Abstract
There is currently no consensus regarding the effects of passive smoking exposure on cognitive function in older adults. We evaluated 7000 permanent residents from six regions within Zhejiang Province, China, aged ≥60 years, without cognitive impairment at baseline and during follow-up examinations for two years. The Chinese version of the Mini-Mental State Examination was used to assess the participants’ cognitive function. Multivariate regression analyses were carried out to calculate the adjusted relative risks (RRs) as measures of the association between passive smoking exposure and cognitive impairment after adjusting for potential confounders. The results showed an association between passive smoking exposure in the living environment and increased risk of cognitive impairment (RR: 1.16; 95% confidence interval (CI): 1.01–1.35). No dose–response relationship between the cumulative dose of passive smoking exposure (days) and cognitive impairment was observed. The results of stratified analyses suggested a harmful effect of passive smoking exposure on cognitive function in non-smokers (RR: 1.24; 95% CI: 1.06–1.46), but not in smokers (RR: 1.11; 95% CI: 0.71–1.92). Therefore, passive smoking exposure increased the risk of cognitive impairment in older adults, especially non-smokers. More effective measures to restrict smoking in the living environment should be developed and implemented.
Keywords: passive smoking, cognitive impairment, longitudinal study, hazard ratio, dementia, older adults, aging
1. Introduction
With increasing population aging worldwide, cognitive impairment is becoming an increasingly common and important health-care challenge for older people, with negative effects on the activities of daily living of older people and burdens on both their families and society. Therefore, identification of the factors associated with cognitive impairment is imperative to reduce risks in older people.
Passive smoking, also called “secondhand smoke,” refers to the mixed smoke released from the tobacco products of other smokers. Secondhand smoke contains more than 7000 harmful chemicals and dozens of carcinogens [1,2]. Exposure to passive smoking can increase the risk of developing or dying from diseases such as cancer (particularly lung and breast cancer) and cardiovascular diseases (e.g., stroke, angina, and hypertension), as well as cognitive impairment [3,4,5]. Positive correlations between tobacco exposure and cognitive impairment, dementia, and other neurodegenerative diseases have also been reported [6,7]. However, some studies have suggested a protective role of nicotine against cognitive impairment [8,9]. Therefore, in this prospective cohort study, we recruited nearly 10,000 older adults from six counties in Zhejiang Province of China, in 2014, to further explore the relationship between passive smoking exposure and cognitive impairment in older adults.
2. Materials and Methods
2.1. Participants
The participants were enrolled in the Zhejiang Major Public Health Surveillance Program (ZJMPHS), a prospective study of health issues among older adults that started in 2014, with follow-up investigations conducted in 2015 and 2016. Detailed information on the program has been provided previously [10,11]. At baseline (2014), the ZJMPHS survey included 9353 permanent residents aged ≥60 years, from six counties in Zhejiang Province. A total of 7947 participants with no cognitive impairment at baseline were followed up for two years. However, 447 (5.6%) participants died, and 500 (6.3%) were lost to follow-up. The remaining 7000 participants who completed the baseline and follow-up investigations were included in the analysis. Written informed consent was obtained from each participant. The program was approved by the Ethics Committee of the Zhejiang Provincial Center for Disease Control and Prevention (2018-50).
2.2. Assessment of Passive Smoking Exposure in Living Environments
Passive smoking exposure was assessed by asking the participants, “Has anyone living with you smoked in recent years?” Participants who answered “yes” were further asked the following questions: (1) “How many days a week are you generally exposed to passive smoking?”; (2) “How often are you exposed to passive smoking on these days?”; and (3) “How many years have you had this exposure to passive smoking?” The cumulative exposures to passive smoking were calculated according to the responses to these three questions, and the participants were further divided into three groups, by the 25th quartile (40 days) and the 75th quartile (300 days).
2.3. Assessment of Cognitive Function
The Chinese version of the Mini-Mental State Examination (CMMSE) was used to assess the cognitive function of the participants. CMMSE was translated from MMSE with full consideration of the Chinese language and culture by a bi-national team of psychiatrists and social scientists in the 1980s. It showed high validity in the identification of cognitive function, with diagnostic sensitivity of 80–90% and specificity of 70–80% [12,13]. It includes a total of 30 items, with a full score of 30, and higher scores indicating better cognitive function. The following education-specific cutoff values were used to define cognitive impairment: ≤17 points for those with no education, ≤20 for those with primary education only, and ≤24 for those with education beyond the primary level [12].
