Abstract
Background
Lung disease in preterm infants is often complicated with lung edema.
Objectives
To assess the risks and benefits of diuretics acting on distal segments of the renal tubule (distal diuretics) in preterm infants with or developing chronic lung disease (CLD).
Search methods
The standard method of the Cochrane Neonatal Review Group were used. Initially, MEDLINE (1966 to November 2001), EMBASE (1974 to November 2001) and the Cochrane Controlled Trials Register (CENTRAL,The Cochrane Library, Issue 4, 2001) were searched. In addition, several abstract books of national and international American and European Societies were hand searched. Updated searches in April 2003, April 2007, and December 2010 did not yield any additional trials.
Selection criteria
Included in this analysis are trials in which preterm infants with or developing CLD and at least five days of age were randomly allocated to receive a diuretic acting on the distal renal tubule. Eligible studies needed to assess at least one of the outcome variables defined a priori for this systematic review.
Data collection and analysis
The standard method for the Cochrane Collaboration described in the Cochrane Collaboration Handbook were used. Two investigators extracted, assessed and coded separately all data for each study. Any disagreement was resolved by discussion. Parallel and cross‐over trials were combined. Whenever possible, baseline and final outcome data measured on a continuous scale was transformed into change scores using Follmann's formula.
Main results
Of the six studies fulfilling entry criteria, most focused on pathophysiological parameters and did not assess effects on important clinical outcomes defined in this review, or the potential complications of diuretic therapy.
In preterm infants > 3 weeks of age with CLD, a four week treatment with thiazide and spironolactone improved lung compliance and reduced the need for furosemide. A single study showed thiazide and spironolactone decreased the risk of death and tended to decrease the risk for remaining intubated after eight weeks in infants who did not have access to corticosteroids, bronchodilators or aminophylline.
Authors' conclusions
In preterm infants > 3 weeks of age with CLD, acute and chronic administration of distal diuretics improve pulmonary mechanics. However, positive effects should be interpreted with caution as the numbers of patients studied are small in surprisingly few randomized controlled trials.
Plain language summary
Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease
There is no strong evidence of benefit from routine use of distal diuretics in preterm infants with chronic lung disease. Lung disease in infants born early (preterm) is often complicated with excess of water. Medications that reduce body water (diuretics) might help the infant recover from lung disease. The review of trials analysed the effects of diuretics working on the end of the small kidney tubes (distal diuretics). It found that diuretic treatment for four weeks improved lung function. Only one study showed long term benefit (decreased rates of death and artificial ventilation). However, the infants in these trials did not receive all the medications that are currently available.
Background
The present review describes the evidence about administration of distal diuretics (i.e., diuretics acting on distal segments of the renal tubule) in preterm infants with or developing chronic lung disease (CLD). Distal diuretics include thiazides, metolazone, and potassium‐sparing diuretics (spironolactone, triamterene and amiloride). This review is part of a group of three closely related reviews on diuretics in preterm infants with CLD, developing CLD or at high risk of CLD (Brion 1999a; Brion 1999b). The other two reviews discuss the use of systemic loop diuretics (Brion 1999a) and the use of the aerosolized diuretics (Brion 1999b). The group of reviews was summarized elsewhere (Brion 2001).
Description of the condition
Factors involved in lung edema in neonates with CLD include increased capillary permeability resulting from lung injury, congestive heart failure due to patent ductus arteriosus, and fluid overload (Brown 1978; Zimmerman 1995). This edema could not only reduce pulmonary compliance (and thus tidal volume if using a pressure‐limited ventilator) but also increase airway resistance by narrowing terminal airways (Northway 1967).
Description of the intervention
One might expect diuretics to reduce lung edema in preterm infants with or developing CLD. However, when administration of a single diuretic is repeated for several days, hormonal and renal adaptation mechanisms eventually will limit the diuretic response. Addition of a diuretic acting upon another segment of the nephron is often able to overcome this tolerance. As an example, the addition of metolazone increases urine output in preterm infants with CLD treated with furosemide (Segar 1992).
Pharmacokinetics and pharmacodynamics of diuretics acting on distal segments of the renal tubule: Because of the long half‐life of many medications in immature infants, a prolonged washout period (without previous drug administration) is needed if one wishes to eliminate any residual drug activity before initiating a clinical trial or between exposures in cross‐over trials.
We found no data on half‐life of thiazides, metolazone or spironolactone by searching MEDLINE using the keywords <exp diuretics> and <half‐life> limited to <newborn infant <birth to 1 month> or infant <1 year>. In adults, the half‐life of hydrochlorothiazide ranges between 3.2 and 13.1 hours (Chen 1992). In preterm infants (mean birthweight 887 g, gestational age 26.8 weeks, postnatal age 4.2 weeks), plasma elimination half‐life of chlorothiazide after the first dose is 5.2 hours, compared with 1.5 ‐ 2.5 hours in adults (Mirochnick 1989). On day 7 of therapy (using an oral dose of 10mg/kg twice a day), a urinary excretion averaged 30.4% of the administered dose.
The half‐life of canrenone, the most important non‐conjugated metabolite of spironolactone, ranges in adults between 12.5 ± 3.4 hours (four doses/day) and 19.2 ± 6.6 hours (one daily dose) (Karim 1976).
Potential complications of distal diuretics include the following:
(1) Electrolyte and acid‐base imbalances including: hyponatremia, hypo or hyperkalemia, hypochloremia, hyperuricemia and metabolic alkalosis or acidosis. Since thiazides do not affect urinary concentration mechanisms (in contrast with loop diuretics), they often cause hyponatremia in very low birth weight infants (Horgan 1996). Thiazides are complicated with hypokalemic hypochloremic metabolic alkalosis. Metabolic alkalosis reduces respiratory drive in adults (Jhaveri 2010). Treatment of this alkalosis may include administration of potassium chloride (with potential risk of hyperkalemia), sodium chloride (with potential risk of sodium overload and vicious cycle of sodium excess and lung edema, and nephrocalcinosis), or arginine chloride. In contrast, potassium‐sparing diuretics such as spironolactone are complicated with hyperkalemia and metabolic acidosis.
(2) Intravascular volume depletion which can contribute to dehydration, hypovolemia, hypotension and pre‐renal failure.
(3) Changes in mineral handling by the renal tubule leading to osteopenia and/or nephrocalcinosis. All diuretics may cause hyperphosphaturia (leading to osteopenia). One controlled clinical trial showed no effect of spironolactone on calciuria or phosphaturia (Sonntag 1996). Thiazides, metolazone and potassium‐sparing diuretics other than spironolactone enhance calcium reabsorption by the renal tubule and thus cause hypocalciuria and even may cause hypercalcemia. Nevertheless, administration of a thiazide diuretic along with salt loading or sodium replacement results in hypercalciuria, no matter whether the thiazide is used alone (Campfield 1997) or simultaneously with spironolactone (Atkinson 1988). The administration of thiazide does not prevent hypercalciuria in patients receiving a loop diuretic (Campfield 1997) nor nephrocalcinosis in those receiving spironolactone (Toffolo 1997).
(4) Decreased alveolar liquid clearance, resulting from inactivation of the epithelial sodium channel (ENaC) by amiloride (Sakuma 1994; O'Brodovich 1990; Jayr 1994). Thus, amiloride, which may increase, rather than decrease, lung water content, is contraindicated in preterm infants with CLD. Chronic administration of spironolactone completely blocks aldosterone‐mediated enhancement of alveolar lung fluid resorption observed in sodium‐depleted adult rats (Suzuki 2001).
(5) Endocrine and metabolic effects: Spironolactone is a non specific aldosterone antagonist and binds to androgen receptors. It is used as antiandrogen in women with hirsutism including those with non classic congenital adrenal hyperplasia (Merke 2002). Spironolactone can cause gynecomastia in men due to antiandrogen effects. There is one case report of ovarian cyst in a neonate on spironolactone (Vachharajani 2001). Spironolactone interferes with 17‐hydroxyprogesterone measurement, which can lead to erroneous interpretations of newborn screening tests, since 17‐hydroxyprogeterone is used to screen neonates for congenital adrenal hyperplasia (Loriaux 1976; Terai 1999). Glucose intolerance is a known side effect of thiazides in adults.
How the intervention might work
Diuretics may improve lung function by (1) an immediate, diuresis‐independent fluid reabsorption (2) a reduction in extracellular volume resulting from increased natriuresis and urine output (Brion 1999a). This may improve lung compliance (and tidal volume with pressure‐limited ventilation), decrease airway resistance and increase expiratory flow. Acute administration of spironolactone decreases lung congestion, hemorrhage and lung fluid (Atalay 2010). Acute administration of distal diuretics reduces lung fluid by the second mechanism, as shown for thiazides (Beyer 1958). Furthermore, some distal diuretics may reduce alveolar lung fluid reabsorption (Sakuma 1994; O'Brodovich 1990; Jayr 1994; Suzuki 2001).
Chronic administration of spironolactone may reduce lung fibrosis, cardiac fibrosis or both (Zhao 1998; Agostini 2005). This may decrease pulmonary or cardiac fibrosis, thereby improving lung diffusion and compliance (Agostini 2005). The reduction in cardiac fibrosis may result from stimulation of cardiac connective tissue growth factor by serum‐ and glucocorticoid‐inducible kinase 1 (Vallon 2006).
Why it is important to do this review
The current review is designed to assess the evidence for short and long‐term benefits and side effects of distal diuretics in these patients.
Objectives
The aim of this systematic review was to assess the risks and benefits of distal diuretics in preterm infants with or developing CLD. Primary objectives were to assess: 1. short‐term improvement: changes in mean airway pressure, need for artificial ventilation, need for continuous positive airway pressure, failure to tolerate extubation, and oxygen supplementation 2. long‐term improvement: mortality, duration of need for oxygen supplementation and respiratory support, bronchopulmonary dysplasia (BPD) (defined as need for oxygen supplementation at 28 days of life), death or BPD, CLD at 36 weeks of postconceptional age (gestational age + postnatal age), length of hospital stay, and number of rehospitalizations during the first year of life.
Methods
Criteria for considering studies for this review
Types of studies
Only randomized controlled studies were included in this analysis. Randomization needed to involve the allocation of all patients either to a specific treatment (patients on diuretic vs. controls on placebo or another therapy), or to a specific time of administration of the diuretic (diuretic first vs. placebo first).
Types of participants
Participants needed to be: (1) Preterm infants (2) With oxygen dependency (> 21% oxygen to maintain pulse oximetry > 90% or PaO2 > 50 mm Hg) or ventilator dependency secondary to lung disease beyond five days of life. Although BPD is usually defined by the need for oxygen supplementation at four weeks of age, the pathophysiology of chronic pulmonary disease has been shown to already start during the first few days of life in patients with RDS (Northway 1967). For the present study, only studies with entry criteria of oxygen or ventilator dependence beyond five days were included in order to avoid overlap with a systematic review of the use of diuretics in patients with RDS (Brion 1999c).
Types of interventions
The design of the study needed to include a randomized allocation to the administration of a distal diuretic. Eligible studies were those that assessed either (1) the administration of one or more distal diuretics compared with placebo (or no treatment), (2) the administration of an additional distal diuretic compared with a single diuretic in controls, (3) the administration of a different diuretic from that in controls, or (4) administration of a diuretic using another mode compared with control therapy.
Types of outcome measures
Outcome measures had to include an assessment of the effect of diuretic administration on at least one of the following variables:
Primary outcomes
1. Primary outcomes: 1.1. Short‐term improvement: changes in mean airway pressure, need for artificial ventilation, need for continuous positive airway pressure, failure to tolerate extubation, and oxygen supplementation 1.2. Long‐term improvement: mortality, duration of need for oxygen supplementation and respiratory support, BPD, death or BPD, chronic lung disease at 36 weeks postmenstrual (gestational age + postnatal age), length of stay, and number of rehospitalizations during the first year of life.
Secondary outcomes
2. Secondary outcomes: 2.1. Potential complications of therapy: hypovolemia, alkalosis, hyponatremia, hypokalemia, hypochloremia, bone demineralization, nephrocalcinosis, nephrolithiasis, cholelithiasis, neurosensory hearing loss. 2.2. Pulmonary function: resistance, compliance, tidal volume for patients on pressure‐limited ventilation, expiratory flow, minute ventilation.
Secondary objectives were to assess potential complications of diuretic therapy and changes in pulmonary mechanics after treatment.
Search methods for identification of studies
See: Cochrane Neonatal Review Group (CNRG) search strategy
1. Published manuscripts: Initially, MEDLINE (1966 ‐ 1998), EMBASE (1974 ‐ 1998) and the Cochrane Controlled Trials Register (CCTR) (The Cochrane Library, Issue 3, 1998) were searched in August 1998. The search was not limited to any language. The following keywords were used: {<bronchopulmonary dysplasia> or <chronic lung disease>} and <explode diuretics>, limited to <human> and limited to <infant, newborn> or <infant>.
The MEDLINE, EMBASE and CCTR searches were repeated in November 2001. These searches did not yield any additional relevant randomized trials.
Additional searches of MEDLINE done using {<bronchopulmonary dysplasia> or <chronic lung disease>} and <diuretic> in April 2003, April 2007 and Deceber 2010 did not yield any additional eligible studies. An additional search of EMBASE in April 2007 and December 2010 did not yield any additional studies.
