Abstract
Background: The benefits of physical activity during pregnancy include lower maternal weight gain, a lower likelihood of gestational diabetes, low back pain, preeclampsia, preterm delivery, caesarian delivery, and macrosomia. This study aimed to examine the factors associated with insufficient leisure-time physical activity (LTPA) during the first trimester. Methods: A cross-sectional study was conducted at the Clinic for Obstetrics and Gynecology of Clinical Center of Serbia, Belgrade, between January and June of 2018. The final analyses included 162/175 pregnant women. The questionnaire was used to obtain social characteristics, pregnancy, and lifestyle characteristics (Pregnancy Risk Assessment Monitoring System—PRAMS), pre-pregnancy LTPA (International Physical Activity Questionnaire—IPAQ), and LTPA during the first trimester (Pregnancy Physical Activity Questionnaire—PPAQ). Women were classified into two groups of sufficient and insufficient LTPA during the first trimester based on the recommendations of the World Health Organization. Multivariate logistic regression analysis was applied. Results: A total of 27.2% of the women had insufficient LTPA during pregnancy. Insufficient LTPA during pregnancy was associated with <12 years of education (OR: 2.3, 95% CI: 1.05–5.04), self-rated financial status as poor (OR: 0.34, 95% CI: 0.14–0.79), and hours spent walking before pregnancy (OR: 0.87, 95% CI: 0.77–0.99). Conclusions: Our results can help direct health care professionals advice for women who are planning pregnancy towards walking as it seems to be sustained during pregnancy.
Keywords: physical activity, pregnancy, factors, walking
1. Introduction
Physical activity (PA) provides health benefits in all stages of life, it can improve quality of life, mood, and general health, manage obesity and obesity-related problems [1,2,3,4,5]. Traditionally, the widespread opinion was that pregnant women should avoid every type of PA, as it was thought that PA reduces placental circulation and therefore, increases the risk for preterm delivery, growth retardation, and miscarriages [6], although The American College of Obstetricians and Gynecologists (ACOG) gave the recommendation for PA during pregnancy back in 1994 [7]. The benefits of PA during pregnancy were confirmed in recent research as well [8,9,10].
The recommendations of ACOG were reaffirmed in 2009 and 2015, stating that all pregnant women without contraindications (medical or obstetric) for PA should engage in 30 min of moderate-intensity PA per day on most or all days a week [11]. Similarly, the Society of Gynecologists and Obstetricians of Canada and the Canadian Society for Exercise physiology gave recommendations for a minimum of 150 min of moderate-intensity PA per week [12]. The World Health Organization (WHO) gave recommendations in 2011 for minimal energy expenditure of 600 MET (metabolic equivalents of task)/min/week for health benefits for all adults, including pregnant women [13].
Studies on the topic of PA during pregnancy showed that it could reduce maternal weight gain and the risk for development of gestational diabetes mellitus (GDM) [14,15,16,17,18]. The studies highlight the importance of early initiation of PA during pregnancy [12]. The initiation of the PA during the first trimester is associated with lower risk for development of gestational diabetes [15,16,17,18]. Additionally, early initiation of PA during pregnancy is associated with a lower likelihood of preeclampsia [19,20] and better sleep quality [21]. This leads to further need for examination of factors that influence the PA level during the first trimester.
Other health benefits of PA during pregnancy include relief of the symptoms of low back pain, which is very common and affects around 60% of pregnant women [22,23,24], a decrease in the likelihood of caesarian delivery and postpartum recovery time [11,25,26,27]. Studies have also shown that physically active pregnant women have a lower chance for the development of depression and have lower anxiety levels [28,29,30,31,32]. The studies on the influence of the in utero environment show that it has effects on infants and throughout childhood into adulthood [33], which is in accordance with developmental origins hypothesis. PA during pregnancy has a positive effect on the offspring which was shown in the Danish National Birth Cohort study, with lower body mass index (BMI) and lower frequency of obesity at the age of 7 [34]. The exposure to exercise during pregnancy is associated with cardiac and neuromotor development of the infants, as studies have shown lower heart rate and increased heart rate variability among infants, which is the same as among adults who are exercised trained [35]. Further, children with slower heart rates are shown to have higher psychomotor and language developmental outcomes at one, two, and three years of age [33].
Most women do not meet pregnancy PA recommendations, but it seems that women who are physically active before pregnancy have a higher likelihood of remaining active during pregnancy [28]. Among factors associated so far with the reduction of PA are lower socioeconomic status and a greater number of children [36]. Additionally, perceived barriers to PA among pregnant women were symptoms of pregnancy as women often state that tiredness and nausea were the factors that hindered PA along with family and household obligations or fear of injuries [37]. Another commonly reported reason for the reduction of PA during pregnancy is discomfort during exercise. This discomfort usually comes during the second trimester, when there are changes in posture, the shift of point of gravity, and progressive lordosis [11].
The majority of studies which examined the influence of PA interventions among pregnant women included participants in the second trimester, or late in the first trimester [38]. Consequently, the factors associated with PA levels in the second trimester are well understood [39]. However, PA in the first trimester is associated with some benefits such as positive effects on prevention of GDM, preeclampsia, and sleep disturbances. It is important to examine which factors are associated with PA levels during the early pregnancy.
The aim of this study was to explore the factors associated with insufficient PA during the first trimester.
2. Methods
This was a cross-sectional study conducted at the Clinic for Obstetrics and Gynecology of Clinical Center of Serbia, Belgrade, between January and June of 2018.
