290-LCA subject S3 (Fig.3A), and NPHP5-LCA subject S6 (Fig.4A). TPLR traces for the entire 15 s recording duration are also shown (Suppl. Fig. 1) but were not further characterized in the current work. Both subjects show no TPLR elicited by the two dimmest red and blue stimuli (−3.3 and −2.3 log phot-cd.m−2). However, starting with the third stimulus (−1.3 log phot-cd.m−2, red and blue), both subjects demonstrate TPLRs with an initial constriction phase followed by redilation. With increasing luminance, there is acceleration of the initial rapid constriction phase and greater delays in the redilation phase (Fig.3A, 4A). Use of two stimulus colors allowed better understanding of the photoreceptor sources of TPLRs. Qualitatively, TPLRs evoked by the lower 3 or 4 stimuli appeared to be better matched photopically as compared to scotopically (Fig.3A, 4A). For higher luminances, qualitative differentiation was not possible and quantitative analyses would be required.
Fig. 3.
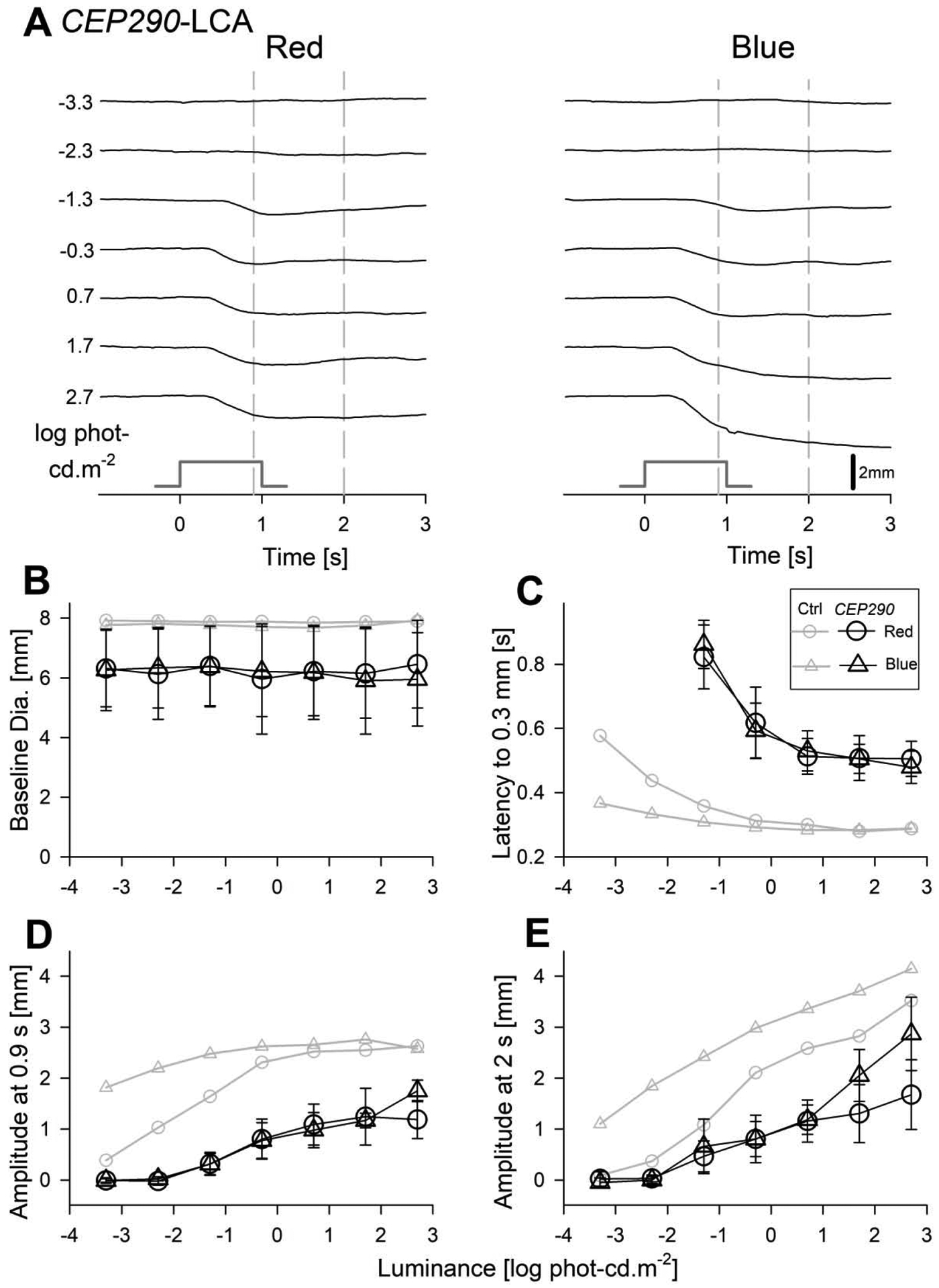
Transient pupillary light responses in dark-adapted CEP290-LCA eyes evoked with red and blue stimuli over a 6-log unit dynamic range. (A) Traces of pupil diameter as a function of time for red (left) and blue (right) stimuli in the right eye of the subject S3. Responses are absent for the two dimmest light levels but detectable thereafter. Time zero corresponds to the onset of 1 second long stimuli (stimulus monitor shown). Vertical gray dashed lines depict the fixed times (0.9 and 2 s) when the amplitude of the pupillary constriction was measured. (B) The baseline pupil diameters are invariant as a function of luminance but were smaller than the control (Ctrl) subjects (gray symbols). (C) The response latency to reach criterion amplitude is similar with red and blue stimuli when plotted on photopic axis. Latency accelerates with increasing luminance to reach a plateau of near 0.5 s which is substantially slower than that of controls. (D, E) Response amplitudes show monotonic increase with luminance at both 0.9 s and 2 s. Amplitudes at all light levels and at both time points are consistently smaller than in controls. Parameters associated with red stimuli are shown with circles, and blue stimuli with triangles. Average data from controls is shown with unfilled gray symbols. Error bars represent ±1 sd.
Fig. 4.
