Abstract
Background
Preterm birth is a major cause of perinatal mortality and morbidity. Cyclo‐oxygenase (COX) inhibitors inhibit uterine contractions, are easily administered and appear to have few maternal side effects. However, adverse effects have been reported in the fetus and newborn as a result of exposure to COX inhibitors.
Objectives
To assess the effects on maternal and neonatal outcomes of COX inhibitors administered as a tocolytic agent to women in preterm labour when compared with (i) placebo or no intervention and (ii) other tocolytics. In addition, to compare the effects of non‐selective COX inhibitors with COX‐2 selective inhibitors.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (24 August 2014). We also contacted recognised experts and searched reference lists of retrieved studies.
Selection criteria
All published and unpublished randomised trials in which COX inhibitors were used for tocolysis for women in labour between 20 and 36 completed weeks' gestation.
Data collection and analysis
Two review authors independently evaluated methodological quality and extracted data. We sought additional information from study authors. Results are presented using risk ratio (RR; dichotomous data) and mean difference (MD; continuous data) with 95% confidence interval (CI). The number needed to treat for benefit (NNTB) and the number needed to treat for harm (NNTH) were calculated for statistically different categorical outcomes.
Main results
With the addition of seven studies with a total of 684 women, this review now includes outcome data from 20 studies including 1509 women. The non‐selective COX inhibitor indomethacin was used in 15 studies. The overall quality of the included studies was considered moderate to low.
Three small studies (102 women), two of which were conducted in the 1980's, compared COX inhibition (indomethacin only) with placebo. No difference was shown in birth less than 48 hours after trial entry (average RR 0.20, 95% CI 0.03 to 1.28; two studies with 70 women). Indomethacin resulted in a reduction in preterm birth (before completion of 37 weeks of gestation) in one small study (36 women) (RR 0.21, 95% CI 0.07 to 0.62; NNTB 2, 95% CI 2 to 4); and an increase in gestational age at birth (average MD 3.59 weeks, 95% CI 0.65 to 6.52; two studies with 66 women) and birthweight (MD 716.34 g, 95% CI 425.52 to 1007.16; two studies with 67 infants). No difference was shown in measures of neonatal morbidity or neonatal mortality.
Compared with betamimetics, COX inhibitors resulted in a reduction in birth less than 48 hours after trial entry (RR 0.27, 95% CI 0.08 to 0.96; NNTB 7, 95% CI 6 to 120; two studies with 100 women) and preterm birth (before completion of 37 weeks of gestation) (RR 0.53, 95% CI 0.28 to 0.99; NNTB 6, 95% CI 4 to 236; two studies with 80 women) although no benefit was shown in terms of neonatal morbidity or mortality. COX inhibition was also associated with fewer maternal adverse affects compared with betamimetics (RR 0.19, 95% CI 0.11 to 0.31; NNTB 3, 95% CI 2 to 3; five studies with 248 women) and maternal adverse effects requiring cessation of treatment (average RR 0.09, 95% CI 0.02 to 0.49; NNTB 5, CI 95% 5 to 9; three studies with 166 women).
No differences were shown when comparing COX inhibitors with magnesium sulphate (MgSO4) (seven studies with 792 women) or calcium channel blockers (CCBs) (two studies with 230 women) in terms of prolonging pregnancy or for any fetal/neonatal outcomes. There were also no differences in very preterm birth (before completion of 34 weeks of gestation) and no maternal deaths occurred in the one study that reported on this outcome. However COX inhibitors resulted in fewer maternal adverse affects when compared with MgSO4 (RR 0.39, 95% CI 0.25 to 0.62; NNTB 11, 95% CI 9 to 17; five studies with 635 women).
A comparison of non‐selective COX inhibitors versus any COX‐2 inhibitor (two studies with 54 women) did not demonstrate any differences in maternal, fetal or neonatal outcomes.
No data were available to assess COX inhibitors compared with oxytocin receptor antagonists (ORAs). Further, no data were available on extremely preterm birth (before 28 weeks of gestation), longer‐term infant outcomes or costs.
Authors' conclusions
In this review, no clear benefit for COX inhibitors was shown over placebo or any other tocolytic agents. While some benefit was demonstrated in terms of postponement of birth for COX inhibitors over placebo and betamimetics and also maternal adverse effects over betamimetics and MgSO4, due to the limitations of small numbers, minimal data on safety, lack of longer‐term outcomes and generally low quality of the studies included in this review, we conclude that there is insufficient evidence on which to base decisions about the role of COX inhibition for women in preterm labour. Further well‐designed tocolytic studies are required to determine short‐ and longer‐term infant benefit of COX inhibitors over placebo and other tocolytics, particularly CCBs and ORAs. Another important focus for future studies is identifying whether COX‐2 inhibitors are superior to non‐selective COX inhibitors. All future studies on tocolytics for women in preterm labour should assess longer‐term effects into early childhood and also costs.
Plain language summary
Cyclo‐oxygenase (COX) inhibitors for treating preterm labour
Not enough evidence on whether COX inhibitors administered to women in threatened premature labour may reduce the risk of babies being born too early.
Babies born too early are at increased risk of serious illness, and often do not survive. COX inhibitors inhibit uterine contractions, so may postpone birth to allow the administration of steroids to the mother to help mature the baby's lungs. COX inhibitors may have adverse effects on the baby's heart, lungs and kidneys, and on the mother. Other drugs given for premature labour also have side effects. This review found that COX inhibitors may be better than no treatment, or other drugs, in reducing the number of babies born too early; however, there is insufficient evidence on possible adverse effects.
Background
Description of the condition
Preterm birth, defined as birth occurring between 20 and 36 weeks of gestation is a major contributor to perinatal mortality and morbidity.
The rate of preterm birth is increasing across low‐ and middle‐income countries, affecting 8.6% of births in high‐income countries and between 7.4% to 13.3% in low‐ and middle‐income countries (WHO 2012). Preterm birth is a leading cause of perinatal morbidity including respiratory distress syndrome, chronic lung disease, intraventricular haemorrhage, sepsis, cerebral palsy and other forms of neuro‐developmental impairment (Gladstone 2011), blindness and deafness. The birth of a preterm infant who requires intensive care for survival is a crisis, not only for the infant, but also for the parents (McCain 1993). The costs to the parents, community and society as a whole, both economic and emotional, are substantial (Petrou 2011).
Approximately 65% to 70% are spontaneous preterm births either following spontaneous preterm labour (40% to 45%) and those following preterm rupture of membranes (25% to 30%) (Goldenberg 2008). While the cause of spontaneous preterm birth is often unclear, some risk factors have been identified including: maternal age (adolescence and advanced age); history of preterm birth; race; multiple pregnancy, short inter‐pregnancy interval; infections; medical conditions; poor nutrition; psychological factors and genetic predisposition (Goldenberg 2008).
Description of the intervention
Despite improvements in standards in obstetric and neonatal care over recent years, no progress has been made over the last two decades in reducing the incidence of preterm birth in high‐income countries. In fact, rates of preterm birth are rising, in part due to increasing obstetric intervention (Goldenberg 2008; Norman 2009). Some benefits have been identified from prolongation of pregnancy, which theoretically allows time for corticosteroids to be administered to the mother to hasten fetal lung maturation (Roberts 2006), to effect transfer to a centre with neonatal intensive care facilities (Powell 1995) and magnesium sulphate (MgSO4) administration to reduce risk of cerebral palsy (Doyle 2009). For these reasons, short‐term tocolytic therapy is commonly used to inhibit preterm labour and postpone preterm birth.
A range of pharmacological agents (tocolytics) have been used to inhibit preterm labour in order to allow time for such co‐interventions to occur (short‐term tocolytic therapy) and are the topics of Cochrane systematic reviews including: nitric oxide donors (glyceryl trinitrate) (Duckitt 2014), oxytocin receptor antagonists (ORAs) (Flenady 2014a), betamimetics (Neilson 2014), MgSO4 (Crowther 2002), calcium channel blockers (CCBs) (Flenady 2014b) and progesterone (Su 2010). A review on combinations of different tocolytics for women in preterm labour is the subject of a recent review (Vogel 2014).
Cyclo‐oxygenase (COX) inhibitors have the advantage of being easily administered (orally or rectally) and have a better maternal side‐effect profile than betamimetics (Babay 1998), however there remains a concern about adverse fetal and neonatal effects particularly at higher gestations (Koren 2006).
Maintenance tocolytic therapy, used to prevent recurrence of preterm labour after an initial course of successful treatment, has not been shown to improve perinatal outcomes or effectively prolong pregnancy and is not widely used (Sanchez‐Ramos 1999). The role of maintenance therapy for women following threatened preterm labour is not addressed in this review.
How the intervention might work
Prostaglandins are hormones that have a multitude of functions. Prostaglandins affect uterine muscle contraction by causing an increase in free intracellular calcium levels and amplifying activation of myosin light chain kinase (Murray 2006). Prostaglandins have an important role in the onset and maintenance of labour (Keirse 1992). COX enzymes are fundamental to the production of prostaglandins and inhibition of COX activity, resulting in reduced production of prostaglandins, reduce uterine contractions (Doret 2002; Van den Veyver 1993). Indomethacin, the prostaglandin inhibitor most frequently used for tocolysis, achieves its effect by reversibly binding to COX.
The recognition of distinct forms of COX, namely COX‐1 and COX‐2, led to the development of COX‐2 specific inhibitors. The expression of COX‐2 has been shown to increase significantly prior to the onset of labour, while the expression of COX‐1 remains unchanged (Slater 1999), indicating a role of COX‐2 in labour induction. It is possible that selective inhibition of COX‐2 could enable more effective tocolysis with reduced adverse events.
Why it is important to do this review
The ideal tocolytic agent is one which is effective in prolonging pregnancy, easy to administer with minimal side effects for the woman or the baby (Crowther 2002; Neilson 2014).The betamimetics, arguably the most commonly used tocolytics (ritodrine, salbutamol and terbutaline), have been shown to be effective in delaying delivery by seven days and longer, although no impact has yet been shown on perinatal mortality (Neilson 2014). Furthermore, betamimetics have a high frequency of unpleasant, sometimes severe maternal side effects including tachycardia, hypotension, tremor and a range of biochemical disturbances, and they have been associated with life‐threatening cardiovascular and respiratory events and deaths (FDA 2011).
Compared with other tocolytic agents (mostly betamimetics), CCBs have been shown to prolong pregnancy and improve short‐term neonatal outcomes, with fewer maternal adverse effects (Flenady 2014b). However, a fifth of women still delivered within 48 hours of CCB treatment so there is still a need for other safe, effective tocolytic agents, particularly at very early gestations.
Another class of tocolytics, ORAs, have been developed specifically as a tocolytic agent, with atosiban being the most researched and used ORA. ORAs are associated with fewer maternal side effects, however no clear benefit has been shown for ORAs as tocolytic agents (Flenady 2014a). Concerns regarding the increase in infant mortality with the use of MgSO4 for treatment of preterm birth rightly limit its utility as a first‐line tocolytic (Crowther 2002).
In a recent network meta‐analysis of tocolytic agents (Haas 2012), prostaglandin inhibitors were shown to be more efficacious in delaying delivery by 48 hours when compared with placebo and had a 96% probability of being ranked in the top three most efficacious tocolytics. However, COX inhibitors freely cross the placenta and can interfere with prostaglandin homeostasis in the fetus (Moise 1990) and concerns remain regarding the potentially deleterious effects of prolonged exposure on the fetal cardiovasculature, gut and kidney (Perron 2013; Walker 2011). Reports from case series have attributed a range of adverse effects in the fetus and neonate to in utero indomethacin exposure (the most common COX inhibitor) during tocolysis (Eronen 1994; Norton 1993; Souter 1998) including oligohydramnios, renal failure, premature closure of the ductus arteriosus with consequent pulmonary hypertension, persistent patent ductus arteriosus, necrotising enterocolitis, and intraventricular haemorrhage.
It is important to undertake a systematic review of all randomised controlled trials of COX inhibitors used in the management of women in preterm labour to determine the relative risks and benefits of this intervention.
Objectives
To assess the effects on maternal, fetal and neonatal outcomes of COX inhibitors administered as a tocolytic agent to women in preterm labour when compared with either placebo or no intervention.
To assess the effects on maternal, fetal and neonatal outcomes of COX inhibitors administered as a tocolytic agent to women in preterm labour when compared with other tocolytic agents by type of other tocolytic as follows: betamimetics, MgSO4, CCBs and ORAs.
To assess the effects on maternal, fetal and neonatal outcomes of non‐selective COX inhibitors administered as a tocolytic agent to women in preterm labour when compared with COX‐2 selective inhibitors.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised and cluster‐randomised trials in which COX inhibitors were used for tocolysis in the management of preterm labour were assessed for eligibility. We excluded studies using quasi‐random allocation. Studies using cross‐over methodology were not eligible for inclusion.
Types of participants
Women assessed as being in preterm labour (between 20 and 36 completed weeks of gestation), and suitable for tocolysis.
Types of interventions
COX inhibitors administered by any route compared with placebo.
COX inhibitors administered by any route compared with other classes of tocolytic agents by type of other tocolytic.
Selective COX‐2 inhibitors administered by any route compared with non‐selective COX inhibitors administered by any route.
Types of outcome measures
In this review we aimed to assess clinically relevant maternal, perinatal and infant short‐ and long‐term outcomes after use of COX inhibitors for threatened preterm labour, as well as tocolytic efficacy of treatment.
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been prespecified following consultation with the editors and authors of the individual reviews. Consensus was reached on a set of ‘core’ outcomes, which are highlighted below. These will be included in all tocolysis reviews. In addition to these core outcomes, individual teams may include other outcomes as necessary.
Primary outcomes
Birth less than 48 hours after trial entry
Extremely preterm birth (before completion of 28 weeks of gestation)
Very preterm birth (before completion of 34 weeks of gestation)
Perinatal mortality (fetal death and neonatal death up to 28 days)
Serious infant outcome ‐ death or major sensorineural disability at two years of age (defined as any one or more of the following: legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy or developmental delay/intellectual impairment (defined as developmental quotient or intelligence quotient less than two standard deviations below the mean))
Serious maternal outcome (defined as death, cardiac arrest, respiratory arrest, admission to intensive care unit)
Secondary outcomes
Maternal
Maternal adverse effects
Maternal adverse effects requiring cessation of treatment
Antepartum haemorrhage
Postpartum haemorrhage
Need for blood transfusion
Caesarean section
Length of postnatal hospital stay
Length of antenatal hospital stay
Satisfaction with the therapy
Infant
Complete course of antenatal steroids
Pregnancy prolongation (interval between randomisation and birth)
Birth less than seven days after trial entry
Preterm birth (before completion of 37 weeks of gestation)
Gestational age at birth
Birthweight
Birthweight less than the third centile for gestational age
Birthweight less than 2500 g
Apgar score less than seven at five minutes
Persistent pulmonary hypertension
Neonatal renal failure
Respiratory distress syndrome
Use of mechanical ventilation
Duration of mechanical ventilation
Intraventricular haemorrhage
Intraventricular haemorrhage ‐ Grades III or IV
Periventricular leukomalacia
Retinopathy of prematurity
Retinopathy of prematurity ‐ Grades III or IV
Chronic lung disease
Necrotising enterocolitis
Neonatal sepsis
Admission to neonatal intensive care unit
Duration of neonatal intensive care unit stay
Oligohydramnios
Premature closure of the ductus arteriosus
Patent ductus arteriosus
In this update, primary and secondary outcomes measures were revised to enhance consistency across Cochrane tocolytic reviews and to better reflect important outcome measures.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (24 August 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains studies identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Studies identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
In addition, the review authors searched reference lists of retrieved studies and sought ongoing and unpublished studies by contacting experts in the field.
We did not apply any language or date restrictions.
Data collection and analysis
For the methods used when assessing the studies identified in the previous version of this review, seeKing 2005.
For this update, we applied the following methods to all previously included and new studies.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors (VF, JS) independently assessed for inclusion all the potential studies identified as a result of the search strategy. All disagreements in assessment of studies were resolved through discussion and if required, a third author (HR) was consulted.
Data extraction and management
A collection form was designed to extract data. For eligible studies, at least two review authors (HR, CR, RS, SK) extracted the data using the agreed form. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy with 100% agreement between the authors. We resolved any disagreements through discussion and/or by consulting a third assessor.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
At least two review authors (HR, CP, CR, RS, VF) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). All disagreement were resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results.
We assessed the methods as:
low, high or unclear risk of bias.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the study authors, we re‐included missing data in the analyses.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias as:
low, high or unclear risk of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI). Calculations for number needed to treat for benefit (NNTB) and number needed to treat for harm (NNTH) were performed and included where applicable.
Continuous data
For continuous data, we used the mean difference (MD) if outcomes were comparable between studies. We planned to use the standardised mean difference (SMD) to combine studies that measured comparable outcomes, but used different methods.
Unit of analysis issues
Cross‐over studies
Cross‐over studies were not eligible for inclusion.
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review, but we may include studies of this type in future updates.
If cluster‐randomised trials are included in future reviews, we plan to include these in the analyses along with individually‐randomised trials. Their sample sizes will be adjusted using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation units.
Multi‐arm studies
To avoid double‐counting we planned to divide out data from the shared group approximately evenly among the comparisons undertaken (described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
One study (Klauser 2012) included three treatment groups: COX inhibitor, CCB and MgSO4. For the analyses undertaken in this review, we split the number of events and number of included infants/women in the COX inhibitor group between the comparison with CCB and MgSO4.
One three‐armed study (Sawdy 2003) used two different non‐selective COX inhibitors, and one COX‐2 selective inhibitor. For this review, we used combined numbers of women/infants and events in the non‐selective COX inhibitor groups in the comparisons with the COX‐2 selective inhibitor.
Multiple pregnancy
The analysis in this review involves multiple pregnancies, therefore we intended to adjust analyses for clustering to take into account the non‐independence of babies from the same pregnancy (Gates 2004). Treating babies from multiple pregnancies as if they are independent, when they are more likely to have similar outcomes than babies from different pregnancies, will overestimate the sample size and give CIs that are too narrow. Each woman can be considered a cluster in multiple pregnancy, with the number of individuals in the cluster being equal to the number of fetuses in her pregnancy. Analysis using cluster trial methods allows calculation of risk ratio and adjustment of CIs. Usually this will mean that the CIs get wider. Although this may make little difference to the conclusion of a study, it avoids misleading results in those studies where the difference may be substantial. We planned to adjust for clustering in the analyses, wherever possible, and to use the inverse variance method for adjusted analyses, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and in Yelland 2011.
Eight studies reported infant outcomes from twin pregnancies (Besinger 1991; Klauser 2012; McWhorter 2004; Morales 1989; Morales 1993; Niebyl 1980; Panter 1999; Parilla 1997). In three studies the inclusion of multiple pregnancies were unclear, however, no infant outcomes were reported (Asgharnia 2002; Kramer 1999; Schorr 1998). Due to the small number of included infants across the comparisons in this review, we have not adjusted for multiple pregnancies. The distribution of twin pregnancies was relatively equally distributed across the trial groups in these studies.