2.1. Population
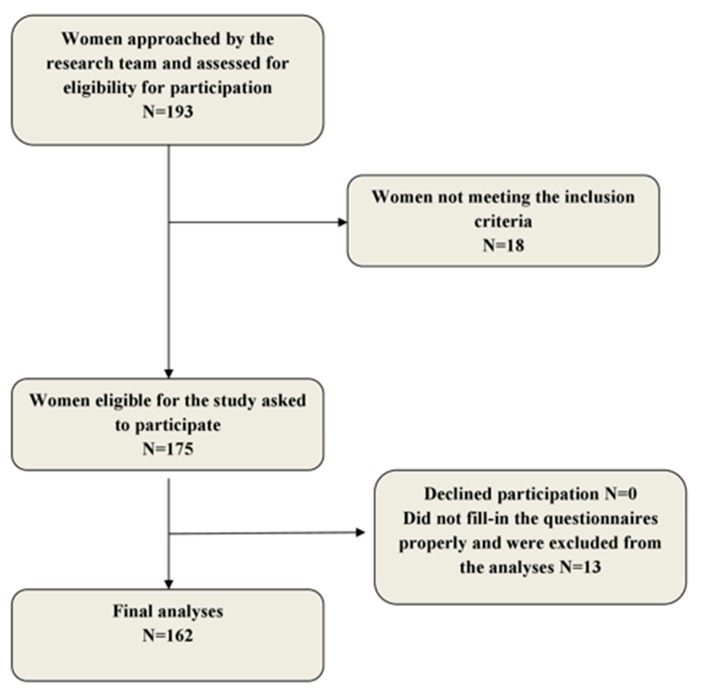
The study included 175 pregnant women who had a regular appointment with the gynecologist, at the end of their first trimester (12th week of gestation). All women with a singleton pregnancy and without existing obstetric and medical contraindications for PA during pregnancy were included in the study. Exclusion criteria were: existing medical or obstetric contraindication for PA during pregnancy [40], heavy smoking, and mental inability to understand and fill in the questionnaire. Eligibility of women was assessed by the members of the research team (lead researcher and on-call gynecologist) Once eligibility was confirmed, potential participants were invited to participate in the study. Participants were asked to fill-in the hard copy of questionnaires. Participants could ask the lead researcher if they had any questions regarding the completion of the questionnaire. The details on the recruitment of the participants are presented in Figure 1.
Figure 1.
Flow-chart of recruitment and participation.
2.2. Ethical Approval
All participants were given an oral and written description of the study, its processes and aims and all of them gave written consent for participation. The ethical committee of the Belgrade Medical Faculty gave consent for the research (No. 2650/IV-12).
2.3. Research Instrument
The questionnaire created was based on the questionnaires from similar research on PA during pregnancy [41,42,43]. The questionnaire consisted of 85 questions divided in five sections: social characteristics (age, place of residence, marital status, education, employment status, self-rated health, financial status), anthropometric characteristics (height, weight, waist circumference), pregnancy and lifestyle characteristics (planning a pregnancy, symptoms present during the first trimester, and diet), and (leisure-time PA in three months before pregnancy and PA during the first trimester).
Sections on social characteristics, anthropometric characteristics, and pregnancy and lifestyle characteristics were adapted from the Pregnancy Risk Assessment Monitoring System Phase Seven questionnaire (PRAMS questionnaire) [42]. PA in the three months before pregnancy was assessed with the International Physical Activity Questionnaire (IPAQ) [43]. PA during the first trimester was assessed with the Pregnancy Physical Activity Questionnaire (PPAQ) [41]. The final questionnaire was modified and translated to Serbian by two independent translators following the standard method of translating (forward and backward translation) [44]. Researchers agreed on the final version of the questionnaire, after which a pre-testing on ten women was done. Pre-testing was done to determine whether the questions were clear, concise, understandable, if their order was adequate, and to determine the time needed to fill in the questionnaire (which was approximately 15 min). Assessment of test–retest reliability was done with 20 pregnant women, with whom the retest was performed two weeks after filling in the initial questionnaire, using kappa coefficients, which were ≥0.90. The test–retest reliability of the Serbian version of IPAQ was published elsewhere [45].
Anthropometric characteristics (weight, height, and waist circumference) were taken after the participants agreed to participate in the study. All the measurements were done by the lead researcher. Waist circumference was measured with the participant in the standing position, at the end of expiration, with inelastic tape, at the nearest 0.5 cm, and the tape positioned at the middle point between the top of the iliac crest and the lowest palpable rib.
2.4. Variables
Level of Leisure-time PA in three months before pregnancy was assessed through total energy expenditure in metabolic equivalents (METS) [43] using the IPAQ. For calculations, it is considered that energy expenditure during walking is 3.3 METs every minute, energy expenditure during moderate-intensity PA is 4 METs, and during vigorous-intensity PA 8 METs. Total weekly energy expenditure is accordingly calculated using the formula:
Total energy expenditure (MET-minutes/week) = Time (in minutes) spent in vigorous physical activity per day × number of days per week with vigorous physical activity × 8 + time (in minutes spent) in moderate Physical activity per day × number of days per week with moderate physical activity × 4 + time (in minutes) spent walking per day × number of days per week walking × 3.3.
Based on total energy expenditure in MET-minutes per week, women were classified into three groups: low (<600 MET-minutes per week), moderate (601–3000 MET-minutes per week), or high (>3000 MET-minutes per week) level of PA [43].
