Abstract
Rice (Oryza sativa L.), a major staple food for billions of people, was assessed for its phytotoxicity of copper oxide nanoparticle (CuO NPs, size < 50 nm). Under hydroponic condition, seven days of exposure to 62.5, 125, and 250 mg/L CuO NPs significantly suppressed the growth rate of rice seedlings compared to both the control and the treatment of supernatant from 250 mg/L CuO NP suspensions. In addition, physiological indexes associated with antioxidants, including membrane damage and antioxidant enzyme activity, were also detected. Treatment with 250 mg/L CuO NPs significantly increased malondialdehyde (MDA) content and electrical conductivity of rice shoots by 83.4% and 67.0%, respectively. The activity of both catalase and superoxide dismutase decreased in rice leaves treated with CuO NPs at the concentration of 250 mg/L, while the activity of the superoxide dismutase significantly increased by 1.66 times in rice roots exposed to 125 mg/L CuO NPs. The chlorophyll, including chlorophyll a and chlorophyll b, and carotenoid content in rice leaves decreased with CuO NP exposure. Finally, to explain potential molecular mechanisms of chlorophyll variations, the expression of four related genes, namely, Magnesium chelatase D subunit, Chlorophyll synthase, Magnesium-protoporphyrin IX methyltransferase, and Chlorophyllide a oxygenase, were quantified by qRT-PCR. Overall, CuO NPs, especially at 250 mg/L concentration, could affect the growth and development of rice seedlings, probably through oxidative damage and disturbance of chlorophyll and carotenoid synthesis.
Keywords: Oryza sativa, CuO nanoparticles, membrane damage, oxidative stress, chlorophyll, carotenoids, gene expression
1. Introduction
Engineered nanoparticles (ENPs) are particles with sizes ranging from 1 to 100 nm [1]. Due to their particular properties, they have been utilized for various purposes, such as in biomedicine, agriculture, and industries [2,3]. However, the extensive utilization of ENPs has resulted in their inevitable and irreversible release into the environment [4]. With ENPs expected to become more widely used, their effect on the environment and organisms has been an increasing cause of concern [5,6,7,8]. As plants interact with the atmosphere, soil, and water, they might be directly contaminated by ENPs. Thus, frequent assessment of the phytotoxicity of ENPs is considered to be of pivotal significance in environmental protection [9,10,11,12].
Among the various types of ENPs, copper oxide nanoparticles (CuO NPs) are extensively applied in various fields, such as high-temperature superconductors [13], batteries [14], gas sensors [15], lubricating oil additives [16], contamination remover [17], and catalysis [18]. It is conservatively estimated that, from 2020 to 2025, the world will consume at least 200,000–830,000 kg of CuO NPs every year [19]. The high demand and application of CuO NPs will greatly increase the possibility of their release into the environment and bioaccumulation into the food chain through plants, causing potential harm to human health [20,21]. Therefore, it is particularly important to evaluate the phytotoxicity of CuO NPs.
CuO NPs have been reported to exhibit phytotoxicity by causing a range of physiological effects, and they are likely to have different toxic effects on different plants at different target concentrations [9,22]. For example, copper oxide nanoparticles could inhibit Brassica juncea L. root and shoot growth in a dose-dependent manner [23]. In two studies, 500 mg/L copper oxide nanoparticles was shown to inhibit maize growth [24], while ˃10 mg/L copper oxide nanoparticles significantly inhibited cotton biomass accumulation [25]. It has also been reported that CuO NPs can exert negative influence on Pisum sativum [26], Schoenoplectus tabernaemontani [27], Hordeum vulgare [28], Landoltia punctate [29], and Oryza sativa (rice) [30,31]. CuO NPs were also found to inhibit root elongation of freshly germinated maize and rice seedlings, even at a low concentration of 25 mg/L [32]. The phytotoxicity of CuO NPs is usually assessed by measuring the phenotypic indexes of plant growth and development, e.g., germination rates, root elongation, and biomass accumulation [9,33,34]. However, compared to other nanoparticles, published papers focusing on the physiological and molecular changes caused by CuO NPs are still limited.
As a vital staple food in our daily lives, the safe production of rice is of critical importance. The phytotoxicity of CuO NPs in rice has been reported, and two main physiological factors are responsible for the phytotoxicity of CuO NPs: oxidative damage and impaired photosynthesis. For example, Shaw and Hossain [30] reported that CuO (<50 nm) at 0.5, 1.0, and 1.5 mM could reduce germination of rice seeds and viability of root cells but increase H2O2 and malondialdehyde (MDA) levels as well as ascorbate peroxidase (APX) and glutathione reductase (GR) activity. Furthermore, CuO NPs at 1000 mg/L could reduce photosynthetic rate, transpiration rate, and photosynthetic pigment content and increase the content of MDA and proline in rice [31]. However, the molecular mechanisms by which CuO NPs cause the phytotoxicity are still unclear. More studies are needed to improve our understanding of the mechanisms.
The aims of this study were to (i) investigate the phenotypic changes caused by short-time exposure of CuO NPs to rice seedlings at early stage under hydroponic culture; (ii) examine the changes of physiological factors, e.g., oxidative damage and impaired photosynthesis, caused by CuO NPs in seedlings; and (iii) evaluate the potential mechanisms of the decreased chlorophyll in rice leaves exposed to CuO NPs at the molecular level.
