Abstract
Background: Mosquito-borne viruses, such as Zika, dengue, yellow fever, and chikungunya, are important causes of human diseases nearly worldwide. The greatest health risk for arboviral disease outbreaks is the presence of the most competent and highly invasive domestic mosquito, Aedes aegypti. In Cabo Verde, two recent arbovirus outbreaks were reported, a dengue outbreak in 2009, followed by a Zika outbreak in 2015. This study is the first entomological survey for Ae. aegypti that includes all islands of Cabo Verde archipelago, in which we aim to evaluate the actual risk of vector-borne arboviruses as a continuous update of the geographical distribution of this species. Methods: In order to assess its current distribution and abundance, we undertook a mosquito larval survey in the nine inhabited islands of Cabo Verde from November 2018 to May 2019. Entomological larval survey indices were calculated, and the abundance analyzed. We collected and identified 4045 Ae. aegypti mosquitoes from 264 positive breeding sites in 22 municipalities and confirmed the presence of Ae. aegypti in every inhabited island. Results: Water drums were found to be the most prevalent containers (n = 3843; 62.9%), but puddles (n = 27; 0.4%) were the most productive habitats found. The overall average of the House, Container, and Breteau larval indices were 8.4%, 4.4%, and 10.9, respectively. However, 15 out of the 22 municipalities showed that the Breteau Index was above the epidemic risk threshold. Conclusion: These results suggest that if no vector control measures are considered to be in place, the risk of new arboviral outbreaks in Cabo Verde is high. The vector control strategy adopted must include measures of public health directed to domestic water storage and management.
Keywords: Aedes aegypti, arboviruses, larval index, surveillance, control, Cabo Verde
1. Introduction
Mosquito-borne arboviral diseases are of global importance. Zika, dengue fever, and chikungunya are currently the most challenging arboviruses to international public health, despite control program efforts and research in new control methodologies [1,2,3]. Four billion people live in geographic areas suitable for dengue virus transmission alone [4,5,6]. The presence and abundance of vector mosquitoes associated with these diseases are the key points for the health risks of arboviral disease outbreaks. Globally, Aedes aegypti (Linnaeus, 1762) (=Stegomyia aegypti) is the primary vector of all these viruses, followed by other Aedes species, namely Aedes albopictus (Skuse, 1895) (=Stegomyia albopicta), which are a competent and epidemiologically significant species [1]. Ae. aegypti is the invasive mosquito species that have caused the most human casualties worldwide. They are a highly anthropophilic, peridomestic, day biting species that usually breed in artificial sites inside or around dwellings [7,8,9,10].
In Cabo Verde, two mosquito-borne virus outbreaks were recently reported for the first time: a dengue outbreak in 2009, with more than 21,000 notified cases, including 174 cases of dengue hemorrhagic fever and four reported deaths. This was followed by a Zika outbreak in 2015, with more than 7500 notified cases and 18 associated cases of microcephaly [11,12,13]. This was the first time that a Zika strain associated with these neurological damages in infants was detected in Africa [12]. In Cabo Verde, Ae. aegypti is so far the only mosquito vector associate of these arboviruses.
The archipelago of Cabo Verde is located on the west coast of Africa, and is composed of 10 islands clustered in two groups: the Barlavento group (comprising the islands of Santo Antão, São Vicente, Santa Luzia, São Nicolau, Sal, and Boavista) and the Sotavento group (comprising the islands of Maio, Santiago, Fogo, and Brava). However, each island has specific topography and displays differences in microclimate and vegetation. Historically, the topography and geographical location of Cabo Verde has promoted and allowed for the active movement of population and goods [14]. This increases the risk of pathogen circulation through infected travelers, which can cause the emergence or re-emergence of arboviral diseases if competent mosquito vectors are present and vector capacity is high [15]. In 2015, Cabo Verde had a passenger volume of more than 7000 travelers from Zika-affected countries, including direct flights from Brazil [13]. The modification of the environment by anthropic actions, disordered urban planning, population growth, and emergent factors related to the globalization process and climate change affect the bionomics of mosquito vectors, increasing their vectorial capacity [16,17,18].
In the archipelago of Cabo Verde, 11 mosquito species belonging to five genera were reported: Aedes caspius (Pallas, 1771), Ae. aegypti (Linnaeus, 1762), Anopheles pretoriensis (Theobald, 1903), Anopheles arabiensis (Patton, 1905), Culex bitaeniorhynchus (Giles, 1901), Culex quinquefasciatus (Say, 1823), Culex pipiens (Linnaeus, 1758), Culex perexiguus (Theobald, 1903), Culex tritaeniorhynchus (Giles, 1901), Lutzia tigripes (de Grandpre and de Charmoy, 1901), and Culiseta longiareolata (Macquart, 1838) [19,20,21,22]. Entomological surveys in Cabo Verde started in the 1920s and Ae. aegypti was reported for the first time by Sant’Anna in 1931 on São Vicente island [19]. The data from these pioneer surveillance operations were compiled with the last countrywide mosquito survey [19]. In 2007, another entomological survey was carried out on the four islands of the Sotavento group: Maio, Santiago, Fogo, and Brava. Ae. aegypti was detected on Santiago, Fogo, and Brava [21]. In 2011, an entomological survey was carried out in Santiago, where Ae. aegypti was reported in the municipalities of Praia and Tarrafal [22]. Phylogeographic and population genetic studies of Cabo Verde’s Ae. aegypti population suggested an ancient West African origin, most likely from Senegal, and a population belonging to the subspecies formosus [23]. Integrated vector control measures, including strategies of source reduction by breeding site elimination, biological control with fish, and chemical control with insecticides, have been used toward controlling malaria and dengue, Anopheles arabiensis, and Ae. aegypti, respectively [24]. Regarding insecticide susceptibility, knockdown resistance (kdr) mutations, genetic mutation conferring resistance to dichlorodiphenyltrichloroethane (DDT), and pyrethroids insecticides were not found in 2007 and 2010 Ae. aegypti samples. This is in line with insecticide susceptibility tests performed on Ae. aegypti from Santiago Island during the dengue outbreak in 2009 [23,25]. However, the situation changed in 2012 and 2014, with the first reports of resistance to these insecticides [26].
