Abstract
Background
Natural resources provide more efficient and safer alternatives in managing diabetes compare to the synthetic oral anti diabetes (OAD). The plants not only have hypoglycemic effect, but also prevent its complications; in which no synthetic drugs provide of both properties. Among antidiabetes plants, mahogany seed (Swietenia macrophylla) has been used as traditional medicine in Indonesia and India, though most popular utilized as timber wood.
Methods
The present study was performed of chemotaxonomic approach to review its phytochemical and anti-diabetic properties of Swietenia mahagoni (L.) Jacq seed/bark/leaves. The qualitative systematic review (SR) was carried out by analysing indexed journals and peer reviewed of Swietenia and Swietenia spp from Scopus, PubMed, Medline, Google Scholar, and Research Gate. Data selection criteria are accordance to botany, phytochemistry, in vitro, in vivo, and clinical test of related subject. The keywords used for the search in the databases were Swietenia, Swietenia mahagony, diabetes, and diabetes plants.
Results
Swietenia mahagoni (L.) Jacq. extracts have shown in vitro, in vivo and limited clinically test of its anti-diabetic properties. Ethanolic/methanolic/aqueous/petroleum/n-hexane extracts of mahagonis's seed/bark or leaves have anti-diabetic activities comparable to the synthetic drug and observed no to relatively mild toxic effect. The hypoglycemic mechanism suggested via reducing blood glucose level, restoring liver and β-cells islet function (might) blocking epinephrine function, inhibiting of α-amylase and β-glucosidase, antioxidant and antihiperlipidemia. Phytochemical compounds of S. mahagoni consist of the phenolics (flavonoids (swietemacrophyllanin, catechins and epichatechins) and tannins), triterpenoids and tetranortriterpenoids (limonoids: mahonin, secomahoganin, swietmanins, swiemahogins, swietenine and swietenolide), saponins and alkaloids which are known as anti-diabetic bioactive principles.
Conclusion
S. mahagoni was potentially used and developed as an antidiabetes source. To use it as an antidiabetic further, more extensive clinical trials and biomarkers of active compounds determination are needed.
Keywords: Toxicology, Diabetes, Hypoglycemic, Mahogany, Meliaceae, Swietenia sp
Toxicology; Diabetes; Hypoglycemic; Mahogany; Meliaceae; Swietenia sp
1. Introduction
Diabetes is endocrine chronic metabolic disorder in insulin production or insulin resistance. It is diagnosed with hyperglycemia and other parameters such as HbA1c. Insulin is one of among the hormones which regulate the blood glucose level (BGL). It facilitates glucose energy source consume in most of the cell and also to store as glycogen in the liver or as a fat in the tissue. Other hormones (glucagon, amylin, cortisol, epinephrine, growth hormone, glucagon-like peptide-1 and polypeptide glucose-dependent insulinotropic) are indirectly influence BGL by leaven of insulin production (Khardori, 2018). Uncontrolled diabetes hyperglycemia overtime, leads to serious impairment of vascularization of the body's systems, reduces the quality of life with disabilities to causes premature death. Micro-vascular diabetes complication damages the nerves (neuropathy), the eyes (retinopathy), the kidneys (nephropathy) and blood vessels to the heart; while macro-vascular complications cause ischemic heart disease, stroke to peripheral vascular disorder. Diabetes prevalence estimates more than two times at 2030 from 171 million people (2000) and will be seventh leading cause of death. It will be global burden for low- and middle-income countries, so diabetes was one of four targeted priorities of non-communicable diseases in the 2011 (World Health Organization, 2016).
The classifications of diabetes are important in managing diabetes and delaying its complication. Diabetes therapy objective is to sustain normal BGL with diet, physical activities, medication; blood glucose level regularly check and complications therapy. For type 2 diabetes, life style management (diet, medical nutrition, weight control, and exercises) is important, before the use of oral anti-diabetic drugs (OAD) and or insulin injections treatment (American Diabetes Association, 2017).
Though the developing of OAD has changed with new drug classes provided and the results of medication are beyond the reach of medical therapy previously; still OADs have limitation on its side or adverse reaction effects. Some of OAD may lose its efficacy in a significant percentage of patients (Pandey et al., 2011). OAD's cause weakness and fatigue to lactic acidosis, weight gain of hyper-insulinemia, nausea, vomiting, diarrhea pancreatitis, to thyroid tumor inhibitor (Khardori, 2018). Regardless the terms of effectiveness and safety; the threat of diabetes to global endemic have encouraged source finding to alternative or complementary in diabetes therapy.
Plants provide great alternatives to manage diabetes. It was used in many developing countries with natural diversity resources. The plants are not only hypoglycemic or insulin mimetic, but also preventing the complications; which no synthetic drug provide both properties. Some have been shown in ß-cells regeneration function and delaying the insulin resistance, while others have antioxidant and cholesterol lowering activities. More than 1200 plants were found in ethno-pharmacological surveys for blood sugar lowering properties (Pandey et al., 2011). Here some of the plants with in the list: Aloe vera and Allium spp (Liliaceae), Bilberry (Moraceae), Bitter melon/Momordica charantia (Cucurbitaceae) (Perumal et al., 2015; Meles et al., 2019), Cinnamon spp (Lauraceae), Ginger/Zingiber officinale (Zingiberaceae), Fenugreek/Trigonella foenum graecum (Fabaceae), Ochra/Abelmoschus esculenthus (Malvaceae) and Brassica juncea (Cruciferae.), Gymnema sylvestre (Apocynaceae) and Azadirachta indica (Meliaceae) (Arumugam et al., 2013; Patel et al., 2012), Moringa oleifera (Amelia et al., 2018), Garcinia mangostana (Ansori et al., 2019; Husen et al., 2017), Tinospora crispa (Arundina et al., 2017; Roestamadji et al., 2017), Cassia fistula (Noorhajati et al., 2012). Even some mangrove plants were used for diabetes such as Acanthus ilicifolius, Hisbiscus tiliaceus, Ipomoea pes-capre (Purnobasuki, 2004) and also bee related product propolis (bee glue) (Trusheva et al., 2013). The plants are used by empirical base and some are supported by preclinical to clinical studies.
Among the plants previously described, mahogany seed (Swietenia macrophylla) has been used as traditional medicine in Indonesia (Kadota et al., 1990) and India (Arumugam et al., 2013; Patel et al., 2012; De et al., 2011). The present study was performed chemotaxonomic approach to review phytochemical and anti-diabetic properties of Swietenia mahagoni (L.) Jacq.
2. Swietenia mahagoni botanical view
Swietenia is genus of chinaberry family (Meliaceae). It was brought into some Asian countries to Bolivia from Caribbean, Mexico and Southern to Central America. Three species based of geographically separated are known; which are Swietenia mahagoni (L.) Jacq (West Indian mahagoni); Swietenia humilis Zucc (Pacific Coast mahagoni); and Swietenia macrophylla King (Honduran mahagoni) (Orwa et al., 2009).
S. mahagoni Jacq. is a small to medium deciduous tree (up to 30 m high), in spherical crown, short and buttressing base with diameter up to 1 m. The bark is smoothing grey and turning to scaly dark reddish-brown with many heavy branches and dense shade. The leaves phylotaxis are even and pinnate, size of 10–18 cm length, with 4–10 pairs of leaflets. The leaves are dark shiny green, lance-shaped of 2.5–5 cm and 0.7–2 cm. Its flowers are unisexual, greenish-yellow, panicles axillary and glabrous appearance. The seed capsules are green to light brown varies, upright stands, its size is about 6–10 cm and 4–5 cm of diameter. The splitting upward valves produced about 20 flat, brown-winged seeds, 4–6 cm of each. Flowering and fruiting of Swietenia are regular and annual. Developing of flower to mature fruit is of 8–10 months. Insect pollination and hybridization among the species is frequent, especially with S. macrophylla. S. mahagoni grows at a moderate rate and its wood mostly used (Orwa et al., 2009) (Divya et al., 2012).