2. Materials and Methods
2.1. CuO NP Characterization
CuO NPs (<50 nm, Product #544868) were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). The morphology of CuO NPs was elliptic or spherical with size ranging from 40 to 80 nm as observed by transmission electron microscope (TEM). The purity of CuO NPs was greater than 97%, and the pH of CuO NP suspensions (2000 mg/L) was 6.36 ± 0.02. Details of the TEM figure and the zeta potential of CuO NP suspensions have been published previously [21].
2.2. Culture of Rice Plants
Seeds of rice (Oryza sativa spp. japonica) were harvested in 2018 and stored in our laboratory. Their germination rate was proved to be higher than 90% before experiments. The seeds were first sterilized using sodium hypochlorite (10%) for 10 min, then rinsed with sterilized deionized (DI) water five times to remove the remaining disinfectants, and germinated in a 100 mm × 15 mm Petri dish containing 5 mL sterilized DI water in a growth chamber in the dark at 25 °C. After four days, 12 germinated seeds were transplanted to a 200 mL beaker with 200 mL Yoshida nutrient solution (pH 5.5) [35]. All the rice seedlings were grown in a chamber under the following conditions: 60%–70% relative humidity, 25/20 °C day/night temperature,14 h photoperiod, and a light intensity of 16,500 lx. In the next 10 days, the Yoshida nutrient solution in the 200 mL beaker was renewed every 2 days.
Before treatment, CuO NP suspensions were diluted in different contents of Yoshida nutrient solution (pH 5.5) at the concentration of 0, 62.5, 125, and 250 mg/L and sonicated using an ultrasonic vibration (100 W, 40 KHz) for 45 min. The supernatant from 250 mg/L CuO NP suspensions (S250) was obtained by sonicating (45 min) in Yoshida nutrient solution (pH 5.5), centrifuging for 30 min at 15,000 rpm, and then filtering through 0.22 μm filters. The 14-day-old rice seedlings were subjected to 0, 62.5, 125, and 250 mg/L CuO NPs and the supernatant from 250 mg/L CuO NP suspensions and renewed every 2 days. All the experiments were conducted with three biological replicates. After another 7 days of exposure, the 21-day-old seedlings from every beaker were harvested and washed thoroughly with DI water. The roots and leaves of the rice seedlings were separated, and their fresh weights (FW) were recorded.
2.3. Integrity of the Cell Membrane System of Rice
To evaluate the integrity of cell membrane, the lipid peroxidation level and ion leakage in rice roots and leaves were quantified. The lipid peroxidation was examined by the MDA method described by Heath and Packer [36] with minor modifications. Briefly, 0.5 g fresh tissue of rice roots and leaves was grounded in 0.1% (w/v) trichloroacetic acid (TCA). After being centrifuged for 20 min at 10,000 g, 20% TCA (1 mL) with 0.5% thiobarbituric acid (TBA) was added to 1 mL of the supernatant. After the addition of 100 μL 4% butylated hydroxyltoluene (BHT, in ethanol), the mixture was immediately heated in a water bath at 95 °C for 30 min, then quickly cooled and centrifuged for 15 min at 10,000 g. The supernatant was taken out to measure the absorbance at 532 and 600 nm with a Beckman Du-640 spectrometer (Beckman Coulter, Brea, CA). Then, 0.25% TBA in 10% TCA was used as a blank; the extinction coefficient was 155 mM−1 cm−1. Ion leakage from the root and leaf was measured following the method by Zhao et al. [37] with minor modifications. Five root segments (around 1 cm long) and 10 leaf segments (around 0.5 cm diameter) were cut and rinsed three times with deionized water to remove the leakage ion due to the immediate injury inflicted on the tissue during cutting. The segments were then incubated in 10 mL vials containing 8 mL of deionized water for 3 h in a rotary shaker at room temperature. After being incubated for 3 h, the conductivity of the solution was measured, and it was then boiled at 100 °C for 30 min. After the solution was cooled to room temperature, the conductivity was measured again, and the results were expressed as relative conductivity [(conductivity after incubation/conductivity before boiling) × 100].
2.4. Antioxidant Enzyme Activity of the Rice Seedlings
To analyze the activity of the antioxidant enzyme in rice exposed to CuO NPs, 1 g of fresh roots and leaves were grounded in 10 mL precooled potassium phosphate buffer (PBS, 100 mM, pH 6.8) and then centrifuged for 20 min at 10,000 g. The supernatant was used to examine the activity of the antioxidant enzymes. The catalase (CAT, EC 1.11.1.6) was measured by monitoring the degradation of H2O2 at 240 nm, whose extinction coefficient was 39.4 mM−1 cm−1 [38], while the superoxide dismutase (SOD, EC 1.11.1.7) activity was quantified by monitoring the inhibition of nitroblue tetrazolium at 560 nm according to Beyer et al. [39]. The activity of guaiacol peroxidase (POD, EC 1.15.1.1) was detected by monitoring the formation of guaiacol dehydrogenation (extinction coefficient 6.39 mM−1 cm−1) at 420 nm [40]. The activities of CAT, SOD, and POD were performed with a Beckman Du-640 spectrometer (Beckman Coulter, Brea, CA, USA) at 25 °C.
