Abstract
Background
Chronic alveolar hypoventilation is a common complication of many neuromuscular and chest wall disorders. Long‐term nocturnal mechanical ventilation is commonly used to treat it. This is a 2014 update of a review first published in 2000 and previously updated in 2007.
Objectives
To examine the effects on mortality of nocturnal mechanical ventilation in people with neuromuscular or chest wall disorders. Subsidiary endpoints were to examine the effects of respiratory assistance on improvement of chronic hypoventilation, sleep quality, hospital admissions and quality of life.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL, MEDLINE and EMBASE on 10 June 2014. We contacted authors of identified trials and other experts in the field.
Selection criteria
We searched for quasi‐randomised or randomised controlled trials of participants of all ages with neuromuscular or chest wall disorder‐related stable chronic hypoventilation of all degrees of severity, receiving any type and any mode of long‐term nocturnal mechanical ventilation. The primary outcome measure was one‐year mortality and secondary outcomes were unplanned hospital admission, short‐term and long‐term reversal of hypoventilation‐related clinical symptoms and daytime hypercapnia, improvement of lung function and sleep breathing disorders.
Data collection and analysis
We used standard Cochrane methodology to select studies, extract data and assess the risk of bias in included studies.
Main results
The 10 eligible trials included a total of 173 participants. Roughly half of the trials were at low risk of selection, attrition or reporting bias, and almost all were at high risk of performance and detection bias. Four trials reported mortality data in the long term. The pooled risk ratio (RR) of dying was 0.62 (95% confidence interval (CI) 0.42 to 0.91, P value = 0.01) in favour of nocturnal mechanical ventilation compared to spontaneous breathing. There was considerable and significant heterogeneity between the trials, possibly related to differences between the study populations. Information on unplanned hospitalisation was available from two studies. The corresponding pooled RR was 0.25 (95% CI 0.08 to 0.82, P value = 0.02) in favour of nocturnal mechanical ventilation. For most of the outcome measures there was no significant long‐term difference between nocturnal mechanical ventilation and no ventilation. Most of the secondary outcomes were not assessed in the eligible trials. Three out of the 10 trials, accounting for 39 participants, two with a cross‐over design and one with two parallel groups, compared volume‐ and pressure‐cycled non‐invasive mechanical ventilation in the short term. From the only trial (16 participants) on parallel groups, there was no difference in mortality (one death in each arm) between volume‐ and pressure‐cycled mechanical ventilation. Data from the two cross‐over trials suggested that compared with pressure‐cycled ventilation, volume‐cycled ventilation was associated with less sleep time spent with an arterial oxygen saturation below 90% (mean difference (MD) 6.83 minutes, 95% CI 4.68 to 8.98, P value = 0.00001) and a lower apnoea‐hypopnoea (per sleep hour) index (MD ‐0.65, 95% CI ‐0.84 to ‐0.46, P value = 0.00001). We found no study that compared invasive and non‐invasive mechanical ventilation or intermittent positive pressure versus negative pressure ventilation.
Authors' conclusions
Current evidence about the therapeutic benefit of mechanical ventilation is of very low quality, but is consistent, suggesting alleviation of the symptoms of chronic hypoventilation in the short term. In four small studies, survival was prolonged and unplanned hospitalisation was reduced, mainly in participants with motor neuron diseases. With the exception of motor neuron disease and Duchenne muscular dystrophy, for which the natural history supports the survival benefit of mechanical ventilation against no ventilation, further larger randomised trials should assess the long‐term benefit of different types and modes of nocturnal mechanical ventilation on quality of life, morbidity and mortality, and its cost‐benefit ratio in neuromuscular and chest wall diseases.
Keywords: Humans; Respiration, Artificial; Respiration, Artificial/mortality; Chronic Disease; Hypoventilation; Hypoventilation/etiology; Hypoventilation/mortality; Hypoventilation/therapy; Motor Neuron Disease; Motor Neuron Disease/complications; Muscular Dystrophy, Duchenne; Muscular Dystrophy, Duchenne/complications; Neuromuscular Diseases; Neuromuscular Diseases/complications; Randomized Controlled Trials as Topic; Sleep; Thoracic Wall; Thoracic Wall/abnormalities; Time Factors
Plain language summary
Mechanical ventilation at night for people with nerve, muscle or chest wall disease who have persistent breathing problems
Review question
We reviewed the evidence about the effect of mechanical ventilation at night in people with chronic (i.e. persistent) difficulties with breathing spontaneously due to diseases of the nerves, muscles or chest wall.
Background
Weakness of muscles of breathing or changes in the control of breathing are major complications of diseases of the nerves or muscles and of the chest wall. These problems can lead to hypoventilation, which is a state in which not enough air enters the lungs. People may then need devices to help them breathe (mechanical ventilation). Mechanical ventilation is widely offered to people with nerve and muscle diseases or chest wall deformities who develop chronic hypoventilation. We wanted to discover whether mechanical ventilation at night improved survival and symptoms of hypoventilation in this group and to compare different types of ventilation.
Study characteristics
This review includes 10 clinical trials involving 173 participants with persistent, stable hypoventilation. Three trials included only participants with motor neuron diseases, in three all participants had chest wall deformation only, and one trial included only participants with Duchenne muscular dystrophy. The other three trials had mixed populations.
The trials were comparisons of mechanical ventilation and standard care (five trials), different methods of ventilation (four trials) or both (one trial).
Key results and quality of the evidence
We found that mechanical ventilation at night may relieve the symptoms of chronic hypoventilation and prolong survival. However, the quality of the studies was very low. The benefit of long‐term mechanical ventilation should be confirmed in further trials.
The evidence is current to June 2014.
Summary of findings
Summary of findings for the main comparison. Nocturnal ventilation versus no ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders.
| Nocturnal ventilation versus no ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders | ||||||
| Patient or population: patients with neuromuscular and chest wall disorders and chronic hypoventilation Settings: ambulatory patients Intervention: nocturnal ventilation versus no ventilation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No ventilation | Nocturnal ventilation | |||||
| Mortality at 1 year (or more) | Study population | RR 0.62 (0.42 to 0.91) | 99 (4 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 521 per 1000 | 323 per 1000 (219 to 474) | |||||
| Moderate | ||||||
| 421 per 1000 | 261 per 1000 (177 to 383) | |||||
| Unplanned admission to hospital | Study population | RR 0.25 (0.08 to 0.82) | 38 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 526 per 1000 | 132 per 1000 (42 to 432) | |||||
| Moderate | ||||||
| 517 per 1000 | 129 per 1000 (41 to 424) | |||||
| No improvement of hypoventilation symptoms: long‐term | Study population | RR 0.43 (0.18 to 1.03) | 51 (3 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 885 per 1000 | 380 per 1000 (159 to 911) | |||||
| Moderate | ||||||
| 900 per 1000 | 387 per 1000 (162 to 927) | |||||
| No improvement of daytime hypercapnia: long‐term | Study population | RR 0.59 (0.41 to 0.86) | 58 (3 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 828 per 1000 | 488 per 1000 (339 to 712) | |||||
| Moderate | ||||||
| 778 per 1000 | 459 per 1000 (319 to 669) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in a footnote3. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Small unblinded trials. 2There was strong heterogeneity across the trials, possibly related to populations with very different severity of illness. 3Assumed control risk is based on actual control group risk as reported in included trials.
Summary of findings 2. Volume‐cycled ventilation versus pressure‐cycled ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders.
| Volume‐cycled ventilation versus pressure‐cycled ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders | ||||||
| Patient or population: patients with neuromuscular and chest wall disorders and chronic hypoventilation Settings: ambulatory patients Intervention: volume‐cycled ventilation versus pressure‐cycled ventilation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Volume‐cycled ventilation | Pressure‐cycled ventilation | |||||
|
Mortality at 1 year (or more) Not measured ‐ data reported for mortality at 3 months |
111 per 1000 | 143 per 1000 (11 to 1000) | RR 1.29 (0.1 to 17.14) | 16 (1 study) | ⊕⊝⊝⊝ very low1 | |
| Unplanned admission to hospital ‐ not measured | ‐ | ‐ | Not estimable | ‐ | ‐ | |
| No improvement of hypoventilation symptoms: long‐term ‐ not measured | ‐ | ‐ | Not estimable | ‐ | ‐ | |
| No improvement of daytime hypercapnia: long‐term ‐ not measured | ‐ | ‐ | Not estimable | ‐ | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes2. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Only one small sized and unblinded study with few events (one death in each group). 2Assumed control risk is based on actual control group risk as reported in included trials.
Background
Long‐term mechanical ventilation in people with neuromuscular conditions was first introduced between 1950 and 1960 in France and Sweden, as a consequence of the poliomyelitis epidemics. During the following decades, the concept of home mechanical ventilation expanded rapidly. Long‐term mechanical ventilation was implemented in many other countries, and for many other conditions including neuromuscular or chest wall disorders, spinal cord injury and chronic obstructive pulmonary disease. Long‐term mechanical ventilation can be delivered via a canula directly inserted into the trachea (invasive mechanical ventilation) or more frequently via face or nasal mask, or via a mouth piece (non‐invasive mechanical ventilation). At the beginning of the 1990s, 26,000 people in France and 11,419 in the USA were receiving long‐term respiratory assistance. About 10% presented with neuromuscular or chest wall disorders (Chailleux 1996; Milligan 1991). A European survey conducted between July 2001 and June 2002 reported the use of home mechanical ventilation in 483 centres in 16 countries (Lloyd‐Owen 2005). That study identified 27,118 participants with long‐term mechanical ventilation for lung or neuromuscular diseases related chronic respiratory failure. Then, the estimated prevalence was 6.6 per 100,000 people. In 2010, the estimated number of ventilator users in the USA was about 11,000, with three‐quarters receiving invasive mechanical ventilation (King 2012). The estimated prevalence of children receiving home mechanical ventilation ranged from 4.7 to 6.4 per 100,000 children, that is a total of 3500 to 4800 children. Prolonged mechanical ventilation is a life‐sustaining technology, which can be delivered at home, and is the fastest growing sector of the home health care economy (Arras 1995; Lewarski 2007). In 1990, the cost in the USA was estimated to be USD 789 per day for each ventilator‐assisted patient by the American Association for Respiratory Care and the Gallup Organization (Milligan 1991). In the USA, a Medicare rough estimate of total payment for home mechanical ventilation was over USD 35 million for 2005 (Lewarski 2007). The average payment for home mechanical ventilation by Medicare, Medicaid or other private insurances varies from USD 500 to 1000 per month (Lewarski 2007).
Chronic alveolar hypoventilation is a state characterised by reduced arterial oxygen tension (PaO2) and increased carbon dioxide tension (PaCO2), which the patient may correct at least partially by voluntary hyperventilation (Newsom‐Davis 1980). In most cases, chronic alveolar hypoventilation leads to daytime fatigue, hypersomnia and changes in psychological function. The mechanisms underlying hypercapnia in people with neuromuscular or chest wall disorders are multiple and not yet fully understood. They may involve impairment of lung mechanics or airway function and cough, ventilation‐perfusion mismatch, blunted central ventilatory drive or respiratory‐muscle fatigue. Abnormalities may occur while awake or during sleep (Loh 1979; Newsom‐Davis 1980; Raphaël 1987; Skatrud 1980; Smith 1987). Numerous observational and uncontrolled studies suggest that nocturnal mechanical ventilation at least partially improves lung mechanics, respiratory muscle strength, or respiratory drive, or reduces ventilation‐perfusion mismatch, by day as well as night (Annane 1999; Bach 1987; Barbé 1996; Carroll 1988; Ellis 1987; Heckmatt 1990; Hoeppner 1984). A multicentre randomised trial of preventive nocturnal non‐invasive positive pressure ventilatory assistance in 70 participants with moderate pulmonary insufficiency due to Duchenne muscular dystrophy (DMD) showed that survival was significantly worse in participants receiving preventive nocturnal ventilatory assistance, although daytime blood gases were improved (Raphaël 1994). Several other randomised trials, performed on parallel groups (Bourke 2006; Pinto 1995; Ward 2005), or based on a cross‐over design and small samples (Ambrosino 1997; Ellis 1987; Laserna 2003; Restrick 1993; Willson 2004), suggested that nocturnal ventilation had beneficial effects on arterial blood gases or sleep parameters. Several reviews emphasise that nocturnal ventilatory assistance may be effective in reducing daytime hypoventilation, improving quality of life and prolonging survival in people with chronic alveolar hypoventilation (Claman 1996; Orlikowski 2005; Simonds 2003). A recent observational study highlighted that ventilator users deserve close monitoring (Chatwin 2010). In that study, 1211 patients requiring home mechanical ventilation had access to online support. There were about 500 patient calls each month, and close to 200 home emergency visits, identifying a ventilator dysfunction in about one‐quarter of cases. A Consensus Conference of the American College of Chest Physicians recommended the implementation of long‐term ventilation in patients with chronic hypercapnia during the day and in those with symptomatic nocturnal hypercapnia, particularly when secondary to neuromuscular or skeletal disorders (Make 1998). Another recent experts' consensus recommended the use of non‐invasive bilevel positive ventilation either part time (< 23 hours) or full time (> 23 hours) in patients with end‐stage chronic respiratory failure related to neuromuscular or chest wall disorders (Bach 2013).