One of the tools used in Ae. aegypti surveillance is the determination of Stegomyia indices, namely the House Index (HI), Container Index (CI), and Breteau Index (BI). These indices measure the abundance, spatial distribution, and provide information about areas or periods of mosquito population growth [27,28,29,30,31].
Most of the studies on Ae. aegypti based on Stegomyia indices support a significant association between these and the transmission risk of arboviruses [32,33,34,35,36]. In studies that did not observe this association, mosquito and human migration were considered as possible factors that affected the lack of association [37,38,39]. Hence, knowledge of these indices allowed for the timely application of control measures and strategies [40].
In this context, we aimed to evaluate the actual risk of vector-borne arboviruses in Cabo Verde based on the Stegomyia indices, as a continuous update of the geographic distribution of Ae. aegypti. To our knowledge, this study represents the first entomological survey for this species that includes all islands of Cabo Verde archipelago.
2. Methods
2.1. Study Area
We collected mosquito larvae in the 22 municipalities of Cabo Verde, a volcanic archipelago with an area of 4033 km2 located about 550 km off the coast of Senegal. The archipelago consists of 10 islands, nine of which are inhabited with approximately 537,660 inhabitants (Figure 1). It has an arid and semi-arid climate, warm and dry, with an average annual temperature of around 25 °C, and low rainfall. Two seasons can be identified: the dry season, from December to June, and the rainy season, from August to October [41,42].
Figure 1.
Geographic localization of Cabo Verde archipelago (A), islands’ distribution in the archipelago; (B), regarding the West Coast of Africa).
In 2010, 141,762 accommodations, including 114,469 buildings, were registered in the country. Of those, 94,894 (82.9%) have one division/room, 10,646 (9.3%) have two divisions/rooms, and 6983 (6.1%) are buildings with three or more rooms. Of the total buildings, 74,404 (65%) are finished, while the remaining are under construction. Regarding the type of habitat, 44,185 (38.6%) of the houses are in urban areas and 30,449 (26.6%) in rural areas [43].
More than 95% of the population use conventional material for construction of their houses, 3.9% use non-conventional material, and 1.3% use a thatched roof, brass, drum plates, or others for cover [44,45].
The most common pavement types (99.4%) are cement and mosaic, and only 0.6% are clay or other [46]. In terms of wall cladding, 66.7% are plastered and painted, while just over 16% do not have any type of coating. Regarding the ceilings, most (79.3%) use reinforced concrete terraces [42].
One-third of Cape Verdeans do not have access to public water [45] and for those who do have access, the distribution is irregular, leading to water storage inside and outside of the homes.
In rural and semi-rural areas, pig pens or henneries are found around the houses, from which additional income is obtained [47].
2.2. Entomological Collections and Sampling Methodology
From November 2018 to May 2019, mosquito larvae were collected in all municipalities of Cabo Verde. We selected the sampling area with each municipal health delegation team, according to the high incidence history of mosquito-borne diseases, Ae. aegypti densities, and the human population. The houses were selected randomly, both in rural and urban areas. In urban areas with two parallel rows of houses, the selection was made by choosing a first house and then skipping four houses, counting a zigzag pattern. All containers, or potential breeding sites with water for larvae, were inspected and recorded (container type, position, vegetation, and sun exposure). The collected larvae were transported to the National Institute of Public Health (INSP) Medical Entomology Laboratory for morphological identification.
2.3. Morphological Identification
Larvae and reared adult mosquitoes were morphologically identified as Ae. aegypti under a stereomicroscope, according to the identification keys of Ribeiro et al. [19,48,49,50]. The larvae were mounted on slides with 2% glycerinated Hoyer’s medium, and adult reared mosquitoes, and stored at −20 °C for further molecular and genetic analysis.
2.4. Statistical Analysis
We compiled the data into a Microsoft Excel database. For the data analysis, the continuous variables were expressed in measures of central tendency and dispersion, and the categorical ones in simple frequency. The chi-square test was used to determine the association between the presence of Ae. aegypti and the type of breeder/container (type, position, and physical characteristics). We considered positive breeding sites for Ae. aegypti where there was at least one larva. The level of significance for statistical analysis was 0.05. We used IBM SPSS Statistics 20 (International Business Machines Corporation, New York City, NY, USA) to analyze the data.
Larval indices were calculated, namely, HI, CI, and BI. The maps were drawn using ArcGIS 10.6 (Environmental Systems Research Institute, Redlands, CA, USA).