3. Ethnomedicinal and pharmacological used of Swietenia spp.
S. mahagoni is used as medicinal plants in India (Ayurvedic system), some African countries, also in Indonesia (Patel et al., 2012.) and Malaysia (Hashim et al., 2013). Traditionally it uses for malaria, hypertension, diabetes and diarrhea, as antipyretic, as bitter tonic and astringent (Bourdy et al., 2000). Table 1 presents plant part and traditional technique on the use of Swietenia spp. Pharmacological activities of S. mahagoni are antimicrobial, anti-inflammatory, hepatoprotective, antidiarrheal, antiulcer, depressant, anticonvulsant and neuropharmacological, anti-diabetic, anti-HIV, immunomodulator, insect repellent and larvicidal, antifungal, antioxidant, analgesic, platelet aggregation inhibitors, antimutagenic and anticancer (Divya et al., 2012; Hashim et al., 2013; Bourdy et al., 2000; Moghadamtousi et al., 2013; Naveen et al., 2014; Bhurat et al., 2011).
Table 1.
Traditional use of Swietenia spp.
| Region | Plants part and method to use |
|---|---|
| Malaysia | Raw seeds of S. macrophylla are chewed/pounded to treat hypertension, diabetes and relieve pain. Decoction of chrushed seed for treating skin ailments and wounds (Kadota et al., 1990). |
| Amazonian Bolivian | Mashed seed of S. macrophylla in water is internal used for abortion, while external use for leishmaniasis (Bourdy et al., 2000) |
| India | - The bark decoction of S. mahagoni is orally taken for diarrhoea, dysentery and haemostyptic since its vitamins and iron contents. It also serves antipyretic and tonic, increasing appetite, restoring in tuberculosis, treating anaemia, fever and toothache (Khare, 2007) - Its leaf decoction is used against nerve ailments, while the leaf or root poultice for bleeding (Anonymous, 1986) - The seed infusion against chest pain (Miroslav, 2005). - The aqueous extract of S. mahagoni seed and bark for psoriasis, diabetes, diarrhea and as an antiseptic in cuts and wounds in East Medinipur (West Bengal), Balasore (Orissa) (Haldar et al., 2011). |
| Indonesia | The crushed of S mahagoni seed water decoction for hypertension, controlling blood glucose, treating constipation, and menstrual pain, eczema and rheumatism, improving fertility and appetizing, relieving fever and cold. It also uses for powder external use as insect repellant (Mardisiswojo and Radjakmangunsudarso, 1965; Kadota et al., 1990). |
4. Phytochemical of Swietenia mahagoni (L.). Jacq
Phytochemicals content of S. mahagoni are phospholipid, alkaloids, phenols, flavonoids, antraquinones, saponins, terpenoids, cardiac glycosides, volatile oils and long chain unsaturated acid. The contents are including 45 limonoids such as swietenolide, swiemahogins A and B, 2-hydroxy-3-O-tigloylswietenolide, andirobin, mexicanolide, gendunin and phragmalin, triterpens, tetranortriterpenes, swietenine dimeric triterpmahonienoid and chlorogenic acid (Patel et al., 2012; Kadota et al., 1990), swietenine acetate; 3,6-di-0-acetylswietenolide, 3-0-tigloylswietenolide, 6-acetyl-3-tigloylswietenolide, 2-α-hydroxymexioanolide, 6-acetylswietenine (Kadota et al., 1990; Naveen et al., 2014; Bhurat et al., 2011). Mostafa et al. (2011) found that S. mahagoni seed oil which has bitter taste, moderate drying oil and high content on unsaturated fatty acid, considered as useful source for soap and dying industries. The unsaturated fatty acid contents as listed in Table 2. Moghadamtousi et al. (2013) reviewed the isolated phytochemicals content from seed, bark, twig, leaves and stem of S. macrophylla. Most of them are limonoids (81.91%), polyphenolics (4.26%), steroids (4.26%), essential oils (6.38%), fatty acids (1.06%), coumarin (1.06%) and lignan (1.06%).
Table 2.
Some of phytochemicals of Swietenia spp.
| Parts of the plant | Contents (%) | References |
|---|---|---|
| Seed of S. mahagoni | Secomahoganin (0.0014), swietemahonin F (0.0036), swietemahonolide (0.0009), swietemahonin C (0.0012), 3-O-tiogloyl-6-O-acetylswietenolide (0.0003), 6-acetoxygedunin (0.0006), 7-deacetoxy-7-oxogedunin (0.0007), methyl angolensate (0.0014), swietemahonin B (0.0023) | Kadota et al. (1990) |
| Seed S. mahagoni | Swietemahonin A (0.0002), D (0.0024), E (0.0003), and G (0.0040) and 3-O-acetylswietenolide (0.0010) and 6-O-acetylswietenolide (0.0015) | Ekimoto et al. (1991) |
| Seed S. mahagoni | Swiemahogins A (0.0010) and B (0.0001) | Chen et al. (2007) |
| Fruits of S. mahagoni | Mexicanolide-type limonoids, swietmanins A (0.00005), B (0.0033), C (0.00008), D (0.00005), E (0.00008), F (0.0003), G (0.0002), H (0.0003), I (0.0003) and J (0.00005). (2-Hydroxy-3-O-isobutyrylproceranolide (0.0005), 2-Hydroxy-3-O-benzoylproceranolide (0.00008), andirobin-type limonoid, swietmanin J, mexicanolide (0.0006), 3,8-hemiketalcarapin (0.0003), 8R-hydroxycarapin (0.00025), and fissinolide (0.0002); khivorin (0.0004), and 2-hydroxyfissinolide (0.0004), 2,3-dihydroxy-3- deoxymexicanolide (0.0004) and 2-hydroxyfissinolid (0.0167), methyl angolensate (0.0002), 6-hydroxyangolensate (0.0005), and 3-deacetylkhivorin (0.0002), 3,7-dideacetylkhivorin (0.0003), 1,3,7-trideacetylkhivorin (0.0003), and 7-deacetoxy-7-oxogedunin (0.0002), and 7-deacetylkhivorin (0.0004), and 1-deacetylkhivorin (0.0005), 2-hydroxy-6-deoxyswietenolide tiglate (0.00025) and seneganolide A (0.0002) | Lin et al. (2011) |
| Seed S. mahagoni | Moisture (14.37), minerals (16.36), fats (19.42), crude fiber 19.60), protein (8.76) and carbohydrate (21.49) Fatty acid of the seed's oil: -linoleic acid (26.00), elaidic acid (24.39), stearic acid (14.32), palmitic acid (12.97), 10-methyl-10-nonadecanol (5.24), ecosanoic acid (2.48), 3-heptyne-2,5-diol-6-methyl-5-(1-methylethyl) (2.03) octadecanoic acid, 9,10,12-trimethoxy (1.90); 1,3-dioxalane-4-ethyl-4-methyl-2-pentadecyl (1.89) and 2-furapentanoic acid (1.03). |
Mostafa et al. (2011) |
| Bark S. macrophylla | Swietemacrophyllanin (2R∗, 3S∗, 7″R∗)-catechin-8,7″-7,2″-epoxy-(methyl-4″,5″-dihydroxyphenylpropanoate) (0.0002), catechin (2,3-trans-5,7,3′,4′-tetrahydroxyflavan-3-ol) (0.0004), and epicatechin (2,3-cis-5,7,3′,4′-tetrahydroxyflavan-3-ol) (0.0003) | Falah et al. (2008) |
| Seed S. macrophylla | Swietemacrophin (0.0012). humilinolide F (0.0011),. 3,6-O,O-diacetylswietenolide (0.0015), 3-O-tigloylswietenolide (0.0012), swietemahonin E (0.0014). swietenine (0.0051) | Chen et al. (2015) |
| Twigs of S. macrophylla | Swietenitins N (0.0002), O (0.0002), P (0.00005), Q (0.00003), R (0.00005), S (0.00005), T (0.00010), U (0.00003), V (0.00005), W (0.00002), X (0.00002), epoxyfebrinin B (0.00005) | Lin et al. (2011) |
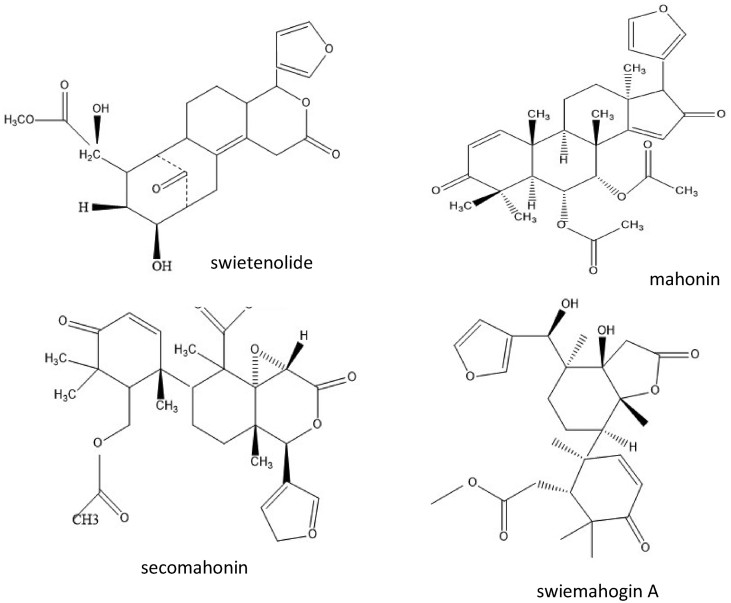
The leaves of S. mahagoni is contain tetracyclic triterpene (cyclomahogenol) (Chakraborty and Basak, 1971). The twigs and leaves produced limonoids, swiemahogins A (Figure 1) and B, which first andirobin and phragmalin types of limonoids (Chen et al., 2007). While Lin et al. (2011) isolated eleven swietenitins N to X limonoids and known epoxyfebrinin B compound from the twigs of S. macrophylla with percentage as written in Table 2.