The determination of the size of any beneficial effect of nocturnal mechanical ventilation on daytime hypoventilation in patients with neuromuscular or chest wall disorders and the comparative effects of different modes of ventilation requires a systematic review.
The primary aim of the present review was to search systematically for, and combine all evidence from, randomised trials relating to the effects of nocturnal mechanical ventilation in people with neuromuscular or chest wall disorders in order to supply the best evidence currently available on which to base recommendations for clinical practice and further research. This is a 2014 update of a review first published in 2000 and previously updated in 2007.
Objectives
To examine the effects on mortality of nocturnal mechanical ventilation in people with neuromuscular or chest wall disorders. Subsidiary endpoints were to examine the effects of respiratory assistance on improvement of chronic hypoventilation, sleep quality, hospital admissions and quality of life.
Methods
Criteria for considering studies for this review
Types of studies
We examined all randomised and quasi‐randomised trials with or without blinding (although blinding seems unlikely to be feasible).
Types of participants
Participants with stable chronic hypoventilation, defined by an arterial CO2 tension above 6 kPa during the day, or symptoms of nocturnal hypercapnia (i.e. any of the following: diurnal hypersomnia, headaches, nightmares, enuresis), including children and adults of all degrees of severity, whether living in institutions or in the community. We excluded studies reporting results for people with chronic obstructive pulmonary disease. We included data from studies of mixed populations if separate data were available from the participants with neuromuscular or chest wall disorders, or when contact with the authors resulted in the provision of the data.
We considered chronic hypoventilation due to one of the following medical conditions, according to the classification of the Consensus Conference of the American College of Chest Physicians.
Central nervous system disorders: Arnold‐Chiari malformation, central nervous system trauma, cerebrovascular disorders, disorders of congenital and acquired central control of breathing, myelomeningocele, spinal cord traumatic injuries.
Neuromuscular disorders: amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), polio and postpolio syndrome, congenital childhood hypotonia, Guillain‐Barré syndrome, infantile botulism, muscular dystrophy, myotonic dystrophy, phrenic nerve paralysis, myasthenia gravis.
Skeletal disorders: kyphoscoliosis, thoracic wall deformities, thoracoplasty.
Types of interventions
Long‐term treatment (at least four weeks) with nocturnal mechanical ventilation (for at least three hours per night) versus no ventilation.
Type of ventilation: invasive techniques, i.e. tracheostomy (intermittent positive pressure) versus non‐invasive techniques using negative pressure or positive pressure. For negative pressure, we considered the four available types of body enclosures, (i) body tanks (iron lungs), (ii) chest shells (rigid domes that fit over the chest and abdomen), (iii) body wraps (nylon or plastic jackets that surround the chest and abdomen), and (iv) abdominal pneumobelts (inflatable rubber bladders held firmly against the abdomen by nylon corsets). For non‐invasive positive pressure ventilation, nasal, mouthpiece and oronasal interfaces were all considered.
Mode of ventilation: volume‐cycled, pressure‐cycled mechanical ventilation or bilevel positive airway pressure (BiPAP).
Types of outcome measures
Primary outcomes
Mortality at one year.
Secondary outcomes
Admission to hospital (other than routine visits).
Reversal of daytime hypoventilation‐related clinical symptoms, i.e. diurnal hypersomnia, headaches, nightmares, enuresis.
Reversal of daytime hypercapnia, i.e. arterial CO2 tension (on room air) below 6 kPa.
Lung function measurements, i.e. forced vital capacity (FVC), respiratory muscle strength, ventilation‐perfusion mismatch.
Sleep studies, i.e. apnoea‐hypopnoea index or mean oxygen saturation or the time spent with an arterial oxygen saturation below 90%.
Quality of life as assessed in individual studies.
We recorded outcomes for short‐term effects (within one month) and the long‐term effects (12 months or more) following implementation of mechanical ventilation.
Search methods for identification of studies
On 10 June 2014, we searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL (2014, Issue 5 in The Cochrane Library), MEDLINE (January 1966 to May 2014) and EMBASE (January 1980 to May 2014).
We checked all references in the identified trials and contacted authors to identify any additional published or unpublished data.
The detailed search strategies are in the appendices: Neuromuscular Disease Group Specialized Register Appendix 1, CENTRAL Appendix 2, MEDLINE Appendix 3 and EMBASE Appendix 4.
Data collection and analysis
Selection of studies
Two review authors (DA, DO) checked titles and abstracts identified from the database searches. We obtained the full text of all studies of possible relevance for independent assessment by both review authors. The review authors decided which trials fitted the inclusion criteria, and graded their risk of bias. Any disagreement was resolved by discussion between the review authors. We contacted the authors of one large trial in DMD to clarify whether the study had included people with chronic hypoventilation (Raphaël 1994).
Data extraction and management
Two review authors independently performed data extraction and the review authors systematically contacted authors of trials to provide missing data where possible. The review authors resolved any disagreement by discussion. One review author entered data into the Cochrane authoring software Review Manager (currently RevMan 5, RevMan 2014), and the other review authors checked this.
Assessment of risk of bias in included studies
We assessed the risk of bias in included studies using the Cochrane 'Risk of bias' tool (Higgins 2011).
We documented the risk of bias using the following criteria: baseline comparison of experimental groups, explicit diagnostic criteria, completeness of follow‐up. The review authors resolved any disagreement by discussion
Measures of treatment effect
For each outcome measure we computed 2 x 2 tables, summarising the number of people who experienced the event or outcome in each comparison group and the total number in each group. These tables were organised so that a beneficial effect of treatment was associated with a risk ratio (RR) < 1. We performed intention‐to‐treat analyses. We performed all statistical calculations using RevMan.
Assessment of reporting biases
We would have sought evidence of publication bias using the funnel plot method but there were too few studies included in any meta‐analysis (the Cochrane Handbook recommends at least 10, "because when there are fewer studies the power of the tests is too low to distinguish chance from real asymmetry" (Higgins 2011)).
Data synthesis
We calculated a weighted treatment effect (using a fixed‐effect model) across trials. We expressed the results as risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous outcomes and mean difference (MD and 95% CI) for continuous outcomes. We considered methods based on random‐effects only when there was heterogeneity.
We included a 'Summary of findings' table for each comparison for which data were available and included the following outcomes:
the number of deaths at one year;
admission to hospital (other than routine visits);
the number of participants with improvement in symptoms of hypoventilation in the long term
the number of participants with improvement in daytime hypercapnia in the long term
We used the GRADE criteria (study limitations (risk of bias), consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the evidence for each outcome. We followed the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and used GRADEpro software (GRADEpro 2008).
Subgroup analysis and investigation of heterogeneity
We carried out a subgroup analysis to consider people with skeletal disorders and those with primary central nervous system or neuromuscular dysfunction separately.
When we found significant heterogeneity, we performed a graphic analysis to identify the trials and the factors responsible (related to participants' selection or treatment).
Sensitivity analysis
We performed sensitivity analyses on the basis of risk of bias to test for heterogeneity in the results.
Results
Description of studies
Results of the search
Our search results are detailed in Figure 1. In June 2014, the updated search revealed 26 possible randomised trials. We excluded 16 trials (see Characteristics of excluded studies). There remained 10 trials that fulfilled the selection criteria (see Characteristics of included studies).
1.
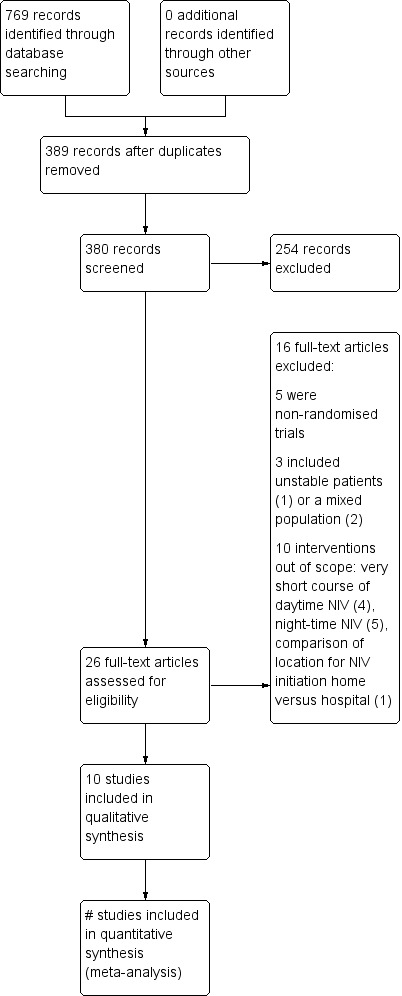
Study flow diagram.
Included studies
Source of information
In addition to the extracted data from the publications, we obtained unpublished information from one trial (Raphaël 1994).
Trial centres and design
There was only one multicentre trial, involving 17 hospitals in France (Raphaël 1994). This trial was performed on two parallel groups. Four were small, single‐centre, cross‐over studies (Jaye 2009; Laserna 2003; Tuggey 2005; Willson 2004).
Age of participants
Two trials enrolled both children and adults (Raphaël 1994; Ward 2005). All remaining trials enrolled only adults.
Trial populations
Three trials included only participants with motor neuron diseases (Bourke 2006; Jackson 2001; Pinto 1995). Three trials included only participants with chest wall deformities (Laserna 2003; Struik 2011; Tuggey 2005). One trial included only participants with DMD (Raphaël 1994). All remaining trials enrolled a mixed population of congenital or acquired neuromuscular diseases or chest wall deformation. All trials included only participants with stable chronic hypoventilation. One trial included both asymptomatic and symptomatic chronic hypoventilation (Raphaël 1994). Contact with the primary trial author resulted in provision of individual data showing that 19 of the 70 participants had symptoms of nocturnal hypoventilation or daytime hypercapnia, 10 in the ventilation group and nine in the control group. Individual data from these 19 participants were available.
Control intervention
None of the studies used sham mechanical ventilation. The control arm was spontaneous ventilation in all except four trials (Jaye 2009; Struik 2011; Tuggey 2005; Willson 2004). In the first trial, the authors compared two different modalities for bilevel positive airway pressure (Jaye 2009). In two trials, the authors compared volume‐targeted to pressure‐targeted non‐invasive ventilation (Struik 2011; Tuggey 2005). In the last trial, the authors compared two different interfaces (nasal versus full face mask) to deliver bilevel positive airway pressure (Willson 2004). In all trials, co‐interventions included, as needed, oxygen, physiotherapy, antibiotics and other palliative care measures.
Nocturnal mechanical ventilation
The experimental treatments were volume‐cycle positive intermittent ventilation in two studies (Laserna 2003; Raphaël 1994), and BiPAP in all remaining trials. One study had three arms (one control arm and two experimental arms) (Laserna 2003).
Three trials compared volume‐cycle versus BiPAP (Laserna 2003; Struik 2011; Tuggey 2005).
Outcomes
Mortality at one year was reported in four trials (Bourke 2006; Pinto 1995; Raphaël 1994; Ward 2005).