3. Results
A total of 2612 houses were surveyed in the 22 municipalities of Cabo Verde, and 6113 containers were inspected. Of these, immature mosquitoes were detected in 7.5% (n = 458) of all inspected containers, the majority (84.3%; n = 386) located outdoors, and the others (15.7%; n = 72) indoors. No significant differences were observed in the distribution of indoor/outdoor and positive/negative breeding sites (p > 0.05 in both cases) (Table 1).
Table 1.
Number of houses, inspected containers, its position (indoor/outdoor), and total number of containers with mosquitoes’ larvae.
| Island | Municipalities | All Species | Ae. aegypti | Total of Inspected Houses | Total of Inspected Containers | ||||
|---|---|---|---|---|---|---|---|---|---|
| Positive Houses | Positive Containers | Positive Houses | Positive Containers | ||||||
| Indoor | Outdoor | Indoor | Outdoor | ||||||
| Santo Antão | Paul | 11 | 2 | 9 | 8 | 2 | 6 | 123 | 231 |
| Porto Novo | 23 | 5 | 26 | 14 | 4 | 11 | 112 | 201 | |
| Ribeira Grande | 18 | 2 | 18 | 8 | 1 | 7 | 125 | 251 | |
| São Vicente | São Vicente | 14 | 8 | 16 | 13 | 6 | 15 | 105 | 354 |
| São Nicolau | Tarrafal | 27 | 0 | 30 | 25 | 0 | 28 | 125 | 302 |
| Ribeira Brava | 56 | 2 | 68 | 14 | 1 | 19 | 103 | 240 | |
| Sal | Sal | 6 | 0 | 7 | 1 | 0 | 1 | 129 | 282 |
| Boavista | Boavista | 13 | 0 | 18 | 4 | 0 | 5 | 107 | 246 |
| Maio | Maio | 25 | 24 | 9 | 22 | 21 | 9 | 101 | 324 |
| Santiago | Tarrafal | 21 | 6 | 21 | 16 | 0 | 21 | 120 | 274 |
| São Miguel | 19 | 4 | 22 | 10 | 4 | 8 | 149 | 276 | |
| Santa Catarina | 8 | 3 | 5 | 4 | 1 | 3 | 117 | 282 | |
| São Salvador do Mundo | 21 | 4 | 21 | 17 | 4 | 16 | 121 | 319 | |
| São Lourenço dos Órgãos | 11 | 2 | 15 | 3 | 0 | 5 | 130 | 406 | |
| Santa Cruz | 3 | 1 | 3 | 2 | 1 | 1 | 142 | 359 | |
| São Domingos | 20 | 1 | 19 | 8 | 1 | 7 | 134 | 281 | |
| Praia | 11 | 2 | 12 | 7 | 1 | 7 | 140 | 386 | |
| Ribeira Grande | 4 | 0 | 4 | 2 | 0 | 3 | 108 | 208 | |
| Fogo | São Filipe | 13 | 4 | 12 | 6 | 3 | 4 | 100 | 151 |
| Mosteiros | 4 | 0 | 4 | 3 | 0 | 3 | 105 | 138 | |
| Santa Catarina | 1 | 0 | 3 | 0 | 0 | 0 | 100 | 242 | |
| Brava | Brava | 35 | 2 | 44 | 29 | 2 | 35 | 116 | 360 |
| Total | 364 | 72 | 386 | 218 | 55 | 209 | 2612 | 6113 | |
Of the 458 containers with immature mosquitoes, 57.6% (n = 264) were positive for Ae. aegypti, of which 20.8% (n = 55) were found inside dwellings, and 79.2% (n = 209) were found outside.
A total of 4045 Ae. aegypti larvae and pupae were collected from the 264 Aedes-positive containers found in all 22 of the country’s municipalities (Table 2). Other mosquito species found across the survey were Aedes caspius, Anopheles pretoriensis, Anopheles arabiensis, Culex bitaeniorhynchus, Culex pipiens s.l. (Culex quinquefasciatus, Culex pipiens), Culex tritaeniorhynchus, Lutzia tigripes, and Culiseta longiareolata. Culex pipiens s.l. and An. pretoriensis were found sharing the same breeding sites with Ae. aegypti.
Table 2.
Positive containers for Aedes aegypti.
