Abstract
A comparison between the most investigated alginate-based encapsulating agents was performed in the current study. Here, the survivability of Lactobacillus plantarum microencapsulated with alginate (Alg) combined with skim milk (Sm), dextrin (Dex), denatured whey protein (DWP) or coated with chitosan (Ch) was evaluated after exposure to different heat treatments and in presence of some food additives, during storage and under simulated gastrointestinal condition. In addition, the encapsulated cells were evaluated for production of different bioactive compounds such as exopolysacchar.
ides and antimicrobial substances compared with the unencapsulated cells. The results showed that only Alg-Sm maintained the viability of the cells >106 cfu/g at the pasteurization temperature (65 °C for 30 min). Interestingly, storage under refrigeration conditions increased the viability of L. plantarum entrapped within all the tested encapsulating agents for 4 weeks. However, under freezing condition, only Alg-DWP and Alg-Sm enhanced the survival of the entrapped cells for 3 months. All the microencapsulated cells were capable of growing at the different NaCl concentrations (1%–5%) except for cells encapsulated with Alg-Dex, showed viability loss at 3% and 5% NaCl concentrations. Tolerance of the microencapsulated cells toward organic acids was varied depending on the type of organic acid. Alg-Ch and Alg-Sm provide better survival for the cells under simulated gastric juice; however, all offer a good survival for the cells under simulated intestinal condition. Our findings indicated that Alg-Sm proved to be the most promising encapsulating combination that maintains the survivability of L. plantarum to the recommended dose level under almost all the stress conditions adopted in the current study. Interestingly, the results also revealed that microencapsulation does not affect the metabolic activity of the entrapped cells and there was no significant difference in production of bioactive compounds between the encapsulated and the unencapsulated cells.
Keywords: Food science, Microbiology, Materials science, Antimicrobial, Extrusion, Microencapsulation, Lactobacillus plantarum, Sodium alginate
Food science; Microbiology; Materials science; Antimicrobial; Extrusion; Microencapsulation; Lactobacillus plantarum; Sodium alginate.
1. Introduction
Nowadays, several studies have highlighted the vital role of the human microbiome, especially the gut microbiome in regulating human health and disease. Probiotics, such as Lactobacillus and Bifidobacterium, are crucial part of human intestinal microbes and probiotic supplements could possibly influence the microbiome composition, and consequently conferring several health benefits to the host (Nie et al., 2019). Baek and Lee (2009) define probiotics as live microorganisms, which can provide a health benefit through improving the host intestinal microbial balance when administered in adequate amounts. These health benefits include alleviation of lactose intolerance, immunomodulation, anti-cancer activity, cholesterol lowering effect, reduction of gastrointestinal disorders including antibiotic-associated diarrhea, increasing the eradication rate of the gastric pathogen Helicobacter pyloi, and modulation of the brain function (Divya et al., 2012; Liu et al., 2015; Zhu and Liu, 2017; Quigley, 2019). It has been reported that probiotics exert not only prophylactic but also therapeutic effects on host gut and immune health, therefore incorporation of probiotic bacteria in fermented food products enhances their value as better functional foods (Moumita et al., 2017; Suez et al., 2019). Dairy products such as yoghurt, cheese, ice cream, desserts, cultured milks, or pasteurized unfermented milk are considered as natural vehicles of probiotics (Giraffa et al., 2010; Homayouni et al., 2012; Castro et al., 2015). However, many factors have been found to influence the survivability and functionality of probiotics in fermented dairy products like heat treatment, storage temperature, presence of some food additives such as salts and organic acids, that are commonly used in food as a taste enhancer or as a preservative, microbiota competition and possible presence of bacteriocins or other antimicrobials (Tripathi and Giri, 2014; Castro et al., 2015; Ilha et al., 2015). Transition through gastrointestinal (GI) tract and other factors are also negatively affecting the viability of the cells (Tripathi and Giri, 2014). The minimum recommended dose level of probiotic bacteria should be at least 106 cfu/g in the fermented food product at the time of consumption in order to confer their beneficial health effects (Kailasapathy and Chin, 2000; Kechagia et al., 2013). Therefore, during the production of probiotic food, the viability of the cells within the food and the bioavailability within the host must be taken into consideration (Corona-Hernandez et al., 2013). Interestingly, microencapsulation appears to be one of the promising techniques in protecting probiotic bacteria (Rokka and Rantamäki, 2010). This process can protect the microbial cells from the adverse environmental conditions through entrapment within a matrix of biopolymeric material (Krasaekoopt et al., 2003; Abd El-Salam and El-Shibiny, 2015).
So far, various techniques have been considered for the encapsulation of probiotics and the most used techniques are extrusion, emulsion, coacervation, freeze drying and spray drying. Among them, the extrusion method probably is mildest one since it does not require high temperature or any solvents to ensure high cell viability (Lee et al., 2019). Extrusion technique is based on mixing the probiotic cells with the polymeric solution, followed by extrusion into a crosslinking solution such as calcium chloride through a syringe needle or nozzle (Rathore et al., 2013). The capsules formed immediately by contacting the cell-polymer droplet with the crosslinking solution. The size of the produced capsules affected by different factors including the nozzle size, viscosity of polymeric solution, and the distance between the syringe and the crosslinking solution (Rathore et al., 2013; Liu et al., 2019).
The material used for microencapsulation of probiotics should be natural, biocompatible, permeable to nutrients and metabolites, to provide optimal conditions for functionality of the entrapped cells, and biodegradable to ensure the release of the entrapped cells in the host colon (Rathore et al., 2013). Several encapsulating agents have been investigated for the microencapsulation process of probiotics, including polysaccharides originated from seaweed (Κ-carrageenan, alginate), plants (pectin, starch and its derivatives, gum Arabic) or bacteria (gellan, xanthan) and animal proteins (milk proteins, gelatin). Among them, sodium alginate is considered to be the common encapsulating agents that usually used for this purpose, it is a linear heteropolysaccharide, naturally derived from various species of algae, and composed of β-D-mannuronic (M) and α-L-guluronic (G) acids. Alginate microcapsules are formed through ionotropic gelation in presence of divalent cations, commonly calcium ions in the form of CaCl2 solution (Rathore et al., 2013). Each calcium ion is coordinated to the carboxyl and hydroxyl groups of four α-L-guluronate (G) monomers from two adjacent chains of the sodium alginate polymer forming what called “egg-box” model (Kühbeck et al., 2015). The advantages of this compound include natural identity, biocompatibility, non-toxicity and the relatively simple application in the encapsulation process (Krasaekoopt et al., 2003; Etchepare et al., 2015). Despite the previously mentioned advantages, some liabilities are also associated with using alginate in microencapsulation. For example, alginate microcapsules possess a porous structure which does not impart an integrity to the capsule wall leading to less efficient encapsulation (Etchepare et al., 2015). Moreover, alginate is sensitive to acidic media which make their capsule vulnerable to the stomach juice leading to pre-mature release of the encapsulated probiotic which is supposed to be released in the intestine (Etchepare et al., 2015).