Unplanned hospital admission was reported in two trials (Raphaël 1994; Ward 2005).
Four trials reported short‐term effects of interventions on arterial blood gas, pulmonary function tests and sleep studies (Jackson 2001; Jaye 2009; Laserna 2003; Tuggey 2005). One study reported only arterial blood gas at hospital discharge and at three months (Struik 2011). We extrapolated short‐term (within one month) values from the three‐month values. One study reported only short‐term effects on sleep studies (Willson 2004).
Long‐term effects of intervention on clinical symptoms of nocturnal hypoventilation were reported in three studies (Jackson 2001; Pinto 1995; Raphaël 1994). Five trials reported long‐term effects of interventions on arterial blood gas, pulmonary function, sleep studies and quality of life (Bourke 2006; Jackson 2001; Pinto 1995; Raphaël 1994; Ward 2005).
Qualitative analysis of included trials
Four were cross‐over trials. A first study compared one‐month effects of volume‐cycle nocturnal intermittent positive ventilation to pressure‐cycle ventilation (i.e. BiPAP) in people with kyphoscoliosis‐related chronic hypoventilation (Laserna 2003). This study showed that, compared to baseline, both modes of nocturnal ventilation improved daytime arterial blood gas and nocturnal mean arterial oxygen saturation. Another study compared the short‐term effects of nocturnal BiPAP delivered through a nasal mask or through a full face mask in people with symptomatic chronic hypoventilation (Willson 2004). This study showed that nocturnal BiPAP was equally effective on nocturnal mean oxygen saturation and nocturnal transcutaneous CO2 tension. This study did not assess daytime or long‐term effects of ventilation. Another study compared a one‐month treatment with two different modes of pressure support ventilation with or without automatic titration in 20 participants with neuromuscular disorders (Jaye 2009). This study showed comparable effects of the two modes of ventilation on nocturnal oxygenation and sleep quality. However, there was a small but significant increase in nocturnal arterial CO2 tension with automatic pressure support titration compared with standard pressure support ventilation (mean transcutaneous CO2 tension: MD ‐0.80, 95% CI ‐1.18 to ‐0.42). The last cross‐over trial compared a one‐month treatment with volume‐cycled or pressure‐cycled nocturnal non‐invasive ventilation in 13 participants with chest wall deformities (Tuggey 2005). This trial found comparable effects of the two modes of ventilation on daytime arterial gas exchange, sleep studies, pulmonary function, psychometric tests and health status.
Six studies were conducted on parallel groups, one in DMD (Raphaël 1994), three in motor neuron disease (Bourke 2006; Jackson 2001; Pinto 1995), one in people with chest wall deformities (Struik 2011), and one in people with various congenital neuromuscular and chest wall diseases (Ward 2005). The first was a multicentre study and included 70 participants (Raphaël 1994). Contact with the primary author resulted in provision of individual data showing that 19 of the 70 participants had symptoms of nocturnal hypoventilation or daytime hypercapnia, 10 in the ventilation group and nine in the control group. Individual data from these 19 participants were available. This study compared nocturnal intermittent positive ventilation versus supportive treatment, i.e. physiotherapy and antibiotics if needed. This study showed that nocturnal mechanical ventilation improved daytime arterial blood gases in the short term. At one year, nocturnal mechanical ventilation improved symptoms related to nocturnal hypoventilation, tended to improve daytime arterial blood gases and had no significant effect on FVC. At one year, one of the 10 participants in the experimental group died, and none of the nine participants in the control group. This study provided no data on sleep studies. The second study included 20 participants in a single centre, 10 in the experimental group, who received nocturnal BiPAP, and 10 in the control group, who received oxygen, bronchodilators and other palliative measures (Pinto 1995). This study provided no data on short‐term effects of nocturnal ventilation. This study showed at one year a significant difference in survival rate in favour of nocturnal ventilation and no significant difference for daytime arterial blood gases and FVC between nocturnal ventilation and supportive treatment. This study provided no data on symptoms related to nocturnal hypoventilation or on sleep studies. The third study included 13 participants with ALS, an FVC of 70% or more, and nocturnal oxygen desaturation of at least one minute or at least two clinical signs of chronic hypoventilation (Jackson 2001). Participants were followed up every three months on the basis of ALS functional rating scale‐respiratory, pulmonary symptom scale, Short Form (SF) 36, pulmonary function testing and nocturnal oxymetry. In this small‐sized study, nocturnal positive pressure ventilation improved the vitality subscale of SF‐36, the mean pulmonary symptom score (before treatment of 72.7 versus after treatment of 80.8, P value = 0.04). The fourth study included 41 participants with ALS and either orthopnoea with maximum inspiratory pressure less than 60% of that predicted or symptomatic hypercapnia (Bourke 2006). Twenty‐two participants were randomly assigned to nocturnal BiPAP and 19 to standard care, that is physiotherapy and assisted cough techniques as needed. In both groups participants received pneumococcal and annual influenza vaccines and had access to palliative care, including hospice facilities, whenever needed. This study showed that nocturnal ventilation improved survival and prolonged quality of life on several scales including the generic SF‐36, and two specific scales, namely the symptoms domain of the sleep apnoea quality of life index and the chronic respiratory disease questionnaire. This study provided no data on short‐term effects of nocturnal ventilation, and no data on arterial blood gas, lung function or sleep studies. The fifth study involved 16 participants with chest wall deformities and compared the effects of volume‐cycled (nine participants) with pressure‐cycled non‐invasive ventilation (seven participants) on the time to achieve normalisation of daytime arterial CO2 tension (Struik 2011). Four participants were lost to follow‐up in the volume‐cycled non‐invasive ventilation arm and one was lost to follow‐up in the pressure‐cycled non‐invasive ventilation arm. Per protocol analysis showed a significant drop of PaCO2 at hospital discharge in the pressure‐cycled non‐invasive ventilation arm (5.3 ± 1.2 versus 7.6 ± 1.2 kPa) but not in the volume‐cycled non‐invasive ventilation arm (6.4 ± 0.9 versus 7.2 ± 0.7 kPa). At three months, PaCO2 dropped significantly from baseline in both arms, 5.6 ± 1.3 versus 7.6± 1.2 kPa and 5.3 ± 1.3 versus 7.2 ± 0.7 kPa. This study provided no information on mortality, unplanned hospitalisation, clinical symptoms, pulmonary function tests or sleep studies.
In the last study, 26 participants with chronic nocturnal hypoventilation and normal daytime CO2 tension were randomly assigned to nocturnal bilevel airway positive pressure (n = 12) or to standard care (n = 14), that is physiotherapy and antibiotics if needed (Ward 2005). Participants were followed up for 24 months. This study showed that nocturnal BiPAP reduced time spent with nocturnal hypercapnia and improved mean nocturnal oxygen saturation. There was no significant difference between groups in daytime arterial CO2 tension variations over time. There were more participants in the control group that met the criteria for mandatory ventilation than in the experimental group. Finally, compared with controls, a gain in SF‐36 general health score was seen in the experimental group by 18 months. In addition, in this study, participants with daytime hypercapnia at the time of randomisation were not included but were similarly followed up. These participants had significant reductions in daytime mean arterial CO2 tension with no significant changes in lung function.
Excluded studies
Five of the 10 excluded studies were non‐randomised studies (Aboussouan 1997; Bach 1998; Fanfulla 2005; Masa 1997; Padman 1998). One study included participants in an unstable condition (i.e. acute respiratory failure) (Celikel 1998). Four studies assessed the effects of a very short course of mechanical ventilation during the daytime and provided no data on nocturnal mechanical ventilation (Ambrosino 1997; Elliott 1994; Hart 2002; Meecham Jones 1993). Five trials compared a short course (one to three nights) of different modes of non‐invasive ventilation with a control night on spontaneous breathing (Ellis 1987; Restrick 1993), or without such a control night (Crescimanno 2011; Highcock 2002; Orlikowski 2009). Another trial compared initiation of non‐invasive nocturnal ventilation at home versus at hospital in people with neuromuscular or chest wall diseases and chronic hypoventilation (Chatwin 2008).
Risk of bias in included studies
The detailed risk of bias in the included trials is reported in the 'Risk of bias' tables (Characteristics of included studies) and in Figure 2.
2.
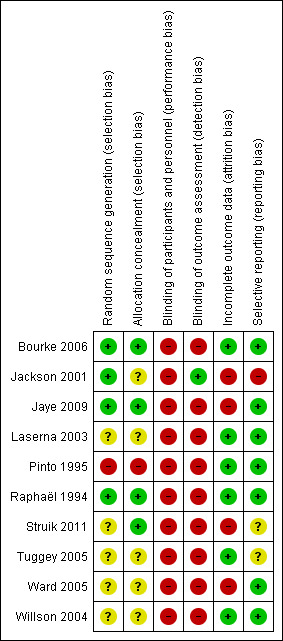
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In four trials, we considered allocation concealment adequate (Bourke 2006; Jaye 2009; Raphaël 1994; Struik 2011). In one trial, participants were alternately assigned to nocturnal BiPAP or supportive treatment and we considered the randomisation concealment inadequate (Pinto 1995). In the remaining trials allocation concealment was unclear.
Blinding
Observer blinding was intended in only one study (Jackson 2001). In one trial, participants were blinded to the intervention they received, i.e. volume‐cycled or pressure‐cycled non‐invasive ventilation (Tuggey 2005). In the remaining trials, participant blinding was felt not to be feasible and none of trials used sham mechanical ventilation.
Incomplete outcome data
In all trials the baseline clinical features were similar in each group. In one trial, two of 12 participants in the experimental group and two of 14 in the control group were lost to follow‐up (Ward 2005). In a second trial, one participant in the experimental arm was lost to follow‐up (Jackson 2001). In a third trial that compared volume‐cycled non‐invasive ventilation to pressure‐cycled non‐invasive ventilation, five participants were lost to follow‐up, four in the volume‐cycled non‐invasive ventilation arm and one in the pressure‐cycled non‐invasive ventilation arm (Struik 2011). Two participants did not tolerate volume‐cycled non‐invasive ventilation and were switched to pressure‐cycled non‐invasive ventilation after two days following randomisation. One participant in each arm died and one participant in the volume‐cycled non‐invasive ventilation arm did not want to return for measurements in hospital at three months. In all other trials, follow‐up was complete and adherence to treatment was good.
Selective reporting
We assessed the trials as at low risk of bias from selective reporting with the exception of three trials. Jackson 2001 was at high risk of bias because the investigators reported only preliminary results. Two trials did not provide information allowing us to assess reporting bias and we considered them at unclear risk (Struik 2011; Tuggey 2005).
Explicit definition
All trials used explicit and internationally accepted diagnostic criteria for DMD, other congenital neuromuscular disease, motor neuron diseases and chest wall disease. All trials used explicit criteria to define chronic nocturnal hypoventilation.
Effects of interventions
Nocturnal ventilation versus no ventilation
Primary outcome measure: mortality at one year
Four studies reported the one‐year mortality rate (Bourke 2006; Pinto 1995; Raphaël 1994; Ward 2005). In Raphaël 1994, one out of 10 participants died in the treatment group compared to none of the control group. In Pinto 1995, three out of 10 participants died in the treatment group compared to nine out of 10 in the control group. In Bourke 2006, 13 out of 22 participants died in the treatment group compared to 16 out of 19 in the control group. In Ward 2005, after 24 months of follow‐up there were no reported deaths. In the pooled analysis, 17 deaths occurred among 51 participants in the nocturnal ventilation group versus 25 deaths among 48 participants in the control group, resulting in a risk ratio (RR) of dying of 0.62 (95% confidence interval (CI) 0.42 to 0.91, P value = 0.01) in favour of the experimental arm (Analysis 1.1). There was some heterogeneity across the studies (I2 = 30%) that may be explained by differences in the rate of disease progression, which was faster for participants with hypercapnic amyotrophic lateral sclerosis (ALS) (Bourke 2006; Pinto 1995) than in participants with Duchenne muscular dystrophy (DMD) (Raphaël 1994) or other slowly progressive diseases (Ward 2005). It was also the case that observed mortality rates were higher in the two studies conducted in ALS participants than in the two other trials.