2.5. Chlorophyll and Carotenoid Content
The content of chlorophyll and carotenoids in rice leaves was detected according to Nair and Chung [41]. Rice leaves with a weight of 0.05 g were incubated in 95% (v/v) ethanol (10 mL) for 3 days in the dark at 4 °C. After centrifugation, the absorption of the supernatant was measured by a Beckman Du-640 spectrometer (Beckman Coulter, Brea, CA, USA) at 470, 665, and 649 nm. Then, the content of chlorophyll and carotenoids was calculated according to Lichtenthaler and Wellburn [42].
2.6. Quantitative Real-Time PCR (qRT-PCR) of the Chlorophyll Synthesis Genes
The RNA was extracted using a TRIzol reagent (Bioteke, Beijing, China) following the manufacturer’s instructions. One microgram of the total RNA was reverse-transcribed into cDNA by a PrimeScript™ RT reagent kit (TaKaRa, Tokyo, Japan) according to the manufacturer’s protocol. Finally, qRT-PCR of four chlorophyll synthesis genes, namely, Magnesium chelatase D subunit (CHLD), Chlorophyll synthase (CHLG), Magnesium-protoporphyrin IX methyltransferase (CHLM), and Chlorophyllide a oxygenase (CAO), were performed. The ACT2 (actin-2, LOC4349087) [3] of rice plants was used as an internal reference. The primer pairs used in qRT-PCR analysis of CHLD, CHLG, CHLM, and CAO have been detailed in a previous study [33]. The qRT-PCR experiment was carried out with an Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). Briefly, a total of 10 μL reaction volume was prepared with 0.2 μM forward and reverse primers, 5 μL SYBR Premix Ex Taq™ II (TaKaRa, Tokyo, Japan), and 1 μL template cDNA. The amplification program was as follows: initial denaturing at 95 °C for 5 min, 40 cycles of 10 s at 95 °C, 10 s at 60 °C, and 20 s at 72 °C. The fluorescence data were collected, and the relative expression levels of these genes were calculated with the formula 2−△△Ct [43]. In the qRT-PCR analysis, the biological replicates were measured three times.
2.7. Statistical Analysis
The results are shown as mean ± standard deviation (SD). After the normality and homogeneity tests, the data were subjected to one-way ANOVA test in the SPSS software (Version 17.0) (SPSS, Chicago, IL, USA). Tukey’s honestly significant difference (HSD) multiple comparisons was utilized to conduct multiple comparisons. The significance level was p < 0.05.
3. Results and Discussion
3.1. Biomass Accumulation of Rice Seedlings
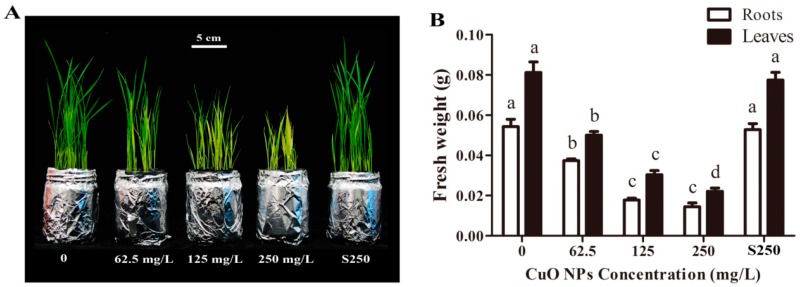
After seven days, the seedlings exposed to CuO NPs showed phenotypic changes compared to the control plants (Figure 1A). Specifically, roots and shoots were shorter than those in the control, suggesting CuO NPs were toxic to rice plants, even at a low concentration of 62.5 mg/L. The statistical results showed that 62.5, 125, and 250 mg/L CuO NPs reduced the weight of rice roots by 31.1%, 67.2%, and 73.5%, respectively, compared to the control (p < 0.05) (Figure 1B). In addition, the weight of rice leaves treated with 62.5, 125, and 250 mg/L CuO NPs was 38.4%, 62.7%, and 72.8% lower, respectively, compared to the control (p < 0.05), suggesting that the phytotoxicity of CuO NPs in rice seedlings was dose-dependent. Indeed, such a dose-dependent manner of phytotoxicity has also been reported in various plants, e.g., Schoenoplectus tabernaemontani [27], Hordeum vulgare L. [28], wheat [44], and rice [30].
Figure 1.
Effects of CuO NPs on the growth of rice seedlings. (A) Phenotype of rice seedlings; (B) Fresh weight of the rice roots and leaves exposed to 62.5, 125 and 250 mg/L CuO NPs, supernatant from 250 mg/L CuO NPs suspensions and without CuO NPs, for 7 days. The bars show the mean and SD of triplicate samples. Different letters represent significant differences between the means of the treatments (p < 0.05, Tukey-HSD).
The toxicity of Cu2+ at a high level in plants has been reported previously [45,46]. It has been found that CuO NPs can release Cu ions in aqueous solutions [47,48], which may be a factor in causing the toxicity of CuO NPs in plants. In this study, the plants treated with supernatant from 250 mg/L CuO NP suspensions showed no observable phenotypic changes and changes in fresh weight of the rice seedlings compared to the control. Shi et al. [29] reported that CuO NP suspension could release 0.16 mg/L of Cu2+, which was not sufficient to lead to the phytotoxicity of CuO NPs in Landoltia punctata. Zhang et al. [27] also showed that the concentration of 0.06 mg/L, the level released from CuO NP suspension, did not affect the growth of Schoenoplectus tabernaemontani. Our previous study showed that the released Cu2+ (0.11 ± 0.04 mg·L−1) of 2000 mg/L CuO NPs showed no significant inhibition effects on the root growth of both maize and rice [32]. Therefore, these results suggest that the release of Cu2+ is probably not the main reason for the phytotoxicity caused by CuO NPs.