Figure 1.
Some of phytochemical content of S. mahagoni L.
The main phytoconstituents of methanolic and water extract of mahagony seeds are tannins, alkaloids, saponins and terpenoids (Hajra et al., 2011), anthraquinones, cardiac glycosides and volatile oils (Sahgal et al., 2009). Limonoids swietenolide (Figure 1) and 2-hydroxy-3-O-tigloylswietenolide were isolated from the methanolic extracts of the seed; which have antimicrobial activity (Rahman et al., 2009). Meanwhile in the ether extract was isolated 28 tetranotriterpenoids; which related to swietenine and swietenolide (Kadota et al., 1990). Some of these compounds are listed in Table 2. Among those substances, swietemahonins A, D, E and G and 3-O-acetyl-swietenolide and 6-O-acetyl-swietenolide inhibited platelet activating factor (PAF), thus induced platelet aggregation activity (Ekimoto et al., 1991). Another two tetranotriterpenoids of the seed were mahonin and secomahoganin (Figure 1).
Thirty compounds were isolated from the S. mahagoni fruits, which consisted of eleven mexicanolide-type limonoids (swietmanins A to I, 2-hydroxy-3-O-isobutyrylproceranolide, 2-hydroxy-3-O-benzoylproceranolide), and andirobin-type limonoid, swietmanin J, and 19 known compounds (Lin et al., 2009). Falah et al. (2008) found no different chemical constituents of Swietenia's bark on methanol to water extract. Some phytochemical compounds such as flavonoids, triterpenoids, alkaloids and phenolics are known as bioactive antidiabetic principles (Nagappa et al., 2003; Battu et al., 2007). Furthermore, flavonoids have known as antioxidant class compound (Falah et al., 2010), which have advantage synergistic role for anti-diabetic.
Other result was reported on the phytochemical qualities parameters of S. mahagoni bark. It described the total and sulphated ash, water soluble and total acid insoluble ash content of the bark to be 22.0%, 14.5%, 1.4% and 0.6% respectively (Sukardiman et al., 2016); and tannin content (15.0%) (Divya et al., 2012). Sukardiman et al. (2016) was report on the quality of Indonesian dried Mahagony seed. The physical characteristic of the bark was described as flat and corrugated shaped, with 3–5 cm to 2–3 cm of the size; while the seed microscopic was observed of testa, endosperm, schlereids, essential oil and amylum fragments. It was also determined its phytochemical parameters and stigmasterol content of dried part and ethanolic extract of Mahagony seed. The study concluded its quality appropriate with Indonesia Herbal Pharmacopeia. Table 2 presents percentage of the phytochemicals isolated from S. mahagoni and S. macrophylla.
5. Swietenia spp. for diabetes
Swietenia seed and bark empirical most used for diabetes. Following presents scientific development on the antidiabetes research on Swietenia spp. In vitro antidiabetic potency was obtained by Hajra et al. (2011), who found ethanolic extract of S. mahagoni seed (EEMS) inhibited α-amylase. Furthermore Wresdiyati et al., 2015 observed its aqueous and ethanol extract (maceration and reflux methods extraction) at doses 100–500 mg/kgBW, have α-glucosidase inhibition and in vivo hypoglycemic activities. The test obtained best α-glucosidase inhibition was produced by ethanolic extract maceration methods. They were also showed in vivo antidiabetes effects.
Raja (1990) did in vivo treatment of ethanolic extract of mahagony seed (91,0 mg/dl) and found that it decreased BGL of the rat at 180 min Li et al. (2005) was shown Swietenia mahagoni extract at the dose of 1000 mg/kg (SmE) have peroxisome proliferator-activated receptor (PPARγ) agonists approximately half of rosiglitazone on diabetic db/db mice. The mechanism was supposed by increasing absorption and the use of glucose in the peripheral cell membrane of insulin gen forming and translocating GLUT (glukosa-transporter) of swietenin activated.
The effect of a methanol extract of the seeds (MEMS) of Swietenia macrophylla King was evaluated to streptozotocin-induced (STZ) diabetic rats with oral glucose tolerance (OGTT) and normo-glycemic activities test. The MEMS was found to be a potential antidiabetic compared to glibenclamide. The results observed reduction on BGL, serum lipids and increasing of liver glycogen level of diabetic rats significantly. Another result observed was the lowering of fasting BGL in normal rats, group of treated extract (300 mg/kg) and glibenclamide group. Improvement of body weight profile was also observed in extract-treated diabetic rats. The research concluded the MEMS had reduction of oxidative stress associated to hypoglycemic as well as hypolipidemic effect (Maiti et al., 2007; Maiti et al., 2008; Maiti et al., 2009a, b).
The antidiabetic and antioxidant activity of the methanol extract of S. mahagoni bark at doses 25 and 50 mg/kg body weight (MEMB) to STZ rats to glibenclamide was evaluated. The results were shown that MEMB reduced significantly BGL and restored the body weight compared to normal rats. Other parameters were observed the decreased of TBARS, but increased of the GSH levels and CAT activities; which indicated of antioxidant activities. These would reduce free radical formation in the liver and kidney tissues of diabetic rats. These findings provoke the hypoglycemic and antioxidant activity of the MEMB extract in diabetic rats (Panda et al., 2010).
