Abstract
Microorganisms area treasure in terms of theproduction of various bioactive compounds which are being explored in different arenas of applied sciences. In agriculture, microbes and their bioactive compounds are being utilized in growth promotion and health promotion withnutrient fortification and its acquisition. Exhaustive explorations are unraveling the vast diversity of microbialcompounds with their potential usage in solving multiferous problems incrop production. Lipopeptides are one of such microbial compounds which havestrong antimicrobial properties against different plant pathogens. These compounds are reported to be produced by bacteria, cyanobacteria, fungi, and few other microorganisms; however, genus Bacillus alone produces a majority of diverse lipopeptides. Lipopeptides are low molecular weight compounds which havemultiple industrial roles apart from being usedas biosurfactants and antimicrobials. In plant protection, lipopeptides have wide prospects owing totheirpore-forming ability in pathogens, siderophore activity, biofilm inhibition, and dislodging activity, preventing colonization bypathogens, antiviral activity, etc. Microbes with lipopeptides that haveall these actions are good biocontrol agents. Exploring these antimicrobial compounds could widen the vistasof biological pest control for existing and emerging plant pathogens. The broader diversity and strong antimicrobial behavior of lipopeptides could be a boon for dealing withcomplex pathosystems and controlling diseases of greater economic importance. Understanding which and how these compounds modulate the synthesis and production of defense-related biomolecules in the plants is a key question—the answer of whichneeds in-depth investigation. The present reviewprovides a comprehensive picture of important lipopeptides produced by plant microbiome, their isolation, characterization, mechanisms of disease control, behavior against phytopathogens to understand different aspects of antagonism, and potential prospects for future explorations as antimicrobial agents. Understanding and exploring the antimicrobial lipopeptides from bacteria and fungi could also open upan entire new arena of biopesticides for effective control of devastating plant diseases.
Keywords: lipopeptides, Bacillus spp., biosurfactant, antimicrobials, biocontrol
1. Introduction
Crop plants are damaged every year by phytopathogens, leading to enormous economic losses to farmers across the world. Currently, themost effective available control measure for plant diseasesthroughchemical pesticideshasresulted intoxic effectsonnon-target organismsandas these compounds arenon-biodegradable in nature, this has become a matter of serious concern for contemporary environmentalists. Since chemical control is not sustainable and is almost certain to cause environmental pollution [1,2,3], different microbiological agents and biologically active molecules are beingexplored for their potential to inhibit thegrowth of phytopathogensand alleviation of other stresses [4,5,6,7,8] to crop plants. These bioactive compounds are produced by microorganisms, specificallyby the Bacillus genus whichis considered one of the most important bioactive compound factories [2,3,8]. Plant diseases caused by phytopathogenshave beenone of the most important and emerging categories of threats to global food security [9,10]. Microbiome associated with the plants is known to produce a structurally diverse group of compounds with hydrophilic and hydrophobic moieties and of which exhibitsbiosurfactant activity. These biosurfactants include lipopeptides, glycolipids, phospholipids, polysaccharide-protein complexes, neutral lipids, and fatty acids [11].
Due to the enormous variation in the chemical structures, lower toxicity to non-targets, biodegradability, and effectiveness to be functional under extreme environmental conditions such as high pH, extreme temperature, salinity, drought, metal stress, etc., these bio-surfactants qualifythe parameters set for asuitable green and eco-friendly alternative as compared to their synthetic counterparts for managing phytopathogens and reduce crop losses therefrom. Duetothese properties, they have gained much attension in applied sectors ranging from pharmaceutical, cosmetics, agriculture, oil recovery, and food industriestothe activities related to environmental remediation [12,13,14,15,16] Lipopeptides are defined as cyclic, low molecular weight compounds withantimicrobial potential largely produced by Bacillus and Pseudomonas spp. [17,18]. In general, the molecular weight of lipopeptides ranges from 1000–2000 Da. They are synthesized by specific gene clusters, namely nonribosomal peptides synthetase (NRPs) via a multi-enzyme biosynthesis pathway [19]. Surfactin, iturin, and fengycin are the three major families reported from Bacillus groups and are mainly composed of a hydrophilic amino acid (7–10 amino acids) linked with a hydrophobic fatty acid tail. Aneurinifactin is a group of lipopeptide reported from marine bacterium Aneurinibacillus aneurinilyticus isolated from the Gulf of Mannar [20]. Moreover, several lipopeptides such as iturin [21], surfactin [22], sophorolipids [23], rhamnolipids [24], trehalose lipid [25], and mannosylerythritol lipids [26] exhibited antifungal, antibacterial, or antitumor activities, signifying their utility as potent alternativesof conventional therapeutic agents and biocontrol agents for use in various biomedical and agriculturalapplications [15,24]. Surfactins consist of seven amino acids linked to one unique hydroxy fatty acid, whereas iturins consist of seven amino acids linked to one unique amino acid. The chemical composition of fengycins reveals that it consists of 10 amino acids linked to one unique hydroxy fatty acid. These lipopeptides are the most important factors contributing to their biocontrol potential in the plant growth-promoting microorganisms [17,18,22].
These cyclic lipopeptides produced by different microorganisms retain antiviral, antifungal, antibacterial, biofilm-forming, and plant resistance-inducing activities. Several reports indicate that these small molecular weight lipopeptides facilitate root colonization in many plants [8,9,10,15,16]. They act as potential antagonists by direct inhibition of phytopathogens through different mechanisms and/or by stimulating and strengthening plant defense machinery of defense-related networks known as induced systemic resistance (ISR) [16]. Recently, biocontrol agents of microbial origin have gained much attention for soil-borne disease control [27]. These bioagents synthesized and secreted several diffusible and volatile organic compounds in the rhizosphere and plant system and these compounds are the most important factors contributing to the biocontrol activity [28] of the producer organism. These biomoleculesare chiefly characterized for their antagonistic activity against plant pathogens of different crops [27,29,30]. There are some cyclic lipopeptides which have recently been identified as elicitors of plant defence response such as ISR [7,16]. These biomolecules play a key role and modulate various mechanisms underlying the defense responses or, more specifically, ISR, both directly and indirectly. Moreover, cyclic lipopeptides may be involved anywhere in the three-step process of ISR, viz. the perception of bacterial elicitor, systemic signal transduction, and, finally, defense gene expression in the host system [8,9,10]. However, the role of plant growth-promoting rhizobacteria (PGPR) in the signaling and induction of defense mechanisms isbeing well documented [31,32]. Until now, very little wasknown about mechanisms as to how the lipopeptides elicit ISR and activate different cascades of pathways after early interaction with the plant cell. Some molecules accountable for the ISR-eliciting activity may be cell-surface components [33,34,35], volatiles [36], iron-regulated metabolites [37,38,39], antibiotics compounds [40,41,42,43], and quorum-sensing signals [44]. Tran et al. [45] reported that “massetolide” produced by Pseudomonas fluorescens SS101 elicit ISR-reactions in tomatoeschallenged with Phytophthora infestans.
Similarly, members of the surfactins and fengycins families also act as elicitors of ISR by supplementing and eliciting the host resistance potential [46]. Members of the surfactin family show structural variability among them with good emulsifying and foaming properties. This is in contrast to the various investigations conducted with some PGPRs and pathogen-associated molecular patterns (PAMPs) used to decipher the events for ISR development taking place after lipopeptide inoculation [47,48,49]. However, very little information is available about the role of lipopeptides as elicitors of plant defense in controlling pathogen invasion. Although, they are known to act as chemical barriers and thus, slow down or inhibit pathogen colonization [50].
2. Isolation and Characterization of Lipopeptides
2.1. Isolation and Purification of Lipopeptides
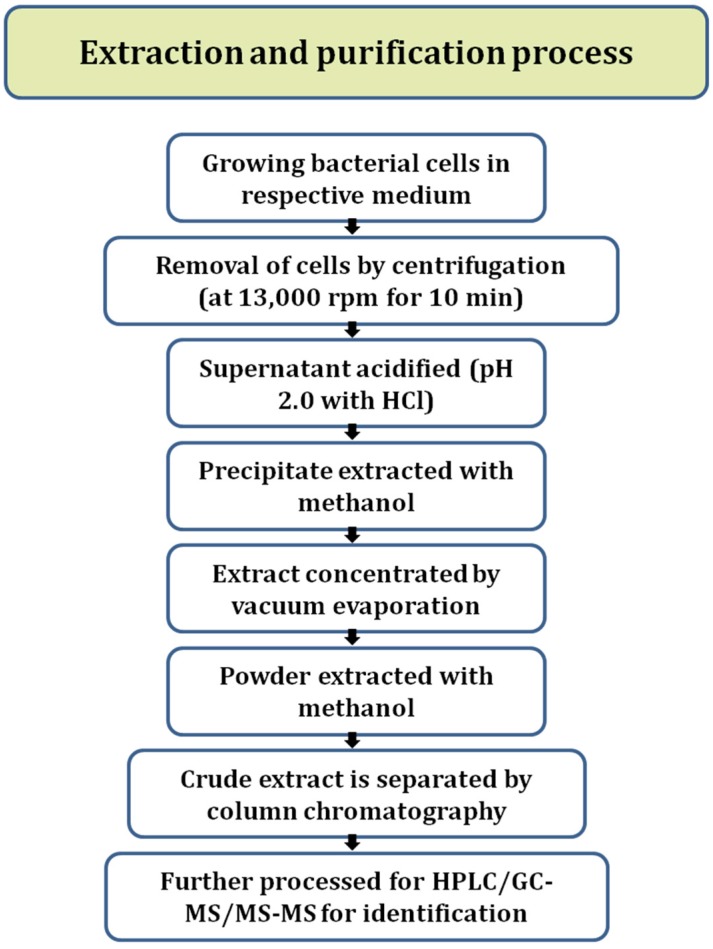
Lipopeptides are chiefly synthesized by microorganisms belongingto bacterial genus Bacillus [51] by multimodular enzymes that are NRPSs. The cells are grown in their respective media and are allowed to produce lipopeptides (Figure 1). These lipopeptides are separated from cells bycentrifugation. Malfanova et al. [52] grew the bacterial cells for 60 h at 28 °C and then centrifuged at 13,000 rpm for 10 min to remove cells and obtain crude lipopeptides. After this, the supernatantwasacidified (pH 2.0) using concentrated HCl and acid precipitate wasextracted with methanol. The extract obtained wasconcentrated by vacuum evaporation. This can also be done by lyophilization. The resulting material wasdissolved in 1/50 of the initial volume of methanol or may be dissolved in PBS for further experimentations [52,53,54]. The crude extract wasthen purified by different methods of chromatography like gel filtration in Sephadex column using methanol, HPLC, etc. and the collected eluent wasused for identification using MALDI-TOF-MS, LC-MS, or MS-MS, comparing retention time and molecular masses. Different elution programs are used for the quantification of different families of lipopeptides [55].
Figure 1.
Flow chart diagram of lipopeptide extraction and the purification process.
2.2. Molecular Characterization of Antifungal Lipopeptides
Lipopeptide producing genes are well characterized and studies on specific lipopeptides can be done with great efficiency by a PCR based approach using specific primer pairs [56,57]. Some of the validated primer sets are listed in Table 1. The presence and expression of these lipopeptide producing genes can be assessed by PCR and real time PCR (qPCR) based studies, respectively. A large population can be screened quickly using these kinds of primer sets for the presence of different lipopeptides producing genes. The expression of these genes during antagonism is determined by RT-PCR. RNA is isolated under different treatment conditions. The purity and concentration of isolated RNA isolates are determined by absorbance recording at 260/280 nm or using nanodrop and are diluted to a common concentration in all the treatments. The RNA is then converted to cDNA, which is further used as a template in RT-PCR flowing standard protocols. After PCR amplification, the PCR products are loaded on to 1.2% agarose gel and the eluted products were applied for Sanger’s dideoxy sequencing and similar sequence can be obtained by using ‘BLAST’ (Basic Local Alignment Search Tool) available in NCBI.
Table 1.
List of various lipopeptides encoding genes and their respective primer set for PCR amplification.
| S.No. | Lipopeptide Class | Gene | Primer Name | Primer Sequences (5′-3′) | Reference |
|---|---|---|---|---|---|
| 1. | Surfactins | ||||
| (a) | Surfactin | sfP | SFP-F1 SFP-R1 |
ATGAAGATTTACGGAATTTA TTATAAAAGCTCTTCGTACG |
[58] |
| (b) | Surfactin | Srfc | Sur3f Sur3r |
ACAGTATGGAGGCATGGTC TTCCGCCACTTTTTCAGTTT |
[57] |
| (c) | Surfactin | SrfA-A | srfAA-Fw srfAA-Rv |
AAAGGATCCAGCCGAAGGGTG TCATGGT AAAAAGCTTGTTTTTCTCAAAGAACCAGCG |
[59] |
| (d) | Surfactin | srfAA (Surfactin synthetase subunit 1) | SRFAF SRFAR |
TCGGGACAGGAAGACATCAT CCACTCAAACGGATAATCCTGA |
[60] |
| (e) | Surfactin | srfA | F3726 R3879 |
GAAGTCTTCAGCGGCGAACTG GGGTGGCTCCGTTTTTCTCG |
[56] |
| (f) | Surfactin | srfDB | SUR3F SUR3R |
ACAGTATGGAGGCATGGTC TTCCGCCACTTTTTCAGTTT |
[61] |
| 2. | Iturins | ||||
| (a) | Iturin A | ItuD | ItuD1f ItuD1r |
GATGCGATCTCCTTGGATGT ATCGTCATGTGCTGCTTGAG |
[57] |
| (b) | Iturin A | Itu-C | ituC-Fw ituC-Rv |
AAAGGATCCAAGCGTGCCTTTTACGGGAAA AAAAAGCTT AATGACGCCAGCTTTCTCTT |
[59] |
| (c) | Iturin | ituC (Iturin A synthetase C) | ITUCF ITUCR |
GGCTGCTGCAGATGCTTTAT TCGCAGATAATCGCAGTGAG |
[60] |
| 3. | Fengycins | ||||
| (a) | Fengycin | FenD | FenD1f FenD1d |
TTTGGCAGCAGGAGAAGTTT GCTGTCCGTTCTGCTTTTTC |
[62] |
| (b) | Fengycin | fenD (Fengycin synthetase) | FENDF FENDR |
GGCCCGTTCTCTAAATCCAT GTCATGCTGACGAGAGCAAA |
[60] |
| (c) | Fengycin | FenE | FenEF FenER |
GTTTCATGGCGGCGAGCACG GATTCGCGGGAAGCGGATTGAGC |
[62] |
| (d) | Fengycin | Fen | Af2-F Tf1-R |
GAATAYMTCGGMCGTMTKGA GCTTTWADKGAATSBCCGCC |
[63] |
| 4. | Bacillomycins | ||||
| (a) | Bacillomycin | bmyB (Bacillomycin L synthetase B) | BMYBF BMYBR |
GAATCCCGTTGTTCTCCAAA GCGGGTATTGAATGCTTGTT |
[60] |
| (b) | Bacillomycin D | BamC | Bacc1f Bacc1r |
GAAGGACACGGAGAGAGTC CGCTGATGACTGTTCATGCT |
[57] |
| (c) | Bacillomycin D | bam D | ITUD-F1 ITUD-R1 |
TTGAAYGTCAGYGCSCCTTT TGCGMAAATAATGGSGTCGT |
[64] |
| 5. | Bacilysin | ||||
| (a) | Bacilysin |
bacAB |
BACD-F1 BAMD-R1 |
AAAAACAGTATTGGTYATCGCTGA CCATGATGCCTTCKATRCTGAT |
[65] |
| (b) | Bacilysin |
bacAB |
BACAB-F1 BACAB-R1 |
CTTCTCCAAGGGGTGAACAG TGTAGGTTTCACCGGCTTTC |
|
| 6. | Ericin | ||||
| Ericin | eriB |
eriBF
eriBR |
GAWKNACWCCWTWTGG CCRCCATATCSWTMTRYYTC |
[66] | |
| 7. | Mersacidin | ||||
| (a) | Mersacidin | mrsA | MRSA-F1 MRSA-R1 |
GGGTATATGCGGTATAAACTTATG GTTTCCCCAATGATTTACCCTC |
[67] |
| (b) | Mersacidin | mrsM | MRSM-F1 MRSM-R1 |
AAATGACCCGGCATATGTTC TGCTGACTAACTGGAATTGGAA |
|
| 8. | Mycosubtilin | ||||
| (a) | Mycosubtilin | fenF | ITUD-F1 ITUD-R1 |
TTGAAYGTCAGYGCSCCTTT TGCGMAAATAATGGSGTCGT |
[68] |
| (b) | Mycosubtilin | mycC | MYCC-F1 MYCC-R1 |
AATCAATTGGCACGAACCTT ATCGCCCGTTTTGTACATTC |
|
| 9. | Zwittermicins | ||||
| (a) | Zwittermicin A | Zwit | ZWITF2 ZWITR1 |
TTGGGAGAATATACAGCTCT GACCTTTTGAAATGGGCGTA |
[61] |
| 10. | Kurstakins | ||||
| (a) | Kurstakins | Kur | Aks-F Tks-R |
TCHACWGGRAATCCAAAGGG CCACCDKTCAAAKAARKWATC |
[69] |
Where H denotes A or C or T, W-A or T, R-A or G, D- A or G or T, K- G or T, Y-C or T.