2. Published abstracts: We searched the abstracts of the following national or international societies (1991 ‐ 1998 unless otherwise specified): # American Academy of Pediatrics 90‐98 (published in American Journal of Perinatology [90‐95] and in Pediatrics [96‐98]) # American Society of Nephrology (published in Journal of the American Society of Nephrology) # American Thoracic Society 91‐98 (published in American Review of Respiratory Disease [91 ‐ 93] and in American Journal of Respiratory and Critical Care Medicine [94 ‐ 98]) # British Paediatric Association (now Royal College of Paediatrics and Child Health [RCPCH] Annual Scientific Meeting) # European Respiratory Society # European Society for Pediatric Research (published in Pediatric Research) # Neonatal Society [UK] # Society for Pediatric Research [US] (published in Pediatric Research). Details of the search can be found in a related review (Brion 1999a). Hand searching of the Neonatal Society [UK] and RCPCH abstracts in April 2007 did not yield any additional eligible study.
3. Database of the Cochrane Neonatal Review Group: All publications coded under diuretics as intervention were initially screened in September, 1998. A search of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2003; Issue 1, 2007; and Issue 4, 2010) did not yield any additional eligible study.
4. Selection process: Only randomized controlled trials fulfilling the selection criteria described in the previous section were selected. Selection was done separately by two investigators; any disagreement was resolved by discussion.
Data collection and analysis
The standard method for the Cochrane Collaboration described in the Cochrane Collaboration Handbook as well as the methods outlined by the Cochrane Neonatal Review Group were used.
Selection of studies
All randomized and quasi‐randomized controlled trials fulfilling the selection criteria described in the previous section were included. The reviewers independently assessed eligibility for inclusion in this review.
Data extraction and management
Two investigators working independently extracted, assessed and coded all data for each study, using a form that had been designed specifically for this review. Graphical data was transformed into numerical data using a millimetric ruler and an electronic spreadsheet. Any standard error of the mean was replaced by the corresponding standard deviation (SD). As much as possible, units of measurement were standardized among studies. In some cases, this required using a specific formula to estimate the SD of a ratio or a product (Baird 1995; Armitage 1994).
In December 1998 and in April 1999, each author was sent an itemized letter requesting additional information about design, patients, methods, or original outcome data (if missing, incomplete or presented in graphical form). Dr. Albersheim confirmed that no patients included in the study ever received postnatal steroids.
All calculations within the spreadsheet and entries into RevMan were done by one review author (LPB) and subsequently checked for accuracy by the other review authors (IAP and RP). Any disagreement was resolved by discussion.
Conversion of values into SI units: To obtain gas pressure in kPa, we multiplied the value in cm H2O by 0.10 or that in mm Hg by 0.13 To obtain calcium:creatinine ratio in mM:mM, we multiplied the ratio in mg:mg by 2.84 To obtain calcium in mM/L, we multiplied the value in mg/dl by 0.25
Assessment of risk of bias in included studies
The standard methods of the Cochrane Neonatal Review Group were used (http://neonatal.cochrane.org/en/index.html) . The methodological quality of the studies was evaluated by assessing the risk for four types of bias (selection, performance, attrition and detection). Each study was assessed separately by two review authors; disagreements were resolved by discussion with the other review authors.
For the update in 2011, review authors independently assessed study quality and risk of bias using the following criteria documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
1) sequence generation: was the allocation sequence adequately generated?
2) allocation concealment: was allocation adequately concealed?
3) blinding of participants, personnel and outcome assessors for each main outcome or class of outcomes: was knowledge of the allocated intervention adequately prevented during the study?
4) incomplete outcome data for each main outcome or class of outcomes: were incomplete outcome data adequately addressed?
5) selective outcome reporting: are reports of the study free of suggestion of selective outcome reporting?
6) other sources of bias: was the study apparently free of other problems that could put it at a high risk of bias? We gave particular attention to completeness of follow up of all randomised infants and to the length of follow‐up studies to identify whether any benefits claimed are robust.
When necessary, we requested additional information and clarification of published data from the authors of individual trials. We assessed each trial for risk of bias based on the criteria listed above and marked as:
a) low risk of bias;
b) unclear risk of bias;
c) high risk of bias.
Measures of treatment effect
As much as possible, outcomes were assessed in all patients entered in each trial on an intent‐to‐treat basis. The total number of patients initially entered in the trial is mentioned as the first item in the column entitled 'participants' in the table of included studies. However, in some studies outcomes were available only in some patients; exclusions are described in the column entitled 'methods.'
Dichotomous variables were analyzed using the relative risk (RR) and the risk difference (RD) with their 95% confidence intervals (CI). For continuous variables, the weighted mean difference (WMD) and its CI between change scores in the treatment group and in the control group were obtained. This was possible only in studies for which the number of patients analyzed remained constant over time. Change scores were obtained either from the mean of individual differences between baseline and final values, from mean and SD (or standard error) values of change (or percent of baseline) provided by the authors, or from the means and SD of baseline and final values. In the latter case, the variance (var) of change was estimated using Follmann's (Follmann 1992) method, described in version 3.0.2 of the Cochrane Collaboration Handbook (page 213): Var(change)=Var(pretest) + Var(posttest) ‐ 2 x SD(pretest) x SD (posttest) x pretest‐posttest correlation coefficient.
The literature was searched for values of pretest‐posttest correlation coefficient (r) for each test, interval and patient group. If such data were not available, we assumed a value of 0.4, and conducted a sensitivity analysis by successively using r = 0.3 and r = 0.5.
For those studies providing mean and SD of baseline values and of the percent change from baseline, the mean change was obtained by multiplying percent change by the average baseline. Variance of change was calculated using established guidelines (Baird 1995; Armitage 1994), as described elsewhere (Brion 1999a).
Dealing with missing data
Noted is section on data extraction. Each author was sent an itemized letter requesting additional information about design, patients, methods, or original outcome data (if missing, incomplete or presented in graphical form).
Assessment of heterogeneity
We planned to estimate the treatment effects of individual trials and examine heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the two formal statistics described below.
1) The Chi2 test, to assess whether observed variability in effect sizes between studies is greater than would be expected by chance. Since this test has low power when the number of studies included in the meta‐analysis is small, we planned to set the probability at the 10% level of significance.
2) The I2 statistic to ensure that pooling of data is valid. We planned to grade the degree of heterogeneity as: 0% to 30%: might not be important; 31% to 50%: moderate heterogeneity; 51% to 75%: substantial heterogeneity; 76% to 100%: considerable heterogeneity.
Where there is evidence of apparent or statistical heterogeneity, we planned to assess the source of the heterogeneity using sensitivity and subgroup analysis looking for evidence of bias or methodological differences between trials.
Data synthesis
The meta‐analysis was performed using Review Manager software (RevMan 5), supplied by the Cochrane Collaboration. For estimates of typical relative risk and risk difference, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. All meta‐analyses were done using the fixed effect model.
Parallel trials and cross‐over trials were combined using Metaview. For trials with a cross‐over design, the number in each arm was used as the total number of patients entered into the trial. In trials with a cross‐over design, many authors have only reported mean change from baseline or percent change from baseline, failing to provide information that would rule out a carry‐over effect (possibly yielding an underestimation of the real effect of diuretic administration) and a period effect.
Subgroup analysis and investigation of heterogeneity
Planned comparisons and subgroup analyses:
The following comparisons based on type of intervention were planned: 1. Comparison of a thiazide versus placebo 2. Comparison of two diuretics versus one diuretic
Subgroup analyses:
1. Average postnatal age: Within each of the above groups, subgroups were determined based on mean postnatal age. The initial plan was to use a cut‐off value of four weeks, based on the usual definition of bronchopulmonary dysplasia. However, using this value would have made it impossible to classify two of the twenty studies included in this group of three related reviews on diuretics in preterm infants with (or developing) CLD (Singhal 1983; Robbins 1993).Therefore, a mean postnatal age of three weeks was selected as the cut‐off for all three reviews.
2. Mean gestational age: Subcategories based on gestational age were planned if differences greater than four weeks were observed among studies.
3. Presence of an endotracheal tube: Pre‐determined subcategories were used for intubated patients vs. non‐intubated patients. The presence of an endotracheal tube is expected to increase total resistance and to decrease dead space. Patients requiring an endotracheal tube are likely to be sicker and thus to have lower pulmonary compliance and to require more oxygen than the other patients.
Results
Description of studies
A total of 17 studies were considered for this review. Ten were eliminated because they did not involve random allocation to a distal diuretic. One (Segar 1992) was eliminated because it did not analyze any of the primary outcomes defined for this review. Thus, six studies were included in the present review. Five studies involved the use of a thiazide with or without spironolactone, and one study involved the use of metolazone with or without furosemide. Details reported by the authors are provided in the table.
In all studies included in this review but two, the authors used dynamic measurements of pulmonary mechanics with or without an esophageal balloon. Static measurements were done in one study (Albersheim 1989). Pulmonary mechanics were not assessed in one study (Segar 1997).
Main categories (intervention) are shown as first entry in the column labeled 'Interventions.' No subcategories were used based on gestational age, because the maximum range of gestational age within each intervention group was four weeks. No subcategories were used based on postnatal age, because the minimum average age in all studies was not less than seven weeks. Main categories (based on intervention) and subcategories (based on postnatal age and mechanical ventilation) are described below:
1. Administration of a thiazide diuretic, versus placebo or no treatment in controls: Four studies were in this group, including three with parallel design (Albersheim 1989; Engelhardt 1989; Kao 1994), and one with cross‐over design (Kao 1984). Average postnatal age was above four weeks in all studies (range 7 to 12 wk), so that only one group of postnatal age was used. Average gestational age ranged between 26 and 29 weeks, so that only one category of gestational age was used.
1.1. Non‐intubated patients:
Engelhardt 1989: parallel design. Patients were randomized to receive either thiazide and spironolactone for one week, or placebo. No washout period is documented, possibly minimizing short‐term effects of diuretics. Change scores were estimated using Follmann's formula. Twenty‐one patients were randomized, 12 into the treatment group and nine into the control group. The average birth weight was 995 ± 262 g in the diuretic group and 920 ± 206 g in the control group. The average gestational age was 28 ± 2.2 weeks and 27 ± 1.7 weeks, respectively. Postnatal age was 7.8 ± 2.1 and 8.8 ± 4.2 weeks, respectively, and postmenstrual age 35.8 ± 3.0 and 35.8 ± 4.5 weeks. At entry into the study, Mean FiO2 was 0.33 ± 0.08 and 0.36 ± 0.11, respectively. Patients in the diuretic group tended to have a 37% higher compliance and a 29% higher resistance than controls; this did not reach statistical significance. Total fluid intake was 135 ± 16 ml/kg/day in the treatment group and 132 ± 10 ml/kg/day in the control group.
Kao 1984: cross‐over design; data during first exposure kept separate from those after cross‐over. Patients were randomized to receive either thiazide and spironolactone for one week followed by placebo for one week, or vice‐versa. A washout period of 48 h was planned before the study, but no washout was planned at the time of cross‐over. Nevertheless, data before and after cross‐over were analyzed and did not show any evidence for a carry‐over effect. Change scores were estimated using Follmann's formula.
Ten patients were included in the study. Mean birth weight was 1.2 ± 0.6 kg, gestational age 29.0 ± 3.2 weeks, average postnatal age 10 weeks, postmenstrual age 39 ± 3.2 weeks, weight at the time of the study 1.8 ± 0.3 kg, duration of ventilation 7 ± 3 weeks, compliance 3.2 ± 0.6 ml/cm H2O, resistance 75.9 ± 2.1 cm H2O/L/sec, specific airway conductance 0.16 ± 0.03, tidal volume 9.2 ± 3.2 ml/kg, and thoracic gas volume 47.0 ± 12.6 ml/kg. Patients did not require oxygen. Baseline values were similar between patients initially started on placebo and those started on diuretics.
Kao 1994: parallel design. Patients were randomized to receive either thiazide and spironolactone, or placebo, until they were weaned off oxygen. Duration of randomization was approximately ten weeks. A washout period of three to five days was planned before the study. Change scores were estimated using Follmann's formula. A total of 43 patients were included in the analysis, including 22 in the diuretic group and 21 in the control group. The average birthweight in the diuretic group was 0.96 ± 0.40 kg vs 1.03 ±. 58 in the control group. Gestational age was 28 ± 3 weeks and 28 ± 4 weeks, respectively. Postnatal age at the time of entry was 12.1 ± 3.9 weeks and 11.1 ± 4.0 weeks, respectively, and postmenstrual age was 40.1 ± 4.9 and 39.1 ± 5.7 weeks, respectively. FiO2 was 0.35 ± 0.10 and 0.37 ± 0.16, respectively. Both groups were similar for all parameters except for maximum expiratory flow at FRC, which was significantly lower in the diuretic group than in controls (0.22 ± 0.14 sec‐1 vs 0.48 ± 0.33 sec‐1, p < 0.005). Resistance tended to be 27% higher in the diuretic group than in controls. Seven patients in each group had received surfactant. Nine of 22 patients in the treatment group and 7/21 patients in the control group received steroids for BPD.