Dealing with missing data
For included studies we noted levels of attrition. We contacted authors for additional data. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each study was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either theTau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Had there been 10 or more studies in the meta‐analysis for any particular outcome, we planned to investigate reporting biases (such as publication bias) using funnel plots. We planned to assess possible asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it. In this review update there were insufficient data to allow for this analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where studies were examining the same intervention, and the studies’ populations and methods were judged sufficiently similar. If the clinical heterogeneity was sufficient to expect that the underlying treatment effects differed between studies, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across studies could be considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between studies. If the average treatment effect was not clinically meaningful, we did not combine studies.
If random‐effects analyses were used, the results are presented as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If substantial heterogeneity had been identified, we planned to investigate it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if so, use random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses, as follows:
women receiving treatment commenced before 28 weeks' gestation versus 28 weeks and above;
women with a multiple pregnancy versus women with a singleton pregnancy;
women with ruptured membranes versus women with intact membranes;
high‐quality versus low‐quality studies (high‐quality studies are defined as studies that employed an adequate method for blinding of randomisation and intervention ‐ seeAssessment of risk of bias in included studies for further details).
We planned to use the following outcomes for the subgroup analyses:
birth less than 48 hours after trial entry;
extremely preterm birth (before completion of 28 weeks of gestation);
very preterm birth (before completion of 34 weeks of gestation);
preterm birth (before completion of 37 weeks of gestation);
perinatal mortality;
respiratory distress syndrome;
serious infant outcome (defined as death or chronic lung disease (need for supplemental oxygen at 28 days of life or later), grade three or four intraventricular haemorrhage or periventricular leukomalacia, major sensorineural disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay/intellectual impairment [defined as developmental quotient (DQ) or intelligence quotient (IQ) less than two standard deviations below mean]));
serious maternal outcome (defined as death, cardiac arrest, respiratory arrest, admission to intensive care unit);
maternal adverse effects requiring cessation of treatment.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014) and to report the results of subgroup analyses quoting the ChiI² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of study quality assessed by concealment of allocation, high attrition rates (greater than 20%), or both, with poor‐quality studies (including those assessed as high or unknown risk of bias) being excluded from the analyses.
Results
Description of studies
Results of the search
In this review, 42 studies were identified as potentially eligible for inclusion. Of these, 20 studies were excluded (Abramov 2000; Bartfield 1998; Bivins 1993; Caballero 1979; Carlan 1992; Carlan 1995; Ehsanipoor 2011; Gamissans 1982; Gamissans 1984; Groom 2005; Hallak 1992; Humphrey 2001; Jain 2006; Katz 1983; Mital 1992; Newton 1991; Rasanen 1995; Rios‐Anez 2001; Spearing 1979; Zuckerman 1984b). For further information please see Characteristics of excluded studies. In addition, two studies are awaiting classification, pending translation of published paper (Mesdaghinia 2012) and additional information from authors (Castillo 1988), please seeCharacteristics of studies awaiting classification for further information.
Therefore, this review update includes a total of 20 studies involving 1509 women testing the effects of COX inhibitors for tocolysis for women with threatened preterm labour. Two included studies (57 women) did not contribute data for any outcomes reported in this review update (Odeh 1997; Purwaka 2004).
Included studies
A total of 1509 women participated in the 20 included studies (Asgharnia 2002; Besinger 1991; Borna 2007; Kashanian 2011; Klauser 2012; Kramer 1999; Kurki 1991; McWhorter 2004; Morales 1989; Morales 1993; Nevils 1994; Niebyl 1980; Odeh 1997; Panter 1999; Parilla 1997; Purwaka 2004; Sawdy 2003; Schorr 1998; Stika 2002; Zuckerman 1984a).
Three of the included studies (involving 102 women) compared COX inhibitors versus placebo (Niebyl 1980; Panter 1999; Zuckerman 1984a), seven studies (involving 792 women) compared COX inhibitors versus magnesium sulphate (Asgharnia 2002; Borna 2007; Klauser 2012; McWhorter 2004; Morales 1993; Parilla 1997; Schorr 1998), seven studies (involving 331 women) compared COX inhibitors versus betamimetics (Besinger 1991; Kramer 1999; Kurki 1991; Morales 1989; Nevils 1994; Odeh 1997; Purwaka 2004), two studies (involving 230 women) compared COX inhibitors versus CCBs (Kashanian 2011; Klauser 2012) and two studies (involving 54 women) compared selective COX‐2 inhibitors versus non‐selective COX inhibitors (Sawdy 2003; Stika 2002).
Participants
The participants included in these studies were reasonably homogeneous. In 14 studies the gestational age at inclusion ranged from a minimum of 20 to 25 weeks to a maximum of 32 to 35 weeks (Asgharnia 2002; Besinger 1991; Borna 2007; Kashanian 2011; Klauser 2012; Kramer 1999; Kurki 1991; McWhorter 2004; Niebyl 1980; Panter 1999; Purwaka 2004; Sawdy 2003; Stika 2002; Zuckerman 1984a). In six studies (Morales 1989; Morales 1993; Nevils 1994; Odeh 1997; Parilla 1997; Schorr 1998), the gestation at inclusion ranged from less than 30 to less than 34 with no lower limit being specified. Preterm labour was reasonably consistently defined across the studies, most excluding those women with a cervical dilatation of greater than 4 cm. Twelve studies reported the exclusion of women with preterm premature rupture of membranes; and one study (Morales 1993) did not report whether this was an exclusion criterion. The standard contraindications for tocolysis were reported as exclusion criteria in the majority of included studies, i.e. fetal distress, chorioamnionitis, severe pre‐eclampsia/eclampsia, abruptio placentae or contraindications to non‐steroidal anti‐inflammatory agents.
Eight studies reported infant outcomes from twin pregnancies (Besinger 1991; Klauser 2012; McWhorter 2004; Morales 1989; Morales 1993; Niebyl 1980; Panter 1999; Parilla 1997). Two of these studies (70 infants) compared COX inhibitors versus placebo (Niebyl 1980; Panter 1999), two studies (142 infants) compared COX inhibitors versus betamimetics (Besinger 1991; Morales 1989), one study (170 infants) compared COX inhibitor versus CCB (Klauser 2012) and four studies (490 infants) compared COX inhibitors versus MgSO4 (Klauser 2012; McWhorter 2004; Morales 1993; Parilla 1997). In three studies the inclusion of multiple pregnancies was unclear, however, no infant outcomes were reported (Asgharnia 2002; Kramer 1999; Schorr 1998). One of these compared COX inhibitors versus MgSO4 (Asgharnia 2002) and excluded high‐order multiples, but it is unclear if any twin pregnancies were included as the numbers of infants in the study were not reported. Another study comparing COX inhibitors versus MgSO4 (Schorr 1998) included singleton and twin pregnancies, however numbers of infants in the study were also not reported. In one study comparing COX inhibitors versus betamimetics (Kramer 1999) it is unclear whether any multiple pregnancies were included as numbers of infants were not reported.
Tocolysis
Of the 20 studies included, 15 used the COX inhibitor indomethacin. Indomethacin dosing regimens were similar across the studies with loading doses of 50 to 100 mg (mostly given rectally) followed by 25 to 50 mg orally every four to six hours (every eight hours for Kurki 1991) for 24 to 48 hours (Besinger 1991; Klauser 2012; Niebyl 1980; Odeh 1997; Panter 1999; Parilla 1997; Stika 2002). Three studies used a similar dosing regimen, but repeated the bolus dose if contractions persisted (Morales 1989; Morales 1993; Zuckerman 1984a). One study administered indomethacin 100 mg rectally every 12 hours to 48 hours (Sawdy 2003) and one study administered one dose of indomethacin 100 mg rectally and repeated after one hour if contractions persisted (Kashanian 2011). One study administered 25 mg every six hours up to a maximum of four doses (Asgharnia 2002) and one study only reported that indomethacin was administered orally for 48 hours (Nevils 1994).
Other COX inhibitors used for comparisons in the studies included in this review were: nimesulide (Purwaka 2004; Sawdy 2003), sulindac (Kramer 1999; Sawdy 2003), ketorolac (Schorr 1998), rofecoxib (McWhorter 2004) and celecoxib (Borna 2007; Stika 2002). Fifteen studies compared COX inhibitors with other tocolytic agents. Seven studies compared with betamimetic agents (three ritodrine (Besinger 1991; Morales 1989; Odeh 1997), two terbutaline (Kramer 1999; Nevils 1994), one nylidrin (Kurki 1991) and one isoxuprine (Purwaka 2004)), seven with MgSO4 (Asgharnia 2002; Borna 2007; Klauser 2012; McWhorter 2004; Morales 1993; Parilla 1997; Schorr 1998) and two with CCBs (nifedipine only; Kashanian 2011; Klauser 2012). Two studies (Sawdy 2003; Stika 2002) compared non‐selective with selective COX inhibitors. One study (Stika 2002) compared indomethacin with the COX‐2 inhibitor celecoxib and the other compared sulindac and indomethacin with nimesulide (Sawdy 2003). Four studies (Besinger 1991; Kramer 1999; Morales 1993; Schorr 1998) reported the use of maintenance tocolysis. Three of the studies reported use of maintenance tocolysis up to 35 to 37 completed weeks of gestation (Besinger 1991; Kramer 1999; Schorr 1998); one study (Morales 1993) did not report an end‐point for maintenance tocolysis. Two studies reported continuation of tocolysis beyond the treatment protocol if the patient required (Borna 2007; McWhorter 2004). Nine studies (Besinger 1991; Kashanian 2011; McWhorter 2004; Morales 1989; Nevils 1994; Niebyl 1980; Parilla 1997; Stika 2002; Zuckerman 1984a) reported using alternative tocolytics after failure of the study treatment (rescue tocolysis), although details on the tocolytics used were largely not reported. It was not possible to ascertain the proportion of women given rescue tocolysis from the various publications. Of the studies included, the majority (16) were from high‐income countries.
Antenatal corticosteroids
Twelve out of 20 studies reported the administration of antenatal corticosteroids for all women enrolled (Borna 2007; Kashanian 2011; Klauser 2012; Kramer 1999; McWhorter 2004; Morales 1989; Morales 1993; Panter 1999; Parilla 1997; Sawdy 2003; Schorr 1998; Stika 2002). However, the majority did not state the proportion of women who actually received corticosteroids. In the remaining studies antenatal corticosteroid use was not reported.
Outcomes
No outcome data were available in any of the included studies for two of the six prespecified primary outcome measures for this review: death or major sensorineural disability at two years of age and extremely preterm birth (before completion of 28 weeks of gestation). The outcome neonatal mortality was reported in 15 studies (Besinger 1991; Kashanian 2011; Klauser 2012; Kramer 1999; Kurki 1991; McWhorter 2004; Morales 1989; Morales 1993; Niebyl 1980; Panter 1999; Parilla 1997; Sawdy 2003; Schorr 1998; Stika 2002; Zuckerman 1984a). Ten studies (Besinger 1991; Borna 2007; Kashanian 2011; Klauser 2012; Kurki 1991; McWhorter 2004; Morales 1993; Panter 1999; Stika 2002; Zuckerman 1984a) reported data for the prespecified primary outcome measure of birth less than 48 hours after trial entry. There was limited reporting for the remaining primary outcome of serious maternal outcome (defined as death, cardiac arrest, respiratory arrest, admission to intensive care unit), with only one study reporting on maternal death (Klauser 2012). Although six studies (Besinger 1991; Klauser 2012; Kramer 1999; Morales 1989; Morales 1993; Schorr 1998) reported maternal adverse drug reactions requiring cessation of treatment, other potential adverse effects such as antepartum and postpartum haemorrhage were reported in only two studies (Panter 1999; Stika 2002). The majority of studies assessed either the efficacy of tocolysis of the COX inhibitor and/or the fetal/neonatal safety aspects of the agent.
Reporting of other prespecified fetal and neonatal outcomes is as follows: respiratory distress syndrome (11 studies), persistent pulmonary hypertension of the newborn (eight studies), intraventricular haemorrhage (10 studies), premature closure of the ductus arteriosis (10 studies), oligohydramnios (five studies) and neonatal renal failure (one study). Clear definitions of outcome measures were often lacking in the study reports.
Please seeCharacteristics of included studies for further details.
Excluded studies
Twenty studies were excluded from this review.
One study was excluded on the basis of quasi‐random allocation (Spearing 1979), one study compared different administration routes (Abramov 2000) and one publication was a book chapter that summarised results from other studies (Gamissans 1984). The remaining 17 studies did not fulfil inclusion criteria as follows: comparison between two types of non‐selective COX inhibitors (Rasanen 1995); did not include a comparison group (Zuckerman 1984b); not described as randomised controlled trials (Caballero 1979; Jain 2006); studies of combination therapy (Gamissans 1982; Katz 1983; Newton 1991; Rios‐Anez 2001); studies of maintenance tocolysis (Bivins 1993; Carlan 1995; Humphrey 2001); assessment of treatment following successful (Bartfield 1998) or failed (Carlan 1992) tocolytic treatment; not described as tocolytic studies (Groom 2005; Hallak 1992; Mital 1992) and excluded women in preterm labour (Ehsanipoor 2011).
Please seeCharacteristics of excluded studies for further details.
Risk of bias in included studies
Overall, the quality of the included studies was moderate to low. Please seeCharacteristics of included studies for further details and Figure 1 and Figure 2 for a summary of 'Risk of bias' assessment.
1.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In 11 of the 20 studies the random sequence generation was considered adequate and as low risk of selection bias (Besinger 1991; Borna 2007; Kashanian 2011; Klauser 2012; Kramer 1999; McWhorter 2004; Morales 1993; Niebyl 1980; Panter 1999; Parilla 1997; Sawdy 2003), and one study was considered high risk of bias (Odeh 1997). In the remaining eight studies the randomisation sequence generation was unclear (Asgharnia 2002; Kurki 1991; Morales 1989; Nevils 1994; Purwaka 2004;Schorr 1998; Stika 2002; Zuckerman 1984a).
Concealment of allocation was considered adequate (low risk bias) in seven studies (Borna 2007; Kramer 1999; McWhorter 2004; Parilla 1997; Sawdy 2003; Schorr 1998; Stika 2002) and deemed as high risk of bias in one study (Odeh 1997); while the allocation concealment processes were unclear in the remaining 12 studies (Asgharnia 2002; Besinger 1991; Kashanian 2011; Klauser 2012; Kurki 1991; Morales 1989; Morales 1993; Nevils 1994; Niebyl 1980; Panter 1999; Purwaka 2004; Zuckerman 1984a).
Blinding
Nine studies described blinding of participants and personnel and were considered low risk of performance bias (Borna 2007; Kramer 1999; Kurki 1991; McWhorter 2004; Niebyl 1980; Panter 1999; Sawdy 2003; Stika 2002; Zuckerman 1984a) and nine studies were deemed high risk (Asgharnia 2002; Besinger 1991; Kashanian 2011; Klauser 2012; Morales 1989; Morales 1993; Parilla 1997; Purwaka 2004; Schorr 1998). The processes for blinding of participants and personnel were unclear in the remaining two studies (Nevils 1994; Odeh 1997).
The risk of detection bias was considered low in eight of the included studies (Borna 2007; Klauser 2012; Kurki 1991; McWhorter 2004; Niebyl 1980; Panter 1999; Sawdy 2003; Stika 2002), high in two studies (Asgharnia 2002; Kashanian 2011) and unclear in 10 studies (Besinger 1991; Kramer 1999; Morales 1989; Morales 1993; Nevils 1994; Odeh 1997; Parilla 1997; Purwaka 2004; Schorr 1998; Zuckerman 1984a).
Incomplete outcome data
The majority of included studies (14 of the 20 included studies) had minimal or no attrition and were assessed as having low risk of bias. One study had high risk of attrition (Nevils 1994): of 45 women randomised, 22 were included in analyses. For the remaining five studies it was unclear whether outcome data were complete (Odeh 1997; Parilla 1997; Purwaka 2004; Stika 2002; Zuckerman 1984a).
Selective reporting
In 10 studies, we found no obvious evidence of reporting bias (Besinger 1991; Klauser 2012; Kurki 1991; McWhorter 2004; Morales 1989; Morales 1993; Niebyl 1980; Panter 1999; Stika 2002; Zuckerman 1984a) and judged these studies to be at low risk of bias. Two studies were deemed to be at high risk of reporting bias. In one study (Sawdy 2003), the objective of the study was to establish fetal side effects of two different drugs after acute maternal exposure for tocolysis; however very limited data on side effects were reported from this study. Instead the authors state: "There were no reports of adverse neonatal complications related to the study drugs". The aim of the other study (Schorr 1998) was to evaluate safety and efficacy of tocolysis; however limited data were available in the report for pregnancy prolongation and maternal, fetal and neonatal side effects. In the remaining eight studies (Asgharnia 2002; Borna 2007; Kashanian 2011; Kramer 1999; Nevils 1994; Odeh 1997; Parilla 1997; Purwaka 2004), it was unclear whether selective reporting bias was present.
Other potential sources of bias
Eighteen of the included 20 studies were assessed as being at low risk of bias for other potential sources of bias based on baseline characteristics being similar between groups and no other bias was apparent (Besinger 1991; Borna 2007; Klauser 2012; Kramer 1999; Kurki 1991; McWhorter 2004; Morales 1989; Morales 1993; Nevils 1994; Niebyl 1980; Odeh 1997; Panter 1999; Parilla 1997; Purwaka 2004; Sawdy 2003; Schorr 1998; Stika 2002; Zuckerman 1984a). In the remaining two studies (Asgharnia 2002; Kashanian 2011), the risk of other sources of bias was unclear.
Effects of interventions
This review includes outcomes data from 20 studies with a total of 1509 women. Due to small numbers, all estimates of effect are therefore imprecise and need to be interpreted with caution. The following comparisons were undertaken:
any COX inhibitor versus placebo;
COX inhibitor versus other tocolytic agents, i.e. betamimetics, MgSO4and CCBs;
any non‐selective COX inhibitor versus any COX‐2 inhibitor.
Any COX inhibitor versus placebo
Three small studies (102 women), two of which were conducted in the 1980s, compared COX inhibition (indomethacin only) with placebo (Niebyl 1980; Panter 1999; Zuckerman 1984a).
Primary outcomes
Birth less than 48 hours after trial entry (Analysis 1.1)
1.1. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 1 Birth less than 48 hours after trial entry.
No difference was shown in birth less than 48 hours after trial entry (average risk ratio (RR) 0.20, 95% confidence interval (CI) 0.03 to 1.28, random‐effects, two studies with 70 women). Statistical heterogeneity was evident (Tau² = 1.18; Chi² = 2.81, df = 1 (P = 0.09); I² = 64%). However, upon exploration of the possible reasons for the heterogeneity by examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis.
Neonatal mortality (Analysis 1.2)
1.2. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 2 Neonatal mortality.