Blending alginate with other biopolymers could serve as a useful approach in strengthening the microcapsule structure (Krasaekoopt et al., 2003; Burgain et al., 2011). Milk proteins are one of the candidates that can be incorporated with alginate to improve the structure characteristics of the microcapsules that envelope probiotic bacteria (Abd El-Salam and El-Shibiny, 2015). Milk proteins are caseins and whey proteins, whey protein induce gelation through heating (Abd El-Salam and El-Shibiny, 2015) and can be used as coat (Gbassi et al., 2009) or in combination with alginate (Rajam et al., 2012) for encapsulation of probiotics. Addition of whey protein isolate to alginate showed 40% improvement in the survivability of L. acidophilus in simulated gastric juice (Dehkordi et al., 2019). Moreover, hydrolysis of milk proteins by digestive enzymes potentially generates bioactive peptides that may exert several physiological effects in vivo (Kilara and Panyam, 2003). Therefore, milk proteins could be considered as good candidates for encapsulation of probiotics, and as such they are attracting more research attention. In addition to milk proteins, chitosan which is a positively charged linear polysaccharide can also be used with alginate to enhance the physical stability and mucoadhesivity of alginate microcapsules in the colon (Chávarri et al., 2010; Fareez et al., 2015). Coating alginate with chitosan improved the survivability of L. plantarum in simulated gastric solution (pH 1.5) by 0.5–2 logs compared to the uncoated capsules (Nualkaekul et al., 2012). Dextrin can also be a promising encapsulating agent since it is biocompatible, inexpensive and biodegradable (Das et al., 2017). Dextrin is a mixture of D-glucose units linked by α-(1→4) or α-(1→6) glycosidic bonds (Umeki and Yamamoto, 1975) and produced by the heating of dry starch in presence of acid or base (Gibbs et al., 1999). Many reports investigated the promising effect of blending alginate with starch (Sultana et al., 2000; Martin et al., 2013). Moreover, Patil et al. (2019) demonstrated that encapsulation using maltodextrin and starch (ratio 2:1) maintained the shelf life stability of four types of lactic acid bacteria, L. plantarum, L. rhamnosus, L. acidophilus, and Bacillus shackletonii till 12 months at 4 °C. However, to date, no reports investigated the efficacy of combing dextrin with alginate for probiotic encapsulation.
Having inspired by the promising outcomes obtained from the use of alginate combined with milk proteins or coated with chitosan as effective protective and delivery systems for probiotics and the necessity of an efficient, cost effective and food-grade encapsulating agent, the current study aimed to evaluate different combinations of sodium alginate and other adjuvant biopolymers, including skim milk, denatured whey protein, chitosan and dextrin, in order to see which combination was the most efficient for improving the survivability of L. plantarum (as a probiotic model) after exposure to different stress factors that may be encountered in real food processing, storage and after ingestion.
2. Materials and methods
2.1. Materials
2.1.1. Microbial strains
The Lactobacillus plantarum EMCC1039 was provided by Cairo MIRCEN (Faculty of Agriculture, Ain Shams University, Egypt). Streptococcus thermophilus CH-1 and Lactobacillus acidophilus CH-2 were provided by Chr. Hansen's Lab., Denmark. Lactobacillus dulbrueckii subsp. bulgaricus Lb-12 DRI-VAC, Lactobacillus rhamnosus B-445, Leuconostoc mesenteroides 12 DRI-VAC, Bacillus cereus B-3711 and Saccharomyces cerevisiae Y-2223 were provided by Northern Regional Research Laboratory. Illinois, USA. Escherichia coli O157: H7 was provided by Ministry of Health and Population, Egypt. Aspergillus niger J5 was provided by Department of Microbiology, Swedish University of Agricultural Sciences. Staphylococcus aureus ATCC 25923 generously provided by Department of Microbiology, Faculty of Agriculture, Cairo University.
2.1.2. Chemicals
Sodium alginate and hydrochloric acid pure (35–38%) were purchased from Loba Chemie, Pvt Ltd - Mumbai, India. Fresh liquid skim milk was provided by Faculty of Agriculture, Cairo University, Egypt. White dextrin (C6H12O5)n.xH2O was provided by Merck (Darmstadt, Germany). Chitosan (Deacetylation 93%; C6H11NO4X2; Molecular weight 161.16) was purchased from Oxford Lab Chem (Thane, Maharashtra, India). Whey protein concentrate 80% was purchased from milkiland Intermarket (Poland). Pepsin (1:3,000), calcium chloride and potassium chloride were purchased from Science Lab (Texas, USA). Pancreatin from hog pancreas (5× USP specifications) and bile salt were purchased from BIOBASIC INC (Canada). Peptone was purchased from S D Fine-Chem Limited (Mumbai, India). Sodium chloride, sodium bicarbonate, trisodium citrate and sodium hydroxide were purchased from El Nasr pharmaceutical chemicals (Cairo, Egypt). Glecial acetic acid (extra pure) was purchased from SHAM LAB (Damascus, Syria). De Man Rogosa and Sharpes (MRS) broth and MRS agar were purchased from CONDA (Spain).
2.2. Growth condition
L. plantarum was grown on MRS broth and incubated under aerobic condition for 24 h at 37 °C. The strain was subcultured two or three times in order to obtain high biomasses in the stationary phase then the cell pellets were harvested by centrifugation at 4000 rpm, for 20 min at 4 °C. The pellets washed by sterile saline solution (0.9% (w/v) NaCl) and recovered under the same centrifugation condition then dissolved with an equal volume of sterile saline solution (Dianawati et al., 2013; Fareez et al., 2015). Afterwards, cell suspension was used for encapsulation.