3. Different Classes of Lipopeptides
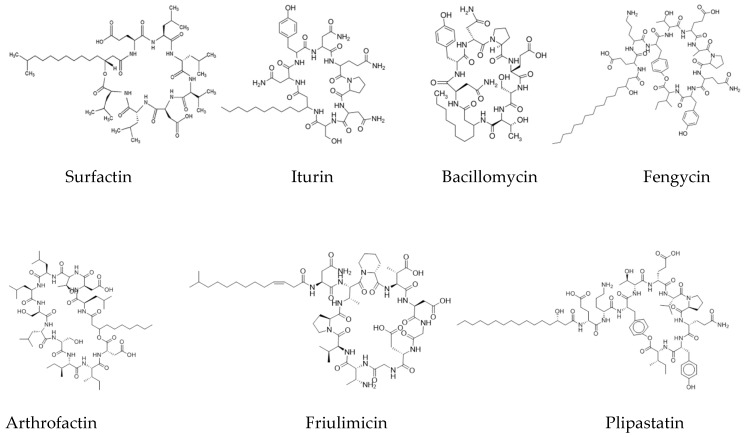
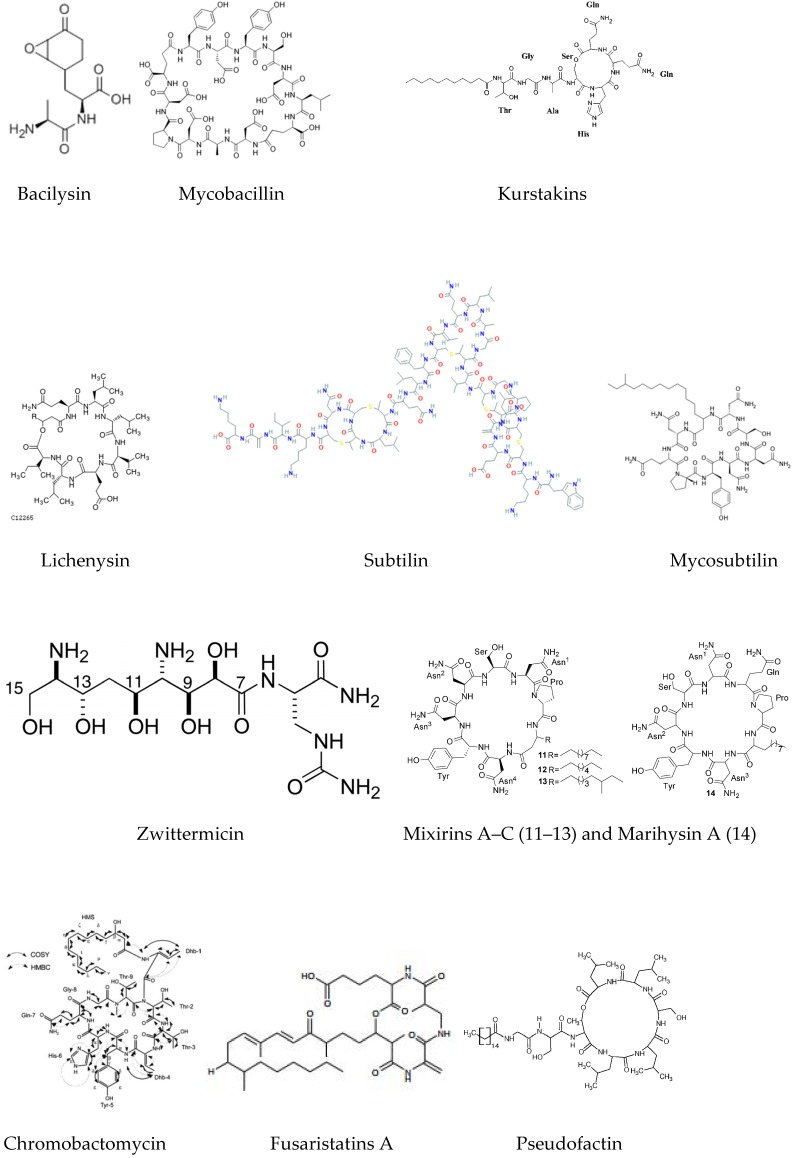
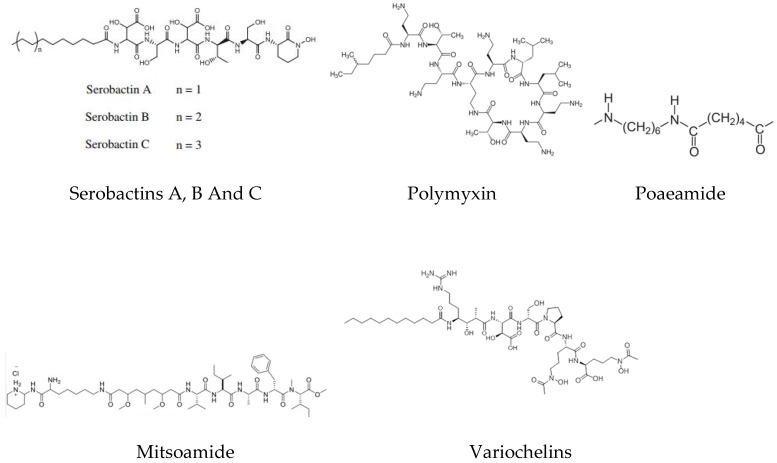
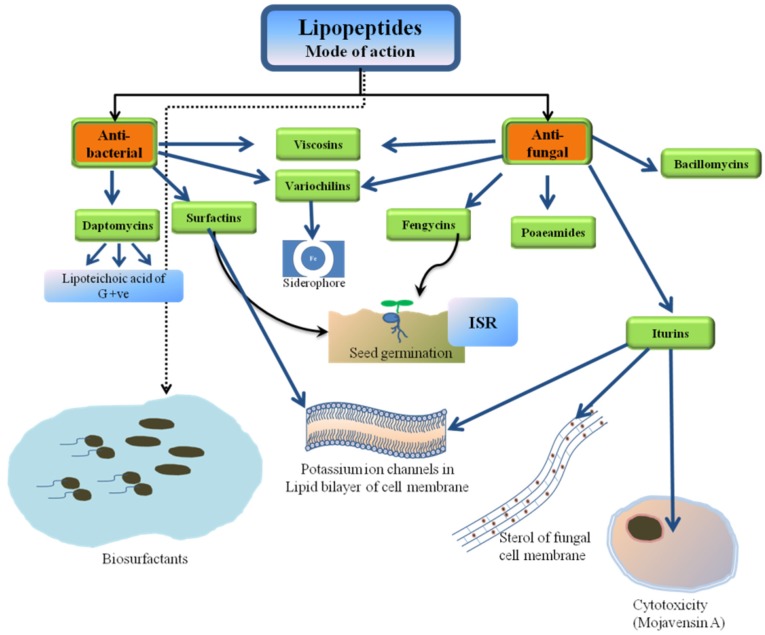
Lipopeptides are a group of microbial surfactants such as surfactin, lichenycin, iturin, and fengycin [70]. From previous studies, lipopeptides from the surfactin, iturin, and fengycins families are characterized in Figure 2. These are majorly produced by Bacillus spp., including B. subtilis, B. amyloliquefaciens, B. licheniformis, B. globigii, B. pumilus, B. cereus, B. megatarium, and B. thurigiensis [71,72,73,74]. Based on amino acid sequences, they have been broadly classified into three major groups: surfactins, iturins, and fengycins. The following are major classes of lipopeptides.
Figure 2.
Chemical structures of some important lipopeptides produced by microbial inoculants in the natural ecosystem. Source: PubChem (https://pubchem.ncbi.nlm.nih.gov).
3.1. Iturins
The antifungal cyclic lipopeptide produced by Bacillus spp. is low molecular weight lipopeptide iturin. Iturin have antimicrobial potential against plant pathogens [75]. The biopesticide and fungicidal properties of iturins are realized by interacting with sterol components of the cell membrane of phytopathogenic fungus. Compounds of the iturin family are characterized by a peptide ring of seven amino acids, which shows high polymorphism resulting in varied biological and physico-chemical properties. The vast diversity of this family includes iturin A, iturin C, iturin D, iturin E, bacillomycin D, bacillomycin F, bacillomycin Lc, mojavensin A, and mycosubtilin [76]. Iturin A causes potassium ion-conducting channels in lipid bilayers as a mechanism of antagonism. Some of the members like mojavensin A, however, show cytotoxic activities [77].
3.2. Surfactins
Surfactins showed high antifungal and antibacterial activities and constitute one of the major classes of antibiotic lipopeptides produced by Bacillus spp. [19,78]. It is biosurfactant and important for biofilm production and its stability. Produced by Bacillus subtilis, it has been proven to be cidal to the bacterial phytopathogen, Pseudomonas syringae pv. tomato in Arabidopsis [79]. In the structure of surfactins, a cyclic lactone ring is formed which contains amphipathic cyclic lipoheptapeptide of Glu-Leu-Leu-Val-Asp-Leu-Leu (ELLVDLL) fatty acid chain and another sequence chiral to this (LLDLLDL) which is interlinked with 12-16-C β-hydroxy [80]. A change in the size and order of amino acids alsocauses variation in the lipid portion [81]. The amino acids and β-hydroxy fatty acids in a given surfactin not only vary with the producer bacterial strain, but the culture conditions also contribute to the diversity [82]. Surfactins affect the lipid bilayer of membranes and thus, are effective against both Gram-positive and Gram-negative bacteria. It is also thought to have anti-mycoplasma, antiviral, and antitumor activities and canalso suppress inflammatory responses through the inhibition of phospholipase A2 [82,83]. In biocontrol, it cansuppress phytopathogens like Pseudomonas syringae, Xanthomonas axonopodis, Sclerotinia sclerotium, Botrytis cinerea, Colletotrichum gloeosporioides, and also activated ISR [84,85].
3.3. Fengycins
Fengycins are a family of lipopeptides produced by members of genera Bacillus and Paenibacillus. They have antifungal activity and affect filamentous fungi [86], the most important group of phytopathogens. Structurally, fengycins are decapeptides, with14-19-C attached to a β-hydroxy fatty acid chain exhibiting strong antifungal activity [87,88]. This third family of lipopeptides is also known as plipastatin. They arealso involved in the triggering systemic response against plant pathogens. There are two classes of Fengycins—Fengycin A and Fengycin B. Both classes differ from each other by the amino acid attached at position 6. Fengycin A contains Ala at position 6, whereas Fengycin B contains Val in this position.
3.4. Pseudofactins
These are a class of lipopeptides produced by Pseudomonas fluorescens. There are of two major types—Pseudofactin I and Pseudofactin II. This class has got a huge therapeutic usage, especially Pseudofactin II, which has got antiadhesive properties [89]. Itsgreater emulsification activity and its stability have made it a compound of choice over other synthetic surfactants; therefore, it is considered potent in bioremediation. Structurally, Pseudofactins are cyclic octapeptides attached to palmitic acid [75].
3.5. Viscosins
Viscosins are also obtained from Pseudomonas fluorescens and have antibacterial and antifungal activity. The specific feature of viscosins is itshigh surface-activeness, which can inhibit the migration of cancer cells. In the case of Pseudomonas, viscosins increase the efficiency of plant roots and also have protective roles for germinating seedlings against plant pathogens [90].
3.6. Daptomycins
Daptomycins are a newer class of lipopeptide antibiotics approved by the US FDA and are effective against Gram-positive bacterial infections [91]. Structurally, daptomycins are a cyclic decanoyl lipid chain with 13 amino acid peptides. Theyexhibit broad-spectrum activity against an array of Gram-positive bacteria such as Staphylococcus, Streptococcus, Pneumococcus, Clostridium, and Enterococcus. The source microorganism for the production of daptomycin is actinobacterium Streptomyces roseosporus [91]. It inhibits the synthesis of lipoteichoic acid and disrupts bacterial membrane potential (depolarization) by the formation of pores which provides itsantimicrobial, antiparasitic, and immuno-suppressor properties. The functioning of daptomycin is calcium ion-dependent [91].