No differences were shown in neonatal mortality (RR 0.80, 95% CI 0.25 to 2.58; three studies with 106 infants). No data were available on stillbirth or perinatal mortality in any of the included studies.
No data were available for the other primary outcomes: extremely preterm birth (before completion of 28 weeks of gestation), very preterm birth (before completion of 34 weeks of gestation), death or major sensorineural disability at two years of age or serious maternal outcome.
Secondary outcomes
For the infant
Preterm birth (before completion of 37 weeks of gestation) (Analysis 1.3)
1.3. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 3 Preterm birth (before completion of 37 weeks of gestation).
When compared with placebo, COX inhibition (indomethacin only) resulted in a statistically significant reduction in the number of preterm births (before completion of 37 weeks of gestation) (RR 0.21, 95% CI 0.07 to 0.62; one study with 36 women) (number needed to treat for benefit (NNTB) 2, 95% CI 2 to 4).
Gestational age at birth (Analysis 1.5) and Birthweight (Analysis 1.6)
1.5. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 5 Gestational age (weeks).
1.6. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 6 Birthweight (g).
Two studies showed an increase in birthweight in the COX inhibitor group versus placebo (MD 716.34 g, 95% CI 425.52 to 1007.16; 67 women) (Analysis 1.6), but no difference in gestational age at birth (mean difference (MD) 3.59 weeks; 95% CI 0.65 to 6.52; 66 women) (Analysis 1.5).
Statistical heterogeneity was evident for the outcome of gestational age at birth (Tau² = 1.44; Chi² = 1.47, df = 1 (P = 0.22); I² = 32%). However, upon exploration of the possible reasons for the heterogeneity by examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis.
No differences were shown in the following neonatal outcome measures.
Birth less than seven days after trial entry: average RR 0.41, 95% CI 0.10 to 1.66; two studies with 70 women. Statistical heterogeneity was evident (Tau² = 0.83; Chi² = 5.36, df = 1 (P = 0.02); I² = 81%) as in other comparisons combining these two studies. However, upon exploration of the possible reasons for the heterogeneity by examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis (Analysis 1.4).
Apgar score less than seven at five minutes: RR 0.53, 95% CI 0.05 to 5.34; one study with 39 infants (Analysis 1.7).
Admission to neonatal intensive care unit: RR 0.80, 95% CI 0.56 to 1.15; one study with 39 infants (Analysis 1.8).
Respiratory distress syndrome: RR 1.00, 95% CI 0.40 to 2.49; three studies with 106 infants (Analysis 1.9).
Chronic lung disease: average RR 0.96, 95% CI 0.07 to 12.37; two studies with 70 infants, random‐effects. Statistical heterogeneity was evident (Tau² = 2.17; Chi² = 2.51, df = 1 (P = 0.11); I² = 60%). However, upon exploration of the possible reasons for the heterogeneity including examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis. (Analysis 1.10).
Persistent pulmonary hypertension of the newborn: three studies with 106 infants reported no events of persistent pulmonary hypertension of the newborn during the trial (Analysis 1.11).
Intraventricular haemorrhage (Grades III or IV): RR 3.15, 95% CI 0.14 to 72.88; one study with 39 infants (Analysis 1.12).
Necrotising enterocolitis: RR 0.97, 95% CI 0.21 to 4.43; two studies with 70 infants (Analysis 1.13).
Neonatal sepsis: RR 0.31, 95% CI 0.01 to 7.15; two studies with 70 infants (Analysis 1.14).
Premature closure of the ductus arteriosus: three studies with 106 infants; no events were reported. (Analysis 1.15).
Patent ductus arteriosus: RR 1.40, 95% CI 0.36 to 5.46; one study with 39 infants (Analysis 1.16).
1.4. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 4 Birth less than 7 days after trial entry.
1.7. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 7 Apgar score less than 7 at 5 minutes.
1.8. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 8 Admission to neonatal intensive care unit.
1.9. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 9 Respiratory distress syndrome.
1.10. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 10 Chronic lung disease.
1.11. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 11 Persistent pulmonary hypertension of the newborn.
1.12. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 12 Intraventricular haemorrhage Grades III or IV.
1.13. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 13 Necrotising enterocolitis.
1.14. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 14 Neonatal sepsis.
1.15. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 15 Premature closure of the ductus arteriosus.
1.16. Analysis.
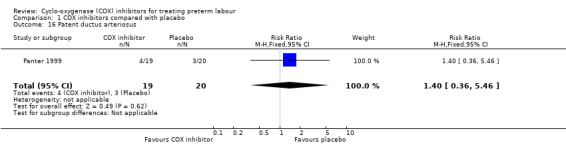
Comparison 1 COX inhibitors compared with placebo, Outcome 16 Patent ductus arteriosus.
For the woman
No differences were shown in the maternal outcomes measures as follows.
Maternal adverse drug reaction: RR 1.58, 95% CI 0.66 to 3.78; three studies with 101 women (Analysis 1.17).
Postpartum haemorrhage: one study with 34 women (Panter 1999) showed a trend towards increased proportion of postpartum haemorrhage for women treated with COX inhibitors compared with placebo (RR 3.94, 95% CI 0.95 to 16.29) (Analysis 1.18).
Chorioamnionitis or endometritis: RR 1.94, 95% CI 0.44 to 8.60; two studies with 64 women (Analysis 1.19).
Caesarean section: RR 1.00, 95% CI 0.07 to 14.79; one study with 36 women (Analysis 1.20).
1.17. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 17 Maternal adverse effects.
1.18. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 18 Postpartum haemorrhage.
1.19. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 19 Chorioamnionitis or endometritis.
1.20. Analysis.

Comparison 1 COX inhibitors compared with placebo, Outcome 20 Caesarean section.
Any COX inhibitor versus any other tocolytic by class of other tocolytic
This comparison includes seven studies with 331 women comparing COX inhibitors with betamimetics (Besinger 1991; Kramer 1999; Kurki 1991; Morales 1989; Nevils 1994; Odeh 1997; Purwaka 2004); seven studies with 792 women comparing COX inhibitors with MgSO4 (Asgharnia 2002; Borna 2007; Klauser 2012; McWhorter 2004; Morales 1993; Parilla 1997; Schorr 1998) and two studies with 230 women (Kashanian 2011; Klauser 2012) comparing COX inhibitors with CCBs.
Primary outcomes
Birth less than 48 hours after trial entry (Analysis 2.1)
2.1. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 1 Birth less than 48 hours after trial entry.
COX inhibitors versus betamimetics: a reduction in birth less than 48 hours after trial entry was shown with the use of COX inhibitors when compared with betamimetics: RR 0.27, 95% CI 0.08 to 0.96; NNTB 7, 95% CI 6 to 120; two studies with 100 women.
COX inhibitors versus MgSO4: no difference was shown in birth within 48 hours of trial entry with the use of COX inhibitors when compared with MgSO4: RR 0.87; 95% CI 0.59 to 1.29; four studies with 547 women.
COX inhibitors versus CCBs: there was no difference between COX inhibitors versus any CCB for the outcome birth within 48 hours of trial entry: RR 1.08; 95% CI 0.58 to 2.01; two studies with 230 women.
Neonatal mortality (Analysis 2.2)
2.2. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 2 Neonatal mortality.
No statistically significant differences were shown in neonatal mortality comparing COX inhibitors with other tocolytic as follows.
COX inhibitors versus betamimetics: RR 0.99, 95% CI 0.27 to 3.57; four studies with 237 infants.
COX inhibitors versus MgSO4: RR 1.59, 95% CI 0.59 to 4.29; five studies with 570 infants
COX inhibitors versus CCBs: RR 2.81, 95% CI 0.82 to 9.62; two studies with 249 infants.
No data were available for stillbirth or perinatal mortality or the other primary outcomes of death, major sensorineural disability at two years of age or serious maternal outcome.
Very preterm birth (before completion of 34 weeks of gestation) (Analysis 2.3)
2.3. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 3 Very preterm birth (before completion of 34 weeks of gestation).
COX inhibitors versus betamimetics: no outcome data were reported.
COX inhibitors versus MgSO4: no difference was shown in very preterm birth: RR 0.84, 95% CI 0.64 to 1.11; one study with 128 women.
COX inhibitors versus CCBs: no difference was shown in preterm birth: RR 0.92, 95% CI 0.70 to 1.21; one study with 148 women.
Maternal death (Analysis 2.4)
2.4. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 4 Maternal death.
COX inhibitors versus betamimetics: no outcome data were reported.
COX inhibitors versus MgSO4: one study with 128 women reported no events in the COX inhibitor group or in the MgSO4 group for this outcome.
COX inhibitors versus CCBs: one study with 148 women reported no events in the COX inhibitor group or in the CCB group for this outcome.
Secondary outcomes
For the infant
Preterm birth (before completion of 37 weeks of gestation) (Analysis 2.5)
2.5. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 5 Preterm birth (before completion of 37 weeks of gestation).
Statistical heterogeneity was evident for this outcome in the comparison of COX inhibitors versus MgSO4 (Tau² = 0.08; Chi² = 1.45, df = 1 (P = 0.23); I² = 31%). However, upon exploration of the possible reasons for the heterogeneity including examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis.
COX inhibitors versus betamimetics: a reduction in preterm birth was shown with the use of COX inhibitors when compared with betamimetics: average RR 0.53, 95% CI 0.28 to 0.99; NNTB 6, 95% CI 4 to 236; two studies with 80 women.
COX inhibitors versus MgSO4: no difference was shown in preterm birth comparing COX inhibitors with MgSO4: average RR 0.88, 95% CI 0.52 to 1.47; two studies with 216 women.
COX inhibitors versus CCBs: no difference was shown in preterm birth comparing COX inhibitors with CCBs: average RR 1.08, 95% CI 0.94 to 1.25; one study with 148 women.
No statistically significant differences were shown for any other reported outcome measures of pregnancy prolongation or neonatal morbidity as follows.
Birth less than seven7 days after trial entry (Analysis 2.6)
2.6. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 6 Birth less than 7 days after trial entry.
COX inhibitors versus betamimetics: RR 0.88, 95% CI 0.52 to 1.46; two studies with 146 women.
COX inhibitors versus MgSO4 : no outcome data were reported.
COX inhibitors versus CCBs: RR 0.26, 95% CI 0.03 to 2.19; one study with 79 women.
Pregnancy prolongation (days) (Analysis 2.7)
2.7. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 7 Pregnancy prolongation (days).
COX inhibitors versus betamimetics: no outcome data were reported.
COX inhibitors versus MgSO4: MD 0.20 days, 95% CI ‐4.66 to 5.06; one study with 101 women.
COX inhibitors versus CCBs: no outcome data were reported.
Gestational age (Analysis 2.8) and Birthweight (Analysis 2.9)
2.8. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 8 Gestational age (weeks).
2.9. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 9 Birthweight (g).
COX inhibitors versus betamimetics: gestational age (MD 1.55 weeks, 95% CI ‐0.41 to 3.50; two studies with 100 women); birthweight (MD 199.40 g; 95% CI ‐176.85 to 575.65; two studies with 105 infants).
COX inhibitors versus MgSO4: gestational age (MD 0.21 weeks, 95% CI ‐0.39 to 0.80; five studies with 538 women); birthweight (MD ‐74.93, 95% CI ‐190.16 to 40.30; five studies with 578 infants).
COX inhibitors versus CCBs: gestational age (MD ‐0.75 weeks, 95% CI ‐1.63 to 0.14; two studies with 230 women); birthweight (MD ‐34.00, 95% CI ‐256.20 to 188.20; one study with 170 infants).
Antenatal corticosteroids (Analysis 2.10)
2.10. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 10 Antenatal corticosteroids ‐ any.
COX inhibitors versus betamimetics: no outcome data were reported.
COX inhibitors versus magnesium sulphate: no outcome data were reported.
COX inhibitors versus any calcium channel blocker: RR 0.26, 95% CI 0.03 to 2.19; one study with 79 women.
Respirory distress syndrome (Analysis 2.11)
2.11. Analysis.
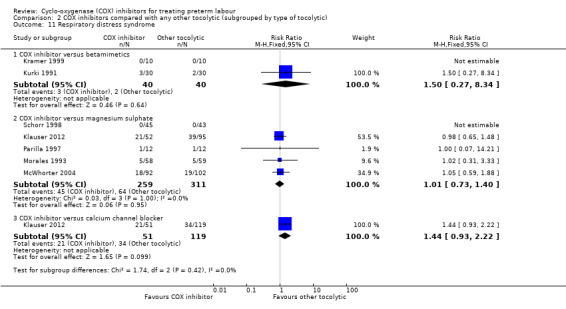
Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 11 Respiratory distress syndrome.
COX inhibitors versus betamimetics: RR 1.50, 95% CI 0.27 to 8.34; two studies with 80 infants. One of these studies (Kramer 1999) reported no events for this outcome in the COX inhibitor group or in the betamimetics group.
COX inhibitors versus MgSO4: RR 1.01, 95% CI 0.73 to 1.40; five studies with 570 infants. One of these studies (Schorr 1998) reported no events for this outcome in the COX inhibitor group or in the MgSO4 group.
COX inhibitors versus CCBs: RR 1.44, 95% CI 0.93 to 2.22; one study with 170 infants.
Apgar score less than seven at five minutes (Analysis 2.12)
2.12. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 12 Apgar score less than 7 at 5 minutes.
COX inhibitors versus betamimetics: RR 3.00, 95% CI 0.13 to 70.83, one study with 60 infants.
COX inhibitors versus magnesium sulphate: RR 0.43, 95% CI 0.16 to 1.15; one study with 194 infants.
COX inhibitors versus any calcium channel blocker: no outcome data were reported.
Admission to neonatal intensive care unit (Analysis 2.13)
2.13. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 13 Admission to neonatal intensive care unit.
COX inhibitors versus betamimetics: no outcome data were reported.
COX inhibitors versus MgSO4: RR 0.83, 95% CI 0.48 to 1.43; one study with 194 infants.
COX inhibitors versus CCBs: no outcome data were reported.
Duration of neonatal intensive care unit stay (Analysis 2.14)
2.14. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 14 Duration of neonatal intensive care unit stay.
COX inhibitors versus betamimetics: no outcome data were reported.
COX inhibitors versus MgSO4: MD ‐2.62 days, 95% CI ‐7.51 to 2.28; two studies with 341 infants.
COX inhibitors versus CCBs: MD ‐3.60 days, 95% CI ‐14.97 to 7.77; one study with 170 infants.
Use of mechanical ventilation (Analysis 2.15)
2.15. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 15 Use of mechanical ventilation.
COX inhibitors versus betamimetics: RR 1.50, 95% CI 0.47 to 4.78; one study with 60 infants.
COX inhibitors versus MgSO4: no outcome data were reported.
COX inhibitors versus CCBs: no outcome data were reported.
Duration of mechanical ventilation (Analysis 2.16)
2.16. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 16 Duration of mechanical ventilation (days).
COX inhibitors versus betamimetics: no outcome data were reported.
COX inhibitors versus MgSO4: MD ‐3.70 days, 95% CI ‐10.02 to 2.62; one study with 147 infants.
COX inhibitors versus CCBs: MD 2.00 days, 95% CI ‐2.58 to 6.58; one study with 170 infants.
Persistent pulmonary hypertension of the newborn (Analysis 2.17)
2.17. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 17 Persistent pulmonary hypertension of the newborn.
COX inhibitors versus betamimetics: RR 5.65, 95% CI 0.31 to 103.46; three studies with 177 infants.
COX inhibitors versus MgSO4: RR 1.78, 95% CI 0.23 to 13.44; two studies with 311 infants.
COX inhibitors versus CCBs: no outcome data were reported.
Intraventricular haemorrhage all grades (Analysis 2.18)
2.18. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 18 Intraventricular haemorrhage ‐ all grades.
COX inhibitors versus betamimetics: RR 5.34, 95% CI 0.66 to 43.10; three studies with 125 infants.
COX inhibitors versus MgSO4: RR 1.00, 95% CI 0.60 to 1.66; five studies with 570 infants.
COX inhibitors versus CCBs: RR 1.63, 95% CI 0.66 to 4.05; one study with 170 infants.
Intraventricular haemorrhage Grades III or IV (Analysis 2.19)
2.19. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 19 Intraventricular haemorrhage Grades III or IV.
COX inhibitors versus betamimetics: one small study including 20 infants reported no events in the COX inhibitor group or in the betamimetics group for this outcome.
COX inhibitors versus MgSO4: RR 0.61, 95% CI 0.08 to 4.40; three studies with 229 infants.
COX inhibitors versus CCBs: no outcome data were reported.
Other major cerebral abnormality (Analysis 2.20)
2.20. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 20 Other major cerebral abnormality.
COX inhibitors versus betamimetics: no outcome data were reported.
COX inhibitors versus MgSO4: RR 5.43, 95% CI 0.23 to 131.06; one study with 147 infants.
COX inhibitors versus CCBs: RR 6.92, 95% CI 0.29 to 167.15; one study with 170 infants.
Retinopathy of prematurity Grades III or IV (Analysis 2.21)
2.21. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 21 Retinopathy of prematurity Grades III or IV.
COX inhibitors versus betamimetics: one small study including 20 infants reported no events in the COX inhibitor group or in the betamimetics group for this outcome.
COX inhibitors versus MgSO4: no outcome data were reported.
COX inhibitors versus CCBs: no outcome data were reported.
Necrotising enterocolitis (Analysis 2.22)
2.22. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 22 Necrotising enterocolitis.
COX inhibitors versus betamimetics: RR 3.00, 95% CI 0.13 to 70.83; two studies with 80 infants.
COX inhibitors versus MgSO4: RR 1.49, 95% CI 0.46 to 4.84; three studies with 365 infants.
COX inhibitors versus CCBs: RR 1.75, 95% CI 0.41 to 7.54; one study with 170 infants.
Neonatal sepsis (Analysis 2.23)
2.23. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 23 Neonatal sepsis.
COX inhibitors versus betamimetics: RR 1.00, 95% CI 0.07 to 15.26; two studies with 80 infants.
COX inhibitors versus MgSO4: RR 1.10, 95% CI 0.42 to 2.85; one study with 147 infants.
COX inhibitors versus CCBs: RR 1.63, 95% CI 0.66 to 4.05; one study with 170 infants.
Oligohydramnios (Analysis 2.24)
2.24. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 24 Oligohydramnios.
COX inhibitors versus betamimetics: RR 2.50, 95% CI 0.75 to 8.33; two studies with 138 infants.
COX inhibitors versus MgSO4: RR 5.30, 95% CI 0.26 to 107.70; two studies with 189 infants.
COX inhibitors versus CCBs: no outcome data were reported.
Premature closure of the ductus arteriosus (Analysis 2.25)
2.25. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 25 Premature closure of the ductus arteriosus.
COX inhibitors versus betamimetics: three studies including 154 infants reported no events for this outcome.
COX inhibitors versus MgSO4: RR 3.05, 95% CI 0.13 to 73.39; two studies with 205 infants. Only one event was reported in the COX inhibitor group in one of the studies (Morales 1993) for this outcome.