2.4. Covariates
The following participant information was collected: (1) demographic data, including age, sex, ethnic group, body mass index (BMI), education level (illiterate or semiliterate, primary school, junior high school, high school graduation or higher), marital status (unmarried, married, widowed, or divorced), job (never worked, farmer, housework, standard work, and other), and family income; (2) potential covariates of passive smoking exposure, including participation in group activities (never, occasionally, and frequently), smoking status (non-smokers, current smokers, and ex-smokers), alcohol consumption (non-drinkers, current drinkers, and ex-drinkers), tea consumption (non-drinkers, current drinkers, and ex-drinkers), physical exercise, or work. We used patient medical records to determine the presence of underlying diseases (stroke, high blood pressure, hyperlipidemia, diabetes, coronary heart disease, chronic bronchitis, gallstones, tumor, arthritis, cataracts, and others).
2.5. Statistical Analysis
We compared the distributions of demographic variables and other covariates between participants with and those without passive smoking exposure, using the t-test or Chi-square test. We used a multivariate regression model to calculate the adjusted relative risks (RRs) and 95% confidence intervals (CIs) of cognitive impairment among participants who were exposed to passive smoking. We further performed multivariate regression analyses to assess the relationship between passive smoking exposure and cognitive impairment among participants with and without active smoking. Statistical testing was conducted with a two-tailed α value of 0.05. All analyses were performed by using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA).
3. Results
Of the 7000 participants in this study, 993 (14.2%) were exposed to passive smoking in the living environment. Compared to those not exposed to passive smoking, these 993 participants were more likely to be women and of younger age; have never worked; have a higher family income; participate in group activities; be tea drinkers; and have underlying diseases, including chronic bronchitis, gallstones, arthritis, and cataract (Table 1). No significant differences in other factors were observed between the exposed and unexposed groups (Table 1).
Table 1.
Participant’s characteristics according to passive smoking status (ZJMPHS Program, Zhejiang, China).
| Variables | Passive Smoking Exposure | p-Value | |||
|---|---|---|---|---|---|
| No | Yes | ||||
| n | % | n | % | ||
| Age (years) | |||||
| Mean (SD) | 70.3 | (7.0) | 69.0 | (6.5) | <0.001 |
| Ethnicity | |||||
| Han | 5794 | 86.4 | 914 | 13.6 | <0.001 |
| Other | 172 | 68.5 | 79 | 31.5 | |
| Sex | |||||
| Female | 2932 | 82.1 | 641 | 17.9 | <0.001 |
| Male | 3035 | 89.6 | 352 | 10.4 | |
| BMI, kg/m2 | |||||
| 18.5–24.99 | 3740 | 86.4 | 589 | 13.6 | 0.434 |
| <18.5 | 299 | 87.7 | 42 | 12.3 | |
| >24.99 | 1663 | 85.5 | 283 | 14.5 | |
| Education | |||||
| Illiterate or semiliterate | 2902 | 85.8 | 482 | 14.2 | 0.448 |
| Primary school | 2616 | 85.3 | 450 | 14.7 | |
| Junior high school | 380 | 88.0 | 52 | 12.0 | |
| High school graduation or higher | 69 | 88.5 | 9 | 11.5 | |
| Marital status | |||||
| Unmarried | 80 | 83.3 | 16 | 16.7 | <0.001 |
| Married | 4637 | 84.6 | 847 | 15.4 | |
| Widowed | 1213 | 90.7 | 125 | 9.3 | |
| Divorced | 25 | 86.2 | 4 | 13.8 | |
| Job | |||||
| Never worked | 1356 | 80.8 | 322 | 19.2 | <0.001 |