3.7. Poaeamides
Poaeamides are known fortheir diverse capability like swarming, biofilm formation, and regulation of attachment-detachment to plant roots, etc., apart from antifungal activity against plant pathogens like Rhizoctonia solani causing damping-off [92,93]. It is produced by Pseudomonas spp. [94] and has got potential pharma and biocontrol properties.
4. Biocontrol Potential of Lipopeptides
Almost all of the Bacillus species produce antimicrobial compounds known as lipopeptides and several strains of B. subtilis and B. amyloliquefaciens are reported to produce lipopeptides. Gong et al. [95] and Qian et al. [96] reported that the crude lipopeptides are stable to heat, pH, and showed high capability as biocontrol agents against various pathogens [97]. Mora et al. [60] observed that the antagonistic activity between plant-associated Bacillus and phyto-pathogens are related to the presence of cyclic lipopeptide genes. However, natural Bacillus strains play an important role in the production of different concentrations of each lipopeptide and thus are crucial for their interaction with plant as well as biofilm formation interaction with plants and the production of biofilms [85,98]. It has been reported that lipopeptides have shown potential antagonistic activity against disease causing bacteria and fungi in vitro and in planta conditions [99]. Cho et al. [100] reported that the Bacillus pumilus strain HY1 hasshown strong biocontrol activity against harmful aflatoxin producing fungi A. flavus and A. parasiticus suppress the fungal species Aspergillus flavus and A. parasiticus, which areproducers of potentially harmful aflatoxins. There are a few key mechanisms discussed in this section by which lipopeptides show antimicrobial activities (Table 2).
Table 2.
Different lipopeptides, their source microorganisms, and the nature of antimicrobial activity.
| S. No. | Source Organism | Lipopeptide Class/Type | Activity/Action | References (Not to Be Attended) |
|---|---|---|---|---|
| 1. | Actinoplanes friuliensis | Friulimicin | Broad range of multi-resistant Gram-positive bacteria | [101] |
| 2. | Arthrobacter spp. MIS38 | Arthrofactin | Bio-surfactant | [102] |
| 3. | Bacillus subtilis | Iturin A, Bacillomycin, Fengycin, Bacillomycin | Antifungal | [57] |
| 4. | B. subtilis HC8 | Surfactin, Fengycin A, Fengycin B, Iturin A, | Antifungal | [52] |
| 5. | B. subtilis K1 | Surfactin, Iturin, Fengycin A and B, Fengycin A2 and B2 | Antifungal | [103] |
| 6. | B. subtilis GA1 | Iturins, Fengycins, Surfactins | Antifungal | [104] |
| 7. | B. subtilis M4 | Fengycin A and B | Antifungal | [30] |
| 8. | B. subtilis B-FS01 | Fengycin | Antifungal | [105] |
| 9. | B. subtilis and B. amyloliquefaciens | Fengycin, Iturins, Surfactins, Bacillomycin | Antibaterial | [60] |
| 10. | B. subtilis SPB1 | Surfactin, Fengycin, Iturins | Antifungal | [106] |
| 11. | B. subtilis EBS05 | Surfactin A | Antifungal | [107] |
| 12. | B. subtilis B49 | Fengycin, Bacillomycin D | Antifungal | [61] |
| 13. | B. subtillis ATCC 13952 | Fengycin | Antifungal | [61] |
| 14. | B. subtillis DF-HO8 | Fengycin | Antifungal | [61] |
| 15. | B. subtilis CMB32 | Iturin A, Fengycin, Surfactin A | Antifungal | [108] |
| 16. | B. subtilis (Marine) | Surfactins and Fengycins | Delayed Germination | [109] |
| 17. | B. subtilis B1 | Iturin C, Surfactin, Fengycin A and B, Bacillomycin D, Bacilysin, Mycobacillin | Antifungal | [110] |
| 18. | B. subtilis JA | Surfactin, Iturin, and Fengycin | Antifungal | [111] |
| 19. | B. subtilis 9407 | Fengycin | Antifungal | [112] |
| 20. | B. subtilis HC8 | Fengycin A and Fengycin B | Antifungal | [52] |
| 21. | B. subtilis S499 | Surfactin, Fengycin A, and Fengycin B | Antifungal | [30] |
| 22. | B. subtilis fmbj | Fengycin A and Fengycin B | Antifungal | [113] |
| 23. | B. subtilis EPCO16 | Iturin A, Surfactin, Zwittwermicin A, Bacillomycin D | Antifungal | [114] |
| 24. | B. subtilis 6051 | Surfactin | Antibacterial activity against P. Syringae | [79] |
| 25. | B. subtilis M4 | Iturin/Fengycin | Antifungal activity against Pythium ultimum causing Damping-off disease of Beans | [30] |
| 26. | B. subtilis M4 | Fengycin | Antifungal activity against Botrytis cinerea causing Gray mold disease of Apples | [30] |
| 27. | B. subtilis | Iturin/Fengycin | Antifungal activity against podosphaera fusca causing Powdery mildew of Cucurbits | [97] |
| 28. | B. subtilis JA; JA026 | Fengycin | Antifungal activity against Gibberella zeae (anamorph of Fusarium graminearum) Fusarium causing head blight (FHB) in Wheat and Barley and Ear rot in Corn | [115] |
| 29. | B. subtilis B-FS01 | Fengycin | Antifungal activity against Fusarium moniliforme causing Seedlingblight, Stalk rot, and Ear rot | [105] |
| 30. | B. subtilis BBG127 and BBG131 | Cyclic lipopeptides | Antifungal activity against Botrytis cinerea 630 causing Necrosis of Grapevines | [116] |
| 31. | B. subtilis 9407 | Fengycin | Antifungal activity against Botryosphaeria dothidea causing Apple ring rot | [112] |
| 32. | B. subtilis GA1 | Iturin, Fengycin, Surfactin | Antifungal activity against Botrytis cinerea causing Grey mould disease of Apples | [104] |
| 33. | B. amyloliquefaciens ES-2 | Fengycin, Surfactin | Antibacterial/anti-fungal | [53] |
| 34. | B. amyloliquefaciens TF28 | Iturin A | Antifungal | [54] |
| 35. | B. amyloliquefaciens ARP23 and MEP218 | Surfactin C15, Fengycins A, Iturin A | Antifungal activity against Sclerotinia sclerotiorum | [59] |
| 36. | B. amylolequifaciens S499 | Fengycin, Iturins, Surfactin | ISR | [117] |
| 37. | B. amyloliquefaciens 32a | Surfactin, Iturin A, Bacillomycin D, Fengycin | Antimicrobial | [118] |
| 38. | B. amyloliquefaciens CC09 | Fengycin, Iturin, Surfactin, Bacillomycin | Antifungal | [18] |
| 39. | B. amyloliquefaciens BO7 | Surfactin | Antifungal | [119] |
| 40. | B. amyloliquefaciens subsp. plantarum SV65 | Fengycin | Antifungal activity | [120] |
| 41. | B. amyloliquefaciens MEP218 | Iturin, Fengycin, Surfactin | Antibacterial, Antifungal | [121] |
| 42. | B. amyloliquefaciens KPS46 | Surfactin | Antibacterial activity against Xanthomonas axonopodis pv. glycines | [122] |
| 43. | B. amylolequifaciens | Lipopeptides, Surfactin, Iturin, Fengycin | Antiviral ActivityagainstRhizomania, an important disease of Sugarbeet | [117] |
| 44. | B. mojavensis RRC101 | Leu7-Surfactin | Antifungal | [123] |
| 45. | B. mojavensis A21 | Surfactin, Fengycin, Pumalicidin | Antimicrobial, Antifungal | [124] |
| 46. | B. mojavensis RRC101 | Surfactin, Fengycin | Antifungal | [125] |
| 47. | B. licheniformis | Lichenysin | Bio-surfactant | [124] |
| 48. | B. licheniformis | Surfactins, Lichenysins | Bio-surfactant | [126] |
| 49. | B. pumilus HY1 | Iturins | Antifungal | [100] |
| 50. | B. pumilus (Marine) | Pumilacidin | Antibacterial activityagainst Staphylococcus aureus | [127] |
| 51. | B. thuringiensis CMB26 | Fengycins | Fungicidal, Bactericidal, andInsecticidal activity | [128] |
| 52. | B. thuringiensis kurstaki HD-1 | Kurstakins | Antifungal activity against Stachybotrys charatum | [129] |
| 53. | B. thuringiensis kurstaki | Kurstakin | Antifungal activity | [130] |
| 54. | B. vallismortis R2 | Surfactins, Iturins, Fengycins | Antifungal activity against Alternaria alternate causing Black point disease of Wheat | [131] |
| 55. | B. cereus DFE4 | Surfactin, Iturin A, Bacillomycin D | Antifungal | [132] |
| 56. | B. methyltrophicus TEB1 | Iturin A, Fengycin, Surfactin | Antifungal | [133] |
| 57. | B. methylotrophicus HC51 | Iturin A, Fengycin | Antifungal | [134] |
| 58. | Bacillus sp.C3 | Iturin A, Surfactin, Subtilosin, Subtilin | Antifungal | [135] |
| 59. | Bacillus sp.BmB9 | Surfactin, Iturin, Fengycin | Antifungal, Antibacterial | [136] |
| 60. | Bacillus sp. FJAT-14262 | Surfactin | Antifungal | [137] |
| 61. | Bacillus sp. CY22 | Iturin | Antifungal | [138] |
| 62. | Bacillus sp. NH-100 | Surfactin A | Antifungal | [139] |
| 63. | Bacillus sp. | Iturin A, Surfactin, Zwittermicin A, Bacillomycin D | Antifungal activity against Fusarium oxysporum f.sp. lycopersici causing Wilt in Tomato | [114] |
| 64. | Bacillus sp. (Marine) | Mixirins A, B, and C | Cytotoxic | [77] |
| 65. | Chromobacterium sp. C61 | Chromobactomycin | Antifungal activity against Magnoporthe grisea causing Rice Blast | [140] |
| 66. | Fusarium sp. YG-45 | Fusaristatins A and B | Antimicrobial | [141] |
| 67. | Fusarium decemcellulare LG53 | Fusaristatin A | Mildantimicrobial | [142] |
| 68. | Geitlerinema sp.(Marine cyanobacterium) | Mitsoamide | Cytotoxic activities | [77] |
| 69. | Herbaspirillum seropedicae Z67 | Serobactins A, B, and C | As aniron source | [143] |
| 70. | Pseudomonas fluorescens 96.578 | Tensin | Antifungal activity against Rhizoctonia solani causing Sugarbeet seed infection | [144] |
| 71. | P. fluorescens BD5 | Pseudofactin II | Anti-adhesive activity | [89] |
| 72. | P. fluorescens SS101 | Massetolide A | Systemic resistance (Late Blight) | [45] |
| 73. | Pseudomonas poae RE*1-1-14 | Poaeamide | Antifungal activity against Rhizoctonia solani causing Damping off and Rootrot in Sugarbeet | [93] |
| 74. | Pseudomonas sp. UCMA 17,988 (Isolated from Bovine raw milk) | Milkisin | Antimicrobial activity against Listeria monocytogenes, Staphylococcus aureus, and Salmonella enteric | [145] |
| 75. | Paenibacillus polymyxa M-1 | Polymyxin, Fusaricidin | Suppress phytopathogenic Erwinia spp. | [146] |
| 76. | Paenibacillus sp. IIRAC-30 | Surfactin | Antifungal | [147] |
| 77. | Scopulariopsis brevicaulis (Marine sponge-derived) | Scopularides A and B | Cytotoxic activities | [77] |
| 78. | Streptomyces canus | Amphomycins | Inhibit bacterial cell wall synthesis | [148,149] |
| 79. | Streptomyces viridochromogenes | Laspartomycins | Antibacterial, Antiherpes activity | [150] |
| 80. | Variovorax boronicumulanss BAM-48 | Variochelins A and B | Siderophore production | [151] |
4.1. Lipopeptides as Biosurfactants Distressing Membrane Integrity and Permeability
Lipopoetide surfactants exhibited a unique pore and ion channels forming property thus may disturb the normal integrity and permeability of lipid bilayer of cell membrane [152]. This ability of disrupting the structural integrity of the biological membrane establishes their primary mode of antimicrobiotic action against bacteria, fungi, virus, mycoplasma, etc. [153]. Surfactins, which are one of the most potent biosurfactants, get inserted into the lipid bilayer after dimerization, chelate mono and divalent positively charged ions disturb the membrane permeability due to trans-membrane ion influxes and, finally, cause cell death as a result of cell disruption [153,154,155,156].
Iturins are strongly mycotoxic against a broad range of yeasts and fungal pathogens including A. flavus and R. solaniin vitro, but possess limited antibacterial and no activity against viruses [77,157,158,159]. Although iturins function through their membrane permeabilization properties, their mode of action is slightly different from surfactins [28]. Iturins cause osmotic disturbance by forming ion-conducting pores in the membrane without disrupting or solublizing them, unlike surfactins [160]. Fengicins are potent antifungal agents which act specifically against filamentous fungi, including phytopathogenic ones, viz. Plasmodiaophora monoliforme, Fusarium moniliforme, Fusarium gramineareum, and Podosphera fusca [161,162,163]. Their interaction with the lipid bilayer depends upon their concentration and they lead the alteration of membrane structural integrity and permeability [164].
Studies using scanning electron and optical microscopy revealed that B. thuringiensis CMB26 derived antibiotic lipopeptide affected the cell surface of plant pathogenic fungus, Colletotrichum gloeosporioides, E. coli O157, and Pieris rapae crucivora (cabbage white butterfly larvae) by acting on plasma membrane [128]. Pseudomonas syringae pv. syringae derived cyclic lipodepsipeptides, Syringopeptin, and Syringomycin also exhibited ion channel formation and lytic against plant and human cells due to their membrane-permeabilizing properties [165,166,167]. A fungal cyclohexadepsipeptides enniatin, derived from Fusarium sp. Verticillium and Halosarpheia, acts as an ionophore which first gets incorporated in the cell membrane and leads to the intracellular ion leakage and cation specific pores formation. This may establish the basis of the mechanism of actionof enniatin as a antimicrobial, anthelmintic, anticancer, and enzyme inhibiting agent [168].
Disruption of the integrity and permeability of biological membranes due to thepore and ion channels forming ability of lipopeptide surfactants has been established as the most important mode or mechanism of their action for explaining their applications as antibacterial, antifungal, antitumor, and hemolytic agents in the agriculture, biomedical, pharmaceutical, and therapeutic sectors. Moreover, the synergistic effect of various lipopeptides (mainly surfactins, iturins, and fengycins) showed antiadhesive activity, resulting in the reduction of colonization and stimulating biofilm dispersion of pathogenic bacteriadue to their amphiphilic surfactant like property [169]. The biosurfactant property of various lipopeptides is important in prohibiting biofilm formation. These biosurfactants are amphiphillic molecules that not only inhibit biofilm formation, but also dislodge the existing biofilm. Two Pseudomonas fluorescens lipopeptides putisovin I and II are reported to suppress the biofilm formation and breakdown of existing biofilm [170]. Abdallah et al. [171] reported the suppression of Agrobacterium tumefaciens biofilm by a mixture of lipopeptides produced by Bacillus amyloliquifaciens. The mixture maybe able to inhibit tumor formation upon pathogen adhesion on a tomato stem. In this experiment, the formation of new biofilm was also inhibited and the old ones dislodged. In agriculture, antiadhesive properties of various lipopeptides biosurfactants may also be used to reduce the attachment of phytopathogens on the plant surface and thus, their colonization efficiency, which is very crucial for the development of various plant diseases.
