Abstract
Transforming growth factor-β (TGFβ) is a secreted cytokine whose aberrant spatiotemporal expression is related to cancer progression and metastasis. While TGFβ acts as a tumor suppressor in normal and premalignant stages, TGFβ functions as a tumor promoter during the malignant phases of tumor progression by prompting cancer cells to undergo epithelial-mesenchymal transition (EMT), which enhances tumor cell invasion and ultimately promotes metastasis to other organs. Extensive studies have been performed to uncover the molecular and cellular mechanisms underlying TGFβ inducing EMT in cancer cells. Here, we suggested that ELK3, which encodes a protein that orchestrates invasion and metastasis of triple negative breast cancer cells, is a downstream target of TGFβ-SMAD3 in MDA-MB231 cells. ELK3 expression was increased in a time-dependent manner upon TGFβ treatment. Chemical and molecular inhibition of the TGFβ receptor blocked the ability of TGFβ to induce ELK3 expression. Small interfering RNA-mediated suppression analysis revealed that SMAD3 induces TGFβ signaling to express ELK3. Moreover, the results of the luciferase reporter assay and chromatin immunoprecipitation analysis showed that SMAD3 directly binds to the SMAD-binding element on the promoter of ELK3 to activate gene expression following TGFβ stimulation. We concluded that ELK3 is a novel downstream target of TGFβ-SMAD3 signaling in aggressive breast cancer cells.
Keywords: TGFβ, SMAD3, ELK3, MDA-MB-231, MCF7, luciferase assay, chromatin immunoprecipitation
Introduction
Cancer metastasis is the process of cancer cells disseminating from the primary tumor to a distal site through lymphatic tissue and blood vessels. Cancer metastasis is responsible for approximately 90% of cancer deaths, indicating that it is the primary cause of morbidity and mortality (1). Even though most solid tumors are now manageable or curable by advances in early cancer detection and treatment, cancers spreading beyond the initial primary site are usually highly incurable (2). Lack of understanding of the mechanism underlying the metastatic process has meant that the predominant cancer treatments focus on inhibition of cancer growth with little emphasis on metastasis, meaning that the overall survival of metastatic cancer patients has not been improved significantly.
Transforming growth factor-β (TGFβ) is one of the master factors of metastasis in that it induces the epithelial-mesenchymal transition (EMT), which is associated with cancer. EMT is the reversible orchestrated transcriptional program in which well-organized, tightly connected epithelial cells transdifferentiate into disorganized and motile mesenchymal cells. TGF-β signaling mediated by SMAD or non-SMAD pathways plays a fundamental role in activating the transcriptional network to induce the expression of mesenchymal components and to suppress the expression of epithelial genes (3,4). As a result, epithelial cancer cells undergo dramatic remodeling of the cytoskeleton along with dissolution of tight junctions to acquire mesenchymal features that exhibit a significantly enhanced metastatic dissemination potential into distal organs. This event is induced by the activity of master regulators of EMT, which include SNAIL, SLUG, ZEB1/delta EF1 and ZEB2/SIP1 (5–8).
ELK3 is an ETS domain-containing protein capable of forming a ternary complex with DNA and serum response factor (9). ELK3 is reported to be involved in the migration and invasion of various cancer cells including aggressive basal-like breast cancer cells and liver cancer stem cells (10,11). Previously, we reported that ELK3 suppression impairs the ability of TGFβ signaling to activate the expression of mesenchymal markers such as Vimentin, Slug and SNAIL in the triple negative breast cancer cells, which suggests that ELK3 is implicated in the TGFβ signaling pathway to regulate the metastatic process of aggressive cancer cells (12,13).
In the present study, to extend our understanding of the molecular implication of ELK3 to the TGFβ signaling pathway in cancer cells, we analyzed the regulatory mechanism of TGFβ signaling on ELK3 expression. We found that TGFβ stimulates the transcriptional expression of ELK3 in the representative triple negative breast cancer cell line, MDA-MB231. Furthermore, based on the biochemical and molecular biology study, we demonstrated that TGFβ-mediated phosphorylation of SMAD3 functions as a transcriptional activator of ELK3. Taken together, our data reveal that ELK3 is a direct downstream target of TGFβ-SMAD3 signaling pathway in MDA-MB231 cells.