| Farmers | 2697 | 88.2 | 361 | 11.8 | |
| Housework | 717 | 87.3 | 104 | 12.7 | |
| Workers | 616 | 84.5 | 113 | 15.5 | |
| Others | 557 | 86.2 | 89 | 13.8 | |
| Family income (1000 ¥/year) | |||||
| <10 | 952 | 90.4 | 101 | 9.6 | <0.001 |
| 10–19 | 1350 | 88.6 | 174 | 11.4 | |
| 20–49 | 1636 | 85.6 | 276 | 14.4 | |
| 50–99 | 992 | 81.4 | 226 | 18.6 | |
| ≥100 | 1032 | 82.8 | 214 | 17.2 | |
| Participation in group activities | |||||
| Never | 3706 | 86.8 | 563 | 13.2 | 0.003 |
| Occasional | 1522 | 83.6 | 298 | 16.4 | |
| Frequent | 735 | 84.8 | 132 | 15.2 | |
| Smoking | |||||
| Non-smokers | 4211 | 85.4 | 718 | 14.6 | 0.284 |
| Current smokers | 1164 | 85.8 | 192 | 14.2 | |
| Ex-smokers | 592 | 87.7 | 83 | 12.3 | |
| Alcohol consumption | |||||
| Nondrinkers | 4012 | 85.9 | 656 | 14.1 | 0.076 |
| Current drinkers | 1569 | 86.1 | 253 | 13.9 | |
| Ex-drinkers | 378 | 82.2 | 82 | 17.8 | |
| Tea drinking | |||||
| Nondrinkers | 4389 | 87.2 | 646 | 12.8 | <0.001 |
| Current drinkers | 1453 | 82.0 | 319 | 18.0 | |
| Ex-drinkers | 110 | 84.6 | 20 | 15.4 | |
| Physical exercise | |||||
| No | 4824 | 86.1 | 776 | 13.9 | 0.064 |
| Yes | 1139 | 84.2 | 214 | 15.8 | |
| Physical work | |||||
| No | 3975 | 86.4 | 628 | 13.6 | 0.112 |
| Yes | 1981 | 84.9 | 351 | 15.1 | |
| Stroke | |||||
| No | 5807 | 85.7 | 971 | 14.3 | 0.314 |
| Yes | 159 | 88.3 | 21 | 11.7 | |
| High blood pressure | |||||
| No | 3140 | 85.2 | 546 | 14.8 | 0.169 |
| Yes | 2826 | 86.3 | 447 | 13.7 | |
| Hyperlipidemia | |||||
| No | 5710 | 85.8 | 946 | 14.2 | 0.437 |
| Yes | 250 | 84.2 | 47 | 15.8 | |
| Diabetes | |||||
| No | 5384 | 85.9 | 887 | 14.1 | 0.329 |
| Yes | 577 | 84.5 | 106 | 15.5 | |
| Coronary heart disease | |||||
| No | 5743 | 85.7 | 960 | 14.3 | 0.552 |
| Yes | 221 | 87.0 | 33 | 13.0 | |
| Chronic bronchitis | |||||
| No | 5851 | 85.9 | 959 | 14.1 | 0.003 |
| Yes | 112 | 77.2 | 33 | 22.8 | |
| Gallstones | |||||
| No | 5774 | 86.0 | 940 | 14.0 | 0.001 |
| Yes | 190 | 78.2 | 53 | 21.8 | |
| Tumor | |||||
| No | 5823 | 85.7 | 973 | 14.3 | 0.497 |
| Yes | 141 | 87.6 | 20 | 12.4 | |
| Arthritis | |||||
| No | 5768 | 85.9 | 946 | 14.1 | 0.017 |
| Yes | 188 | 80.3 | 46 | 19.7 | |
| Cataract | |||||
| No | 5687 | 85.9 | 930 | 14.1 | 0.020 |
| Yes | 276 | 81.4 | 63 | 18.6 | |
| Depressive symptoms | |||||
| Normal | 5157 | 85.6 | 869 | 14.4 | 0.524 |
| Mild depression | 607 | 86.3 | 96 | 13.7 | |
| Moderate depression | 164 | 86.8 | 25 | 13.2 | |
| Heavy depression | 39 | 92.9 | 3 | 7.1 | |
ZJMPHS Program: Zhejiang Major Public Health Surveillance Program.
Overall, 7000 participants with normal cognitive function at baseline were followed up for two years, of whom 1224 (17.5%) developed cognitive impairment. Table 2 shows the association between passive smoking exposure and cognitive impairment. After adjusting for covariates, including sex, age, body mass index (BMI), education, marital status, job type, family income, living or eating alone, participation in group activities, sleep quality, napping, alcohol consumption, tea consumption, water consumption, physical exercise, and work, the association between passive smoking exposure and cognitive impairment remained significant (RR: 1.16; 95% CI: 1.01–1.35). However, we did not observe a dose–response relationship between the cumulative dose of exposure (days) and cognitive impairment.
Table 2.
Adjusted relative risks (RRs) for the association between passive smoking exposure and cognitive impairment (ZJMPHS Program, Zhejiang, China).