1.2. Intubated patients:
Albersheim 1989: parallel design. Patients were randomized to receive either thiazide and spironolactone for eight weeks, or placebo. Patients received neither steroids, nor bronchodilators, nor theophylline during the study. Pulmonary function measurements were only obtained in intubated patients. Because the number of intubated patients decreased with time, we were unable to validly calculate change scores for pulmonary mechanics, and used instead values of pulmonary function at various time points as outcome data. No washout period was planned before the study, possibly minimizing the effects of diuretic administration, especially on short‐term outcome. A total of 34 premature infants were available for the analysis. The average birth weight was 838 ± 224 g in the treatment group and 876 ± 195 g in the control group. The average gestational age was 25.8 ± 1.1 weeks in the treatment group and 26.4 ± 1.2 weeks in the control groups. The average postnatal age at the time of entry into the study was 40.5 ± 9.0 days and 36.5 ± 4.7 days, respectively. Mean postmenstrual age was 31.6 ± 1.7 weeks and 31.6 ± 1.4 weeks, respectively. Both groups were similar for all parameters assessed, except for gender: 8/19 patients in the treatment group were girls, compared to 1/15 in the control group (p = 0.047). Total fluid intake was 160 ± 13 ml/kg/day in the treatment group and 160 ± 18 ml/kg/day in the control group. Baseline FiO2 was 0.73 ± 0.19 and 0.74 ± 0.17, respectively, peak inspiratory pressure 22.1 ± 5.0 and 23.5 ± 6.8 cm H2O, and mean airway pressure 10.3 ± 3.6 and 11.6 ±4 .5 cm H2O. Baseline values of pulmonary function were similar in both groups.
2. Administration of thiazide with spironolactone vs. thiazide alone in controls:
Hoffman 2000: parallel design. Patients were randomized to receive either thiazide and spironolactone for two weeks or thiazide only. A washout period of three to five days was used for patients who had received furosemide before enrollment. Change scores were estimated using Follmann's formula. A total of 33 infants were randomized, 17 into the treatment group (four of which were intubated upon enrolment) and 16 into the placebo group (two of which were intubated upon enrolment). Mean birth weight was, respectively, 838 ± 204 and 859 ± 160 g, gestational age 26.1 ± 1.4 and 26.2 ± 1.7 weeks, postnatal age 6.7 and 6.5 weeks, postmenstrual age 32.8 ± 3.5 and 32.7 ± 1.7 weeks, FiO2 0.31 ± 0.11 and 0.27 ± 0.06, lung compliance 1.14 ± 0.37 and 1.15 ± 0.40 ml/cm H2O/kg, tidal volume 6.9 ± 1.7 and 6.0 ± 1.7 ml, and total lung resistance 52.5 ± 24.3 and 56.8 ± 20.1 cm H2O/L/sec. Serum electrolytes (sodium and potassium) were similar in the two groups. Total fluid intake was similar in the two groups and ranged from 140 to 160 ml/kg/day. 3. Administration of furosemide with metolazone vs. furosemide alone:
Segar 1997: parallel design. Patients were randomized to receive either furosemide with metolazone, or furosemide alone. Change scores were estimated using Follmann's formula. This study assessed none of the primary outcome variables selected for the current review and did not assess pulmonary mechanics. Patients who had received diuretics within the previous seven days or indomethacin within 14 days were not eligible. Seven patients were allocated to the treatment group and five to the control group. The average birthweight was 778 ± 119 g in the treatment group and 810 ± 96 g in the control group. Gestational age was 26.0 ± 5.6 weeks and 26.6 ± 3.4 weeks, respectively. Postnatal age at the time of study entry was 42 ± 13 days and 37 ± 13 days, respectively. Weight at the time of study entry was 1201 ± 286 g and 1278 ± 418 g, respectively. In the treatment group, all patients were receiving mechanical ventilation, whereas in the control group, 2/7 patients required positive airway pressure without mechanical ventilation. Fluid intake was not significantly different between the two groups. The percent inspiratory oxygen was > 0.35 in all patients.
Risk of bias in included studies
1. Administration of a thiazide diuretic, versus placebo or no treatment in controls:
1.1. Non‐intubated patients: Engelhardt 1989: The method of randomization is not provided. Intervention was not blinded. Blinding of outcome was inadequate: investigators performing pulmonary function measurements were not blinded, while those performing final calculations and data analysis were blinded. All patients were followed.
Kao 1984: Randomization was blinded; the code was compiled using a table of randomly assorted digits. Intervention was blinded adequately. Diuretics and placebo were each labeled using a code ("drug 1" or "drug 2"), the identity of which was unknown to nurses who administered the drugs. Outcome was blinded. All patients were followed.
Kao 1994: Blinding of randomization, intervention and outcome is unclear: all investigators but one were blinded. The role of this investigator in the study and in patient care is not clearly stated. Of 49 patients entered into the study, six were eliminated, three in each group, including five at the request of the parents and one because parents moved out of state. Blinding of outcome is not documented.
1.2. Intubated patients: Albersheim 1989: The authors used blinded randomization, specifically, a blocked randomization by the pharmacy. Intervention and outcome were also blinded. Of 35 patients entered into the study, one was withdrawn at the parents' request. Pulmonary function was measured only in patients remaining on artificial ventilation, resulting in progressively decreasing the numbers of patients especially in the treated group. This may have resulted in underestimation of effect of diuretics on pulmonary mechanics and ventilatory requirements in the treated group, because sicker patients were likely to remain intubated for a longer duration.
2. Administration of thiazide with spironolactone vs thiazide only: Hoffman 2000: This study included intubated patients, patients on nasal continuous positive airway pressure and patients receiving oxygen without positive pressure. Blinding of randomization, intervention and outcome was adequate. All patients were followed.
3. Administration of furosemide with metolazone vs furosemide only: Segar 1997: Blinding was not documented in this study. All patients were followed.
Effects of interventions
Of six studies fulfilling entry criteria, most focused on pathophysiological parameters (pulmonary mechanics) and failed to assess the primary outcomes defined in this review (e.g., need for and duration for mechanical ventilation or oxygen supplementation, BPD, mortality) or the potential complications of diuretic therapy.
Furthermore, for most outcomes, only one to three studies provided data that could be merged into a meta‐analysis, so that only a small number of patients was included in each analysis. Therefore, it is possible that real differences due to furosemide administration could have been missed. For each analysis we report the number of studies and the number of patients in which the particular outcome is reported.
ADMINISTRATION OF THIAZIDE‐SPIRONOLACTONE VERSUS PLACEBO OR NO TREATMENT IN CONTROLS (Comparison 1): 1.1. Non‐intubated patients:
1.1.1. Effect of a one‐week diuretic course: The effect of a one‐week diuretic course on pulmonary mechanics was analyzed in two studies (Engelhardt 1989; Kao 1984) (n=31 patients).
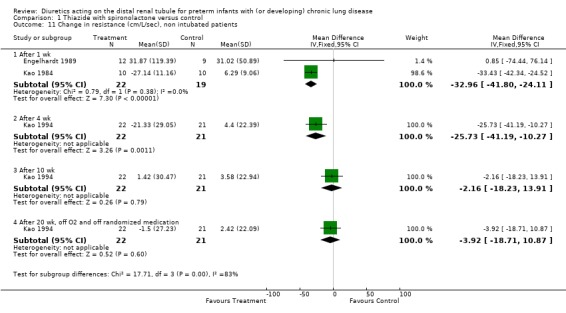
Effects on pulmonary mechanics: In Engelhardt's study, there was no significant difference in dynamic lung compliance, resistance and oxygenation between the two groups on day 8. In Kao's study, the authors reported that diuretic administration decreased resistance, increased compliance and airway conductance, but did not affect total gas volume. Summary statistics showed no significant effect of diuretic administration on the change in lung compliance (WMD 0.44 ml/cm H2O/kg, 95%CI ‐0.05 to +0.93) but a significant decrease in airway resistance (WMD ‐33.0 cm/L/sec, 95%CI ‐41.8 to ‐24.1). Diuretic administration improved dynamic compliance and resistance compared with placebo in one study (cross‐over trial, n = 10 patients) (Kao 1984) but not in the other study (parallel design, n = 21 patients) (Engelhardt 1989). Heterogeneity test reached significance for compliance (chi‐square = 8.71) but not for resistance (chi‐square = 0.82). This heterogeneity may have resulted from differences in severity of lung disease (21% inhaled oxygen in Kao's study vs. 35% oxygen for Engelhardt's study), study design (cross‐over with adequate demonstration of lack of carry‐over or period effect vs parallel), blinding (adequate vs. none), washout period before study entry (48 hr versus none). Lack of significance of the heterogeneity test for resistance may have resulted from the large CI for Engelhardt study compared to Kao's study, possibly related to differences in method of measurement (pneumotachygraph, transducer and esophageal catheter, vs. plethysmograph, respectively).
Effects on urine output and calcium excretion: In Engelhardt's study, diuretic administration significantly increased urine output vs baseline after one and two days of treatment, but not after three and four days. A one‐week administration of diuretics tended to decrease calcium excretion (Kao 84) (n = 10 patients). In Kao's study, diuretic administration increased urine output, fluid excretion, excretion of potassium, excretion of phosphate, and osmolal clearance, and decreased urinary excretion of calcium, but did not affect creatinine clearance. Comparison of first‐exposure data between diuretic and placebo yielded results that were identical to the above, except that the difference in urinary excretion of phosphate and calcium and the osmolal clearance did not reach statistical significance. Weight gain was less during each of the first three days of diuretic administration than during the corresponding days of placebo.
1.1.2. Effect of chronic diuretic administration: The effects of chronic distal diuretic administration were analyzed in a single study involving 43 randomized patients (Kao 1994). Total duration of the course was approximately 20 weeks in both groups (138 ± 58 days in the treatment group and 148 ± 49 days in the control group).
The percent inspiratory oxygen after four weeks was lower in the diuretic group than in the control group (0.23 ± 0.02 vs 0.29 ± 0.11, p < 0.01). However, distal diuretic administration did not significantly affect the change in percent inspiratory oxygen or the duration of supplemental oxygen.
Distal diuretic administration did not significantly affect the length of hospital stay after study entry. Chronic distal diuretic administration significantly increased the percentage of patients needing rehospitalization for respiratory deterioration until one year of corrected age (RR 2.23, 95%CI 1.06 to 4.70; RD 0.35, 95%CI 0.07 to 0.63), but did not affect the mean number of hospitalizations per patient (one in the treatment group vs. 0.9 in controls).
Diuretic administration did not affect survival rate.
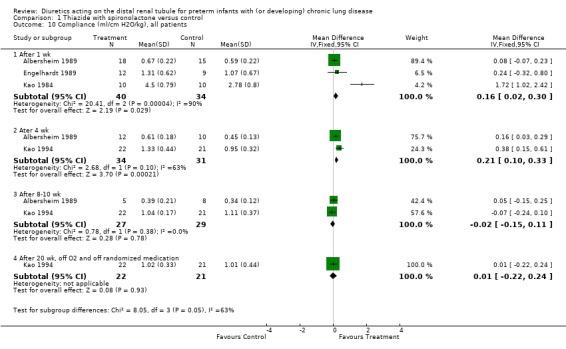
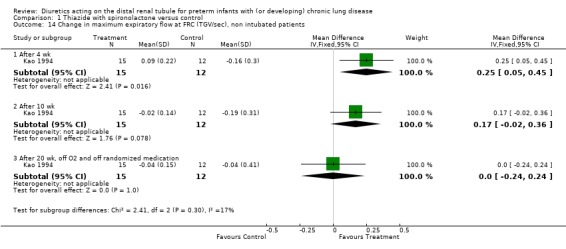
Chronic diuretic administration significantly improved lung compliance at four weeks (WMD 0.62 ml/cm H2O/kg, 95%CI 0.38 to 0.86, n = 43) and at 20 weeks (after stopping therapy, WMD 0.25 ml/cm H2O/kg, 95%CI 0.02 to 0.48) and tended to improve compliance at ten weeks (WMD 0.17 ml/cm H2O/kg, 95%CI ‐ 0.03 to +0.37). Distal diuretic administration improved airway resistance at four weeks (WMD ‐26 cm/L/sec, CI ‐41 to ‐10) but not at ten weeks or at 20 weeks. Distal diuretic administration improved maximum expiratory flow at functional residual capacity at four weeks (WMD 0.25 total gas volume/sec, 95%CI 0.05 to 0.45) but not at ten weeks and 20 weeks.
The number of patients given at least 10 doses of furosemide for clinical indication was lower in the diuretic group than in the control group (5/21 vs. 0/22, p < 0.05). Chronic diuretic administration reduced the risk for needing at least one dose of furosemide (RR 0.32, 95%CI 0.10 to 1.02; RD ‐0.29, 95%CI ‐0.55 to +‐0.04, n = 43).
Distal diuretic administration did not affect the incidence of nephrocalcinosis (RR 1.34, 95%CI 0.50 to 3.56; RD 0.08, 95%CI ‐0.19 to +0.35, n = 43), the incidence of hearing loss or body size at one year. Diuretic administration did not affect weight and length at one year of age
1.2. Intubated patients: One study was included in this category (Albersheim 1989) (n = 34 patients). At eight weeks, 5/19 patients in the treatment group required ventilation, compared with 8/14 in the control group (p = 0.15). Diuretic administration tended to decrease the risk for lack of extubation after eight weeks (RR 0.46, 95%CI 0.19 to 1.11; RD ‐0.31, 95%CI ‐0.63 to +0.02). Diuretic therapy did not affect the total number of ventilator days or length of hospital stay (non‐parametric analysis performed by the authors, details not provided).