Materials and methods
Plasmids, siRNA and primers
Information on the plasmids and siRNAs is summarized in the supplementary Tables SI and SII.
Cell culture and transfection
The triple negative breast cancer cell line MDA-MB231 and the human breast adenocarcinoma cell line MCF7 and the human embryonic kidney 293T cells were purchased from American Type Culture Collection (Manassas, VA, USA). These cells were maintained in DMEM (Gibco BRL Life Technologies, Rockville, MD, USA) containing 10% (v/v) heat-inactivated fetal bovine serum (Gibco BRL). 293T cells were used for the luciferase assay with the pGL3-ELK3 plasmid. Transient transfection of plasmid DNA or siRNA was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocols.
RNA extraction and reverse transcription-quantitative (RT-q)PCR
Total RNA was extracted by manual methods using TRIZol (Invitrogen), and 1 µg of cDNA was synthesized using the LeGene Express 1st Strand cDNA Synthesis System (LeGene Biosciences Inc., San Diego, CA, USA) according to the manufacturer's instructions. RT-qPCR was performed using synthetic cDNAs and TOPreal™ qPCR 2X PreMIX (Enzynomics, Daejeon, Korea). The expression of the target genes was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The PCR primers are listed in Table SIII.
Immunoblot analysis
Cells were lysed with RIPA buffer (Cell Signaling Technology, Beverly, MA, USA) and total cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). The membranes were blotted with the indicated primary antibodies at 4°C overnight. After washing with TBST, the membranes were incubated for 1 h at room temperature with secondary antibodies. Immunoreactivity was detected with an ECL kit (Thermo Scientific, Rochester, NY, USA). The antibodies used in this study are summarized in Table SIV.
Luciferase assay
The 293T cells were transfected with the indicated plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocols. Cells were harvested 48 h after transfection, and luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's protocols. The values of firefly luciferase were normalized to the respective values of Renilla luciferase.
Chromatin immunoprecipitation
In brief, 37% formaldehyde was added to the cell culture medium to a final concentration of 1% and incubated for 15 min at RT. Glycine was added to a final concentration of 125 mM for 5 min at RT, and the cells were washed three times with cold PBS. The cells were lysed in 400 µl of 1X cell lysis buffer (Cell Signaling) containing protease/phosphatase inhibitor cocktail (Pierce Biotechnology). After eight rounds of sonication, the lysates were cleared by centrifugation at 13,000 rpm for 15 min at 4°C. The supernatants were mixed with 40 µl of Dynabead protein G and 2 µg of primary antibodies for 2 h at RT or overnight at 4°C. The complexes were washed sequentially with 1X RIPA buffer, 1X RIPA buffer (500 mM NaCl), LiCl buffer and TE buffer twice for 10 min each. Then, 3 µl of 10% SDS and 5 µl of 20 mg/ml proteinase K were added to separate the DNA-protein complex. The DNA was purified by the phenol/chloroform extraction method, and then it was used in PCR with primers targeting the ELK3 promoter.
Statistical analysis
Samples were analyzed with Student's t-test or ANOVA with Duncan's multiple range procedure for multiple comparisons. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Prism, USA) or the SigmaPlot 11.2 program (Systat Software, USA). All statistical analyses were performed using GraphPad Prism 5 (GraphPad Prism, USA). The error bars represent the standard errors from three independent experiments, which were each performed using triplicate samples. P-values less than 0.05 were considered statistically significant.