3.2. Integrity of the Cell Membrane System of Rice
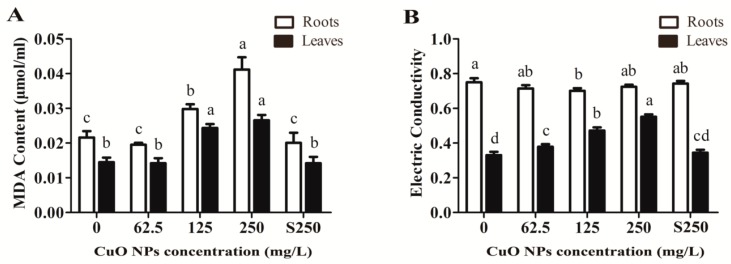
Under adverse conditions, plants can produce reactive oxygen species (ROS) that can cause damage to biological macromolecules and the cell membrane system [26]. As shown in Figure 2A, 125 and 250 mg/L CuO NPs significantly increased the MDA content of the rice shoots by 69.7% and 83.4%, respectively, compared to unexposed plants (control) (p < 0.05). Correspondingly, the levels of electrical conductivity in rice leaves treated with 62.5, 125, and 250 mg/L CuO NPs also increased by 14.6%, 42.7%, and 67.0%, respectively, compared to the control (p < 0.05) (Figure 2B). On the other hand, MDA content was relatively higher in rice roots exposed to 125 and 250 mg/L CuO NPs. Previous studies have also found decreases in plasma membrane integrity induced by CuO NPs in other plants, e.g., Arabidopsis Thaliana [49] and Hordeum vulgare L. [28]. Shaw and Hossain also reported that MDA content significantly increased in rice leaves exposed to CuO NPs for both 7 and 14 days [30]. Only 125 mg/L CuO NPs significantly reduced the electrical conductivity of rice roots by 6.5% (0.70 ± 0.01) compared to the control (0.75 ± 0.02), while the electrical conductivity showed unremarkable changes in rice roots exposed to 62.5 (0.72 ± 0.02) and 250 mg/L (0.72 ± 0.01) CuO NPs. Thus, it is supposed that rice roots may have probably generated a defense system to maintain ionic balance inside and outside the cells.
Figure 2.
The effects of CuO NPs on the integrity of rice seedlings cells membrane system. (A) MDA content; (B) the electrical conductivity of rice seedlings exposed to 62.5, 125 and 250 mg/L CuO NPs, supernatant from 250 mg/L CuO NPs suspensions and have no CuO NPs for 7 days. The bars indicates the mean and SD of triplicate samples. Different letters represent significant differences between the means of treatments (p < 0.05, Tukey-HSD).
3.3. Antioxidant Enzyme Activity of Rice Seedlings
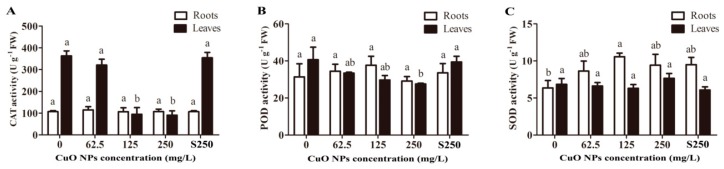
The integrity destruction of the rice cell membrane system was probably caused by increased reactive oxygen species under CuO NP treatment. As shown in Figure 3A, 125 and 250 mg/L CuO NPs significantly decreased the CAT activity of the rice leaves by 73.9% (94.84 ± 53.17) and 75.0% (90.71 ± 34.39), respectively, compared to the unexposed plants (363.18 ± 39.44) (p < 0.05). Only a high concentration of 250 mg/L CuO NPs significantly decreased the POD activity of the rice leaves by 32.3% (27.57 ± 0.69), while 62.5 (33.45 ± 0.85) and 125 mg/L (29.72 ± 4.13) CuO NPs showed no significant change compared to the control (Figure 3C), suggesting that rice leaves may have other active defense systems to protect them from ROS-mediated oxidative stress. Additionally, unremarkable changes were found in CAT and POD activities between the roots exposed to CuO NPs and the control roots (Figure 3A,C). SOD activity showed different trends to CAT and POD activity in rice seedlings. As shown in Figure 3B, the SOD activity was significantly upregulated by 166% (p < 0.05) in the rice roots treated with 125 mg/L CuO NPs, but no changes were observed in the rice leaves. Based on previous studies, many factors have been suggested as potential mechanisms of CuO NPs causing phytotoxicity, e.g., DNA damage, metal ions released from NPs, ROS generation, and oxidative stress [21]. Among them, oxidative stress has attracted considerable attention [28,30]. To protect themselves from the harm of excess H2O2, plants usually activate their defense system, including enzymatic (CAT, POD, etc.) and nonenzymatic (cytochrome f, proline, carotenoids, etc.) ways [50]. As important antioxidant enzymes, SOD can catalyze O2− to H2O2, while CAT and POD can catalyze H2O2 to H2O [51]. The increasing SOD activity in the rice roots exposed to CuO NPs suggested that rice seedlings could generate significant amounts of ROS. However, there was no change in the activity of CAT and POD in rice roots exposed to CuO NPs. This result can probably be explained by the excess H2O2 existing in rice roots, which exceeded the maximum catalytic ability of CAT and POD.