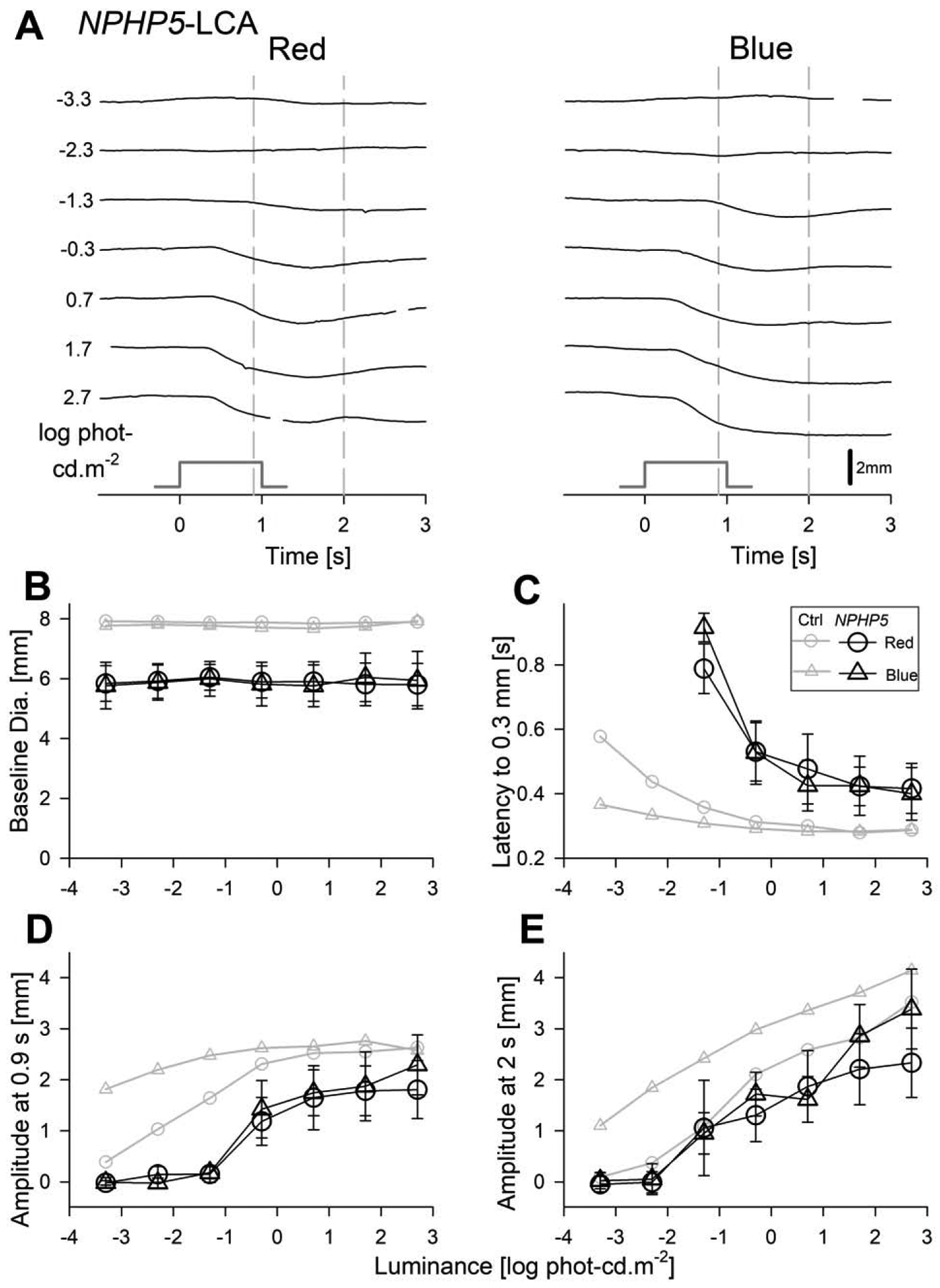
Transient pupillary light responses in dark-adapted NPHP5-LCA subjects evoked with red and blue stimuli over a 6-log unit dynamic range. (A) Traces of pupil diameter as a function of time for red (left) and blue (right) in the right eye of the subject S6. The responses are absent for the two dimmest light levels but detectable thereafter. Time zero corresponds to the onset of 1 second long stimuli (stimulus monitor shown). Vertical gray dashed lines depict the fixed times (0.9 and 2 s) when the amplitude of the pupillary constriction was measured. (B) The baseline pupil diameters are invariant as a function of luminance but were smaller than the control (Ctrl) subjects (gray symbols). (C) The response latency to reach criterion amplitude is similar with red and blue stimuli when plotted on photopic axis. Latency accelerates with increasing luminance to reach a plateau of near 0.4 s which is slower than that of controls.(D, E) Response amplitudes show monotonic increase with luminance when measured at both 0.9 s and 2 s. Amplitudes at both time points were in general consistently smaller than in controls except for the red amplitudes at 2 s that are close to controls. Parameters associated with red stimuli are shown with circles, and blue stimuli with triangles. Average data from controls is shown with unfilled gray symbols. Error bars represent ±1 sd.
Four parameters defining salient features of the TPLR as a function of luminance were quantified, summarized across the two cohorts of LCA patients and compared to control eyes (Fig.3B–E, 4B–E). The first parameter was pupil diameter in darkness. After complete binocular adaptation to darkness, LCA pupils were well dilated. Throughout the experimental protocol, the inter-stimulus periods were kept long enough with increasingly brighter stimuli to allow for the baseline pupil diameter to recover to dark adapted values (Fig.3B, 4B). LCA pupil diameters across all recordings (mean=6.06 mm, sd=1.18 and 0.38 mm for between- and within-subject variability, respectively) were slightly smaller than controls and would be expected to cause, on average, 0.2 log relative reduction in retinal illuminance.