Results
TGFβ induces accumulation of ELK3 in the nucleus of MDA-MB231 cells, but not in MCF7 cells
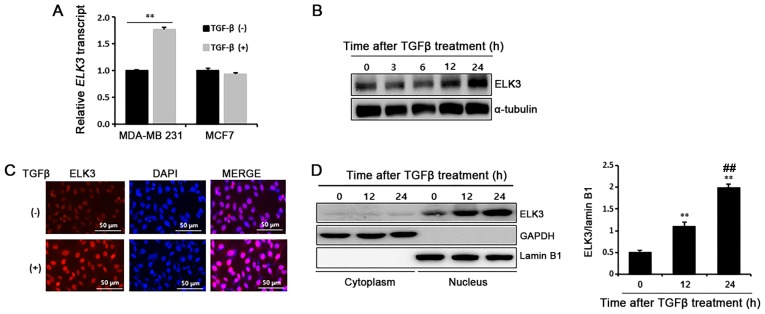
Cancer cells treated with TGFβ undergo the EMT process by developing a fibroblast-like morphological appearance and changing epithelial and mesenchymal phenotype marker expression. Unlike MDA-MB231 cells, TGFβ-treated MCF7 cells that display morphological changes of EMT do not show suppression of E-cadherin, a typical epithelial phenotype marker (14). Recently, we reported that ELK3 is highly expressed in TNBC-like MDA-MB231 cells, where it functions as a transcriptional repressor of E-cadherin by collaborating with ZEB1 (15). Therefore, we hypothesized that ELK3 is the missing link that explains the different molecular responses of MDA-MB231 and MCF7 cells when they are treated with TGFβ. We first compared the expression of ELK3 between MDA-MB231 and MCF7 cells following TGFβ treatment. As expected, TGFβ stimulated ELK3 expression in MDA-MB231 cells but not in MCF7 cells (Fig. 1A). Consistently, ELK3 protein was also accumulate in the TGFβ-treated MDA-MB231 cells (Fig. 1B). Immunocytochemical analysis and subcellular fractionation assays of the cytosol and nucleus confirmed that ELK3 accumulates in the TGFβ-treated MDA-MB231 cells (Fig. 1C and D). Overall, these data indicate that TGFβ induces transcriptional activation of ELK3 in MDA-MB231 cells but not in MCF7 cells.
Figure 1.
TGFβ induces accumulation of ELK3 in the nuclei of MDA-MB231 cells. (A) Effect of TGFβ on the expression of ELK3 in MDA-MB231 and MCF7 cells was compared by RT-qPCR of cancer cells treated with TGFβ (5 ng/ml) for 24 h. **P<0.01. (B) The increase of ELK3 protein (right panel) upon TGFβ treatment (5 ng/ml) for the indicated time was analyzed by immunoblot assay. (C) Nuclear accumulation of ELK3 upon TGFβ treatment was examined in MDA-MB231 cells by immunocytochemical staining. Cells were treated with 5 ng/ml TGFβ for 12 h. (D) Nuclear accumulation of ELK3 was examined by immunoblot of nuclear and cytoplasmic fractions from MDA-MB231 cells treated with TGFβ for the indicated times. A loading control of the cytoplasmic fraction was estimated by GAPDH and a loading control of the nuclear fraction was estimated by Lamin B1 (left panel). For quantitative analysis, the mean density of each band was measured with Multi Gauge V3.0 software, and the band density of EL3 was divided by Lamin B1 to obtain the normalized band intensity (right panel). The error bars represent the standard errors from three independent experiments, which were each performed using triplicate samples. TGFβ, transforming growth factor-β; RT-qPCR, reverse transcription-quantitative PCR. **P<0.01 vs. 0 h; ##P<0.01 vs. 12 h.