1.1. Analysis.
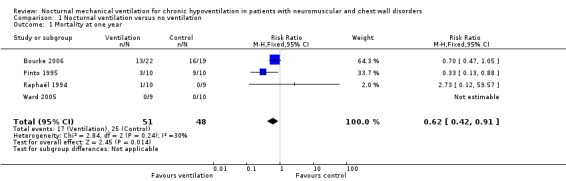
Comparison 1 Nocturnal ventilation versus no ventilation, Outcome 1 Mortality at one year.
Secondary outcome measures
Admission to hospital (other than routine visits)
Two studies, accounting for 38 participants, reported this outcome in the long term (Raphaël 1994; Ward 2005). Two of 19 participants in the nocturnal ventilation group had unplanned hospital admission versus 10 of 19 in the control group, resulting in a RR of 0.25 (95% CI 0.08 to 0.82, P value = 0.02) (Analysis 1.2). There was no heterogeneity across the studies (I2 = 0%).
1.2. Analysis.
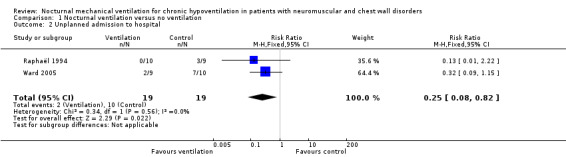
Comparison 1 Nocturnal ventilation versus no ventilation, Outcome 2 Unplanned admission to hospital.
Reversal of daytime hypoventilation‐related clinical symptoms
None of the trials provided data for this outcome in the short term.
Three trials, accounting for 51 participants, reported this outcome in the long term (Jackson 2001; Raphaël 1994; Ward 2005). In Raphaël 1994, four out of 10 participants in the treatment group did not have improved clinical symptoms at one year compared to seven out of nine participants in the control group. The estimated risk of "no improvement of hypoventilation related clinical symptoms" in this study was not significant (RR 0.51, 95% CI 0.22 to 1.19). Jackson 2001 reported failure to improve hypoventilation‐related clinical symptoms at three months in three out of six participants in the experimental arm and all seven controls. In this study, the pulmonary symptom score improved from baseline in the experimental arm (80.8 versus 72.7, P value = 0.04) (RR 0.53, 95% CI 0.25 to 1.14). In Ward 2005, at 24 months there was an absence of improvement in clinical symptoms in none of the nine treated participants and nine of 10 participants in the control arm (RR 0.06, 95% CI 0.00 to 0.87).
Combining these three trials, seven of 25 participants in the nocturnal ventilation group did not experience improvement in hypoventilation‐related symptoms versus 23 of 26 participants in the control group. The RR was 0.43 (95% CI 0.18 to 1.03, P value = 0.06) (Analysis 1.3).
1.3. Analysis.

Comparison 1 Nocturnal ventilation versus no ventilation, Outcome 3 No improvement of hypoventilation symptoms: long‐term.
Reversal of daytime hypercapnia
One study provided data on this outcome in the short term (Raphaël 1994). Three out of seven participants in the treatment group did not have improved daytime hypercapnia compared to nine out of nine in the control group (RR 0.33, 95% CI 0.14 to 0.80, P value = 0.01) (see Analysis 1.4).
1.4. Analysis.
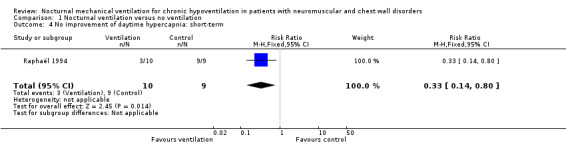
Comparison 1 Nocturnal ventilation versus no ventilation, Outcome 4 No improvement of daytime hypercapnia: short‐term.
Three studies, accounting for 58 participants, reported the proportion of participants who did not experience an improvement in daytime hypercapnia (Pinto 1995; Raphaël 1994; Ward 2005). There were 14 of 29 such participants in the experimental arm versus 24 of 29 in the control arm. The pooled RR was 0.59 (95% CI 0.41 to 0.86, P value = 0.006) in favour of nocturnal ventilation (Analysis 1.5). There was significant heterogeneity across the studies (I² = 94%). This heterogeneity may be explained by differences in the speed of disease progression: slow in two trials (Raphaël 1994; Ward 2005) versus rapid in one trial (Pinto 1995).
1.5. Analysis.
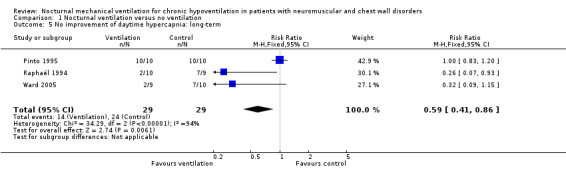
Comparison 1 Nocturnal ventilation versus no ventilation, Outcome 5 No improvement of daytime hypercapnia: long‐term.
However, at one year, from 60 participants in these three studies (Pinto 1995; Raphaël 1994; Ward 2005), the mean difference (MD) of the arterial CO2 tension was ‐0.26 kPa (95% CI ‐0.61 to 0.09 kPa), which means that it was lower in the treated group but not significantly (see Analysis 1.6).
1.6. Analysis.
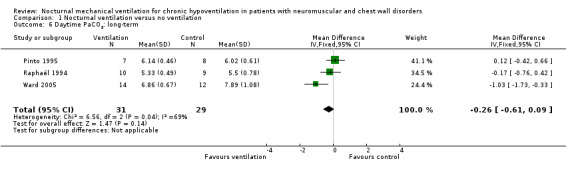
Comparison 1 Nocturnal ventilation versus no ventilation, Outcome 6 Daytime PaCO2: long‐term.
Lung function measurements
None of the studies assessed this outcome in the short term. Two studies reported one‐year values for forced vital capacity (FVC) (Pinto 1995; Raphaël 1994), for 13 participants in the treated group and 15 participants in the control group. The MD of the FVC in these two trials was 159 mL (95% CI ‐84 to 401 mL), non‐significantly more in the treated than the control group (see Analysis 1.7). At 24 months of follow‐up, one study showed no significant difference in changes in sniff nasal inspiratory pressure (SNIP), which was 1.62 (6.1) in the control group versus ‐4.1 (14.1) cmH2O in the treated group (see Analysis 1.8), or changes in cough peak flow, which was 43.3 (52.2) L/min in the control group versus ‐0.9 (34.4) L/min in the treated group (Ward 2005). The other subcategory outcome, namely ventilation‐perfusion mismatch, was not available.
1.7. Analysis.
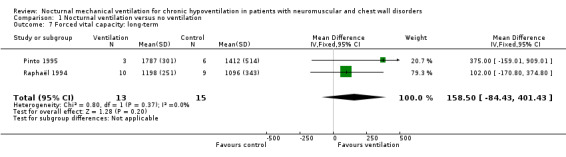
Comparison 1 Nocturnal ventilation versus no ventilation, Outcome 7 Forced vital capacity: long‐term.
1.8. Analysis.
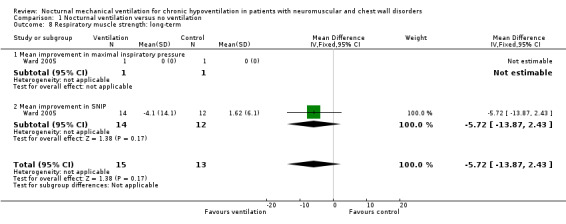
Comparison 1 Nocturnal ventilation versus no ventilation, Outcome 8 Respiratory muscle strength: long‐term.
Sleep studies
One study assessed this outcome in the long term, at 24 months, in 14 treated and 12 control participants (Ward 2005). This study showed that mean nocturnal oxygen saturation increased from 93.5 (1.9)% to 96.6 (1.2)% in the treated group versus 95.2 (2.1)% to 93.6 (1.7)% in the control group, and the MD of nocturnal oxygen saturation in this study was 3.00% (95% CI 1.85% to 4.15%) (see Analysis 1.9). There was no significant difference between the two groups for the minimum overnight oxygen saturation.
1.9. Analysis.
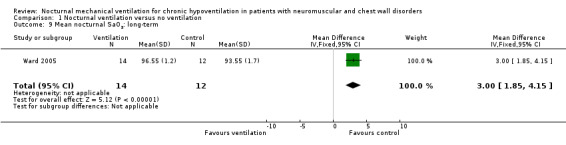
Comparison 1 Nocturnal ventilation versus no ventilation, Outcome 9 Mean nocturnal SaO2: long‐term.
Quality of life
In a small‐sized study, nocturnal positive pressure ventilation improved the vitality subscale of SF‐36, the mean pulmonary symptom score (before treatment of 72.7 versus after treatment of 80.8, P value = 0.04) (Jackson 2001). In a second trial of 41 participants with ALS, nocturnal ventilation improved survival and prolonged quality of life on several scales including the generic SF‐36, and two specific scales, namely the symptoms domain of the sleep apnoea quality of life index and the chronic respiratory disease questionnaire (Pinto 1995).
Invasive ventilation versus non‐invasive ventilation
We found no study that compared invasive to non‐invasive ventilation.
Intermittent positive pressure ventilation versus negative pressure ventilation
We found no study that compared invasive ventilation versus non‐invasive ventilation.
Volume‐cycled versus pressure‐cycled ventilation
Primary outcome: mortality at one year
We found only one study of volume‐cycled versus pressure‐cycled ventilation, which provided mortality data after three months following randomisation (Struik 2011). In this small (16 participants), single‐centre trial only two deaths were reported, one in each group (see Analysis 2.1).
2.1. Analysis.
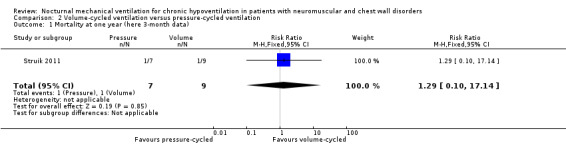
Comparison 2 Volume‐cycled ventilation versus pressure‐cycled ventilation, Outcome 1 Mortality at one year (here 3‐month data).
Secondary outcome measures
Admission to hospital (other than routine visits)
This outcome was not available.
Reversal of daytime hypoventilation‐related clinical symptoms
This outcome was not available.
Reversal of daytime hypercapnia
One study reported in the short term, i.e. at one month, that six of 10 participants in the pressure‐cycled non‐invasive ventilation group and six of 10 participants in the volume‐cycled non‐invasive ventilation group did not improve daytime hypercapnia (RR 1.00, 95% CI 0.49 to 2.05, P value = 1.00) (see Analysis 2.2) (Laserna 2003).
2.2. Analysis.
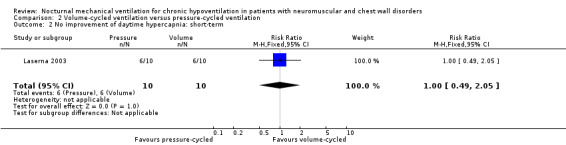
Comparison 2 Volume‐cycled ventilation versus pressure‐cycled ventilation, Outcome 2 No improvement of daytime hypercapnia: short‐term.
Three studies, accounting for 39 participants, reported daytime arterial oxygen tension after one month of intervention (Laserna 2003; Struik 2011; Tuggey 2005). The MD in arterial CO2 tension was 0.45 kPa (95% CI ‐0.21 to 1.10, P value = 0.18) in favour of volume‐cycled ventilation with significant heterogeneity across the studies (I2 = 93%) (see Analysis 2.3).
2.3. Analysis.
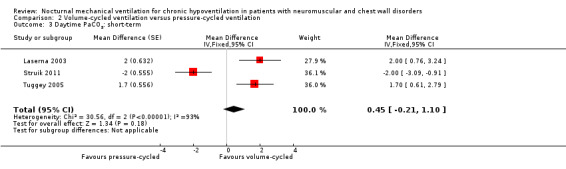
Comparison 2 Volume‐cycled ventilation versus pressure‐cycled ventilation, Outcome 3 Daytime PaCO2: short‐term.
Lung function measurements
This outcome was not available in the long term.