| Covariates | Cognitive Impairment | Multivariate Adjusted Regression Analyses | ||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| n | % | n | % | Adjusted RR (95% CI) a | p-Value | |
| Age (years) | ||||||
| Mean (SD) | 69.4 | (6.5) | 73.3 | (7.8) | 1.04 (1.03–1.05) | <0.001 |
| Male sex | 2883 | 85.1 | 504 | 14.9 | 0.85 (0.73–0.969) | 0.040 |
| Han ethnicity | 5486 | 81.8 | 1222 | 18.2 | 1.30 (0.92–1.84) | 0.141 |
| BMI, kg/m2 | ||||||
| 18.5–24.99 | 3565 | 82.4 | 764 | 17.6 | 1.00 | |
| <18.50 | 241 | 70.7 | 100 | 29.3 | 1.21 (1.05–1.39) | 0.007 |
| >24.99 | 1635 | 84.0 | 311 | 16.0 | 0.94 (0.84–1.06) | 0.305 |
| Education | ||||||
| Illiterate or semiliterate | 2647 | 78.2 | 737 | 21.8 | 1.00 | |
| Primary school | 2612 | 85.2 | 454 | 14.8 | 0.99 (0.89–1.12) | 0.964 |
| Junior high school | 372 | 86.1 | 60 | 13.9 | 1.03 (0.80–1.33) | 0.825 |
| High school or higher | 69 | 88.5 | 9 | 11.5 | 0.86 (0.49–1.51) | 0.605 |
| Marital status | ||||||
| Married | 83 | 86.5 | 13 | 13.5 | 1.00 | |
| Unmarried | 4599 | 83.9 | 885 | 16.1 | 1.31 (0.77–2.25) | 0.319 |
| Widowed | 986 | 73.7 | 352 | 26.3 | 1.27 (0.74–2.19) | 0.390 |
| Divorced | 23 | 79.3 | 6 | 20.7 | 1.56 (0.57–4.24) | 0.388 |
| Job | ||||||
| Never worked | 1254 | 74.7 | 424 | 25.3 | 1.00 | |
| Farmers | 2545 | 83.2 | 513 | 16.8 | 0.88 (0.78–0.99) | 0.035 |
| Housework | 657 | 80.0 | 164 | 20.0 | 0.83 (0.71–0.98) | 0.023 |
| Workers | 642 | 88.1 | 87 | 11.9 | 0.78 (0.62–0.99) | 0.038 |
| Others | 577 | 89.3 | 69 | 10.7 | 0.77 (0.60–0.99) | 0.043 |
| Family income (1000 ¥/year) | ||||||
| <10 | 734 | 69.7 | 319 | 30.3 | 1.00 | |
| 10–19 | 1175 | 77.1 | 349 | 22.9 | 0.93 (0.82–1.06) | 0.273 |
| 20–49 | 1673 | 87.5 | 239 | 12.5 | 0.62 (0.52–0.73) | <0.001 |
| 50–99 | 1026 | 84.2 | 192 | 15.8 | 0.74 (0.62–0.87) | <0.001 |
| ≥100 | 1087 | 87.2 | 159 | 12.8 | 0.58 (0.48–0.69) | <0.001 |
| Participation in group activities | ||||||
| Never | 3359 | 78.7 | 910 | 21.3 | 1.00 | |
| Occasionally | 1559 | 85.7 | 261 | 14.3 | 0.75 (0.66–0.85) | <0.001 |
| Frequently | 778 | 89.7 | 89 | 10.3 | 0.56 (0.46–0.69) | <0.001 |
| Smoking | ||||||
| Non-smokers | 3951 | 80.2 | 978 | 19.8 | 1.00 | |
| Current smokers | 1163 | 85.8 | 193 | 14.2 | 1.01 (0.84–1.21) | 0.923 |
| Ex-smokers | 586 | 86.8 | 89 | 13.2 | 0.88 (0.69–1.12) | 0.295 |
| Alcohol consumption | ||||||
| Non-drinkers | 3751 | 80.4 | 917 | 19.6 | 1.00 | |
| Current drinkers | 1554 | 85.3 | 268 | 14.7 | 1.13 (0.97–1.30) | 0.109 |
| Ex-drinkers | 389 | 84.6 | 71 | 15.4 | 0.95 (0.74–1.20) | 0.648 |
| Tea consumption | ||||||
| Non-drinkers | 4032 | 80.1 | 1003 | 19.9 | 1.00 | |
| Current drinkers | 1534 | 86.6 | 238 | 13.4 | 0.81 (0.70–0.94) | 0.004 |
| Ex-drinkers | 117 | 90.0 | 13 | 10.0 | 0.49 (0.27–0.88) | 0.016 |
| Physical exercise | 1198 | 88.5 | 155 | 11.5 | 0.68 (0.58–0.81) | <0.001 |
| Physical work | 2083 | 89.3 | 249 | 10.7 | 0.70 (0.61–0.81) | <0.001 |
| Passive smoking exposure | 819 | 82.5 | 174 | 17.5 | 1.16 (1.01–1.35) | 0.047 |
| Cumulative dose of passive smoking exposure (days) | ||||||
| <40 | 174 | 81.3 | 40 | 18.7 | 1.00 | |
| 40–299 | 336 | 80.8 | 80 | 19.2 | 1.17 (0.62–1.46) | 0.277 |
| ≥300 | 199 | 87.3 | 29 | 12.7 | 0.72 (0.41–1.32) | 0.421 |
a Adjusted for age, sex, ethnicity, body mass index (BMI), education, marital status, job type, family income, participation in group activities, smoking, alcohol consumption, tea consumption, physical exercise, or work. RR: relative risk; CI: confidence interval; ZJMPHS Program: Zhejiang Major Public Health Surveillance Program.