Patients in the treatment group had a significantly lower FiO2 than those in the control group on weeks four (p < 0.01) and eight (p = 0.05), but similar mean airway pressure; data are not provided.
Survival at discharge was more frequent in the treatment group than in controls (16/19 or 84% vs 7/15 or 47%, p = 0.025); however, this did not reach statistical significance among boys (9/11 vs 7/14, p = 0.21). Thus, an eight week diuretic treatment significantly decreased death rate before discharge (RR 0.30, 95%CI 0.09 to 0.93, RD ‐0.38, 95%CI ‐0.68 to ‐0.07), and tended to decrease death rate in boys (RR 0.36, 95%CI 0.09 to 1.41; RD ‐0.32, 95%CI ‐0.67 to +0.03).
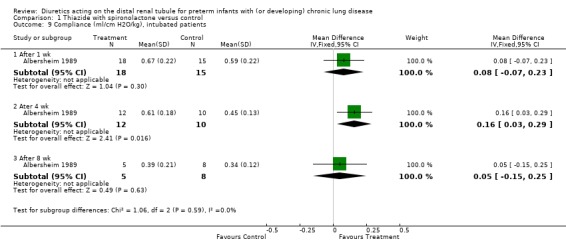
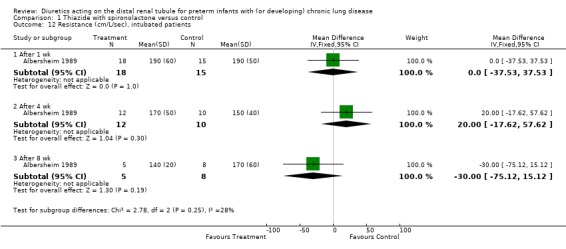
Pulmonary mechanics was assessed only in intubated patients; change scores could not be calculated because the number of patients remaining intubated decreased with time; WMD was calculated using mean values at each time point. Diuretic administration significantly improved lung compliance in intubated patients after four weeks (WMD 0.16 ml/cm H2O/kg, CI 0.03 to 0.29, n = 22) but not after one week (WMD +0.08 ml/cm H2O/kg, CI ‐0.07 to +0.23) or eight weeks (WMD 0.05 ml/cm H2O/kg, 95%CI ‐0.15 to +0.25, n = 13). Diuretic administration did not significantly affect airway resistance in intubated patients at one week, four weeks or eight weeks.
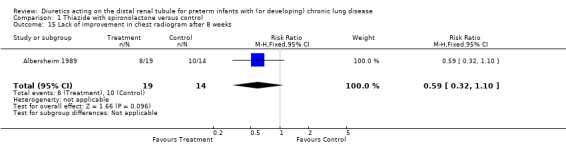
Chest radiographs improved over the course of the study in 11/19 patients in the treatment group and in 4/14 in the control group (p = 0.09), yielding a RR of 0.59, 95%CI 0.32 to 1.10 and a RD of ‐0.29, 95%CI ‐0.62 to +0.03.
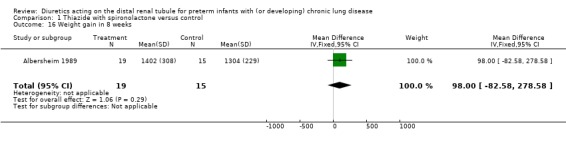
Diuretic administration did not affect weight gain in eight weeks. Total amount of furosemide administered by the clinicians in the treatment group was 3.7 ± 7.3 mg/kg, compared to 12.0 ± 9.7 mg/kg in the control group, p = 0.08, WMD ‐8.3 mg/kg, CI ‐14.2 to ‐2.4.
One patient in the treatment group and none in the control group developed severe electrolyte abnormality requiring withdrawal from the study (RR 2.40, 95%CI 0.10 to 55.03; RD 0.05, 95%CI ‐0.10 to +0.21). Seventeen patients in the treatment group and 15 in the control group required Na or K supplementation (RR 1.92, 95%CI 1.09 to 3.37; RD 0.43, 95%CI 0.14 to 0.72).
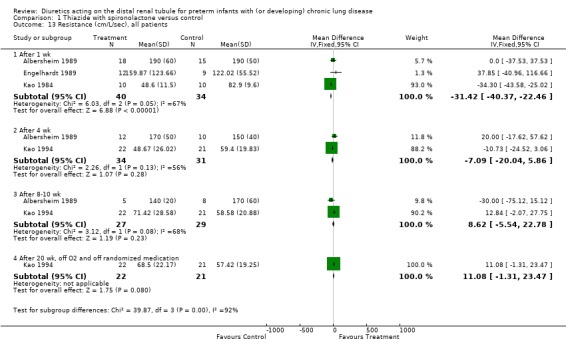
1.3. Overall summary effects of thiazide‐spironolactone: Summary effects could be calculated for mortality rate, compliance, resistance and need for furosemide administration. Limitations of summary effects on compliance and resistance include (1) expected differences in pulmonary mechanics between intubated and non intubated patients (Brion 1999a), (2) differences in baseline values between the two groups in Kao's study (3) attrition bias in Albersheim's study.
Chronic administration of thiazide‐spironolactone significantly decreased mortality rate (RR 0.30, 95%CI 0.09 to 0.93; RD ‐0.17, 95%CI ‐0.31 to ‐0.02, n=77 patients) (Albersheim 1989; Kao 1994). Test for heterogeneity was highly significant (chi‐square 15.88); this heterogeneity may have resulted from difference in patient population (intubated in Albersheim's study vs not intubated in Kao's study), or age at entry (11 ‐ 12 weeks versus seven weeks).
Administration of distal diuretics significantly improved compliance after 1 wk (WMD 0.16 ml/cm H2O/kg, 95%CI 0.02 to 0.30, n = 74) (Albersheim 1989; Engelhardt 1989; Kao 1984) and 4 wk (WMD 0.21 ml/cm H2O/kg, 95%CI 0.10 to 0.33, n = 65) (Albersheim 1989; Kao 1994) of therapy. No effect was observed at 8 ‐ 10 wk (Albersheim 1989; Kao 1994) or after stopping the treatment (Kao 1994). The test for heterogeneity was highly significant at one week (chi‐square = 20.41, df = 2); this could be explained by reasons explained above (see 1.1), by expected differences between intubated and non intubated patients, by attrition bias in Albersheim's study (possibly reducing the effect of diuretics), and by difference in method of measuring pulmonary mechanics (static in Albersheim's study vs dynamic in the other studies).
Administration of distal diuretics improved airway resistance after one week (WMD ‐31.4 cm/L/sec, 95%CI ‐40.4 to ‐22.5, n = 74) (Albersheim 1989; Engelhardt 1989; Kao 1984), but not at four weeks (n = 65) (Albersheim 1989; Kao 1994), and tended to increase resistance at 8 ‐ 10 wk (Albersheim 1989; Kao 1994) and after stopping therapy (Kao 1994). The test for heterogeneity was significant at one week (chi‐square = 6.03); this could be explained by reasons discussed for compliance (see above).
Chronic administration of thiazide‐spironolactone reduced the use of furosemide given on clinical indication, regardless of the need for mechanical ventilation at study entry. Patients treated with thiazide‐spironolactone for 8‐20 weeks tended to be at less risk for receiving at least one dose of furosemide (RR 0.32, 95%CI 0.10 to 1.02; RD ‐0.29, 95%CI ‐0.55 to ‐0.04, n = 43) (Kao 1994) and were at significantly less risk for receiving at least 10‐12 doses (RR 0.12, 95%CI 0.02 to 0.65; RD ‐0.26, 95%CI ‐0.42 to ‐0.10, n = 77) during the study.
Thus, the only consistent overall effects of chronic administration of distal diuretics were an improvement in compliance at four weeks of treatment and a decrease in furosemide administration on clinical indication. For the other outcomes, there was substantial heterogeneity across studies.
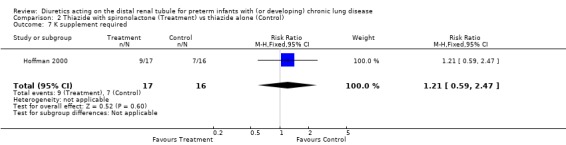
ADMINISTRATION OF THIAZIDE AND SPIRONOLACTONE VS. THIAZIDE ALONE (Comparison 2): A single study involving a total of 33 patients was included in this comparison (Hoffman 2000). Failure of extubation was observed in 1/4 patients in the treatment group and 2/2 in the control group (RR 0.25, 95%CI 0.05 to 1.36; RD ‐0.53, 95%CI ‐1.12 to +0.05). The use of spironolactone did not affect the change in percent inspiratory oxygen.
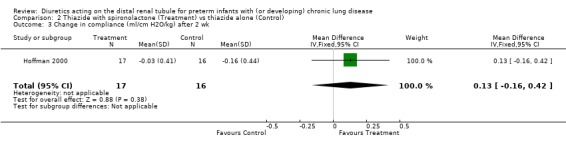
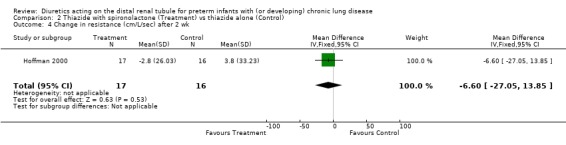
The use of spironolactone did not affect the change in compliance, resistance or tidal volume within two weeks.
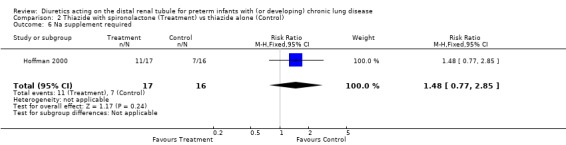
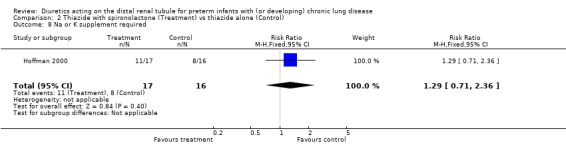
Using spironolactone in addition to thiazide did not significantly affect the risk of needing sodium supplementation (RR 1.48, 95%CI 0.77 to 2.85; RD +0.21, 95%CI ‐0.12 to +0.54), the risk of needing potassium supplementation (RR 1.21, 95%CI 0.59 to 2.47; RD +0.09, CI ‐0.25 to +0.43), or the risk of needing either sodium or potassium supplementation (RR 1.29, 95%CI 0.71 to 2.36; RD +0.15, 95%CI ‐0.19 to +0.48).
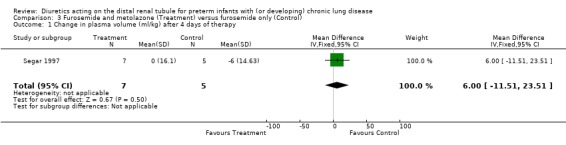
ADMINISTRATION OF FUROSEMIDE AND METOLAZONE VS. FUROSEMIDE ALONE (Comparison 03): The single study in this group (Segar 1997) provided information on only one of the outcomes defined for this review: plasma volume. Metolazone addition to furosemide did not significantly affect the change in plasma volume after a four‐day treatment. Plasma volume did not change significantly during treatment in either group. Extracellular water volume decreased similarly in both groups. Body weight decreased by 11 ± 27g in the treatment group and increased by 63 ± 69 g in the control group (p<0.05). The lower weight gain and the increased urine output in patients on furosemide‐metolazone compared with those on furosemide resulted from a decrease in interstitial fluid. Two‐way analysis of variance showed a transient increase in urine output in the treatment group but not in the control group (p<0.05).
Discussion
1. Limitations 1.1. Limitations of the studies included in this review: Design: Blinding was either not documented or inadequate in half the studies available for this review; this could have biased the results toward an increase in the effects of diuretics on outcome.
1.2. Limitations of this review: It is possible that the incidence of lung edema in preterm infants may have decreased as a result of changes in therapy introduced in the last decade (Gortner 1991, Sonntag 1996).
Outcomes analyzed: Most studies focused on pulmonary mechanics and did not assess the most important outcomes defined in this review.
Methods used for the analysis: The methods recommended by the Cochrane Neonatal Review Group were used. For almost all studies, the review authors did not have access to the original data. Therefore, multiple transformations using formulae established by or derived from Follmann, Baird, and Armitage were used. Calculations using Baird and Armitage overestimate the SD of the final variable if the numerator is related to the denominator, because they assume lack of correlation between them. For calculations using Follman's formula, a pretest‐posttest correlation of 0.4 was assumed and a sensitivity analysis was conducted using a correlation coefficient of 0.3 ‐ 0.5. The sensitivity analysis yielded limited variation, no substantive change in the results and no change in the conclusions. Limited data in the literature (Kugelman 1997) suggest that this value is close to the observed correlation for dynamic resistance (0.4 for a two‐hour, 0.7 for a one‐hour period) but lower than observed for dynamic compliance (0.8 for a one‐hour or a two‐hour period) . A high mean correlation coefficient (Cronbach alpha) was noted for serial dynamic measurements of tidal volume (r = 0.72), minute ventilation (r = 0.84), compliance (r = 0.92) and resistance (r = 0.80) obtained in full‐term infants over a period of 66 hours (Goyal 1995). To the review authors' knowledge, there are no such values in preterm infants. Therefore, the SD of change scores for compliance may be over‐estimated. Thus, results are likely to be conservative, i.e., confidence intervals are wider than they should be.