Leisure-time physical activity (LTPA)during pregnancy was presented in MET-minutes per week and calculated based on the recommended calculations for PPAQ of duration and intensity of each type of PA [41]:
Total energy expenditure (MET-minutes/week) = (duration × 3.2 for walking slowly for fun/exercise+ duration × 4.6 for walking quickly for fun exercise + duration × 6.5 for walking up-hill quickly for fun/exercise + duration × 7.0 for jogging + duration × 3.5 for prenatal exercise class + duration × 6.0 for swimming + duration × 4.5 for dancing) × 60.
Duration of each leisure-time PA was given in six categories: none; less than thirty minutes; more than thirty minutes, but less than one hour; between one and two hours; between two and three hours; and more than three hours. These categories were translated into durations: 0, 0.25, 0.75, 1.5, 2.5, 3, which were then computed into average weekly time in these activities. The intensity was calculated based on the specific MET values assigned to each activity (walking slowly for fun/exercise—3.2, walking quickly for fun exercise—4.6, walking up-hill quickly for fun/exercise—6.5, jogging—7.0, prenatal exercise class—3.5, swimming—6.0, and dancing—4.5) [41]. Women were classified into two groups: a group of sufficient and a group of insufficient PA. Insufficient PA was defined as PA of less than 600 MET-minute/week, based on recommendations by WHO which state that the minimal PA for adults, including pregnant women, is 600 MET-minutes/week [46].
The sedentary time before pregnancy was assessed with the question ‘How many minutes per day do you on average spend sitting?’ using the IPAQ [43]. All values were then translated into hours per week. The sedentary time during pregnancy was assessed with questions from PPAQ regarding sedentary activities (‘How many hours per day do you on average spend watching TV/being on the computer outside of work/working on the computer at work, reading books, driving?’) [41]. In the PPAQ questionnaire duration was given in six categories: none; less than thirty minutes; more than thirty minutes, but less than one hour; between one and two hours; between two and three hours; and more than three hours. These categories were translated into durations: 0, 0.25, 0.75, 1.5, 2.5, 3, which were then computed into average weekly time in these activities.
Education was assessed as the number of years of education, more than 12 years (college/faculty), or 12 years or less (primary/secondary education). Participants were asked to name their place of residence which was then classified by researchers into urban and rural according to the division made by the statistical office of Serbia [47]. Self-perceived financial status was assessed with the question: ‘How would you describe your financial status?’ (good, average, poor).
2.5. Analyzed Variables
A total of 22 variables were included in the analyses to assess their association with insufficient LTPA. These were: age, nationality, place of residence, years of education, marital status, working status, self-rated financial status, if the pregnancy was planned, parity, nausea in pregnancy, daily fruit intake, daily vegetable intake, chronic disease, height, weight, waist circumference, BMI, PA category based on METS pre-pregnancy, total time before pregnancy spent sedentary, spent walking, spent in moderate or vigorous LTPA before pregnancy.
2.6. Statistical Analyses
Statistical analyses were done with methods of analytical and descriptive statistics. Normality was tested using the Shapiro–Wilk test. Significance of differences between normally distributed continuous variables was examined using the T-test. Chi-square test was used to assess differences in social characteristics, medical, and dietary between the groups of insufficient and sufficient LTPA. All variables which were shown to be significant (p < 0.05) were entered into a multivariate logistic regression model with insufficient LTPA as an outcome variable. Analyses were done using Statistical Package for Social Science SPSS for Windows 21.0 (Armonk, NY, USA).
3. Results
A total of 175 women were included in the study. Thirteen questionnaires were not properly filled in, and our final analysis included 162 pregnant women. A total of 44/162 (27.2%) women had insufficient LTPA during the first trimester of pregnancy (<600 MET-minutes/week). Average MET-minutes/week during pregnancy in a group of sufficient PA was 1482.88 ± 728.71, while in a group of insufficient LTPA was 372.99 ± 24.09 MET-minutes/week.
The total of 39.5% of women in a group of insufficient LTPA had a low level of PA before pregnancy, compared to 18.9% of women from the sufficient pregnancy LTPA group, p = 0.027.
The groups differed significantly in frequency of more than 12 years of education, self-rated financial status, and total LTPA level before pregnancy (Table 1).
Table 1.
Sufficiently and insufficiently active participants.
| Sufficient LTPA | Insufficient LTPA | p-Value | |
|---|---|---|---|
| Age (mean ± SD) | 31.42 ± 5.29 | 32.16 ± 4.99 | 0.426 |
| Nationality N (%) | |||
| Serbian | 117 (99.2) | 44 (100) | |
| Other | 1 (0.8) | 0 (0) | 0.540 |
| Place of residence N (%) | |||
| Urban | 110 (94.8) | 38 (86.4) | |
| Rural | 6 (5.2) | 6 (13.6) | 0.07 |
| Marital status N (%) | |||
| Married/permanent relationship | 118 (100) | 44 (100) | |
| Single | 0 | 0 | / |
| Working status N (%) | |||
| Employed | 98 (83.1) | 39 (88.6) | |
| Unemployed | 20 (16.9) | 5 (11.4) | 0.381 |
| Years of education N (%) | |||
| ≤12 years of education | 38 (32.2) | 23 (52.3) | |
| >12 years of education | 80 (67.8) | 21 (47.7) | 0.019 |
| Self-rated financial status N (%) | |||
| Poor | 14 (12.2) | 7 (16.3) | |
| Average | 66 (57.4) | 14 (32.6) | |
| Good | 35 (30.4) | 22 (51.2) | 0.019 |
| Planned pregnancy N (%) | 85 (72) | 37 (84.1) | 0.113 |
| Nausea N (%) | 80 (67.8) | 33 (75.0) | 0.375 |
| Has children N (%) | 55 (46.6) | 25 (56.8) | 0.248 |
| Daily fruit intake N (%) | 79 (66.9) | 31 (70.5) | 0.671 |
| Daily vegetables intake N (%) | 67 (56.8) | 22 (50.0) | 0.440 |
| Chronic disease N (%) | 34 (28.8) | 13 (29.5) | 0.927 |
| Height in cm (mean ± SD) | 167.65 ± 6.43 | 168.14 ± 5.73 | 0.660 |
| Weight in kg (mean ± SD) | 68.53 ± 15.96 | 66.81 ± 14.35 | 0.533 |
| Waist circumference in cm (mean ± SD) | 78.88 ± 11.90 | 77.25 ± 12.42 | 0.569 |
| BMI in kg/m2 (mean ± SD) | 24.29 ± 4.95 | 23.64 ± 5.05 | 0.457 |
| PA level before pregnancy N (%) | |||
| Low | 21 (18.9) | 17 (39.5) | |
| Moderate | 63 (56.8) | 17 (39.5) | |
| High | 27 (24.3) | 9 (20.0) | 0.027 |
LTPA = leisure time physical activity; SD = standard deviation; BMI = body mass index; PA = physical activity; All bold values are statistically significant.