To quantify the early time-course of rapid constriction, we evaluated the response latency to reach a criterion constriction of 0.3 mm. Response latency was indeterminate for the two dimmest light levels (−3.3 and −2.3 log phot-cd.m−2, Fig.3C, 4C). Starting with the third stimulus (1.3 log phot-cd.m−2), the latency was detectable but exceedingly slow for both red and blue stimuli and both cohorts of patients (Fig.3C, 4C). With higher red and blue luminances, response latencies rapidly decreased and appeared to asymptote to a plateau level which was ~0.5 s in CEP290-LCA (Fig.3C) and ~0.4 s in NPHP5-LCA (Fig.4C). The latencies for photopically paired red and blue stimuli were well matched throughout the luminance range (Fig.3C, 4C) supporting a dominant L/M-cone photoreceptor input driving the early time course of rapid pupillary constriction in both CEP290- and NPHP5-LCA. Variability of the latency was quantified (over the 0.7 to 2.7 log phot-cd.m−2 luminance range), yielding a 95% confidence interval for intra-visit test-retest difference of ±0.072 s. The larger latencies in LCA subjects compared to controls (Fig.3C, 4C) could not be accounted by small differences in baseline pupil diameter between the groups or by variability.
The amplitude of pupil constriction was quantified at fixed times. Early following the onset of the stimulus at 0.9 s, both with red and blue stimuli, a criterion of 0.3 mm was reached −1.3 and −0.3 log phot-cd.m−2 for CEP290- and NPHP5-LCA, respectively. These results suggested loss of light sensitivity as well as reduction in early constriction amplitude (Fig.3D,4D). At the later time point of 2 s, discordance between normal and CEP290-LCA amplitudes was retained throughout the luminance range for red and blue stimuli (Fig.3E). For NPHP5-LCA, red TPLR amplitudes tended to be like normal, whereas blue amplitudes tended to be generally smaller than normal except for the highest intensities (Fig.4E).
3.4. Pupillometry and Perception
To better understand the relation between perceptual measures of subjective vision loss and objective measure of TPLR, we considered full-field stimulus testing (FST) and static perimetry thresholds in our cohort of LCA patients. We first compared the TPLR amplitude thresholds with FST thresholds, both obtained with two colors under dark-adapted conditions. TPLR amplitude thresholds for red and blue stimuli were −1.23±0.44 and −1.23±0.33 log phot-cd.m−2, respectively, and were closely matched to the corresponding FST thresholds of −1.19±0.21 and −1.21±0.14 log phot-cd.m−2 for red and blue stimuli, respectively (Fig.5A). Red TPLR amplitude thresholds driven by cones were 2.3 log units elevated compared to normal thresholds driven by rods (Fig.5A). The loss of red TPLR sensitivity was close to the rod sensitivity loss of 2.6 log units and unlike the cone sensitivity loss of 0.8 log units with red FST (Fig.5A). With blue stimuli both TPLR amplitude thresholds and FST thresholds were elevated by ~5 log units (Fig.5A).
Fig.5.
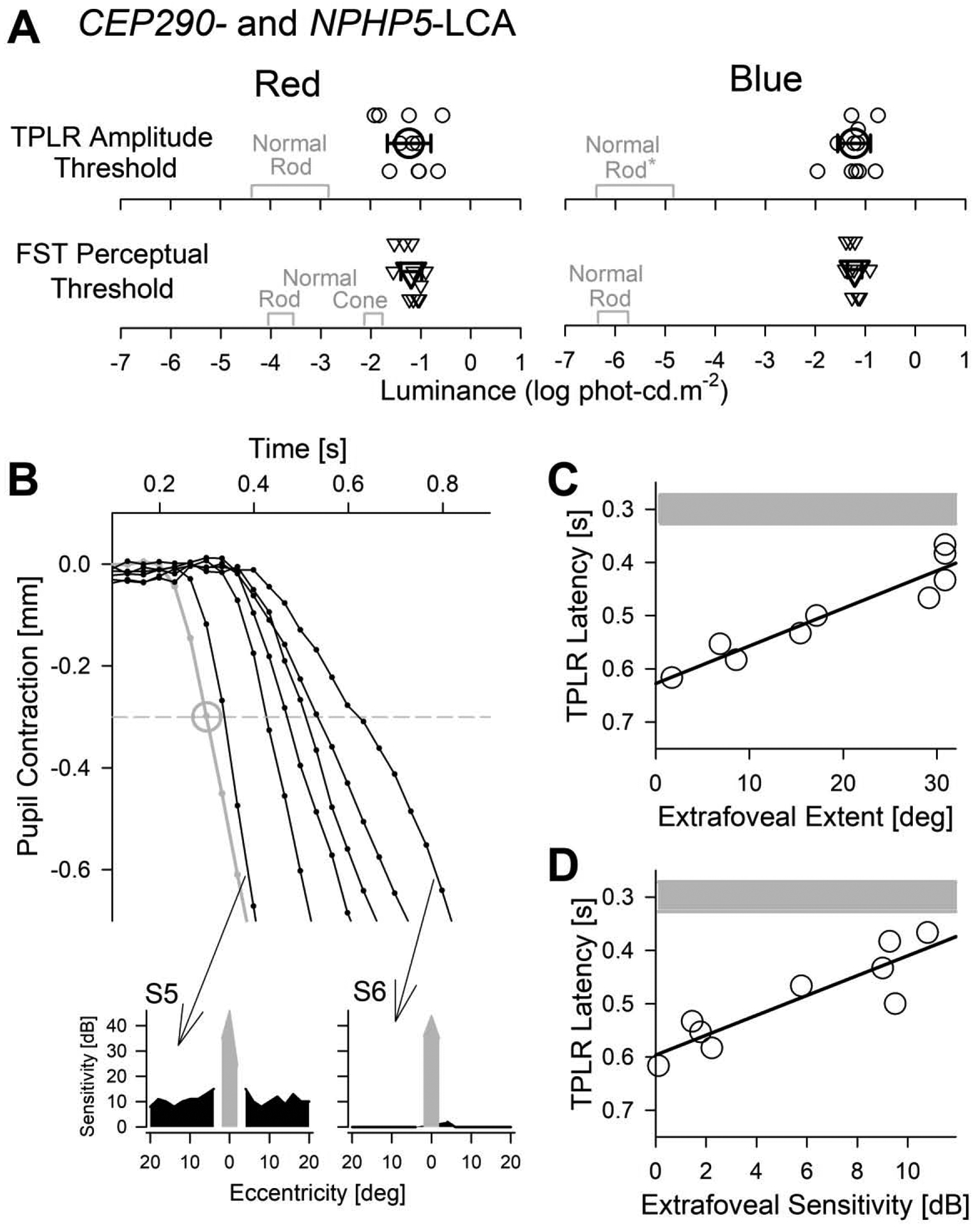
Comparison of TPLR parameters with perception. (A) TPLR amplitude (at 0.9 s) thresholds to reach a criterion of 0.3 mm compared to perceptual full-field stimulus testing (FST) thresholds for red (left) and blue stimuli (right). Values from individual eyes are shown with small symbols and average across all eyes as large symbols (error bars=±1 sd). Normal values driven by the rod system in TPLR, and by rod and cone systems in FST are shown (gray). Normal blue TPLR values (*) are assumed to be equal to red TPLR thresholds in scotopic units. (B) Enlarged view of the initial TPLR constriction phase with the 0.7 log phot-cd.m−2 (5 cd.m−2) red stimulus in a representative normal (gray) and one representative trace from each of the six patients (black). Large gray circle shows the average normal (with ±1 sd error bars). Insets below show the perimetric thresholds with white stimuli in dark-adapted conditions obtained along the horizontal profile in two patients (S5,S6) with the slowest and the fastest TPLR latencies; parafoveal remnant vision shown with black, foveal vision with gray. (C, D) TPLR latency for the 0.7 log phot-cd.m−2 (5 cd.m−2) red stimulus as a function of the extent of extrafoveal vision (C) and average extrafoveal retinal sensitivity (D) as estimated with dark-adapted static perimetry with a white stimulus. Note the inverted y-axis. Linear regression lines (black) and normal range (mean±sd) of latencies (gray) are shown.