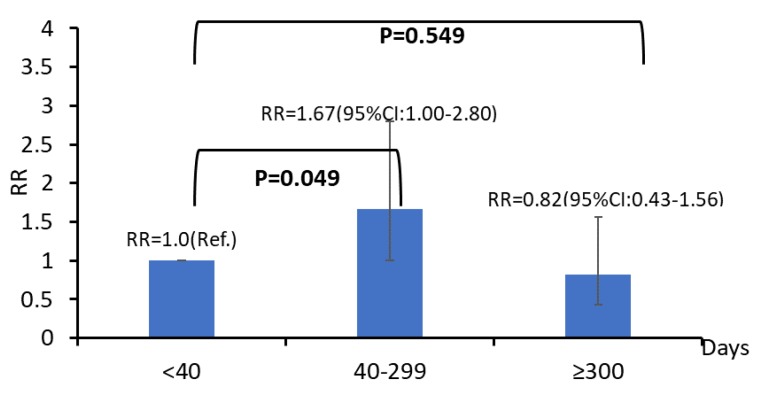
We further analyzed the relationship between passive smoking exposure and cognitive impairment among smoking and non-smoking participants (Table 3). The harmful effects of passive smoking on cognitive impairment were observed in non-active smokers (RR: 1.24; 95% CI: 1.06–1.46), but not in active smokers (RR: 1.11; 95% CI: 0.71–1.92). Among non-smokers, those exposed to passive smoking for a cumulative dose of 40–299 days had a higher risk of cognitive impairment than that in participants with exposures of less than 40 days (RR: 1.67; 95% CI: 1.00–2.80). However, no such harmful effect was observed in participants with cumulative doses over 300 days (Figure 1). An adjusted RR was not obtained for active smokers, because of the insufficient sample size.
Table 3.
Stratified analysis of the relationship between passive smoking exposure and cognitive impairment with respect to active smoking (ZJMPHS Program, Zhejiang, China).
| Active Smoking | Passive Smoking Exposure | Cognitive Impairment | Multivariate Adjusted Regression Analyses | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| n | % | n | % | Adjusted RR a (95% CI) | p-Value | ||
| No | No | 3884 | 80.9 | 919 | 19.1 | 1.00 | |
| Yes | 653 | 81.5 | 148 | 18.5 | 1.24 (1.06–1.46) | 0.008 | |
| Yes | No | 997 | 85.7 | 167 | 14.3 | 1.00 | |
| Yes | 166 | 86.5 | 26 | 13.5 | 1.11 (0.71–1.92) | 0.610 | |
a Adjusted for age, sex, ethnicity, body mass index (BMI), education, marital status, job type, family income, participation in group activities, smoking, alcohol consumption, tea consumption, physical exercise, or work. RR: relative risk; CI: confidence interval; ZJMPHS Program: Zhejiang Major Public Health Surveillance Program.
Figure 1.
Relationship between the cumulative dose (days) of passive smoking exposure and cognitive impairment among non-smokers (ZJMPHS Program, Zhejiang, China). Adjusted for age, sex, ethnicity, body mass index, education, marital status, job type, family income, participation in group activities, smoking, alcohol consumption, tea consumption, physical exercise, and work. RR: relative risk; CI: confidence interval; ZJMPHS Program: Zhejiang Major Public Health Surveillance Program.
4. Discussion
This prospective study assessed the relationship between passive smoking exposure in the living environment and the incidence of cognitive impairment among older adults. Our results revealed that passive smoking exposure exerted significant harmful effects on cognitive function, particularly in non-smokers.
Previous studies have shown that passive smoking can increase the risk of cognitive impairment or dementia, which is a possible outcome of cognitive impairment [14,15,16]. The results of our study also provided evidence of the harmful effect of passive smoking exposure on cognitive function. Passive smoking can damage the cardiovascular system by increasing platelet coagulability, leading to endothelial dysfunction [17,18]. Endothelial dysfunction might be related to the faulty clearance of amyloid beta-peptide across the blood-brain barrier, which plays an essential role in cognitive impairment [19]. Furthermore, the results of animal experiments have demonstrated that chronic smoking exposure can suppress synaptic function or cause other neuropathological changes, which might explain the early phases of neurodegeneration in brains [20]. Tobacco-specific procarcinogens may reduce neuronal mass in specific regions of the brain related to learning and memory [21]. A third important explanation is that the carbon monoxide (CO) in tobacco smoke interferes with the flow of oxygen through the blood to the brain, which may, in turn, impair cognitive function.