Kurniawati (2010) was tested of 1574.9 mg/kg body weight EEMS (ethanolic extract of Mahagony seed) to alloxan induced diabetic rat. The decreasing of BGL observed better compare to glibenclamida. It concluded the activities was supposed of flavonoid and saponin content of mahagony's seed extract. Al-Hasan et al. (2011) also observed in vivo different method of EEMS at 1000 mg/kg orally. It found no significant of the BGL treatment group compared to positive control group; but a significant reduction compared to diabetic control. The histological examination of pancreas showed destruction, retaining of islets and few de-granulations of beta cells of pancreas. These observations and results provide information that EEMS has hypoglycemic effect in experimentally induced diabetic rats.
Antidiabetic, antioxidative and antihyperlipidemic activities of 60% methanolic of mahagony seed studied at 250 mg/kg body weight to STZ rats. The seed extract was given for 21 days. The lowered of BGL as well as the glycogen level in liver observed; while improving of antioxidant enzymes (catalase/cat, peroxidase/perox) and radicals (conjugated diene/CD and thiobarbituric acid/TBAR) level in liver, kidney and skeletal muscles were obtained. The extract was also improving serum urea, uric acid, creatinine, cholesterol, triglyceride and lipoproteins level. The results indicated the MEMS potential use for the diabetes therapy, oxidative stress and hyperlipidemia related complications (De, et al., 2011). Another observation was made by Dutta et al. (2014) on increasing of plasma H2S (hydrogen sulfide) level as H2S synthesis activity in plasma of water extract of S. macrophylla to lowering FBG in streptozotocin induced diabetic rats. H2S plasma functions as a neuro-modulator and a neuro-protectant against diabetes related oxidative stress.
Bera et al. (2012) found in vivo effects of MEMS (60% methanolic, at the 250 mg/kg body weight) to STZ rats compare to metformin. The extract and metformin were administered orally once a day. The results showed a significant reduction in the activities of hepatic hexokinase and glucose-6-phosphate dehydrogenase sequence to elevation in glucose-6-phosphatase were noted in diabetes animals compare to control animals. Level of fasting blood glucose (FBG) was elevated in diabetes animals. Activities of CAT, PEROX and SOD were diminished significantly with the elevation in TBAR. This result suggested diabetic mechanism action of the MEMS is the regeneration of acini and islets cells of the pancreas that are damaged by STZ. The extract constituents sensitized and/regenerated the ß cells that increasing insulin level serum and rectifying glycated Hb level, regulating carbohydrate enzymes metabolic activities and FBG level, respectively. Furthermore no general toxic effect was observed by SGOT, SGPT and histology to body weight of the extract-treated diabetic group. Furthermore, La Basy et al. (2015) showed EEMS improving renal dysfunction of streptozotocin-induced diabetic rats at a dose of 50, 100 and 200 mg/kg body weight for 21 days.
Suryani et al. (2013) treated the MLD-STZ rat with EEMS. It found that 250 mg/kg body weigh dose approached to control effect. It increased insulin level; decrease of TNF-⍺ and regenerate pancreatic islet. Noormalasari (2015) found that ethanolic extract of Mahagony seed would decrease BGL (347–179 mg/dl), decrease food intake and inhibit damage rate of Langerhans island and β cell of the pancreas.
Kalaivanan and Pugalendi (2011) observed of EESM (at doses 50, 100, and 200 mg/kg BW) and glibenclamide on streptozotocin-diabetic rats. They found increasing of hemoglobin (Hb) level and decreasing of glycosylated Hb level. Thus pancreas histological and biochemical finding supported insulin secretion mechanism.
Hashim et al. (2013) was also test of antidiabetic effect from various solvents of S. macrophylla seeds to normoglycaemic and STZ rats. It was used petroleum ether (PE), chloroform (CE) and methanol (ME). The results were shown none of the extracts had a significant effect on the BGL of 60 randomly selected normoglycaemic and diabetic rats. PE extract (at doses 500 mg/kg and 1000 mg/kg), however, significantly reduced BGL in 30 randomly selected normoglycaemic rats with IPGTT (intraperitoneal glucose tolerance tests) 30–120 min after glucose administration. PE significantly increased glucose uptake on abdominal muscle with or without insulin presence. GC-MS analysis of phytochemical content indicated terpens of diterpenes, triterpenoids and phytosterols (fucosterol and β-sitosterol), fatty acid methyl esters and aldehydes might act as principle compounds for the hypoglycemic effect of PE extract. Maiti et al. (2009a, b) isolated swietenine, a tetranortriterpenoids from chloroform fraction of hydroalcohol extract of S. macrophylla seeds, whose antidiabetic comparable to that of human insulin (p < 0.01).
The potent of aqueous extract of S. mahagoni leaf (AEML) as anti-diabetic was tested by oral administration to diabetic rats at 500 mg/kg for 45 days. The extract lowered the fasting BGL. There was also observation of an improvement in antioxidant glutathione components, decreasing activity of liver enzymes in the serum and reduction in body mass loss in treated groups. Thus, it can be proposed that the effect may be mediated through increasing the antioxidant strength, improving liver glycogen content, balancing the lipid components in serum, decreasing the muscle protein catabolism and improved overall health (Naveen and Urooj, 2015).
Hypoglycemic activity was also observed from ethanolic dried extract of S. mahagoni (500 mg and 1000 mg/kg body weigh) to alloxan-induced mice much greater compare to glibenclamide (Wardani, 2016). Alloxan and streptozotocin chemicals are usually used for the induction of diabetes mellitus in vivo experimental, since both are destructive to Langerhans ß cells of the islets. Histopathological observation on the restoring of the density and percentage of ß cells diabetic extract treated might indicated the regeneration. Some plant extracts observed summation to regenerate of ß cells, restore insulin secretion from surviving ß cells of the islets of Langerhans and lowering of BGL (Szkdelski, 2001; Singh and Gupta, 2001; Yadav et al., 2008). The water infusion and methanol extracts of mahogany bark (Falah et al., 2010) exerted regenerating of ß cells, restoring insulin of the cell (pancreatrophic) producing or the extract may have insulin like substances with dose-dependent manner (Adewole and Ojewole, 2007).
The result of in vivo antidiabetic of EEMS to alloxan intraperitoneal administration to mice; showed dose-independent manner of antidiabetic activity compare to glibenclamide. This research supported the developing of mahagoni seed extract to pharmaceutical formulation, since the mahagoni extract used in dried formulation (filling with avicel: cab-o-sil by ratio of 70:30 and dried 4:1) (Sukardiman et al., 2017).
Limited clinical study of S. mahagoni was observed in 68 type II diabetic patients with pre- and post-test control group design experimental. Result was shown that 85.3% treatment group has glucose blood level 90–199 mg/dl. Bivariate analysis result was shown the potential of Mahagoni seed to reducing BGL compare to glimepiride (Astuti et al., 2017).
Sukardiman et al. (2013) tested the combination of herbal tea of Andrographis paniculata herbs and S. mahagoni seeds (2:1), which has given for seven days at the dose of 0.4 ml/20g body weight; resulted the highest BGL reduction (88.20 ± 43.16 mg/dl) of alloxan diabetic mice compare to other ratios.
Govindappa (2007) reviewed 419 plant species of 133 families of plant extracts or phytochemicals to in-vitro and in-vivo of antidiabetes therapy. It resumed that the plant extracts involved different mechanisms in diabetes. Antidiabetic molecules from different parts of the plant extracts produced signal transduction in restoring insulin production or normalize BGL. The Azadirachta indica A. Juss and Trichilia emetica of Meliaceae's family, were include on the list, but none of Mahagony. The review also suggested the antidiabetes mechanism of the phytochemicals. Azadirachtin and nimbin (bioactive compound of A. indica seed) are supposed increased peripheral glucose uptake by inhibiting of epinephrine on glucose metabolism (Donga et al., 2011) while nimbidin, nimbin, nimbidol and nimbosterol (leaves extracts content) have glycogenolytic of epinephrine blocked (Chattopadhyay, 2007). Both extracts increased insulin secretion (Tripathi et al., 2007). The flavonoid-rich fractions of T. emetica extract was shown antidiabetic, anti-lipidemic and antihypertensive activities (Konaté; et al., 2014). Mendes and Bogle (2015) was also proposed mechanism of alkaloids, saponins, vitamins, polyphenols, flavonoids and limonoids as hypoglycemic bioactive component. Limonoids content of the hexane extract of S. humilis also noted as hypoglycemic, reducing serum triglycerides and uric acid. This result suggested the insulin sensitizing mechanism and glycogen synthesis activating, abdominal fat rats eliminating, blood triglycerides eliminating and increasing adipose tissue glucose uptake (Magallanes et al., 2015).