Next, we explored the relationship between TPLR latencies obtained with red supra-threshold (i.e. bright) stimuli and perception by considering the extent of remnant extrafoveal vision and average extrafoveal retinal sensitivity measured with dark-adapted perimetry. The dimmest red stimulus that resulted in an early constriction greater than criteria (0.3 mm) in all the 11 eyes was −0.3 log phot-cd.m−2. Red TPLR latencies for the next brighter (0.7 log phot-cd.m−2) stimulus showed a large spectrum of delays between patients (Fig.5B). Some patients (such as S5) had red TPLR latencies very close to normal whereas others (such as S6) had very delayed latencies. All patients showed large perceptual sensitivity deficits with white dark-adapted perimetry but there were some differences among patients. The extent of extrafoveal remnant vision ranged from 1.6 to 30.9° and the average extrafoveal retinal sensitivity (within ± 20° of fixation excluding 4° foveal region) ranged from 0.1 to 10.8 dB. The TPLR latency for the red 0.7 log phot-cd.m−2 stimulus showed a linear relationship with extent of extrafoveal vision diameter (r2 = 0.90; Fig. 5C) and there was a similar correlation with average extrafoveal retinal sensitivity (r2 = 0.80; Fig. 5D). When using the red 1.7 log phot-cd.m−2 stimulus, there were similar distribution of latencies and a similar correlation between latency and visual function. Nearly identical thresholds between FST and TPLR, together with a strong relationship between central perimetric function and TPLR latency suggested that a short protocol involving one to three red TPLR stimuli can be used as an ancillary objective outcome measure of efficacy in future clinical trials evaluating cone function in CEP290- or NPHP5-LCA patients.
4. Discussion
LCA is a genetically heterogeneous inherited retinal disease caused by mutations in more than 20 genes (Chacon-Camacho & Zenteno, 2015; den Hollander et al., 2008). There is always severe and early loss of rod and cone function but the relation between visual function and retinal structure can be complex and variable. Four retinal structural phenotypes of LCA have been described (Jacobson et al., 2016), and CEP290- and NPHP5-LCA represent the phenotype with a central island of retained cone photoreceptors (Cideciyan et al., 2007a, 2011; Cideciyan & Jacobson, 2019; Downs et al., 2016; Jacobson et al., 2017b; Stone et al., 2011). Functional assessment of the retained cone photoreceptors in CEP290- and NPHP5-LCA with dark-adapted two-color perimetry shows a spectrum of localized sensitivity losses (Sumaroka et al., 2019) topographically defining the potential for subjective vision improvement with successful treatments (Cideciyan et al., 2019). The current work assessed the TPLR in a special cohort of CEP290- and NPHP5-LCA patients retaining central cone function in order to evaluate an objective test of visual function and better interpret possible changes occurring in future interventional clinical trials.
TPLR is a simple yet powerful and objective technique to explore the status of the visual pathway including the retina and optic nerve (Loewenfeld, 1993). In general, abnormal visual function in LCA is associated with abnormal TPLRs (Aguirre et al., 2007; Aleman et al., 2004; Charng et al., 2017; Cideciyan, 2010; Jacobson et al., 2009a, 2011, 2013, 2017a, 2017b). Importantly, successful gene therapy improving visual function in RPE65-LCA has been shown to also improve TPLR in dogs (Acland et al., 2001; Aguirre et al., 2007) and in patients (Cideciyan et al., 2008; Jacobson et al., 2012). The current study extends the previous work by using a commercially available pupillometer that can produce chromatic narrow-band stimuli over a 6-log unit dynamic range to evaluate the constriction latency and amplitude and their relation to central cone function in a selected cohort of patients.
Rods, S-, M- and L-cones of the outer retina, and ipRGCs of the inner retina can drive TPLRs (Dacey et al., 2005; Gamlin et al., 2007; Hattar et al., 2003; Lucas, Douglas, & Foster, 2001; Lucas et al., 2014). Differences in sensitivity to spectrally distinct stimuli and differences in TPLR response dynamics can be used to estimate contributions from different photoreceptor populations in normal human eyes (McDougal & Gamlin, 2010). Alternatively, or additionally, results from molecularly and phenotypically well clarified retinal diseases can be used to better understand and interpret human TPLRs. An example of the latter was clarification of the details of the human TPLR likely driven exclusively by ipRGC receptors in patients with retained retinal structure but no visual function (Charng et al., 2017). The current work constitutes another example where a special cohort of patients allows evaluation of exclusive input from central cones driving TPLRs evoked by red stimuli in the dark. Input from rods is ruled out due to the structural and perceptual lack of rod function as previously described (Cideciyan et al., 2007a, 2011; Cideciyan & Jacobson, 2019; Downs et al., 2016; Jacobson et al., 2017b; Stone et al., 2011). Input from S-cones and ipRGCs are unlikely contributors to the TPLR evoked by red stimuli used in the current work due to the wide spectral separation between the stimuli and peak absorptions of the S-opsin and melanopsin as well as the relative insensitivity of the latter receptors.