One trial, which was a cross‐over study with 13 participants, reported in the short term (at one month) (Tuggey 2005). The trial found no significant difference between pressure‐ and volume‐cycled non‐invasive ventilation for FVC with a mean difference of 40.00% (95% CI ‐7.04 to 87.04, P value = 0.10) (see Analysis 2.4), maximal inspiratory pressure (mean difference ‐2.20 cmH2O (95% CI ‐4.92 to 0.52, P value = 0.11) and sniff nasal inspiratory pressure (SNIP) (mean difference ‐1.70 cm H2O (95% CI ‐4.70 to 1.30, P value = 0.06) (see Analysis 2.5).
2.4. Analysis.
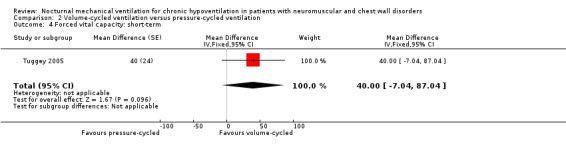
Comparison 2 Volume‐cycled ventilation versus pressure‐cycled ventilation, Outcome 4 Forced vital capacity: short‐term.
2.5. Analysis.
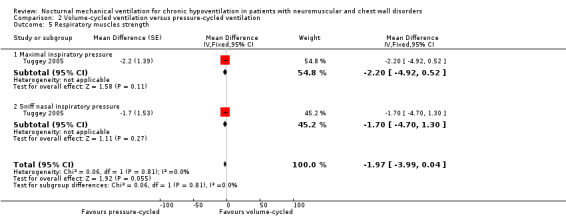
Comparison 2 Volume‐cycled ventilation versus pressure‐cycled ventilation, Outcome 5 Respiratory muscles strength.
Sleep studies
One study, which was a cross‐over study with 13 participants, provided treatment effects on mean nocturnal SaO2 in the short term (Tuggey 2005). There was no difference between pressure‐ and volume‐cycled non‐invasive ventilation, with a mean difference of 1.20 (95% CI ‐0.70 to 3.10, P value = 0.22) (see Analysis 2.6).
2.6. Analysis.
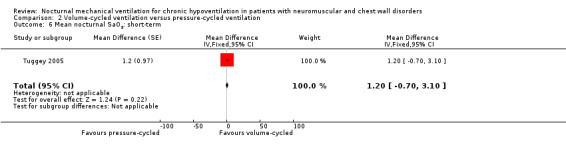
Comparison 2 Volume‐cycled ventilation versus pressure‐cycled ventilation, Outcome 6 Mean nocturnal SaO2: short‐term.
Two studies, accounting for 23 participants, reported treatment effects on the time spent with nocturnal SaO2 below 90%, in the short term (Laserna 2003; Tuggey 2005). The MD was 6.83 minutes (95% CI 4.68 to 8.98 minutes, P value < 0.00001) in favour of the volume‐cycled non‐invasive ventilation. However, there was strong heterogeneity across the studies (I2 = 99%) (see Analysis 2.7).
2.7. Analysis.
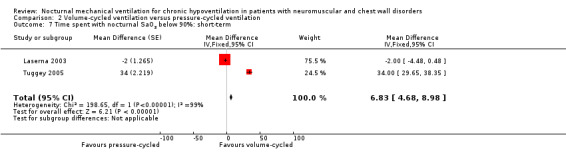
Comparison 2 Volume‐cycled ventilation versus pressure‐cycled ventilation, Outcome 7 Time spent with nocturnal SaO2 below 90%: short‐term.
Two studies, accounting for 23 participants, reported the apnea‐hypopnoea index per hour of sleep in the short term (Laserna 2003; Tuggey 2005). The MD was ‐0.65 (95% CI ‐0.84 to ‐0.46, P value < 0.00001) in favour of the volume‐cycled non‐invasive ventilation. There was no heterogeneity across the studies (I2 = 0%) (see Analysis 2.8).
2.8. Analysis.
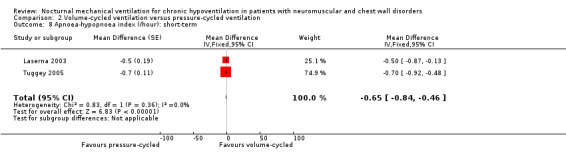
Comparison 2 Volume‐cycled ventilation versus pressure‐cycled ventilation, Outcome 8 Apnoea‐hypopnoea index (/hour): short‐term.
Quality of life
In one study, which was a cross‐over study with 13 participants, there were no differences between volume‐cycled and pressure‐cycled ventilated patients in any of the components of the SF‐36 scale.
Discussion
A huge number of reports of uncontrolled trials claim beneficial effects of nocturnal mechanical ventilation for chronic hypoventilation related to neuromuscular or chest wall disorders (Claman 1996; Make 1998). By contrast, we found a limited number of randomised studies. Only one study was a multicentre trial (Raphaël 1994). This trial enrolled 70 participants with Duchenne muscular dystrophy (DMD) during a five‐year period. The study was designed to assess whether implementation of nocturnal mechanical ventilation early in the course of the disease prevents respiratory failure and prolongs survival. At randomisation, only 19 out of the 70 participants met the criteria currently proposed for long‐term mechanical ventilation, namely symptoms of nocturnal hypoventilation or daytime hypercapnia (Make 1998). Three studies were based on a cross‐over design and the population size was very small (Laserna 2003; Tuggey 2005; Willson 2004). The allocation concealment was adequate in only four of the 10 studies. Thus, the overall methodological quality of these trials was weak.
This systematic review suggests that, in people with chronic hypoventilation related to neuromuscular or chest wall diseases, nocturnal mechanical ventilation improves hypoventilation‐related clinical symptoms, nocturnal mean oxygen saturation, daytime hypercapnia and quality of life, and reduces the risk of unplanned hospitalisation and mortality, compared to no ventilation. However, taking into account the small number of participants, the lack of observer blinding and the presence of significant heterogeneity across the studies, any conclusions must be drawn very cautiously. Data on the effects of nocturnal mechanical ventilation on lung function measurements and on sleep studies are too scarce to allow any definite conclusion. A large, Italian, single‐centre, retrospective cohort study of patients with DMD showed a progressive increase in crude survival (Passamano 2012). The survival rate in DMD at the age of 20 years was 23.3% in the 1960s, 54% in patients born in the 1970s and 59.8% in those born in the 1980s (P value < 0.001). In this observational study, the crude mean age for respiratory‐related death was 17.7 years in patients who did not receive mechanical ventilation and 27.9 years in mechanically ventilated patients (P value < 0.001). Similar findings in patients with DMD were found in other, single‐centre, retrospective observational studies from the United Kingdom (Eagle 2002), the United States of America (Bach 2011), Canada (McKim 2013), Belgium (Toussaint 2006), and Germany (Wollinsky 2012). An observational study of 144 children (47% with neuromuscular disorders, including DMD, spinal muscular atrophy (SMA) type 1 and type 2, and congenital myopathy) showed 94% and 91% survival rates at 5 and 10 years, respectively, with long‐term mechanical ventilation (McDougall 2013).
It seems likely that hypoventilation‐related symptoms may reflect different degrees of respiratory failure more in rapidly progressive diseases than in less rapidly progressive neuromuscular disorders. Subsequently, the effects of nocturnal mechanical ventilation should be different. So, it seems likely that the criteria for nocturnal mechanical ventilation should differ in rapidly progressive and less rapidly progressive neuromuscular disorders (Raphaël 1999). Similarly, correcting nocturnal hypoventilation in people with diurnal hypercapnia may yield a more rapid, clinically evident benefit than in people with normal daytime hypercapnia.
Data from two small cross‐over studies showed that volume‐cycled and pressure‐cycled ventilation improved symptoms related to chronic hypoventilation and daytime hypercapnia significantly and similarly, at least in the short term (Laserna 2003; Tuggey 2005). Volume‐cycled non‐invasive ventilation may be associated with less nocturnal desaturation and apneas. However, taking into account the small number of participants, any conclusion should be drawn very cautiously.
There were no available data from randomised trials to decide on comparisons between invasive ventilation and non‐invasive ventilation, or between intermittent positive ventilation and negative pressure ventilation. Observational studies have suggested that, in DMD with nocturnal mechanical ventilation and diurnal hypercapnia, daytime non‐invasive ventilation may prolonged survival and may be safe even when patients required 24‐hour continuous respiratory support, thus preventing tracheostomy (Bach 2011; McKim 2013; Toussaint 2006). Likewise, an observational study of 144 children requiring long‐term respiratory support, including patients with DMD (n = 18), SMA type 1 (n = 6) and type 2 (n = 14), and other congenital myopathies (n = 18), showed that five‐year survival was 94% overall and was significantly higher for patients on non‐invasive ventilation (97%) than invasively ventilated patients (84%) (McDougall 2013). Another study of 194 infants with SMA type 1, found that in comparison with invasive mechanical ventilation, non‐invasive ventilation with mechanical cough assist was associated with lower survival rates at 24 months of life (68% versus 95%, P value < 0.001) and at 48 months of life (45% versus 89%, P value < 0.001) (Gregoretti 2013).
The lack of assessment of adverse events associated with long‐term mechanical ventilation is a limitation of the current review. Unpublished information was available for only one trial. Subsequently, there were missing data for several outcomes of interest.
Authors' conclusions
Implications for practice.
Current evidence about the therapeutic benefit of mechanical ventilation is of very low quality, but directionally consistent, suggesting alleviation of the symptoms of chronic hypoventilation, improvement in survival and reduced risk of unplanned hospitalisation.
Implications for research.
Large randomised trials are needed to confirm the possible long‐term beneficial effects of nocturnal mechanical ventilation on hypoventilation‐related symptoms, quality of life, unplanned hospital admission rate and mortality, and to evaluate its cost‐effectiveness in people with chest wall disorders and in people with neuromuscular diseases other than motor neuron disease or Duchenne muscular dystrophy, because natural history studies have shown substantial improvements in survival. Future trials should be stratified according to the type of disease and whether the course is rapidly progressive or not. The comparisons between invasive and non‐invasive ventilation, and between volume‐cycled and pressure‐cycled ventilation, also require well‐designed, multicentre, randomised trials. Finally, particular attention may be given to new modes of ventilation, such as auto‐titrating pressure support devices, which are increasingly used.
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2014 | New search has been performed | The review has been updated, with inclusion of new trials We have re‐arranged the outcomes to move up mortality at one year (or more) to be the primary outcome We have excluded trials that have investigated short‐course and short‐term effects (from a few minutes to a few nights) as this review aims to summarise the effects of long‐term mechanical ventilation |
| 8 August 2013 | New citation required but conclusions have not changed | We have included two new trials |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 20 June 2008 | Amended | Converted to new review format. |
| 21 August 2007 | New citation required and conclusions have changed | The searches for MEDLINE, EMBASE and the Cochrane Neuromuscular Disease Group Trials Register were updated in June 2006. Four new trials were identified. |
Acknowledgements
We acknowledge the work of JC Chevrolet and the late JC Raphael on earlier versions of this review.
The authors thank Miss S. Loiseau from the Association Française de lutte contre la Myopathie for her technical assistance in searching for articles.
The Cochrane Neuromuscular Disease Group (CNMDG) Trials Search Co‐ordinator, Angela Gunn, provided searches.
The editorial base of the CNMDG receives funding from the MRC Centre for Neuromuscular Diseases and the Motor Neuron Disease Association.