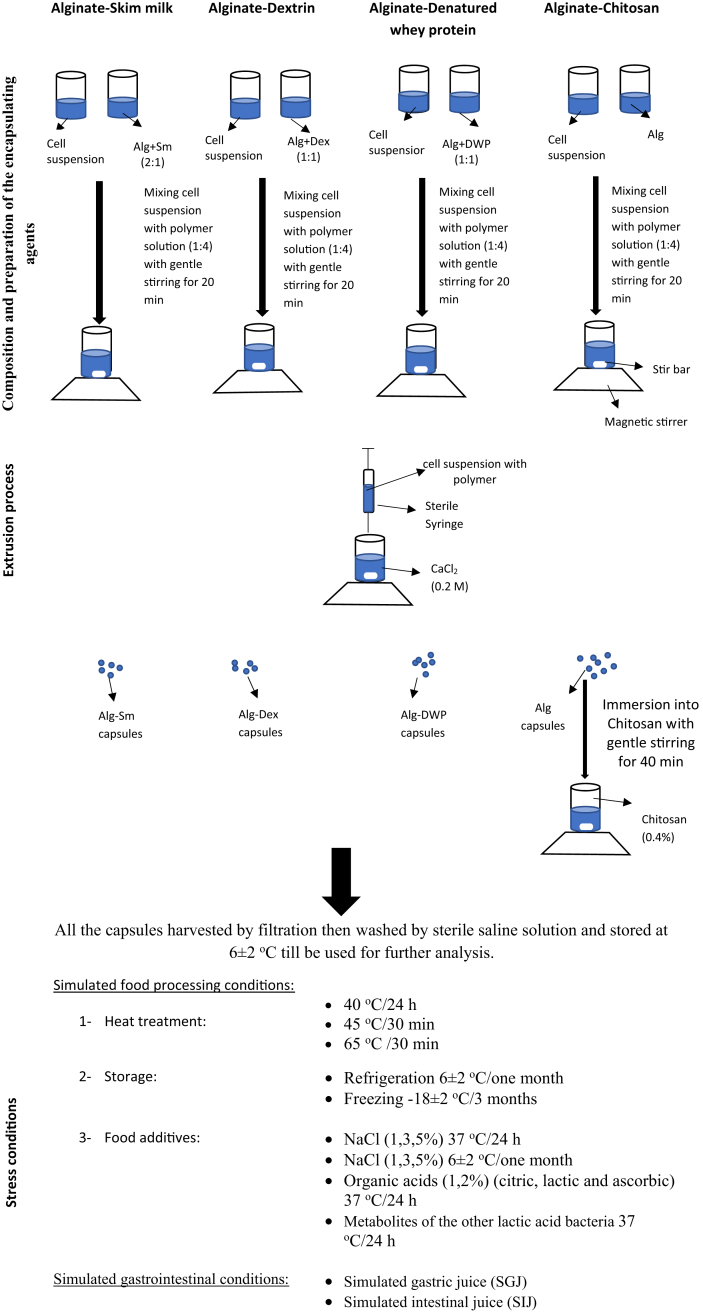
2.3. Composition and preparation of the encapsulating agents
Four combinations of the encapsulating agents were prepared based on alginate as principle biopolymer combined with another adjuvant biopolymer as follows (all the concentrations that used in this study were chosen after different preliminary optimization experiments):
-
1
Sodium alginate-skim milk (Alg-Sm) was prepared according to Shi et al. (2013) with some modifications. One part of fresh skim milk was sterilized by autoclaving at 121 °C for 5 min then mixed with 2 parts of 3% (w/v) alginate solution that was sterilized by autoclaving at 121 °C for 15 min.
-
2
Sodium alginate-dextrin (Alg-Dex) was prepared according to Mirzaei et al. (2012) with some modifications. 3 g (Alg) was dissolved with an equal amount of (Dex) in 100 ml distilled water then the solution was sterilized by autoclaving at 121 °C for 15 min.
-
3
Sodium alginate-chitosan (Alg-Ch) capsule. Chitosan was prepared as the method described by Zhou et al. (1998). In details, 4% chitosan was prepared by dissolving 0.4 g in 100 ml sterilized distilled water acidified with 0.4 ml of glacial acetic acid because chitosan is insoluble at pH levels above 6.5. After dissolution, pH was adjusted to (5.7–6) by 1 N NaOH. This solution was filtered to remove any remaining insoluble materials.
-
4
Sodium alginate-denatured whey protein (Alg-DWP) was prepared by mixing equal volume of 10% (w/v) freshly prepared (DWP) with sterile 3% (w/v) alginate. Briefly, DWP was prepared according to Rajam et al. (2012) by dissolving 10 g of whey protein concentrate 80% in 100 ml sterile distilled water. The solution was stirred gently for 2 h then rest for 1 h. After that, the solution was exposed to heat treatment in water bath at 90 °C for 30 min.
2.4. Microencapsulation procedure
Generally, the microencapsulation process was performed using the extrusion technique (Feucht and Kwak, 2013). One part of the cell suspension was mixed separately with four parts of the freshly prepared encapsulating agents with gentle stirring for 10–20 min. The mixture was then extruded into the hardening solution (CaCl2, 0.2 M) through sterile syringe (25 G, 0.5 mm) with gentle stirring for 30 min to ensure complete solidification. In case of Alg-Ch, after extruding the alginate-cells mixture into the crosslinking solution, the harvested alginate microcapsules were then coated with chitosan by immersing (about 12 g capsules) in 100 ml of chitosan solution with gentle stirring for 40 min. The formed microcapsules were harvested by filtration then washed by sterile saline solution and stored at 6 ± 2 °C till be used for further analysis (see Figure 1).
Figure 1.
Schematic representation of the encapsulation system and the adopted stress conditions.
2.5. Enumeration of the microencapsulated cells
The viability of L. plantarum was assessed as described by (Chávarri et al., 2010). In details, one gram of the microcapsules was dissolved in 9 ml of sterile 2% (w/v) tri-sodium citrate solution and vortexed till complete dissolution. After that, the samples were serially diluted to appreciate concentration using 0.1% (w/v) peptone and pour plated in MRS agar. The plates were incubated at 37 °C for 48 h under anaerobic condition. The viable cell number was expressed as colony forming unit per gram of microcapsule (cfu/g).
Encapsulation efficiency (EE) was determined by using the following equation as described by (Fareez et al., 2015):
| (1) |
Where
N = the number of the bacterial cells loaded inside the microcapsules.
No = the number of the free bacterial cells added to the biopolymer mixture during the preparation of the microcapsules.
2.6. Morphology and size of capsules
The surface morphology of the capsules was determined by scanning electron microscope (SEM) (model Quanta 250, high resolution field emission gun (HRFEG, Czech). Before SEM analysis, the samples were immersed at first in buffer glutaraldehyde (0.1 M) for 2 h at 4 °C (pH = 7.3), then were post fixed by osmium tetraoxide (0.1 M) for 1 h at 4 °C. After that, the samples were consecutively dehydrated by 30, 50 and 70% ethyl alcohol for 2 min each and remained in 100% ethyl alcohol for 30 min at 4 °C. The samples were then mounted on a piece of adhesive paper and gold coated using a vacuum sputtering coater (Edwards S150A, England).