| Container | No. of Containers with Ae. aegypti (%) |
No. of Ae. aegypti (%) |
No. of Containers with Other Species (%) |
|---|---|---|---|
| Ceramic pots | 5 (1.9) | 101 (2.5) | 1 (0.5) |
| Buckets | 10 (3.8) | 102 (2.5) | 2 (1.0) |
| Cisterns | 8 (3.0) | 107 (2.6) | 6 (3.1) |
| Water drums | 162 (61.4) | 1904 (46.1) | 53 (27.6) |
| Flowerpots | 38 (14.4) | 1455 (36.0) | 9 (4.7) |
| Puddles | 3 (1.1) | 26 (0.6) | 6(3.1) |
| Tanks | 21 (8.0) | 249 (6.2) | 70 (36.5) |
| Tires | 2 (0.8) | 11 (0.3) | 0 (0.0) |
| Other | 8 (3.0) | 47 (1.2) | 45 (23.4) |
| Water fountains | 7 (2.7) | 43 (1.1) | 0 (0.0) |
| Total | 264 | 4045 | 192 |
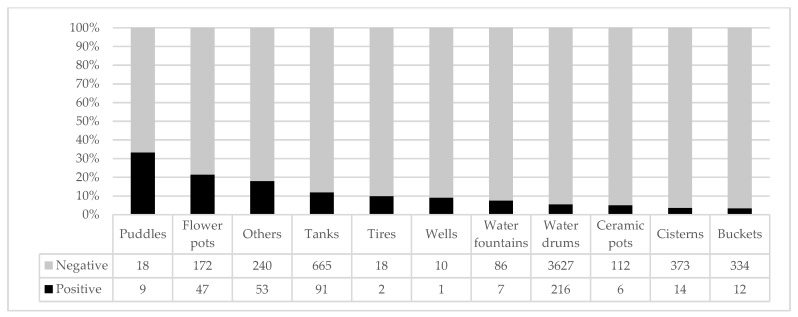
Water drums (50–200 L) were the most common breeding sites (62.9%; n = 3843), followed by tanks (1000–5000 L) (12.4%; n = 756), cisterns (5000–10,000 L) (6.3%; n = 387), and other types of containers that comprised 18.4% of total breeding sites. From all inspected containers, 458 (7.5%) were positive, and the productivity proportion analysis for each container showed puddles as the most productive habitat (33%; n = 9), followed by flowerpots (21%; n = 47), other (18%; n = 53), and tanks (12%; n = 91). All other types of containers showed that productivity equaled less than 10% (Figure 2).
Figure 2.
Number and proportion of positive and negative breeding sites inspected during the study.
The four most productive containers for Ae. aegypti were water drums (61.4%), flowerpots (14.4%), tanks (8.0%), and buckets (3.8%) (Table 2). A positive association between the container type and the presence of Ae. aegypti was observed (χ2 = 133.816, p < 0.001). The frequency of other breeding sites was relatively low, 3% for cisterns and other containers, and lower for drinking fountains, puddles, pots, and tires (Table 2).
Entomological Indices
The average HI, CI, and BI were 8.4%, 4.4%, and 10.9, respectively. Fifteen out of the 22 studied municipalities presented a BI above five. The maximum values were in the municipality of Brava (HI = 25%; CI = 9.7% and BI = 30.2) and the minimum values, below 1%, were found in Sal. In the municipality of Santa Catarina (Fogo island), despite no Ae. aegypti larvae being found, adults were recorded during the survey (Table 3).
Table 3.
Entomological indices in municipalities.
| Island | Municipalities | Entomological Indices | ||
|---|---|---|---|---|
| HI (%) | CI (%) | BI | ||
| Santo Antão | Paul | 6.5 | 3.5 | 6.5 |
| Porto Novo | 12.5 | 7.5 | 13.4 | |
| Ribeira Grande | 6.4 | 6.0 | 12.0 | |
| São Vicente | São Vicente | 12.4 | 5.9 | 20.0 |
| São Nicolau | Tarrafal | 20.0 | 9.27 | 22.4 |
| Ribeira Brava | 13.6 | 8.3 | 19.4 | |
| Sal | Sal * | 0.8 | 0.4 | 0.8 |
| Boavista | Boavista * | 3.7 | 2.0 | 4.7 |
| Maio | Maio | 21.8 | 9.3 | 29.7 |
| Santiago | Tarrafal | 13.3 | 7.7 | 17.5 |
| São Miguel | 6.7 | 4.3 | 8.1 | |
| Santa Catarina | 3.4 | 1.4 | 3.4 | |
| São Salvador do Mundo | 14.1 | 6.3 | 16.5 | |
| São Lourenço dos Órgãos * | 2.3 | 1.2 | 3.8 | |
| Santa Cruz * | 1.4 | 0.6 | 1.4 | |
| São Domingos | 6.0 | 2.8 | 6.0 | |
| Praia | 5.0 | 2.1 | 5.7 | |
| Ribeira Grande * | 1.9 | 1.4 | 2.7 | |
| Fogo | São Filipe | 6.0 | 4.6 | 7.0 |
| Mosteiros * | 2.9 | 2.2 | 2.9 | |
| Santa Catarina ** | 0.0 | 0.0 | 0.0 | |
| Brava | Brava | 25.0 | 10.3 | 31.9 |
* Municipalities with Breteau Index (BI) <5; ** No Ae. aegypti larva found; ** Ae. aegypti adult mosquitoes were recorded during survey. House Index (HI), Container Index (CI).
4. Discussion
This study represents the first archipelago-wide analysis of the Ae. aegypti breeding sites in Cabo Verde, which are exclusively domestic containers. This domestic mosquito is one of the most important arthropod vectors of arboviruses worldwide, namely dengue, Zika, chikungunya, and yellow fever [51]. Although there has been an ongoing focus on vaccine development for prevention of these diseases, vector control has been the key strategy to control or prevent the transmission of mosquito-borne arbovirus infections [52]. In previous studies, Ae. aegypti populations infected with DENV-2 and DENV-4 were found in Cabo Verde, with high vector competence to transmit DENV-2 and DENV-3, and to be infected with and transmit chikungunya and yellow fever [53,54,55].