Appendices
Appendix 1. NMD Register (CRS) search strategy
#1 MeSH DESCRIPTOR Musculoskeletal Diseases Explode All [REFERENCE] [STANDARD] #2 MeSH DESCRIPTOR Nervous System Diseases Explode All [REFERENCE] [STANDARD] #3 "central nervous" or neuromuscular or muscular or motor or musculo* or musculoskeletal* or bone* or "chest wall" [REFERENCE] [STANDARD] #4 #1 or #2 or #3 [REFERENCE] [STANDARD] #5 Hypoventilat* or ((gas or oxygen* or "carbon dioxide") and tension*) [REFERENCE] [STANDARD] #6 (respirat* or breath* or ventilat*) NEAR10 (insufficienc* or failur* or deficienc* or disorder* or difficult* or depress*) [REFERENCE] [STANDARD] #7 #5 or #6 [REFERENCE] [STANDARD] #8 MeSH DESCRIPTOR Ventilators, Mechanical Explode All [REFERENCE] [STANDARD] #9 MeSH DESCRIPTOR Respiration, Artificial Explode All [REFERENCE] [STANDARD] #10 ventilat* or NIPPV or ((positiv* or negativ* or inspirator*) NEAR10 pressur*) [REFERENCE] [STANDARD] #11 #8 or #9 or #10 [REFERENCE] [STANDARD] #12 #4 and #7 and #11 [REFERENCE] [STANDARD] #13 (#4 and #7 and #11) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Musculoskeletal Diseases] explode all trees
#2 MeSH descriptor: [Nervous System Diseases] explode all trees
#3 "central nervous system" or neuromuscular* or muscul* or motor or musculoskeletal or bone* or chest wall
#4 #1 or #2 or #3
#5 (Hypoventilat* or ((oxygen* or carbon dioxid*) and tensio*))
#6 ((respirat* or breath* or ventilat*) near/10 (insufficienc* or failur* or deficienc* or disorder* or difficult* or depress*))
#7 #5 or #6
#8 MeSH descriptor: [Ventilators, Mechanical] explode all trees
#9 MeSH descriptor: [Respiration, Artificial] this term only
#10 ventilat* or NIPPV or ((positiv* or negativ* or inspirator*) and pressur*)
#11 #8 or #9 or #10
#12 #4 and #7 and #11
Appendix 3. MEDLINE (OvidSP) Search Strategy
Database: Ovid MEDLINE(R) <1946 to May Week 4 2014>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 randomized controlled trial.pt. (374960)
2 controlled clinical trial.pt. (88427)
3 randomized.ab. (273382)
4 placebo.ab. (146420)
5 drug therapy.fs. (1704068)
6 randomly.ab. (193925)
7 trial.ab. (283482)
8 groups.ab. (1246094)
9 or/1‐8 (3198880)
10 exp animals/ not humans.sh. (3947165)
11 9 not 10 (2722297)
12 exp musculoskeletal diseases/ or exp nervous system diseases/ (2679148)
13 (central nervous$ or neuromuscular$ or muscul$ or motor or skelet$ or musculoskeletal$ or bone$ or chest wall$).mp. (1487938)
14 12 or 13 (3617875)
15 (Hypoventilat$ or ((gas$2 or oxygen$ or carbon dioxid$) adj10 tension$)).mp. (19652)
16 ((respirat$ or breath$ or ventilat$) adj10 (insufficienc$ or failur$ or deficienc$ or disorder$ or difficult$ or depress$)).mp. (79973)
17 15 or 16 (97242)
18 exp Ventilators, Mechanical/ or Respiration, Artificial/ (44530)
19 (ventilat$ or ((positiv$ or negativ$ or inspirator$) and pressur$) or NIPPV).mp. (213803)
20 18 or 19 (225419)
21 11 and 14 and 17 and 20 (1592)
22 remove duplicates from 21 (1568)
Appendix 4. EMBASE (OvidSP) search strategy
Database: EMBASE <1980 to 2014 Week 23>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 crossover‐procedure.sh. (39104)
2 double‐blind procedure.sh. (113517)
3 single‐blind procedure.sh. (18341)
4 randomized controlled trial.sh. (343012)
5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1030618)
6 trial.ti. (157233)
7 or/1‐6 (1161845)
8 (animal/ or nonhuman/ or animal experiment/) and human/ (1263730)
9 animal/ or nonanimal/ or animal experiment/ (3217383)
10 9 not 8 (2697085)
11 7 not 10 (1066806)
12 limit 11 to embase (882304)
13 exp Motor Dysfunction/ (495826)
14 exp musculoskeletal disease/ or exp central nervous system disease/ or exp neuromuscular disease/ or exp neuropathy/ or exp radiculopathy/ (3197454)
15 (central nervous$ or neuromuscular$ or motor or muscular or muscul$ skelet$ or musculoskeletal$ or bone$ or chest wall$ or nocturnal hypoventilation).mp. (1921421)
16 or/13‐15 (4373364)
17 exp Hypoventilation/ (5545)
18 ((gas$2 or oxygen$ or carbon dioxid$) adj10 tension$).mp. (49894)
19 ((respirat$ or breath$) adj10 (insufficienc$ or failur$ or deficienc$ or disorder$ or difficult$)).mp. (106507)
20 sleep apnoea.mp. (6018)
21 or/17‐20 (159898)
22 exp artificial ventilation/ (118120)
23 (ventilat$ or NIPPV or ((positiv$ or negativ$ or inspirator$) adj10 pressur$)).mp. (243294)
24 22 or 23 (248409)
25 16 and 21 and 24 (12441)
26 12 and 25 (700)
27 remove duplicates from 26 (698)
Data and analyses
Comparison 1. Nocturnal ventilation versus no ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at one year | 4 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.91] |
| 2 Unplanned admission to hospital | 2 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.82] |
| 3 No improvement of hypoventilation symptoms: long‐term | 3 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.18, 1.03] |
| 4 No improvement of daytime hypercapnia: short‐term | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.80] |
| 5 No improvement of daytime hypercapnia: long‐term | 3 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.86] |
| 6 Daytime PaCO2: long‐term | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.61, 0.09] |
| 7 Forced vital capacity: long‐term | 2 | 28 | Mean Difference (IV, Fixed, 95% CI) | 158.50 [‐84.43, 401.43] |
| 8 Respiratory muscle strength: long‐term | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐5.72 [‐13.87, 2.43] |
| 8.1 Mean improvement in maximal inspiratory pressure | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Mean improvement in SNIP | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐5.72 [‐13.87, 2.43] |
| 9 Mean nocturnal SaO2: long‐term | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [1.85, 4.15] |
Comparison 2. Volume‐cycled ventilation versus pressure‐cycled ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at one year (here 3‐month data) | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.10, 17.14] |
| 2 No improvement of daytime hypercapnia: short‐term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.49, 2.05] |
| 3 Daytime PaCO2: short‐term | 3 | Mean Difference (Fixed, 95% CI) | 0.45 [‐0.21, 1.10] | |
| 4 Forced vital capacity: short‐term | 1 | Mean Difference (Fixed, 95% CI) | 40.00 [‐7.04, 87.04] | |
| 5 Respiratory muscles strength | 1 | Mean Difference (Fixed, 95% CI) | ‐1.97 [‐3.99, 0.04] | |
| 5.1 Maximal inspiratory pressure | 1 | Mean Difference (Fixed, 95% CI) | ‐2.2 [‐4.92, 0.52] | |
| 5.2 Sniff nasal inspiratory pressure | 1 | Mean Difference (Fixed, 95% CI) | ‐1.7 [‐4.70, 1.30] | |
| 6 Mean nocturnal SaO2: short‐term | 1 | Mean Difference (Fixed, 95% CI) | 1.2 [‐0.70, 3.10] | |
| 7 Time spent with nocturnal SaO2 below 90%: short‐term | 2 | Mean Difference (Fixed, 95% CI) | 6.83 [4.68, 8.98] | |
| 8 Apnoea‐hypopnoea index (/hour): short‐term | 2 | Mean Difference (Fixed, 95% CI) | ‐0.65 [‐0.84, ‐0.46] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bourke 2006.
| Methods | Single‐centre, open, parallel‐group study with a follow‐up period of at least 1 year | |
| Participants | 41 participants with ALS with or without bulbar features, meeting definite diagnostic criteria, who had orthopnoea with inspiratory maximal pressure less than 60% of predicted or symptomatic daytime hypercapnia | |
| Interventions | Control: standard care
Experimental: non‐invasive nasal nocturnal ventilation with bilevel airway pressure At least 1 year follow‐up period |
|
| Outcomes | Survival time Quality of life SF‐36 mental component summary Sleep apnoea quality of life index symptoms domain |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Computer‐generated list; centralised randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No party was blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessor was not blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes presented in the results section matched those reported in the methods |
Jackson 2001.
| Methods | Single‐centre, single‐blind study, randomisation stratified according to bulbar versus limb onset | |
| Participants | 13 adults with ALS of less than 3 years duration, with an FVC ≥ 70% and nocturnal oxygen desaturation for ≥ 1 minute or at least 2 clinical symptoms of chronic hypoventilation | |
| Interventions | Control: no respiratory support until FVC fell below 50% Experimental: nocturnal positive pressure ventilation |
|
| Outcomes | No clear primary outcome
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes were assessed by a blinded clinical evaluator |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One participant out of 7 in the experimental arm was lost to follow‐up at 3 months |
| Selective reporting (reporting bias) | High risk | Only preliminary data were published |
Jaye 2009.
| Methods | Single‐centre, randomised, cross‐over trial | |
| Participants | 25 adults meeting the following criteria:
|
|
| Interventions | Control: 1‐month treatment with conventional nocturnal positive pressure support ventilation Experimental: 1‐month treatment with auto‐titrated nocturnal positive pressure ventilation |
|
| Outcomes | Primary outcome: mean overnight oxygen saturation Secondary outcomes:
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Centralised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessor was not blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 out of 25 randomised participants were dropped from the analysis for incomplete data |
| Selective reporting (reporting bias) | Low risk | Outcomes presented in the results section matched those reported in the methods |
Laserna 2003.
| Methods | Single‐centre, open, cross‐over study | |
| Participants | 10 participants with kyphoscoliosis and chronic hypercapnic respiratory failure | |
| Interventions | Control: spontaneous ventilation Experimental 1: nocturnal ventilation with bilevel airway pressure Experimental 2: nocturnal volume‐cycle ventilation | |
| Outcomes | 1‐month improvement in sleep parameters, clinical symptoms and arterial blood gas | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was not provided |
| Allocation concealment (selection bias) | Unclear risk | Information was not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No party was blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessor was not blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes presented in the results section matched those reported in the methods |
Pinto 1995.
| Methods | Single‐centre, open, parallel‐group study with a 3‐year follow‐up period | |
| Participants | 20 participants having ALS with bulbar features (definite diagnostic criteria) and with chronic hypercapnic respiratory failure | |
| Interventions | Control: oxygen, bronchodilators and other palliative measures Experimental group: non‐invasive nasal nocturnal ventilation with bilevel airway pressure | |
| Outcomes | Mortality at 1 and 3 years Survival time Bulbar and spinal Norris scores Quality of life Modified Barthel score Arterial blood gases Respiratory function tests |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were alternately assigned to experimental or control arms |
| Allocation concealment (selection bias) | High risk | Participants were alternately assigned to experimental or control arms |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No party was blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessor was not blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes presented in the results section matched those reported in the methods |
Raphaël 1994.
| Methods | Multicentre (17 centres in France), open, parallel‐group study | |
| Participants | Children and adults with Duchenne muscular dystrophy 19 of the 70 included participants presenting with symptoms of nocturnal hypoventilation or daytime PaCO2 ≥ 6 kPa and were evaluable | |
| Interventions | Control group: combination of antibiotic and physiotherapy, if needed Experimental group: nocturnal intermittent positive ventilation through a nose mask or a mouthpiece | |
| Outcomes | Short‐term (24 hours): arterial blood gases Long‐term (6 months to 1 year):
|
|
| Notes | Individual data were available from the co‐ordinating centre. 5‐year inclusion period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Computer‐generated list, centralised randomisation, stratified for centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No party was blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessor was not blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up Only a subgroup of participants had symptoms of chronic hypoventilation |
| Selective reporting (reporting bias) | Low risk | Outcomes presented in the results section matched those reported in the methods |
Struik 2011.
| Methods | Single‐centre, open‐label trial with 2 parallel groups | |
| Participants | 16 adults with chest wall deformities and daytime PaCO2 > 45 mm Hg, and one of the following symptoms of nocturnal hypoventilation: daytime sleepiness, fatigue, morning headaches and/or dyspnea, or weight loss. All the participants were NIV‐naïve | |
| Interventions | Control treatment: volume‐targeted NIV during the night‐time Experimental treatment: pressure‐targeted NIV during the night‐time In both arms NIV was titrated to decrease PaCO2 to 45 mmHg, maintain SaO2 above 90%, and improve symptoms while maintaining patient comfort |
|
| Outcomes | The number of days needed to successfully establish the patient on NIV, defined as adequate patient adjustment to NIV and effective ventilation (PaCO2 < 45 mm Hg). NIV adjustment was considered adequate when the patient could sleep with the NIV for at least 6 hours per night | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method for generating the sequence of randomisation was not available |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the participants nor the personnel were blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants did not tolerate volume NIV and switched to pressure NIV after 2 days. 3 participants dropped out: 2 died (1 from cancer, the other from pneumothorax), and 1 did not want to return for measurements in hospital at 3 months |
| Selective reporting (reporting bias) | Unclear risk | No information available |
Tuggey 2005.