COX inhibitors versus CCBs: no outcome data were reported.
Patent ductus arteriosus (Analysis 2.26)
2.26. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 26 Patent ductus arteriosus.
COX inhibitors versus betamimetics: RR 1.66, 95% CI 0.36 to 7.66; three studies with 125 infants.
COX inhibitors versus MgSO4: RR 0.65, 95% CI 0.28 to 1.49; two studies with 235 infants.
COX inhibitors versus CCBs: RR 1.27, 95% CI 0.50 to 3.26; one study with 170 infants.
Neonatal renal failure (Analysis 2.27)
2.27. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 27 Neonatal renal failure.
COX inhibitors versus betamimetics: one small study, including 20 infants, reported no events in the COX inhibitor group or in the betamimetics group for this outcome.
COX inhibitors versus MgSO4: no outcome data were reported.
COX inhibitors versus CCBs: no outcome data were reported.
For the woman
Maternal adverse effects (Analysis 2.28)
2.28. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 28 Maternal adverse effects.
COX inhibitors versus betamimetics: a reduction in maternal adverse effects was shown with the use of COX inhibitors when compared with betamimetics: RR 0.19, 95% CI 0.11 to 0.31, NNTB 3, 95% CI 2 to 3; five studies with 248 women.
COX inhibitors versus MgSO4: a reduction in maternal adverse effects was shown with the use of COX inhibitors when compared with MgSO4: RR 0.39, 95% CI 0.25 to 0.62; NNTB 11, 95% CI 9 to 17; five studies with 635 women.
COX inhibitors versus CCBs: no statistically significant differences was shown in maternal adverse effects with the use of COX inhibitors when compared with CCBs: RR 0.67, 95% CI 0.36 to 1.23; two studies with 227 women.
Maternal adverse drug reaction requiring cessation of treatment (Analysis 2.29)
2.29. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 29 Maternal adverse effects requiring cessation of treatment.
Statistical heterogeneity was evident for this outcome in the comparison of COX inhibitors versus MgSO4 (Tau² = 12.58; Chi² = 6.81, df = 1 (P = 0.009); I² = 85%). However, upon exploration of the possible reasons for the heterogeneity by examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis.
COX inhibitors versus betamimetics: three studies including 166 women showed a reduction in maternal adverse drug reaction requiring cessation of treatment with the use of COX inhibitors when compared with betamimetics (average RR 0.09, 95% CI 0.02 to 0.49; NNTB 5, CI 95% 5 to 9).
COX inhibitors versus MgSO4: there was no difference comparing COX inhibitors versus MgSO4: average RR 0.91, 95% CI 0.00 to 186.10; three studies with 317 women.
COX inhibitors versus CCBs: no difference was shown in maternal adverse effects comparing COX inhibitors with CCBs: average RR 1.18, 95% CI 0.31 to 4.51; one study with 148 women.
No statistically significant differences were shown for any of the following secondary maternal outcome.
Chorioamnionitis or endometritis (Analysis 2.30)
2.30. Analysis.
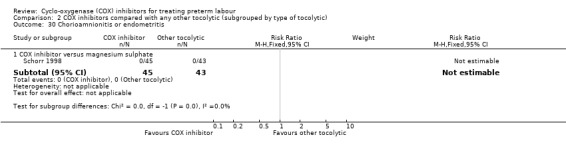
Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 30 Chorioamnionitis or endometritis.
COX inhibitors versus betamimetics: no outcome data were reported.
COX inhibitors versus MgSO4: one study with 88 women reported no events in the COX inhibitor group or in the MgSO4 group for this outcome.
COX inhibitors versus CCBs: no outcome data were reported.
Caesarean section (Analysis 2.31)
2.31. Analysis.

Comparison 2 COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic), Outcome 31 Caesarean section.
COX inhibitors versus betamimetics: no outcome data were reported.
COX inhibitors versus MgSO4: RR 1.23, 95% CI 0.70 to 2.13; one study with 194 women.
COX inhibitors versus CCBs: no outcome data were reported.
Due to insufficient data, the planned subgroup analyses (treatment commenced before 28 weeks' gestation; and studies enrolling women with a multiple pregnancy) could not be performed.
Any non‐selective COX inhibitor versus any COX‐2 inhibitor
A comparison of any non‐selective COX inhibitor (indomethacin and sulindac) versus any COX‐2 inhibitor (celecoxib and nimesulide) including two studies with 54 women (Sawdy 2003; Stika 2002) showed no differences in any reported maternal or neonatal outcomes.
Primary outcomes
Birth less than 48 hours after trial entry (Analysis 3.1)
3.1. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 1 Birth less than 48 hours after trial entry.
One study (24 women) (Stika 2002) reported no births less than 48 hours after trial entry in either group.
Neonatal mortality (Analysis 3.2)
3.2. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 2 Neonatal mortality.
Both included studies reported no events for this outcome in the non‐selective COX inhibitor group or COX‐2 inhibitor group.
No data were available for the other primary outcome measures of extremely preterm birth (before completion of 28 weeks of gestation), very preterm birth (before completion of 34 weeks of gestation), death or major sensorineural disability at two years of age or serious maternal outcome.
Secondary outcomes
For the infant/child
No differences were shown in the following neonatal outcome measures.
Preterm birth (before completion of 37 weeks of gestation): RR 1.00, 95% CI 0.31 to 3.19; two studies with 54 women (Analysis 3.3).
Birth less than seven days after trial entry: RR 3.00, 95% CI 0.13 to 67.06; one study with 24 women (Analysis 3.4).
Gestational age at birth: MD 0.00 weeks, 95% CI ‐2.81 to 2.81; one study with 24 women (Analysis 3.5).
Birthweight: MD 27.00 g, 95% CI ‐549.58 to 603.58; one study with 24 infants (Analysis 3.6).
Respiratory distress syndrome: RR 1.00, 95% CI 0.07 to 14.21; one study with 24 infants (Analysis 3.7).
Intraventricular haemorrhage Grades III or IV: no events reported in one study with 24 infants (Analysis 3.8).
Intraventricular haemorrhage ‐ all grades: RR 0.50, 95% CI 0.05 to 4.81; one study with 24 infants (Analysis 3.9).
Necrotising enterocolitis: no events reported in one study with 24 infants (Analysis 3.10).
Apgar score less than seven at five minutes: RR 3.00, 95% CI 0.13 to 67.06; one study with 24 infants (Analysis 3.11).
Admission to neonatal intensive care unit: RR 1.00, 95% CI 0.34 to 2.91; two studies with 54 infants (Analysis 3.12).
Use of mechanical ventilation: RR 1.00, 95% CI 0.07 to 14.21; one study with 24 infants (Analysis 3.13).
Neonatal sepsis: RR 0.33, 95% CI 0.01 to 7.45; one study with 24 infants (Analysis 3.14).
Premature closure of the ductus arteriosus: no events reported in two studies with 54 infants (Analysis 3.15).
Oligohydramnios: RR 4.00, 95% CI 0.52 to 30.76; one study with 24 infants (Analysis 3.16).
3.3. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 3 Preterm birth (before completion of 37 weeks of gestation).
3.4. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 4 Birth less than 7 days after trial entry.
3.5. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 5 Gestational age (weeks).
3.6. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 6 Birthweight (g).
3.7. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 7 Respiratory distress syndrome.
3.8. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 8 Intraventricular haemorrhage Grades III or IV.
3.9. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 9 Intraventricular haemorrhage ‐ all grades.
3.10. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 10 Necrotising enterocolitis.
3.11. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 11 Apgar score less than 7 at 5 minutes.
3.12. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 12 Admission to neonatal intensive care unit.
3.13. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 13 Use of mechanical ventilation.
3.14. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 14 Neonatal sepsis.
3.15. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 15 Premature closure of the ductus arteriosus.
3.16. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 16 Oligohydramnios.
For the woman
No differences were shown in the maternal outcomes measures as follows.
Maternal adverse effects: two studies with 54 women reported no maternal adverse effects (Analysis 3.17).
Antepartum haemorrhage: RR 0.33, 95% CI 0.01 to 7.45; one study with 24 women (Analysis 3.18).
Chorioamnionitis or endometritis: RR 2.00, 95% CI 0.21 to 19.23; one study with 24 women (Analysis 3.19).
Caesarean section: RR 0.67, 95% CI 0.13 to 3.30; one study with 24 women (Analysis 3.20).
3.17. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 17 Maternal adverse effects.
3.18. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 18 Antepartum haemorrhage.
3.19. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 19 Chorioamnionitis or endometritis.
3.20. Analysis.

Comparison 3 Non‐selective COX inhibitors compared with selective COX‐2 inhibitors, Outcome 20 Caesarean section.
Discussion
Summary of main results
With the addition of seven studies with a total of 684 women, this review now includes outcome data from 20 studies including 1509 women. The non‐selective cyclo‐oxygenase (COX) inhibitor indomethacin was used in 15 studies. Three small studies (106 women), two of which were conducted in the 1980s, compared COX inhibition (indomethacin only) with placebo. A trend towards fewer births less than 48 hours after trial entry, which was not statistically significant was shown when combining the results of two small studies; however a reduction in preterm birth (before completion of 37 weeks of gestation) and less than 48 hours after trial entry was shown in one of these studies (36 women). Some benefit was also shown in gestational age (four weeks on average) and birthweight (716 g on average) (two studies with 67 women). However, despite this modest improvement in prolongation of pregnancy no difference was shown in important neonatal outcomes. No benefit was shown in measures of neonatal morbidity or neonatal mortality in any of the comparisons in this review. Further, no data were available on longer‐term infant outcomes and potential adverse effects of COX inhibition on the fetus, newborn or mother could not be adequately assessed due to insufficient data.
Compared to betamimetics, COX inhibitors resulted in a reduction in birth less than 48 hours after trial entry combing the results of two small studies yielding a NNTB of 7 on average, however confidence intervals (CIs) were wide (95% CI 6 to 120) (100 women) and preterm birth (before 37 weeks of gestation) (80 women); NNTB of 6, again with wide CIs (95% CI 4 to 236). No differences were shown when comparing COX inhibitors with magnesium sulphate (MgSO4) (four studies with 508 women), or calcium channel blockers (CCBs) (one study with 79 women) in terms of prolongation of pregnancy or fetal/neonatal outcomes. No data were available to assess COX inhibitors compared with oxytocin receptor antagonists (ORAs). No conclusions could be drawn on the relative benefits of non‐selective COX inhibitors over COX‐2 inhibitors (two studies with 54 women). COX inhibition was associated with fewer maternal adverse affects than betamimetics and MgSO4 but not when compared with CCBs.
Overall completeness and applicability of evidence
Because these conclusions are based on small numbers, they must be interpreted with caution. Despite some positive findings of prolongation of pregnancy, COX inhibitors were not associated with improvements in neonatal mortality or any markers of neonatal morbidity. There are only three studies (Niebyl 1980; Panter 1999; Zuckerman 1984a) comparing COX inhibition with placebo, with a total of 102 participants. Two of these studies (Niebyl 1980; Zuckerman 1984a) included participants up to 35 weeks' gestation, when the incidence of neonatal adverse events is low. The small number of adverse events reported allows no meaningful conclusion to be drawn. Inclusion criteria of very low gestational age (≤ 24 weeks) in studies (20 weeks in one study) again raises concerns about the validity of extrapolating results of tocolytic efficacy and other outcomes as the pathophysiology of parturition at these extreme gestations may be very different compared to later in pregnancy (e.g. cervical incompetence). There was a paucity of more recent studies included in this review (the oldest study being almost 34 years old), which may diminish applicability to current clinical practice for women in preterm labour.
In 12 of the 20 included studies, administration of antenatal corticosteroids to all women enrolled was stated in the methodology; however the majority did not report the proportion of women who completed the course of antenatal steroids.
Eleven studies included multiple pregnancy, which may have different preterm labour aetiologies and therefore extrapolation of the efficacy of COX inhibitors in this group of women has to be guarded. Notwithstanding these caveats, what is quite apparent from this review is the reassuring data on maternal safety and low incidence of side effects with the use of COX inhibitors for the inhibition of preterm labour.
A major clinical issue regarding the use of COX inhibitors has been concerned about possible effects on the fetal ductus arteriosus; in particular, antenatal premature constriction and closure leading to postnatal persistent patent ductus. In this review, premature closure of the ductus arteriosus was reported in 10 studies with 287 infants exposed to short‐term tocolysis (up to 48 hours) with COX inhibitors (mainly indomethacin); only one event of premature closure of the ductus arteriosus was reported. Further, in six studies with 232 infants exposed to short‐term tocolysis with COX inhibitors there were 20 events of patent ductus arteriosus; no significant increase in the incidence of patent ductus arteriosus postnatally for infants exposed to COX inhibitors was noted. These data suggest that, for short‐term COX inhibition, the concern about ductal effects of COX inhibitors may have been overstated. In addition, the data also suggest that with short‐term use of COX inhibitors, maternal side effects and symptoms appear to be minimal. Given the benefits shown in this review (pregnancy prolongation and less maternal adverse effects than betamimetics), the role of COX inhibitors for women in preterm labour warrants further attention in the form of further high‐quality studies.
Quality of the evidence
The overall quality of the included studies was considered moderate to low. Only half (11) of the included studies reported adequate random sequence generation. One study (Odeh 1997) was considered high risk and in the remaining studies, the randomisation sequence generation was unclear. Similarly, concealment of allocation was considered low risk of bias in less than half (seven of the 20 studies). One study (Odeh 1997) was considered high risk of bias for allocation concealment while 12 studies were unclear. Importantly, Odeh 1997 did not contribute data to the outcomes assessed in this review. Nine studies employed blinding of participants and personnel and nine studies did not. Eight studies had adequate blinding of outcome assessment while two studies were considered high risk. The majority (14) of included studies had minimal or no attrition and were assessed as having low risk of bias; only one study was considered to have high risk of attrition bias. In 10 studies, we found no obvious evidence of reporting bias and judged these studies to be at low risk of bias. Two studies (Sawdy 2003; Schorr 1998) were deemed to be at high risk of reporting bias with minimal data reported as expected. The majority of studies were assessed as being at low risk of bias for other potential sources of bias.
Potential biases in the review process
We are aware that the review process itself is subject to bias, and we took steps to minimise bias. At least two review authors carried out data extraction and independently assessed risk of bias; however, a different review team may not have made identical decisions.
Agreements and disagreements with other studies or reviews
Our finding of a reduction in births less than 48 hours after trial entry randomisation and less preterm births with the use of COX inhibitors (mainly indomethacin) is consistent with a network meta‐analysis (Haas 2012) showing COX inhibitors tended to have the best probability of delaying birth for 48 hours or more. Further, this review reported that nifedipine (the most commonly used CCB) may be superior in preventing neonatal complications, which is consistent with the recently updated Cochrane review on CCBs (Flenady 2014b), when compared to other tocolytics. Similar to our review, this review found limited study outcome data comparing COX inhibitors with CCBs although these tocolytics appear to have a similarly lower adverse effect profile for women. However, the effect on neonatal mortality or morbidity is not clear when comparing CCBs and COX inhibitors. We were not able to identify any studies comparing ORAs with COX inhibitors. The most commonly used COX inhibitor for tocolysis is indomethacin, which has the advantage of ease of administration and less cost compared with some other tocolytic agents, e.g. ORA. Consistent with our conclusions, Haas et al (Haas 2012) supported further well‐designed, randomised, placebo‐controlled studies to evaluate COX inhibitors in prolonging pregnancy for women in preterm labour.
Authors' conclusions
Implications for practice.
There is insufficient information on which to base decisions about the value of COX inhibition for women in preterm labour.
Implications for research.
On the basis of the results of this review, further well‐designed placebo controlled studies are justified to assess the potential benefits and safety of COX inhibition for women in preterm labour, particularly addressing longer‐term outcomes for infants.