Heterogeneity: Within each subgroup, a chi‐square analysis was used to test for statistical evidence of heterogeneity among studies. When chi‐square analysis was significant, differences (in patient selection, baseline values, bias, design and methods) among studies that could possibly explain the heterogeneity were analyzed. Because of the long half‐life of thiazides and spironolactone a prolonged washout period is needed if one wishes to eliminate any residual diuretic activity before initiating a clinical trial or between exposures in cross‐over trials. However, a prolonged washout period may not be possible or ethically acceptable for patients considered clinically to require diuretics. Several studies had a short or no washout period, thereby possibly decreasing the apparent effect of diuretic administration on short‐ and long‐term outcome. All but one cross‐over trials failed to provide information that would rule out a carry‐over effect and a period effect; this could also have reduced the apparent effect of diuretics on outcome.
Sample size: Because of small sample size in most of the subgroups, any real effects of furosemide may have remained undetected.
2. Group‐specific comments: Overall, long‐term administration of thiazides improves lung compliance at four weeks of therapy and reduces administration of furosemide in preterm infants with CLD, regardless of need for mechanical ventilation at the onset of the treatment. The single study (Kao 1994) assessing the effect of chronic thiazide therapy in non‐intubated patients showed a significant increase in the risk of at least one rehospitalization for respiratory deterioration until one year of corrected age, but not in the number of rehospitalizations per infant. It also showed an improvement in lung compliance (significant at four weeks and 20 weeks) and a transient improvement in airway resistance and expiratory flow at four weeks. The single study (Albersheim 1989) assessing the effect of chronic thiazide therapy in intubated patients showed a significant decrease in mortality. A similar trend was observed in males but the percentage of males was not similar in the two groups. Pulmonary mechanics were obtained only in intubated patients, probably resulting in attrition bias and thus underestimating the effect of distal diuretics on lung function. The study design excluded the administration of corticosteroids, bronchodilators and aminophylline. Finally, the study was conducted before the era of prenatal steroids and surfactant (Sonntag 1996).
The single study (Hoffman 2000) comparing thiazide‐spironolactone combination with thiazide alone showed no difference in oxygen requirement, pulmonary mechanics or need for electrolyte supplementation. The numbers requiring mechanical ventilation at study entry (n = 6) were too small to yield useful data about this outcome. Further studies are needed to compare the effect of these two therapeutic modalities on the severity and the prognosis of CLD, bone demineralization and renal toxicity (nephrocalcinosis, nephrolithiasis).
The single study (Segar 1997) comparing furosemide‐metolazone with furosemide alone showed no difference in the change in plasma volume. Neither this study, nor a related study by the same group (Segar 1992), assessed the effects of metolazone on any of the primary outcomes defined for this review. Thus, neither study showed any evidence supporting the hypothesis that increasing urine output by adding metolazone to furosemide improves the outcome of preterm infants with CLD. Studies are needed to assess whether metolazone has any beneficial effect on the severity and the prognosis of CLD.
Authors' conclusions
Implications for practice.
Despite the widespread use of distal diuretics in preterm infants with CLD, the clinical impact of their use has been studied in surprisingly few infants in randomized clinical trials. In infants > 3 weeks of age with CLD, chronic administration of thiazide and spironolactone improves lung compliance at four weeks of treatment and reduces need for furosemide. Nevertheless, the role of adding spironolactone to hydrochlorothiazide remains to be investigated.
The single study assessing the effect of distal diuretics in intubated patients showed a significant decrease in mortality and a trend toward a decreased risk of failure to extubate within 8 weeks of therapy. In contrast, there is little or no evidence to support any benefit of diuretic administration in non‐intubated patients.
Implications for research.
Studies are needed to assess (1) whether thiazide administration improves mortality, duration of O2 dependency, duration of ventilator‐dependency, length of hospital stay and long‐term outcome in patients exposed to current therapy and (2) whether adding spironolactone to thiazides or adding metolazone to furosemide modifies the risks and benefits.
What's new
| Date | Event | Description |
|---|---|---|
| 28 December 2010 | New search has been performed | This updates the review "Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease", published in the Cochrane Database of Systematic Reviews (Brion 2008).
Searches of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 4, 2010), MEDLINE, EMBASE and CINAHL in December 2010 did not yield any additional eligible studies. Additional searches in clinicaltrials.gov and controlled‐trials.com did not yield any eligible studies. No change to conclusions. |
| 28 December 2010 | New citation required but conclusions have not changed | Change in authorship. |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 3 June 2008 | Amended | Converted to new review format. |
| 30 April 2007 | New search has been performed | This review updates the previously published review "Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease", The Cochrane Library, Issue 3, 2003 (Brion 2003). Searches of MEDLINE in April 2007, Embase in April 2007 and the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 1, 2007) did not yield any additional eligible studies. Hand searching of the Neonatal Society [UK] and RCPCH abstracts in April 2007 did not yield any additional eligible studies. No substantive changes were made to the review. |
| 24 November 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
This review is part of a group of three closely related reviews on diuretics in preterm infants with (or developing) chronic lung disease (Brion 1999 a,b). Angel Rios and Jean‐Yves Pauchard participated in the editing of the protocol used in these three reviews. We wish to thank Dr. Albersheim for providing us with additional data. The reader is also referred to a systematic review on furosemide in indomethacin‐treated patients (Brion 1998) and a systematic review on diuretics in preterm infants with respiratory distress syndrome (Brion 1999c).
Data and analyses
Comparison 1. Thiazide with spironolactone versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lack of extubation after 8 weeks of treatment | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.19, 1.11] |
| 2 Change in % inspiratory O2 after 4 wk | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐11.65, 3.65] |
| 3 Duration of O2 supplementation (days) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐12.00 [‐51.58, 23.58] |
| 4 Length of hospital stay (days) after study entry | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐32.01, 26.01] |
| 5 Rehospitalization(s) for respiratory deterioration | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.06, 4.70] |
| 6 Death before discharge | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.09, 0.93] |
| 6.1 Non intubated patients | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Intubated patients | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.09, 0.93] |
| 7 Death before discharge in males (intubated patients) | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.09, 1.41] |
| 8 Change in compliance (ml/cm H2O/kg), non intubated patients | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 After 1 wk | 2 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [‐0.05, 0.93] |
| 8.2 After 4 wk | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [0.38, 0.86] |
| 8.3 After 10 wk | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.03, 0.37] |
| 8.4 After 20 wk, off O2 and off randomized medication | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.02, 0.48] |
| 9 Compliance (ml/cm H2O/kg), intubated patients | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 After 1 wk | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.07, 0.23] |
| 9.2 Ater 4 wk | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.03, 0.29] |
| 9.3 After 8 wk | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.15, 0.25] |
| 10 Compliance (ml/cm H2O/kg), all patients | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 After 1 wk | 3 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.02, 0.30] |
| 10.2 Ater 4 wk | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [0.10, 0.33] |
| 10.3 After 8‐10 wk | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.15, 0.11] |
| 10.4 After 20 wk, off O2 and off randomized medication | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.22, 0.24] |
| 11 Change in resistance (cm/L/sec), non intubated patients | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 After 1 wk | 2 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐32.96 [‐41.80, ‐24.11] |
| 11.2 After 4 wk | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐25.73 [‐41.19, ‐10.27] |
| 11.3 After 10 wk | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐2.16 [‐18.23, 13.91] |
| 11.4 After 20 wk, off O2 and off randomized medication | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐3.92 [‐18.71, 10.87] |
| 12 Resistance (cm/L/sec), intubated patients | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 After 1 wk | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐37.53, 37.53] |
| 12.2 After 4 wk | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 20.0 [‐17.62, 57.62] |
| 12.3 After 8 wk | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐30.0 [‐75.12, 15.12] |
| 13 Resistance (cm/L/sec), all patients | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 After 1 wk | 3 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐31.42 [‐40.37, ‐22.46] |
| 13.2 After 4 wk | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐7.09 [‐20.04, 5.86] |
| 13.3 After 8‐10 wk | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | 8.62 [‐5.54, 22.78] |
| 13.4 After 20 wk, off O2 and off randomized medication | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 11.08 [‐1.31, 23.47] |
| 14 Change in maximum expiratory flow at FRC (TGV/sec), non intubated patients | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 After 4 wk | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.05, 0.45] |
| 14.2 After 10 wk | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.02, 0.36] |
| 14.3 After 20 wk, off O2 and off randomized medication | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.24, 0.24] |
| 15 Lack of improvement in chest radiogram after 8 weeks | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.10] |
| 16 Weight gain in 8 weeks | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 98.0 [‐82.58, 278.58] |
| 17 Need for at least 1 dose of furosemide during the study | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.10, 1.02] |
| 18 Need for at least 10‐12 doses of furosemide during the study | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.65] |
| 19 Total dose of furosemide required in 8 weeks (mg/kg) | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐8.3 [‐14.21, ‐2.39] |
| 20 Severe electrolyte anomaly requiring withdrawal from the study | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [0.10, 55.03] |
| 21 Need for Na or K supplementation | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.09, 3.37] |
| 22 Calcium excretion (%) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐5.27, 1.87] |
| 23 Nephrocalcinosis | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.50, 3.56] |
| 24 Hearing loss | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.19, 19.52] |
| 25 Weight (Kg) at one year postterm corrected age | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.61, 1.01] |
| 26 Length (cm) at one year postterm corrected age | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.81, 2.21] |
1.1. Analysis.
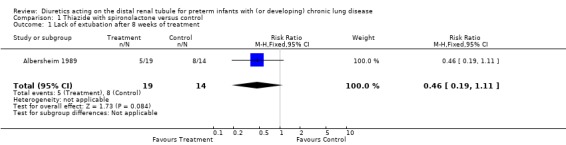
Comparison 1 Thiazide with spironolactone versus control, Outcome 1 Lack of extubation after 8 weeks of treatment.
1.2. Analysis.
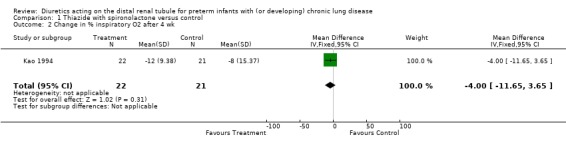
Comparison 1 Thiazide with spironolactone versus control, Outcome 2 Change in % inspiratory O2 after 4 wk.
1.3. Analysis.
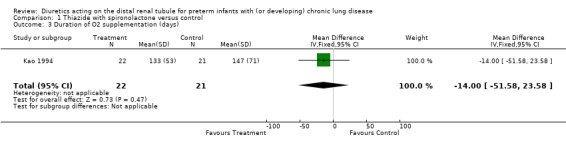
Comparison 1 Thiazide with spironolactone versus control, Outcome 3 Duration of O2 supplementation (days).
1.4. Analysis.
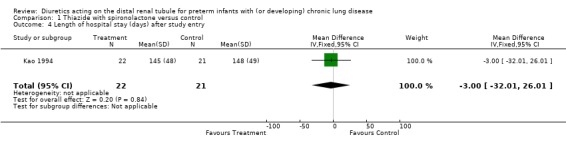
Comparison 1 Thiazide with spironolactone versus control, Outcome 4 Length of hospital stay (days) after study entry.
1.5. Analysis.
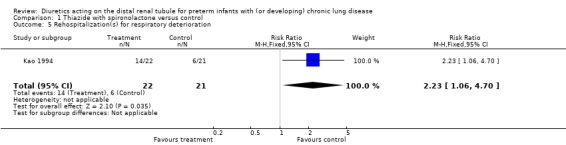
Comparison 1 Thiazide with spironolactone versus control, Outcome 5 Rehospitalization(s) for respiratory deterioration.
1.6. Analysis.
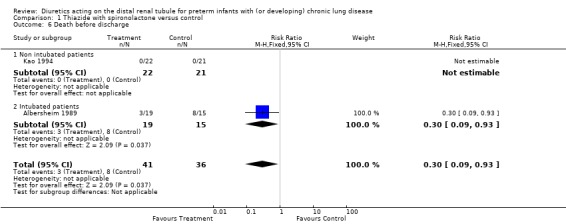
Comparison 1 Thiazide with spironolactone versus control, Outcome 6 Death before discharge.
1.7. Analysis.
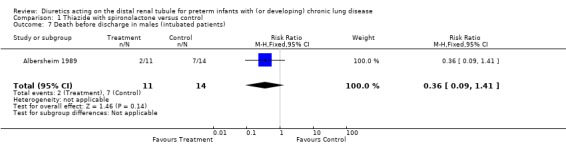
Comparison 1 Thiazide with spironolactone versus control, Outcome 7 Death before discharge in males (intubated patients).