The average number of hours per week spent in walking before pregnancy was significantly higher in the group of sufficient LTPA during the first trimester (4.73 ± 0.44 hours/week vs. 3.17 ± 0.48 hours/week, p = 0.006 (Table 2).
Table 2.
Physical activity and sedentary time before and during pregnancy among sufficiently and insufficiently active participants.
| Sufficient LTPA (Mean ± SD) | Insufficient LTPA (Mean ± SD) | p-Value | |
|---|---|---|---|
| Sedentary before pregnancy (Hours/week) | 25.88 ± 2.62 | 22.97 ± 4.06 | 0.942 |
| Walking before pregnancy (Hours/week) | 4.73 ± 0.44 | 3.17 ± 0.48 | 0.006 |
| Moderate recreational physical activity before pregnancy (Hours/week) | 5.37 ± 0.5 | 4.83 ± 0.74 | 0.326 |
| Vigorous physical activity before pregnancy (Hours/week) | 4.80 ± 0.44 | 3.57 ± 0.54 | 0.379 |
LTPA = leisure time physical activity; All bold values are statistically significant.
Multivariate logistic regression model showed that women with 12 or less years of education had 2.3 (OR: 2.30, 95% CI: 1.05–5.04) times higher odds for having insufficient LTPA during the first trimester. Women who rated their financial status as poor had lower odds for insufficient LTPA (OR: 0.34, 95% CI: 0.14–0.79) compared to women who rated their financial status as good. More hours spent walking before pregnancy were associated with lower odds for insufficient LTPA during pregnancy (OR: 0.87, 95% CI: 0.77–0.99) (Table 3).
Table 3.
Logistic regression analyses with insufficient leisure-time physical activity during pregnancy as an outcome variable.
| Characteristic | OR (95% CI) |
|---|---|
| Years of education | |
| ≤12 years of education | 2.30 (1.05–5.04) |
| >12 years of education | 1.0 (reference category) |
| Self-rated financial status | |
| Poor | 0.34 (0.14–0.79) |
| Average | 0.85 (0.27-2.69) |
| Good | 1.0 (reference category) |
| PA level before pregnancy | |
| Low | 1.01 (0.26-3.91) |
| Moderate | 0.54 (0.18-1.57) |
| High | 1.0 (reference category) |
| Walking before pregnancy (Hours/week) | 0.87 (0.77–0.99) |
All bold values are statistically significant.
4. Discussion
Our study showed that almost one-third of women in their first trimester (27.2%) had insufficient LTPA. Insufficient LTPA was associated with lower educational level, higher self-perceived financial status, and time spent walking during the three months before pregnancy.
More than one-quarter of the pregnant women (27.2%) were insufficiently active during early pregnancy in our study, which is similar to the results from the Omega study in which 25% of women were insufficiently active during early pregnancy [16]. Other studies found a higher prevalence of insufficient PA during pregnancy [32,48]. A study among pregnant women in Singapore found insufficient PA among 34.3% of participants [32], while a national study in the United States showed that the prevalence of insufficient LTPA during early pregnancy is 43% [48]. The higher prevalence of insufficient PA in United States can be associated with an increase in the prevalence of physical inactivity among the adult female population in the U.S. from 19.1% in 1988 to 51.7% in 2010 [49]. However, the data from Serbia differ. The prevalence of physical inactivity among adults in Serbia is 68% [50], just over one-quarter of our sample were insufficiently active. It was suggested that pregnancy might trigger the adoption of the risk-reducing behaviors, like a lifestyle change, same as other life changing events [51], which might explain the difference between the studies.
One of the most commonly identified factors associated with LTPA level during pregnancy, in all trimesters, including the first [52], was LTPA level before pregnancy. That being said, a prospective study conducted among 1175 healthy pregnant women in Spain showed that almost one-half of pregnant women who were sufficiently active before pregnancy did not stay active during pregnancy [53]. Our results stressed the association between sufficient/insufficient LTPA in pregnancy and only hours spent walking before pregnancy, not spent in moderate or vigorous PA. Our results might suggest that women, who used to walk before pregnancy, can continue this throughout the pregnancy, while those who engage in moderate or vigorous-intensity exercises tend to stop this. This might be due to fear of injuries, but also due to the feeling of decreased maneuverability during pregnancy, when women do not feel safe exercising [37,54].Another reason might be that there is a strong traditional belief that walking in pregnancy can make childbirth easier.