Similar to other studies, we also performed dose–response tests of passive smoking [22,23,24]. However, in contrast to previous results, we did not observe a risk trend between exposure dose and cognitive impairment. To assess the threshold effect of cumulative passive smoke exposure and to control for the possible short-term beneficial effects on cognitive function (especially on memory and attention) caused by nicotine [25], we filtered out participants who had passively smoked for more than 10 years before calculating and dividing the total exposure time and into four levels. Higher exposure to passive smoking did not show a significant risk effect on cognitive function. While several studies have shown no association between passive smoking and dementia, others have shown an inverse relationship between serum cotinine level and cognitive function; i.e., the effect was more significant at lower levels of exposure [26]. These conflicting results may be because of differences in the ventilation of the living environment of passive smokers, the actual effective dose of passive smoking exposure, insufficient sample size, recall bias from participants, and other factors related to individual differences.
The results of the independent analyses of participants who actively smoked and for those who did not revealed that the influence of the passive smoking on cognitive impairment was not significant in participants who were actively smoking but was significant in those who were not actively smoking. This finding suggests that passive smoking exposure was more harmful to non-active smokers than to active smokers. Previous studies have reported the negative effect of exposure to secondhand smoke on cognitive function among non-smokers [22,24]. One possible explanation is that nicotine reduces the activity of monoamine oxidase, which can cause nerve damage, allowing short-term cognitive improvements in people with cognitive impairment to mask some symptoms of the disease [27,28]. This makes it difficult to observe the harmful effects of passive smoking on the cognitive function in active smokers. In contrast, the cover-up effect is weaker in non-smokers, allowing the harmful effects of passive smoking to be observed in non-smokers. However, the different harmful effects and potential mechanisms of passive smoking on cognitive function between smokers and non-smokers have rarely been assessed. Further research into these differences is warranted, especially regarding genetic susceptibilities.
Nicotine may have some beneficial short-term effects on the cognitive function, particularly in areas related to memory and attention [25]. However, effects of smoking on the cognitive function is primarily observed in people with impaired cognitive function, particularly in those with neurological or psychiatric disorders and not in people with normal cognitive function. However, smoking tends to only improve the cognitive function in the short-term. Although nicotinoid nerve excitation mediated by nicotine receptors can improve the cognitive function, nicotine has many negative effects, such as damaging the blood vessels, increasing the oxidative stress, affecting mitochondrial energy metabolism, decreasing the function of synaptic network connectivity, and influencing metabolic enzymes related to A beta or tau protein. Therefore, in the long-term, smoking harms the cognitive function [29,30].
Our findings were based on a prospective design. This ensured a causal relationship between passive smoking exposure and cognitive impairment. We also compared the effects of passive smoking exposure on cognitive function among smokers and non-smokers, which has been rarely reported previously. Nevertheless, this study has several limitations. First, investigations based on self-reports may lead to overestimation or underestimation of the passive smoking exposure [31,32], which might affect the accuracy of the harmful effects of this exposure on cognitive impairment. The multiple regression analysis also suggested that the association between passive smoking and cognitive impairment may be weak, because at least ten confounding factors influence the detrimental effect. The other limitation is that our study only calculated passive smoking exposure time in living environments. Other possible exposures include unconscious exposure in public spaces. Some studies using serum cotinine as a biomarker for passive smoking exposure [26,33] have reported its significant negative association with cognitive performance. In contrast, urine sampling is much less invasive and can also be used to quantify cotinine levels [34]. Continuous long-term monitoring of urine or serum nicotine levels and epidemiological investigation are required to objectively assess passive smoking exposure.
5. Conclusions
Our findings appear to indicate that there is an association between passive smoking exposure in the living environment and an increased risk of cognitive impairment among older adults, with a greater harmful effect in non-smokers compared to that in smokers. Considering that more than 90% of the world’s population is not completely protected by smoke-free public health regulations [35], we should appeal to families and societies to reduce the exposure to passive smoking, especially for older adults. The Chinese government has taken strict measures to limit smoking in public places in recent years and has made great progress. However, smokers may increase the amount of smoke in the family household and other living environments, which increases the exposure of the co-dwellers to passive smoking. In light of this study, we suggest that the government establish a community supervision mechanism and take the family as a unit to persuade smokers to quit or control smoking. In addition, further studies are required to assess the dose–response relationship between passive smoking exposure and cognitive impairment and to determine the potential mechanisms in smokers and non-smokers.