In Cabo Verde, recent entomological data on the mosquito species distribution in the nine inhabited islands are missing, and data regarding Ae. aegypti are scarce. Although several factors influence a breeding site’s availability and mosquito distribution, in this study, Ae. aegypti was mostly found in water drums used in water storage by the population, which corroborates previous results [56,57,58,59,60].
High vector density and susceptible human population are key factors to arbovirus disease outbreaks. Between these two, the first one is the major contributor and can be estimated by Aedes indices, such as CI, HI, and BI [61]. These larval indices provide useful information to plan, evaluate, and monitor the efficacy of vector control interventions. The BI is the most used, considering the number of positive containers and searched houses. We noticed variation in the indices among the municipalities, with some municipalities showing BI as high as 31.9 and others 0. This variation is highly dependent on a container’s availability, which can be affected by numerous factors, such as seasonality (rainy or dry season), local population habits, customs, and traditions, and local microclimate. The irregularities in the water distribution force people to store water in containers; thus, this factor plays an important role in the ecology of larval mosquito habitats [59]. Positive containers found outside of dwellings were associated with domestic animals and agriculture (not statistically tested), but further studies should be done to approve or disprove this claim.
In this study, the fieldwork occurred during the dry season and we observed that Ae. aegypti is extremely adapted to domestic habitats. It is also important to note that no correlation was found between indoor and outdoor containers in this study.
Thirteen municipalities presented a BI above the epidemic risk threshold [40,62]. Our results suggest that Ae. aegypti is well established in all archipelago islands, and several municipalities in Cabo Verde are at risk of arboviral disease outbreaks.
5. Conclusions
Aedes aegypti is a major threat to public health in Cabo Verde, considering the values of larval indices found in this study associated with previous studies showing Ae. aegypti vector competence to diseases registered and not registered in Cabo Verde [54,63], as well as resistance to insecticides in the archipelago [25,26]. Nevertheless, more studies are crucial to evaluate this species’ resistance to more insecticides used in the public health context. We also recommend implementation of a countrywide vector control strategy with environmental management and modification, according to general international guidelines [31]. A program for monitoring Ae. aegypti, carried out by each municipality’s health delegation, with the support of the Ministry of Health, is also desirable.
Acknowledgments
We acknowledge the National Institute of Public Health of Cabo Verde for supporting the study, and also to all the Health facilities, health delegates, control vector agents, drivers, and administrators that provided their support during the entomological survey. We are also grateful to the National Program for vector control. We acknowledge Cláudia Samira Varela for laboratory work support, Kátia Euriza Jesus Pereira Batalha for statistical analysis support, and Jailson José Tavares Varela, from the National Institute of Territorial Management, for the map drawing.
Author Contributions
Design of the study, S.D.V.L. and I.B.F.V.; Collections and laboratory work, D.D.S.M., C.M.R.d.S., A.A.L.G., and I.B.F.V.; Data analyses, I.B.F.V., A.A.L.G., M.J.A., H.C.O., and S.D.V.L. Original Draft Preparation, I.B.F.V., A.A.L.G., M.J.A., H.C.O., and S.D.V.L.; Writing—Review and Editing, I.B.F.V., A.A.L.G., M.J.A., H.C.O., and S.D.V.L.; Revision and Supervision, A.J.D.P., M.d.L.L.M., and M.J.A. All authors read and approved the final manuscript.
Funding
This research as also the APC were funded by the National Institute of Public Health of Cabo Verde grant number 65.06.01.04.29.01.-Reforço Do Laboratório Nacional De Saúde Pública.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Weaver S.C., Reisen W.K. Present and future arboviral threats. Antivir. Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A., Lal S.K. Zika virus: Transmission, detection, control, and prevention. Front. Microbiol. 2017;8:110. doi: 10.3389/fmicb.2017.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilder-Smith A., Gubler D.J., Weaver S.C., Monath T.P., Heymann D.L., Scott T.W. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 2017;17:e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- 4.Brady O.J., Gething P.W., Bhatt S., Messina J.P., Brownstein J.S., Hoen A.G., Moyes C.L., Farlow A.W., Scott W.T., Hay S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leta S., Beyene T.J., De Clercq E.M., Amenu K., Kraemer M.U., Revie C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018;67:25–35. doi: 10.1016/j.ijid.