What's new
| Date | Event | Description |
|---|---|---|
| 24 August 2014 | New search has been performed | With the addition of seven studies with a total of 684 women, this review now includes outcome data from 20 studies including 1509 women. The conclusions of the previous review remain unchanged. |
| 24 August 2014 | New citation required but conclusions have not changed | We searched the Cochrane Pregnancy and Childbirth Group's Trial Register and identified additional studies for inclusion. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 1 October 2009 | Amended | Search updated. Seven reports added to Studies awaiting classification (Bartfield 1998a; Borna 2007a; Caballero 1979a; Gamissans 1984a; Groom 2005a; Jain 2006a; Shrivastava 2008a). |
| 12 May 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Steve Carlan for assistance in providing unpublished information from the McWhorter 2004 study. We would also like to thank Steve Thornton, James King and Steve Colewho who were authors on previous versions of this review. We would also like to thank Katie Welsh for assistance with reference management
As part of the pre‐publication editorial process, this review has been commented on by three peers (2005 version) (an editor and two referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. COX inhibitors compared with placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth less than 48 hours after trial entry | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.03, 1.28] |
| 2 Neonatal mortality | 3 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.25, 2.58] |
| 3 Preterm birth (before completion of 37 weeks of gestation) | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.62] |
| 4 Birth less than 7 days after trial entry | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.10, 1.66] |
| 5 Gestational age (weeks) | 2 | 66 | Mean Difference (IV, Random, 95% CI) | 3.59 [0.66, 6.52] |
| 6 Birthweight (g) | 2 | 67 | Mean Difference (IV, Fixed, 95% CI) | 716.34 [425.52, 1007.16] |
| 7 Apgar score less than 7 at 5 minutes | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.34] |
| 8 Admission to neonatal intensive care unit | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.15] |
| 9 Respiratory distress syndrome | 3 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.40, 2.49] |
| 10 Chronic lung disease | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.07, 12.37] |
| 11 Persistent pulmonary hypertension of the newborn | 3 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Intraventricular haemorrhage Grades III or IV | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.14, 72.88] |
| 13 Necrotising enterocolitis | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.21, 4.43] |
| 14 Neonatal sepsis | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.15] |
| 15 Premature closure of the ductus arteriosus | 3 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Patent ductus arteriosus | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.36, 5.46] |
| 17 Maternal adverse effects | 3 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.66, 3.78] |
| 18 Postpartum haemorrhage | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.94 [0.95, 16.29] |
| 19 Chorioamnionitis or endometritis | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.44, 8.60] |
| 20 Caesarean section | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.79] |
Comparison 2. COX inhibitors compared with any other tocolytic (subgrouped by type of tocolytic).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth less than 48 hours after trial entry | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 COX inhibitor versus betamimetics | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.08, 0.96] |
| 1.2 COX inhibitor versus magnesium sulphate | 4 | 547 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.29] |
| 1.3 COX inhibitor versus calcium channel blocker | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.58, 2.01] |
| 2 Neonatal mortality | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 COX inhibitor versus betamimetics | 4 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.27, 3.57] |
| 2.2 COX inhibitor versus magnesium sulphate | 5 | 570 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.59, 4.29] |
| 2.3 COX inhibitor versus calcium channel blocker | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.82, 9.62] |
| 3 Very preterm birth (before completion of 34 weeks of gestation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 COX inhibitor versus magnesium sulphate | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.64, 1.11] |
| 3.2 COX inhibitor versus calcium channel blocker | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.21] |
| 4 Maternal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 COX inhibitor versus magnesium sulphate | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 COX inhibitor versus calcium channel blocker | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Preterm birth (before completion of 37 weeks of gestation) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 COX inhibitor versus betamimetics | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.28, 0.99] |
| 5.2 COX inhibitor versus magnesium sulphate | 2 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.52, 1.47] |
| 5.3 COX inhibitor versus calcium channel blocker | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.25] |
| 6 Birth less than 7 days after trial entry | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 COX inhibitor versus betamimetics | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.52, 1.46] |
| 6.2 COX inhibitor versus calcium channel blocker | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.19] |
| 7 Pregnancy prolongation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 COX inhibitor versus magnesium sulphate | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐4.66, 5.06] |
| 8 Gestational age (weeks) | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 COX inhibitor versus betamimetics | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 1.55 [‐0.41, 3.50] |
| 8.2 COX inhibitor versus magnesium sulphate | 5 | 538 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.39, 0.80] |
| 8.3 COX inhibitor versus calcium channel blocker | 2 | 230 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.63, 0.14] |
| 9 Birthweight (g) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 COX inhibitor versus betamimetics | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | 199.40 [‐176.85, 575.65] |
| 9.2 COX inhibitor versus magnesium sulphate | 5 | 578 | Mean Difference (IV, Fixed, 95% CI) | ‐74.93 [‐190.16, 40.30] |
| 9.3 COX inhibitor versus calcium channel blocker | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐34.0 [‐256.20, 188.20] |
| 10 Antenatal corticosteroids ‐ any | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 COX inhibitor versus calcium channel blocker | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.19] |
| 11 Respiratory distress syndrome | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 COX inhibitor versus betamimetics | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.34] |
| 11.2 COX inhibitor versus magnesium sulphate | 5 | 570 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.40] |
| 11.3 COX inhibitor versus calcium channel blocker | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.93, 2.22] |
| 12 Apgar score less than 7 at 5 minutes | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 COX inhibitor versus betamimetics | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
| 12.2 COX inhibitor versus magnesium sulphate | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.16, 1.15] |
| 13 Admission to neonatal intensive care unit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 COX inhibitor versus magnesium sulphate | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.48, 1.43] |
| 14 Duration of neonatal intensive care unit stay | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 COX inhibitor versus magnesium sulphate | 2 | 341 | Mean Difference (IV, Fixed, 95% CI) | ‐2.62 [‐7.51, 2.28] |
| 14.2 COX inhibitor versus calcium channel blocker | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐14.97, 7.77] |
| 15 Use of mechanical ventilation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 COX inhibitor versus betamimetics | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.47, 4.78] |
| 16 Duration of mechanical ventilation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 COX inhibitor versus magnesium sulphate | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐10.02, 2.62] |
| 16.2 COX inhibitor versus calcium channel blocker | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐2.58, 6.58] |
| 17 Persistent pulmonary hypertension of the newborn | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 COX inhibitor versus betamimetics | 3 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.65 [0.31, 103.46] |
| 17.2 COX inhibitor versus magnesium sulphate | 2 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.23, 13.44] |
| 18 Intraventricular haemorrhage ‐ all grades | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 COX inhibitor versus betamimetics | 3 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.34 [0.66, 43.10] |
| 18.2 COX inhibitor versus magnesium sulphate | 5 | 570 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.60, 1.66] |
| 18.3 COX inhibitor versus calcium channel blocker | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.66, 4.05] |
| 19 Intraventricular haemorrhage Grades III or IV | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 COX inhibitor versus betamimetics | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 COX inhibitor versus magnesium sulphate | 3 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.08, 4.40] |
| 20 Other major cerebral abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 COX inhibitor versus magnesium sulphate | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.43 [0.23, 131.06] |
| 20.2 COX inhibitor versus calcium channel blocker | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.92 [0.29, 167.15] |
| 21 Retinopathy of prematurity Grades III or IV | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 COX inhibitor versus betamimetics | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Necrotising enterocolitis | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 COX inhibitor versus betamimetics | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
| 22.2 COX inhibitor versus magnesium sulphate | 3 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.46, 4.84] |
| 22.3 COX inhibitor versus calcium channel blocker | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.41, 7.54] |
| 23 Neonatal sepsis | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 COX inhibitor versus betamimetics | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 23.2 COX inhibitor versus magnesium sulphate | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.42, 2.85] |
| 23.3 COX inhibitor versus calcium channel blocker | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.66, 4.05] |
| 24 Oligohydramnios | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 COX inhibitor versus betamimetics | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [0.75, 8.33] |
| 24.2 COX inhibitor versus magnesium sulphate | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.3 [0.26, 107.70] |
| 25 Premature closure of the ductus arteriosus | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 COX inhibitor versus betamimetics | 3 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 COX inhibitor versus magnesium sulphate | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.13, 73.39] |
| 26 Patent ductus arteriosus | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 COX inhibitor versus betamimetics | 3 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.36, 7.66] |
| 26.2 COX inhibitor versus magnesium sulphate | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.28, 1.49] |
| 26.3 COX inhibitor versus calcium channel blocker | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.50, 3.26] |
| 27 Neonatal renal failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 COX inhibitor versus betamimetics | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Maternal adverse effects | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 COX inhibitor versus betamimetics | 5 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.11, 0.31] |
| 28.2 COX inhibitor versus magnesium sulphate | 5 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.25, 0.62] |
| 28.3 COX inhibitor versus calcium channel blocker | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.36, 1.23] |
| 29 Maternal adverse effects requiring cessation of treatment | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 29.1 COX inhibitor versus betamimetics | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.02, 0.49] |
| 29.2 COX inhibitor versus magnesium sulphate | 3 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.00, 186.10] |
| 29.3 COX inhibitor versus calcium channel blocker | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.31, 4.51] |
| 30 Chorioamnionitis or endometritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 COX inhibitor versus magnesium sulphate | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Caesarean section | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31.1 COX inhibitor versus magnesium sulphate | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.70, 2.13] |
Comparison 3. Non‐selective COX inhibitors compared with selective COX‐2 inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth less than 48 hours after trial entry | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Neonatal mortality | 2 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Preterm birth (before completion of 37 weeks of gestation) | 2 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.19] |
| 4 Birth less than 7 days after trial entry | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 67.06] |
| 5 Gestational age (weeks) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.81, 2.81] |
| 6 Birthweight (g) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 27.0 [‐549.58, 603.58] |
| 7 Respiratory distress syndrome | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.21] |
| 8 Intraventricular haemorrhage Grades III or IV | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Intraventricular haemorrhage ‐ all grades | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.81] |
| 10 Necrotising enterocolitis | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Apgar score less than 7 at 5 minutes | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 67.06] |
| 12 Admission to neonatal intensive care unit | 2 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.91] |
| 13 Use of mechanical ventilation | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.21] |
| 14 Neonatal sepsis | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.45] |
| 15 Premature closure of the ductus arteriosus | 2 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Oligohydramnios | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.52, 30.76] |
| 17 Maternal adverse effects | 2 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Antepartum haemorrhage | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.45] |
| 19 Chorioamnionitis or endometritis | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.21, 19.23] |
| 20 Caesarean section | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asgharnia 2002.
| Methods | Randomised controlled trial. | |
| Participants | 120 women in preterm labour between 24‐32 weeks' gestation with intact membranes and cervical dilation ≥ 2 cm. Exclusion criteria: PROM, complete cervical dilatation, severe haemorrhage, chorioamnionitis, high‐order multiple pregnancy (≥ triplets), fetal or placental abnormality or sensitivity to tocolytic drugs. |
|
| Interventions | COX group: indomethacin. 25 mg every 6 h (p.o.) up to maximum of 4 doses. Control group: MgSO4. Bolus dose of 4 g (i.v.) followed by 2 g/h (i.v.) given in 5% dextrose at 10‐25 µg/min (i.v.). Both groups: antibiotics as required and 5 mg dexamethasone every 12 h up to 4 doses. |
|
| Outcomes | Gestational age, mode of delivery, frequency of uterine contractions, dilatation and effacement and delivery delay. | |
| Notes | This paper was published in a non‐English language and has been translated for the purpose of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to determine how randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, but there is no mention of simultaneous placebo treatment to adjust for differences in drug administration and it is likely that participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated, but there is no mention of simultaneous placebo treatment to adjust for differences in drug administration and it is likely that participants and personnel were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were included in reporting. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine. |
| Other bias | Unclear risk | Unable to determine. |
Besinger 1991.
| Methods | Randomised controlled trial. | |
| Participants | 43 women with preterm labour between 23 to 34 weeks' gestation with intact membranes and contractions at a rate of ≥ 4/20 min or ≥ 8/60 min and cervical dilatation > 2 cm or effacement > 75%. Singleton and multiple pregnancies were included. Exclusion criteria: cervical dilatation > 4 cm, or with ruptured membranes or if they had a cervical cerclage. |
|
| Interventions | COX group: indomethacin. Initial dose of 50 mg (p.o.), then 25‐50 mg (p.o.) every 4 h until contractions ceased, thereafter 25 mg (p.o.) every 4‐6 h. Control group: ritodrine. 100‐350 μg/min (i.v.) continued until 8‐12 h after contractions ceased. Terbutaline (dosing as required to prevent recurrent uterine activity, 2.5‐5 g) were then given every 4‐6 h. Both groups: maintenance fluid 125 mL/h. All tocolytic treatments were discontinued at 35 weeks' gestation. Patients with initial treatment failure (progressive preterm labour or patient intolerance) received MgSO4 (bolus dose: 4 g (i.v.) followed by 2‐4 g/h (i.v.)) for 8‐12 h after contractions ceased. |
|
| Outcomes | Efficacy and side effects of treatment. Maternal: birth (> 35 weeks), delayed delivery > 48 h and > 7 days, side effects, interval from treatment to delivery, gestational age at delivery. Neonatal: birth weight, 5 min Apgar score, umbilical cord pH, days in NICU, days on ventilator, primary pulmonary hypertension, PDA, IVH, neonatal death. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly assigned by a table of random numbers via a sealed envelope..." |
| Allocation concealment (selection bias) | Unclear risk | "... via a sealed envelope.." The authors consider this approach unclear risk as it is not stated whether the envelopes were "Sequentially numbered, opaque, sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Postrandomisation exclusions: 3 women ‐ 1 in indomethacin group for 'evidence of a concealed abruptio placentae' and 2 in ritodrine group for protocol errors. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as expected. |
| Other bias | Low risk | None apparent. |
Borna 2007.
| Methods | Randomised controlled trial. | |
| Participants | 104 women with preterm labour (defined as progressive cervical dilatation or effacement associated with regular uterine contractions at a rate of ≥ 4/20 min or ≥ 8/60 min) with intact membranes and cervical dilatation ≤ 4 cm between 24‐34 weeks' gestation with a singleton pregnancy. Exclusion criteria: cervical dilatation > 4 cm, non‐reassuring FHR or other contra‐indications to tocolysis (including intra‐amniotic infection), fetal anomaly, placenta previa, renal or hepatic dysfunction, thrombocytopenia, coagulation disorder, history of peptic ulcer disease or use of fluconazole. |
|
| Interventions | COX group: celecoxib. 100 mg every 12 h (p.o.) up to 48 h. Simultaneous administration of physiological saline (80 mL/h) for the duration of study. Control group: MgSO4. Bolus dose of 4‐6 g (i.v.) followed by 2‐4 g/h (i.v.) up to 48 h. Simultaneous administration of placebo tablet (p.o.) every 12 h. Both groups: electrical fetal monitoring. 12 mg betamethasone (12 mg) every 24 h up to 2 doses. Continuing treatment if required. |
|
| Outcomes | Delay in delivery for 48 h, maternal and neonatal side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each participant in the study was randomly assigned by the pharmacy with a random number table [...]." The authors consider this approach low risk. |
| Allocation concealment (selection bias) | Low risk | "The hospital pharmacy supplied the patient with the medications [...]." The authors consider this approach low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The hospital pharmacy supplied the patient with the medications and the investigators and patients were blinded as to which preparation the patient was taking." The authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "At no time before data analysis did any clinical investigator have access to or knowledge of the identity of the assigned drug." The authors consider this approach low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised have been included in analyses. |
| Selective reporting (reporting bias) | Unclear risk | Maternal and neonatal side effects: Although maternal side effects were reported in the text, it was stated: "There were no severe maternal or neonatal complications in either group believed to be related to study medications." |
| Other bias | Low risk | None apparent. |
Kashanian 2011.
| Methods | Randomised controlled trial, block randomisation | |
| Participants | 80 nulliparous women between 26‐34 weeks' gestation documented by a definite LMP and sonography in the first trimester. Preterm labour defined as contraction rate of 4/20 min or 8/60 min, cervical dilation of ≥ 1 cm and cervical effacement of ≥ 50%. Exclusions: PROM, vaginal bleeding, multiple pregnancy, fetal distress or fetal death, IUGR, a history of trauma, cervical dilation > 4 cm, systemic disorders of the mother including pre‐eclampsia, a known uterine or fetal anomaly, smoking, use of other drugs, polyhydramnios, suspected intrauterine infection, prior use of tocolytics and BP < 90/50. |
|
| Interventions | COX group: indomethacin. 100 mg suppository, repeated after 1 h if contractions continued, maximum dose of 200 mg. Control group: nifedipine. 10 mg (p.o.) every 20 mins until cessation of contractions or up to 40 mg total followed by 20 mg every 6 h for 24 h, then 20 mg every 8 h for 24 h, then 10 mg every 8 h for 24 h. Both groups: 12 mg of betamethasone every 24 h up to 48 h. Alternative treatment was used if treatment failed. |
|
| Outcomes | Maternal: cessation of contractions after 2 h, delivery within 48 h, delivery within 7 days, adverse drug reactions. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, 4 part, ABCD. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women in nifedipine group excluded post‐randomisation due to hypotension, 1 woman in indomethacin group excluded ‐ unexplained. |
| Selective reporting (reporting bias) | Unclear risk | Minimal neonatal outcome data reported. |
| Other bias | Unclear risk | Unable to determine. |
Klauser 2012.
| Methods | 3‐armed randomised controlled trial. | |
| Participants | 276 women in preterm labour (uterine contractions ≤ 5 min apart, cervical dilatation ≥ 1 cm or a change form the previous vaginal examination) between 20‐32 weeks' gestation with intact membranes and vertex presentations with cervical dilatation from 1 to 6 cm as well as sufficient effacement and decrease in station. Exclusion criteria: high‐order multiple pregnancy (≥ triplets), women with prior significant medical/surgical reasons for early delivery such as severe pre‐eclampsia, abruptio placenta, fetal malformations inconsistent with life, chorioamnionitis, IUGR (< 5th percentile), non‐reassuring FHR or refusal to participate in the study. |
|
| Interventions | COX group: indomethacin. 100 mg suppository, which was repeated after 2 h if required, then 50 mg (p.o.) every 6 h until contractions had been extinguished for 12 h or up to a maximum of 48 h. Pepcid (20 mg) was given p.o. twice daily. Control group: MgSO4. 6 g over 20 min (i.v.), thereafter 4‐6 g/h until contractions had been stopped for 1‐ 2 h. CCB group: nifedipine. Bolus dose 30 mg (p.o.) followed by 20‐30 mg every 4‐6 h for 24 h until contractions abated. All groups: antenatal steroids were started and no antibiotics were used. |
|
| Outcomes | Maternal: proportion of women undelivered after 48 h and 7 days, delivery after 37, 34 and 30 weeks' gestation, maternal drug side effects, tachycardia, fetal ductal constriction, oligohydramnios. Neonatal: composite measure of "adverse neonatal morbidity" (including RDS, PDA, sepsis, IVH, PVL, NEC), gestational age at birth, birthweight, acidosis, days of mechanical ventilation, neonatal length of stay. | |
| Notes | For subgroup comparisons, the authors of this review have split data from the COX arm into 2 groups with smaller sample size, to achieve reasonably independent comparisons (i.e., (1) COX versus MgSO4, and (2) COX versus CCB). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drugs were distributed by the University pharmacy by opening consecutive opaque envelopes containing a card advising group allocation. The cards were "generated by random selection" but no details as to how. The authors consider this approach low risk. |
| Allocation concealment (selection bias) | Unclear risk | Sequentially opened opaque envelopes. Probably low risk, but no details as to whether envelopes were sealed and tamper‐proof. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, diverging treatment regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Those assessing outcomes were not privy to group assignment as they were not involved in their clinical care." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25 of 301 women were lost to attrition, reasons for each loss are provided (e.g., delivered elsewhere, no medications available). |
| Selective reporting (reporting bias) | Low risk | Neonatal and maternal outcomes reported in separate papers, all as expected. |
| Other bias | Low risk | None evident. |
Kramer 1999.
| Methods | Randomised controlled study. | |
| Participants | 20 women ≥ 17 years with preterm labour (defined as uterine contractions at a rate of 4/20 min or 8/60 min in conjunction with cervical dilatation ≥ 2 cm, cervical effacement ≥ 80%, or documented cervical change on serial examinations) between 24‐35 weeks' gestation. Exclusion criteria: maternal medical illness, contraindications to tocolytic therapy, ruptured membranes, fetal anomalies, IUGR, oligohydramnios, evidence of bacteria or white blood cells, glucose concentration < 15 mg/dL, lecithin/sphingomyelin ratio > 1.7 and presence of phosphatidylglycerol. |
|
| Interventions | COX group: sulindac. 200 mg (p.o.) every 12 h for 6 doses. Every 4 h, when not receiving COX, a placebo tablet was given. Control group: terbutaline. 5 mg (p.o.) every 4 h for 72 h. Both groups: all study medications were ground to powder form and combined with glucose‐based powder. 12 mg betamethasone (i.m.) was given with the first study medication and again at 24 h. No other medications given. After 72 h all women received 5 mg terbutaline (p.o.) every 4 h until delivery or until 36 weeks' gestation. |
|
| Outcomes | Fetal cardiovascular function (by studying the waveforms in the ductus arteriosus, middle cerebral artery and umbilical artery), fetal urinary function (by studying the renal arteries, fetal urinary output and amniotic fluid output). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated random table." The authors consider this low risk. |
| Allocation concealment (selection bias) | Low risk | "All study medications, including placebos, were contained in identical opaque capsules in previously prepared blister packs. [...] which was given to the pharmacist responsible for dispensing the medications." The authors consider this approach low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both the principal investigator and the patient were unaware of the type of medication given." Although it is not known if other personnel involved in patient care were blinded, the authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "All infants were assessed at birth by a neonatologist and managed accordingly." It is unknown if the neonatologist was blinded to study medications. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women appear to be included in analyses. |
| Selective reporting (reporting bias) | Unclear risk | Minimal data were reported for neonatal outcomes and efficacy of drug treatment; however this was not the aim of this study. |
| Other bias | Low risk | None evident. |
Kurki 1991.