4.2. Lipopeptides as Siderophores
Iron is an essential nutrient that is required for normal functioning of important physiological processes including biological nitrogen fixation, transportation of oxygen, methane production, and DNA biosynthesis [172]. Microbes, including many bacteria and fungi, produce siderophores, a class of low molecular weight compounds with astrong tendency to form complexes with inorganic iron ions, thus making it biologically available to carry out cellular functions [173,174]. Several lipopeptides are also known to function as siderophores. Variochelins, a class of photoreactive lipopeptide siderophores, are mainly produced by specific genera of marine bacteria, viz. Halomonas, Marinobacter, Ochrobactrum, Synechococcus, and Vibrio [175,176,177,178]. Using a genome mining strategy, Variovorax boronicumulans BAM-48, a plant-associated terrestrial bacterium wasalso found to produce variochelins [151]. These siderophores support the growth of the producing organism by making important nutrients such as iron biologically available to them and also play a crucial role in determining the microbial community structure [179,180]. Some lipopeptide siderophores also act as chemical mediators for bacteria−algal interactions in the ocean [181]. These molecules have iron-binding α-hydroxycarboxylate ligand groups triggering a ligand-to-metal charge transfer reaction by absorbing photons in UV light, making iron available to surrounding microalgae, which, in turn, provides organic matter to the siderophore producing bacteria [181,182,183,184,185]. This mutualism has important ecological implications [186].
Moreover, Herbaspirillum seropedicae Z67 (class Betaproteobacterium), a N2 fixing and growth promoting endophyte, inhabiting many important crop has been reported to secrete a class of amphiphillic lipopeptidal siderophore called serobactins [187,188,189,190]. Serobactins employs a similar mechanism of increasing the bioavailability of inorganic iron common to many siderophores produced by aquatic bacteria [173]. The lipopeptide siderophores producing plant-associated bacteria can significantly influence the microbial community structure and thus, contribute to plant growth and health by modulating plant-microbe as well as plant-pathogen interactions.
4.3. Lipopeptides ISR-Inducer in Plants
Some beneficial bacteria indirectly protect plants from disease-causing microbes through the stimulation of inducible defense mechanisms which are systemic in nature and called ISR, which are effective in the management of several plant diseases [191]. Lipopeptides also showed antifungal activity and are involved in ISR activation and defense responses [116]. They are less toxic, biodegradable, and environmentally-friendly, with a broad range of target phytopathogens and, thus, have huge potential for plant diseases management. Many strains of Bacillus spp. strains are known to induce defense responses in plants, but knowledge about the molecular determinants of the Bacillus mediated ISR is very limited [36,192]. Ongena et al. [46] showed that surfactins and fengycins lipopeptides protect bean and tomato plants through ISR and, thus, represent a novel class of microbial-associated molecular patterns (MAMPs) which play an important role in the activation of the defense signalling pathway. They also revealed that surfactin and fengycin encoding genes when overexpressed in Bacillus subtilis strain 168 (poor producer) elevated the ISR potential of derivatives in tomato and bean plants. Moreover, increase in the activity of the main enzymes of the lipoxygenase pathway were observed in resistant plants when challenged with lipopeptide overproducers. They also hypothesized that surfactins and fengycins should have a distinct mechanism for ISR-induction in plants.
Surfactin and fengycin caused ISR induction in the plants against phytopathogenic fungi, but showed varying responses in different plant cell types [28]. Now, it is well established that surfactin is crucial for ISR induction, colonization of root and biofilm formation and extracellular matrix formation in B. subtilis [193]. The binding of surfactin molecules to the plant cell membrane is mainly responsible for ISR-induction [194]. Enhanced resistance has been reported againstgrey leaf spot disease caused by Magnaporthe oryzae due to H2O2-mediated defense responses sensitized by ISR responses elicited by semi-purified surfactin lipopeptides in perennial ryegrass [195]. The Pseudomonas-derived cyclic lipopeptide orfamide was shown to act as an ISR elicitor, triggering early defense events and inducing expression of defence related genes without causing cell death in rice against the brown spot disease fungus Cochliobolus miyabeanus [196]. Endophytic bacteria showed antifungal activity, which played an important role indefending plants against invading fungal pathogens. Endophytic Bacillus sp. has been utilized in protecting maize and horse-bean fungal pathogens. Gond et al. [57] reported that through lipopeptide production, the maize associated symbiotic bacteria directly inhibits the potential pathogens and also induce host defense gene activation against fungal pathogens. The possible mechanisms of lipopeptide mediated plant disease management are summarized in Figure 3.
Figure 3.
The possible mechanisms of lipopeptide mediated plant disease management.
5. Future prospects of Microbial Lipopeptides in Plant Disease Management
Cropdestruction by existing and emerging phytopathogens is an important issue of contemporary agriculture that needs to be addressed properly to optimize gains from this age-old enterprise. As the population continues to build up, losses due to plant diseases are emerging as a threat to global food security. Plant diseases affect agricultural produce not only quantitatively, but also qualitatively, hence affecting the gains from marketable produce at two distinct levels. Not only this, but food safety is also a concern that is related to microbial infestation of agricultural produce [197]. Many of the currently available antimicrobial products that areused in agriculture are highly toxic and non-biodegradable and, thus, cause extended environmental pollution. Moreover, increasing resistance to existing antimicrobial agents among the phytopathogens is also a matter of great concern for the future of agriculture [84,198]. On the other hand, extensive use of agrochemicals has disturbed the ecological balance by creating non-target toxicity, development of resistance among pathogens, contamination of reservoirs and groundwater, obvious health risks to humans and other living beings, and increases in the cost of cultivation. In such a situation, the challenge before microbiologists and plant pathologists in the future is to control the stronger pathosystems using environmentally-friendly alternatives. This would require exploring non-conventional and newer approaches to combat a variety of crop diseases [46]. Plant resistance has been exploited for along timeand in response, a large number of resistant strains of plant pathogens have been reported. The current scenario of antimicrobial agents involvesthe problem of toxic effects on non-target organisms, including human consumers and the environment, and this toxicity, coupled with low biodegradability, has made this solution poor [199] enough to search for alternatives.
Microbial agents for biological control are being explored extensively in different crops against an array of pathogens as they exhibit a wider range of antimicrobial activities. Some microbial agents directly act upon the pathogens by millions of antimicrobial compounds, nutrient quenching, competition for space, etc. Whereas, others interact with plants with long-distance signaling by eliciting defense responses using microbe-associated molecular patterns and their compounds or priming plants without making any direct interaction to the pathogen in question. These beneficial microbes modulate the growth condition of the surrounding region so that the pathogen could not thrive and develop in numbers to cause economically significant damage to plants.
Antagonistic microbes control the pathogens via hyperparasitism and antibiosis in direct interaction. This involves multiple mechanisms and compounds generated through highly regulated cascades. To use these antimicrobial metabolites for the control of pathogens on the field requires a stringent and exhaustive registration process to avoid any non-target effects on the other constituents of the environment. In this aspect, currently, risks associated with antagonistic microbial metabolites are often assessed similar to that of single-molecule fungicides. Since the nature of the compound and mode of actions are different, thisrequires a re-thinking of data requirements for the registration of microbial agents as biopecticides. Endless research data indicated the enormous antimicrobial capabilities of the genus Bacillus, Pseudomonas, and Trichoderma. These three genera are considered as factories for the production of biologically active molecules which are potent growth inhibitors of phytopathogens. Lipopeptides, a subclass of antimicrobial peptides, arenow emerging as an attractive alternative for the development of new peptide-based biopesticides [84,200]. The usefulness of lipopeptides is being evaluated as a potent versatile weapon for the control of a wide range of phytopathogens. The three broad families of Bacillus lipopeptides, surfactins, iturins, and fengycins, along with other new groups of antimicrobial lipopetides are being explored for their effectiveness against a wide range of phytopathogens including bacteria, fungi, and viruses [84]. Shafi et al. [201] advocated that the Bacillus species has numbers of antagonizing attributes against plant pathogens including the production of lipopeptides, enzymes, and plant growth promotion. Hazarika et al. [202] studied the role of lipopeptides produced by leaf endophytes against 10fungal species.
Apart from bacterial lipopetides, fungi-derived lipopeptide antibiotics are also gaining importance. These lipopetides have four categories: cyclic depsipeptides, peptaibiotics, non-depsipeptide cyclic lipopeptides, and non-peptaibiotic linear lipopeptides [203,204]. These compounds have greater future significance due to their varied bioactivity and diversity. The major fungal genera producing lipopetides are Acremonium, Aspergillus, Alternaria, Metarhizium, Beauveria, Fusarium, Penicillium, etc. They exhibit cytotoxic, antimicrobial, antiviral, insecticidal, antitumoral, and enzyme-inhibitory activities which could be potential weapon against plant pathogens of economic importance [203]. Fungi derived antimicrobial lipopetides like peptaibols, lipoaminopeptides, lipopeptaibols, echinocandins, aspochracins, etc. could be tested against new and emerging pathosystems for preparing biofungicides of tomorrow.
This could open an entire new arena of biopesticides since the whole of society is concerned about green chemicals. These biodegradable biosurfactants could be environmentally-friendly with low toxicity andalternatives to highly toxic synthetic chemical pesticides [205]. The implications of overwintering and the resting stages of pathogens could also be worked out to reduce the inoculum loads from croplands. The microbial strain may be engineered for the novel structure of surfactin production [206]. Further, advancement in the bioinformatics assisted molecular and dynamics simulation studies can also help to decipher the molecular and biochemical mechanisms. Moreover, several reports have already been initiated on the structure prediction and their possible interaction with different cellular protein/enzyme targets [207,208,209,210]. Cob-Calan et al. [211] demonstrated the interactions of the cyclic lipopeptides iturin A, fengycin, and surfactin with β-tubulin using molecular docking and molecular dynamics simulation. A comparative study had shown that iturin A and fengycin had higher binding affinity as compared to surfactin for the catalytic site of β-tubulin [210,211]. With the help of advancements in genetic engineering and synthetic biology, improved lipopeptide production with higher efficiency could be developed, targeting important pathosystems. The work on reducing the cost of industrial production is also an area to be explored with greater efficiency. With all these initiatives, the lipopeptides of microbial origin could form a useful and wider base to combat the plant pathogens of today and tomorrow.
Acknowledgments
Authors gratefully acknowledge their respective institutions for providing the necessary facilities.