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paixão E.S., Teixeira M.G., Rodrigues L.C. Zika, Chikungunya and dengue: The causes and threats of new and re-emerging arboviral diseases. BMJ Glob. Health. 2018;3(Suppl. 1):e000530. doi: 10.1136/bmjgh-2017-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gloria-Soria A., Ayala D., Bheecarry A., Calderon-Arguedas O., Chadee D.D., Chiappero M., Coetzee M., Elahee K.B., Fernandez-Salas I., Kamal H.A., et al. Global genetic diversity of Aedes aegypti. Mol. Ecol. 2016;25:5377–5395. doi: 10.1111/mec.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braga I.A., Valle D. Aedes aegypti: Inseticidas, mecanismos de ação e resistência. Epidemiol. Serv. Saúde. 2007;16:279–293. doi: 10.5123/S1679-49742007000400006. [DOI] [Google Scholar]
- 9.Maciel-de-Freitas R., Marques W.A., Peres R.C., Cunha S.P., Lourenço-de-Oliveira R. Variation in Aedes aegypti (Diptera: Culicidae) container productivity in a slum and a suburban district of Rio de Janeiro during dry and wet seasons. Memórias Inst. Oswaldo Cruz. 2007;102:489–496. doi: 10.1590/S0074-02762007005000056. [DOI] [PubMed] [Google Scholar]
- 10.Andrew J., Bar A. Morphology and morphometry of Aedes aegypti adult mosquito. Annu. Rev. Res. Biol. 2013;3:52–69. [Google Scholar]
- 11.World Health Organization . Dengue Fever in Cape Verde. WHO; Geneva, Switzerland: 2009. [(accessed on 3 June 2019)]. update 1. Available online: http://www.who.int/csr/don/2009_11_18/en/index.html. [Google Scholar]
- 12.World Health Organization . Zika Virus Infection—Cape Verde. WHO; Geneva, Switzerland: 2015. [(accessed on 4 June 2019)]. Available online: http://www.who.int/csr/don/21-december-2015-zika-cape-verde/en/ [Google Scholar]
- 13.Lourenço J., de Lourdes Monteiro M., Valdez T., Rodrigues J.M., Pybus O., Faria N.R. Zika virus outbreak in Cabo Verde Islands, West Africa: Early epidemiological findings. bioRxiv. 2017:198952. doi: 10.1371/currents.outbreaks.19433b1e4d007451c691f138e1e67e8c. [DOI] [Google Scholar]
- 14.Lobban R.A. Cape Verde: Crioulo Colony to Independent Nation. Routledge; Abingdon-on-Thames, UK: 2018. [Google Scholar]
- 15.Aagaard-Hansen J., Nombela N., Alvar J. Population movement: A key factor in the epidemiology of neglected tropical diseases. Trop. Med. Int. Health. 2010;15:1281–1288. doi: 10.1111/j.1365-3156.2010.02629.x. [DOI] [PubMed] [Google Scholar]
- 16.Githeko A.K., Lindsay S.W., Confalonieri U.E., Patz J.A. Climate change and vector-borne diseases: A regional analysis. Bull. World Health Organ. 2000;78:1136–1147. [PMC free article] [PubMed] [Google Scholar]
- 17.McMichael A.J., Woodruff R.E., Hales S. Climate change and human health: Present and future risks. Lancet. 2006;367:859–869. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- 18.Gould E., Pettersson J., Higgs S., Charrel R., de Lamballerie X. Emerging arboviruses: Why today? One Health. 2017;4:1–13. doi: 10.1016/j.onehlt.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro H., Ramos H.D.C., Capela R.A., Pires C.A. Os mosquitos de Capo Verde (Diptera: Culicidae). Sistematica, distribuição, bioecologia e importância médica. Junta Investig. Cient. Ultramar Estud. Ens. Dot. Lisboa. 1980;135:141. [Google Scholar]
- 20.Cambournac F.J.C., Oliveira M.C., Correia A., Coutinho M.A., Tourinho J., Soares B. Culex (Lutzia) tigripes (Grandpré); mais uma espécie nova para Cabo Verde. An. do Inst. de Hig. e Med. Trop. 1984;10:41–46. [Google Scholar]
- 21.Alves J., Gomes B., Rodrigues R., Silva J., Arez A.P., Pinto J., Sousa C.A. Mosquito fauna on the Cabo Verde Islands (West Africa): An update on species distribution and a new finding. J. Vector Ecol. 2010;35:307–312. doi: 10.1111/j.1948-7134.2010.00087.x. [DOI] [PubMed] [Google Scholar]
- 22.Alves J., Pina A.D., Diallo M., Dia I. First report of Culex (Culex) tritaeniorhynchus Giles, 1901 (Diptera: Culicidae) in the Cabo Verde Islands. Zool. Caboverdiana. 2014;5:4–19. [Google Scholar]
- 23.Salgueiro P., Serrano C., Gomes B., Alves J., Sousa C.A., Abecasis A., Pinto J. Phylogeography and invasion history of Aedes aegypti, the Dengue and Zika mosquito vector in Cape Verde islands (West Africa) Evol. Appl. 2019;12:1797–1811. doi: 10.1111/eva.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministry of Health Cape Verde. WHO. University of California . Eliminating Malaria: Case Study 2. Moving Towards Sustainable Elimination in Cape Verde. WHO; Geneva, Switzerland: 2012. [(accessed on 1 November 2019)]. Available online: http://www.who.int/malaria/publications/atoz/9789241504386/en/ [Google Scholar]