Figure 3.
Effects of CuO NPs exposed to antioxidant enzyme activities. (A) CAT; (B) SOD, and (C) POD are the activities in the roots and shoots of rice seedlings exposed to 62.5, 125 and 250 mg/L CuO NPs, supernatant from 250 mg/L CuO NPs suspensions and without treatment for 7 days. The bars indicate the mean and SD of triplicate samples. Different letters represent significant differences between the means of treatments (p < 0.05, Tukey-HSD).
3.4. Chlorophyll and Carotenoid Content of Rice Leaves
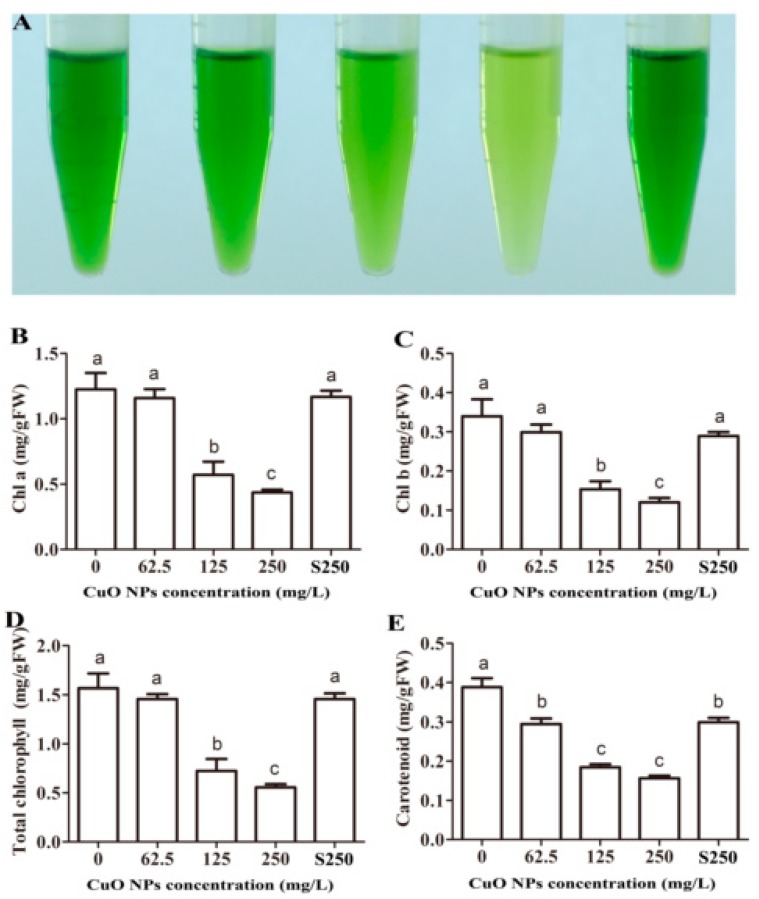
The rice leaves exposed to increasing concentrations of CuO NPs gradually turned to yellow (Figure 1A). Chlorophyll and carotenoid extracts from exposed rice leaves also exhibited different colors compared to the control group and the plants treated with supernatant from 62.5 to 250 mg/L CuO NP suspensions (Figure 4A). The chlorophyll a (Chl-a) content in rice leaves treated with 125 and 250 mg/L CuO NPs decreased significantly by 53.5% and 70.4%, respectively, while the chlorophyll b (Chl-b) content was 54.8% and 64.7% lower, respectively, than the control (p < 0.05) (Figure 4B,C). The total chlorophyll content exhibited the same pattern as both Chl-a and Chl-b (Figure 4D). The carotenoid content decreased significantly by 24.1%, 52.5%, and 59.7% in rice leaves exposed to 62.5, 125, and 250 mg/L CuO NPs, respectively (p < 0.05) (Figure 4E). The photosynthetic yield and the content of Chl-a and Chl-b are considered important indicators in assessing the phytotoxicity of NPs, e.g., Ag NPs [52] and ZnO NPs [1,33]. Shaw et al. also reported that Hordeum vulgare L. with exposure to 0.5, 1.0, and 1.5 mM CuO NPs for 20 days had significantly decreased chlorophyll content [28]. Consistent with these reports, the decrease in chlorophyll content was also found in rice leaves exposed to CuO NPs in our study. Furthermore, photosynthesis in the rice seedlings was constrained, and plant growth was inhibited.
Figure 4.
Effects of CuO NPs on the chlorophyll and carotenoid contents in rice leaves. (A) Photographs of chlorophyll extracted solution of 21-days-old rice leaves treated with 0, 62.5, 125, 250 mg/L CuO NPs, and supernatant from 250 mg/L CuO NPs (from left to right); (B) Chl-a contents; (C) Chl-b contents; (D) Total chlorophyll contents; (E) Carotenoid contents. The bars indicate the mean and SD of triplicate samples. Different letters represent significant differences between the means of the treatments (p < 0.05, Tukey-HSD).