Author Contributions
P.K.S., U.B.S., H.V.S. and G.P.B. conceived the idea and designed the layout of manuscript. A.R.G. collected the information form different resources. P.K.S., U.B.S., H.V.S. and S.P. curated the informations and analysed the content. D.M., S.P., S.S., A.G., and M.K. wrote the original draft. D.P. and J.P.R. edited the final version of manuscript. D.M. and S.S. corrected the proof. U.B.S., P.K.S. and S.P. proof read the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Brahmaprakash G.P., Sahu P.K. Biofertilizers for sustainability. J. Indian Inst. Sci. 2012;92:37–62. [Google Scholar]
- 2.Singh U.B., Malviya D., Singh S., Imran M., Pathak N., Alam M., Rai J.P., Singh R.K., Sarma B.K., Sharma P.K., et al. Compatible salt-tolerant rhizosphere microbe-mediated induction of phenylpropanoid cascade and induced systemic responses against Bipolaris sorokiniana (Sacc.) Shoemaker causing spot blotch disease in wheat (Triticum aestivum L.) Appl. Soil Ecol. 2016;108:300–306. doi: 10.1016/j.apsoil.2016.09.014. [DOI] [Google Scholar]
- 3.Singh U.B., Malviya D., Singh S., Pradhan J.K., Singh B.P., Roy M., Imram M., Pathak N., Baisyal B.M., Rai J.P., et al. Bio-protective microbial agents from rhizosphere eco-systems trigger plant defense responses provide protection against sheath blight disease in rice (Oryza sativa L.) Microbiol. Res. 2016;192:300–312. doi: 10.1016/j.micres.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Meena K.K., Sorty A.M., Bitla U.M., Choudhary K., Gupta P., Pareek A., Singh D.P., Ratna P., Sahu P.K., Gupta V.K., et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017;8:1–25. doi: 10.3389/fpls.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair S.S., Sahu P.K., Brahmaprakash G.P. Microbial inoculants for agriculture in changing climates. Mysore J. Agril. Sci. 2017;51:27–44. [Google Scholar]
- 6.Sahu P.K., Brahmaprakash G.P. Modified liquid dual culture methodology for screening bacterial endophytes against fungal pathogens. Mysore J. Agril. Sci. 2018;52:234–240. [Google Scholar]
- 7.Sahu P.K., Singh D.P., Prabha R., Meena K.K., Abhilash P.C. Connecting microbial capabilities with the soil and plant health: Options for agricultural sustainability. Ecol. Indic. 2019;105:601–612. doi: 10.1016/j.ecolind.2018.05.084. [DOI] [Google Scholar]
- 8.Sahu P.K., Singh S., Gupta A., Singh U.B., Brahmaprakash G.P., Saxena A.K. Antagonistic potential of bacterial endophytes and induction of systemic resistance against collar rot pathogen Sclerotium Rolfsii in tomato. Biol. Control. 2019;137:104014. doi: 10.1016/j.biocontrol.2019.104014. [DOI] [Google Scholar]
- 9.Singh U.B., Malviya D., Singh S., Kumar M., Sahu P.K., Singh H.V., Kumar S., Roy M., Imran M., Rai J.P., et al. Trichoderma harzianum-and methyl jasmonate-induced resistance to Bipolaris sorokiniana through enhanced phenylpropanoid activities in bread wheat (Triticum aestivum L.) Front. Microbiol. 2019;10:1697. doi: 10.3389/fmicb.2019.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh U.B., Singh S., Malviya D., Karthikeyan N., Imran M., Chaurasia R., Alam M., Singh P., Sarma B.K., Rai J.P., et al. Integration of anti-penetrant tricyclazole, signaling molecule salicylic acid and root associated Pseudomonas fluorescens enhances suppression of Bipolaris sorokiniana in bread wheat (Triticum aestivum L.) J. Plant Pathol. 2019;101:943–954. doi: 10.1007/s42161-019-00296-5. [DOI] [Google Scholar]
- 11.Gudina E.J., Teixeira J.A., Rodrigues L.R. Biosurfactants produced by marine microorganisms with therapeutic applications. Mar. Drugs. 2016;14:38. doi: 10.3390/md14020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitschke M., Costa S.G.V.A.O. Biosurfactants in food industry. Trends Food Sci. Technol. 2007;18:252–259. doi: 10.1016/j.tifs.2007.01.002. [DOI] [Google Scholar]
- 13.Pacwa-Płociniczak M., Płaza G.A., Piotrowska-Seget Z., Cameotra S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011;12:633–654. doi: 10.3390/ijms12010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sachdev D.P., Cameotra S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013;97:1005–1016. doi: 10.1007/s00253-012-4641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudina E.J., Rangarajan V., Sen R., Rodrigues L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013;34:667–675. doi: 10.1016/j.tips.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Shekhar S., Sundaramanickam A., Balasubramanian T. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015;45:1522–1544. doi: 10.1080/10643389.2014.955631. [DOI] [Google Scholar]
- 17.Peypoux F., Bonmatin J.M., Wallach J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 1999;51:553–563. doi: 10.1007/s002530051432. [DOI] [PubMed] [Google Scholar]
- 18.Cai X.C., Li H., Xue Y.R., Liu C.H. Study of endophytic Bacillus amyloliquefaciens CC09 and its antifungal CLPs. J. Appl. Biol. Biotechnol. 2013;1:1–5. [Google Scholar]
- 19.Stein T. Bacillus subtilis antibiotics: Structure, syntheses and specific functions. Mol. Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 20.Balan S.S., Kumar C.G., Jayalakshmi S. Aneurinifactin, a new lipopeptide biosurfactant produced by a marine Aneurinibacillus aneurinilyticus SBP-11 isolated from Gulf of Mannar: Purification, characterization and its biological evaluation. Microbiol. Res. 2017;194:1–9. doi: 10.1016/j.micres.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Arrebola E., Jacobs R., Korsten L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against post harvest fungal pathogens. J. Appl. Microbiol. 2010;108:386–395. doi: 10.1111/j.1365-2672.2009.04438.x. [DOI] [PubMed] [Google Scholar]
- 22.Cao X.H., Wang A.H., Wang C.L., Mao D.Z., Lu M.F., Cui Y.Q., Jiao R.Z. Surfactin induces apoptosis in human breast cancer MCF-7 cells through aROS/JNK-mediated mitochondrial/caspase pathway. Chem. Biol. Interact. 2010;183:357–362. doi: 10.1016/j.cbi.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Hirata Y., Ryu M., Oda Y., Igarashi K., Nagatsuka A., Furuta T., Sugiura M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, next term as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009;108:142–146. doi: 10.1016/j.jbiosc.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Cortes-Sanchez A.J., Hernandez-Sanchez H., Jaramillo-Flores M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013;168:22–32. doi: 10.1016/j.micres.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Christova N., Lang S., Wray V., Kaloyanov K., Konstantinov S., Stoineva I. Production, structural elucidation, and in vitro antitumor activity of trehalose lipid biosurfactant from Nocardia farcinica strain. J. Microbiol. Biotechnol. 2015;25:439–447. doi: 10.4014/jmb.1406.06025. [DOI] [PubMed] [Google Scholar]
- 26.Arutchelvi J., Bhaduri S., Uppara P.V., Doble M. Mannosylerythritol lipids: A review. J. Ind. Microbiol. Biotechnol. 2008;35:1559–1570. doi: 10.1007/s10295-008-0460-4. [DOI] [PubMed] [Google Scholar]
- 27.Leclere V., Bechet M., Adam A., Guez J.S., Wathelet B., Ongena M., Thonart P., Gancel F., Chollet-Imbert M., Jacques P. Myco-subtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl. Environ. Microbiol. 2005;71:4577–4584. doi: 10.1128/AEM.71.8.4577-4584.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ongena M., Jacques P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16:115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Zahir Z.A., Arshad M., Frankenberger W.T. Plant growth promoting rhizobacteria: Applications and perspectives in agriculture. Adv. Agron. 2004;81:97–168. [Google Scholar]
- 30.Ongena M., Jacques P., Touré Y., Destain J., Jabrane A., Thonart P. Involvement of fengycin-type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. Appl. Microbiol. Biotechnol. 2005;69:29–38. doi: 10.1007/s00253-005-1940-3. [DOI] [PubMed] [Google Scholar]
- 31.Pieterse C.M.J., Van Pelt J.A., Van Wees S.C.M., Ton J., Leon-Kloosterziel K.M., Keurentjes J.J.B., Verhagen B.W.M., Knoester M., Van der Sluis I., Bakker P.A.H.M., et al. Rhizobacteria-mediated induced systemic resistance: Triggering, signalling and expression. Eur. J. Plant. Pathol. 2001;107:51–61. doi: 10.1023/A:1008747926678. [DOI] [Google Scholar]
- 32.Van Loon L.C., Bakker P.A.H.M. Induced systemic resistance as a mechanism of disease suppression by rhizobacteria. In: Siddiqui Z.A., editor. PGPR: Biocontrol and Biofertilization. Springer; Dordrecht, The Netherlands: 2006. pp. 39–66. [Google Scholar]
- 33.Coventry H.S., Dubery I.A. Lipopolysaccharides from Burkholderia cepacia contribute to an enhanced defensive capacity and the induction of pathogenesis-related proteins in Nicotianae tabacum. Physiol. Mol. Plant Pathol. 2001;58:149–158. doi: 10.1006/pmpp.2001.0323. [DOI] [Google Scholar]
- 34.Reitz M., Oger P., Meyer A., Niehaus K., Farrand S.K., Hallmann J., Sikora R.A. Importance of the O-antigen, core-region and lipid A of rhizobial lipopolysaccharides for the induction of systemic resistance in potato to Globodera pallida. Nematology. 2002;4:73–79. doi: 10.1163/156854102760082221. [DOI] [Google Scholar]
- 35.Meziane H., Van der Sluis I., Van Loon L.C., Höfte M., Bakker P.A.H.M. Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol. Plant Pathol. 2005;6:177–185. doi: 10.1111/j.1364-3703.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 36.Ryu C.M., Farag M.A., Hu C.H., Reddy M.S., Kloepper J.W., Pare P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004;134:1017–1026. doi: 10.1104/pp.103.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Audenaert K., Pattery T., Cornelis P., Höfte M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic acid, pyochelin, and pyocyanin. Mol. Plant Microbe Interact. 2002;15:1147–1156. doi: 10.1094/MPMI.2002.15.11.1147. [DOI] [PubMed] [Google Scholar]
- 38.Ongena M., Jourdan E., Schafer M., Kech C., Budzikiewicz H., Luxen A., Thonart P. Isolation of an n-alkylated benzylamine derivative from Pseudomonas putida BTP1 as elicitor of induced systemic resistance in bean. Mol. Plant Microbe Interact. 2005;18:562–569. doi: 10.1094/MPMI-18-0562. [DOI] [PubMed] [Google Scholar]
- 39.Ran L.X., Li Z.N., Wu G.J., Van Loon L.C., Bakker P.A.H.M. Induction of systemic resistance against bacterial wilt in Eucalyptus urophylla by fluorescent Pseudomonas spp. Eur. J. Plant. Pathol. 2005;113:59–70. doi: 10.1007/s10658-005-0623-3. [DOI] [Google Scholar]
- 40.Iavicoli A., Boutet E., Buchala A., Métraux J.P. Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Mol. Plant Microbe Interact. 2003;16:851–858. doi: 10.1094/MPMI.2003.16.10.851. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui I.A., Shaukat S.S. Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: Importance of bacterial secondary metabolite, 2,4-diacetylpholoroglucinol. Soil Biol. Biochem. 2003;35:1615–1623. doi: 10.1016/j.soilbio.2003.08.006. [DOI] [Google Scholar]
- 42.De Vleesschauwer D., Cornelis P., Höfte M. Redox-active pyocyanin secreted by Pseudomonas aeruginosa 7NSK2 triggers systemic resistance to Magnaporthe grisea but enhances Rhizoctonia solani susceptibility in rice. Mol. Plant Microbe Interact. 2006;19:1406–1419. doi: 10.1094/MPMI-19-1406. [DOI] [PubMed] [Google Scholar]
- 43.Raaijmakers J.M., de Bruijn I., de Kock M.J.D. Cyclic lipopeptide production by plant-associated Pseudomonas spp.: Diversity, activity, biosynthesis, and regulation. Mol. Plant Microbe Int. 2006;19:699–710. doi: 10.1094/MPMI-19-0699. [DOI] [PubMed] [Google Scholar]
- 44.Schuhegger R., Ihring A., Gantner S., Bahnweg G., Knappe C., Vogg G., Hutzler P., Schmid M., Van Breusegem F., Eberl L., et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006;29:909–918. doi: 10.1111/j.1365-3040.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 45.Tran H., Ficke A., Asiimwe T., Höfte M., Raaijmakers J.M. Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol. 2007;175:731–742. doi: 10.1111/j.1469-8137.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 46.Ongena M., Jourdan E., Adam A., Paquot M., Brans A., Joris B., Arpigny J.L., Thonart P. Surfactin and fengycinlipopeptides of Bacillus subtilis as elicitors of induced systemic resistance inplants. Environ. Microbiol. 2007;9:1084–1090. doi: 10.1111/j.1462-2920.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- 47.Gómez-Gómez L. Plant perception systems for pathogen recognition and defense. Mol. Immunol. 2004;41:1055–1062. doi: 10.1016/j.molimm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Zhao J., Davis L.C., Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Brugger A., Lamotte O., Vandelle E., Bourque S., Lecourieux D., Poinssot B., Wendehenne D., Pugin A. Early signalling events induced by elicitors of plant defenses. Mol. Plant-Microbe Interact. 2006;19:711–724. doi: 10.1094/MPMI-19-0711. [DOI] [PubMed] [Google Scholar]
- 50.Jourdan E., Henry G., Duby F., Dommes J., Barthelemy J.P., Thonart P., Ongena M.A.R.C. Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol. Plant-Microbe Int. 2009;22:456–468. doi: 10.1094/MPMI-22-4-0456. [DOI] [PubMed] [Google Scholar]
- 51.Ghribi D., Abdelkefi-Mesrati L., Mnif I., Kammoun R., Ayadi I., Saadaoui I., Maktouf S., Chaabouni-Ellouze S. Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J. Biomed. Biotechnol. 2012 doi: 10.1155/2012/373682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malfanova N., Franzil L., Lugtenberg B., Chebotar V., Ongena M. Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch. Microbiol. 2012;194:893–899. doi: 10.1007/s00203-012-0823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun L., Lu Z., Bie X., Lu F., Yang S. Isolation and characterization of a co-producer of fengycins and surfactins, endophytic Bacillus amyloliquefaciens ES-2, from Scutellaria baicalensis Georgi. World J. Microbiol. Biotechnol. 2006;22:1259–1266. doi: 10.1007/s11274-006-9170-0. [DOI] [Google Scholar]
- 54.Zhang S.M., Wang Y.X., Meng L.Q., Li J., Zhao X.Y., Cao X., Chen X.L., Wang A.X., Li J.F. Isolation and characterization of antifungal lipopeptides produced by endophytic Bacillus amyloliquefaciens TF28. Afri. J. Microbiol. Res. 2012;6:1747–1755. [Google Scholar]