Acknowledgments
We would like to thank the study participants and the investigators from the six selected counties for their assistance with the investigation and data collection. In addition, thank you to Editage (www.editage.cn) for English language editing.
Author Contributions
Conceptualization, G.Z. and J.L.; methodology, F.H.; software, F.H. and T.L.; validation, F.L., and Y.Z.; formal analysis, T.Z.; investigation, X.G.; resources, F.H.; data curation, T.Z.; writing—original draft preparation, F.H.; writing—review & editing, G.Z. and J.L.; funding acquisition, G.Z., F.H. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R & D Program of China (2017YFC0907000) and the Zhejiang Provincial Public Welfare Technology Application Research Project of China (LGF19H260003).
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.Centers for Disease Control and Prevention A Report of the Surgeon General, How Tobacco Smoke Causes Disease: What it Means to You. [(accessed on 14 September 2019)];2010 Available online: https://www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf.
- 2.Hecht S.S. Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob. Res. 2012;14:18–28. doi: 10.1093/ntr/ntq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim A.S., Ko H.J., Kwon J.H., Lee J.M. Exposure to Secondhand Smoke and Risk of Cancer in Never Smokers: A Meta-Analysis of Epidemiologic Studies. Int. J. Environ. Res. Public Health. 2018;15:E1981. doi: 10.3390/ijerph15091981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee W., Hwang S.H., Choi H., Kim H. The association between smoking or passive smoking and cardiovascular diseases using a Bayesian hierarchical model: Based on the 2008–2013 Korea Community Health Survey. Epidemiol. Health. 2017;39:e2017026. doi: 10.4178/epih.e2017026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiue I. Modeling the effects of indoor passive smoking at home, work, or other households on adult cardiovascular and mental health: The Scottish Health Survey, 2008–2011. Int. J. Environ. Res. Public Health. 2014;11:3096–3107. doi: 10.3390/ijerph110303096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stirland L.E., O’Shea C.I., Russ T.C. Passive smoking as a risk factor for dementia and cognitive impairment: Systematic review of observational studies. Int. Psychogeriatr. 2018;30:1177–1187. doi: 10.1017/S1041610217002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khorasanchi Z., Bahrami A., Avan A., Jaberi N., Rezaey M., Bahrami-Taghanaki H., Ferns G.A., Ghayour-Mobarhan M. Passive smoking is associated with cognitive and emotional impairment in adolescent girls. J. Gen. Psychol. 2019;146:68–78. doi: 10.1080/00221309.2018.1535485. [DOI] [PubMed] [Google Scholar]
- 8.Newhouse P., Kellar K., Aisen P., White H., Wesnes K., Coderre E., Pfaff A., Wilkins H., Howard D., Levin E.D. Nicotine treatment of mild cognitive impairment: A 6-month double-blind pilot clinical trial. Neurology. 2012;78:91–101. doi: 10.1212/WNL.0b013e31823efcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White H.K., Levin E.D. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer’s disease. Psychopharmacology (Berl) 1999;143:158–165. doi: 10.1007/s002130050931. [DOI] [PubMed] [Google Scholar]
- 10.Sheng Y., He F., Lin J.F., Shen W., Qiu Y.W. Tea and risk of age-related cataracts: A cross-sectional study in Zhejiang Province, China. J. Epidemiol. 2016;26:587–592. doi: 10.2188/jea.JE20150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F.D., He F., Chen T.R., Xiao Y.Y., Lin S.T., Shen W., Wang X.Y., Zhai Y.J., Shang X.P., Lin J.F. Reproductive history and risk of cognitive impairment in elderly women: A cross-sectional study in Eastern China. J. Alzheimers Dis. 2016;49:139–147. doi: 10.3233/JAD-150444. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z.Y., Zhang M.Y., Zhai G.Y., Chen J.X., Zhao J. Application of the Chinese version of the Mini-Mental State Examination. Shanghai Arch. Psychiatr. 1989;7:108–111. [Google Scholar]
- 13.Zhang Z.J. Handbook of Behavioral Medical Scales. Chinese Medical Multimedia Press; Beijing, China: 2005. [Google Scholar]