3.5. Synthesis of Chlorophyll and Carotenoid in Rice Leaves
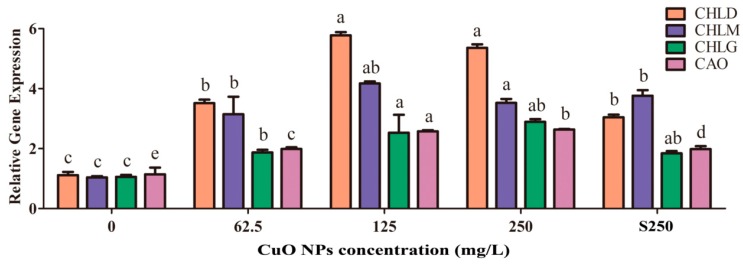
Four chlorophyll biosynthesis genes, namely, CHLD, CHLG, CHLM, and CAO, were found to be significantly upregulated according to different magnitudes of CuO NPs at 62.5, 125, and 250 mg/L. Specifically, 125 mg/L CuO NPs exhibited the maximum variation, with the level of CHLD, CHLG, CHLM, and CAO genes increasing by 5.18, 2.39, 4.02, and 2.25 times, respectively, compared to the control (p < 0.05) (Figure 5). Although the chlorophyll content in rice leaves treated with 250 mg/L CuO NPs was significantly less than those treated with 125 mg/L CuO NPs, the level of these four genes in plants treated with 250 mg/L CuO NPs was quite similar to that treated with 125 mg/L CuO NPs. In addition, the levels of CHLD, CHLG, CHLM, and CAO genes in plants treated with the supernatant of 250 mg/L CuO NPs also increased by 2.72, 1.74, 3.62, and 1.73 times compared to the control. Such a different pattern of chlorophyll content and expression level suggests that these rice seedlings exposed to CuO NPs could regulate the expression of some genes to adapt to stressed environment.
Figure 5.
Effects of CuO NPs on the Chlorophyll synthesis genes in rice leaves treated with 0, 62.5, 125, 250 mg/L CuO NPs, or supernatant of 250 mg/L CuO NPs. The bars provide the mean and SD of triplicate samples. Different letters represent significant differences between the means of the treatments (p < 0.05, Tukey-HSD).
4. Conclusions
This study aimed to assess the effects of CuO NPs on rice seedlings by estimating phenotypic changes and the relevant physiological responses. The results indicated that 62.5, 125, and 250 mg/L CuO NPs suspended in Yoshida nutrient solution could significantly inhibit the growth of rice seedlings and chlorophyll content. The oxidative damage was also seen in rice shoots exposed to CuO NPs, and the MDA content and electrical conductivity were significantly upregulated compared to the control. The activity of SOD was also greater in rice roots exposed to 125 mg/L CuO NPs. The content of chlorophyll, including Chl-a and Chl-b, and carotenoids decreased, while four chlorophyll synthesis genes, namely, CHLD, CHLG, CHLM, and CAO, significantly increased. Overall, the findings in this study could provide evidence for risk assessment and guide applications of CuO NPs in agriculture. However, the toxicity in rice seedlings was obtained by short exposure to high levels of CuO NPs, which may not exist in the natural environment. The potential impacts of long-term exposure to CuO NPs at low concentrations on edible plants and their bioaccumulation in edible portions need to be investigated in further research.
Author Contributions
J.C. and Y.G. conceived and designed the experiments; Z.Y. and Y.X. performed the experiments; T.J. and Y.Z. analyzed the data; J.C. wrote the paper; and Y.G. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (31570452), Ministry of Science and Technology (2014DFA31740), the Bureau of Science and Technology of Jilin Province (20190201264JC and 20180101347JC), and the Bureau of Education of Jilin Province (JJKH20190263KJ).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wang X., Yang X., Chen S., Li Q., Wei W., Hou C., Xiao G., Li W., Wang S. Zinc Oxide Nanoparticles Affect Biomass Accumulation and Photosynthesis in Arabidopsis. Front. Plant Sci. 2015;6:559. doi: 10.3389/fpls.2015.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White J.C., Gardeatorresdey J. Achieving food security through the very small. Nat. Nanotechnol. 2018;13:627–629. doi: 10.1038/s41565-018-0223-y. [DOI] [PubMed] [Google Scholar]
- 3.Nair P.M., Chung I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere. 2014;112:105–113. doi: 10.1016/j.chemosphere.2014.03.056. [DOI] [PubMed] [Google Scholar]
- 4.Peralta-Videa J.R., Zhao L., Lopez-Moreno M.L., de la Rosa G., Hong J., Gardea-Torresdey J.L. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011;186:1–15. doi: 10.1016/j.jhazmat.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs R., Meesters J.A., Ter Braak C.J., Van D.M.D., Van D.V.H. Combining exposure and effect modelling into an integrated probabilistic environmental risk assessment for nanoparticles. Environ. Toxicol. Chem. 2016;35:2958–2967. doi: 10.1002/etc.3476. [DOI] [PubMed] [Google Scholar]
- 6.Dev A., Srivastava A.K., Karmakar S. Nanomaterial toxicity for plants. Environ. Chem. Lett. 2017;16:1–16. doi: 10.1007/s10311-017-0667-6. [DOI] [Google Scholar]
- 7.Nowack B., Bucheli T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007;150:5–22. doi: 10.1016/j.envpol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Mckee M.S., Filser J. Impacts of metal-based engineered nanomaterials on soil communities. Environ. Sci. Nano. 2016;3:506–533. doi: 10.1039/C6EN00007J. [DOI] [Google Scholar]