Although we did not examine PA levels during the second trimester, some studies have shown an increase in LTPA during the second trimester, like research done on pregnant women in all three trimesters in Denmark which showed a higher number of footsteps per day during the second trimester compared to both third and first trimester [55]. Others have shown a progressive decline in the prevalence of sufficient LTPA throughout the pregnancy [56,57,58]. Having that in mind, and a possibility that the prevalence of insufficient LTPA might only increase later in pregnancy, the exact identification of walking time as an independent predictor of sufficient LTPA in pregnancy can direct health promotion efforts and physician guidelines for LTPA in pregnancy, and furthermore, improve the prevalence of women who comply with those guidelines.
A low educational level was previously associated with a decrease of LTPA during pregnancy, both in high and in middle-income countries [53] and our study showed that it is associated not only with LTPA level decrease but with it being insufficient to maintain health. Similar to our results, studies have also associated low pregnancy LTPA with higher income [56,59]. These two findings might only seem conflicting. Pregnant women of poor self-perceived socio-economic status might have to walk more for transportation, rather than use a car or even public transport, and therefore report a higher number of minutes spent walking during pregnancy. Women who have a higher education likely have a higher consciousness about the necessity of a healthy lifestyle during pregnancy.
Parity and pre-pregnancy BMI were also identified as factors associated with the lifestyle of pregnant women [58,59]. Women with children are expected to have less time for LTPA, while women with higher BMI are thought to more often have a sedentary lifestyle, and are expected to continue with a sedentary lifestyle in pregnancy as well [52]. Our study did not find these associations since both pre-pregnancy BMI and parity were not associated with insufficient LTPA during pregnancy. We did not find any association with pregnancy symptoms, such as nausea and reduction of LTPA, although the prevalence of nausea was a bit higher in the insufficient PA group. Our finding differs to the study on 1171 women in Singapore which showed that nausea in the first trimester is associated with the reduction of LTPA during pregnancy [60].
The main limitation of this study is its retrospective design as there could have been a recall bias for leisure-time PA. Another limitation is that the study was conducted in only one center in Serbia, and the sample might not be representative of the entire population. We relied on self-reported data, so there could be some bias, as women might have overestimated their LTPA levels both in pregnancy and before pregnancy. Another limitation is a cross-sectional design that does not allow the establishment of a causal relationship between the variables. Nonetheless, this is one of the few studies that have examined pre-pregnancy and first trimester LTPA at the 12th week of gestation and not during the 24–28th week period or postpartum and these data can be very valuable.
5. Conclusions
Results from this study show that almost one third of the pregnant women in Serbia are insufficiently active during the first trimester. Sufficient LTPA was associated with hours spent walking before pregnancy. This allows health care professionals to design their advice for women in their reproductive period or women who are planning pregnancy and direct them to walking as it seems to be the type of LTPA that is usually sustained during pregnancy. Walking is a type of PA that is easily integrated into daily routines and is resistant to the common barriers to PA that many pregnant women face. Due to this resistance to common barriers, walking can provide very important benefits for maternal and child health. It is the ideal type of PA for all women, including those who were insufficiently active or sedentary before pregnancy. Community-based approaches to create communities that foster walking for the entire population may be needed to support the conditions for walking among pregnant women. Our study also showed the association of insufficient LTPA during the first trimester with educational level and self-perceived financial status. Health care professionals can easily identify women with a higher likelihood for insufficient LTPA through questions on social characteristic described in this study and pay more attention to advising these women on a healthy lifestyle during pregnancy.
Author Contributions
J.T. was involved in the study design, data curation, data analysis, writing the article, revising the article and approving the final version of the article. Z.T.-S. was involved in the study supervision, study design, data analysis and writing the final version of the article. V.B.-M. was involved in the study design, study supervision, writing the article and approving the final version of the article. P.P. was involved in data curation, data analysis, drafting the article and revising the article. S.D. was involved in data curation, data analysis, data interpretation and writing the article. M.G.-D. was involved in study design, study supervision and writing the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 41004, Contract No. 175042 (2011-2014), and Grant No.175046).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Warburton D.E.R., Bredin S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017;32:1–16. doi: 10.1097/HCO.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 2.Shin C., Lee Y., Belyea M. Physical activity, benefits, and barriers across the aging continuum. Appl. Nurs. Res. 2018;44:107–112. doi: 10.1016/j.apnr.