Swietenine, a limonoid from S. macrophylla, have moderate hypoglycemic and reduce triglycerides in blood diabetic rat model (Dewanjee et al., 2009; Maiti et al., 2009a, b). Antidiabetic synergistic mechanism and hypolipidemic activity on different molecular targets was also proposed (Mendes and Bogle, 2015) (Table 3). A mixture of many components in the extract of the plant would enhance the bioavailability of one or several compounds of the extract, thus improving its pharmacological action. Synergistic effect made difficult to prove mechanism and might produce contrary result in some research. Medicinal chemistry-based analysis of the phytochemical can promote the prospects as natural products in managing diabetes.
Table 3.
Proposed synergistically mechanism of antidiabetic and hypolipidemic of phytochemicals class.
|
Molecular Target |
Compound Class | Mechanisms |
|---|---|---|
| Enzyme (α-glucosidase, α-amylase) inhibition activity | Flavonoids, sterols, terpens | Inhibition of glycogen phosphorylases and glucose 6-phospatase, inhibit lipid peroxidation, stimulate insulin secretion, protective regeneration cell β function |
| Acetyl CoA activation | Cathechin | Inhibit lipid peroxidation, stimulate insulin secretion, protective regeneration cell β function, activate of AMPK, increase expression level of GLUT4 transporter |
| α-glucosidase inhibition activity | Saponins | Decrease insulin resistance and inhibits carbohydrates absorption, increase expression level of GLUT4 transporter, inhibition of glycogen phosphorylases and glucose 6-phospatase |
| Inhibition of mitochondrial function/stimulating of glycolysis | Berberine | Inhibit lipid peroxidation, stimulate insulin secretion, protective regeneration cell β function, activate of AMPK |
| Insulin sensitizising, α-amylase inhibition |
Limonoids | – |
Molecular targeting approached of antidiabetic mechanisms were proposed by some researchers. Molecular target on SGLT2 (Vigneshwaran and Lalitha, 2016), peroxisome proliferator-activated receptor gamma (PPARɤ) (Jian et al., 2018), and α-amylase inhibitor (Ponnusamy et al., 2010) have been done. These studies were exploring the potency of hypoglycemic phytochemical, or improving the activity of provided antidiabetic substances on its specific target. Zapata-Sudo et al. (2012) and Jian et al. (2018) examined the N-benzylbenzamide or sulphonylhydrazone antidiabetic derivatives on PPARɤ agonist; while Ponnusamy et al. (2010) was working with gedunin and azadiradione limonoids of A. indica on α-amylase inhibitor, so did Vigneshwaran and Lalitha (2016) with antidiabetic phytocehmical of the Swietenia mahagoni seeds. Jian et al. (2018) found the possible binding active site of PPARɤ on the residues of His323, Tyr473, Ser289 and Ser342 with the hydrogen bond interactions. Zapata-Sudo et al. (2012) was found LASSBio-1471, a novel sulfonylhydrazone derivative ligand, have least theoretical energy binding for PPARɤ among all new compounds. It was effective on reducing BGL from 548.4 ± 26.0 to 259.6 ± 73.1 mg/dL and paw withdrawal from 21.9 ± 1.7 to 36.7 ± 1.2 g of STZ-diabetic rats’ neuropathy (20 mg/kg, i. p.) for 7 days. The structure of LASSBio-1471 was of 1,3-benzodioxole subunit which inhibited and/or induced CYP450. Furthermore, Vigneshwaran and Lalitha (2016) were analyzed of S. mahagoni seed 18 compounds and dapaglifozin reference antidiabetic drug to SGLT2 targeted molecule. The content included Mahonin, Sweitenin B–F, Secomahoganin, Swietenollide, Swietemahonin A-G, Swietemahonolide, and oleanolic acid. It was found that all compounds have lesser, and oleanolic acid has the least binding energy compare to the synthetic reference dapaglifozin (-7.77 kcal/mol). This new approach not only provided supportive information for empirical or scientific factual, but also offered effective screening methods, as versatile tools for antidiabetic natural substances drug discovery experiments.
The toxicity of the seed and ethanolic extract of Swietenia macrophylla and Swietenia mahagoni were tested with in vitro and in vivo. The result was obtained that S macrophylla seed orally given of 2 g/kg BW is safe to rats (not influenced either by food or water intake, weight and histology of vital organ, the biochemical and hematological parameters as well); neither observed of toxicity signs nor deaths during the toxicity study period (Balijepalli et al., 2015). Furthermore, the toxicity of S. mahagoni seed extract showed safe to mild toxic range. Brine shrimp lethality in vitro test resulted LD50 of methanolic extract S. mahagoni Jacq. seed (MEMS) is more than 2500 mg/kg; which is classified as a relatively nontoxic (Sahgal et al., 2010). This result was also supported by Ghosh et al. (2009) which found increasing dose of intraperitoneal injection of S. mahagoni seed extract to mice was non-toxic (up to 1.2 g/kg body weigh up to a day). Tough in vivo acute toxicity test of mahagoni ethanolic extract (LD50 7.998 g/kg body weigh) was observed the changes on the kidney and liver histology; thus categorized as mild toxic of (Saputri, 2014).
6. Conclusion
S. mahogani is one of among three plants of Swietenia's Meliaceae family, which has more potential to develop as antidiabetic phytomedicine agent. Its seed/bark or leaves of ethanolic/methanolic/aqueous/petroleum/hexane extracts have shown antidiabetes activities by reducing BGL, restoring liver and ß-cells islet function, blocking epinephrine function, inhibiting of α-amylase and β-glucosidase, antioxidant and antihiperlipidemia mechanisms. Phytochemical compounds of S. mahogani consist of the phenolic (flavonoids (swietemacrophyllanin, catechin and epichatechin) and tannins), triterpenoids, tetranortriterpenoid (limonoid: mahonin, secomahoganin, swietmanins, swiemahogins, swietenine and swietenolide), saponins and alkaloids which are known as antidiabetic bioactive principles. To use it as an antidiabetic further, more extensive clinical trials and biomarkers of active compounds determination are required.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Universitas Airlangga Grant for Article Review Program 2019
Competing Interest Statement
The authors declare no conflict of interest.
Additional Information
No additional information is available for this paper.
Acknowledgements
Thanks to the Faculty of Pharmacy Universitas Airlangga for the supporting facilities.