TGFβ activates ELK3 expression via SMAD3
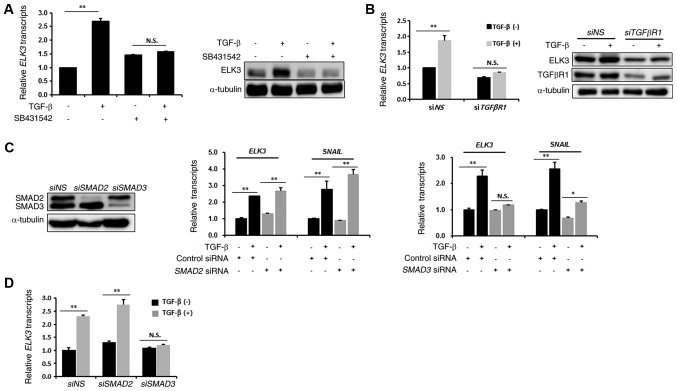
To understand the underlying mechanism of ELK3 activation by TGFβ treatment, we examined the effect of SB431542, an inhibitor of TGFβ type I receptor, on the TGFβ-mediated ELK3 expression. As shown in Fig. 2A, pretreatment with SB431542 inhibited mRNA and protein accumulation of ELK3 in the TGFβ-treated MDA-MB231 cells. Consistent with the result of chemical inhibition, transfection of siRNA targeting TGFβ type I receptor abolished the effect of TGFβ on the transcriptional activation of ELK3 (Fig. 2B). We next questioned whether ELK3 expression is regulated by SMAD2 or SMAD3. Like SNAIL, which is a downstream target of SMAD3, transfection of an siRNA targeting SMAD3 (siSMAD3) hindered the TGFβ-mediated expression of ELK3, whereas an siRNA targeting SMAD2 (siSMAD2) did not interfere with the TGFβ effect on ELK3 or SNAIL expression (Fig. 2C). The increase in expression of ELK3 over time in TGFβ-treated MDA-MB231 cells was similar between the control and siSMAD2 transfected MDA-MB231 cells, whereas siSMAD3 transfection abolished the effect of TGFβ on ELK3 expression (Fig. 2D). Taken together, these results suggest that TGFβ-mediated transcriptional activation of ELK3 is mediated by SMAD3.
Figure 2.
Effect of TGFβ on ELK3 expression is mediated by SMAD3 but not by SMAD2. (A) Effect of chemical inhibition of the TGFβ receptor on the TGFβ-mediated activation of ELK3. Cells were pre-incubated with 10 µM SB431542 for 1 h and then treated with 10 ng/ml TGFβ for 24 h. Relative transcripts and protein levels of ELK3 and TGFβ receptor-I were analyzed by RT-qPCR (left panel) and immunoblot analysis (right panel), respectively. (B) Effect of molecular inhibition of TGFβ receptor-I on the TGFβ-mediated activation of ELK3 expression. Non-specific or TGFβ receptor-I targeting siRNAs were transfected into MDA-MB231 cells for 24 h followed by treatment with 5 ng/ml of TGFβ for 24 h. Relative transcripts and protein levels of ELK3 and TGFβ receptor-I were analyzed by RT-qPCR (left panel) and immunoblot analysis (right panel), respectively. (C) Effect of molecular inhibition of SMAD2 or SMAD3 on the TGFβ-mediated activation of ELK3 expression. Non-specific, SMAD2 or SMAD3 targeting siRNAs were transfected into MDA-MB231 cells for 24 h, which was followed by treatment with 5 ng/ml of TGFβ for 24 h. Knockdown effect of SMAD2 and SMAD3 was analyzed by immunoblot analysis (left panel). Relative transcripts and protein levels of ELK3 and SNAIL, which is a target of SMAD3, were analyzed by RT-qPCR (middle and right panel). (D) Effect of molecular inhibition of SMAD2 or SMAD3 on the TGFβ-treated, time-dependent activation of ELK3 expression. Nonspecific, SMAD2 or SMAD3 targeting siRNAs were transfected into MDA-MB231 cells for 24 h, which was followed by treatment with 5 ng/ml of TGFβ for additional 24 h. The expression of ELK3 was quantified by RT-qPCR. The error bars represent the standard errors from three independent experiments, which were each performed using triplicate samples. *P<0.05, **P<0.01. N.S, not significant; TGFβ, transforming growth factor-β; RT-qPCR, reverse transcription-quantitative PCR; si, small interfering; NS, non-specific.