- 55.Arguelles-Arias A., Ongena M., Halimi B., Lara Y., Brans A., Joris B., Fickers P. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb. Cell Fact. 2009;8:63. doi: 10.1186/1475-2859-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao J., Li Y., Zhang C., Yao Z., Zhang L., Bie X., Lu F., Lu Z. Genome shuffling of Bacillus amyloliquefaciens for improving antimicrobial lipopeptide production and an analysis of relative gene expression using FQ RT-PCR. J. Ind. Microbiol. Biot. 2012;39:889–896. doi: 10.1007/s10295-012-1098-9. [DOI] [PubMed] [Google Scholar]
- 57.Gond S.K., Bergen M.S., Torres M.S., White J.F., Jr. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015;172:79–87. doi: 10.1016/j.micres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Chung S., Kong H., Buyer J.S., Lakshman D.K., Lydon J., Kim S.D., Roberts D.P. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soil borne pathogens of cucumber and pepper. Appl. Microbiol. Biotechnol. 2008;80:115–123. doi: 10.1007/s00253-008-1520-4. [DOI] [PubMed] [Google Scholar]
- 59.Alvarez F., Castro M., Príncipe A., Borioli G., Fischer S., Mori G., Jofré E. The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of Sclerotinia stem rot disease. J. Appl. Microbiol. 2012;112:159–174. doi: 10.1111/j.1365-2672.2011.05182.x. [DOI] [PubMed] [Google Scholar]
- 60.Mora I., Cabrefiga J., Montesinos E. Cyclic lipopeptide biosynthetic genes and products, and inhibitory activity of plant-associated Bacillus against phytopathogenic bacteria. PLoS ONE. 2015;10:e0127738. doi: 10.1371/journal.pone.0127738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramarathnam R., Bo S., Chen Y., Fernando W.D., Xuewen G., De Kievit T. Molecular and biochemical detection of fengycin-and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can. J. Microbiol. 2007;53:901–911. doi: 10.1139/W07-049. [DOI] [PubMed] [Google Scholar]
- 62.Athukorala S.N.P., Fernando W.G.D., Rashid K.Y. Identification of antifungal antibiotics of Bacillus species isolated from different microhabitats using polymerase chain reaction and MALDI-TOF mass spectrometry. Can. J. Microbiol. 2009;55:1021–1032. doi: 10.1139/W09-067. [DOI] [PubMed] [Google Scholar]
- 63.Beltran-Gracia E., Macedo-Raygoza G., Villafaña-Rojas J., Martinez-Rodriguez A., Chavez-Castrillon Y.Y., Espinosa-Escalante F.M., Di Mascio P., Ogura T., Beltran-Garcia M.J. Fermentation Processes. InTech; Rijeka, Croatia: Production of lipopeptides by fermentation processes: Endophytic bacteria, fermentation strategies and easy methods for bacterial selection; p. 12. [Google Scholar]
- 64.Moyne A.L., Cleveland T.E., Tuzun S. Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiol. Lett. 2004;234:43–49. doi: 10.1111/j.1574-6968.2004.tb09511.x. [DOI] [PubMed] [Google Scholar]
- 65.Steinborn G., Hajirezaei M.R., Hofemeister J. bac genes for recombinant bacilysin and anticapsin production in Bacillus host strains. Arch. Microbiol. 2005;183:71–79. doi: 10.1007/s00203-004-0743-8. [DOI] [PubMed] [Google Scholar]
- 66.Stein T., Borchert S., Conrad B., Feesche J., Hofemeister B., Hofemeister J., Entian K.D. Two different lantibiotic-like peptides originate from the ericin gene cluster of Bacillus subtilis A1/3. J. Bacterial. 2002;184:1703–1711. doi: 10.1128/JB.184.6.1703-1711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herzner A.M., Dischinger J., Szekat C., Josten M., Schmitz S., Yakéléba A., Reinartz R., Jansen A., Sahl H.G., Piel J., et al. Expression of the lantibiotic mersacidin in Bacillus amyloliquefaciens FZB42. PLoS ONE. 2011;6:e22389. doi: 10.1371/journal.pone.0022389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duitman E.H., Hamoen L.W., Rembold M., Venema G., Seitz H., Saenger W., Bernhard F., Reinhardt R., Schmidt M., Ullrich C. The mycosubtilin synthetase of Bacillus subtilis ATCC6633: A multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc. Natl. Acad. Sci. USA. 1999;96:13294–13299. doi: 10.1073/pnas.96.23.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abderrahmani A., Tapi A., Nateche F., Chollet M., Leclère V., Wathelet B., Hacene H., Jacques P. Bioinformatics and molecular approaches to detect NRPS genes involved in the biosynthesis of kurstakin from Bacillus thuringiensis. Appl. Microbiol. Biotechnol. 2011;92:571–581. doi: 10.1007/s00253-011-3453-6. [DOI] [PubMed] [Google Scholar]
- 70.Das P., Mukherjee S., Sen R. Genetic regulations of the biosynthesis of microbial surfactants: An overview. Biotechnol. Genet. Eng. Rev. 2008;25:165–186. doi: 10.5661/bger-25-165. [DOI] [PubMed] [Google Scholar]
- 71.Yu G.Y., Sinclair J.B., Hartman G.L., Bertagnolli B.L. Production of Iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol. Biochem. 2002;34:955–963. doi: 10.1016/S0038-0717(02)00027-5. [DOI] [Google Scholar]
- 72.Vater J., Kablitz B., Wilde C., Franke P., Mehta N., Cameotra S.S. Matrix-assisted laser desorption ionization-time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl. Environ. Microbiol. 2002;68:6210–6219. doi: 10.1128/AEM.68.12.6210-6219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pueyo M.T., Bloch C., Jr., Carmon-Ribeiro A.M., di Mascio P. Lipopeptides produced by a soil Bacillus megaterium Strain. Microb. Ecol. 2005;57:367–378. doi: 10.1007/s00248-008-9464-x. [DOI] [PubMed] [Google Scholar]
- 74.Nihorimbere V., Cawoy H., Seyer A., Brunelle A., Thonart P., Ongena M. Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol. Ecol. 2012;79:176–191. doi: 10.1111/j.1574-6941.2011.01208.x. [DOI] [PubMed] [Google Scholar]
- 75.Patel S., Ahmed S., Eswari J.S. Therapeutic cyclic lipopeptides mining from microbes: Latest strides and hurdles. World J. Microbiol. Biotechnol. 2015;31:1177–1193. doi: 10.1007/s11274-015-1880-8. [DOI] [PubMed] [Google Scholar]
- 76.Ali S., Hameed S., Imran A., Iqbal M., Lazarovits G. Genetic, physiological and biochemical characterization of Bacillus sp. strain RMB7 exhibiting plant growth promoting and broad spectrum antifungal activities. Microbial. Cell Factories. 2014;13:144. doi: 10.1186/PREACCEPT-6657919731258908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma Z., Wang N., Hu J., Wang S. Isolation and characterization of a new iturinic lipopeptide, mojavensin A produced by a marine-derived bacterium Bacillus mojavensis B0621A. J. Antibiot. 2012;65:317. doi: 10.1038/ja.2012.19. [DOI] [PubMed] [Google Scholar]
- 78.Koumoutsi A., Chen X.H., Henne A., Liesegang H., Hitzeroth G., Franke P., Vater J., Borriss R. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 2004;186:1084–1096. doi: 10.1128/JB.186.4.1084-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bais H.P., Fall R., Vivanco J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004;134:307–319. doi: 10.1104/pp.103.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seydlov’a G., ˇCabala R., Svobodov´a J. Surfactin—Novel Solutions for Global Issues. Vol. 13. InTech; Rijeka, Croatia: 2011. Biomedical engineering, trends, research and technologies; pp. 306–330. [Google Scholar]
- 81.Korenblum E., de Araujo L.V., Guimarães C.R., De Souza L.M., Sassaki G., Abreu F., Nitschke M., Lins U., Freire D.M.G., Barreto-Bergter E. Purification and characterization of a surfactin-like molecule produced by Bacillus sp. H2O-1 and its antagonistic effect against sulfate reducing bacteria. BMC Microbiol. 2012;12:252. doi: 10.1186/1471-2180-12-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim K., Jung S.Y., Lee D.K., Jung J.K., Park J.K., Kim D.K., Lee C.H. Suppression of inflammatory responses by surfactin, 1 a selective inhibitor of platelet cytosolic phospholipase A2. Biochem. Pharmacol. 1998;55:975–985. doi: 10.1016/S0006-2952(97)00613-8. [DOI] [PubMed] [Google Scholar]
- 83.Hamley I.W. Lipopeptides: From self-assembly to bioactivity. Chem. Commun. 2015;51:8574–8583. doi: 10.1039/C5CC01535A. [DOI] [PubMed] [Google Scholar]
- 84.Meena K.R., Kanwar S.S. Lipopeptides as the antifungal and antibacterial agents: Applications in food safety and therapeutics. Biomed. Res. Int. 2015;2015:473050. doi: 10.1155/2015/473050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cawoy H., Mariutto M., Henry G., Fisher C., Vasilyeva N., Thonart P., Dommes J., Ongena M. Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol. Plant–Microbe Interact. 2014;27:87–100. doi: 10.1094/MPMI-09-13-0262-R. [DOI] [PubMed] [Google Scholar]
- 86.Deleu M., Paquot M., Nylander T. Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes. Biophys. J. 2008;94:2667–2679. doi: 10.1529/biophysj.107.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu C.Y., Chen C.L., Lee Y.H., Cheng Y.C., Wu Y.C., Shu H.Y. Non ribosomal synthesis of fengycin on an enzyme complex formed by fengycin synthetases. J. Biol. Chem. 2007;282:5608–5616. doi: 10.1074/jbc.M609726200. [DOI] [PubMed] [Google Scholar]
- 88.Li X.Y., Wang Y.H., He Y.Q. Diversity and active mechanism of fengycin-type cyclopeptides from Bacillus subtilis XF-1 against Plasmodiophora brassicae. J. Microbiol. Biotechnol. 2013;23:313–321. doi: 10.4014/jmb.1208.08065. [DOI] [PubMed] [Google Scholar]
- 89.Janek T., Łukaszewicz M., Krasowska A. Antiadhesive activity of the biosurfactant pseudofactin II secreted by the Arctic bacterium Pseudomonas fluorescens BD5. BMC Microbiol. 2012;12:24. doi: 10.1186/1471-2180-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alsohim A.S., Taylor T.B., Barrett G.A., Gallie J., Zhang X.X., Altamirano-Junqueira A.E., Johnson L.J., Rainey P.B., Jackson R.W. The biosurfactant viscosin produced by Pseudomonas fluorescens SBW 25 aids spreading motility and plant growth promotion. Environ Microbiol. 2014;16:2267–2281. doi: 10.1111/1462-2920.12469. [DOI] [PubMed] [Google Scholar]
- 91.Enoch D.A., Bygott J.M., Daly M.L., Karas J.A. Daptomycin. J. Infect. 2007;55:205–213. doi: 10.1016/j.jinf.2007.05.180. [DOI] [PubMed] [Google Scholar]
- 92.Raaijmakers J.M., De Bruijn I., Nybroe O., Ongena M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010;34:1037–1062. doi: 10.1111/j.1574-6976.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 93.Zachow C., Jahanshah G., de Bruijn I., Song C., Ianni F., Pataj Z., Gerhardt H., Pianet I., Lämmerhofer M., Berg G., et al. The novel lipopeptide poaeamide of the endophyte Pseudomonas poae RE* 1-1-14 is involved in pathogen suppression and root colonization. Mol. Plant–Microbe Interact. 2015;28:800–810. doi: 10.1094/MPMI-12-14-0406-R. [DOI] [PubMed] [Google Scholar]
- 94.Xue Y., Wang M., Zhao P., Quan C., Li X., Wang L., Gao W., Li J., Zu X., Fu D., et al. Gram-negative bacilli-derived peptide antibiotics developed since 2000. Biotechnol. Lett. 2018;40:1271–1287. doi: 10.1007/s10529-018-2589-1. [DOI] [PubMed] [Google Scholar]
- 95.Gong M., Wang J.D., Zhang J., Yang H., Lu X.F., Pei Y., Cheng J.Q. Study of the antifungal ability of Bacillus subtilis strain PY-1 in vitro and identification of its antifungal substance (iturin A) Acta Biochem. Biophysiol. Sin. 2006;38:233–240. doi: 10.1111/j.1745-7270.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 96.Qian C.D., Li B.Q., Zhao T., Guo Q.G., Lu X.Y., Li S.Z., Ma P. Isolation and stability analysis of lipopeptides produced by Bacillus subtilis strain BAB21. China J. Agric. Sci. Technol. 2009;11:69–74. [Google Scholar]
- 97.Romero D., de Vicente A., Rakotoaly R.H., Dufour S.E., Veening J.W., Arrebola E., Pérez-García A. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol. Plant–Microbe Interact. 2007;20:430–440. doi: 10.1094/MPMI-20-4-0430. [DOI] [PubMed] [Google Scholar]
- 98.Debois D., Jourdan E., Smargiasso N., Thonart P., De Pauw E., Ongena M. Spatiotemporal monitoring of the antibiome secreted by Bacillus biofilms on plant roots using MALDI mass spectrometry imaging. Anal. Chem. 2014;86:4431–4438. doi: 10.1021/ac500290s. [DOI] [PubMed] [Google Scholar]
- 99.Makovitzki A., Viterbo A., Brotman Y., Chet I., Shai Y. Inhibition of fungal and bacterial plant pathogens in vitro and in planta with ultrashort cationic lipopeptides. Appl. Environ. Microbiol. 2007;73:6629–6636. doi: 10.1128/AEM.01334-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho K.M., Math R.K., Hong S.Y., Islam S.M.A., Mandanna D.K., Cho J.J., Yun M.G., Kim J.M., Yun H.D. Iturin produced by Bacillus pumilus HY1 from Korean soybean sauce (kanjang) inhibits growth of aflatoxin producing fungi. Food Control. 2009;20:402–406. doi: 10.1016/j.foodcont.2008.07.010. [DOI] [Google Scholar]
- 101.Aretz W., Meiwes J., Seibert G., Vobis G., Wink J. Friulimicins: Novel lipopeptide antibiotics with peptidoglycan synthesis inhibiting activity from Actinoplanes friuliensis sp. nov. J. Antibiot. 2000;53:807–815. doi: 10.7164/antibiotics.53.807. [DOI] [PubMed] [Google Scholar]
- 102.Morikawa M., Daido H., Takao T., Murata S., Shimonishi Y., Imanaka T. A new lipopeptide biosurfactant produced by Arthrobacter sp. strain MIS38. J. bacterial. 1993;175:6459–6466. doi: 10.1128/JB.175.20.6459-6466.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pathak K.V., Keharia H., Gupta K., Thakur S.S., Balaram P. Lipopeptides from the banyan endophyte, Bacillus subtilis K1: Mass spectrometric characterization of a library of fengycins. J. Am. Soc. Mass Spectrom. 2012;23:1716–1728. doi: 10.1007/s13361-012-0437-4. [DOI] [PubMed] [Google Scholar]
- 104.Toure Y., Ongena M.A.R.C., Jacques P., Guiro A., Thonart P. Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J. Appl. Microbiol. 2004;96:1151–1160. doi: 10.1111/j.1365-2672.2004.02252.x. [DOI] [PubMed] [Google Scholar]
- 105.Hu L.B., Shi Z.Q., Zhang T., Yang Z.M. Fengycin antibiotics isolated from B-FS01 culture inhibit the growth of Fusarium moniliforme Sheldon ATCC 38932. FEMS Microbiol. Lett. 2007;272:91–98. doi: 10.1111/j.1574-6968.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- 106.Mnif I., Grau-Campistany A., Coronel-León J., Hammami I., Triki M.A., Manresa A., Ghribi D. Purification and identification of Bacillus subtilis SPB1 lipopeptide biosurfactant exhibiting antifungal activity against Rhizoctonia bataticola and Rhizoctonia solani. Environ. Sci. Poll. Res. 2016;23:6690–6699. doi: 10.1007/s11356-015-5826-3. [DOI] [PubMed] [Google Scholar]