References
- Adewole S., Ojewole J. Insulin-induced immunohistochemical and morphological changes in pancreatic β-cells of streptozotocin-treated diabetic rats. Meth. Fund. Exp. Clin. Pharm. 2007;29:447–455. doi: 10.1358/mf.2007.29.7.1119168. [DOI] [PubMed] [Google Scholar]
- Al-Hasan S., Khan M., Umar B. Effect of ethanolic extract of Swietenia mahagoni seeds on experimentally induced diabetes mellitus in rats. Faridpur Med. Coll. J. 2011;6(2):70–73. doi: 10.3329/bmrcb.v39i1.15790. [DOI] [PubMed] [Google Scholar]
- Amelia D., Santoso B., Purwanto B., Miftahussurur M., Joewono H.T., Budiono Effects of Moringa oleifera on insulin levels and folliculogenesis in polycystic ovary syndrome model with insulin resistance. Immunol. Endocr. Metab. Agents Med. Chem. 2018;18:22–30. doi: 10.2174/1871522218666180426100754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care. 2017;40(1):11–24. [Google Scholar]
- Anonymous. Publications & Information Directorate, Council of Scientific and Industrial Research; New Delhi: 1986. The Useful Plants of India; pp. 967–968. [Google Scholar]
- Ansori A.N.M., Susilo R.J.K., Hayaza S., Winarni D., Husen S.A. Renoprotection by Garcinia mangostana L. pericarp extract in streptozotocin-induced diabetic mice. Iraqi J. Vet. Sci. 2019;33(1):13–19. [Google Scholar]
- Arumugam G., Manjula P., Paari N. A review: anti diabetic medicinal plants used for diabetes mellitus. Journal of Acute Dis. 2013:196–200. [Google Scholar]
- Arundina I., Diyatri I., Budhy T., Jit F. The effect of brotowali stem extract (Tinospora crispa) towards increasing number of lymphocytes in the healing process of traumatic ulcer on diabetic Wistar rat. Journal of International Dental and Medical Research. 2017;10(3):975–980. ISSN 1309-100X. [Google Scholar]
- Astuti A., Antriana N., Zelpia Mahagony seed (Swietenia mahagoni) lowering blood glucose on type II Diabetes Melitus, Jurnal IPTEKS Terapan. Res. Appl. Sci. Educ. (translate from Indonesian language) 2017;11(3):187–193. [Google Scholar]
- Balijepalli M.K., Suppaiah V., Chin A., Buru A.S., Sagineedu S.R., Pichika M.R. Acute oral toxicity studies of Swietenia macrophylla seeds in Sprague Dawley rats. Pharmacogn. Res. 2015;7(1):38–44. doi: 10.4103/0974-8490.147197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battu G.R., Mamidipalli S.N., Parimi R., Viriyala R.K., Patchula R.P., Mood L.R. Hypoglycemic and antihyperglycemic effect of alcoholic extract of benincasa hispida in normal and in alloxan-induced diabetic rats. Phcog. Mag. 2007:101–105. [Google Scholar]
- Bera T.K., Chatterjee K., Jana K., Ali K.M., De D., Maiti S., Ghosh D. Antihyperglycemic and antioxidative effect of hydro - methanolic (2:3) extract of the seed of Swietenia mahagoni (L.) Jacq. in Streptozotocin-Induced Diabetic Male Albino. Biomarkers Health Sci. 2012:107–117. [Google Scholar]
- Bhurat M., Bavaskar S., Agrawal A., Bagad Y. Swietenia mahagoni Linn. – a phytopharmacological review. Asian J. Pharmaceut. Res. 2011;1(1):1–4. [Google Scholar]
- Bourdy G., De Walt S.J., De Michel L.R.C., Roca A., Deharo E., Munõz V., Balderrama L., Quenevo C., Gimenez A. Medicinal plants uses of the Tacana, an Amazonian Bolivian ethnic group. J. Ethnopharmacol. 2000;70:87–109. doi: 10.1016/s0378-8741(99)00158-0. [DOI] [PubMed] [Google Scholar]
- Chakraborty D., Basak S. Cyclomahogenol, a new tetracyclic triterpene from Swietenia mahagoni. Phytochemistry (Oxf.) 1971;10(6):1367–1372. [Google Scholar]
- Chattopadhyay R. Possible mechanism of antihyperglycemic effect of Azadirachta indica leaf extract. Gen. Pharmacol. 2007;IV(27):431–434. doi: 10.1016/0306-3623(95)02070-5. [DOI] [PubMed] [Google Scholar]
- Chen Y.Y., Wang X.N., Fan C.Q., Yin S., Yue J.M. Swiemahogins A and B, two novel limonoids from Swietenia mahogany. Tetrahedron Lett. 2007;48(42):7480–7484. [Google Scholar]
- Chen L.C., Liao H.R., Chen P.Y., Kuo W.L., Chang T.H., Sung P.J., Wen Z.H., Chen J.J. Limonoids from the seeds of Swietenia macrophylla and their anti-inflammatory activities. Molecules. 2015;20:18551–18564. doi: 10.3390/molecules201018551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De D., Chatterjee K., Ali K.M., Bera T.K., Ghos D. Antidiabetic potentiality of the aqueous-methanolic extract of seed of Swietenia mahagoni (L.) Jacq. in streptozotocin-induced diabetic male albino rat: a correlative and evidence-based approach with antioxidative and antihyperlipidemic activities. Ev. Bas. Comp. Alt. Med. 2011:11. doi: 10.1155/2011/892807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewanjee S., Maiti A., Dias A.M.S., Dey S. Swietenine: a potential oral hypoglycemic from Swietenia macrophylla seed. Fitoterapia. 2009;80:249–251. doi: 10.1016/j.fitote.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Divya K., Pradeep H.R., Kumar K.K., Venkatesh K.R.H., Jyothi T. Herbal drug Swietenia mahagoni Jacq. - a review. Glob. J. Res. Med. Plants Indig. Med. 2012;1(10):557–567. [Google Scholar]
- Donga J., Surani V.S., Sailor G.U., Chauhan S.P., Seth A.K. A systematic review on natural medicine used for therapy of diabetes mellitus of some Indian medicinal plants. Pharma Sci. Monit. 2011;2:36–72. [Google Scholar]
- Dutta M., Biswas u.K., Chakraborty R., Banerjee P., Raychaudhuri U., Kuma A. Evaluation of plasma H2S levels and H2S synthesis in streptozotocin induced Type-2 diabetes-an experimental study based on Swietenia macrophylla seeds. Asian Pac. J. Trop. Biomed. 2014;4(suppl 1):S483–S487. doi: 10.12980/APJTB.4.201414B58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekimoto H., Irie Y., Araki Y., Han G.Q., Kadota S., Kikuchi T. Platelet aggregation inhibitors from the seeds of Swietenia mahagoni: inhibition of in vitro and in vivo platelet-activating factor-induced effects of tetranortriterpenoids related to swietenine and swietenolide. Planta Med. 1991;57(1):56–58. doi: 10.1055/s-2006-960018. [DOI] [PubMed] [Google Scholar]
- Falah S., Safithri M., Katayama T., Suzuki T. Hypoglycemic effect of mahogany (Swietenia macrophylla King) bark extracts in alloxan-induced diabetic rats. Wood Res. J. 2010;1(2):89–94. ISSN 2087-3840. [Google Scholar]
- Falah S., Suzuki T., Katayama T. Chemical constituents from Swietenia macrophylla bark and their antioxidant activity. Pakistan J. Biol. Sci. 2008;16:7–12. doi: 10.3923/pjbs.2008.2007.2012. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Besra S.E., Roy K., Gupta J.K., Vedasiromoni J.R. Pharmacological effects of methanolic extract of Swietenia mahagoni Jacq (Meliaceae) seeds. Int. J. Green Pharm. 2009;3:206–210. [Google Scholar]
- Govindappa M. A Review on role of plant(s) extracts and its phytochemicals for the management of diabetes. J. Diabetes Metabol. 2007;6:565. [Google Scholar]