Particle size of the manufactured dry microcapsules was determined using static laser scattering device (Master sizer 2000, Malvern, UK). The hydrodynamic particle diameter was expressed as volume weighted mean size distribution % (d4,3). The diameter of twenty wet capsules were also evaluated using Calliper (Powerfix, Germany) and the average diameter was measured and recorded.
2.7. Survivability of microencapsulated cells under simulated food processing conditions
2.7.1. Different heat treatments
Sterilized fresh liquid skim milk was inoculated by 10% of the microcapsules then subjected to three different heat treatments that may stimulate the potential stress that can encounter the cells during food manufacturing. The heat treatments include:
-
1.
High incubation temperature (40 °C for 24 h).
-
2.
Scalding temperature of cheese production (45 °C for 30 min).
-
3.
Pasteurization temperature (65 °C for 30 min).
One gram was taken from the sample and the viable cell count was determined as mentioned above in section 2.5, the survivability of the encapsulated cells was determined using the following equation as described by Brinques and Ayub (2011):
| (2) |
Where Ni and Nt are the number of the viable cell (cfu/g) at the zero time (initial count) and at various storage time, respectively.
2.7.2. Refrigerated storage
The viability of the encapsulated cells under refrigeration was evaluated by inoculating sterilized skim milk with 10% microcapsules and kept in the refrigerator at 6 ± 2 °C for one month. The viable cell count and survivability were determined everyone week as mentioned above.
2.7.3. Freezing storage
The viability of the encapsulated cells under freezing was evaluated by inoculating 10% of the microcapsules into sterilized skim milk and kept in the ordinary freezer at -18 ± 2 °C for three months. The viable cell count was determined everyone month as mentioned above.
2.7.4. Different NaCl concentrations
Sterilized salted skim milk was prepared by adding sodium chloride at concentrations (1%, w/v), (3%, w/v) and (5%, w/v). Each of the three concentrations was inoculated by 10% of the microcapsules then incubated at 37 °C for 24 h, the viable cell count was determined as mentioned above.
2.7.5. Refrigerated storage in different NaCl concentrations
Sterilized salted skim milk was prepared by adding sodium chloride at concentrations (1%, w/v), (3%, w/v) and (5%, w/v). Each of the three concentrations was inoculated by 10% of the microcapsules then stored at refrigerator for one month at 6 ± 2 ᵒC. The viable count was determined every week as mentioned above.
2.7.6. Different concentrations of food-applied organic acids
Sterilized skim milk supplemented with (1%, w/v) and (2%, w/v) of lactic, citric and ascorbic acids was inoculated by 10% of the microcapsules. The samples were incubated at 37 °C for 24 h, the viable cell count was determined as mentioned above.
2.7.7. Metabolites of the other lactic acid bacteria (LAB)
In this experiment, the effect of the cell free supernatant of L. acidophilus, L. bulgaricus, L. rhamnosus, S. thermophilus and Leuconostoc mesenteroides, separately was studied on the microencapsulated L. plantarum.
-
a)
Preparation of cell free supernatant (CFS)
According to Vinderola et al. (2002), all LAB were grown in 11% reconstituted skim milk (obtained from local market) at 37 °C for 24 h. The CFS were obtained by centrifugation (4000 rpm, for 20 min at 4 °C) and sterilized by filtration through a 0.22 μm pore filter. Then CFS was kept frozen.
-
b)
For testing the survivability of the microencapsulated cells in presence of the CFS of the other LAB, ten ml of 15% sterilized skim milk (standardized by the CFS to reach 11% total solid) was inoculated with 10% of encapsulated L. plantarum. After incubation 24 h at 37 °C, one gram was taken from the sample then the viable cell count and the survivability were determined as described above.
2.8. Survivability of the microencapsulated cells in simulated gastrointestinal tract conditions
2.8.1. Simulated gastric juice (SGJ)
SGJ was prepared according to the method of Chávarri et al. (2010). Saline solution (9 g/L NaCl) was adjusted to pH 2.5 (pH of stomach with meal) with 1 N HCl and sterilized by autoclaving at 121 °C for 15 min then pepsin was suspended in the solution to final concentration 3 g/L. SGJ was inoculated with 10% of the microcapsules and incubated at 37 °C. Viable cell count was assessed after 5, 30, 60 and 120 min as described earlier.
2.8.2. Simulated intestinal juice (SIJ)
SIJ was prepared according to the method of Chávarri et al. (2010) and Gbassi et al. (2009). A solution of 6.5 g/L NaCl, 0.835 g/L KCl, 0.22 g/L CaCl2, 1.386 g/L NaHCO3 and 3 g/L bile salt was adjusted to pH 7.5 and sterilized by autoclaving at 121 °C for 15 min then pancreatin was suspended in the solution to final concentration 10 g/L. SIJ was inoculated with 10% of the microcapsules and incubated at 37 °C. Viable cell count was assessed after 5, 60, 90 and 120 min as described earlier.
2.9. Production of bioactive compounds
2.9.1. Exopolysaccharides (EPS)
Isolation of the exopolysaccharides was performed according to Zisu and Shah (2003): Sterilized skim milk was inoculated by 2% of microcapsules or unencapsulated cells and incubated for 72 h at 37 °C. Twenty mls of 20% trichloroacetic acid was added to the culture in order to precipitate casein followed by centrifugation at 4000 rpm for 20 min at 4 °C. The supernatant was harvested and neutralized to pH 6.8 with 1 N NaOH and subjected to heat treatment at 100 °C for 30 min then recentrifuged at 4000 rpm for 20 min at 4 °C to remove the remaining precipitated insoluble proteins. An equal volume of chilled absolute ethanol was added to the supernatant to precipitate EPS and stored overnight at 6 ± 2 °C. The crude EPS pellet was recovered by centrifugation at 4000 rpm for 20 min at 4 °C and washed with distilled water. The crude EPS was transferred to pre-weighed petriplates and put in the oven at 50 °C to remove the remaining water then weighed. After that, the actual weight of the EPS was calculated by subtracting the weight of the plate with EPS from the weight of the empty plate.