- 107.Wen C.Y., Yin Z.G., Wang K.X., Chen J.G., Shen S.S. Purification and structural analysis of surfactin produced by endophytic Bacillus subtilis EBS05 and its antagonistic activity against Rhizoctonia cerealis. Plant Pathol. J. 2011;27:342–348. doi: 10.5423/PPJ.2011.27.4.342. [DOI] [Google Scholar]
- 108.Kim P.I., Ryu J., Kim Y.H., Chl Y.T. Production of biosurfactant lipopeptides iturin A, fengycin, and surfactin A from Bacillus subtilis CMB32 for control of colletotrichum gloeosporioides. J. Microbiol. Biotechnol. 2010;20:138–145. doi: 10.4014/jmb.0905.05007. [DOI] [PubMed] [Google Scholar]
- 109.Nair D., Vanuopadath M., Nair B.G., Pai J.G., Nair S.S. Identification and characterization of a library of surfactins and fengycins from a marine endophytic Bacillus sp. J. Basic Microbial. 2016;56:1159–1172. doi: 10.1002/jobm.201600029. [DOI] [PubMed] [Google Scholar]
- 110.Sajitha K.L., Dev S.A., Florence E.M. Identification and characterization of lipopeptides from Bacillus subtilis B1 against sapstain fungus of rubberwood through MALDI-TOF-MS and RT-PCR. Curr. Microbiol. 2016;73:46–53. doi: 10.1007/s00284-016-1025-9. [DOI] [PubMed] [Google Scholar]
- 111.Chen H., Wang L., Su C.X., Gong G.H., Wang P., Yu Z.L. Isolation and characterization of lipopeptide antibiotics produced by Bacillus subtilis. Lett. Appl. Microbiol. 2008;47:180–186. doi: 10.1111/j.1472-765X.2008.02412.x. [DOI] [PubMed] [Google Scholar]
- 112.Fan H., Ru J., Zhang Y., Wang Q., Li Y. Fengycin produced by Bacillus subtilis 9407 plays a major role in the biocontrol of apple ring rot disease. Microbiol. Res. 2017;199:89–97. doi: 10.1016/j.micres.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 113.Bie X., Lu Z., Lu F. Identification of fengycin homologues from Bacillus subtilis with ESI–MS/CID. J. Microbiol. Methods. 2009;79:272–278. doi: 10.1016/j.mimet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 114.Prabhukarthikeyan S.R., Karthikeyan G., Jeyarani S., Raguchander T. PCR based identification and characterization of lipopeptides producing Bacillus against Fusarium oxysporum f. sp. lycopersici. Biochem. Cell. Arch. 2014;14:133–140. [Google Scholar]
- 115.Liu J., Liu M., Wang J., Yao J.M., Pan R.R., Yu Z.L. Enhancement of the Gibberella zeae growth inhibitory lipopeptides from a Bacillus subtilis mutant by ion beam implantation. Applied Microbiol. Biotechnol. 2005;69:223–228. doi: 10.1007/s00253-005-1981-7. [DOI] [PubMed] [Google Scholar]
- 116.Farace G., Fernandez O., Jacquens L., Coutte F., Krier F., Jacques P., Dorey S. Cyclic lipopeptides from Bacillussubtilis activate distinct patterns of defense responses in grapevine. Mol. Plant Pathol. 2015;16:177–187. doi: 10.1111/mpp.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Desoignies N., Schramme F., Ongena M., Legrève A. Systemic resistance induced by Bacillus lipopeptides in Beta vulgaris reduces infection by the rhizomania disease vector Polymyxa betae. Mol. Plant Pathol. 2013;14:416–421. doi: 10.1111/mpp.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ben A.D., Frikha-Gargouri O., Tounsi S. Bacillus amyloliquefaciens strain 32a as a source of lipopeptides for biocontrol of Agrobacterium tumefaciens strains. J. Appl. Microbiol. 2015;119:196–207. doi: 10.1111/jam.12797. [DOI] [PubMed] [Google Scholar]
- 119.Vitullo D., Di Pietro A., Romano A., Lanzotti V., Lima G. Role of new bacterial surfactins in the antifungal interaction between Bacillus amyloliquefaciens and Fusarium oxysporum. Plant Pathol. 2012;61:689–699. doi: 10.1111/j.1365-3059.2011.02561.x. [DOI] [Google Scholar]
- 120.Abdallah R.A.B., Stedel C., Garagounis C., Nefzi A., Jabnoun-Khiareddine H., Papadopoulou K.K., Daami-Remadi M. Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato. Crop Prot. 2017;99:45–58. doi: 10.1016/j.cropro.2017.05.008. [DOI] [Google Scholar]
- 121.Medeot D.B., Bertorello-Cuenca M., Liaudat J.P., Alvarez F., Flores-Cáceres M.L., Jofré E. Improvement of biomass and cyclic lipopeptides production in Bacillus amyloliquefaciens MEP218 by modifying carbon and nitrogen sources and ratios of the culture media. Biol. Cont. 2017;115:119–128. doi: 10.1016/j.biocontrol.2017.10.002. [DOI] [Google Scholar]
- 122.Preecha C., Sadowsky M.J., Prathuangwong S. Lipopeptide surfactin produced by Bacillus amyloliquefaciens KPS46 is required for biocontrol efficacy against Xanthomonasaxonopodis pv. glycines. Kasetsart J. (Nat. Sci.) 2010;44:84–99. [Google Scholar]
- 123.Snook M.E., Mitchell T., Hinton D.M., Bacon C.W. Isolation and characterization of Leu7-surfactin from the endophytic bacterium Bacillus mojavensis RRC 101, a biocontrol agent for Fusarium verticillioides. J. Agric. Food Chem. 2009;57:4287–4292. doi: 10.1021/jf900164h. [DOI] [PubMed] [Google Scholar]
- 124.Ayed H.B., Hmidet N., Béchet M., Chollet M., Chataigné G., Leclère V., Jacques P., Nasri M. Identification and biochemical characteristics of lipopeptides from Bacillus mojavensis A21. Process Biochem. 2014;49:1699–1707. doi: 10.1016/j.procbio.2014.07.001. [DOI] [Google Scholar]
- 125.Blacutt A.A., Mitchell T.R., Bacon C.W., Gold S.E. Bacillus mojavensis RRC101 lipopeptides provoke physiological and metabolic changes during antagonism against Fusarium verticillioides. Mol. Plant Microbe Interact. 2016;29:713–723. doi: 10.1094/MPMI-05-16-0093-R. [DOI] [PubMed] [Google Scholar]
- 126.Favaro G., Bogialli S., Di Gangi I.M., Nigris S., Baldan E., Squartini A. Characterization of lipopeptides produced by Bacillus licheniformis using liquid chromatography with accurate tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2016;30:2237–2252. doi: 10.1002/rcm.7705. [DOI] [PubMed] [Google Scholar]
- 127.Saggese A., Culurciello R., Casillo A., Corsaro M.M., Ricca E., Baccigalupi L. A Marine isolate of Bacillus pumilus secretes a pumilacidin active against Staphylococcus aureus. Mar. Drugs. 2008;16:180. doi: 10.3390/md16060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim P.I., Bai H., Bai D., Chae H., Chung S., Kim Y., Park R., Chi Y.T. Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J. Appl. Microbiol. 2004;97:942–949. doi: 10.1111/j.1365-2672.2004.02356.x. [DOI] [PubMed] [Google Scholar]
- 129.Hathout Y., Ho Y.P., Ryzhov V., Demirev P., Fenselau C. Kurstakins: A new class of lipopeptides isolated from Bacillus thuringiensis. J. Nat. Prod. 2000;63:1492–1496. doi: 10.1021/np000169q. [DOI] [PubMed] [Google Scholar]
- 130.Béchet M., Caradec T., Hussein W., Abderrahmani A., Chollet M., Leclère V., Dubois T., Lereclus D., Pupin M., Jacques P. Structure, biosynthesis, and properties of kurstakins, non-ribosomal lipopeptides from Bacillus spp. Appl. Microbiol. Biotechnol. 2012;95:593–600. doi: 10.1007/s00253-012-4181-2. [DOI] [PubMed] [Google Scholar]
- 131.Kaur P.K., Joshi N., Singh I.P., Saini H.S. Identification of cyclic lipopeptides produced by Bacillus vallismortis R2 and their antifungal activity against Alternaria alternata. J. Appl. Microbiol. 2017;122:139–152. doi: 10.1111/jam.13303. [DOI] [PubMed] [Google Scholar]
- 132.Ramarathnam R., Fernando W.D. Molecular and biochemical detection of lipopeptide antibiotics producing Bacillus spp., antagonistic to common fungal pathogens of canola (Brassica napus L.); Proceedings of the 12th International Rapeseed Congress; Wuhan, China. 26–30 March 2007; p. 180. [Google Scholar]
- 133.Kalai-Grami L., Karkouch I., Naili O., Slimene I.B., Elkahoui S., Zekri R.B., Touati I., Mnari-Hattab M., Hajlaoui M.R., Limam F. Production and identification of iturin A lipopeptide from Bacillus methyltrophicus TEB1 for control of Phoma tracheiphila. J. Basic Microbiol. 2016;56:864–871. doi: 10.1002/jobm.201500683. [DOI] [PubMed] [Google Scholar]
- 134.Pramudito T.E., Agustina D., Nguyen T.K.N., Suwanto A. A Novel variant of narrow-spectrum antifungal bacterial lipopeptides that strongly inhibit Ganoderma boninense. Probiotics Antimicrob. Proteins. 2017;10:110–117. doi: 10.1007/s12602-017-9334-2. [DOI] [PubMed] [Google Scholar]
- 135.Perez K.J., Viana J.D.S., Lopes F.C., Pereira J.Q., dos Santos D.M., Oliveira J.S., Velho R.V., Crispim S.M., Nicoli J.R., Brandelli A. Bacillus spp. isolated from puba as a source of biosurfactants and antimicrobial lipopeptides. Front. Microbiol. 2017;8:61. doi: 10.3389/fmicb.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jasim B., Sreelakshmi K.S., Mathew J., Radhakrishnan E.K. Surfactin, iturin, and fengycin biosynthesis by Endophytic Bacillus sp. from Bacopa monnieri. Microb. Ecol. 2016;72:106–119. doi: 10.1007/s00248-016-0753-5. [DOI] [PubMed] [Google Scholar]
- 137.Chen Q., Liu B., Wang J., Che J., Liu G., Guan X. Antifungal lipopeptides produced by Bacillus sp. FJAT-14262 isolated from rhizosphere soil of the medicinal plant Anoectochilus roxburghii. Appl. Biochem. Biotechnol. 2017;182:155–167. doi: 10.1007/s12010-016-2317-z. [DOI] [PubMed] [Google Scholar]
- 138.Cho S.J. Identification and molecular characterization of three isoforms of iturin produced by endophytic Bacillus sp. CY22. J. Life Sci. 2005;15:1005–1012. [Google Scholar]
- 139.Sarwar A., Hassan M.N., Imran M., Iqbal M., Majeed S., Brader G., Sessitsch A., Hafeez F.Y. Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol. Res. 2018;209:1–13. doi: 10.1016/j.micres.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 140.Kim H.J., Choi H.S., Yang S.Y., Kim I.S., Yamaguchi T., Sohng J.K., Park S.K., Kim J.C., Lee C.H., Gardener B.M. Both extracellular chitinase and a new cyclic lipopeptide, chromobactomycin, contribute to the biocontrol activity of Chromobacterium sp. C61. Mol. Plant Pathol. 2014;15:122–132. doi: 10.1111/mpp.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shiono Y., Tsuchinari M., Shimanuki K., Miyajima T., Murayama T., Koseki T., Laatsch H., Funakoshi T., Takanami K., Suzuki K. A and B, two new cyclic lipopeptides from an endophytic Fusarium sp. J. Antibiot. 2007;60:309. doi: 10.1038/ja.2007.39. [DOI] [PubMed] [Google Scholar]
- 142.Li G., Kusari S., Golz C., Strohmann C., Spiteller M. Three cyclic pentapeptides and a cyclic lipopeptide produced by endophytic Fusarium decemcellulare LG53. RSC Adv. 2016;6:54092–54098. doi: 10.1039/C6RA10905E. [DOI] [Google Scholar]
- 143.Rosconi F., Davyt D., Martínez V., Martínez M., Abin-Carriquiry J.A., Zane H., Butler A., de Souza E.M., Fabiano E. Identification and structural characterization of serobactins, a suite of lipopeptide siderophores produced by the grass endophyte Herbaspirillum seropedicae. Environ. Microbiol. 2013;15:916–927. doi: 10.1111/1462-2920.12075. [DOI] [PubMed] [Google Scholar]
- 144.Nielsen T.H., Thrane C., Christophersen C., Anthoni U., Sørensen J. Structure, production characteristics and fungal antagonism of tensin–a new antifungal cyclic lipopeptide from Pseudomonas fluorescens strain 96.578. J. Appl. Microbiol. 2000;89:992–1001. doi: 10.1046/j.1365-2672.2000.01201.x. [DOI] [PubMed] [Google Scholar]
- 145.Schlusselhuber M., Godard J., Sebban M., Bernay B., Garon D., Seguin V., Oulyadi H., Desmasures N. Characterization of milkisin, a novel lipopeptide with antimicrobial properties produced by Pseudomonas sp. UCMA 17988 isolated from bovine raw milk. Front. Microbiol. 2018;9:1030. doi: 10.3389/fmicb.2018.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Niu B., Rueckert C., Blom J., Wang Q., Borriss R. The genome of the plant growth-promoting rhizobacterium Paenibacillus polymyxa M-1 contains nine sites dedicated to nonribosomal synthesis of lipopeptides and polyketides. J. Bacteriol. 2011;193:5862–5863. doi: 10.1128/JB.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Canova S.P., Petta T., Reyes L.F., Zucchi T.D., Moraes L.A., Melo I.S. Characterization of lipopeptides from Paenibacillus sp.(IIRAC30) suppressing Rhizoctonia solani. World J. Microbiol. Biotechnol. 2010;26:2241–2247. doi: 10.1007/s11274-010-0412-9. [DOI] [Google Scholar]
- 148.Heinemann B., Kaplan M., Muir R., Hooper I. Amphomycin, a new antibiotic. Antibiot. Chemother. 1953;3:1239–1242. [PubMed] [Google Scholar]
- 149.Tanaka H., Oiwa R., Matsukura S., Omura S. Amphomycin inhibits phospho-N-acetylmuramyl-pentapeptide translocase in peptidoglycan synthesis of Bacillus. Biochem. Biophys. Res. Commun. 1979;86:902–908. doi: 10.1016/0006-291X(79)91797-2. [DOI] [PubMed] [Google Scholar]
- 150.Wang Y., Chen Y., Shen Q., Yin X. Molecular cloning and identification of the laspartomycin biosynthetic gene cluster from Streptomyces viridochromogenes. Gene. 2011;483:11–21. doi: 10.1016/j.gene.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kurth C., Schieferdecker S., Athanasopoulou K., Seccareccia I., Nett M. Variochelins, lipopeptide siderophores from Variovorax boronicumulans discovered by genome mining. J. Nat. Prod. 2016;79:865–872. doi: 10.1021/acs.jnatprod.5b00932. [DOI] [PubMed] [Google Scholar]
- 152.Inès M., Dhouha G. Lipopeptide surfactants: Production, recovery and pore forming capacity. Peptides. 2015;71:100–112. doi: 10.1016/j.peptides.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 153.Fracchia L., Cavallo M., Martinotti M.G., Banat I.M. Biosurfactants and bioemulsifiers biomedical and related applications-present status and future potentials. Biomed. Sci. Eng. Technol. 2012;14:326–335. [Google Scholar]
- 154.Bernheimer A.W., Avigad L.S. Nature and properties of a cytolytic agent produced by Bacillus subtilis. J. Gen. Microbiol. 1970;6:361–366. doi: 10.1099/00221287-61-3-361. [DOI] [PubMed] [Google Scholar]
- 155.Carrillo C., Teruel J.A., Aranda F.A., Ortiz A. Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochem. Biophys. Acta. 2003;1611:91–97. doi: 10.1016/S0005-2736(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 156.Mangoni M.L., Shai Y. Short native antimicrobial peptides and engineered ultrashort lipopeptides: Similarities and differences in cell specificities and modes of action. Cell. Mol. Life Sci. 2011;68:2267–2280. doi: 10.1007/s00018-011-0718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moyne A.L., Shelby R., Cleveland T.E., Tuzun S. Bacillomycin D: An iturin with antifungal activity against Aspergillus flavus. J. Appl. Microbiol. 2001;90:622–629. doi: 10.1046/j.1365-2672.2001.01290.x. [DOI] [PubMed] [Google Scholar]