- 25.Dia I., Diagne C.T., Ba Y., Diallo D., Konate L., Diallo M. Insecticide susceptibility of Aedes aegypti populations from Senegal and Cabo Verde Archipelago. Parasit Vectors. 2012;5:238. doi: 10.1186/1756-3305-5-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocha H.D.R., Paiva M.H.S., Silva N.M., de Araújo A.P., da Moura A.J.F., Gómez L.F., Ayres C.F.J., de Melo Santos M.A.V. Susceptibility profile of Aedes aegypti from Santiago Island, Cabo Verde, to insecticides. Acta Trop. 2015;152:66–73. doi: 10.1016/j.actatropica.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Connor M.E., Monroe W.M. Stegomyia indices and their value in Yellow Fever control. Am. J. Trop. Med. Hyg. 1923;3:9–19. doi: 10.4269/ajtmh.1923.s1-3.9. [DOI] [Google Scholar]
- 28.Breteau H. La fièvre jaune en Afrique-occidentale française: Un aspect de la médecine préventive massive. Bull. World Health Organ. 1954;11:453. [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization A system of world-wide surveillance for vectors. Wkly. Epidemiol. Record. 1972;47:73–80. [Google Scholar]
- 30.Nathan M.B. Critical review of Aedes aegypti control programs in the Caribbean and select neighboring countries. J. Am. Mosq. Control. Assoc. 1993;9:1–7. [PubMed] [Google Scholar]
- 31.World Health Organization . Entomological Surveillance for Aedes spp. in the Context of Zika Virus: Interim Guidance for Entomologists. WHO; Geneva, Switzerland: 2016. No. WHO/ZIKV/VC/16.2. [Google Scholar]
- 32.Fofana D., Beugré J.M.V., Yao-Acapovi G.L., Lendzele S.S. Risk of Dengue Transmission in Cocody (Abidjan, Ivory Coast) J. Parasitol. Res. 2019;2019:4914137. doi: 10.1155/2019/4914137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferede G., Tiruneh M., Abate E., Kassa W.J., Wondimeneh Y., Damtie D., Tessema B. Distribution and larval breeding habitats of Aedes mosquito species in residential areas of northwest Ethiopia. J. Epidemiol. Health. 2018;40:e2018015. doi: 10.4178/epih.e2018015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan J., Mathiarasan L. Prevalence of disease vectors in Lakshadweep Islands during post-monsoon season. J. Vector Borne Dis. 2018;55:189–196. doi: 10.4103/0972-9062.249127. [DOI] [PubMed] [Google Scholar]
- 35.Thammapalo S., Nagao Y., Sakamoto W., Saengtharatip S., Tsujitani M., Nakamura Y., Coleman P.G., Davies C. Relationship between transmission intensity and incidence of dengue hemorrhagic fever in Thailand. PLoS Negl. Trop. Dis. 2008;2:e263. doi: 10.1371/journal.pntd.0000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez L., Cortinas J., Pelaez O., Gutierrez H., Concepción D., Van Der Stuyft P. Breteau Index threshold levels indicating risk for dengue transmission in areas with low Aedes infestation. Trop. Med. Int. Health. 2010;15:173–175. doi: 10.1111/j.1365-3156.2009.02437.x. [DOI] [PubMed] [Google Scholar]
- 37.Wijegunawardana N.D.A.D., Gunawardene Y.I.N., Chandrasena T.G.A.N., Dassanayake R.S., Udayanga N.W.B.A.L., Abeyewickreme W. Evaluation of the Effects of Aedes Vector Indices and Climatic Factors on Dengue Incidence in Gampaha District, Sri Lanka. Biomed. Res. Int. 2019;2019:2950216. doi: 10.1155/2019/2950216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Focks D.A. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors. World Health Organization; Geneva, Switzerland: 2004. No. TDR/IDE/DEN/03.1. [Google Scholar]
- 39.Sulaiman S., Pawanchee Z.A., Arifin Z., Wahab A. Relationship between Breteau and House indices and cases of dengue/dengue hemorrhagic fever in Kuala Lumpur, Malaysia. J. Am. Mosq. Control. Assoc. 1996;12:494–496. [PubMed] [Google Scholar]
- 40.Sanchez L., Vanlerberghe V., Alfonso L., del Carmen Marquetti M., Guzman M.G., Bisset J., Van Der Stuyft P. Aedes aegypti larval indices and risk for dengue epidemics. Emerg. Infect. Dis. 2006;12:800–806. doi: 10.3201/eid1205.050866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Instituto Nacional de Estatística . Projeção Demográficas de Cabo Verde, 2010–2030. INE—Instituto Nacional da Estatística de Cabo Verde; Praia, Cabo Verde: 2013. [Google Scholar]
- 42.Instituto Nacional de Estatística . PIB e Componentes (Anual): Contas Nacionais, 2007–2015. Instituto Nacional da Estatística de Cabo Verde; Praia, Cabo Verde: 2017. [Google Scholar]
- 43.Instituto Nacional de Estatística . Recenseamento Geral da População (RGPH) 2010. Projeções Demográficas 2010–2030; Instituto Nacional da Estatística de Cabo Verde; Praia, Cabo Verde: 2010. [Google Scholar]
- 44.Instituto Nacional de Estatística . Inquérito às Despesas e Receitas das Famílias (IDRF) Instituto Nacional da Estatística de Cabo Verde; Praia, Cabo Verde: 2015. [Google Scholar]
- 45.Instituto Nacional de Estatística . Inquérito Multi-Objectivo Contínuo (IMC-CV) 2017. Instituto Nacional da Estatística de Cabo Verde; Praia, Cabo Verde: 2017. [Google Scholar]
- 46.Instituto Nacional de Estatística . Inquérito Multi-Objectivo Contínuo 2016. Instituto Nacional da Estatística de Cabo Verde; Praia, Cabo Verde: 2016. [Google Scholar]