2.9.2. Antimicrobial substances
Sterilized skim milk was inoculated by 2% of microcapsules or unencapsulated cells and incubated for 18 h, 48 h and 72 h at 37 °C. Also, MRS broth was used for evaluation of antimicrobial production using the same inoculation size and incubation conditions. Culture of the L. plantarum was centrifuged at 4000 rpm for 20 min at 4 °C. The cell-free supernatant (CFS) was recovered and sterilized by filtration through syringe filter (nylon) 0.22 μm. The filtered CFS was sequentially treated to assay bacteriocin production, the pH was adjusted to 6.0–6.5 with 1 N NaOH followed by heat treatment at 70 °C for 30 min (Barbosa et al., 2016).
The food-borne pathogens that used were Escherichia coli O157:H7, Staphylococcus aureus, Bacillus cereus and Aspergillus niger and Saccharomyces cerevisiae as a food spoilage microorganism. All strains were grown in Tryptone soya broth and the pathogenic bacteria incubated at 37 °C for 18 h while the yeast and mould at 25 °C for 3–5 days.
The antimicrobial activity was evaluated by agar well-diffusion method according to Mallesha et al. (2010) by inoculating 10 μL of fresh cultures of the pathogenic organism onto previously poured plate count agar plates for bacteria and potato dextrose agar for yeast and mould using spread plate method. After incubation in the refrigerator for 2 h, five wells of 5 mm diameter aseptically made in each plate and filled with 100 μl of the filtered crude and treated CFS. The plates incubated overnight at 37 °C before measuring the zones of inhibition for bacteria and 72 h at 25 °C for yeast and mould.
2.10. Statistical analysis
The data analysis was carried out using CoStat software (StatSoft Inc., Tulsa, USA). Analysis of variance (ANOVA) was applied to determine significant differences (P < 0.05) between results and LSD (Least significant difference) was used to compare means values. The data were expressed as mean ± standard error. All experiments were repeated 3 times (n = 3).
3. Results
3.1. Encapsulation efficiency, particle size and capsule morphology
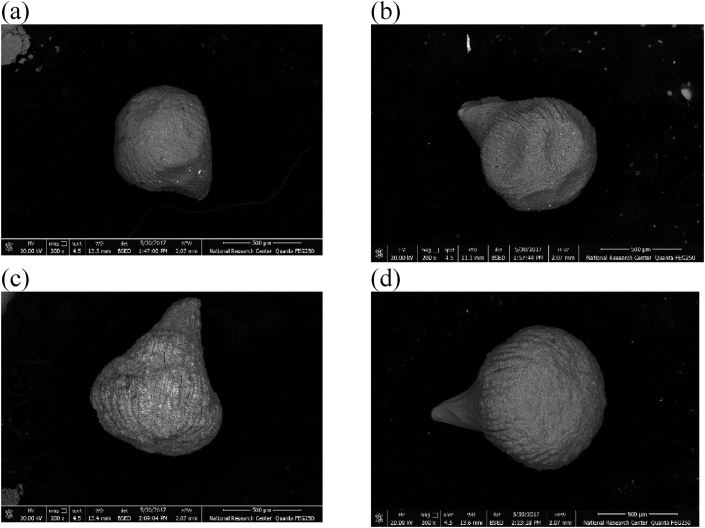
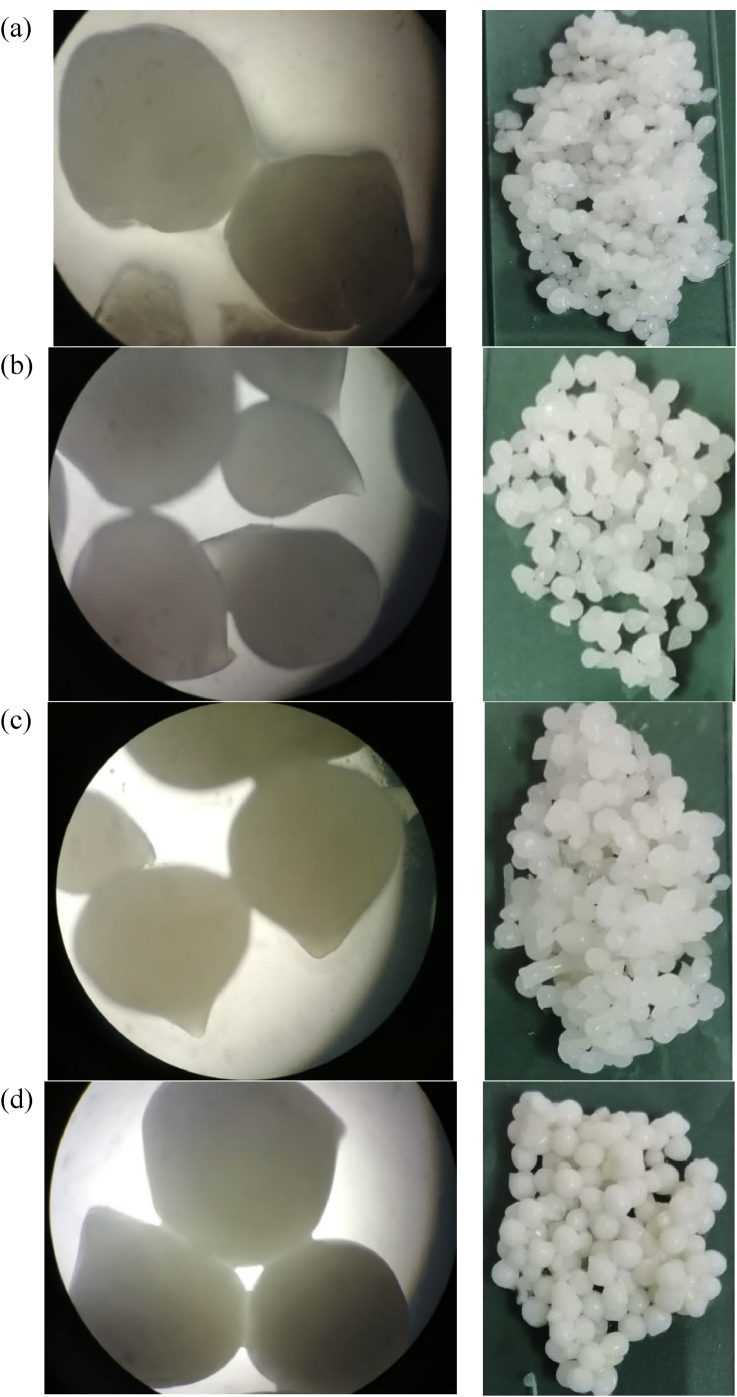
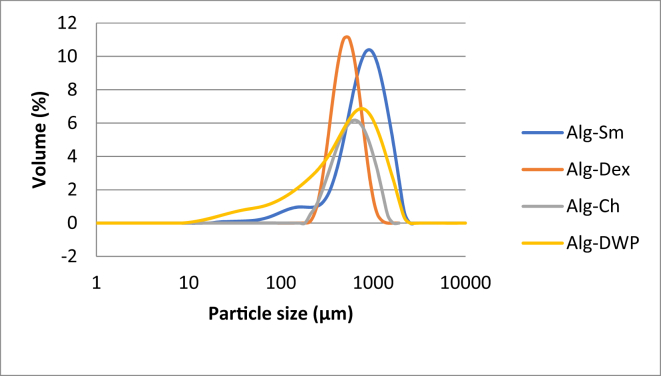
The encapsulation efficiency of the capsules was ranged between 98.11 ± 0.57% and 94.94 ± 1.78% with no significant difference between the different encapsulating agents. Also, the capsule size of the four formulated microcapsules (Alg-Sm, Alg-Dex, Alg-Ch, and Alg-DWP) loaded with L. plantarum in the dry state ranged between 501.54 and 800.739 μm while in the wet state it ranged between 1492 and 1635 μm see Table 1 and Figures 2, 3 and 4. The SEM images of the microcapsules loaded with L. plantarum in Figure 2 indicates that all the produced capsules were irregular in shape with rough surface and appeared as drop-like shape with small tail.