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Wu X.Y., Han L.H., Zhang J.H., Luo S., Hu J.W., Sun K. The influence of physical activity, sedentary behavior on health-related quality of life among the general population of children and adolescents: A systematic review. PLoS ONE. 2017;12:e0187668. doi: 10.1371/journal.pone.0187668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puciato D., Rozpara M., Borysiuk Z. Physical Activity as a Determinant of Quality of Life in Working-Age People in Wrocław, Poland. Int. J. Environ. Res. Phys. Act. 2018;15:623–638. doi: 10.3390/ijerph15040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puciato D., Borysiuk Z., Rozpara M. Quality of life and physical activity in an older working-age population. Clin. Interv. Aging. 2017;12:1627–1634. doi: 10.2147/CIA.S144045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schramm W., Stockbauer J., Hoffman H. Exercise, Employment, Other Daily Activities, and Adverse Pregnancy Outcomes. Am. J. Epidemiol. 1996;143:211–218. doi: 10.1093/oxfordjournals.aje.a008731. [DOI] [PubMed] [Google Scholar]
- 7.ACOG Technical Bulletin Number 189 February 1994 Exercise during pregnancy and the postpartum period. Int. J. Gynecol. Obstet. 1994;45:65–70. doi: 10.1016/0020-7292(94)90773-0. [DOI] [PubMed] [Google Scholar]
- 8.Sanabria-Martínez G., García-Hermoso A., Poyatos-Leõn R., Álvarez-Bueno C., Sánchez-Lõpez M., Martínez-Vizcaíno V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: A meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015;122:1167–1174. doi: 10.1111/1471-0528.13429. [DOI] [PubMed] [Google Scholar]
- 9.Domenjoz I., Kayser B., Boulvain M. Effect of physical activity during pregnancy on mode of delivery. Am. J. Obstet. Gynecol. 2014;211:401.el–401.e11. doi: 10.1016/j.ajog.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Mottola M.F. Physical activity and maternal obesity: Cardiovascular adaptations, exercise recommendations, and pregnancy outcomes. Nutr. Rev. 2013;71(Suppl. 1):31–36. doi: 10.1111/nure.12064. [DOI] [PubMed] [Google Scholar]
- 11.The American College of Obstetricians and Gynecologists Committee opinion summary No.650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet. Gynecol. 2015;126:1326–1327. doi: 10.1097/AOG.0000000000001209. [DOI] [PubMed] [Google Scholar]
- 12.Mottola M.F., Davenport M.H., Ruchat S., Davies G.A., Poitras V.J., Gray C.E., Jaramillo Garcia A., Barrowman N., Adamo K.B., Duggan M., et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sport Med. 2018;40:1339–1346. doi: 10.1136/bjsports-2018-100056. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . Information Sheet: Global Recommendations on Physical Activity for Health 18-64 Years Old. World Health Organization; Geneva, Switzerland: 2011. p. 1. [Google Scholar]
- 14.Streuling I., Beyerlein A., Rosenfeld E., Hofmann H., Schulz T., Von Kries R. Physical activity and gestational weight gain: A meta-analysis of intervention trials. BJOG Int. J. Obstet. Gynaecol. 2011;118:278–284. doi: 10.1111/j.1471-0528.2010.02801.x. [DOI] [PubMed] [Google Scholar]
- 15.Tobias D., Zhang C., van Dam R.M., Bower K., Hu F. Physical Activity Before and During Pregnancy and Risk of Gestational. Diabetes Care. 2011;34:223–229. doi: 10.2337/dc10-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badon S., Wartko P., Qiu C., Sorensen T., Williams M., Enquobahrie D. Leisure-time physical activity and gestational diabetes mellitus in the Omega study. Med. Sci. Sports Exerc. 2016;48:1044–1052. doi: 10.1249/MSS.0000000000000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harizopoulou V.C., Kritikos A., Papanikolaou Z., Saranti E., Vavilis D., Klonos E., Papadimas I., Goulis D.G. Maternal physical activity before and during early pregnancy as a risk factor for gestational diabetes mellitus. Acta Diabetol. 2010;47(Suppl. 1):83–89. doi: 10.1007/s00592-009-0136-1. [DOI] [PubMed] [Google Scholar]
- 18.Momeni Javid F., Simbar M., Dolatian M., Alavi Majd H. Comparison of Lifestyles of Women With Gestational Diabetes and Healthy Pregnant Women. [(accessed on 5 March 2019)];Glob. J. Health Sci. 2014 7:162–169. doi: 10.5539/gjhs.v7n2p162. Available online: http://www.ccsenet.org/journal/index.php/gjhs/article/view/39368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aune D., Saugstad O.D., Henriksen T., Tonstad S. Physical Activity and the Risk of Preeclampsia. [(accessed on 3 February 2020)];Epidemiology. 2014 25:331–343. doi: 10.1097/EDE.0000000000000036. Available online: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00001648-201405000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Catov J.M., Parker C.B., Gibbs B.B., Bann C.M., Carper B., Silver R.M., Simhan H.N., Parry S., Chung J.H., Haas D.M., et al. Patterns of leisure-time physical activity across pregnancy and adverse pregnancy outcomes. Int. J. Behav. Nutr. Phys. Act. 2018;15:68–78. doi: 10.1186/s12966-018-0701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker J.H., Rothenberger S.D., Kline C.E., Okun M.L. Exercise during Early Pregnancy is Associated with Greater Sleep Continuity. Behav. Sleep Med. 2018;16:482–493. doi: 10.1080/15402002.2016.1228649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S.-M., Dezinno P., Maranets I., Berman M.R., Caldwell-Andrews A.A., Kain Z.N. Low Back Pain During Pregnancy. [(accessed on 5 March 2019)];Obstet. Gynecol. 2004 104:65–70. doi: 10.1097/01.AOG.0000129403.54061.0e. Available online: http://insights.ovid.com/crossref?an=00006250-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Patrícia V., De Sousa S., Cury A., Eufrásio L.S. The influence of gestational trimester, physical activity practice and weight gain on the low back and pelvic pain intensity in low risk pregnant women. J. Back Musculoskelet. Rehabil. 2019;32:671–676. doi: 10.3233/BMR-171006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorell E., Kristiansson P. Pregnancy related back pain, is it related to aerobic fitness? A longitudinal cohort study. [(accessed on 5 March 2019)];BMC Pregnancy Childbirth. 2012 12:30. doi: 10.1186/1471-2393-12-30. Available online: http://www.biomedcentral.com/1471-2393/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor P., Ko Y., Chen C., Lin P. Physical activities during pregnancy and type of delivery in nulliparae. Eur. J. Sport Sci. 2016;16:374–380. doi: 10.1080/17461391.2015.1028468. [DOI] [PubMed] [Google Scholar]