TPLRs evoked by blue stimuli in CEP290- and NPHP5-LCA were mostly comparable to those evoked by red stimuli when matched by the photopic luminosity function. Exceptions were larger TPLR amplitudes recorded with the brightest blue stimuli which likely included a melanopic component. The difference between blue and red TPLR amplitudes was smaller at early times (Fig.3D, 4D) and larger at later times (Fig.3E, 4E) consistent with the relative slowness of the isolated human melanopic TPLRs recorded previously (Charng et al., 2017). Latency of the blue responses was well matched to latency of red responses throughout the measured dynamic range including the brightest blue stimuli (Fig.3C,4C) providing further support to the lack of measurable S-cone or melanopic contribution at the earliest times even when using blue stimuli.
CEP290- and NPHP5-LCA patients showed TPLR responses that were significantly slower than normal control eyes (Fig.3C, 4C). In general, such a finding may not be considered unexpected since ‘sluggish pupils’ are often included in the list of clinical signs of LCA (Henderson, Lorenz, & Moore, 2006). However, the pathophysiology of the slow TPLR is rarely evaluated in LCA. In RPE65- and AIPL1-LCA, for example, slow TPLRs are secondary to loss of light sensitivity such that response dynamics with bright stimuli in patients match closely to normal responses evoked by appropriately dim lights (Aguirre et al., 2007; Jacobson et al., 2011). In GUCY2D-LCA, TPLR responses can be near normal in terms of amplitude and timing at least in some patients with well retained rod function (Jacobson et al., 2013; Jacobson et al., 2017a). Previous work evaluating TPLR in CEP290-LCA has not assessed the latency of the responses (Collison et al., 2015; Jacobson et al., 2017b) and we are not aware of any TPLR recordings published in patients with NPHP5-LCA and retained vision.
The functional relationship of latency and luminance can be very helpful in attempting to understand pathophysiology of TPLR. The shape of this function was substantially different in CEP290- and NPHP5-LCA as compared to controls such that no amount of sensitivity adjustment by lateral movement of the curves along the luminance axis could bring the results in congruence (Fig.3C, 4C). Thus, major loss of sensitivity did not explain the delayed responses. Among the remaining possibilities was the fact that TPLRs from controls were dominated by activation of retina-wide rods whereas patients’ TPLRs were dominated by the activation of central cones (at least for the red stimuli). Thus, the slower latency could originate due to the differences in the photoreceptor systems, the differences in spatial integration, abnormality of the partially functioning cone system, or a complex combination of some of these possibilities. PLR latency of ~0.3 s with high luminance stimuli recorded in our control subjects was comparable to those reported by others (Bergamin and Kardon, 2003; Schatz et al., 2019) even though the latter were recorded with different stimuli and analyzed with different methods. Similar to the current results in LCA patients, cone-dominant PLRs in congenital stationary night blindness (CSNB) were recently shown to be slower than rod-dominant PLRs in controls (Schatz et al., 2019), however the extent of the slowing (~40 ms) was substantially smaller than the slowing measured in the current work possibly due to retained retina-wide cone function expected in CSNB patients compared to the foveal cone function in our LCA patients.
Even though the TPLR in all eyes of the current cohort of LCA subjects was slow, there were some differences in the extent of slowness between the eyes. To attempt to explain the variation, we evaluated the correlation between the TPLR latency and other visual function measures. Red TPLR latency decreased (became faster) with increasing extent of extrafoveal remnant vision and with increasing extrafoveal remnant retinal sensitivity as measured with static perimetry under dark-adapted conditions. Slowest TPLR was seen in subjects with a small foveal island of vision and no detectable sensitivity beyond 2° eccentric. These results suggest that visual function originating from extrafoveal cones may accelerate the evoked TPLR as compared to foveal cones only. Consistent with this hypothesis is faster multi-focal pupil perimetry results originating from parafoveal rings compared to foveal stimulation (Wilhelm et al., 2000). It is important to note that differences in transmission of retinal signals to the central nervous system have been reported, with fovea being slower than periphery (Stephen et al., 2002) and photoreceptor transduction is slower in foveal cones compared to peripheral cones (Sinha et al., 2017). Taken together with the literature, our results can be considered to provide a rare view of TPLR dominated solely by central (mostly foveal) cone photoreceptors in dark-adapted conditions.
Treatment approaches that are currently in clinical trials for the cohort of patients considered in this study include the antisense oligonucleotides (Cideciyan et al., 2019) and gene editing (Maeder et al., 2019). In planning stages are other approaches based on successful preclinical results (Hardcastle et al, 2018; Hanke-Gogokhia et al., 2018; Zhang et al., 2018). TPLR could be used as an objective outcome measure in such current and future trials for CEP290- and NPHP5-LCA. Previously, TPLR has been used as an objective outcome in the gene therapy trial for RPE65-LCA where major improvement in rod photoreceptor sensitivity was associated with significant changes in the threshold stimulus to evoke criterion pupillary contraction amplitude (Cideciyan et al., 2008; Jacobson et al., 2012). In CEP290- and NPHP5-LCA on the other hand, improvements are expected to be in the central cones since majority of the patients lack rod photoreceptors (Cideciyan et al., 2019). TPLRs driven by normal dark-adapted cones were not able to be determined with the current methods. Thus, the extent of cone-driven TPLR amplitude threshold improvement that is hypothetically possible in patients is unknown. Extrapolating from dark-adapted cone FST measures (collected during the cone-plateau period of dark-adaptation) this range is expected to be less than 1 log unit (Fig.5A) which may be difficult to detect considering the variability of the PLR amplitudes in LCA patients. TPLR latency with supra-threshold bright stimuli on the other hand is abnormal and appears to have more room for detecting improvement beyond variability. In addition, intra-individual differences appear to suggest that a visual field expansion from fovea to the parafoveal area would be expected to correspond to a faster TPLR. This is especially promising based on our recent work suggesting that the parafoveal area has the greatest treatment potential in CEP290- and NPHP5-LCA (Sumaroka et al., 2019). Future work will have to define the normal cone TPLR latency under dark-adapted conditions as well as the inter-visit repeatability of the PLR latency in LCA patients. In the meantime, we propose measuring the PLR latency with 0.7 log phot-cd.m−2 (5 phot-cd.m−2) or brighter red stimuli to obtain a convenient and objective measure of central cone function in most patients with CEP290- and NPHP5-LCA taking part in clinical trials.