Table 1.
Encapsulation efficiency and particle size of different microcapsules.
| Encapsulating agent | Encapsulation efficiency (%) | Particle size (μm) |
|
|---|---|---|---|
| Dry | Wet | ||
| Alg-Sm | 96.27 ± 1.63a | 800.739 | 1553 |
| Alg-Dex | 98.11 ± 0.57a | 501.541 | 1583 |
| Alg-Ch | 97.26 ± 1.42a | 519.481 | 1492 |
| Alg-DWP | 94.94 ± 1.78a | 571.101 | 1635 |
Data were expressed as mean ± standard error.
Values with different superscript letters are significantly different (P < 0.05).
Figure 2.
Scanning electron micrograph of (a) Alg-Sm; (b) Alg-Dex; (c) Alg-Ch, and (d) Alg-DWP capsules loaded with L. plantarum.
Figure 3.
Microscopic images of (a) Alg-Sm; (b) Alg-Dex; (c) Alg-Ch, and (d) Alg-DWP capsules loaded with L. plantarum.
Figure 4.
Particle size distribution of the microcapsules in dry state.
3.2. Survivability of the microencapsulated cells under simulated food processing conditions
3.2.1. Different heat treatments
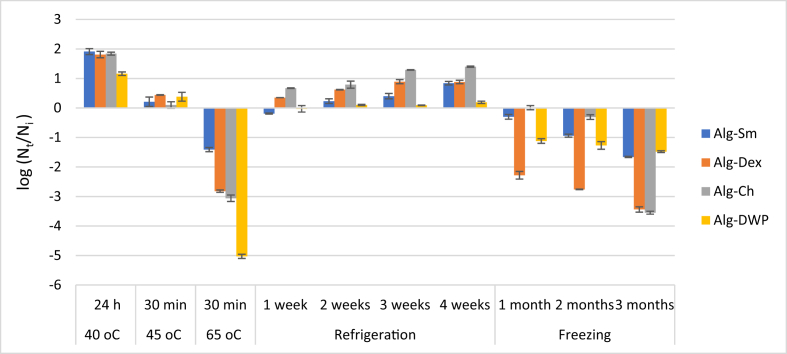
Data recorded in Figure 5 revealed that the viability of the all microencapsulated L. plantarum increased over the initial count, which was around 108 cfu/g sample, after exposure to 40 °C for 24 h and 45 °C for 30 min. In contrast, the viability of the encapsulated cells drastically decreased (relative to initial count 108 cfu/g sample) after exposure to 65 °C for 30 min. The viability loss of the microencapsulated cells using Alg-Sm was only 1.41 log cycle. However, the viability loss was 2.82, 3.06 and 5.03 log cycle using Alg-Dex, Alg-Ch, and Alg-DWP, respectively. Therefore, Alg-Sm was significantly (P < 0.05) the most effective encapsulating agent in securing L. plantarum from the lethal effect of the high temperature.
Figure 5.
Survivability of microencapsulated L. plantarum after different heat treatments at 40 °C for 24 h; 45 °C for 30 min; 65 °C for 30 min (initial cell count: ≈108 cfu/g), during refrigerated storage at 6 ± 2 ᵒC for one month (initial cell count: ≈109 cfu/g) and freezing storage at -18 ± 2 °C for three months (initial cell count: ≈108 cfu/g). Error bars represent the standard error of the mean.
3.2.2. Refrigerated storage
Figure 5 shows that the viability of all the encapsulated L. plantarum increased throughout the storage period over the initial count, which was approximately 109 cfu/g sample. At the end of the storage period (4 weeks), the viable cell count of the encapsulated L. plantarum increased by 1.40, 0.88, 0.84 and 0.19 log cycle using Alg-Ch, Alg-Dex, Alg-Sm and Alg-DWP, respectively. From this result we can figure out that all the encapsulating agents keep the viability of the encapsulated cells under the refrigeration condition; however, Alg-Ch significantly (P < 0.05) the most appropriate encapsulating agent for this mission.
3.2.3. Freezing storage
Obtained data in Figure 5 show that the cell load within all the encapsulating agents significantly () decreased throughout the storage period. At the end of the storage period, the maximum viability loss relative to the initial count (approximately 108 cfu/g sample) was about 1.66 and 1.48 log cycle for the cells entrapped within Alg-Sm and Alg-DWP, respectively. On the other hand, the viability loss was 3.44 log and 3.55 log cycle for the cells entrapped within Alg-Dex and Alg-Ch, respectively. Therefore, Alg-DWP and Alg-Sm were significantly (P < 0.05) the most effective encapsulating agents for enhancing the survivability of L. plantarum during freezing storage up to 3 months.