- 158.Hiradate S., Yoshida S., Sugie H., Yada H., Fujii Y. Mulberry anthracnose antagonists (iturins) produced by Bacillus amyloliquefaciens RC-2. Phytochemistry. 2002;61:693–698. doi: 10.1016/S0031-9422(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 159.Phae C.G., Phae C.G., Shoda M.l., Kubota H. Suppressive effect of Bacillus subtilis and its products on phytopathogenic microorganisms. J. Ferment. Bioeng. 1990;69:1–7. doi: 10.1016/0922-338X(90)90155-P. [DOI] [Google Scholar]
- 160.Aranda F.J., Teruel J.A., Ortiz A. Further aspects on the hemolytic activity of the antibiotic lipopeptide iturin A. Biochim. Biophys. Acta Biomembr. 2005;1713:51–56. doi: 10.1016/j.bbamem.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 161.Vanittanakom N., Loeffler W., Koch U., Jung G. Fengycin—A novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J. Antibiot. 1986;39:888–901. doi: 10.7164/antibiotics.39.888. [DOI] [PubMed] [Google Scholar]
- 162.Hofemeister J., Conrad B., Adler B., Hofemeister B., Feesche J., Kucheryava N., Steinborn G., Franke P., Grammel N., Zwintscher A., et al. Genetic analysis of the biosynthesis of nonribosomal peptide- and polyketide-like antibiotics, iron uptake and biofilm formation by Bacillus subtilis A1/3. Mol. Genet. Genom. 2004;272:363–378. doi: 10.1007/s00438-004-1056-y. [DOI] [PubMed] [Google Scholar]
- 163.Sur S., Romo T.D., Grossfield A. Selectivity and mechanism of Fengycin, an antimicrobial lipopeptide, from molecular dynamics. J. Phys. Chem. B. 2018;122:2219–2226. doi: 10.1021/acs.jpcb.7b11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Deleu M., Paquot M., Nylander T. Fengycin interaction with lipid monolayers at the air–aqueous interface-implications for the effect of fengycin on biological membranes. J. Colloid Interface Sci. 2005;283:358–365. doi: 10.1016/j.jcis.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 165.Hutchison M.L., Gross D.C. Lipopeptide phytotoxins produced by Pseudomonas syringae pv. syringae: Comparison of the biosurfactant and ion channel-forming activities of syringopeptin and syringomycin. Mol. Plant Microbe Interact. 1997;10:347–354. doi: 10.1094/MPMI.1997.10.3.347. [DOI] [PubMed] [Google Scholar]
- 166.Agner G., Kaulin Y.A., Gurnev P.A., Szabo Z., Schagina L.V., Takemoto J.Y., Blasko K. Membrane-permeabilizing activities of cyclic lipodepsipeptides, syringopeptin 22A and syringomycin E from Pseudomonas syringae pv. syringae in human red blood cells and in bilayer lipid membranes. Bioelectrochemistry. 2000;52:161–167. doi: 10.1016/S0302-4598(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 167.Carpaneto A., Dalla Serra M., Menestrina G., Fogliano V., Gambale F. The phytotoxic lipodepsipeptide syringopeptin 25A from Pseudomonas syringae pv. syringae forms ion channels in sugar beet vacuoles. J. Membr. Biol. 2002;188:237–248. doi: 10.1007/s00232-001-0187-x. [DOI] [PubMed] [Google Scholar]
- 168.Sy-Cordero A.A., Pearce C.J., Oberlies N.H. Revisiting the enniatins: A review of their isolation, biosynthesis, structure determination, and biological activities. J. Antibiot. 2012;65:541–549. doi: 10.1038/ja.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moryl M., Spętana M., Dziubek K., Paraszkiewicz K., Różalska S., Płaza G.A., Różalski A. Antimicrobial, antiadhesive and antibiofilm potential of lipopeptides synthesised by Bacillus subtilis on uropathogenic bacteria. Acta Biochim. Polonica. 2015;62:725–732. doi: 10.18388/abp.2015_1120. [DOI] [PubMed] [Google Scholar]
- 170.Kuiper I., Lagendijk E.L., Pickford R., Derrick J.P., Lamers G.E., Thomas-Oates J.E., Lugtenberg B.J., Bloemberg G.V. Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Mol. Microbiol. 2004;51:97–113. doi: 10.1046/j.1365-2958.2003.03751.x. [DOI] [PubMed] [Google Scholar]
- 171.Abdallah D.B., Tounsi S., Gharsallah H., Hammami A., Frikha-Gargouri O. Lipopeptides from Bacillus amyloliquefaciens strain 32a as promising biocontrol compounds against the plant pathogen Agrobacterium tumefaciens. Environ. Sci. Pollut. Res. 2018;25:36518–36529. doi: 10.1007/s11356-018-3570-1. [DOI] [PubMed] [Google Scholar]
- 172.Andrews S.C., Robinson A.K., Rodríguez-Quiñones F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 173.Sandy M., Butler A. Microbial iron acquisition: Marine and terrestrial siderophores. Chem Rev. 2009;109:4580–4595. doi: 10.1021/cr9002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hider R.C., Kong X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 175.Gauglitz J.M., Zhou H., Butler A. A suite of citrate-derived siderophores from a marine Vibrio species isolated following the Deepwater Horizon oil spill. J. Inorg. Biochem. 2012;107:90–95. doi: 10.1016/j.jinorgbio.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Martinez J.S., Zhang G.P., Holt P.D., Jung H.-T., Carrano C.J., Haygood M.G., Butler A. Self-assembling amphiphilic siderophores from marine bacteria. Science. 2000;287:1245–1247. doi: 10.1126/science.287.5456.1245. [DOI] [PubMed] [Google Scholar]
- 177.Ito Y., Butler A. Structure of synechobactins, new siderophores of the marine cyanobacterium synechococcus sp. PCC 7002. Limnol. Oceanogr. 2005;50:1918–1923. doi: 10.4319/lo.2005.50.6.1918. [DOI] [Google Scholar]
- 178.Homann V.V., Sandy M., Tincu J.A., Templeton A.S., Tebo B.M., Butler A. Loihichelins A−F, a suite of amphiphilic siderophores produced by the marine bacterium Halomonas LOB-5. J. Nat. Prod. 2009;72:884–888. doi: 10.1021/np800640h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Traxler M.F., Seyedsayamdost M.R., Clardy J., Kolter R. Interspecies modulation of bacterial development through iron competition and siderophore piracy. Mol. Microbiol. 2012;86:628–644. doi: 10.1111/mmi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Böttcher T., Clardy J. A chimeric siderophore halts swarming Vibrio. Angew. Chem. Int. Ed. 2014;53:3510–3513. doi: 10.1002/anie.201310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Barbeau K., Rue E.L., Bruland K.W., Butler A. Photochemical cycling of iron in the surface ocean mediated by microbial iron (III)-binding ligands. Nature. 2001;413:409. doi: 10.1038/35096545. [DOI] [PubMed] [Google Scholar]
- 182.Butler A., Theisen R.M. Iron (III)–siderophore coordination chemistry: Reactivity of marine siderophores. Coord. Chem. Rev. 2010;254:288–296. doi: 10.1016/j.ccr.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Küpper F.C., Carrano C.J., Kuhn J.U., Butler A. Photoreactivity of iron (III)-aerobactin: Photoproduct structure and iron (III) coordination. Inorg. Chem. 2006;45:6028–6033. doi: 10.1021/ic0604967. [DOI] [PubMed] [Google Scholar]
- 184.Yarimizu K., Polido G., Gardes A., Carter M.L., Hilbern M., Carrano C.J. Evaluation of photo-reactive siderophore producing bacteria before, during and after a bloom of the dinoflagellate Lingulodinium polyedrum. Metallomics. 2014;6:1156–1163. doi: 10.1039/C4MT00053F. [DOI] [PubMed] [Google Scholar]
- 185.Amin S.A., Green D.H., Hart M.C., Küpper F.C., Sunda W.G., Carrano C.J. Photolysis of iron–siderophore chelates promotes bacterial–algal mutualism. Proc. Natl. Acad. Sci. USA. 2009;106:17071–17076. doi: 10.1073/pnas.0905512106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Amin S.A., Parker M.S., Armbrust E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012;76:667–684. doi: 10.1128/MMBR.00007-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Baldani J., Baldani V.L.D., Seldin L., Döbereiner J. Characterization of Herbaspirillumseropedicae gen. Nov., a root-asociated nitrogen-fixing bacterium. Int. J. Syst. Bacteriol. 1986;36:86–93. doi: 10.1099/00207713-36-1-86. [DOI] [Google Scholar]
- 188.James E.K., Olivares F.L., Baldani J.I., Döbereiner J. Herbaspirillum, an endophytic diazotroph colonizing vascular tissue Sorghum bicolor L. Moench. J. Exp. Bot. 1997;48:785–798. doi: 10.1093/jxb/48.3.785. [DOI] [Google Scholar]
- 189.Gyaneshwar P., James E.K., Reddy P.M., Ladha J.K. Herbaspirillum colonization increases growth and nitrogen accumulation in aluminium-tolerant rice varieties. New Phytol. 2002;154:131–145. doi: 10.1046/j.1469-8137.2002.00371.x. [DOI] [Google Scholar]
- 190.Roncato-Maccari L.D., Ramos H.J., Pedrosa F.O., Alquini Y., Chubatsu L.S., Yates M.G., Rigo L.U., Steffens M.B.R., Souza E.M. Endophytic Herbaspirillum seropedicae expresses nif genes in gramineous plants. FEMS Microbiol. Ecol. 2003;45:39–47. doi: 10.1016/S0168-6496(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 191.Van Loon L.C., Bakker P.A.H.M., Pieterse C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 192.Kloepper J.W., Ryu C.M., Zhang S.A. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- 193.Pérez-García A., Romero D., de Vicente A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011;22:187–193. doi: 10.1016/j.copbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 194.Henry G., Deleu M., Jourdan E., Thonart P., Ongena M. The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell. Microbiol. 2011;13:1824–1837. doi: 10.1111/j.1462-5822.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- 195.Rahman A., Uddin W., Wenner N.G. Induced systemic resistance responses in perennial ryegrass against Magnaporthe oryzae elicited by semi-purified surfactin lipopeptides and live cells of Bacillus amyloliquefaciens. Mol. Plant Pathol. 2015;16:546–558. doi: 10.1111/mpp.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ma Z., Ongena M., Höfte M. The cyclic lipopeptide orfamide induces systemic resistance in rice to Cochliobolus miyabeanus but not to Magnaporthe oryzae. Plant Cell Rep. 2017;36:1731–1746. doi: 10.1007/s00299-017-2187-z. [DOI] [PubMed] [Google Scholar]
- 197.Mansfield J., Genin S., Magori S., Citovsky V., Sriariyanum M., Ronald P., Dow M.A.X., Verdier V., Beer S.V., Machado M.A., et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012;13:614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McManus P.S., Stockwell V.O., Sundin V.O., Jones A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002;46:443–465. doi: 10.1146/annurev.phyto.40.120301.093927. [DOI] [PubMed] [Google Scholar]
- 199.Montesinos E., Badosa E., Cabrefiga J., Planas M., Feliu L., Bardají E. Antimicrobial peptides for plant disease control. From discovery to application. In: Rajasekaran K., Cary J.W., Jaynes J.M., Montesinos E., editors. Small Wonders: Peptides for Disease Control. American Chemical Society; Washington, DC, USA: 2012. pp. 235–262. (ACS Symposium Series). [Google Scholar]
- 200.Oliveras À., Baró A., Montesinos L., Badosa E., Montesinos E., Feliu L., Planas M. Antimicrobial activity of linear lipopeptides derived from BP100 towards plant pathogens. PLoS ONE. 2018;13:e0201571. doi: 10.1371/journal.pone.0201571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shafi J., Tian H., Mingshan J. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017;31:446–459. doi: 10.1080/13102818.2017.1286950. [DOI] [Google Scholar]
- 202.Hazarika D.J., Goswami G., Gautom T., Parveen A., Das P., Barooah M., Boro R.C. Lipopeptide mediated biocontrol activity of endophytic Bacillus subtilis against fungal phytopathogens. BMC Microbiol. 2019;19:71. doi: 10.1186/s12866-019-1440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang X., Gong X.P., Li D., Lai I. Structural diversity and biological activities of cyclic depsipeptides from fungi. Molecules. 2018;23:169. doi: 10.3390/molecules23010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhao P., Xue Y., Li X., Li J., Zhao Z., Quan C., Gao W., Zu X., Bai X., Feng S. Fungi-derived lipopeptide antibiotics developed since 2000. Peptides. 2019;113:52–65. doi: 10.1016/j.peptides.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 205.Deravel J., Lemiere S., Coutte F., Krier F., Van Hese N., Bechet M., Sourdeau N., Hofte M., Lepretre A., Jacques P. Mycosubtilin and surfactin are efficient, low ecotoxicity molecules for the biocontrol of lettuce downy mildew. Appl. Environ. Microbiol. 2014;98:6255–6264. doi: 10.1007/s00253-014-5663-1. [DOI] [PubMed] [Google Scholar]
- 206.Hu F., Liu Y., Li S. Rational strain improvement for surfactin production: Enhancing the yield and generating novel structures. Microb. Cell factories. 2004;18:42. doi: 10.1186/s12934-019-1089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vindya N.G., Sharma N., Yadav M., Ethiraj K.R. Tubulins—The target for anticancer therapy. Curr. Top. Med. Chem. 2015;15:73. doi: 10.2174/1568026615666150112115805. [DOI] [PubMed] [Google Scholar]
- 208.Lone M.Y., Athar M., Manhas A., Jha P.C., Bhatt S., Shah A. In Silico exploration of Vinca domain tubulin inhibitors: A combination of 3D-QSAR-Based pharmacophore modeling, docking and molecular dynamics simulations. Chem. Sel. 2017;2:10848–10853. doi: 10.1002/slct.201701971. [DOI] [Google Scholar]
- 209.Shanmugam G., Lee S., Jeon J. Identification of potential nematicidal compounds against the pine wood nematode, Bursaphelenchus xylophilus through an in Silico approach. Molecules. 2018;23:1828. doi: 10.3390/molecules23071828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li Y., Héloir M., Zhang X., Geissler M., Trouvelot S., Jacquens L., Henkel M., Su X., Fang X., Wang Q., et al. Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol. Plant Pathol. 2019;20:1037–1050. doi: 10.1111/mpp.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cob-Calan N.N., Chi-Uluac L.A., Ortiz-Chi F., Cerqueda-García D., Navarrete-Vázquez G., Ruiz-Sánchez E., Hernández-Núñez E. Molecular docking and dynamics simulation of protein β-tubulin and antifungal cyclic lipopeptides. Molecules. 2019;24:3387. doi: 10.3390/molecules24183387. [DOI] [PMC free article] [PubMed] [Google Scholar]