| Methods | Single‐centre, single‐blinded, cross‐over study | |
| Participants | 13 adults with chronic respiratory failure related to chest wall deformities and with stable clinical status on long‐term night‐time mechanical ventilation | |
| Interventions | Control treatment: 4‐week course of night‐time ventilation with volume‐targeted NIV Experimental treatment: 1‐week course of night‐time ventilation with pressure‐targeted NIV |
|
| Outcomes | Primary outcome: daytime arterial blood gas tensions during spontaneous breathing Secondary outcomes:
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for generating the random sequence generation was not available |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment was not available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded to treatment received in an appropriate manner, i.e. use of same ventilator and ventilator setting masked to the participant. Personnel were not blinded to experimental treatments |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessor was not blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed treatments sessions and none was lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information available |
Ward 2005.
| Methods | Single‐centre, open, parallel‐group study with a 2‐year follow‐up period | |
| Participants | n = 26 adults and children with congenital neuromuscular and chest wall disease Chronic respiratory failure with diurnal normocapnia | |
| Interventions | Control: spontaneous ventilation Experimental: nocturnal ventilation with bilevel airway pressure | |
| Outcomes | Peak transcutaneous arterial CO2 tension
Number of participants who developed criteria for mandatory mechanical ventilation
Changes in pulmonary function and respiratory muscles strength SF‐36 quality of life domain |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was not provided |
| Allocation concealment (selection bias) | Unclear risk | Information was not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No party was blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessor was not blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 2/12 and 2/14 participants lost to follow‐up in the control and experimental arms, respectively Half way through the follow‐up period (12 months), 70% of control participants received the experimental treatment NIV, as they worsened to meet criteria for mechanical ventilation |
| Selective reporting (reporting bias) | Low risk | Outcomes presented in the results section matched those reported in the methods |
Willson 2004.
| Methods | Single‐centre, cross‐over, open study | |
| Participants | 16 participants with neuromuscular diseases (n = 3), chest wall diseases (n = 5) or non‐neuromuscular and non‐chest wall diseases (n = 8) | |
| Interventions | Control: nocturnal ventilation with bilevel airway pressure using nasal mask Experimental: nocturnal ventilation with bilevel airway pressure using full face mask | |
| Outcomes | Short‐term (1‐night treatment) effects on sleep parameters | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was not provided |
| Allocation concealment (selection bias) | Unclear risk | Information was not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No party was blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessor was not blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes presented in the results section matched those reported in the methods |
ALS: amyotrophic lateral sclerosis CO2: carbon dioxide FVC: forced vital capacity NIV: non‐invasive ventilation PaCO2: carbon dioxide tension SaO2: oxygen saturation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aboussouan 1997 | Non‐randomised, open study |
| Ambrosino 1997 | Mechanical ventilation was implemented for 1‐hour run during the daytime. No data are available for the effects of nocturnal ventilation |
| Bach 1998 | Non‐randomised, open study |
| Celikel 1998 | Participants in an unstable condition, i.e. acute respiratory failure |
| Chatwin 2008 | This trial compared initiation of NIV at home versus in hospital |
| Crescimanno 2011 | This trial compared the very short‐term (over 3 nights) effect on gas exchange, sleep and respiratory function of 3 different modes of ventilation |
| Elliott 1994 | Mixed population, i.e. COPD and only 4 participants with chest wall disorders. Mechanical ventilation was implemented for 5‐minute courses during daytime. No data were available for night‐time ventilation effects |
| Ellis 1987 | This trial compared short‐term (24‐hour) effects of 1 night on positive‐ or negative‐pressure ventilation or spontaneous breathing |
| Fanfulla 2005 | Non‐randomised, open study |
| Hart 2002 | This trial compared a very short course (i.e. 40 minutes) of proportional assisted ventilation and pressure support ventilation |
| Highcock 2002 | This trial compared the short‐term (overnight) effects on sleep and gas exchange of 2 different ventilators for pressure support ventilation in participants with chronic respiratory failure due to chest wall deformities |
| Masa 1997 | Non‐randomised, open study |
| Meecham Jones 1993 | Mixed population, i.e. COPD and only 3 participants with neuromuscular or chest wall disorders. Mechanical ventilation was implemented for a 2‐hour course during daytime. No data were available for the effects of nocturnal ventilation |
| Orlikowski 2009 | This trial compared overnight effects on air leaks of 2 modes of ventilation with or without automatic air leak compensation |
| Padman 1998 | Non‐randomised, open study. Participants were in an unstable condition, i.e. acute respiratory failure |
| Restrick 1993 | This trial compared the short‐term effects (24‐hour) of 1 overnight session of volume‐cycled or pressure‐cycled NIV or spontaneous breathing |
COPD: chronic obstructive pulmonary disease NIV: non‐invasive ventilation
Differences between protocol and review
Change in authorship: JC Raphael (deceased) and JC Chevrolet are no longer authors of the review for this update.
We included 'Summary of findings' tables.
We have re‐arranged the outcomes to move up mortality at one year (or more) to be the primary outcome.
Contributions of authors
All authors contributed to the design of the protocol for this review. All authors contributed to the search for published and unpublished trials. D Annane and S Chevret were responsible for data extraction and entry onto the computer, and for data analyses. All authors contributed to the interpretation of data and D Annane and S Chevret wrote the final review.
Sources of support
Internal sources
Assistance Publique Hôpitaux de Paris, France.
University of Versailles SQY, France.
External sources
Association Française de lutte contre la Myopathie, France.
Declarations of interest
D Annane: my group was the co‐ordinating centre of a publicly funded multicentre trial of non‐invasive ventilation in DMD that has been included in this review (Raphaël 1994), and we are co‐ordinating an ongoing, publicly funded, multicentre trial of non‐invasive ventilation in adults with myotonic dystrophy type 1.
S Chevret: none known.
D Orlikowski declares the following:
grants from AFM for a randomised study of non‐invasive ventilation in myotonic dystrophy type 1 and from Phillips for a study of Average Volume Assured Pressure Support (AVAPS) in myotonic dystrophy type 1;
fees from IP Santé à domicile;
he has acted as a medical referee for Foyer ADEP Evry.
All the activities mentioned are in the field of care and research conducted in neuromuscular diseases and home ventilation.
Outside this work, he received funding from Covidien and from Genzyme to attend the AAN conference 2012.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bourke 2006 {published data only}
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non‐invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurology 2006;5(2):140‐7. [PUBMED: 16426990] [DOI] [PubMed] [Google Scholar]
Jackson 2001 {published data only}
- Jackson CE, Rosenfeld J, Moore DH, Bryan WW, Barohn RJ, Wrench M, et al. A preliminary evaluation of a prospective study of pulmonary function studies and symptoms of hypoventilation in ALS/MND patients. Journal of the Neurological Sciences 2001;191(1‐2):75‐8. [PUBMED: 11676995] [DOI] [PubMed] [Google Scholar]
Jaye 2009 {published data only}
- Jaye J, Chatwin M, Dayer M, Morrell MJ, Simonds AK. Autotitrating versus standard noninvasive ventilation: a randomised crossover trial. European Respiratory Journal 2009;33(3):566‐71. [PUBMED: 19251798 ] [DOI] [PubMed] [Google Scholar]
Laserna 2003 {published data only}
- Laserna E, Barrot E, Beiztegui A, Quintana E, Hernandez A, Castillo J. Non‐invasive ventilation in kyphoscoliosis. A comparison of a volumetric ventilator and a BiPAP support pressure device [Ventilacion no invasiva en cifoescoliosis. Estudio comparativo entre respirador volumetrico y soporte de presion (BiPAP)]. Archivos de Bronconeumologia 2003;39(1):13‐8. [PUBMED: 12550014] [DOI] [PubMed] [Google Scholar]
Pinto 1995 {published data only}
- Pinto AC, Evangelista T, Carvalho M, Alves MA, Sales Luis ML. Respiratory assistance with a non‐invasive ventilator (Bipap) in MND/ALS patients: survival rates in a controlled trial. Journal of the Neurological Sciences 1995;129(Suppl):19‐26. [PUBMED: 7595610] [DOI] [PubMed] [Google Scholar]
Raphaël 1994 {published and unpublished data}
- Raphaël JC, Chevret S, Chastang C, Bouvet F, and the French Multicenter Cooperative Group on Home Mechanical Ventilation Assistance in Duchenne de Boulogne Muscular Dystrophy. Randomised trial of preventive nasal ventilation in Duchenne muscular dystrophy. Lancet 1994;343(8913):1600‐4. [PUBMED: 7911921 ] [DOI] [PubMed] [Google Scholar]
Struik 2011 {published data only}
- Struik FM, Duiverman ML, Meijer PM, Nieuwenhuis JA, Kerstjens HA, Wijkstra PJ. Volume‐targeted versus pressure‐targeted noninvasive ventilation in patients with chest‐wall deformity: a pilot study. Respiratory Care 2011;56(10):1522–5. [PUBMED: 21513604] [DOI] [PubMed] [Google Scholar]
Tuggey 2005 {published data only}
- Tuggey JM, Elliott MW. Randomised crossover study of pressure and volume non‐invasive ventilation in chest wall deformity. Thorax 2005;60(10):859–64. [PUBMED: 12550014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2005 {published data only}
- Ward S, Chatwin M, Heather S, Simonds AK. Randomised controlled trial of non‐invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime normocapnia. Thorax 2005;60(12):1019‐24. [PUBMED: 16299118] [DOI] [PMC free article] [PubMed] [Google Scholar]
Willson 2004 {published data only}
- Willson GN, Piper AJ, Norman M, Chaseling WG, Milross MA, Collins ER, et al. Nasal versus full face mask for noninvasive ventilation in chronic respiratory failure. European Respiratory Journal 2004;23(4):605‐9. [PUBMED: 15083762] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aboussouan 1997 {published data only}
- Aboussouan LS, Khan SU, Meeker DP, Stelmach K, Mitsumoto H. Effect of noninvasive positive‐pressure ventilation on survival in amyotrophic lateral sclerosis. Annals of Internal Medicine 1997;127(6):450‐3. [DOI] [PubMed] [Google Scholar]
Ambrosino 1997 {published data only}
- Ambrosino N, Vitacca M, Polese G, Pagani M, Foglio K, Rossi A. Short term effects of nasal proportional assist ventilation in patients with chronic hypercapnic respiratory insufficiency. European Respiratory Journal 1997;10(12):2829‐34. [DOI] [PubMed] [Google Scholar]
Bach 1998 {published data only}