1.8. Analysis.
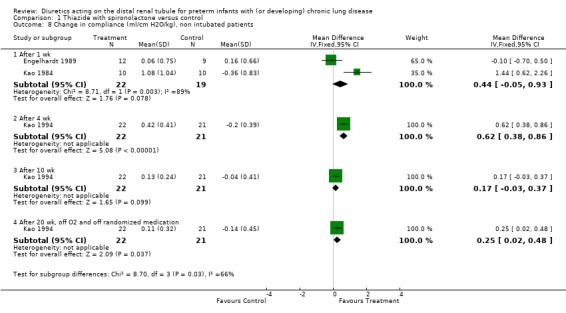
Comparison 1 Thiazide with spironolactone versus control, Outcome 8 Change in compliance (ml/cm H2O/kg), non intubated patients.
1.9. Analysis.
Comparison 1 Thiazide with spironolactone versus control, Outcome 9 Compliance (ml/cm H2O/kg), intubated patients.
1.10. Analysis.
Comparison 1 Thiazide with spironolactone versus control, Outcome 10 Compliance (ml/cm H2O/kg), all patients.
1.11. Analysis.
Comparison 1 Thiazide with spironolactone versus control, Outcome 11 Change in resistance (cm/L/sec), non intubated patients.
1.12. Analysis.
Comparison 1 Thiazide with spironolactone versus control, Outcome 12 Resistance (cm/L/sec), intubated patients.
1.13. Analysis.
Comparison 1 Thiazide with spironolactone versus control, Outcome 13 Resistance (cm/L/sec), all patients.
1.14. Analysis.
Comparison 1 Thiazide with spironolactone versus control, Outcome 14 Change in maximum expiratory flow at FRC (TGV/sec), non intubated patients.
1.15. Analysis.
Comparison 1 Thiazide with spironolactone versus control, Outcome 15 Lack of improvement in chest radiogram after 8 weeks.
1.16. Analysis.
Comparison 1 Thiazide with spironolactone versus control, Outcome 16 Weight gain in 8 weeks.
1.17. Analysis.
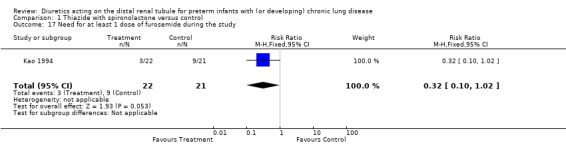
Comparison 1 Thiazide with spironolactone versus control, Outcome 17 Need for at least 1 dose of furosemide during the study.
1.18. Analysis.
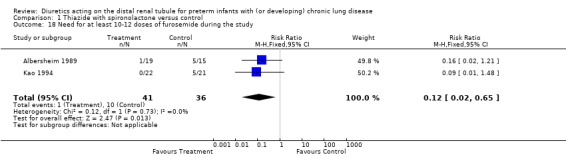
Comparison 1 Thiazide with spironolactone versus control, Outcome 18 Need for at least 10‐12 doses of furosemide during the study.
1.19. Analysis.
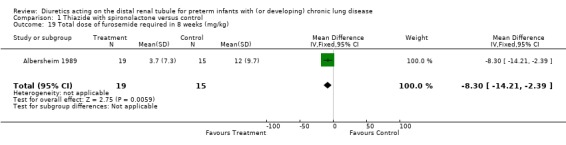
Comparison 1 Thiazide with spironolactone versus control, Outcome 19 Total dose of furosemide required in 8 weeks (mg/kg).
1.20. Analysis.
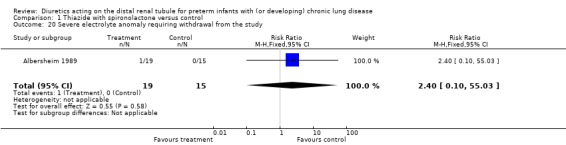
Comparison 1 Thiazide with spironolactone versus control, Outcome 20 Severe electrolyte anomaly requiring withdrawal from the study.
1.21. Analysis.
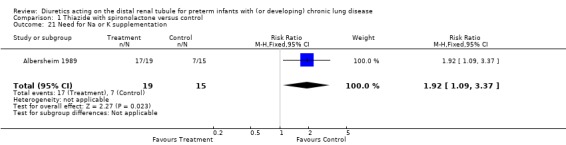
Comparison 1 Thiazide with spironolactone versus control, Outcome 21 Need for Na or K supplementation.
1.22. Analysis.
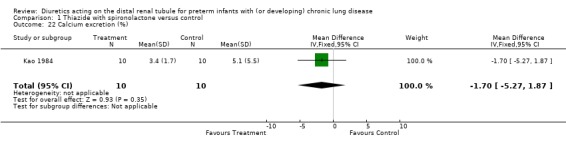
Comparison 1 Thiazide with spironolactone versus control, Outcome 22 Calcium excretion (%).
1.23. Analysis.
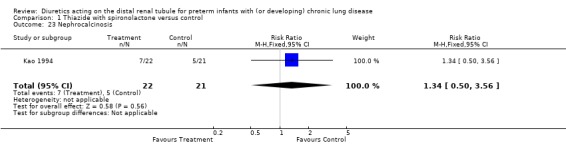
Comparison 1 Thiazide with spironolactone versus control, Outcome 23 Nephrocalcinosis.
1.24. Analysis.
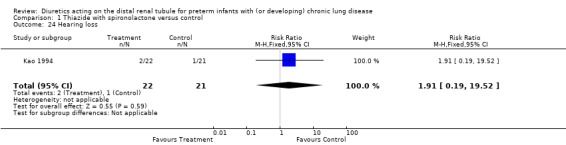
Comparison 1 Thiazide with spironolactone versus control, Outcome 24 Hearing loss.
1.25. Analysis.
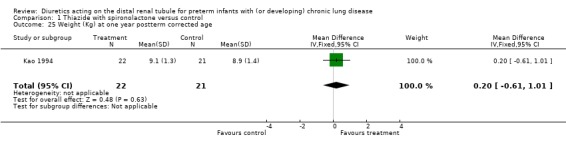
Comparison 1 Thiazide with spironolactone versus control, Outcome 25 Weight (Kg) at one year postterm corrected age.
1.26. Analysis.
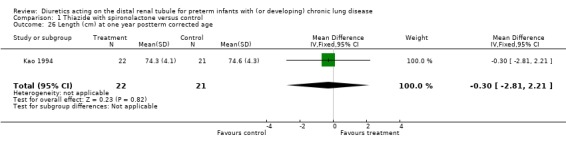
Comparison 1 Thiazide with spironolactone versus control, Outcome 26 Length (cm) at one year postterm corrected age.
Comparison 2. Thiazide with spironolactone (Treatment) vs thiazide alone (Control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lack of extubation after 2 wk | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.09, 1.51] |
| 2 Change in % O2 after 2 wk | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 3 Change in compliance (ml/cm H2O/kg) after 2 wk | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.16, 0.42] |
| 4 Change in resistance (cm/L/sec) after 2 wk | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐6.6 [‐27.05, 13.85] |
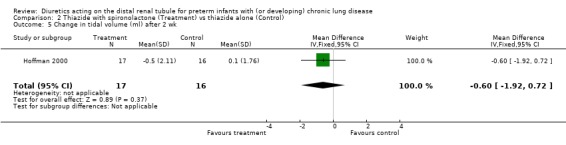
| 5 Change in tidal volume (ml) after 2 wk | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐1.92, 0.72] |
| 6 Na supplement required | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.77, 2.85] |
| 7 K supplement required | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.59, 2.47] |
| 8 Na or K supplement required | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.71, 2.36] |
2.1. Analysis.
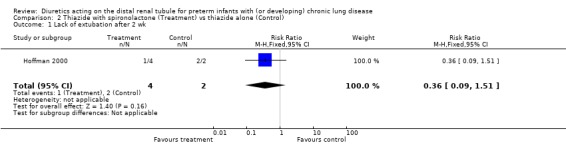
Comparison 2 Thiazide with spironolactone (Treatment) vs thiazide alone (Control), Outcome 1 Lack of extubation after 2 wk.
2.2. Analysis.
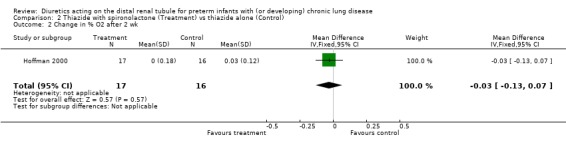
Comparison 2 Thiazide with spironolactone (Treatment) vs thiazide alone (Control), Outcome 2 Change in % O2 after 2 wk.
2.3. Analysis.
Comparison 2 Thiazide with spironolactone (Treatment) vs thiazide alone (Control), Outcome 3 Change in compliance (ml/cm H2O/kg) after 2 wk.
2.4. Analysis.
Comparison 2 Thiazide with spironolactone (Treatment) vs thiazide alone (Control), Outcome 4 Change in resistance (cm/L/sec) after 2 wk.
2.5. Analysis.
Comparison 2 Thiazide with spironolactone (Treatment) vs thiazide alone (Control), Outcome 5 Change in tidal volume (ml) after 2 wk.
2.6. Analysis.
Comparison 2 Thiazide with spironolactone (Treatment) vs thiazide alone (Control), Outcome 6 Na supplement required.
2.7. Analysis.
Comparison 2 Thiazide with spironolactone (Treatment) vs thiazide alone (Control), Outcome 7 K supplement required.
2.8. Analysis.
Comparison 2 Thiazide with spironolactone (Treatment) vs thiazide alone (Control), Outcome 8 Na or K supplement required.
Comparison 3. Furosemide and metolazone (Treatment) versus furosemide only (Control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in plasma volume (ml/kg) after 4 days of therapy | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐11.51, 23.51] |
3.1. Analysis.
Comparison 3 Furosemide and metolazone (Treatment) versus furosemide only (Control), Outcome 1 Change in plasma volume (ml/kg) after 4 days of therapy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albersheim 1989.
| Methods | Blinding of randomization: yes (blocked randomization by the pharmacy) Blinding of intervention: yes Complete follow‐up: no. Of 35 patients recruited into the study, one was withdrawn from the study at parents request. Of 19 patients in the treatment group, 16 completed the study, 2 were discharged from the hospital during week 8 of the study and 1 patient was transferred to another hospital. Of 15 patients in the control group, 11 completed the study, 1 died on day 20 of the study, 2 were discharged from the hospital during week 8 of the study and 1 during week 6. Blinding of outcome: yes. Randomized controlled trial, parallel design No washout period | |
| Participants | Patients entered into the study: n=35 Mechanical ventilation. Entry criteria included: chest radiograph compatible with BPD, postnatal age at least 1 month, ventilator dependency, oxygen requirement at least 30% O2, and full enteral feedings by gavage. The only exclusion criterion was failure to obtain informed consent from the parents. | |
| Interventions | Thiazide and spironolactone vs placebo Patients were randomly allocated to receive either treatment (hydrochlorothiazide 2 mg/kg/12 hours enterally and spironolactone 1.5 mg/kg/12 hours enterally for 8 weeks) (N=19) or placebo (similar solution with same amount of sodium) (N=15). The study protocol was transiently discontinued if serum sodium concentration <125 mM/L or potassium < 3 mM/L, until electrolyte values returned to the normal range. Patients were allowed to receive furosemide if necessary, i.e., if the patient had excessive weight gain, clinical edema and deterioration or absence of expected improvement in respiratory status. Patients on the study were prevented from receiving corticosteroids, theophylline, or aerosolized bronchodilators. | |
| Outcomes | Main outcome: survival at discharge Secondary outcomes: total numbers of ventilator days and of hospital days, pulmonary function, oxygen requirement, chest radiogram assessed using Edwards' criteria, number of doses of furosemide needed clinically. | |
| Notes | Measurements of pulmonary function and FiO2 were obtained at baseline and at 1 week, 4 weeks and 8 weeks. Pulmonary mechanics were measured by the passive occlusion technique. Only intubated patients had pulmonary function tests performed. Fluid intake on weeks 4 and 8 of the study was 13% higher in the treatment group than in the control group (p<0.01 and <0.02, respectively, at 4 and 8 weeks). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized controlled trial, parallel design Blinding of randomization: yes (blocked randomization by the pharmacy) |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes (blocked randomization by the pharmacy) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no. Of 35 patients recruited into the study, one was withdrawn from the study at parents request. Of 19 patients in the treatment group, 16 completed the study, 2 were discharged from the hospital during week 8 of the study and 1 patient was transferred to another hospital. Of 15 patients in the control group, 11 completed the study, 1 died on day 20 of the study, 2 were discharged from the hospital during week 8 of the study and 1 during week 6 |
| Other bias | High risk | Attrition bias: pulmonary mechanics were obtained only in intubated patients. |
Engelhardt 1989.