| Methods | Randomised controlled trial in 2 hospitals. | |
| Participants | 60 women with imminent preterm labour (defined as painful uterine contractions at least at 10 min intervals and cervical dilatation 2‐4 cm), Bishop score of 1‐9, between 25‐34 weeks' gestation with intact membranes, normal amniotic fluid volume and lack of fetal abnormality as assessed by ultrasound. Exclusion criteria: multiple pregnancies, IUGR (< 10th percentile), cervical dilatation > 4 cm, ruptured membranes, placenta previa, previous tocolysis in current pregnancy or contraindication to tocolysis (including suspected chorioamnionitis, cardiovascular disease or aspirin allergy). |
|
| Interventions | COX group: indomethacin. 100 mg suppository, then 50 mg (p.o.) every 8 h for 24 h followed by 50 mg 3 times daily for 48 h. Simultaneous infusion of physiological saline (i.v.; initial dose 30 mL/h, maintenance 90 mL/h). Control group: nylidrin. Initial dose 50 μg/min in physiological saline (initial 30 mL/h; i.v.), after 30 min increased to maintenance dose 100‐150 μg/min (in 90 mL/h). Simultaneous administration of placebo suppository and oral tablets. Both treatments continued until cessation of contractions or up to a maximum of 3 days. |
|
| Outcomes | Maternal: vital signs (including BP, pulse rate, respiration rate and side effects), uterine activity, effect of tocolysis (by assessing postponed delivery and frequency of preterm deliveries), treatment failure (defined as delivery during tocolysis or recurrence of uterine contractions within 48 h of cessation of treatment). Neonatal: placental histology, respiratory distress, PDA, septicaemia, IVH, NEC. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects [...] were treated in randomized order (choice by a sealed envelope)..." |
| Allocation concealment (selection bias) | Unclear risk | "Choice by a sealed envelope." The authors consider this approach unclear risk as it is not stated whether the envelopes were "Sequentially numbered, opaque, sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The double‐dummy technique was followed..." The authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated; however the concomitant placebo treatment to all participants which was identical in appearance to study drugs suggests low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were included in analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None evident. |
McWhorter 2004.
| Methods | Randomised controlled trial. | |
| Participants | 214 women with preterm labour (defined as progressive cervical dilatation or effacement associated with regular uterine contractions) between 22‐34 weeks' gestation with intact membranes and cervical dilatation of ≤ 4 cm. Exclusion criteria: non‐reassuring fetal surveillance, IUGR, lethal congenital anomalies, allergy to NSAIDs, contraindications to tocolysis. | |
| Interventions | COX group: rofecoxib. 50 mg (p.o.) daily. Simultaneous treatment with physiologic saline (80 mL/h) was given for the duration of the study. Control group: MgSO4. Bolus dose (4‐6 g; i.v.) followed by 2‐4 g/h. Simultaneos placebo treatment with tablet identical in appearance to study drug (with lactose filler) was administered daily. All women had urogenital cultures taken at randomisation and were started on ampicillin (2 g every 6 h, i.v.) until cultures returned. If cultures were positive women were treated appropriately for up to 7 days and if cultures were negative antibiotic treatment was terminated. Treatment was continued until cessation of contractions or up to 48 h, longer if required. All women were offered betamethasone (12 mg, i.m.) every 24 h for 2 doses. No participants received aspirin or other tocolysis. |
|
| Outcomes | Primary: delay in delivery for 48 h. Secondary: maternal and neonatal side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each participant [...] was randomly assigned by the pharmacy with a random table number." |
| Allocation concealment (selection bias) | Low risk | Not stated; however the hospital pharmacy supplied the patient with the medication. The authors consider this approach to be low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The pharmacy supplied the patient with the medications, and the investigators and patients were blinded as to which preparation the patient was taking." The authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "At no time before data analysis did any clinical investigator have access to or knowledge of the identity of the assigned drug." The authors consider this approach low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 women in the COX group and 3 women in the control group were excluded post‐randomisation (2 membrane rupture prior to medication start, 1 chorioamnionitis, 1 patient refusal, 1 physician error). However all women randomised were included in analyses. In addition there was no information available for 13 patients in the COX group and 7 patients in the control group for neonatal outcomes. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Morales 1989.
| Methods | Randomised controlled trial. | |
| Participants | 106 women with preterm labour (defined as progressive cervical dilatation or effacement associated with regular uterine contractions at a rate of 4/20 min) less than 32 weeks' gestation with intact membranes. Exclusion criteria: cervical dilatation > 4 cm, clinical evidence of intra‐amniotic infection or pyelonephritis, medical complications contraindicating tocolysis, IUGR and congenital anomalies inconsistent with life. |
|
| Interventions | COX group: indomethacin. 100 mg suppository, which was repeated 1‐2 h later if required, followed by 25 mg (p.o.) every 4 h up to 48 h. Control group: ritodrine. 50 μg/min (i.v.) increasing by 50 μg/min every 20 min until contractions stopped or a maximum of 350 μg/min reached. Treatment was discontinued if patients displayed changes in ECG, had persistent chest pain, persistent HR > 140 bpm or persistent hypotension (defined as diastolic BP < 40 mmHg). Both groups: MgSO4 (i.v.) was administered to women with persistent uterine contractions, progressive cervical dilatation or serious side effects as a bolus dose (5 g; i.v.) followed by 2‐4 g/h as required. At admission a single dose of Vitamin K (10 mg; i.m.) and 2 doses of betamethasone (12 mg; i.m.) 24 h apart were administered. |
|
| Outcomes | Maternal: effectiveness of tocolysis, side effects. Neonatal: RDS, IVH, PDA, platelet count, creatinine, blood urea nitrogen, glucose levels. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized by means of sealed envelopes..." |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomized by means of sealed envelopes..." The authors consider this approach unclear risk as it is not stated whether the envelopes were "Sequentially numbered, opaque, sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however considering the different administration routes for the treatment drugs it is likely that participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were included in data analyses. For neonatal outcomes data from 7 infants in the COX group and 8 infants in the control group were missing. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Morales 1993.
| Methods | Randomised controlled trial. | |
| Participants | 114 women with singleton pregnancies with preterm labour (defined as contraction rate 4/20 min associated with progressive cervical dilatation or effacement or cervical dilatation > 2 cm) less than 32 weeks' gestation. Exclusion criteria: multiple pregnancy, ruptured membranes, IUGR, congenital anomalies incompatible with life, cervical dilatation > 4 cm. |
|
| Interventions | COX group: indomethacin. 100 mg suppository, which was repeated 1‐2 h later if required, followed by 25 mg (p.o.) every 4 h up to 48 h. Control group: MgSO4. Bolus dose (6 g over 30 min) followed by 2 g/h (50 g MgSO4 in 500 mL 5% dextrose in water) increasing by 1 g/h every 30 min up to 5 g/h. Serum magnesium concentration measurements were taken from patients with clinically relevant symptoms. Both groups: at admission a single dose of Vitamin K (10 mg; i.m.) and 2 doses of betamethasone (12 mg; i.m.) 24 h apart were administered. Women < 30 weeks' gestation with failure of tocolysis received phenobarbital (780 mg over 30 min, i.v.). After cessation of contractions (< 4 contractions/h) all patients received terbutaline (5 mg every 6 h; p.o.). |
|
| Outcomes | Maternal: effectiveness of tocolysis, side effects. Neonatal: RDS, IVH, pulmonary hypertension, death, blood urea nitrogen, creatinine, urine output. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomized [...] for tocolysis by means of random number tables. Subjects assigned odd integers were allocated to the MgSO4 group." |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes are mentioned. The authors consider this approach unclear risk as it is not stated whether the envelopes were "Sequentially numbered, opaque, sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however considering the different administration routes for the treatment drugs it is likely that participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 women in the COX group and 6 in the control group were excluded due to failure to adhere to the study protocol. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Nevils 1994.
| Methods | Randomised controlled trial of a rescue tocolytic. | |
| Participants | 45 women between 24‐34 weeks' gestation with preterm labour. | |
| Interventions | COX group: indomethacin (p.o.). Control group: terbutaline (i.v.). Both groups: treatment up to 48 h. Alternative treatment was used if tocolysis was unsuccessful. |
|
| Outcomes | Efficacy of indomethacin as a rescue tocolytic including maternal side effects, cervical dilatation, delayed delivery and neonatal outcomes. | |
| Notes | Only abstract available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 women did not meet inclusion criteria and only 9 women the COX group and 13 in the control group were included in analyses. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine. |
| Other bias | Low risk | None apparent. |
Niebyl 1980.
| Methods | Randomised placebo‐controlled trial. | |
| Participants | 32 women between 24‐33 weeks' gestation with preterm labour (defined as cervical dilatation of ≥ 2 cm or 75% effaced or any cervical change during a period of observation together with painful/regular contractions). Exclusion criteria: ruptured membranes, cervical dilatation > 4 cm, FGR, salicylate sensitivity or peptic ulcer disease history, intrauterine infection and IUGR. |
|
| Interventions | COX group: indomethacin. Bolus dose 50 mg (p.o.) followed by 25 mg every 4 h up to 24 h. If contractions recurred after discontinuation, the protocol was repeated with the same study drug for another 24 h. Control group: placebo. Lactose capsule (p.o.). Both groups: alternative therapy was used for treatment failure (defined as cervical dilatation > 4 cm > 2 h after initial dose). |
|
| Outcomes | Maternal: mean plasma concentration of treatment drug, gestational age. Fetal/neonatal: Side effects. Benign: Bleeding, NEC, respiratory distress, hyperbilirubinaemia, metabolic abnormality. Life‐threatening: pneumothorax, BPD, severe RDS, persistent fetal circulation, PDA with congestive heart failure, bradycardia and premature atrial contractions, chronic hepatitis, hydrocephalus. Causes of death: NEC, sepsis, stillbirth. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Capsules were placed in envelopes and allocated to patients according to a table of random numbers..." |
| Allocation concealment (selection bias) | Unclear risk | "Capsules were placed in envelopes..." The authors consider this approach unclear risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Capsules were allocated to patients [...] in a double‐blind fashion." The authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Based on blinding of participants and personnel, the authors consider this low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women were excluded from the indomethacin group: 1 woman vomited the initial capsule treatment and no detectable levels of indomethacin were achieved and 1 woman was treated in error. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Odeh 1997.
| Methods | Randomised controlled trial. | |
| Participants | 25 women in preterm labour (≥ 6 uterine contractions/h and cervical dilatation > 4 cm) with intact membranes and singleton pregnancy. | |
| Interventions | COX group: indomethacin. 100 mg suppository and 25 mg (p.o.) followed by 25 mg (p.o.) every 6 h. Control group: ritodrine. 0.1 mg/min and increased by 0.05 mg/min every 10 min until inhibition of labour and then maintained for 24 h. Thereafter 20 mg every 4 h (p.o.). Both groups: no antenatal steroid treatment. Treatment was continued up to 48 h. |
|
| Outcomes | Concentration of progesterone after drug administration. | |
| Notes | This study did not contribute with data to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "[...] randomly and consecutively selected for treatment [...] (the first with ritodrine, the second with indomethacin etc)." The authors consider this approach high risk. |
| Allocation concealment (selection bias) | High risk | "[...] randomly and consecutively selected for treatment [...] (the first with ritodrine, the second with indomethacin etc)." The authors consider this approach high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unknown how many women were randomised; only how many women were included in analyses. |
| Selective reporting (reporting bias) | Unclear risk | No neonatal or maternal outcomes were reported and no measures of efficacy of treatment were included; these were not within the scope of this study. |
| Other bias | Low risk | None apparent. |
Panter 1999.
| Methods | Randomised placebo‐controlled study. | |
| Participants | 34 women between 23‐30 weeks' gestation with preterm labour (defined as regular painful contractions with cervical dilatation or effacement). Exclusion criteria: ruptured membranes, SGA, chorioamnionitis, oligohydramnios, maternal or fetal contraindications to indomethacin or tocolysis. |
|
| Interventions | COX group: indomethacin. Bolus dose 50 mg (p.o.) given followed by 25 mg every 6 h for 48 h. Control group: placebo. Tablets of identical appearance distributed in the same fashion as treatment. Both groups: analgesia and i.v. fluids as required, attempted completion of complete course of betamethasone (2 doses of 12 mg (i.m.) 12 h apart). |
|
| Outcomes | Maternal: side effects, prolongation of gestation, gestational age at delivery, postpartum haemorrhage, endometritis, wound infection, amniotic fluid volume. Fetal/neonatal: oxygen dependency at 36 weeks' gestation, perinatal or neonatal death prior to discharge, NEC, IVH (grade 4), PVL, chronic lung disease, admission to NICU, PDA, RDS, surfactant use, days in oxygen, days ventilated, seizures, oliguria, time in hospital. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were randomised [...] in random blocks of 2 and four with stratification for study centre. Randomisation was centrally controlled." The authors consider this approach low risk. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...double‐blind fashion...". In addition, the appearance of the placebo and COX inhibitor tablets was identical. The authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, however based on the blinded approach of participants and personnel the authors consider this low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were included in analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Parilla 1997.
| Methods | Randomised controlled trial. | |
| Participants | 24 women with preterm labour (defined as regular contractions and progressive cervical dilatation and effacement) < 30 weeks' gestation. Multiples included. Exclusion criteria: ruptured membranes, uterine bleeding, IUGR, chorioamnionitis, preeclampsia, or abnormal FHR. |
|
| Interventions | COX group: indomethacin. Bolus dose 50‐100 mg (p.o. or rectally) followed 25‐50 mg (p.o.) every 4‐6 h up to 24‐48 h. Control group: MgSO4. 8 g (i.v.) over 60 min followed by 4 g over 60 min, then 2.5 g/h for 12 h after cessation of contractions. Both groups: betamethasone (2 doses of 12.5 mg 24 h apart). Alternative treatment was used if tocolysis was unsuccessful. |
|
| Outcomes | Maternal: gestational age at delivery. Fetal/neonatal: fetal cerebral blood flow, IVH, RDS, NEC, neonatal death. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was based on a computer‐generated series of random numbers balanced every six patients for a 1:1 ratio." The authors consider this approach low risk. |
| Allocation concealment (selection bias) | Low risk | "...randomised by opaque sealed envelopes..." The authors consider this approach low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded; the drugs were administered via different routes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unknown how many women were randomised and thus the authors consider this unclear risk. |
| Selective reporting (reporting bias) | Unclear risk | No data on tocolytic efficacy and pregnancy prolongation were included. Only minimal data on neonatal and maternal outcomes were included; it appears this was outside the scope of the study. |
| Other bias | Low risk | None apparent. |
Purwaka 2004.
| Methods | Randomised controlled trial. | |
| Participants | 32 women in threatened preterm labour between 24‐32 weeks' gestation. | |
| Interventions | COX group: nimesulide. 4 times 100 mg (p.o.) up to 24 h then 2 times 100 mg per day for 6 days. Control group: isoxsuprine. Given parenterally and followed by 3 times 20 mg (p.o.) per day for 7 days. |
|
| Outcomes | Efficacy of tocolysis (including delivery delay and time for omitting contractions). | |
| Notes | Only abstract available. This study did not contribute with data to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[...] then simply randomized to [...]." |
| Allocation concealment (selection bias) | Unclear risk | "[...] then simply randomized to [...]." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, different administration routes for the treatment drugs. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine. |
| Other bias | Low risk | None apparent. |
Sawdy 2003.
| Methods | 3‐armed randomised trial. | |
| Participants | 30 women between 28‐32 weeks' gestation with preterm labour (contractions ≥ 30 sec at least 5 min apart and cervical dilation ≥ 2 cm). Exclusion criteria: multiple pregnancy, ruptured membranes, advance cervical dilatation, maternal contraindications to NSAIDs. |
|
| Interventions | COX group: sulindac. 200 mg (p.o.) and 1 placebo suppository.
COX group: indomethacin. 100 mg (rectally) and 1 placebo capsule (p.o.). Control group: nimesulide. 200 mg (rectally) and 1 placebo capsule (p.o.). All groups: 1 suppository and 1 oral capsule every 12 h for 4 doses. Genital tract swabs, a midstream urine specimen, a full blood count, and a C‐reactive protein sample were taken and 2 doses of dexamethasone (12.5 mg, i.m.) was given 12 h apart. Antibiotic prophylaxis (i.v.) with either coamoxiclav (1.5 g twice daily) or cefuroxime (500 mg 3 times daily) was given for 24 h. |
|
| Outcomes | Maternal: amniotic fluid index, gestational age at delivery, prolongation of delivery. Fetal neonatal: fetal urine output, Doppler pulsatile index, fetal side effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "To prevent observer bias, drugs were prepared by the pharmacy department in sealed boxes and divided into three equal groups; block randomization was performed to assign the package number. Blocks of 15 patients at a time were produced to ensure that drug expiration dates (1 year) were not violated." |
| Allocation concealment (selection bias) | Low risk | "To prevent observer bias, drugs were prepared by the pharmacy department in sealed boxes and divided into three equal groups;.." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Placebo and active suppository or capsule were designed to have identical appearance". In addition, drugs were prepared by the pharmacy in order to "avoid observer bias". The authors consider this approach low risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and personnel were blinded in this study; the authors consider this low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were included in analyses. |
| Selective reporting (reporting bias) | High risk | The objective of this study was to establish fetal side effects of 2 different drugs after acute maternal exposure for tocolysis; however very limited data on side effects were reported form this study. It is also stated: "There were no reports of adverse neonatal complications related to the study drugs". The authors consider this to be high risk of reporting bias. |
| Other bias | Low risk | None apparent. |
Schorr 1998.
| Methods | Prospective randomised controlled trial. | |
| Participants | 88 women ≤ 32 weeks with preterm labour (defined as contraction rate ≥ 12/60 min, cervical dilatation ≥ 2 cm and 50% effaced or cervical change from earlier examination). Singleton and twin pregnancies included. Exclusion criteria: ruptured membranes, cervical dilatation > 4 cm, significant maternal disease/obstetric disorders or maternal history of peptic ulcer, asthma, bleeding diathesis, thrombocytopenia, NSAID sensitivity, fetal malformations, chorioamnionitis, oligohydramnios, FGR, non reassuring fetal status. |
|
| Interventions | COX group: ketorolac. Bolus dose 60 mg (i.m.) then 30 mg every 6 h for 24 h. Control group: MgSO4. 6 g (i.v.) over 20 min, followed by 2‐6 g/h continuous infusion until 2 h after cessation of uterine contractions, thereafter slow tapering for 3‐4 h before stopping. Both groups: oral tocolysis with magnesium gluconate (2 g every 4 h) after parenteral agents were discontinued up to 37 completed weeks. Betamethasone (12 mg, i.m.) was given and repeated 12 h later. |
|
| Outcomes | Maternal: time to uterine quiescence, recurrent preterm labour, delivery during current admission, readmission for preterm labour, preterm delivery < 37 weeks, amniotic fluid volume, oligohydramnios, side effects. Neonatal: side effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomised by pharmacy personnel who selected a sealed opaque envelope...". Random sequence generation is not mentioned. |
| Allocation concealment (selection bias) | Low risk | "...sealed opaque envelope..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. Drugs were administered via different routes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were included in analyses. However, 7 eligible women did not participate; 4 declined entry to the study, 1 had placental abruption and 2 had advanced cervical dilatation. |
| Selective reporting (reporting bias) | High risk | The aim of this study was to evaluate safety and efficacy of tocolysis; however, limited data were available in the report for pregnancy prolongation and maternal, fetal and neonatal side effects. |
| Other bias | Low risk | None apparent. |
Stika 2002.