3.2.4. Different NaCl concentrations
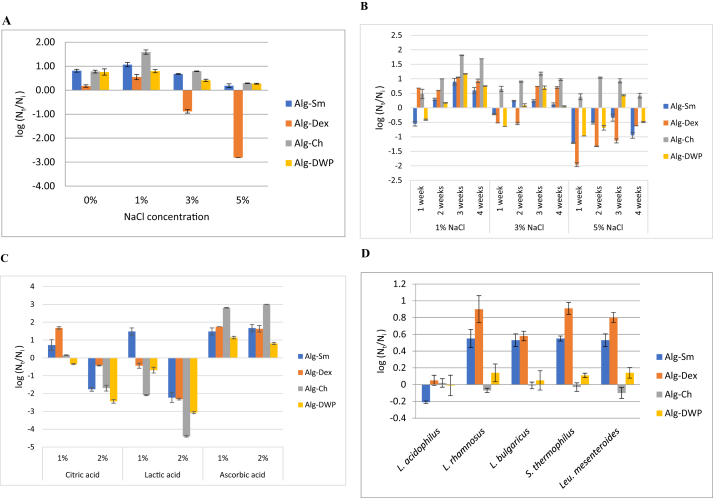
Results in Figure 6A show that all the encapsulated cells were able to grow in milk salted with 1% NaCl. However, the capability of the encapsulated cells to grow in 3% and 5% NaCl decreased relative to 1% NaCl. Only L. plantarum entrapped within Alg-Dex showed cell reduction by 0.89 and 2.81 log cycle in 3% and 5% NaCl, respectively relative to the initial count (around 109 cfu/g sample). Therefore, Alg-Sm, Alg-Ch or Alg-DWP were the most appropriate encapsulating agents to enhance the tolerance of L. plantarum to NaCl concentrations up to 5%.
Figure 6.
Survivability of the microencapsulated L. plantarum (A) after incubation at 37 °C for 24 h in different NaCl concentrations (B) during refrigerated storage at 6 ± 2 °C for one month in different NaCl concentrations. (C) after incubation at 37 °C for 24 h in different concentrations of food-applied organic acids. (D) after incubation at 37 °C for 24 h with the metabolites of the other LAB. Error bars represent the standard error of the mean. Error bars represent the standard error of the mean. Initial cell count: ≈109 cfu.
3.2.5. Refrigerated storage for one month in different NaCl concentrations
Figure 6B shows that the survivability of the encapsulated L. plantarum increased over the initial count, which was approximately 109 cfu/g, during the refrigerated storage in different NaCl concentration. However, by increasing the salt concentration, the survivability of the encapsulated cells decreased. Our results revealed that at the end of the storage period, in 1% NaCl the viable cell count of the encapsulated L. plantarum increased by 1.69, 0.93, 0.75 and 0.60 log cycle using Alg-Ch, Alg-Dex, Alg-DWP and Alg-Sm, respectively. While, in 3% NaCl, the viability also increased by 0.97, 0.70, 0.12 and 0.05 log cycle for Alg-Ch, Alg-Dex, Alg-Sm and Alg-DWP, respectively. On the other hand, in 5% NaCl, only the cells entrapped within Alg-Ch increased by 0.42 log cycle; however the cells using the other encapsulating agents showed slightly viable cell reduction by 0.49, 0.62, 0.95 log cycles for Alg-DWP, Ag-Dex and Alg-Sm, respectively. Based on the above mentioned, all the encapsulating agents secured 107 cfu/g sample in presence of NaCl concentration up to 5% under refrigerated storage for 4 weeks; however, Alg-Ch appeared to be the most appropriate one.
3.2.6. Different concentrations of food-applied organic acids
Figure 6C describes the tolerance of the encapsulated L. plantarum to different organic acids (including citric, lactic and ascorbic acids) after incubation at 37 °C for 24 h. In case of 1% citric acid (pH 3.46), L. plantarum encapsulated with Alg-Dex, Alg-Sm and Alg-Ch grew, and the viable count increased over the initial count, which was approximately 109 cfu/g, by 1.68, 0.72 and 0.15 log, respectively. However, the number of cells entrapped within Alg-DWP decreased by 0.35 log cycle. On the other hand, in case of 2% citric acid (pH 3.02) the viable count of L. plantarum in all microcapsules declined, the minimum cell reduction was 0.43 log cycle for Alg-Dex while the maximum cell reduction was 2.44 cycle for Alg-DWP. It appears that Alg-Dex showed higher relative cell viability in 1% and 2% citric acid than the other encapsulating agents.
In case of 1% lactic acid (pH 3.17), Alg-Sm was the only encapsulating agent that permitted the growth of L. plantarum where the viable count increased by 1.48 log cycle over the initial count, which was approximately 109 cfu/g. However, the cell count within the Alg-Dex, Alg-DWP and Alg-Ch decreased by 0.44, 0.69 and 2.09 log cycle, respectively. In case of 2% lactic acid (pH 2.87), the number of all encapsulated L. plantarum sharply declined by 2 log cycle for Alg-Sm and Alg-Dex, 3 log cycle for Alg-DWP and 4.4 log cycle for Alg-Ch. From the above mentioned, Alg-Sm was the most appropriate encapsulating agent for protection of L. plantarum from lactic acid containing environment up to 2%.
Regarding ascorbic acid, all the encapsulated L. plantarum showed better growth in skim milk containing (1–2%) ascorbic acid (pH 4.44 and 3.78, respectively) than citric and lactic acids. However, encapsulation using Alg-Ch increased the growth of L. plantarum by 2.81 and 3.00 log cycle over the initial count that was 109 cfu/g in 1 and 2% ascorbic acid, respectively. This growth enhancement was greater than that occurred in the other encapsulating agents that used in this study.
3.2.7. Metabolites of the other lactic acid bacteria
All the encapsulating agents maintained the viability of the entrapped L. plantarum after incubation with the metabolites of the other LAB. However, encapsulation using Alg-Dex significantly (P < 0.05) enhanced the growth of the entrapped L. plantarum than the other encapsulating agents see Figure 6D.