| Methods | Blinding of randomization: can't tell. The method of randomization is not provided. Blinding of intervention: no Complete follow‐up: yes Blinding of outcome: inadequate. Investigators performing pulmonary function measurements were not blinded, while those performing final calculations and data analysis were blinded. Randomized controlled trial, parallel design. No washout period documented | |
| Participants | Patients entered into the study: n=21 No mechanical ventilation. Entry criteria included: history of respiratory failure at birth, requiring mechanical ventilation and O2, postnatal age >28 days, O2 needed to maintain oxygen saturation >90%, radiographic evidence of chronic lung disease, no evidence of cor pulmonale or pulmonary infection, no tracheal intubation or mechanical ventilation at the time of the study, full enteral feedings. | |
| Interventions | Thiazide versus no medication. Patients were randomly allocated to receive either treatment (hydrochlorothiazide 3 mg/kg/day and spironolactone 3 mg/kg/day administered q 12 hours) (N=12) or no medication (control group) for 1 week (N=9). | |
| Outcomes | Main outcome: lung mechanics (dynamic lung compliance and resistance) and oxygenation; secondary outcome: urine output. | |
| Notes | Pulmonary mechanics and oxygenation were measured on the first day and after 1 week of treatment. Dynamic pulmonary mechanics were measured using an esophageal balloon. For patients randomized to thiazide, measurements were started 6 hours after the last diuretic dose. Urine output was obtained at baseline and after 1,2,3,and 4 days of treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomization is not provided |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomization: can't tell |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no Blinding of outcome: inadequate. Investigators performing pulmonary function measurements were not blinded, while those performing final calculations and data analysis were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
Hoffman 2000.
| Methods | Blinding of randomization: yes Blinding of intervention: yes Complete follow up: yes Blinding of outcome: yes Randomized controlled trial, parallel design Washout period: 3‐5 days after last dose of furosemide | |
| Participants | Patients entered into the study: n=33 The need for mechanical ventilation was not used as a criterion. Entry criteria: Infants with postconceptional age 26‐33 weeks, chronic lung disease (oxygen dependency beyond 28 days), enteral feeding, decision by the attending to prescribe oral diuretic therapy. Exclusion criteria: renal anomalies known, i.v. fluids, furosemide or early portion of a course of dexamethasone (0.1 to 0.5 mg/kg/day) | |
| Interventions | Thiazide and spironolactone vs thiazide and placebo Patients were randomly allocated to receive either chlorothiazide 20 mg/kg/day with spironolactone 3 mg/kg/day for 2 weeks (N=17), or chlorothiazide 20 mg/kg/day with placebo for 2 weeks (N=16). | |
| Outcomes | Main outcome: need for electrolyte supplementation; secondary outcome: pulmonary function, serum electrolyte concentration | |
| Notes | Dynamic pulmonary mechanics were measured using an esophageal balloon. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized controlled trial, parallel design |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow up: yes |
Kao 1984.
| Methods | Blinding of randomization: yes. The code of randomization was compiled using a table of randomly assorted digits. Blinding of intervention: yes. Diuretics and placebo were each labeled using a code ("drug 1" or "drug 2"), the identity of which was unknown to nurses who administered the drugs. Complete follow up: yes Blinding of outcome: yes Randomized clinical trial with cross‐over design; data during first exposure kept separate from those after cross‐over. Washout period: 48 hr before the study, none at cross‐over | |
| Participants | Patients entered into the study: n=10 No mechanical ventilation, no O2 requirement. Entry criteria: (1) Gestational age < 34 weeks; (2) history of RDS defined as air bronchogram and reticulogranular pattern on initial radiographs, cyanosis in room air, and grunting and retractions within the first 24 hours of life; (3) FiO2 > 0.40 and mechanical ventilation during the first 5 days of life; (4) continued need for mechanical ventilation, FiO2 > 0.30 and radiographic evidence of stage 3 or 4 of BPD by 1 month of age. Exclusion criteria: patent ductus arteriosus, mechanical ventilation or oxygen therapy at the time of study, other lung disease (e.g., pneumonia, meconium aspiration, pulmonary hypoplasia). | |
| Interventions | Thiazide and spironolactone vs thiazide and placebo Diuretic and bronchodilator therapy were discontinued 48 hours before beginning study. Patients were then randomly allocated to receive either: (1) chlorothiazide 40 mg/kg/day and spironolactone 3 mg/kg/day, both divided q 12 hours for one week, followed by a second week of placebo (N=5), or (2) placebo first, followed by diuretics (N=5). Patients received Enfamil 20 cal/oz (67 cal/100 ml) approximately 165 ml/kg/day. | |
| Outcomes | Main outcome: pulmonary mechanics Secondary outcome: fluid and mineral handling, renal function | |
| Notes | The authors reported data on fluids and electrolytes, as well as pulmonary function tests on day 6 and on day 7, respectively, of administration of drug 1 and drug 2. The authors measured thoracic gas volume and airway resistance by plethysmography and dynamic compliance using an esophageal balloon. They used appropriate statistical methods for two‐period cross‐over trial and non‐parametric analysis for weight gain. There was no period effect and no evidence for carry‐over effect. For meta‐analysis, we pooled data obtained during first exposure and those obtained after cross‐over. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized clinical trial with cross‐over design; data during first exposure kept separate from those after cross‐over. The code of randomization was compiled using a table of randomly assorted digits. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes. Diuretics and placebo were each labeled using a code ("drug 1" or "drug 2"), the identity of which was unknown to nurses who administered the drugs. Blinding of outcome: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow up: yes |
Kao 1994.
| Methods | Blinding of randomization: unclear: All investigators but one were blinded. The role of this investigator in the study and in patient care is not clearly stated. Blinding of intervention: unclear (see above). Complete follow‐up: no: 43 out of 49 patients completed the study. Six were eliminated, three in each group, including 5 at the request of the parents and 1 because parents moved out of state. Blinding of outcome: unclear (see above). Randomized trial, parallel design. Washout period: 3‐5 days before the study | |
| Participants | Patients entered into the study: n=49 No mechanical ventilation. Entry criteria: typical radiographic appearance of Northway stage II or IV BPD, history of mechanical ventilatory support for > 1 month, stability after extubation for > week, weight > 1.5 kg, fractional excretion of O2 (FiO2) 0.30‐0.50. Exclusion criteria: rib cage deformities, congenital heart disease, chromosomal abnormalities. | |
| Interventions | Thiazide vs placebo. Patients were randomly allocated to receive either treatment (chlorothiazide 40 mg/kg/day and spironolactone 4 mg/kg/day divided in 2 doses per day) (N=25) or placebo (N=24). The medications were continued until the infant was weaned off O2. When the infant was stable without supplemental O2 for 1 week, the dose was tapered over a 1‐2 week period and then discontinued. Total duration of randomized therapy was similar in the treatment group (138±58 days) and in the control group (148±49 days). | |
| Outcomes | Main outcome: to determine whether patients benefit from continuing diuretic administration until they no longer require oxygen. Secondary outcome: natural history of BPD, by measuring pulmonary mechanics during the first year of life. | |
| Notes | The authors measured thoracic gas volume and airway resistance by plethysmography and dynamic compliance using an esophageal balloon. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized trial, parallel design |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomization: unclear: All investigators but one were blinded. The role of this investigator in the study and in patient care is not clearly stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: unclear Blinding of outcome: unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no: 43 out of 49 patients completed the study. Six were eliminated, three in each group, including 5 at the request of the parents and 1 because parents moved out of state. |
Segar 1997.
| Methods | Blinding of randomization: not documented Blinding of intervention: no. Control patients did not receive a placebo. Complete follow‐up: yes Blinding of outcome: not documented Washout period: 7 days | |
| Participants | Patients entered into the study: n=12 Either mechanical ventilation or continuous positive airway pressure; oxygen supplementation Entry criteria: CLD, gestational age 24‐28 weeks, postnatal age 21‐68 days Exclusion criteria: diuretic administration within 7 days, indomethacin administration within 14 days, underlying renal disease, serum creatinine > 0.7 mg/dl (0.062 mmol/L), major congenital anomalies | |
| Interventions | Furosemide and metolazone vs furosemide alone Patients were randomly allocated to receive either four doses of 1 mg/kg of furosemide intravenously every 24 hours with four doses of 0.2 mg/kg of metolazone enterally every 24 hours (treatment group, N=7), or the same doses of furosemide alone (control, N=5). | |
| Outcomes | Primary outcome: plasma volume, extracellular volume Secondary outcomes: weight, urine output | |
| Notes | Respiratory parameters were not assessed as part of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomization: not documented |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no. Control patients did not receive a placebo. Blinding of outcome: not documented |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atkinson 1988 | Not a randomized study |
| Braden 1997 | Not a randomized study |
| Campfield 1997 | Not a randomized study |
| Giacoia 1991 | Not a randomized study |
| Horgan 1996 | Not a randomized study |
| Kao 1987 | Complex design using randomization into two groups with pre‐determined (not random) sequence of event. Comparison between simultaneous events in the two groups would allow to compare theophylline with placebo (or theophylline and diuretic vs diuretic alone) rather than diuretic with placebo. |
| Kao 1988 | Complex design using randomization into two groups with pre‐determined (not random) sequence of event. Comparison between simultaneous events in the two groups would allow to compare theophylline with placebo (or theophylline and diuretic vs diuretic alone) rather than diuretic with placebo. |
| Kazzi 1992 | Not a randomized study. Patients included in the study were infants with BPD who were started on diuretic therapy upon decision by the clinician. Controls were infants without BPD who did not receive diuretic therapy. |
| Perlman 1986 | Not a randomized study |
| Segar 1992 | This study does not provide any information on the outcome variables selected for this systematic review. |
| Sonntag 1996 | Not a randomized study |
| Verma 1994 | Not a randomized study |
Contributions of authors
LPB, RAP and IAP performed the searches, wrote the review and revised prior reviews. We would like to thank Dr. Primhak for his past contribution to reviews and revisions.
ALS and LPB revised the most recent review update (2011).
In prior versions, all calculations within the spreadsheet and entries into RevMan were done by one review author (LPB) and subsequently checked for accuracy by the other review authors (IAP and RP). Any disagreement was resolved by discussion.
Sources of support
Internal sources
Albert Einstein Coll. Med & Montefiore Med. Ctr., Div. Neonatology, USA.