| Methods | Randomised controlled trial. | |
| Participants | 24 women ≥ 18 years old between 24‐34 weeks' gestation with singleton pregnancy and intact membranes with preterm labour (regular, painful contraction unresponsive to initial i.v. hydration and progressive dilatation or effacement). Exclusion criteria: cervical dilation ≥ 4 cm, non‐reassuring FHR or other contraindication to tocolysis (including intra‐amniotic infection), fetal anomalies, amniotic fluid index < 8, placenta previa, renal or hepatic dysfunction, thrombocytopenia (< 150,000 platelets), platelet dysfunction or coagulation disorder, history of peptic ulcer disease, sensitivity to NSAIDs, use of fluconazole. |
|
| Interventions | COX group: indomethacin. 100 mg (rectally) followed by 50 mg (p.o.) every 6 h up to 48 h. Simultaneous administration of placebo capsule (p.o.). Control group: celecoxib. 100 mg (p.o.) every 12 h up to 48 h. Placebo suppository at start of trial, thereafter 1 capsule every 12 h (p.o.; alternating with active drug). Both groups: administration of MgSO4 (i.v.) prior to and up to 2 h after study drug administration. All women received 1 course of corticosteroid treatment during the first 48 h. Continued cervical change and contraction within 6 h of trial entry was considered treatment failure and MgSO4 (i.v.) was resumed. Recurrent preterm labour episodes after completion of study were treated with MgSO4 (i.v.). Additional NSAIDs were prohibited. |
|
| Outcomes | Maternal: gestational age at delivery, mode of delivery, Apgar score. Neonatal: weight, duration of stay in NICU, duration of ventilatory support, sepsis, hyaline membrane disease, transient hypertension, IVH, PVL. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...women were stratified by gestational age [...]. Women were randomised [...] by sealed envelopes...". |
| Allocation concealment (selection bias) | Low risk | "Women were randomized to tocolytic therapy with either celecoxib or indomethacin by sealed envelopes that were kept in each institution’s pharmacy." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both groups were administered simultaneous placebo to ensure blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of women randomised not reported; only how many women were enrolled. All women enrolled were included in analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
Zuckerman 1984a.
| Methods | Randomised controlled trial. | |
| Participants | 36 women between 25‐35 weeks' gestation with preterm labour (painful, regular uterine contractions at a rate of > 2/10 min for at least 30 min, or accompanied by effacement and/or cervical dilatation ≥ 1‐2 cm and ≤ 4 cm. Exclusion criteria: pre‐eclampsia, diabetes mellitus, twin pregnancy, cardiac disease, APH, ruptured membranes, intra‐uterine infections, IUGR. |
|
| Interventions | COX group: indomethacin. 100 mg (rectally) followed by 25 mg (p.o.) 4 times daily up to 24 h. Additional 100 mg suppository if required. Indications of preterm labour and progressive cervical dilatation within 2 h after trial entry was considered treatment failure. Control group: placebo. Treatment at the same time‐intervals and fashion as the COX group. Both groups: alternative treatment was given if tocolysis was unsuccessful. |
|
| Outcomes | Maternal: delivery within 48 h or 7 days, preterm delivery, gestational age at delivery, side effects. Neonatal: weight, Apgar score, side effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Indomethacin and placebo were allocated at random." Unclear how sequence generation was done. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo controlled trial. "If uterine contractions continued after the insertion of the first suppository, the patient received an additional 100 mg suppository (from the same envelope) after 1 h, and then the treatment was continued orally." Participants and personnel were likely to be blinded to treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear. "The code key was not available to the investigators before completion of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unknown how many women were randomised; only how many women were included in analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as expected. |
| Other bias | Low risk | None apparent. |
BP: blood pressure BPD: bronchopulmonary dysplasia CCB: calcium channel blocker cm: centimetre COX: cyclo‐oxygenase FGR: fetal growth restriction FHR: fetal heart rate g: gram h: hour/s i.m.: intramuscular IUGR: intrauterine growth restriction i.v.: intravenous IVH: intraventricular haemorrhage LMP: last menstrual period mg: milligram MgSO4: magnesium sulphate min: minutes mL: millilitre μg: microgram NEC: necrotising enterocolitis NICU: neonatal intensive care unit NSAID: nonsteroidal anti‐inflammatory drug p.o.: orally PDA: patent ductus arteriosus PROM: premature rupture of membranes PVL: periventricular haemorrhage RDS: respiratory distress syndrome SGA: small for gestational age
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abramov 2000 | This study compared different routes of administration of indomethacin for the treatment of women with preterm labour: intravaginal versus intrarectal plus oral. |
| Bartfield 1998 | This study included pregnant women with an episode of preterm labour successfully treated testing drugs for follow‐up prevention of preterm labour. |
| Bivins 1993 | A study of maintenance therapy following successful tocolysis: indomethacin versus terbutaline. |
| Caballero 1979 | Not a randomised trial. |
| Carlan 1992 | Women were randomised following failed tocolysis to either indomethacin or sulindac. |
| Carlan 1995 | A study of maintenance therapy following successful tocolysis: sulindac versus placebo. |
| Ehsanipoor 2011 | This study investigated the role of indomethacin for women with preterm premature rupture of the membranes and excluded women in preterm labour. |
| Gamissans 1982 | Combination tocolytic therapy comparison: ritodrine versus ritodrine with indomethacin. |
| Gamissans 1984 | This is a book chapter with no original data, summarising results from clinical studies. |
| Groom 2005 | This randomised clinical trial was not a tocolytic study: high‐risk women were enrolled from 16 to 26 weeks for prevention of preterm labour. |
| Hallak 1992 | This was not a tocolytic study: women with a low‐risk pregnancy were randomly assigned to either indomethacin or terbutaline to assess the effects on fetal breathing and body movements. |
| Humphrey 2001 | A study of maintenance therapy following successful tocolysis: sulindac versus placebo. |
| Jain 2006 | This study is not described as a randomised controlled trial. |
| Katz 1983 | This study assessed combination tocolytic therapy: ritodrine versus ritodrine with indomethacin. |
| Mital 1992 | Women were not in preterm labour at enrolment. This study assessed the effects of mefenamic acid in the prevention of preterm labour for women at increased risk of preterm birth. |
| Newton 1991 | 3‐armed combination tocolytic therapy comparison: indomethacin, MgSO4 with antibiotics versus MgSO4 alone. |
| Rasanen 1995 | 2 non‐selective COX inhibitors were compared for the treatment of women in preterm labour: indomethacin versus sulindac. |
| Rios‐Anez 2001 | Combination tocolytic therapy comparison: fenoterol alone versus fenoterol with indomethacin. |
| Spearing 1979 | Quasi‐random combination tocolytic study comparing ethanol, ethanol with indomethacin versus salbutamol. |
| Zuckerman 1984b | Prospective uncontrolled study to evaluate efficacy and safety in women treated with indomethacin for preterm labour. No comparison group included. |
COX: cyclo‐oxygenase MgSO4: magnesium sulphate
Characteristics of studies awaiting assessment [ordered by study ID]
Castillo 1988.
| Methods | Study of women in threatening preterm labour. |
| Participants | 35 pregnant women. |
| Interventions | COX group: indomethacin (n = 10). Betamimetics group: ritodrine (n = 10). Combination group: ritodrine and indomethacin (n = 10). Control group: bedrest with infusion of 1000 cc of physiologic serum (i.v.; n = 5). |
| Outcomes | Biophysical and biochemical modifications. |
| Notes | It is unclear why the number of participants was significantly different in the bedrest group. We will await further clarification if this was a randomised controlled trial. |
Mesdaghinia 2012.
| Methods | Randomised clinical study in Iran. |
| Participants | 60 women between 24‐32 weeks' gestation at risk of preterm labour. |
| Interventions | COX group: indomethacin (rectally). Control group: MgSO4 (i.v.). |
| Outcomes | Maternal: delayed preterm labour, morbidity. |
| Notes | It is unclear whether women were in preterm labour at time of enrolment. This study is published in a non‐English language and we are awaiting translation to enable inclusion assessment. |
COX: cyclo‐oxygenase i.v.: intravenous MgSO4: magnesium sulphate
Differences between protocol and review
In this update, primary and secondary outcomes measures were revised to enhance consistency across Cochrane tocolytic reviews and to better reflect important outcome measures.
Contributions of authors
For this update (2014) João Paulo Souza and Vicki Flenady screened studies for inclusion. Hanna Reinebrant, Rafaela Neman dos Santos and Carla Romero performed data extraction and assessment of quality of the new studies for inclusion; Cynthia Pileggi‐Castro, Hanna Reinebrant and Vicki Flenady also assessed study quality and Hanna Reinebrant and Vicki Flenady completed all 'Risk of bias' tables, revised the analyses and results section based on recent guidelines from the Pregnancy and Childbirth Group. Sailesh Kumar revised the characteristics of the studies tables and accompanying text. All review authors reviewed the final version of the review and approved the final version.
Sources of support
Internal sources
Centre for Clinical Studies ‐ Women's and Children's Health, Mater Mothers' Hospital, South Brisbane, Queensland, Australia.
Department of Perinatal Medicine, Royal Women's Hospital, Melbourne, Victoria, Australia.
Department of Biological Sciences, University of Warwick, Coventry, UK.
External sources
Department of Health and Ageing, Commonwealth Government, Canberra ‐ Supporting the Centre for Clinical Studies, Mater Hospital, Australia.
Declarations of interest
All authors declared no conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Asgharnia 2002 {published data only}
- Asgharnia M, Sobhani A, Omidvar‐Jalali Z. Comparison of Mg‐Sulfate and indomethacin in management of women with preterm labor. Journal of Gorgan University of Medical Sciences 2002;4(2):7‐12. [Google Scholar]
Besinger 1991 {published data only}
- Besinger RE, Niebyl JR, Keyes WG, Johnson TR. Randomized comparative trial of indomethacin and ritodrine for the long‐term treatment of preterm labor. American Journal of Obstetrics and Gynecology 1991;164(4):981‐8. [DOI] [PubMed] [Google Scholar]
Borna 2007 {published data only}
- Borna S, Saeidi FM. Celecoxib versus magnesium sulfate to arrest preterm labor: randomized trial. Journal of Obstetrics and Gynaecology Research 2007;33(5):631‐4. [DOI] [PubMed] [Google Scholar]
Kashanian 2011 {published data only}
- Kashanian M, Bahasadri S, Zolali B. Comparison of the efficacy and adverse effects of nifedipine and indomethacin for the treatment of preterm labor. International Journal of Gynecology & Obstetrics 2011;113(3):192‐5. [DOI] [PubMed] [Google Scholar]
Klauser 2012 {published data only}
- Klauser CK, Briery CM, Keiser SD, Martin RW, Kosek MA, Morrison JC. Effect of antenatal tocolysis on neonatal outcomes. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(12):2778‐81. [DOI] [PubMed] [Google Scholar]
- Klauser CK, Briery CM, Martin RW, Langston L, Magann EF, Morrison JC. A comparison of three tocolytics for preterm labor: a randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(8):801‐6. [DOI] [PubMed] [Google Scholar]
Kramer 1999 {published data only}
- Kramer W, Saade G, Belfort M, Dorman K, Mayes MMK. Randomized double‐blind study comparing sulindac to terbutaline: fetal cardiovascular effects. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):326. [DOI] [PubMed] [Google Scholar]
- Kramer WB, Saade GR, Belfort M, Dorman K, Mayes M, Moise KJ. A randomized double‐blind study comparing the fetal effects of sulindac to terbutaline during the management of preterm labor. American Journal of Obstetrics and Gynecology 1999;180(2):396‐401. [DOI] [PubMed] [Google Scholar]
Kurki 1991 {published data only}
- Eronen M. The hemodynamic effects of antenatal indomethacin in a beta‐sympathomimetic agent on the fetus and the newborn: a randomized study. Pediatric Research 1993;33(6):615‐9. [DOI] [PubMed] [Google Scholar]
- Eronen M, Pesonen E, Kurki T, Ylikorkala O, Hallman M. The effects of indomethacin and beta‐sympathomimetic agent on the fetal ductus arteriosus during treatment of premature labor: A randomized double‐blind study. American Journal of Obstetrics and Gynecology 1991;164:141‐6. [DOI] [PubMed] [Google Scholar]
- Eronen N, Pesonen E, Kurki T, Teramo K, Ylikorkala O, Hallman M. Increased incidence of bronchopulmonary dysplasia after antenatal administration of indomethacin to prevent preterm labor. Journal of Pediatrics 1994;124:782‐8. [DOI] [PubMed] [Google Scholar]
- Kurki T, Eronen M, Lumme R, Ylikorkala O. A randomized double‐dummy comparison between indomethacin and nylidrin in threatened preterm labor. Obstetrics & Gynecology 1991;78(6):1093‐7. [PubMed] [Google Scholar]
- Kurki T, Laatikainen T, Salminen‐Lappalainen K, Ylikorkala O. Maternal plasma corticotrophin‐releasing hormone ‐ elevated in preterm labour but unaffected by indomethacin or nylidrin. British Journal of Obstetrics and Gynaecology 1991;98:685‐91. [DOI] [PubMed] [Google Scholar]
- Kurki T, Schultz E, Linden IB, Ylikorkala O. Catechol‐O‐methyltransferase activity in red blood cells in threatened preterm labor: effect of indomethacin and nylidrin. Acta Obstetricia et Gynecologica Scandinavica 1991;70:187‐91. [DOI] [PubMed] [Google Scholar]
- Lumme R, Kurki T, Pyorala T, Ylikorkala O. Indomethacin is more effective than nylidrine in arresting preterm labor. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:202.
- Salokorpi T, Eronen M, Wendt L. Growth and development until 18 months of children exposed to tocolytics indomethacin or nylidrin. Neuropediatrics 1996;27:147‐7. [DOI] [PubMed] [Google Scholar]
McWhorter 2004 {published data only}
- McWhorter J, Carlan S, O'Leary T, Richici K, O'Brien W. Rofecoxib versus magnesium sulfate to arrest preterm labour: a randomised trial. Obstetrics & Gynecology 2004;103:923‐30. [DOI] [PubMed] [Google Scholar]
- McWhorter J, Carlan SJ, O'Leary TD. Rofecoxib versus magnesium sulfate to arrest preterm labor: a randomized double‐blind trial. Obstetrics & Gynecology 2002;99(4 Suppl 1):2S. [DOI] [PubMed] [Google Scholar]
Morales 1989 {published data only}
- Morales WJ, Smith SG, Angel JL, O'Brien WF. Efficacy and safety of indomethacin vs ritodrine in the management of preterm labor: a randomized study. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans, Louisiana, USA. 1989:35.
- Morales WJ, Smith SG, Angel JL, O'Brien WF, Knuppel RA. Efficacy and safety of indomethacin versus ritodrine in the management of preterm labor: a randomized study. Obstetrics & Gynecology 1989;74(4):567‐72. [PubMed] [Google Scholar]
Morales 1993 {published data only}
- Morales WJ, Madhav H. Efficacy and safety of indomethacin compared with magnesium sulfate in the management of preterm labor: a randomized study. JAMA 1993;270:2676. [DOI] [PubMed] [Google Scholar]
- Morales WJ, Madhav H. Efficacy and safety of indomethacin compared with magnesium sulfate in the management or preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1993;169(1):97‐102. [DOI] [PubMed] [Google Scholar]
- Morales WJ, Madhav H. Efficacy and safety of indomethacin vs. magnesium in the management of preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1991;164:280. [DOI] [PubMed] [Google Scholar]
Nevils 1994 {published data only}
- Nevils B, Curet L, Izquierdo L, Chatterjee M, Gilson G, Maciulla J, et al. Indomethacin as a "rescue" tocolytic in pre‐term labor. American Journal of Obstetrics and Gynecology 1994;170:378. [DOI] [PubMed] [Google Scholar]
Niebyl 1980 {published data only}
- Blake DA, Niebyl JR, White RD, Kumor KM, Dubin NH, Robinson JC, et al. Treatment of premature labor with indomethacin. Advances in prostaglandin, thromboxane and leukotriene research 1980;8:1465‐7. [PubMed] [Google Scholar]
- Niebyl JR, Blake DA, White RD, Kumor KM, Dubin NH, Robinson JC, et al. The inhibition of premature labor with indomethacin. American Journal of Obstetrics and Gynecology 1980;136(8):1014‐9. [DOI] [PubMed] [Google Scholar]
Odeh 1997 {published data only}
- Odeh M, Kaiis M, Markowitz J, Oettinger M. Progesterone levels in preterm labor are not affected by ritodrin treatment. Gynecologic and Obstetric Investigation 1997;43(1):34‐6. [DOI] [PubMed] [Google Scholar]
Panter 1999 {published data only}
- Cavalle‐Garrido T, Panter K, Smallhorn J, Seaward D, Farine D. A RCT of indomethacin for preterm labor: effects on fetal heart and ductus arteriosus. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S46. [Google Scholar]
- Panter K, Hannah M, Farine D, Amankwah K, Jefferies A, Ohlsson A. The effect of indomethacin tocolysis of preterm labor on perinatal outcome: a RCT. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S46. [DOI] [PubMed] [Google Scholar]
- Panter KR, Hannah ME, Amankwah KS, Ohlsson A, Jefferies AL, Farine D. The effect of indomethacin tocolysis in preterm labour on perinatal outcome: a randomised placebo‐controlled trial. British Journal of Obstetrics and Gynaecology 1999;106(5):467‐73. [DOI] [PubMed] [Google Scholar]
Parilla 1997 {published data only}
- Parilla BV, Tamura RK, Cohen LS, Clark E. Lack of effect of antenatal indomethacin on fetal cerebral blood flow. American Journal of Obstetrics and Gynecology 1997;176(6):1166‐71. [DOI] [PubMed] [Google Scholar]
Purwaka 2004 {published data only}
- Purwaka BT, Abadi A, Prasetyadi FOH. Comparison of efficacy between oral nimesulide and parenteral‐oral isoxsuprine to delay threatened preterm labour. Journal of Obstetrics and Gynaecology Research 2004;30(3):264. [Google Scholar]
Sawdy 2003 {published data only}
- Sawdy RJ, Lye S, Fisk NM, Bennett PR. A double‐blind randomized study of fetal side effects during and after the short‐term maternal administration of indomethacin, sulindac, and nimesulide for the treatment of preterm labor. American Journal of Obstetrics and Gynecology 2003;188:1046‐51. [DOI] [PubMed] [Google Scholar]
Schorr 1998 {published data only}
- Schorr SJ, Ascarelli MH, Rust OA, Ross EL, Calfee EL, Perry KG Jr, et al. A comparative study of ketorolac (Toradol) and magnesium sulfate for arrest of preterm labor. Southern Medical Journal 1998;91(11):1028‐32. [DOI] [PubMed] [Google Scholar]
- Schorr SJ, Ascarelli MH, Rust OA, Ross EL, Calfee EL, Perry KG Jr, et al. Ketorolac is a safe and effective drug for acute tocolysis. American Journal of Obstetrics and Gynecology 1997;176(1):S7. [Google Scholar]
Stika 2002 {published data only}
- Stika CS, Gross GA, Leguizamon G, Gerber S, Levy R, Mathur A, et al. A prospective randomized safety trial of celecoxib for treatment of preterm labor. American Journal of Obstetrics and Gynecology 2002;187(3):653‐60. [DOI] [PubMed] [Google Scholar]
Zuckerman 1984a {published data only}
- Zuckerman H, Shalev E, Gilad G, Katzuni E. Further study of the inhibition of premature labor by indomethacin. Part II double‐blind study. Journal of Perinatal Medicine 1984;12(1):25‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abramov 2000 {published data only}
- Abramov Y, Nadjari M, Weinstein D, Ben‐Shachar I, Plotkin V, Ezra Y. Indomethacin for preterm labor: a randomized comparision of vaginal and rectal‐oral routes. Obstetrics & Gynecology 2000;95(4):482‐6. [DOI] [PubMed] [Google Scholar]
Bartfield 1998 {published data only}
- Bartfield M, Carlan SJ. The safety and efficacy of prolonged outpatient sulindac to prevent the recurrence of preterm labor: a prospective double‐blind study. Primary Care Update for Ob/Gyns 1998;5(4):178. [DOI] [PubMed] [Google Scholar]
Bivins 1993 {published data only}
- Bivins HA, Newman RB, Fyfe DA, Campbell BA, Stramm SL. Randomized comparative trial of indomethacin and terbutaline for the long term treatment of preterm labor. American Journal of Obstetrics and Gynecology 1993;168(1 Pt 2):375. [DOI] [PubMed] [Google Scholar]
- Bivins HA, Newman RB, Fyfe DA, Campbell BA, Stramm SL. Randomized trial of oral indomethacin and terbutaline sulfate for the long‐term suppression of preterm labor. American Journal of Obstetrics and Gynecology 1993;169:1065‐70. [DOI] [PubMed] [Google Scholar]
Caballero 1979 {published data only}
- Caballero A, Tejerina A, Dominguez A, Nava JM, Caballero AJ. Indomethacine alone or associated to ritodrine in the prevention of premature labour [abstract]. 9th World Congress of Gynecology and Obstetrics; 1979 Oct 26‐31;Tokyo, Japan. 1979:300.