SMAD3 binds to the ELK3 promoter to activate the transcription of ELK3 upon TGFβ treatment
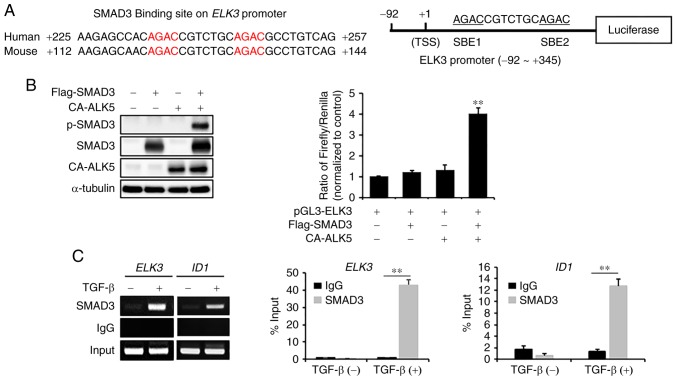
To analyze whether SMAD3 functions as a direct transcriptional activator of ELK3, we examined the sequences of mouse and human ELK3 promoters from −2,000 bp to the first exon region. We found that two SMAD3 binding sites (SBE) are conserved at the first exon of the ELK3 promoter in the human and mouse genomes. Therefore, we constructed a luciferase reporter construct containing the promoter region of ELK3 from −92 bp to +345 bp (Fig. 3A). To assess whether SMAD3 functions as a direct transcriptional activator of the ELK3 promoter, the reporter plasmid containing the ELK3 promoter was cotransfected into 293T cells with plasmids encoding a constitutively active form of the TGFβ type I receptor (CA-ALK5) or SMAD3. As shown in Fig. 3B, the ELK3 reporter plasmid is activated only when ectopically expressed SMAD3 is phosphorylated by cotransfection of the CA-ALK5-expressing plasmid in 293T cells. To confirm that SMAD3 directly binds to the SBE of the ELK3 promoter, we performed chromatin immunoprecipitation (ChIP) analysis with anti-SMAD3 antibody against genomic DNA of MDA-MB231 with or without TGFβ treatment. Since ID1, an inhibitor of differentiation, is a direct downstream target of SMAD3 (16), it was used as a positive control in the ChIP analysis. Like ID1, the SBE region of the ELK3 promoter was significantly enriched by immunoprecipitation with the anti-SMAD3 antibody upon TGFβ stimulation (Fig. 3C). Taken together, we concluded that SMAD3 activates transcription of ELK3 by directly binding to the SBE region of the ELK3 promoter following TGFβ treatment.
Figure 3.
SMAD3 binds to the ELK3 promoter to activate transcription upon TGFβ treatment. (A) Schematics of luciferase assay promoter construct of ELK3 (−92 bp - +345 bp) (left panel). The right panel shows the DNA binding motif of SMAD3 in the ELK3 promoter region of human (+225 bp - +257 bp) and mouse (+112 bp - +144 bp) genomes. (B) Effect of cotransfection of SMAD3 and constitutively active form of the TGFβ receptor-I (CA-ALK5) on the activity of the ELK3 promoter. Reporter plasmid containing the ELK3 promoter (pGL3-ELK3) was cotransfected into 293T cells with CA-ALK5 and SMAD3 expressing plasmids for 24 hrs. The expression of p-SMAD3, SMAD3 and CA-ALK5 was analyzed by immunoblot (left panel), and the activity of the ELK3 promoter was analyzed by luciferase assay (right panel). (C) Chromatin from TGFβ-treated or nontreated MDA-MB231 cells were immunoprecipitated (ChIP) with antibodies against SMAD3 and IgG. The PCR results for the ELK3 promoter region (+225 bp - +257 bp) are presented. SMAD3 binding to the ID1 promoter was used as a positive control of the ChIP experiment. The error bars represent the standard errors from three independent experiments, which were each performed using triplicate samples. **P<0.01. TGFβ, transforming growth factor-β; CA-ALK5, constitutively active form of TGFβ receptor-I; p, phosphorylated; ChIP, chromatin immunoprecipitation.