- Haldar P.K., Adhikari S., Bera S., Bhattacharya S., Panda S.P., Kandar C.C. Hepatoprotective efficacy of Swietenia Mahagoni L. Jacq. (Meliaceae) bark against paracetamol induced hepatic damage in rats. Ind. J. Pharm. Edu. Res. 2011;45(2):108–113. [Google Scholar]
- Hajra S., Mehta A., Pandey P., Vyas S. Antioxidant and antidiabetic potential of ethanolic extract of Swietenia mahagoni (Linn.) seeds. Int. J. Pharmaceut. Res. Dev. 2011;3(3):180–186. [Google Scholar]
- Hashim M., Hashim M.A., Yam M.F., Hor S.Y., Lim C.P., Asmawi M.Z., Sadikun A. Anti-hyperglycemic activity of Swietenia macrophylla King (Meliaceae) seed extracts in normoglycaemic rats undergoing glucose tolerance tests. Chin. Med. 2013;8:11. doi: 10.1186/1749-8546-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husen S.A., Winarni D., Khaleyla F., Kalqutny S.H., Ansori A.N.M. Activity assay of mangosteen (Garcinia mangostana L.) pericarp extract for decreasing fasting blood cholesterol level and lipid peroxidation in type-2 diabetic mice. AIP Conf. Proc. 2017;1888 [Google Scholar]
- Jian Y., He Y., Yang J., Han W., Zhai X., Zhao Y., Li Y. Molecular modeling study for the design of novel peroxisome proliferator-activated receptor gamma agonists using 3D-QSAR and molecular docking. Int. J. Mol. Sci. 2018;19(630):15. doi: 10.3390/ijms19020630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota S., Marpaung L., Kikuchi T., Ekimoto H. Constituents of the Swietenia mahagoni Jacq. Isolation, structures and 1H and 13C-Nuclear magnetic resonance signal assignments of new tetranortriterpenoids related to Swietenine, and Swietenolide. Chem. Soc. Japan. Chem. and Pharm. Bull. 1990;38(3):639–651. [Google Scholar]
- Kalaivanan K., Pugalendi K. Antihyperglycemic effect of the alcoholic seed extract of Swietenia macrophylla on streptozotocin-diabetic rats. Pharmacogn. Res. 2011;3(1):67–71. doi: 10.4103/0974-8490.79119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khardori R. 2018. Medication summary diabetes.https://emedicine.medscape.com/article/117853-overview [Online] Available at: [Google Scholar]
- Khare C.P. Springer; New Delhi: 2007. Indian Medicinal Plants – an Illustrated Dictionary; pp. 633–634. [Google Scholar]
- Konaté K., Yomalan K., Sytar O., Zerbo P., Brestic M., Patrick V.D., Gagniuc P., Barro N. Free radicals scavenging capacity, antidiabetic and antihypertensive activities of flavonoid rich fractions from leaves of Trichilia emetica and Opilia amentacea in an animal model of type 2 diabetes mellitus. Evid Based Complement Alternat Med. 2014:867075. doi: 10.1155/2014/867075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawati D. Ethanolic extract of Physalis minima Linn., Psidium guajava Linn., Swietenia mahagoni Jacq to lowering blood glucose level (translate from Indonesia language) J. Medika Planta. 2010;1(2):55–60. [Google Scholar]
- La Basy L., Lestari S., Kadarsih S. The effects of the ethanolic extract of mahogany seeds (Swietenia macrophylla King) on the renal function of streptozotocin-induced diabetic rats. J. Med. Sci. 2015;47(2):51–58. [Google Scholar]
- Li D.D., Chen J.H., Chen Q., Li G.W., Chen J., Yue J.M., Chen M.L., Wang X.P., Shen J.H., Shen X., Jiang H.L. Swietenia mahagony extract shows agonistic activity to PPARγ and gives ameliorative effects on diabetic db/db mice. Acta Pharmacol. Sin. 2005;26(2):220–222. doi: 10.1111/j.1745-7254.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- Lin B.D., Yuan T., Zhang C.R., Dong L., Zhang B., Wu Y., Yue J.M. Structurally diverse limonoids from the fruits of Swietenia mahagoni. J. Nat. Prod. 2009;72(12):2084–2090. doi: 10.1021/np900522h. [DOI] [PubMed] [Google Scholar]
- Lin B.D., Zhang C.R., Yang S.P., Wu Y., Yue J.M. Phragmalin-type limonoid orthoesters from the twigs of Swietenia macrophylla. Chem. Pharm. Bull. 2011;59(4):458—465. doi: 10.1248/cpb.59.458. [DOI] [PubMed] [Google Scholar]
- Magallanes O., Campos B., Chaverri O., Mata R. Hypoglycemic and antihyperglycemic effects of phytopreparations and limonoids from Swietenia humilis. Phytochemistry. 2015;110:111–119. doi: 10.1016/j.phytochem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Maiti A., Dewanjee S., Jana G., Mandal S. Hypoglycemic effect of Swietenia macrophylla seeds against type II diabetes. Int. J. Green Pharm. 2008;2(4):224–227. [Google Scholar]
- Maiti A., Dewanjee S., Kundu M., Mandal S. Protective effect of methanol extract of Swietenia macrophylla seeds on oxidative states associated with streptozotocin induced diabetic rats. Nat. Prod. Sci. 2007;13(4):295–299. [Google Scholar]
- Maiti A., Dewanjee S., Kundu M., Mandal S. Evaluation of antidiabetic activity of the seeds of Swietenia macrophylla in diabetic rats. Pharm. Biol. 2009;47(2):132–136. [Google Scholar]
- Maiti A., Dewanjee S., Sahu R. Isolation of hypoglycemic phytoconstituent from Swietenia macrophylla seeds. Phytother Res. 2009;23:1731–1733. doi: 10.1002/ptr.2821. [DOI] [PubMed] [Google Scholar]
- Mardisiswojo S., Radjakmangunsudarso H. 1965. Cabe puyang warisan nenek moyang, Djakarta: Prapantja. [Google Scholar]
- Meles D.K., Adnyana D.P.A., Rinaldhi C.P., Octaviani R.R., Cempaka D.K.S. The antidiabetic effect of bitter melon (Momordica charantia L.) extracts towards glucose concentration, langerhans islets, and leydig cells of hyperglycemic mice (Rattus norvegicus) Eur Asia J. BioSci. 2019;13:757–762. [Google Scholar]
- Mendes M., Bogle I. Evaluation of the effects and mechanisms of bioactive components present in hypoglycemic plants. Int. J. Chem. Biomol. Sci. 2015;1(3):167–178. [Google Scholar]
- Miroslav M.G. Elsevier Inc.; London: 2005. Elsevier's Dictionary of Trees. I; p. 381. [Google Scholar]
- Moghadamtousi S.Z., Goh B.H., Chan C.K., Shabab T., Kadir H.A. Review biological activities and phytochemicals of Swietenia macrophylla King. Molecules. 2013;18:10465–10483. doi: 10.3390/molecules180910465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa M., Jahan I.A., Riaz M., Hossain H., Nimmi I., Miah A.S., Chowdhury J.U. Comprehensive analysis of the composition of seed cake and its fatty oil from Swietenia mahagoni Jacq. growing in Bangladesh. Dhaka Univ. J. Pharm. Sci. 2011;10(1):49–52. [Google Scholar]
- Nagappa A., Thakurdesai P., Rao N., Singh J. Antidiabetic activity of Terminalia catappa Linn fruits. J. Ethnopharmacol. 2003;88:45–50. doi: 10.1016/s0378-8741(03)00208-3. [DOI] [PubMed] [Google Scholar]
- Naveen Y., Rupini G., Ahmed F., Urooj A. Pharmacological effects and active phytoconstituents of Swietenia mahagoni: a review. J. Integ. Med. 2014;12(2):86–93. doi: 10.1016/S2095-4964(14)60018-2. [DOI] [PubMed] [Google Scholar]
- Naveen Y., Urooj A. Amelioration of diabetes by Swietenia mahagoni in streptozotocin-induced diabetes rats. IJPSR. 2015;6(9):3892–3900. [Google Scholar]