- 9.Lin D., Xing B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H., Lu L., Zhao X., Zhao S., Gu X., Du W., Wei H., Ji R., Zhao L. Metabolomics Reveals the “Invisible” Responses of Spinach Plants Exposed to CeO2 Nanoparticles. Environ. Sci. Technol. 2019;53:6007–6017. doi: 10.1021/acs.est.9b00593. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Tappero R.V., Acerbo A.S., Yan H., Unrine J.M. Effect of CeO2 nanomaterial surface functional groups on tissue and subcellular distribution of Ce in tomato (Solanum lycopersicum) Environ. Sci. Nano. 2019;6:273–285. doi: 10.1039/C8EN01287C. [DOI] [Google Scholar]
- 12.Štefaniæ P.P., Jarnević M., Cvjetko P., Biba R., Šikić S., Tkalec M., Cindrić M., Letofsky-Papst I., Balen B. Comparative proteomic study of phytotoxic effects of silver nanoparticles and silver ions on tobacco plants. Environ. Sci. Pollut. Res. 2019;26:22529–22550. doi: 10.1007/s11356-019-05552-w. [DOI] [PubMed] [Google Scholar]
- 13.Dar M.A., Kim Y.S., Kim W.B., Sohn J.M., Shin H.S. Structural and magnetic properties of CuO nanoneedles synthesized by hydrothermal method. Appl. Surf. Sci. 2008;254:7477–7481. doi: 10.1016/j.apsusc.2008.06.004. [DOI] [Google Scholar]
- 14.Da-Wei Z., Tang-Hong Y., Chun-Hua C. Cu nanoparticles derived from CuO electrodes in lithium cells. Nanotechnology. 2005;16:2338–2341. doi: 10.1088/0957-4484/16/10/057. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhuri A., Gupta V., Sreenivas K., Kumar R., Patanjali P.K. Response speed of SnO2-based H2S gas sensors with CuO nanoparticles. Appl. Phys. Lett. 2004;84:1180–1182. doi: 10.1063/1.1646760. [DOI] [Google Scholar]
- 16.Jatti V.S., Singh T.P. Copper oxide nano-particles as friction-reduction and anti-wear additives in lubricating oil. J. Mech. Sci. Technol. 2015;29:793–798. doi: 10.1007/s12206-015-0141-y. [DOI] [Google Scholar]
- 17.Reddy K.J., McDonald K.J., King H. A novel arsenic removal process for water using cupric oxide nanoparticles. J. Colloid Interf. Sci. 2013;397:96–102. doi: 10.1016/j.jcis.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 18.Suribabu J., Sekarpandi S., Laxmidhar R., Tathagata M., Santu M., Raja M., Prasenjit S., Tharmalingam P. CuO nanoparticles catalyzed C-N, C-O, and C-S cross-coupling reactions: Scope and mechanism. J. Org. Chem. 2010;40:1971–1976. doi: 10.1021/jo8024253. [DOI] [PubMed] [Google Scholar]
- 19.The Global Market Forecast from 2010 to 2025: Production Volumes, Prices, Future Projections and End User Markets. [(accessed on 15 February 2020)]; Available online: https://www.futuremarketsinc.com/
- 20.Nair R., Varghese S.H., Nair B.G., Maekawa T., Yoshida Y., Kumar D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010;179:154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
- 21.Liu J., Dhungana B., Cobb G.P. Environmental Behavior, Potential Phytotoxicity, and Accumulation of Copper Oxide Nanoparticles and Arsenic in Rice Plants. Environ. Toxicol. Chem. 2018;37:11–20. doi: 10.1002/etc.3945. [DOI] [PubMed] [Google Scholar]
- 22.Farzad A., Samira B., Nurhidayatullaili M.J., Shukor J.A., Golestan H.F.S., Ali B. Effects of Engineered Nanomaterials on Plants Growth: An Overview. Sci. World J. 2014;2014:641759. doi: 10.1155/2014/641759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair P.M.G., Chung I.M. Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.) Ecotoxicol. Environ. Saf. 2015;113:302–313. doi: 10.1016/j.ecoenv.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Sui H.J., Zhang J.Z., Wang Z.Y. Toxicity of Copper Oxide Engineered Nanoparticles to Maize (Zea mays L.) at Different Aging Times. Adv. Mater. Res. 2014;881:972–975. doi: 10.4028/www.scientific.net/AMR.881-883.972. [DOI] [Google Scholar]
- 25.Van N., Ma C., Shang J., Rui Y., Liu S., Xing B. Effects of CuO nanoparticles on insecticidal activity and phytotoxicity in conventional and transgenic cotton. Chemosphere. 2016;144:661–670. doi: 10.1016/j.chemosphere.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Ochoa L., Medina-Velo I.A., Barrios A.C., Bonilla-Bird N.J., Hernandez-Viezcas J.A., Peralta-Videa J.R., Gardea-Torresdey J.L. Modulation of CuO nanoparticles toxicity to green pea (Pisum sativum Fabaceae) by the phytohormone indole-3-acetic acid. Sci. Total. Environ. 2017;598:513–524. doi: 10.1016/j.scitotenv.2017.04.063. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D., Tao H., Fei X., Chen C., Gersberg R.M., Yu L., Ng W.J., Tan S.K. Uptake and accumulation of CuO nanoparticles and CdS/ZnS quantum dot nanoparticles by Schoenoplectus tabernaemontani in hydroponic mesocosms. Ecol. Eng. 2014;70:114–123. doi: 10.1016/j.ecoleng.2014.04.018. [DOI] [Google Scholar]
- 28.Shaw A.K., Ghosh S., Kalaji H.M., Bosa K., Brestic M., Zivcak M., Hossain Z. Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.) Environ. Exp. Bot. 2014;102:37–47. doi: 10.1016/j.envexpbot.2014.02.016. [DOI] [Google Scholar]