External sources
Grant‐in‐Aid 9650445N of the American Heart Association (LPB), USA.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Albersheim 1989 {published data only}
- Albersheim S, Sharma A, Solimano A, Smyth J, Rotschild A. A randomized controlled trial of long‐term diuretic therapy in bronchopulmonary dysplasia (BPD). Pediatric Research 1988;23:497A. [DOI] [PubMed] [Google Scholar]
- Albersheim SG, Solimano AJ, Sharma AK, Smyth JA, Rotschild A, Wood BJ, et. al. Randomized, double‐blind, controlled trial of long‐term diuretic therapy for bronchopulmonary dysplasia. Journal of Pediatrics 1989;15:615‐20. [DOI] [PubMed] [Google Scholar]
Engelhardt 1989 {published data only}
- Engelhardt B, Blalock WA, DonLevy S, Rush M, Hazinski TA. Effect of spironolactone‐hydrochlorothiazide on lung function in infants with chronic bronchopulmonary dysplasia. Journal of Pediatrics 1989;114:619‐24. [DOI] [PubMed] [Google Scholar]
Hoffman 2000 {published data only}
- Hoffman DJ, Abbasi S, Cnaan A, Gerdes JS. Effect of spironolactone on pulmonary function and electrolyte balance in infants with chronic lung disease [abstract]. Pediatric Research 1994;35:337A. [Google Scholar]
- Hoffman DJ, Abbasi S, Sivieri EM, Deuber C, Bhutani VK, Gerdes JS. Pulmonary function and electrolyte balance following spironolactone treatment in preterm infants with chronic lung disease [abstract]. Pediatric Research 1997;41:56A. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Gerdes JS, Abbasi S. Pulmonary function and electrolyte balance following spironolactone treatment in preterm infants with chronic lung disease: A double‐blind, placebo‐controlled, randomized trial. Journal of Perinatology 2000;20:41‐5. [DOI] [PubMed] [Google Scholar]
Kao 1984 {published data only}
- Kao LC, Warburton D, Cheng MH, Cedeno C, Platzker ACG, Keens TG. Effect of oral diuretics on pulmonary mechanics in infants with chronic bronchopulmonary dysplasia: Results of a double‐blind crossover sequential trial. Pediatrics 1984;74:37‐44. [PubMed] [Google Scholar]
Kao 1994 {published data only}
- Kao LC, Durand DJ, McCrea RC, Birch M, Powers RJ, Nickerson BG. Randomized trial of long‐term diuretic therapy for infants with oxygen‐dependent bronchopulmonary dysplasia. Journal of Pediatrics 1994;124:772‐81. [DOI] [PubMed] [Google Scholar]
Segar 1997 {published data only}
- Segar JL, Chemtob S, Bell EF. Changes in body water compartments with diuretic therapy in infants with chronic lung disease. Early Human Development 1997;48:99‐107. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Atkinson 1988 {published data only}
- Atkinson SA, Shah JK, McGee C, Steele BT. Mineral excretion in premature infants receiving various diuretic therapies. Journal of Pediatrics 1988;113:540‐5. [DOI] [PubMed] [Google Scholar]
Braden 1997 {published data only}
- Braden G, Campfield T, Flynn‐Valone P, Bednarek F, Pappagallo M. Nephrocalcinosis in very low birthweight infants: a prospective multicenter study of risk factors and complications [abstract]. Journal of the American Society of Nephrology 1997;8:122A. [Google Scholar]
Campfield 1997 {published data only}
- Campfield T, Braden G, Flynn‐Valone P, Powell S. Effect of diuretics on urinary oxalate, calcium, and sodium excretion in very low birth weight infants. Pediatrics 1997;99:814‐8. [DOI] [PubMed] [Google Scholar]
Giacoia 1991 {published data only}
- Giacoia GP, Pineda R. Diuretics, hypochloremia, and outcome in bronchopulmonary dysplasia patients. Developmental Pharmacology and Therapeutics 1991;4:212‐20. [PubMed] [Google Scholar]
Horgan 1996 {published data only}
- Horgan MJ. Thiazide‐induced hyponatremia in <1500 gm infants with bronchopulmonary dysplasia [abstract]. Pediatric Research 1996;38:216A. [Google Scholar]
- Horgan MJ. Thiazide‐induced hyponatremia in infants with bronchopulmonary dysplasia [abstract]. Pediatric Research 1991;29:218A. [Google Scholar]
Kao 1987 {published data only}
- Kao LC, Durand DJ, Phillips BL, Nickerson BG. Oral theophylline and diuretics improve pulmonary mechanics in infants with bronchopulmonary dysplasia. Journal of Pediatrics 1987;111:439‐44. [DOI] [PubMed] [Google Scholar]
Kao 1988 {published data only}
- Kao LC, Durand DJ, Nickerson BG. Improving pulmonary function does not decrease oxygen consumption in infants with bronchopulmonary dysplasia. Journal of Pediatrics 1988;112:616‐21. [DOI] [PubMed] [Google Scholar]
Kazzi 1992 {published data only}
- Kazzi NJ, Morbach CA, Brans YW. Does chronic thiazide diuretic therapy cause hyperlipidemia infants with bronchopulmonary dysplasia? [abstract]. Pediatric Research 1991;29:220A. [Google Scholar]
- Kazzi NJ, Morbach CA, Brans YW. Effects of thiazide diuretics on the lipid profile of infants with bronchopulmonary dysplasia. Biology of the Neonate 1992;61:318‐25. [DOI] [PubMed] [Google Scholar]
- Kazzi NJ, Morbach CA, Brans YW. Effects of thiazide diuretics on the lipid profile of infants with bronchopulmonary dysplasia [abstract]. Pediatric Research 1991;29:219A. [DOI] [PubMed] [Google Scholar]
Perlman 1986 {published data only}
- Perlman JM, Moore V, Siegel MJ, Dawson J. Is chloride depletion an important contributing cause of death in infants with bronchopulmonary dysplasia?. Pediatrics 1986;77:121‐216. [PubMed] [Google Scholar]
Segar 1992 {published data only}
- Segar JL, Robillard JE, Johnson KJ, Bell EF, Chemtob S. Addition of metolazone to overcome tolerance to furosemide in infants with bronchopulmonary dysplasia. Journal of Pediatrics 1992;120:966‐73. [DOI] [PubMed] [Google Scholar]
Sonntag 1996 {published data only}
- Sonntag J. Effect of dexamethasone and spironolactone therapy on diuresis and creatinine clearance in premature infants with a birth weight below 1,500 g [Einfluss einer Dexamethason‐ und Spironolactontherapie auf die Diurese und Kreatininclearance bei Fruhgeborenen mit einem Geburtsgewicht unter 1500 g]. Klinische Padiatrie 1996;208(6):319‐22. [DOI] [PubMed] [Google Scholar]
- Sonntag J, Gaude M. Effect of dexamethasone and spironolactone therapy in calcium and phosphate homeostasis in premature infants with a birth weight under 1,500 g. Klinische Padiatrie 1998;210(5):354‐7. [DOI] [PubMed] [Google Scholar]
Verma 1994 {published data only}
- Verma RP, Fornell JE, Vidyasagar D. Body fluid electrolytes in bronchopulmonary dysplasia and the effects of diuretic therapy. Indian Journal of Pediatrics 1994;61:213‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Agostini 2005
- Agostoni P, Magini A, Andreini D, Contini M, Apostolo A, Bussotti M, Cattadori G, Palermo P. Spironolactone improves lung diffusion in chronic heart failure. European Heart Journal 2005;26:159‐64. [DOI] [PubMed] [Google Scholar]
Armitage 1994
- Armitage P, Berry G. Sampling. In: Armitage P, Berry G editor(s). Statistical Methods in Medical Research. Oxford: Blackwell Scientific Publications, 1994:78‐92. [Google Scholar]
Atalay 2010
- Atalay C, Dogan N, Aykan S, Gundogdu C, Keles MS. The efficacy of spironolactone in the treatment of acute respiratory distress syndrome‐induced rats. Singapore Medical Journal 2010;51:501‐5. [PubMed] [Google Scholar]
Baird 1995
- Baird DC. Statistics of observation. In: Baird DC editor(s). Experimentation. An introduction to measurement theory and experiment design.. New Jersey: Prentice Hall, 1995:29‐56. [Google Scholar]
Beyer 1958
- Beyer KH. The mechanism of action of chlorothiazide. Annals of the NY Academy of Sciences 1958;71:363‐79. [DOI] [PubMed] [Google Scholar]
Brion 1998
- Brion LP, Campbell DE. Furosemide in indomethacin‐treated infants with symptomatic patent ductus arteriosus (Cochrane Review). Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD001148] [DOI] [Google Scholar]
Brion 1999a
- Brion LP, Primhak RA. Intravenous or enteral administration of a loop diuretic in preterm infants with (or developing) chronic lung disease (Cochrane Review). Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001453] [DOI] [Google Scholar]
Brion 1999b
- Brion LP, Yong SC, Primhak RA. Aerosolized furosemide in preterm infants with or developing chronic lung disease (Cochrane Review). Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001694.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brion 1999c
- Brion LP, Soll RF. Diuretics for respiratory distress syndrome in preterm infants (Cochrane Review). Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001454.pub2] [DOI] [PubMed] [Google Scholar]
Brown 1978
- Brown ER, Stark A, Sosenko I, Lawson EE, Avery ME. Bronchopulmonary dysplasia: possible relationship to pulmonary edema. Journal of Pediatrics 1978;92:982‐4. [DOI] [PubMed] [Google Scholar]
Chemtob 1989
- Chemtob S, Kaplan BS, Sherbotie JR, Aranda JV. Pharmacology of diuretics in the newborn. Pediatric Clinics of North America 1989;36:1231‐50. [DOI] [PubMed] [Google Scholar]
Chen 1992
- Chen TM, Chiou WL. Large differences in the biological half‐life and volume of distribution of hydrochlorothiazide in normal subjects from eleven studies. Correlation with their last blood sampling times. International Journal of Clinical Pharmacology, Therapy, and Toxicology 1992;30:34‐7. [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769‐73. [DOI] [PubMed] [Google Scholar]
Gortner 1991
- Gortner L, Bernsau U, Hellwege HH, Hieronimi G, Jorch G, Reiter HL. Does prophylactic use of surfactant change drug utilization in very premature infants during the neonatal period?. Developmental Pharmacology and Therapeutics 1991;16:1‐16. [MEDLINE: ] [PubMed] [Google Scholar]
Goyal 1995
- Goyal M, Suresh BR, Reinersman G, Gewolb IH, Brion LP. Evolution and variability of pulmonary mechanics during postnatal transition in full‐term infants. Journal of Perinatology 1995;15:441‐8. [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jayr 1994
- Jayr C, Garat C, Meignan M, Pittet JF, Zelter M, Matthay MA. Alveolar liquid and protein clearance in anesthetized ventilated rats. Journal of Applied Physiology 1994;76:2636‐42. [DOI] [PubMed] [Google Scholar]
Jhaveri 2010
- Jhaveri N, Soll RF, Clyman RI. Feeding Practices and Patent Ductus Arteriosus Ligation Preferences—Are They Related?. American Journal of Perinatology 2010;27:667‐674. [DOI] [PubMed] [Google Scholar]
Karim 1976
- Karim A, Zagarella J, Hutsell TC, Dooley M. Spironolactone. III. Canrenone‐‐maximum and minimum steady‐state plasma levels. Clinical Pharmacology and Therapeutics 1976;19:177‐82. [DOI] [PubMed] [Google Scholar]
Kugelman 1997
- Kugelman A, Durand M, Garg M. Pulmonary effect of inhaled furosemide in ventilated infants with severe bronchopulmonary dysplasia. Pediatrics 1997;99:71‐5. [DOI] [PubMed] [Google Scholar]
Loriaux 1976
- Loriaux DL. Spironolactone and endocrine dysfunction. Annals of Internal Medicine 1976;85:630‐6. [DOI] [PubMed] [Google Scholar]
Merke 2002
- Merke DP. Future Directions in the Study and Management of Congenital Adrenal Hyperplasia due to 21‐Hydroxylase Deficiency. Annals of Internal Medicine 2002;136:320‐334. [DOI] [PubMed] [Google Scholar]
Mirochnick 1989
- Mirochnick M, Kramer P, Chapron D. Chlorothiazide pharmacology in preterm infants. Pediatric Research 1989;25:71A. [Google Scholar]
Northway 1967
- Northway WH, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline membrane disease: bronchopulmonary dysplasia. New England Journal of Medicine 1967;276:357‐68. [DOI] [PubMed] [Google Scholar]
O'Brodovich 1990
- O'Brodovich H, Hannam V, Seear M, Mullen JB. Amiloride impairs lung water clearance in newborn guinea pigs. Journal of Applied Physiology 1990;68:1758‐62. [DOI] [PubMed] [Google Scholar]
Robbins 1993
- Robbins G, Tayaba R, Ochshorn IL, Green RS, Holzman IR. Effects of nebulized furosemide vs normal saline on pulmonary mechanics in bronchopulmonary dysplasia [abstract]. Pediatric Research 1993;33:233A. [Google Scholar]
Sakuma 1994
- Sakuma T, Okaniwa G, Nakada T, Nishimura T, Fujimura S, Matthay MA. Alveolar fluid clearance in the resected human lung. American Journal of Respiratory and Critical Care Medicine 1994;150:305‐10. [DOI] [PubMed] [Google Scholar]
Singhal 1983
- Singhal N, McMillan DD, Rademaker AW. Furosemide improves lung compliance in infants with bronchopulmonary dysplasia [abstract]. Pediatric Research 1983;17:336A. [Google Scholar]
Soll 1992
- Soll RF, McQueen MC. Respiratory distress syndrome. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:325‐58. [Google Scholar]
Suzuki 2001
- Suzuki S, Tsubochi H, Suzuki T, Darnel AD, Krozowski ZS, Sasano H, Kondo T. Modulation of transalveolar fluid absorption by endogenous aldosterone in adult rats. Experimental Lung Research 2001;27:143‐55. [DOI] [PubMed] [Google Scholar]
Terai 1999
- Terai I, Yamano K, Ichihara N, Arai J, Kobayashi K. Influence of spironolactone on neonatal screening for congenital adrenal hyperplasia. Archives of Disease in Childhood Fetal and Neonatal ed 1999;81:F179‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toffolo 1997
- Toffolo A, Trevisanuto D, Meneghetti S, Talenti E, Zacchello G, Zanardo V. Non‐furosemide‐related renal calcifications in premature infants with bronchopulmonary dysplasia. Acta Paediatrica Japonica 1997;39:433‐6. [DOI] [PubMed] [Google Scholar]
Vachharajani 2001
- Vachharajani AJ, Shah JK, Paes BA. Ovarian Cyst in a Premature Infant Treated with Spironolactone. American Journal of Perinatology 2001;18:353‐56. [DOI] [PubMed] [Google Scholar]
Vallon 2006
- Vallon V, Wyatt AW, Klingel K, Huang DY, Hussain A, Berchtold S, Friedrich B, Grahammer F, Belaiba RS, Görlach A, Wulff P, Daut J, Dalton ND, Ross J Jr, Flögel U, Schrader J, Osswald H, Kandolf R, Kuhl D, Lang F. SGK1‐dependent cardiac CTGF formation and fibrosis following DOCA treatment. Journal of Molecular Medicine 2006;84:396‐404. [DOI] [PubMed] [Google Scholar]
Zhao 1998
- Zhao L, Zhao M, Fang Q. Spironolactone ameliorates rat pulmonary fibrosis induced by bleomycin A5. Zhao Zhonghua Jie He He Hu Xi Za Zhi 1998;21:300‐2. [PubMed] [Google Scholar]
Zimmerman 1995
- Zimmerman JJ. Bronchoalveolar inflammatory pathophysiology of bronchopulmonary dysplasia. Clinics in Perinatology 1995;22:429‐56. [PubMed] [Google Scholar]
References to other published versions of this review
Brion 1999
- Brion LP, Primhak RA, Ambrosio‐Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001817] [DOI] [Google Scholar]
Brion 2000
- Brion LP, Primhak RA, Ambrosio‐Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD001817] [DOI] [PubMed] [Google Scholar]
Brion 2001
- Brion LP, Yong SC, Perez IA, Primhak R. Diuretics and chronic lung disease of prematurity. Journal of Perinatology 2001;21:269‐71. [DOI] [PubMed] [Google Scholar]
Brion 2002
- Brion LP, Primhak RA, Ambrosio‐Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001817] [DOI] [PubMed] [Google Scholar]
Brion 2003
- Brion LP, Primhak RA, Ambrosio‐Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001817] [DOI] [Google Scholar]
Brion 2008
- Brion LP, Primhak RA, Ambrosio‐Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001817] [DOI] [Google Scholar]