- 14.Chen R., Wilson K., Chen Y., Zhang D., Qin X., He M., Hu Z., Ma Y., Copeland J.R. Association between environmental tobacco smoke exposure and dementia syndromes. Occup. Environ. Med. 2013;70:63–69. doi: 10.1136/oemed-2012-100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orsitto G., Turi V., Venezia A., Fulvio F., Manca C. Relation of secondhand smoking to mild cognitive impairment in older inpatients. Sci. World J. 2012;2012:726948. doi: 10.1100/2012/726948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes D.E., Haight T.J., Mehta K.M., Carlson M.C., Kuller L.H., Tager I.B. Secondhand smoke, vascular disease, and dementia incidence: Findings from the cardiovascular health cognition study. Am. J. Epidemiol. 2010;171:292–302. doi: 10.1093/aje/kwp376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention A Report of the Surgeon General, The Health Consequences of Involuntary Exposure to Tobacco Smoke. [(accessed on 15 September 2019)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK44324/pdf/Bookshelf_NBK44324.pdf. [PubMed]
- 18.Zlokovic B.V. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Barnoya J., Glantz S.A. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 20.Ho Y.S., Yang X., Yeung S.C., Chiu K., Lau C.F., Tsang A.W., Mak J.C., Chang R.C. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS ONE. 2012;7:e36752. doi: 10.1371/journal.pone.0036752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh D., Mishra M.K., Das S., Kaushik D.K., Basu A. Tobacco carcinogen induces microglial activation and subsequent neuronal damage. J. Neurochem. 2009;110:1070–1081. doi: 10.1111/j.1471-4159.2009.06203.x. [DOI] [PubMed] [Google Scholar]
- 22.Llewellyn D.J., Lang I.A., Langa K.M., Naughton F., Matthews F.E. Exposure to secondhand smoke and cognitive impairment in non-smokers: National cross-sectional study with cotinine measurement. BMJ. 2009;338:b462. doi: 10.1136/bmj.b462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R., Zhang D., Chen Y., Hu Z., Wilson K. Passive smoking and risk of cognitive impairment in women who never smoke. Arch. Intern. Med. 2012;172:271–273. doi: 10.1001/archinternmed.2011.762. [DOI] [PubMed] [Google Scholar]
- 24.Chen R., Clifford A., Lang L., Anstey K.J. Association of passive smoking with cognitive impairment in nonsmoking older adults: A systematic literature review and a new study of Chinese cohort. J. Geriatr. Psychiatry Neurol. 2013;26:199–208. doi: 10.1177/0891988713496165. [DOI] [PubMed] [Google Scholar]
- 25.Newhouse P.A., Potter A., Singh A. Effects of nicotinic stimulation on cognitive performance. Curr. Opin. Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Yolton K., Dietrich K., Auinger P., Lanphear B.P., Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ. Health Perspect. 2005;113:98–103. doi: 10.1289/ehp.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray K.N., Abeles N. Nicotine’s effect on neural and cognitive functioning in an aging population. Aging Ment. Health. 2002;6:129–138. doi: 10.1080/13607860220126808. [DOI] [PubMed] [Google Scholar]
- 28.Teaktong T., Graham A.J., Johnson M., Court J.A., Perry E.K. Selective changes in nicotinic acetylcholine receptor subtypes related to tobacco smoking: An immunohistochemical study. Neuropathol. Appl. Neurobiol. 2004;30:243–254. doi: 10.1046/j.0305-1846.2003.00528.x. [DOI] [PubMed] [Google Scholar]
- 29.Cervilla J.A., Prince M., Mann A. Smoking, drinking, and incident cognitive impairment: A cohort community based study included in the Gospel Oak project. J. Neurol. Neurosurg. Psychiatry. 2000;68:622–626. doi: 10.1136/jnnp.68.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill R.D., Nilsson L.G., Nyberg L., Backman L. Cigarette smoking and cognitive performance in healthy Swedish adults. Age Ageing. 2003;32:548–550. doi: 10.1093/ageing/afg067. [DOI] [PubMed] [Google Scholar]
- 31.DeLorenze G.N., Kharrazi M., Kaufman F.L., Eskenazi B., Bernert J.T. Exposure to environmental tobacco smoke in pregnant women: The association between self-report and serum cotinine. Environ. Res. 2002;90:21–32. doi: 10.1006/enrs.2001.4380. [DOI] [PubMed] [Google Scholar]
- 32.Ling J., Heffernan T. The cognitive deficits associated with second-hand smoking. Front. Psychiatry. 2016;7:46. doi: 10.3389/fpsyt.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benowitz N.L. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol. Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 34.Bono R., Bellisario V., Tassinari R., Squillacioti G., Manetta T., Bugiani M., Migliore E., Piccioni P. Bisphenol a, tobacco smoke, and age as predictors of oxidative stress in children and adolescents. Int J. Environ. Res. Public Health. 2019;16:E2025. doi: 10.3390/ijerph16112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oberg M., Jaakkola M.S., Woodward A., Peruga A., Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet. 2011;377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]