- Noorhajati H., Tanjung M., Aminah N., Suwandi J.S. Antioxidant activities of extracts of trengguli stem bark (Cassia fistula L.) Int. J. Basic Appl. Sci. 2012;12(4):85–89. [Google Scholar]
- Noormalasari T. Faculty of Veterinary. Bogor Institute of Agricultural; Bogor: 2015. Influence of mahagony seed ethanolic extract (Swietenia mahagoni Jacq.) to beta pancreas cell profile on diabetes mellitus rat model (translate from Indonesia language) Bachelor Thesis. [Google Scholar]
- Orwa C., Mutua A., Kindt R., Jamnadass R., Anthony S. 2009. Swietenia Mahagoni. Agroforestree Database: a Tree Reference and Selection Guide Version 4.0; pp. 1–5.http://www.worldagroforestry.org/sites/treedbs/treedata bases.asp [Google Scholar]
- Panda S.P., Haldar P.K., Bera S., Adhikary S., Kandar C.C. Antidiabetic and antioxidant activity of Swietenia mahagoni in streptozotocin-induced diabetic rats. Pharm. Biol. 2010;48(9):974–979. doi: 10.3109/13880200903390051. [DOI] [PubMed] [Google Scholar]
- Pandey A., Tripathi P., Pandey R., Srivatava R., Goswami S. Alternative therapies useful in the management of diabetes: a systematic. J Pharm Bioall Sci. 2011:504–512. doi: 10.4103/0975-7406.90103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D., Prasad S., Kumar R. Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property Asian Pacific. J. Trop. Biomed. 2012;2(4):320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal V., Khoo W.C., Abdul-Hamid A., Ismail A., Saari K., Murugesu S., Abas F., Ismail I.S., Lajis N.H., Mushtaq M.Y., Khatib A. Evaluation of antidiabetic properties of Momordica charantia in streptozotocin-induced diabetic rats using metabolomics approach. Int. Food Res. J. 2015;22(3):1298–1306. [Google Scholar]
- Ponnusamy S., Haldar S., Mulani F., Zinjarde S. Gedunin and azadiradione: human pancreatic alpha-amylase inhibiting limonoids from neem (Azadirachta indica) as anti-diabetic agents. PloS One. 2010;10(10) doi: 10.1371/journal.pone.0140113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnobasuki H. Prospect of mangrove as herbal medicine. Biota. 2004;IX(2):125–126. [Google Scholar]
- Rahman A.K., Chowdhury A.K., Ali H.A., Raihan S.Z., Ali M.S., Nahar L., Sarker S.D. Antibacterial activity of two limonoids from Swietenia mahagoni against multiple-drug-resistant (MDR) bacterial strains. J. Nat. Med. 2009;63(1):41–45. doi: 10.1007/s11418-008-0287-3. [DOI] [PubMed] [Google Scholar]
- Raja L. Bachelor Thesis (Translate from Indonesia Language) Faculty Pharmacy of Sumatera Utara University; Medan: 1990. Ethanolic extract of Mahagony seed (Swietenia mahagoni Jacq) test to lowering blood glucose level on white rat. [Google Scholar]
- Roestamadji R., Arundina I., Diyatri I. Brotowali extract (Tinospora crispa) for oral traumatic ulcer in diabetes mellitus wistar rat. J. Int. Dental and Medical Res. 2017;10(3):991–996. [Google Scholar]
- Sahgal G., Ramanathan S., Sasidharan S., Mordi M.N., Ismail S., Mansor S.M. Phytochemical and antimicrobial activity of Swietenia mahagoni crude methanolic seed extract. Trop. Biomed. 2009;26(3):274–279. PMID: 20237441. [PubMed] [Google Scholar]
- Sahgal G., Ramanathan S., Sasidharan S., Mordi M.N., Ismail S., Mansor S.M. Brine shrimp lethality and acute oral toxicity studies on Swietenia mahagoni (Linn.) Jacq. seed methanolic extract. Pharma Res. 2010;2(4):215–220. doi: 10.4103/0974-8490.69107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saputri M. Faculty of Veterinary, Bogor Institute of Agricultural; Bogor: 2014. Acute toxicity test of Mahagony seed extract (Swietenia mahagoni Jacq.) on LD50 parameter on rat (Rattus norvegicus). Indonesia. Bachelor Thesis. [Google Scholar]
- Singh N., Gupta M. Effect of ethanolic extract of Syzygium cumini (Linn.) seed powder on pancreatic islets of alloxan diabetic rats. Indian J. Exp. Biol. 2001;45:861–867. PMID: 17948734. [PubMed] [Google Scholar]
- Sukardiman, Arifianti L., Praharsiwi W. Identification of standard parameter of mahagony’s seed (Swietenia mahagoni Jacq.) based on Indonesian Herbal Pharmacopeia for anti-hyperglycemic medicaments. World J. Pharm. Pharmaceut. Sci. 2016;5(12):1403–1409. [Google Scholar]
- Sukardiman, Rahman A., Studiawan H., Santosa M.H., Widyawaruyanti A., Firdaus R. Hypoglycemic activity herbal tea combination of Andrographis paniculata herbs and Swietenia mahagoni seeds. E-Journal Planta Husada. 2013;1(4–6) ISSN 2338-7130. [Google Scholar]
- Sukardiman, Pegin N., Studiawan H., Arifianti L. Antidiabetic activity of dry extracts of Swietenia mahagoni seeds in alloxan-induced diabetic Balb/C. World J. Pharmaceut. Res. 2017;6(2):1334–1339. [Google Scholar]
- Suryani N., Endang H., Aulanni’am A. Diabetes effect of methanolic Swietenia mahagoni seed extracts in increasing insulin level, decreasing TNF- α expression. J. Kedokteran Brawijaya. 2013;27(3):137–145. [Google Scholar]
- Szkdelski T. The mechanism of alloxan and streptozotocin action in β cells of the rat pancreas. J. Physiol. Res. 2001;50:536–546. PMID: 11829314. [PubMed] [Google Scholar]
- Tripathi A.K., Bhoyar P.K., Baheti J.R., Biyani D.M., Khalique M. Herbal antidiabetics: a review. Int. J. Res. Pharm. Sci. 2007;2:30–37. [Google Scholar]
- Trusheva B., Popova M., Koendhori E.B., Tsvetkova I., Naydenski C., Bankova V. Indonesian propolis: chemical composition, biological activity and botanical origin. Nat. Prod. Res. 2013;25(6):606–613. doi: 10.1080/14786419.2010.488235. [DOI] [PubMed] [Google Scholar]
- Vigneshwaran L., Lalitha K. In silico evaluation of antidiabetic molecules of the seeds of Swietenia mahagoni Jacq. Int. J. Pharm. Phytopharmacol. Res. 2016;6(1):41–49. [Google Scholar]
- Wardani G. Faculty of Pharmacy Airlangga University; Surabaya, Indonesia: 2016. Activity test of antidiabetes of mahagony seed standardized dried extract (Swietenia mahagoni Jacq.) to alloxan induced mice. Bachelor Thesis (Indonesia Language) [Google Scholar]
- World Health Organization Global report on diabetes. 2016. http://www.who.int
- Wresdiyati T., Sa’diah S., Winarto A., Febriyani V. Alpha-glucosidase inhibition and hypoglycemic activities of Swietenia mahagoni seed extract. Hayati J. Biosci. 2015;22(2):73–78. [Google Scholar]
- Yadav J., Saini S., Kalia A., Dangi A. Hypoglycemic activity of ethanolic extract of Salvadora oleoides in normal and alloxan-induced diabetes rats. Indian J. Pharmacol. 2008;40:23–27. doi: 10.4103/0253-7613.40485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata-Sudo G., Lima L.M., Pereira S.L., Trachez M.M., Da Costa F.P., Souza B.J., Monteiro C.E.S., Romeiro N.C., D’Andréa E.D., Sudo R.T., Barreir E.J. Docking, synthesis and anti-diabetic activity of novel sulfonylhydrazone derivatives designed as PPAR-gamma agonists. Curr. Top. Med. Chem. 2012;12(19) doi: 10.2174/156802612804910205. [DOI] [PubMed] [Google Scholar]