- 47.Centeio R.A.G. Master’s Thesis. Universidade Lusófona de Humanidades e Tecnologias; Lisboa, Portugal: 2015. [(accessed on 13 June 2019)]. A Construção e Arquitetura Sustentável em Cabo Verde: Habitação Unifamiliar em Santiago. Available online: http://recil.grupolusofona.pt/handle/10437/6936. [Google Scholar]
- 48.Ribeiro H., de Cunha Ramos H. Boletim da Sociedade Portuguesa de Entomologia. Sociedade Portuguesa de Entomologia (SPEN); Lisboa, Portugal: 1995. Guia ilustrado para a identificação dos mosquitos de Angola: (Diptera: Culcidae) [Google Scholar]
- 49.Azari-Hamidian S., Harbach R.E. Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae) Zootaxa. 2009;2078:1–33. doi: 10.11646/zootaxa.2078.1.1. [DOI] [Google Scholar]
- 50.Dehghan H., Sadraei J., Moosa-Kazemi S.H., Abolghasemi E., Solimani H., Nodoshan A.J., Najafi M.H. A pictorial key for Culex pipiens complex (Diptera: Culicidae) in Iran. J. Arthropod Borne Dis. 2016;10:291–302. [PMC free article] [PubMed] [Google Scholar]
- 51.Powell J.R., Tabachnick W.J. History of domestication and spread of Aedes aegypti—A review. Memórias do Instituto Oswaldo Cruz. 2013;108:11–17. doi: 10.1590/0074-0276130395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powell J.R. Mosquito-borne human viral diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg. 2018;98:1563–1565. doi: 10.4269/ajtmh.17-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vazeille M., Yébakima A., Lourenço-de-Oliveira R., Andriamahefazafy B., Correira A., Rodrigues J.M., Veiga A., Moreira A., Leparc-Goffart I., Grandadam M., et al. Oral receptivity of Aedes aegypti from Cabo Verde for Yellow Fever, dengue, and Chikungunya viruses. Vector Borne Zoonotic Dis. 2013;13:37–40. doi: 10.1089/vbz.2012.0982. [DOI] [PubMed] [Google Scholar]
- 54.Da Moura A.J.F., de Melo Santos M.A.V., Oliveira C.M.F., Guedes D.R.D., de Carvalho-Leandro D., da Cruz Brito M.L., Rocha H.D.R., Gómez L.F., Ayres C.F.J. Vector competence of the Aedes aegypti population from Santiago Island, Cabo Verde, to different serotypes of dengue virus. Parasite Vector. 2015;8:114. doi: 10.1186/s13071-015-0706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guedes D.R., Gomes E.T., Paiva M.H., Melo-Santos M.A.D., Alves J., Gómez L.F., Ayres C.F. Circulation of DENV2 and DENV4 in Aedes aegypti (Diptera: Culicidae) mosquitoes from Praia, Santiago Island, Cabo Verde. J. Insect Sci. 2017;17:86. doi: 10.1093/jisesa/iex057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koh B.K., Ng L.C., Kita Y., Tang C.S., Ang L.W., Wong K.Y., James L., Goh K.T. The 2005 dengue epidemic in Singapore: Epidemiology, prevention and control. Ann. Acad. Med. Singap. 2008;37:538–545. [PubMed] [Google Scholar]
- 57.Ler T.S., Ang L.W., Yap G.S.L., Ng L.C., Tai J.C., James L., Goh K.T. Epidemiological characteristics of the 2005 and 2007 dengue epidemics in Singapore–similarities and distinctions. West. Pac. Surveill. Response J. 2011;2:24–29. doi: 10.5365/WPSAR.2010.1.1.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duarte E.H., Correia E.E., Varela C.E., Varela A. Reproduction of mosquitoes (Diptera: Culicidae) in Santa Cruz, Santiago island, Cabo Verde Islands. Zool Caboverdiana. 2012;3:29–36. [Google Scholar]
- 59.Duarte E.H., Pereira J., Oliveira H.D., Lima H.S., Perez A., Pile E. Aedes (Stegomyia) aegypti (Diptera: Culicidae) em algumas ilhas de Cabo Verde: Tipologia dos criadouros e sua relação com a presença larval. Arquivos do Instituto Biológico. 2013;80:359–362. doi: 10.1590/S1808-16572013000300015. [DOI] [Google Scholar]
- 60.Balasubramanian R., Anukumar B., Nikhil T.L. Stegomyia indices of Aedes mosquito infestation and container productivity in Alappuzha district Kerala. Int. J. Mosq. Res. 2015;2:148. [Google Scholar]
- 61.Hapuarachchi H.C., Koo C., Rajarethinam J., Chong C.S., Lin C., Yap G., Liu L., Lai Y.L., Ooi P.L., Jeffery Cutter J., et al. Epidemic resurgence of dengue fever in Singapore in 2013–2014: A virological and entomological perspective. BMC Infect. Dis. 2016;16:300. doi: 10.1186/s12879-016-1606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bowman L.R., Runge-Ranzinger S., McCall P.J. Assessing the relationship between vector indices and dengue transmission: A systematic review of the evidence. PLoS Negl. Trop. Dis. 2014;8:e2848. doi: 10.1371/journal.pntd.0002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diagne C.T., Faye O., Guerbois M., Knight R., Diallo D., Faye O., Ba Y., Dia I., Faye O., Weaver S.C., et al. Vector competence of Aedes aegypti and Aedes vittatus (Diptera: Culicidae) from Senegal and Cabo Verde archipelago for West African lineages of Chikungunya virus. Am. J. Trop. Med. Hyg. 2014;91:635–641. doi: 10.4269/ajtmh.13-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]