Discussion
During cancer development and progression in malignancy, the TGFβ signaling pathway acts as a tumor promotor by driving EMT, which induces tumor cell migration, invasion and ultimately metastasis to distant organs. ELK3 is constitutively activated in basal triple negative breast cancer cells (TNBCs) and functions as a master regulator of cancer metastasis (10,12). Previously, we suggested that the TGFβ signaling pathway is interconnected with ELK3 activity, based on the fact that ELK3 knockdown in TNBCs induces collapse of TGFβ signaling (12). In this study, we demonstrated that ELK3 is transcriptionally activated by TGFβ treatment in TNBCs. Pharmacological and molecular analysis revealed that ELK3 is a direct downstream target of SMAD3. In addition, TGFβ induced migration was decreased in ELK3 knockdowned MDA-MB231 cells (data not shown).
There are numerous reports that the TGFβ signaling pathway is strictly regulated by a finely tuned system of negative and positive feedback loops. The expression of SMAD7, a representative inhibitory SMAD, is stimulated by TGFβ treatment and forms a complex with E3 ubiquitin ligase to degrade the TGFβ receptor, which results in the SMAD pathway inhibiting hyper activation of TGFβ signaling (17). During late stages of colorectal cancer, TGFβ activates miR-1269a expression targeting SMAD7, hence forming a positive feedback loop to promote metastasis (18). Since TGFβ signaling is impaired by ELK3 suppression and ELK3 expression is increased by TGFβ treatment, we suggest that TGFβ and ELK3 might form a positive autofeedback loop to promote the EMT process.
Numerous studies have shown that inhibition of EMT is considered an appropriate approach towards the prevention of metastasis of cancer. Since TGFβ functions as an inducer of EMT, blocking the TGFβ pathway is considered a promising strategy to inhibit EMT in cancer cells; cytotoxic drugs such as paclitaxel, which targets TGFβ receptor kinase, have been used to target the metastatic potential of breast cancer cells to colonize the lung (19). In line with this concept, ELK3 can be a prominent therapeutic target to prevent TGFβ-mediated metastasis of cancer cells. The potential value of ELK3 as a target of anticancer drug development is supported by the fact that TNBCs with reduced ELK3 activity completely lost their metastatic characteristics (12). It was shown that small molecule based inhibition of Ras/ERK-mediated ELK3 activity results in the inhibition of prostate cancer progression and metastasis in mice (20). It would be interesting to investigate whether simultaneous inhibition of the TGFβ pathway and ELK3 activity produces clinically effective therapeutic outcomes.
In summary, we suggest that ELK3 is a novel downstream target of the TGFβ-SMAD3 signaling pathway and that it performs a major role in directing the metastasis of cancer. TGF-β1 is preferentially expressed at the advancing tumor edges, where it promotes malignant progression and metastasis (21–23). To strengthen our findings, follow-up immunohistochemical studies are needed to demonstrate the accumulation of ELK3 at the site of excessive TGFβ expression on invasive tumors.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Seong-Jin Kim (Seoul National University) for providing the expression plasmids containing FLAG-SMAD3 and constitutively active ALK5 (CA-ALK5).
Glossary
Abbreviations
- TGFβ
transforming growth factor-β
- SBE
SMAD binding element
- TNBCs
triple negative breast cancer cells
- EMT
epithelial-mesenchymal transition
- siTGFR1
small interfering RNA targeting to TGFβ receptor R1
- CA-ALK5
constitutively active form of TGFβ receptor-I
Funding
The present study was supported by The Ministry of Education, Science, and Technology (NRF-2019R1A2C1003581) and by Basic Science Research Program through The National Research Foundation of Korea (NRF) funded by The Ministry of Education (grant no. 2019R1A6A1A03032888).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JHP designed the experiment and performed all experiments. KSP made substantial contributions to the analysis and interpretation of data. KSP has also been involved in drafting the manuscript and revising it critically for important intellectual content. JHP agreed to the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43–73. doi: 10.1615/CritRevOncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells A, Grahovac J, Wheeler S, Ma B, Lauffenburger D. Targeting tumor cell motility as a strategy against invasion and metastasis. Trends Pharmacol Sci. 2013;34:283–289. doi: 10.1016/j.tips.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 5.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 6.Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 8.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/S1097-2765(01)00260-X. [DOI] [PubMed] [Google Scholar]