Carlan 1992 {published data only}
- Carlan SJ, O'Brien WF, O'Leary TD, Mastrogiannis D. Randomized comparative trial of indomethacin and sulindac for the treatment of refactory preterm labor. Obstetrics & Gynecology 1992;79(2):223‐8. [PubMed] [Google Scholar]
- Carlan SJ, O'Brien WF, O'Leary TD, Mastrogiannis DS. A randomized comparative trial of indomethacin and sulindac for the treatment of refactory preterm labor. American Journal of Obstetrics and Gynecology 1992;166:361. [PubMed] [Google Scholar]
Carlan 1995 {published data only}
- Carlan S, Jones M, Schorr S, McNeill T, Rawji H, Clark K. Oral sulindac to prevent recurrence of preterm labor. American Journal of Obstetrics and Gynecology 1994;170:381. [DOI] [PubMed] [Google Scholar]
- Carlan SJ, O'Brien WF, Jones MH, O'Leary TD, Roth L. Outpatient oral sulindac to prevent recurrence of preterm labor. Obstetrics & Gynecology 1995;85:769‐74. [DOI] [PubMed] [Google Scholar]
- Jones M, Carlan S, Schorr S, McNeill T, Rawji H, Clark K, et al. Oral sulindac to prevent recurrence of preterm labor. American Journal of Obstetrics and Gynecology 1995;172:416. [DOI] [PubMed] [Google Scholar]
Ehsanipoor 2011 {published data only}
- Ehsanipoor RM, Shrivastava VK, Lee RM, Chan K, Galyean AM, Garite TJ, et al. A randomized, double‐masked trial of prophylactic indomethacin tocolysis versus placebo in women with premature rupture of membranes. American Journal of Perinatology 2011;28(6):473‐8. [DOI] [PubMed] [Google Scholar]
- Shrivastava V, Ehsanipoor R, Lee RM, Chan K, Gaylean A, Garite T, et al. Randomized double‐blinded trial of indomethacin tocolysis versus expectant management in patients with premature rupture of membranes at 24‐32 weeks of gestation. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S59. [Google Scholar]
Gamissans 1982 {published data only}
- Gamissans O, Canas E, Cararach V, Ribas J, Puerto B, Edo A. A study of indomethacin combined with ritodrine in threatened preterm labor. European Journal of Obstetrics & Gynecology and Reproductive Biology 1978;8:123‐8. [DOI] [PubMed] [Google Scholar]
- Gamissans O, Canas E, Cararach V, Ribas J, Puerto B, Edo A. A study of indomethacin combined with ritodrine in threatened preterm labor. Proceedings of 6th European Congress of Perinatal Medicine; 1978 Aug 29‐Sept 1 Vienna, Austria. 1978:Abstract no: 165. [DOI] [PubMed]
- Gamissans O, Cararach V, Serra J. The role of prostaglandin‐inhibitors, beta‐adrenergic drugs and glucocorticoids in the management of threatened preterm labor. In: Jung H, Lamberti G editor(s). Beta‐Mimetic Drugs in Obstetrics and Perinatology. Stuttgart: Georg Thieme, 1982:71‐84. [Google Scholar]
Gamissans 1984 {published data only}
- Gamissans O, Balasch J. Prostaglandin synthetase inhibitors in the treatment of preterm labor. In: Fuchs F, Stubblefield PG editor(s). Preterm Birth: Causes, Prevention and Management. New York: Macmillan, 1984:223‐48. [Google Scholar]
Groom 2005 {published data only}
- Groom KM, Shennan AH, Jones BA, Seed P, Bennett PR. Tocox‐‐a randomised, double‐blind, placebo‐controlled trial of rofecoxib (a cox‐2‐specific prostaglandin inhibitor) for the prevention of preterm delivery in women at high risk. BJOG: an international journal of obstetrics and gynaecology 2005;112:725‐30. [DOI] [PubMed] [Google Scholar]
Hallak 1992 {published data only}
- Hallak M, Moise KJ, Lira N, Dorman K, O'Brian Smith E, Cotton DB. The effect of tocolytic agents (indomethacin and terbutaline) on fetal breathing (FBM) and body movements (FM): a prospective, randomized, double blind, placebo‐controlled clinical trial. American Journal of Obstetrics and Gynecology 1992;166(1 Pt 2):375. [DOI] [PubMed] [Google Scholar]
- Hallak M, Moise KJ, Lira N, Dorman KF, O'Brian Smith E, Cotton DB. The effect of tocolytic agents (indomethacin and terbutaline) on fetal breathing and body movements: a prospective, randomized, double‐blind, placebo‐controlled clinical trial. American Journal of Obstetrics and Gynecology 1992;167(4):1059‐63. [DOI] [PubMed] [Google Scholar]
- Hallak M, Moise KJ, O'Brian Smith E, Cotton DB. The effects of indomethacin and terbutaline on human fetal umbilical artery velocimetry: a randomized double‐blind study. American Journal of Obstetrics and Gynecology 1993;168(1 Pt 2):348. [DOI] [PubMed] [Google Scholar]
- Hallak M, Moise KJ, O'Brian Smith E, Cotton DB. The effects of indomethacin and terbutaline on human fetal umbilical artery velocimetry: a randomized, double‐blind study. American Journal of Obstetrics and Gynecology 1993;168(3):865‐8. [DOI] [PubMed] [Google Scholar]
Humphrey 2001 {published data only}
- Humphrey RG, Bartfield MC, Carlan SJ, O'Brien WF, O'Leary TD, Triana T. Sulindac to prevent recurrent preterm labor: a randomized controlled trial. Obstetrics & Gynecology 2001;98(4):555‐62. [DOI] [PubMed] [Google Scholar]
Jain 2006 {published data only}
- Jain N, Gahlot D. A comparative study of isoxuprine versus ritodrine hydrochloride in the management of preterm labour [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:148.
Katz 1983 {published data only}
- Katz Z, Lancet M, Yemini M, Mogilner BM, Feigl A, Ben‐Hur H. Treatment of premature labor contractions with combined ritodrine and indomethacine. International Journal of Gynecology & Obstetrics 1983;21:337‐42. [DOI] [PubMed] [Google Scholar]
Mital 1992 {published data only}
- Khuteta RP, Garg S, Bhargava A. Mefanimic acid in prevention of premature labour. 12th FIGO World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:222.
- Mital P, Garg S, Khuteta RP, Khuteta S, Mital P. Mefenamic acid in prevention of premature labour. Journal of the Royal Society of Health 1992;112(5):214‐6. [DOI] [PubMed] [Google Scholar]
Newton 1991 {published data only}
- Newton ER, Shields L, Ridgway LE, Berkus MD, Elliott BD. Combination antibiotics and indomethacin in idiopathic preterm labor: a randomized double‐blind clinical trial. American Journal of Obstetrics and Gynecology 1991;165(6):1753‐9. [DOI] [PubMed] [Google Scholar]
- Ridgway L, Wright J, Newton E. The effect of indomethacin on fetal heart biometry. American Journal of Obstetrics and Gynecology 1991;164:349. [Google Scholar]
Rasanen 1995 {published data only}
- Jouppila P, Rasanen J. Response of the fetal hemodynamics to different maternal prostaglandin synthetase inhibitors. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:Abstract no: 269.
- Jouppila P, Rasanen J. Response to the fetal hemodynamics to different maternal prostaglandin synthetase inhibitors. International Journal of Gynecology & Obstetrics 1994;46:48. [Google Scholar]
- Rasanen J, Jouppila P. Fetal cardiac function and ductus arteriosus during indomethacin and sulindac therapy for threatened preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1995;173(1):20‐5. [DOI] [PubMed] [Google Scholar]
Rios‐Anez 2001 {published data only}
- Rios‐Anez R, Santos‐Luque M, Noguera M. Use of an antagonist of the prostaglandins associated to a b‐sympathomimetic agent in the treatment of pre‐term labour. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):165. [Google Scholar]
Spearing 1979 {published data only}
- Spearing G. Alcohol, indomethacin and salbutamol. A comparative trial of their use in preterm labour. Obstetrics & Gynecology 1979;53:171‐4. [PubMed] [Google Scholar]
Zuckerman 1984b {published data only}
- Zuckerman H, Shalev E, Gilad G, Katzuni E. Further study of the inhibition of premature labor by indomethacin Part I. Journal of Perinatal Medicine 1984;12:19‐23. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Castillo 1988 {published data only}
- Castillo JM, Alonso J, Hernandez‐Garcia JM, Sancho B, Martinez V. Study of biochemical and biophysical modifications produced on pregnant women treated in threatened of premature labor with ritodrine and indometacine. 12th World Congress of Gynecology and Obstetrics; 1988 Oct 23‐28; Rio de Janeiro, Brazil. 1988:20.
Mesdaghinia 2012 {published data only}
- Mesdaghinia E, Mesdaghinia A, Hashemi T, Sooky Z, Mousavi GA. Comparing the effects fo indomethacin and magnesium‐sufate in the treatment of preterm labor. Feyz, Journal of Kashan University of Medical Sciences 2012;16(2):95‐101. [Google Scholar]
- Sooky Z. Comparison of delaying in premature labour by indomethacin and Mg sulphate in pregnant women referred to maternity hospital. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
Additional references
Babay 1998
- Babay Z, Lange IR, Amin H. Neonatal and maternal complications after administration of indomethacin for preterm labour between 24 and 30 weeks gestation. Journal of the Society of Obstetricians and Gynaecologists of Canada 1998;20(5):505‐11. [Google Scholar]
Crowther 2002
- Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001060] [DOI] [PubMed] [Google Scholar]
Doret 2002
- Doret M, Mellier G, Benchaib M, Piacenza JM, Gharib C, Pasquier JC. In vitro study of tocolytic effect of rofecoxib, a specific cyclo‐oxygenase 2 inhibitor. Comparison and combination with other tocolytic agents. BJOG: an international journal of obstetrics and gynaecology 2002;109:983‐8. [DOI] [PubMed] [Google Scholar]
Doyle 2009
- Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004661.pub3] [DOI] [PubMed] [Google Scholar]
Duckitt 2014
- Duckitt K, Thornton S, O'Donovan OP, Dowswell T. Nitric oxide donors for treating preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD002860.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eronen 1994
- Eronen M, Pesonen E, Kurki T, Teramo K, Ylikorkola O, Hallman M. Increased incidence of bronchopulmonary dysplasia after antenatal administration of indomethacin to prevent preterm labor. Journal of Pediatrics 1994;124(5):783‐8. [DOI] [PubMed] [Google Scholar]
FDA 2011
- U.S. Food, Drug Administration. Drug Safety Communication: New warnings against use of terbutaline to treat preterm labor. http://www.fda.gov/Drugs/DrugSafety/ucm243539.htm.
Flenady 2014a
- Flenady V, Reinebrant HE, Liley HG, Tambimuttu EG, Papatsonis DN. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD004452.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flenady 2014b
- Flenady V, Wojcieszek AM, Papatsonis DNM, Stock OM, Murray L, Jardine LA, et al. Calcium channel blockers for inhibiting preterm labour and birth. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD002255.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gates 2004
- Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed?. BJOG: an international journal of obstetrics and gynaecology 2004;111(3):213‐9. [DOI] [PubMed] [Google Scholar]
Gladstone 2011
- Gladstone M, Neilson JP, White S, Kafulafula G, Broek N. Post‐neonatal mortality, morbidity, and developmental outcome after ultrasound‐dated preterm birth in rural Malawi: a community‐based cohort study. PLoS Medicine 2011;8:e1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldenberg 2008
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haas 2012
- Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta‐analysis. BMJ 2012;345:e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Keirse 1992
- Keirse MJNC. Inhibitors of prostaglandin synthesis for treatment of preterm labour. In: Drife JO, Calder AA editor(s). Prostaglandins and the Uterus. London: Springer‐Verlag, 1992. [Google Scholar]
Koren 2006
- Koren G, Florescu A, Costei AM, Boskovic R, Moretti ME. Nonsteroidal antiinflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta‐analysis. Annals of Pharmacotherapy 2006;40(5):824‐9. [DOI] [PubMed] [Google Scholar]
McCain 1993
- McCain GC, Deatrick JA. The experience of high‐risk pregnancy. Journal of Obstetric, Gynecologic and Neonatal Nursing 1993;23:421‐7. [DOI] [PubMed] [Google Scholar]
Moise 1990
- Moise KJ, Ou C‐N, Kirshon B, Cano LE, Carpenter RJ. Placental transfer of indomethacin in the human pregnancy. American Journal of Obstetrics and Gynecology 1990;162:549‐54. [DOI] [PubMed] [Google Scholar]
Murray 2006
- Murray M. Antepartal and Intrapartal Fetal Monitoring. Springer Publishing Company, 2006. [Google Scholar]
Neilson 2014
- Neilson JP, West HM, Dowswell T. Betamimetics for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD004352.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norman 2009
- Norman JE, Morris C, Chalmers J. The effect of changing patterns of obstetric care in Scotland (1980–2004) on rates of preterm birth and its neonatal consequences: perinatal database study. PLoS Medicine 2009;6(9):e1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norton 1993
- Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. New England Journal of Medicine 1993;329(22):1602‐7. [DOI] [PubMed] [Google Scholar]
Perron 2013
- Perron N, Tremblay E, Ferretti E, Babakissa C, Seidman EG, Levy E, et al. Deleterious effects of indomethacin in the mid‐gestation human intestine. Genomics 2013;101(3):171‐7. [DOI] [PubMed] [Google Scholar]
Petrou 2011
- Petrou S, Eddama O, Mangham L. A structured review of the recent literature on the economic consequences of preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(3):F225–32. [DOI] [PubMed] [Google Scholar]
Powell 1995
- Powell SL, Holt VL, Hickok DE, Easterling T, Connell FA. Recent changes in delivery site of low‐birth‐weight infants in Washington: impact on birth weight‐specific mortality. American Journal of Obstetrics and Gynecology 1995;173(5):1585‐92. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2006
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Sanchez‐Ramos 1999
- Sanchez‐Ramos L, Kaunitz AM, Gaudier FL, Delke I. Efficacy of maintenance therapy after acute tocolysis: a meta‐analysis. American Journal of Obstetrics and Gynecology 1999;181:484‐90. [DOI] [PubMed] [Google Scholar]
Slater 1999
- Slater DM, Dennes WJB, Campa JS, Poston L, Bennett PR. Expression of cyclo‐oxygenase types‐1 and ‐2 in human myometrium throughout pregnancy. Molecular Human Reproduction 1999;5(9):880‐4. [DOI] [PubMed] [Google Scholar]
Souter 1998
- Souter D, Harding J, McCowan L, O'Donnell C, McLeay E, Baxendale H. Antenatal indomethacin‐adverse fetal effects confirmed. Australian and New Zealand Journal of Obstetrics and Gynaecology 1998;38(1):11‐6. [DOI] [PubMed] [Google Scholar]
Su 2010
- Su LL, Samuel M, Chong YS. Progestational agents for treating threatened or established preterm labour. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006770.pub2] [DOI] [PubMed] [Google Scholar]
Van den Veyver 1993
- Veyver IB, Moise KJ. Prostaglandin synthetase inhibitors in pregnancy. Obstetrical & Gynecological Survey 1993;48(7):493‐502. [DOI] [PubMed] [Google Scholar]
Vogel 2014
- Vogel JP, Nardin JM, Dowswell T, West HM, Oladapo OT. Combination of tocolytic agents for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD006169.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2011
- Walker MW, Clark RH, Spitzer AR. Elevation in plasma creatinine and renal failure in premature neonates without major anomalies: terminology, occurrence and factors associated with increased risk. Journal of Perinatology 2011;31(3):199‐205. [DOI] [PubMed] [Google Scholar]
WHO 2012
- Howson CP, Kinney MV, Lawn JE. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012. [Google Scholar]
Yelland 2011
- Yelland LN, Saltera AB, Ryana P, Makrides M. Analysis of binary outcomes from randomised trials including multiple births: when should clustering be taken into account?. Paediatric and Perinatal Epidemiology 2011;25:283–97. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
King 2005
- King JF, Flenady V, Cole S, Thornton S. Cyclo‐oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001992.pub2] [DOI] [PubMed] [Google Scholar]