Supplementary Material
Highlights:
Cohort of CEP290 and NPHP5-LCA subjects with preserved central function
Transient pupillary light reflex driven by central L/M cones
Objective pupillary results correlate with subjective visual function
Latency of the pupillary contraction can be used as a potential outcome measure
Supported by:
National Institutes of Health (EY-017549; P30-EY001583); ProQR Therapeutics; Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: S.G.J. and A.V.C. are listed as co-inventors on a patent application for NPHP5 gene therapy.
References
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, … Bennett J (2001). Gene therapy restores vision in a canine model of childhood blindness. Nature Genetics, 28, 92–95. 10.1038/88327 [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Komaromy AM, Cideciyan AV, Brainard DH, Aleman TS, Roman AJ, … Jacobson SG (2007). Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation. PLoS Medicine, 4, e230 10.1371/journal.pmed.0040230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman TS, Jacobson SG, Chico JD, Scott ML, Cheung AY, Windsor EAM, … Cideciyan AV (2004). Impairment of the transient pupillary light reflex in Rpe65−/− mice and humans with leber congenital amaurosis. Investigative Ophthalmology and Visual Science, 45, 1259–1271. 10.1167/iovs.03-1230 [DOI] [PubMed] [Google Scholar]
- Barricks ME, Flynn JT, & Kushner BJ (1977). Paradoxical pupillary responses in congenital stationary night blindness. Archives of Ophthalmology, 95, 1800–1804. 10.1001/archopht.1977.04450100102012 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 1(1). Retrieved from https://www.jstatsoft.org/v067/i01 [Google Scholar]
- Bergamin O, Kardon RH (2003). Latency of the pupil light reflex: Sample rate, stimulus intensity, and variation in normal subjects. Investigative Ophthalmology and Visual Science, 44:1546–1554. doi: 10.1167/iovs.02-0468. [DOI] [PubMed] [Google Scholar]
- Ba-Ali S, Christensen SK, Sander B, Rosenberg T, Larsen M, & Lund-Andersen H (2017). Choroideremia: melanopsin-mediated postillumination pupil relaxation is abnormally slow. Acta Ophthalmologica, 95, 809–814. 10.1111/aos.13394 [DOI] [PubMed] [Google Scholar]
- Chacon-Camacho OF, & Zenteno JC (2015). Review and update on the molecular basis of Leber congenital amaurosis. World Journal of Clinical Cases, 3, 112–124. 10.12998/wjcc.v3.i2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charng J, Jacobson SG, Heon E, Roman AJ, McGuigan DB, Sheplock R, … Cideciyan AV (2017). Pupillary light reflexes in severe photoreceptor blindness isolate the melanopic component of intrinsically photosensitive retinal ganglion cells. Investigative Ophthalmology & Visual Science, 58, 3215–3224. 10.1167/iovs.17-21909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV (2010). Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Progress in Retinal and Eye Research, 29, 398–427. 10.1016/j.preteyeres.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, & Jacobson SG (2019). Leber congenital amaurosis (LCA): Potential for improvement of vision. Investigative Ophthalmology and Visual Science, 60, 1680–1695. 10.1167/iovs.19-26672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Jacobson SG, Khanna H, Sumaroka A, Aguirre GK, … Swaroop A (2007a). Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis. Human Mutation, 28, 1074–1083. 10.1002/humu.20565 [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Swider M, Aleman TS, Roman MI, Sumaroka A, Schwartz SB, … Jacobson SG (2007b). Reduced-illuminance autofluorescence imaging in ABCA4associated retinal degenerations. Journal of the Optical Society of America A, 24, 1457 10.1364/josaa.24.001457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, … Hauswirth WW (2008). Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proceedings of the National Academy of Sciences of the United States of America, 105, 15112–15117. 10.1073/pnas.0807027105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Rachel RA, Aleman TS, Swider M, Schwartz SB, Sumaroka A, … Swaroop A (2011). Cone photoreceptors are the main targets for gene therapy of NPHP5 (IQCB1) or NPHP6 (CEP290) blindness: Generation of an all-cone Nphp6 hypomorph mouse that mimics the human retinal ciliopathy. Human Molecular Genetics, 20, 1411–1423. 10.1093/hmg/ddr022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG, Drack AV, Ho AC, Charng J, Garafalo AV, … Russell SR (2019). Effect of an intravitreal antisense oligonucleotide on vision in Leber congenital amaurosis due to a photoreceptor cilium defect. Nature Medicine, 25, 225–228. 10.1038/s41591-018-0295-0 [DOI] [PubMed] [Google Scholar]
- Collison FT, Park JC, Fishman GA, McAnany JJ, & Stone EM (2015). Full-field pupillary light responses, luminance thresholds, and light discomfort thresholds in CEP290 leber congenital amaurosis patients. Investigative Ophthalmology and Visual Science, 56, 7130–7136. 10.1167/iovs.15-17467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison FT, Park JC, Fishman GA, Stone EM, & McAnany JJ (2016). Two-color pupillometry in enhanced S-cone syndrome caused by NR2E3 mutations. Documenta Ophthalmologica, 132, 157–166. 10.1007/s10633-016-9535-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison FT, Park JC, Fishman GA, Stone EM, & McAnany JJ (2019). Two-color pupillometry in KCNV2 retinopathy. Documenta Ophthalmologica, 139, 11–20. 10.1007/s10633-019-09691-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Liao H-W, Peterson BB, Robinson FR, Smith VC, Pokorny J, … Gamlin PD (2005). Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature, 433, 749–754. 10.1038/nature03387 [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KEJ, … Cremers FPM (2006). Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. American Journal of Human Genetics, 79, 556–561. 10.1086/507318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, Roepman R, Koenekoop RK, & Cremers FPM (2008). Leber congenital amaurosis: Genes, proteins and disease mechanisms. Progress in Retinal and Eye Research, 27, 391–419. 10.1016/j.preteyeres.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Downs LM, Scott EM, Cideciyan AV, Iwabe S, Dufour V, Gardiner KL, … Aguirre GD (2016). Overlap of abnormal photoreceptor development and progressive degeneration in Leber congenital amaurosis caused by NPHP5 mutation. Human Molecular Genetics, 25, 4211–4226. 10.1093/hmg/ddw254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Cuzcano A, Roepman R, Cremers FPM, den Hollander AI, & Mans DA (2012). Non-syndromic retinal ciliopathies: translating gene discovery into therapy. Human Molecular Genetics, 21, R111–24. 10.1093/hmg/dds298 [DOI] [PubMed] [Google Scholar]