- 9.Buchwalter G, Gross C, Wasylyk B. Ets ternary complex transcription factors. Gene. 2004;324:1–14. doi: 10.1016/j.gene.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Heo SH, Lee JY, Yang KM, Park KS. ELK3 expression correlates with cell migration, invasion, and membrane type 1-matrix metalloproteinase expression in MDA-MB-231 breast cancer cells. Gene Expr. 2015;16:197–203. doi: 10.3727/105221615X14399878166276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Hur W, Hong SW, Kim JH, Kim SM, Lee EB, Yoon SK. ELK3 promotes the migration and invasion of liver cancer stem cells by targeting HIF-1α. Oncol Rep. 2017;37:813–822. doi: 10.3892/or.2016.5293. [DOI] [PubMed] [Google Scholar]
- 12.Kong SY, Kim KS, Kim J, Kim MK, Lee KH, Lee JY, Oh N, Park JI, Park JH, Heo SH, et al. The ELK3-GATA3 axis orchestrates invasion and metastasis of breast cancer cells in vitro and in vivo. Oncotarget. 2016;7:65137–65146. doi: 10.18632/oncotarget.11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KS, Kim J, Oh N, Kim MY, Park KS. ELK3-GATA3 axis modulates MDA-MB-231 metastasis by regulating cell-cell adhesion-related genes. Biochem Biophys Res Commun. 2018;498:509–515. doi: 10.1016/j.bbrc.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Lv ZD, Kong B, Li JG, Qu HL, Wang XG, Cao WH, Liu XY, Wang Y, Yang ZC, Xu HM, Wang HB. Transforming growth factor-beta 1 enhances the invasiveness of breast cancer cells by inducing a Smad2-dependent epithelial-to-mesenchymal transition. Oncol Rep. 2013;29:219–225. doi: 10.3892/or.2012.2111. [DOI] [PubMed] [Google Scholar]
- 15.Cho HJ, Oh N, Park JH, Kim KS, Kim HK, Lee E, Hwang S, Kim SJ, Park KS. ZEB1 collaborates with ELK3 to repress E-cadherin expression in triple negative breast cancer cells. Mol Cancer Res. 2019;17:2257–2266. doi: 10.1158/1541-7786.MCR-19-0380. [DOI] [PubMed] [Google Scholar]
- 16.Liang YY, Brunicardi FC, Lin X. Smad3 mediates immediate early induction of Id1 by TGF-beta. Cell Res. 2009;19:140–148. doi: 10.1038/cr.2008.321. [DOI] [PubMed] [Google Scholar]
- 17.Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. 2000;6:1365–1375. doi: 10.1016/S1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 18.Bu P, Wang L, Chen KY, Rakhilin N, Sun J, Closa A, Tung KL, King S, Kristine Varanko A, Xu Y, et al. miR-1269 promotes metastasis and forms a positive feedback loop with TGF-β. Nat Commun. 2015;6:6879. doi: 10.1038/ncomms7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SY, Kim MJ, Park SA, Kim JS, Min KN, Kim DK, Lim W, Nam JS, Sheen YY. Combinatorial TGF-β attenuation with paclitaxel inhibits the epithelial-to-mesenchymal transition and breast cancer stem-like cells. Oncotarget. 2015;6:37526–37543. doi: 10.18632/oncotarget.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenchenko K, Wasylyk C, Cheung H, Tourrette Y, Maas P, Schalken JA, van der Pluijm G, Wasylyk B. XRP44X, an inhibitor of Ras/Erk activation of the transcription factor Elk3, inhibits tumour growth and metastasis in mice. PLoS One. 2016;11:e0159531. doi: 10.1371/journal.pone.0159531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickup M, Novitskiy S, Moses HL. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalal BI, Keown PA, Greenberg AH. Immunocytochemical localization of secreted transforming growth factor-beta 1 to the advancing edges of primary tumors and to lymph node metastases of human mammary carcinoma. Am J Pathol. 1993;143:381–389. [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner MS, Zhou ZZ, Tonb DC, Barrack ER. Expression of transforming growth factor-beta 1 in prostate cancer. Endocrinology. 1994;135:2240–2247. doi: 10.1210/endo.135.5.7956947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.