- 26.Takami M., Tsuchida A., Takamori A., Aoki S., Ito M. Effects of physical activity during pregnancy on preterm delivery and mode of delivery: The Japan Environment and Children’s Study, birth cohort study. PLoS ONE. 2018;13:e02066160. doi: 10.1371/journal.pone.0206160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajabi A., Maharlouei N., Rezaianzadeh A. Physical activities ( exercises or choreses ) during pregnancy and mode of delivery in nulliparous women: A prospective cohort study. Taiwan J. Obstet. Gynecol. 2018;57:18–22. doi: 10.1016/j.tjog.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Cid M., González M. Potential benefits of physical activity during pregnancy for the reduction of gestational diabetes prevalence and oxidative stress. Early Hum. Dev. 2016;94:57–62. doi: 10.1016/j.earlhumdev.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Barakat R., Perales M., Garatachea N., Ruiz J.R., Lucia A. Exercise during pregnancy. A narrative review asking: What do we know? Br. J. Sports Med. 2015;49:1377–1381. doi: 10.1136/bjsports-2015-094756. [DOI] [PubMed] [Google Scholar]
- 30.Vargas-terrones M., Barakat R., Santacruz B., Fernandez-buhigas I., Mottola M.F. Physical exercise programme during pregnancy decreases perinatal depression risk: A randomised controlled trial. Br. J. Sports Med. 2018;53:348–353. doi: 10.1136/bjsports-2017-098926. [DOI] [PubMed] [Google Scholar]
- 31.Szegda K., Bertone-johnson E.R., Pekow P., Powers S., Markenson G., Dole N., Chasan-Taber L. Physical activity and depressive symptoms during pregnancy among Latina women: A prospective cohort study. BMC Pregnancy Childbirth. 2018;18:252–263. doi: 10.1186/s12884-018-1839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padmapriya N., Bernard J.Y., Liang S., Loy S.L., Shen Z., Kwek K., Godfrey K.M., Gluckman P.D., Chong S.M. Association of physical activity and sedentary behavior with depression and anxiety symptoms during pregnancy in a multiethnic cohort of Asian women. Arch Womens Ment. Health. 2016;19:1119–1128. doi: 10.1007/s00737-016-0664-y. [DOI] [PubMed] [Google Scholar]
- 33.May L., Moyer C., Roldán Reoyo O. The Influence of Prenatal Exercise on Offspring Health: A Review. [(accessed on 5 March 2019)];Clin. Med. Insights Women’s Health. 2016 9:85. doi: 10.4137/CMWH.S34670. Available online: http://www.la-press.com/the-influence-of-prenatal-exercise-on-offspring-health-a-reviewsupplem-article-a5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schou Andersen C., Juhl M., Gamborg M., Sørensen T.I.A., Nohr E.A. Maternal Recreational Exercise during Pregnancy in relation to Children’s BMI at 7 Years of Age. [(accessed on 5 March 2019)];Int. J. Pediatr. 2012 2012:1–8. doi: 10.1155/2012/920583. Available online: http://www.hindawi.com/journals/ijpedi/2012/920583/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May L.E., Scholtz S.A., Suminski R., Gustafson K.M. Aerobic exercise during pregnancy influences infant heart rate variability at one month of age. Early Hum Dev. 2014;90:33–38. doi: 10.1016/j.earlhumdev.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Nascimento S.L., Surita F.G., Godoy A.C., Kasawara K.T., Morais S.S. Physical activity patterns and factors related to exercise during pregnancy: A cross sectional study. PLoS ONE. 2015;10:1–14. doi: 10.1371/journal.pone.0128953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall E.S., Bland H., Melton B. Perceived barriers to physical activity among pregnant women living in a rural community. Public Health Nurs. 2012;30:361–369. doi: 10.1111/phn.12006. [DOI] [PubMed] [Google Scholar]
- 38.Currie S., Sinclair M., Murphy M.H., Madden E., Dunwoody L., Liddle D. Reducing the Decline in Physical Activity during Pregnancy: A Systematic Review of Behaviour Change Interventions. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0066385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kikuchi-Noguchi H., Shiraishi M., Matsuzaki M. Physical activity levels in the second trimester of pregnancy and related demographic factors. Cogent. Med. 2019;6:1–11. doi: 10.1080/2331205X.2019.1704607. [DOI] [Google Scholar]
- 40.Savvaki D., Taousani E., Goulis D.G., Tsirou E., Voziki E., Douda H., Nikolettos N., Tokmakidis S.P. Guidelines for exercise during normal pregnancy and gestational diabetes: A review of international recommendations American College of Sport Medicine Canadian Society for Exercise Physiology. Hormones. 2018;17:521–529. doi: 10.1007/s42000-018-0085-6. [DOI] [PubMed] [Google Scholar]
- 41.Chasan-Taber L., Schmidt M.D., Roberts D.E., Hosmer D., Markenson G., Freedson P.S. Development and validation of a pregnancy physical activity questionnaire. Med. Sci. Sports Exerc. 2004;36:1750–1760. doi: 10.1249/01.MSS.0000142303.49306.0D. [DOI] [PubMed] [Google Scholar]
- 42.Centers For Disease Control and Prevention PRAMS Phase 7 Questionnaire Topic Reference 1. [(accessed on 11 December 2017)];2012 Available online: https://www.cdc.gov/prams/pdf/questionnaire/Phase-7-Topics-Reference_508tagged.pdf.