- 29.Jiyan S., Abid A.D., Kennedy I.M., Hristova K.R., Silk W.K. To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ. Pollut. 2011;159:1277–1282. doi: 10.1016/j.envpol.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw A.K., Hossain Z. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere. 2013;93:906–915. doi: 10.1016/j.chemosphere.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 31.Da Costa M.V.J., Sharma P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica. 2016;54:110–119. doi: 10.1007/s11099-015-0167-5. [DOI] [Google Scholar]
- 32.Yang Z., Chen J., Dou R., Gao X., Mao C., Wang L. Assessment of the Phytotoxicity of Metal Oxide Nanoparticles on Two Crop Plants, Maize (Zea mays L.) and Rice (Oryza sativa L.) Int. J. Environ. Res. Public Health. 2015;12:15100–15109. doi: 10.3390/ijerph121214963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J., Dou R., Yang Z., You T., Gao X., Wang L. Phytotoxicity and bioaccumulation of zinc oxide nanoparticles in rice (Oryza sativa L.) Plant Physiol. Biochem. 2018;130:604–612. doi: 10.1016/j.plaphy.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Castiglione M.R., Giorgetti L., Geri C., Cremonini R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanopart. Res. 2011;13:2443–2449. doi: 10.1007/s11051-010-0135-8. [DOI] [Google Scholar]
- 35.Shouichi Y. Laboratory manual for physiological studies of rice. Int. Rice Res. Inst. 1976 doi: 10.1007/978-3-642-22067-8_9. [DOI] [Google Scholar]
- 36.Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhao L., Peng B., Hernandez-Viezcas J.A., Rico C., Sun Y., Peralta-Videa J.R., Tang X., Niu G., Jin L., Varela-Ramirez A., et al. Stress response and tolerance of Zea mays to CeO2 nanoparticles: Cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano. 2012;6:9615–9622. doi: 10.1021/nn302975u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallego S.M., Benavídes M.P., Tomaro M.L. Effect of heavy metal ion excess on sunflower leaves: Evidence for involvement of oxidative stress. Plant Sci. 1996;121:151–159. doi: 10.1016/S0168-9452(96)04528-1. [DOI] [Google Scholar]
- 39.Beyer W.F., Jr., Fridovich I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 40.Egley G.H., Paul R.N., Jr., Vaughn K.C., Duke S.O. Role of peroxidase in the development of water-impermeable seed coats in Sida spinosa L. Planta. 1983;157:224–232. doi: 10.1007/BF00405186. [DOI] [PubMed] [Google Scholar]
- 41.Nair P.M., Chung I.M. Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ. Sci. Pollut. Res. Int. 2014;21:8858–8869. doi: 10.1007/s11356-014-2822-y. [DOI] [PubMed] [Google Scholar]
- 42.Lichtenthaler H.K., Wellburn A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983;11:591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- 43.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Dimkpa C.O., Mclean J.E., Latta D.E., Manangón E., Britt D.W., Johnson W.P., Boyanov M.I., Anderson A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012;14:1–15. doi: 10.1007/s11051-012-1125-9. [DOI] [Google Scholar]
- 45.Mocquot B., Vangronsveld J., Clijsters H., Mench M. Copper toxicity in young maize (Zea mays L.) plants: Effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil. 1996;182:287–300. doi: 10.1007/BF00029060. [DOI] [Google Scholar]
- 46.Khatun S., Ali M.B., Hahn E.-J., Paek K.-Y. Copper toxicity in Withania somnifera: Growth and antioxidant enzymes responses of in vitro grown plants. Environ. Exp. Bot. 2008;64:279–285. doi: 10.1016/j.envexpbot.2008.02.004. [DOI] [Google Scholar]
- 47.Wang Z., von dem Bussche A., Kabadi P.K., Kane A.B., Hurt R.H. Biological and Environmental Transformations of Copper-Based Nanomaterials. ACS Nano. 2013;7:8715–8727. doi: 10.1021/nn403080y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunawan C., Teoh W.Y., Marquis C.P., Amal R. Cytotoxic Origin of Copper(II) Oxide Nanoparticles: Comparative Studies with Micron-Sized Particles, Leachate, and Metal Salts. Acs Nano. 2011;5:7214–7225. doi: 10.1021/nn2020248. [DOI] [PubMed] [Google Scholar]
- 49.Nair P.M.G., Chung I.M. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ. Sci. Pollut. Res. 2004;21:12709–12722. doi: 10.1007/s11356-014-3210-3. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi M.A., Asada K. Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch. Biochem. Biophys. 1983;226:558. doi: 10.1016/0003-9861(83)90325-9. [DOI] [PubMed] [Google Scholar]
- 51.Foyer C.H., Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100. doi: 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navarro E., Wagner B., Odzak N., Sigg L., Behra R. Effects of Differently Coated Silver Nanoparticles on the Photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Technol. 2015;49:8041–8047. doi: 10.1021/acs.est.5b01089. [DOI] [PubMed] [Google Scholar]