- Bach JR, Rajaraman R, Ballanger F, Tzeng AC, Ishikawa Y, Kulessa R, et al. Neuromuscular ventilatory insufficiency: effect of home mechanical ventilator use versus oxygen therapy on pneumonia and hospitalization rates. American Journal of Physical Medicine and Rehabilitation 1998;77(1):8‐19. [DOI] [PubMed] [Google Scholar]
Celikel 1998 {published data only}
- Celikel T, Sungur M, Ceyhan B, Karakurt S. Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failure. Chest 1998;114:1636‐42. [DOI] [PubMed] [Google Scholar]
Chatwin 2008 {published data only}
- Chatwin M, Nickol AH, Morrell MJ, Polkey MI, Simonds AK. Randomised trial of inpatient versus outpatient initiation of home mechanical ventilation in patients with nocturnal hypoventilation. Respiratory Medicine 2008;102(11):1528‐35. [DOI] [PubMed] [Google Scholar]
Crescimanno 2011 {published data only}
- Crescimanno G, Maronne O, Vianello A. Efficacy and comfort of volume‐guaranteed pressure support inpatients with chronic ventilatory failure of neuromuscular origin. Respirology 2011;16(4):672–9. [DOI] [PubMed] [Google Scholar]
Elliott 1994 {published data only}
- Elliott MW, Aquilina R, Green M, Moxham J, Simonds AK. A comparison of different modes of noninvasive ventilatory support: effects on ventilation and inspiratory muscle effort. Anaesthesia 1994;49(4):279‐83. [DOI] [PubMed] [Google Scholar]
Ellis 1987 {published data only}
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease: positive pressure ventilation through a nose mask. American Review Respiratory Disease 1987;135(1):148‐52. [DOI] [PubMed] [Google Scholar]
Fanfulla 2005 {published data only}
- Fanfulla F, Delmastro M, Berardinelli A, Lupo ND, Nava S. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. American Journal of Respiratory and Critical Care Medicine 2005;172(5):619‐24. [DOI] [PubMed] [Google Scholar]
Hart 2002 {published data only}
- Hart N, Hunt A, Polkey MI, Fauroux B, Lofaso F, Simonds AK. Comparison of proportional assist ventilation and pressure support ventilation in chronic respiratory failure due to neuromuscular and chest wall deformity. Thorax 2002;57:979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Highcock 2002 {published data only}
- Highcock MP, Morrish E, Jamieson S, Shneerson JM, Smith IE. An overnight comparison of two ventilators used in the treatment of chronic respiratory failure. European Respiratory Journal 2002;20:942–5. [DOI] [PubMed] [Google Scholar]
Masa 1997 {published data only}
- Masa JF, Celli BR, Riesco JA, Sanchez de Cos J, Disdier C, Sojo A. Noninvasive positive pressure ventilation and not oxygen may prevent overt ventilatory failure in patients with chest wall diseases. Chest 1997;112(1):207‐13. [DOI] [PubMed] [Google Scholar]
Meecham Jones 1993 {published data only}
- Meecham Jones DJ, Wedzicha JA. Comparison of pressure and volume preset nasal ventilator systems in stable chronic respiratory failure. European Respiratory Journal 1993;6(7):1060‐4. [PubMed] [Google Scholar]
Orlikowski 2009 {published data only}
- Orlikowski D, Mroue G, Prigent H, Moulin C, Bohic M, Ruquet M, et al. Automatic air‐leak compensation in neuromuscular patients: a feasibility study. Respiratory Medicine 2009;103(2):173‐9. [DOI] [PubMed] [Google Scholar]
Padman 1998 {published data only}
- Padman R, Lawless ST, Kettrick RG. Noninvasive ventilation via bilevel positive airway pressure support in pediatric practice. Critical Care Medicine 1998;26(1):169‐73. [DOI] [PubMed] [Google Scholar]
Restrick 1993 {published data only}
- Restrick LJ, Fox NC, Braid G, Ward EM, Paul EA, Wedzicha JA. Comparison of nasal pressure support ventilation with nasal intermittent positive pressure ventilation in patients with nocturnal hypoventilation. European Respiratory Journal 1993;6(3):364‐70. [PubMed] [Google Scholar]
Additional references
Annane 1999
- Annane D, Quera‐Salva MA, Lofaso F, Vercken JB, Lesieur O, Fromageot C, et al. Mechanisms underlying effects of nocturnal ventilation on daytime blood gases in neuromuscular diseases. European Respiratory Journal 1999;13(1):157‐62. [DOI] [PubMed] [Google Scholar]
Arras 1995
- Arras JD. Bringing the Hospital Home: Ethical and Social Implications of High‐tech Home Care. Baltimore: The Johns Hopkins University Press, 1995. [080184990xISBN 080184990x] [Google Scholar]
Bach 1987
- Bach JR, Alba A, Mosher R, Delaubier A. Intermittent positive pressure ventilation via nasal access in the management of respiratory insufficiency. Chest 1987;92(1):169‐70. [DOI] [PubMed] [Google Scholar]
Bach 2011
- Bach JR, Martinez D. Duchenne muscular dystrophy: continuous noninvasive ventilatory support prolongs survival. Respiratory Care 2011;56(6):744–50. [DOI] [PubMed] [Google Scholar]
Bach 2013
- Bach JR, Gonçalves MR, Hon A, Ishikawa Y, Vito EL, Prado F, et al. Changing trends in the management of end‐stage neuromuscular respiratory muscle failure: recommendations of an international consensus. American Journal of Physical Medicine & Rehabilitation 2013;92(3):267‐77. [PUBMED: 23051760] [DOI] [PubMed] [Google Scholar]
Barbé 1996
- Barbé F, Quera‐Salva MA, Lattre J, Gajdos P, Agusti AGN. Long term effects of nasal intermittent positive‐pressure ventilation on pulmonary function and sleep architecture in patients with neuromuscular diseases. Chest 1996;110(5):1179‐83. [DOI] [PubMed] [Google Scholar]
Carroll 1988
- Carroll N, Branthwaite MA. Control of nocturnal hypoventilation by nasal intermittent positive pressure ventilation. Thorax 1988;43(5):349‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chailleux 1996
- Chailleux E, Fauroux B, Binet F, Dautzenberg B, Polu JM for the Observatory Group of ANTADIR. Predictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation. Chest 1996;109(3):741‐9. [DOI] [PubMed] [Google Scholar]
Chatwin 2010
- Chatwin M, Heather S, Hanak A, Polkey MI, Simonds AK. Analysis of home support and ventilator malfunction in 1,211 ventilator‐dependent patients. European Respiratory Journal 2010;35(2):310‐6. [DOI] [PubMed] [Google Scholar]
Claman 1996
- Claman DM, Piper A, Sanders MH, Stiller RA, Votteri BA. Nocturnal non‐invasive positive pressure ventilatory assistance. Chest 1996;110(6):1581‐8. [DOI] [PubMed] [Google Scholar]
Eagle 2002
- Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscular Disorders 2002;12(10):926‐9. [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Gregoretti 2013
- Gregoretti C, Ottonello G, Chiarini Testa MB, Mastella C, Ravà L, Bignamini E, et al. Survival of patients with spinal muscular atrophy type 1. Pediatrics 2013;131(5):e1509‐14. [DOI] [PubMed] [Google Scholar]
Heckmatt 1990
- Heckmatt JZ, Loh L, Dubowitz V. Night time nasal ventilation in neuromuscular disease. Lancet 1990;335(8689):579‐82. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoeppner 1984
- Hoeppner VH, Cockcroft DW, Dosman JA, Cotton DJ. Nighttime ventilation improves respiratory failure in secondary kyphoscoliosis. American Review of Respiratory Disease 1984;129(2):240‐3. [PubMed] [Google Scholar]
King 2012
- King AC. Long‐term home mechanical ventilation in the United States. Respiratory Care 2012;57(6):921‐30. [PUBMED: 22663967] [DOI] [PubMed] [Google Scholar]
Lewarski 2007
- Lewarski JS, Gay PC. Current issues in home mechanical ventilation. Chest 2007;132(2):671–6. [PUBMED: 17699139] [DOI] [PubMed] [Google Scholar]
Lloyd‐Owen 2005
- Lloyd‐Owen SJ, Donaldson GC, Ambrosino N, Escarabill J, Farre R, Fauroux B, et al. Patterns of home mechanical ventilation use in Europe: results of the European Survey. European Respiratory Journal 2005;25(6):1025‐31. [DOI] [PubMed] [Google Scholar]
Loh 1979
- Loh L, Hughes JM, Davis J. Gas exchange problems in bilateral diaphragm paralysis. Bulletin Européen de Physiopathologie Respiratoire 1979;15(Suppl):137‐41. [PubMed] [Google Scholar]
Make 1998
- Make BJ, Hill NS, Goldberg AI, Bach JR, Criner GJ, Dunne PE, et al. Mechanical ventilation beyond the intensive care unit: report of a consensus conference of the American College of Chest Physicians. Chest 1998;113(5 Suppl):289S‐344S. [DOI] [PubMed] [Google Scholar]
McDougall 2013
- McDougall CM, Adderley RJ, Wensley DF, Seear MD. Long‐term ventilation in children: longitudinal trends and outcomes. Archives of Disease in Childhood 2013;98(9):660‐5. [DOI] [PubMed] [Google Scholar]
McKim 2013
- McKim DA, Griller N, LeBlanc C, Woolnough A, King J. Twenty‐four hour noninvasive ventilation in Duchenne muscular dystrophy: a safe alternative to tracheostomy. Canadian Respiratory Journal 2013;20(1):e5‐e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milligan 1991
- Milligan S. American Association for Respiratory Care and Gallup estimate numbers and costs of caring for chronic ventilator patients. American Association for Respiratory Care Times 1991;15:30‐6. [Google Scholar]
Newsom‐Davis 1980
- Newsom‐Davis J. The respiratory system in muscular dystrophy. British Medical Bulletin 1980;36(2):135‐8. [DOI] [PubMed] [Google Scholar]
Orlikowski 2005
- Orlikowski D, Prigent H, Gonzalez J, Sharshar T, Raphaël JC. Long term domiciliary mechanical ventilation in patients with neuromuscular diseases (indications, establishment and follow up) [Ventilation mécanique à domicile et au long cours des patients neuromusculaires (indications, mise en place et surveillance)]. Revue des Maladies Respiratoires 2005;22(6 Pt 1):1021‐30. [DOI] [PubMed] [Google Scholar]
Passamano 2012
- Passamano L, Taglia A, Palladino A, Viggiano E, D'Ambrosio P, Scutifero M, et al. Improvement of survival in Duchenne Muscular Dystrophy: retrospective analysis of 835 patients. Acta Myologica 2012;31(2):121‐5. [PUBMED: 23097603] [PMC free article] [PubMed] [Google Scholar]
Raphaël 1987
- Raphaël JC, Gajdos P, Lattre J. Chronic respiratory insufficiency related to neuromuscular diseases, therapeutic perspectives [Handicape respiratoire chronique d’origine neuromusculaire: physiopathologie, perspectives thérapeutiques]. Fonction diaphragmatique ‐ Travail respiratoire. Monographie de la Société de Réanimation de Langue Française. Paris: Expansion Scientifique Française, 1987:203‐28. [Google Scholar]
Raphaël 1999
- Raphaël JC, Chevret S, Annane D. Is early noninvasive mechanical ventilation of first choice in stable restrictive patients with chronic respiratory failure?. Monaldi Archives of Chest Disease 1999;54(1):90‐7. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Simonds 2003
- Simonds AK. Home ventilation. European Respiratory Journal. Supplement 2003;22(Suppl 47):38S‐46S. [DOI] [PubMed] [Google Scholar]
Skatrud 1980
- Skatrud J, Iber C, McHugh W, Rasmussen H, Nichols D. Determinants of hypoventilation during wakefulness and sleep in diaphragmatic paralysis. American Review of Respiratory Disease 1980;121(3):587‐93. [DOI] [PubMed] [Google Scholar]
Smith 1987
- Smith PE, Calverley PM, Edwards RH, Evans GA, Campbell EJ. Practical problems in the respiratory care of patients with muscular dystrophy. New England Journal of Medicine 1987;316(19):1197‐205. [DOI] [PubMed] [Google Scholar]
Toussaint 2006
- Toussaint M, Steens M, Wasteels G, Soudon P. Diurnal ventilation via mouthpiece: survival in end‐stage Duchenne patients. European Respiratory Journal 2006;28(3):549–55. [DOI] [PubMed] [Google Scholar]
Wollinsky 2012
- Wollinsky KH, Kutter B, Geiger PM. Long‐term ventilation of patients with Duchenne muscular dystrophy:experiences at the Neuromuscular Centre Ulm. Acta Myologica 2012;31(3):170‐8. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Annane 2000
- Annane D, Chevrolet JC, Chevret S, Raphaël JC. Nocturnal mechanical ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001941] [DOI] [PubMed] [Google Scholar]
Annane 2007
- Annane D, Orlikowski D, Chevret S, Chevrolet JC, Raphaël JC. Nocturnal mechanical ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001941.pub2] [DOI] [PubMed] [Google Scholar]