- 43.Hagströmer M., Oja P., Sjöström M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. [(accessed on 10 September 2016)];Public Health Nutr. 2006 9:755–762. doi: 10.1079/PHN2005898. Available online: http://www.journals.cambridge.org/abstract_S1368980006001261. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization Process of Translation and Adaptation of Instruments. [(accessed on 25 December 2017)];2008 Available online: http://www.who.int/substance_abuse/research_tools/translation/en/index.html.
- 45.Todorovic J., Terzic-Supic Z., Djikanovic B., Nesic D., Piperac P., Stamenkovic Z. Can social media intervention improve physical activity of medical students? Public Health. 2019;174:69–73. doi: 10.1016/j.puhe.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization . Physical Activity and Adults: Recommended Levels of Physical Activity for Adults Aged 18–64 Years. World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
- 47.Nikitovic V. In: Population of Serbia at the Beggining of the 21st Century. Nikitovic V., editor. Republic Institute of Statistics; Belgrade, Serbia: 2015. Populacija Srbije Pocetkom 21 Veka. [Google Scholar]
- 48.Evenson K.R., Wen F. National trends in self-reported physical activity and sedentary behaviors among pregnant women: NHANES 1999–2006. Prev. Med. (Baltim.) 2010;50:123–128. doi: 10.1016/j.ypmed.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 49.Ladabaum U., Mannalithara A., Myer P.A., Singh G. Obesity, Abdominal Obesity, Physical Activity, and Caloric Intake in US Adults: 1988 to 2010. Am. J. Med. 2010;127:717–727. doi: 10.1016/j.amjmed.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loyen A., Verloigne M., Van Hecke L., Hendriksen I., Lakerveld J., Steene-Jochannessen J., Koster A., Donnelly A., Ekelund U., Deforche B., et al. Variation in population levels of sedentary time in European adults according to cross-European studies: A systematic literature review within DEDIPAC. Int. J. Behav. Nutr. Phys. Act. 2016;13:71. doi: 10.1186/s12966-016-0397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McBride M., Emmons M., Lipkus M. Understanding the potential of teachable moments: The case of smoking cessation. Health Educ. Res. 2003;18:156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- 52.Bacchi E., Bonin C., Zanolin M.E., Zambotti F., Livornese D., Donic S., Bonora E., Baldisser G., Ihnatava T., Di Sarra D., et al. Physical activity patterns in normal-weight and overweight/obese pregnant women. PLoS ONE. 2016;11:1–11. doi: 10.1371/journal.pone.0166254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amezcua-Prieto C., Olmedo-Requena R., Jiménez-Mejías E., Mozas-Moreno J., Lardelli-Claret P., Jiménez-Moleón J.J. Factors associated with changes in leisure time physical activity during early pregnancy. Int. J. Gynecol. Obstet. 2013;121:127–131. doi: 10.1016/j.ijgo.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 54.Cioffi J., Schmied V., Dahlen H., Mills A., Thornton C., Duff M., Cummings J., Kolt G.S. Physical activity in pregnancy: Women’s perceptions, practices, and influencing factors. J. Midwifery Women’s Health. 2010;55:455–461. doi: 10.1016/j.jmwh.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Renault K., Nørgaard K., Andreasen K.R., Secher N.J., Nilas L. Physical activity during pregnancy in obese and normal-weight women as assessed by pedometer. Acta Obstet. Gynecol. Scand. 2010;89:956–961. doi: 10.3109/00016341003792459. [DOI] [PubMed] [Google Scholar]
- 56.Domingues M., Santos I., Matijasevich A., Horta B.L., Hallal P.C., Coll C. Changes in Leisure-Time Physical Activity from the Prepregnancy to the Postpartum Period: 2004 Pelotas (Brazil) Birth Cohort Study. J. Phys. Act. Health. 2016;13:361–365. doi: 10.1123/jpah.2015-0324. [DOI] [PubMed] [Google Scholar]
- 57.Sui Z., Moran L.J., Dodd J.M. Physical activity levels during pregnancy and gestational weight gain among women who ara overweight or obese. Physicl. Act. Diet. 2013;24:206–213. doi: 10.1071/HE13054. [DOI] [PubMed] [Google Scholar]
- 58.Lof M., Forsum E. Activity pattern and energy expenditure due to physical activity before and during pregnancy in healthy Swedish women. [(accessed on 25 December 2017)];Br. J. Nutr. 2006 95:296. doi: 10.1079/BJN20051497. Available online: http://www.who.int/substance_abuse/research_tools/translation/en/index.html. [DOI] [PubMed] [Google Scholar]
- 59.Hegaard H.K., Pedersen B.K., Nielsen B.B., Damm P. Leisure time physical activity during pregnancy and impact on gestational diabetes mellitus, pre-eclampsia, preterm delivery and birth weight: A review. Acta Obstet. Gynecol. Scand. 2007;86:1290–1296. doi: 10.1080/00016340701647341. [DOI] [PubMed] [Google Scholar]
- 60.Padmapriya N., Shen L., Soh S., Shen Z., Kwek K., Godfrey K., Gluckman P.D., Chong Y.S., Saw S.M., Müller-Riemenschneider F. Physical Activity and Sedentary Behavior Patterns Before and During Pregnancy in a Multi-ethnic Sample of Asian Women in Singapore. Matern. Child Health J. 2015;19:2523–2535. doi: 10.1007/s10995-015-1773-3. [DOI] [PubMed] [Google Scholar]