- Feigl B, Mattes D, Thomas R, & Zele AJ (2011). Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Investigative Ophthalmology and Visual Science, 52, 4362–4367. 10.1167/iovs.10-7069 [DOI] [PubMed] [Google Scholar]
- Feigl B, Zele AJ, Fader SM, Howes AN, Hughes CE, Jones KA, & Jones R (2012). The post-illumination pupil response of melanopsin-expressing intrinsically photosensitive retinal ganglion cells in diabetes. Acta Ophthalmologica, 90, e230–4. 10.1111/j.1755-3768.2011.02226.x [DOI] [PubMed] [Google Scholar]
- Feigl B, & Zele AJ (2014). Melanopsin-expressing intrinsically photosensitive retinal ganglion cells in retinal disease. Optometry and Vision Science, 91, 894–903. 10.1097/OPX.0000000000000284 [DOI] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, & Dacey DM (2007). Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Research, 47, 946–954. 10.1016/j.visres.2006.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke-Gogokhia C, Chiodo VA, Hauswirth WW, Frederick JM, & Baehr W (2018). Rescue of cone function in cone-only Nphp5 knockout mouse model with Leber congenital amaurosis phenotype. Molecular Vision, 24, 834–846. [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, … Yau K-W (2003). Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature, 424, 76–81. 10.1038/nature01761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle AJ, Sieving PA, Sahel JA, Jacobson SG, Cideciyan AV, Flannery JG, … Aguirre GD (2018). Translational retinal research and therapies. Translational Vision Science and Technology, 7, 1–19. 10.1167/tvst.7.5.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R, Lorenz B, & Moore AT (2006). Clinical and molecular genetic aspects of leber’s congenital amaurosis. Pediatric Ophthalmology, Neuro-Ophthalmology, Genetics, 157–177. 10.1007/3-540-31220-x_10 [DOI] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Windsor EAM, … Stone EM (2009a). Leber congenital amaurosis caused by Lebercilin (LCA5) mutation: Retained photoreceptors adjacent to retinal disorganization. Molecular Vision, 15, 1098–1106. [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Roman AJ, Sumaroka A, Windsor EAM, … Stone EM (2009b). Defining the residual vision in leber congenital amaurosis caused by RPE65 mutations. Investigative Ophthalmology and Visual Science, 50, 2368–2375. 10.1167/iovs.08-2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Roman AJ, Swider M, … Stone EM (2011). Human retinal disease from AIPL1 gene mutations: foveal cone loss with minimal macular photoreceptors and rod function remaining. Investigative Ophthalmology & Visual Science, 52, 70–79. 10.1167/iovs.10-6127 [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, … Hauswirth WW (2012). Gene therapy for leber congenital amaurosis caused by RPE65 mutations: Safety and efficacy in 15 children and adults followed up to 3 years. Archives of Ophthalmology, 130, 9–24. 10.1001/archophthalmol.2011.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Peshenko IV, Sumaroka A, Olshevskaya EV, Cao L, … Dizhoor AM (2013). Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human leber congenital amaurosis en route to therapy: Residual cone-photoreceptor vision correlates with biochemical properties of the mutants. Human Molecular Genetics, 22, 168–183. 10.1093/hmg/dds421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Huang WC, Sumaroka A, Nam HJ, Sheplock R, Schwartz SB (2016). Leber congenital amaurosis: Genotypes and retinal structure phenotypes in: Rickman CB, LaVail MM, Anderson RE, Grimm C, Hollyfield J, Ash J, (Eds.), Advances in Experimental Medicine and Biology (Vol. 854): Retinal degenerative diseases mechanisms and experimental therapy. Springer International Publishing, Switzerland: pp. 169–176. 10.1192/bjp.112.483.211-a [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Sumaroka A, Roman AJ, Charng J, Lu M, … Boye SE (2017a). Defining outcomes for clinical trials of leber congenital amaurosis caused by GUCY2D mutations. American Journal of Ophthalmology, 177, 44–57. 10.1016/j.ajo.2017.02.003 [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Sumaroka A, Roman AJ, Charng J, Lu M, … Fishman GA (2017b). Outcome measures for clinical trials of leber congenital amaurosis caused by the intronic mutation in the CEP290 gene. Investigative Ophthalmology and Visual Science, 58, 2609–2622. 10.1167/iovs.17-21560 [DOI] [PubMed] [Google Scholar]
- Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, & Kawasaki A (2011). Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology, 118, 376–381. 10.1016/j.ophtha.2010.06.033 [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Crippa SV, Kardon R, Leon L, & Hamel C (2012). Characterization of pupil responses to blue and red light stimuli in autosomal dominant retinitis pigmentosa due to NR2E3 mutation. Investigative Ophthalmology and Visual Science, 53, 5562–5569. 10.1167/iovs.12-10230 [DOI] [PubMed] [Google Scholar]
- Kelbsch C, Maeda F, Lisowska J, Lisowski L, Strasser T, Stingl K, … Peters T (2017). Analysis of retinal function using chromatic pupillography in retinitis pigmentosa and the relationship to electrically evoked phosphene thresholds. Acta Ophthalmologica, 95, e261–e269. 10.1111/aos.13259 [DOI] [PubMed] [Google Scholar]
- Kelbsch C, Strasser T, Chen Y, Feigl B, Gamlin PD, Kardon R, … Wilhelm BJ (2019). Standards in pupillography. Frontiers in Neurology, 10, 1–26. 10.3389/fneur.2019.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon L, Crippa SV, Borruat FX, & Kawasaki A (2012). Differential effect of long versus short wavelength light exposure on pupillary re-dilation in patients with outer retinal disease. Clinical and Experimental Ophthalmology, 40, 16–24. 10.1111/j.1442-9071.2011.02665.x [DOI] [PubMed] [Google Scholar]
- Lisowska J, Lisowski L, Kelbsch C, Maeda F, Richter P, Kohl S, … Kahle NA (2017). Development of a chromatic pupillography protocol for the first gene therapy trial in patients with CNGA3-linked achromatopsia. Investigative Ophthalmology and Visual Science, 58, 1274–1282. 10.1167/iovs.16-20505 [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE, (1993). The light reflex, in: Loewenfeld IE & Lowenstein O (Eds.) The Pupil: Anatomy, Physiology and Clinical Applications. Iowa State University Press, Iowa, pp. 193–267. [Google Scholar]
- Lorenz B, Strohmayr E, Zahn S, Friedburg C, Kramer M, Preising M, & Stieger K (2012). Chromatic pupillometry dissects function of the three different light-sensitive retinal cell populations in RPE65 deficiency. Investigative Ophthalmology and Visual Science, 53, 5641–5652. 10.1167/iovs.12-9974 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, & Foster RG (2001). Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nature Neuroscience, 4, 621–626. 10.1038/88443 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, … Brainard GC. (2014). Measuring and using light in the melanopsin age. Trends Neurosci. 37, 1–9. 10.1016/j.tins.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder ML, Stefanidakis M, Wilson CJ, Baral R, Barrera LA, Bounoutas GS, … Jiang H (2019). Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nature Medicine, 25, 229–233. 10.1038/s41591-018-0327-9 [DOI] [PubMed] [Google Scholar]
- McDougal DH, & Gamlin PD (2010). The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Research, 50, 72–87. 10.1016/j.visres.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, … Hildebrandt F (2005). Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nature Genetics, 37, 282–288. 10.1038/ng1520 [DOI] [PubMed] [Google Scholar]
- Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, & Hood DC (2011). Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Investigative Ophthalmology and Visual Science, 52, 6624–6635. 10.1167/iovs.11-7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I, Delphin N, Hanein S, Gerber S, Dufier J-L, Roche O, … Rozet J-M (2007). Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Human Mutation, 28, 416 10.1002/humu.9485 [DOI] [PubMed] [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- Reiter JF, & Leroux MR (2017). Genes and molecular pathways underpinning ciliopathies. Nature Reviews. Molecular Cell Biology, 18, 533–547. 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P, Wilhelm H, Peters T, Luedtke H, Kurtenbach A, Jaegle H, & Wilhelm B (2017). The diagnostic accuracy of chromatic pupillary light responses in diseases of the outer and inner retina. Graefe’s Archive for Clinical and Experimental Ophthalmology, 255, 519–527. 10.1007/s00417-016-3496-6 [DOI] [PubMed] [Google Scholar]
- Roman AJ, Schwartz SB, Aleman TS, Cideciyan AV, Chico JD, Windsor EAM, … Jacobson SG (2005). Quantifying rod photoreceptor-mediated vision in retinal degenerations: Dark-adapted thresholds as outcome measures. Experimental Eye Research, 80, 259–272. 10.1016/j.exer.2004.09.008 [DOI] [PubMed] [Google Scholar]
- Roman AJ, Cideciyan AV, Aleman TS, & Jacobson SG (2007). Full-field stimulus testing (FST) to quantify visual perception in severely blind candidates for treatment trials. Physiological Measurement, 28 10.1088/0967-3334/28/8/N02 [DOI] [PubMed] [Google Scholar]
- Schatz A, Kelbsch C, Zeitz C, Kohl S, Zrenner E, Gekeler F, … Willmann G (2019). Disinhibition of intrinsic photosensitive retinal ganglion cells in patients with X-linked congenital stationary night blindness. Graefe’s Archive for Clinical and Experimental Ophthalmology, 257, 1207–1215. 10.1007/s00417-019-04319-w [DOI] [PubMed] [Google Scholar]
- Sinha R, Hoon M, Baudin J, Okawa H, Wong ROL, & Rieke F (2017). Cellular and circuit mechanisms shaping the perceptual properties of the primate fovea. Cell, 168, 413–426. 10.1016/j.cell.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Aine CJ, Christner RF, Ranken D, Huang M, & Best E (2002). Central versus peripheral visual field stimulation results in timing differences in dorsal stream sources as measured with MEG. Vision Research, 42, 3059–3074. 10.1016/S0042-6989(02)00415-7 [DOI] [PubMed] [Google Scholar]
- Stingl KT, Kuehlewein L, Weisschuh N, Biskup S, Frans P, Cremers M, … Stingl K (2019). Chromatic full-field stimulus threshold and pupillography as functional markers for late-stage, early-onset retinitis pigmentosa caused by CRB1 mutations. Translational Vision Science & Technology, 8(6), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Cideciyan AV, Aleman TS, Scheetz TE, Sumaroka A, Ehlinger MA, … Jacobson SG (2011). Variations in NPHP5 in patients with nonsyndromic leber congenital amaurosis and Senior-Loken syndrome. Archives of Ophthalmology, 129, 81–87. 10.1001/archophthalmol.2010.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumaroka A, Garafalo AV, Semenov EP, Sheplock R, Krishnan AK, Roman AJ, … Cideciyan AV (2019). Treatment potential for macular cone vision in leber congenital amaurosis due to CEP290 or NPHP5 mutations: Predictions from artificial intelligence. Investigative Ophthalmology & Visual Science, 60, 2551–2562. 10.1167/iovs.19-27156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm H, Wilhelm B, Beuel S, & Lu H (2000). Pupil perimetry using m-sequence stimulation technique. Investigative Ophthalmology and Visual Science, 41, 1229–1238. [PubMed] [Google Scholar]
- Zhang W, Li L, Su Q, Gao G, & Khanna H (2018).Gene therapy using a minicep290 fragment delays photoreceptor degeneration in a mouse model of leber congenital amaurosis. Human Gene Therapy, 29, 42–50. 10.1089/hum.2017.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