3.3. Survivability of the microencapsulated cells in simulated gastrointestinal conditions
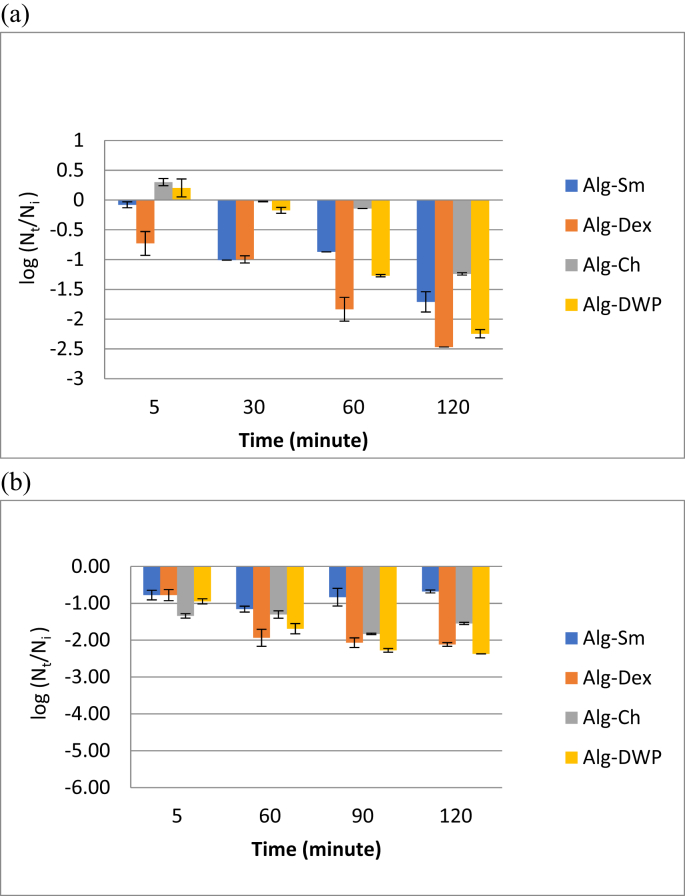
Figure 7 presents that after exposure to SGJ for 120 min, the viable cell count of the all encapsulated L. plantarum reduced from the initial count (around 108 cfu/g). The viability loss was 1.24 and 1.71 log cycle for the cell entrapped within Alg-Ch and Alg-Sm, respectively. However, the cell reduction was about 2.24 and 2.47 log cycle for Alg-DWP and Alg-Dex, respectively Therefore, Alg-Ch was the most efficient encapsulating agent for maintaining the viability L. plantarum under simulated gastric condition followed by Alg-Sm. However, after exposure to SIJ for 120 min, the viable cell count of the encapsulated L. plantarum reduced from the initial count (around 109 cfu/g) by only 0.68 log cycle for the cells entrapped within Alg-Sm while the reduction was 1.55, 2.12 and 2.37 log cycle for the cell entrapped within Alg-Ch, Alg-Dex and Alg-DWP, respectively. Based on that, Alg-Sm microcapsules appeared to be significantly (P < 0.05) the most efficient encapsulating agent for maintaining the survivability of L. plantarum up to 107 cfu/g capsule under simulated intestinal condition.
Figure 7.
Survivability of the microencapsulated cells after exposure to simulated gastric juice (SGJ) (a) and simulated intestinal juice (SIJ) (b). Error bars represent the standard error of the mean. Initial cell count: ≈108 cfu/g.
3.4. Production of bioactive compounds
L. plantarum was capable of excreting polysaccharides with no significant difference between the unencapsulated cells and all the tested encapsulating agents. The amount of the exopolysaccharide produced by the unencapsulated cells was 0.44 ± 0.16 g/100 ml skim milk and it was about 0.38 ± 0.01, 0.34 ± 0.02, 0.35 ± 0.02, and 0.465 ± 0.09 g/100 ml skim milk for the cells encapsulated with Alg-Sm, Alg-Dex, Alg-Ch and Alg DWP, respectively (Table 2).
Table 2.
Exopolysaccharide production by L. plantarum in skim milk (g/100 ml skim milk).
| Encapsulating agent | Exopolysaccharide yield |
|---|---|
| Unencapsulated cells | 0.44 ± 0.16a |
| Alg-Sm | 0.38 ± 0.01a |
| Alg-Dex | 0.34 ± 0.02a |
| Alg-Ch | 0.35 ± 0.02a |
| Alg-DWP | 0.465 ± 0.09a |
Data were expressed as meansstandard error of three replicates ().
Value represents no significant difference between the data ).
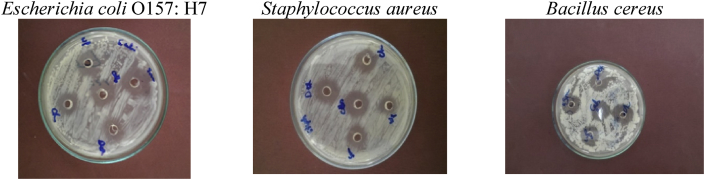
However, the CFS of L. plantarum grown in skim milk did not show any antimicrobial activity against the tested pathogens. Due to this result, the unencapsulated and the encapsulated L. plantarum were evaluated for production of antimicrobial substances in MRS broth. The results showed that only the crude CFS of L. plantarum grown in MRS broth for 72 h at 37 °C was effective against B. cereus, E. coli O157: H7 and Staph. aureus, but it did not show any antimicrobial activity against Sacch. cereviciae and A. niger. In addition, it could be confirmed that, there was no significant difference between the unencapsulated and the entrapped cell in production of the antimicrobial substances. Also, there was no significant difference between using all the encapsulating agents (Table 3 and Figure 8).
Table 3.
Antimicrobial activity of cell free supernatant of L. plantarum grown in MRS broth media for 72 h at 37 °C.
| Encapsulating agent | Size of Inhibition Zones (mm) |
||||
|---|---|---|---|---|---|
| B. cereus | E. coli O157: H7 | Staph. aureus | Sacch. cerevisiae | A. niger | |
| Unencapsulated cells | 20 ± 0.00a | 15.67 ± 2.33ab | 17.33 ± 1.45ab | 0.0 | 0.0 |
| Alg-Sm | 20 ± 0.00a | 15.67 ± 2.33ab | 16.67 ± 0.88ab | 0.0 | 0.0 |
| Alg-Dex | 18.33 ± 0.88ab | 16.33 ± 1.86ab | 15 ± 2.89b | 0.0 | 0.0 |
| Alg-Ch | 20 ± 0.00a | 14.67 ± 1.45b | 15.67 ± 0.67ab | 0.0 | 0.0 |
| Alg-DWP | 17.33 ± 1.45ab | 16 ± 2.08ab | 15 ± 0.00b | 0.0 | 0.0 |
Data were expressed as meansstandard error of three replicates ().
a-bValues with different superscript letters are significantly different (P < 0.05).
Figure 8.
Antimicrobial activity of cell free supernatant of free and microencapsulated L. plantarum.
4. Discussion
Microencapsulation technology is regarded as one of the most useful methods for enhancing the stability and viability of probiotics under harsh environmental conditions, facilitating handling of cells with constant characteristics and allowing a controlled dosage (Rokka and Rantamäki, 2010; Huq et al., 2013; Kavitake et al., 2018). Several studies have verified that encapsulated bacteria survive better than unencapsulated cells during exposure to different lethal conditions, through high temperature (Fareez et al., 2015), freezing (Homayouni et al., 2008), during storage (Chen et al., 2017a), during gastric transit (Shori, 2017) and in high bile salt concentration (Chávarri et al., 2010). Several encapsulating materials have been tested for their ability to improve the viability of many probiotic strains. However, few studies have investigated the comparison between different encapsulating agents in different harsh conditions and there are still challenges to find the proper encapsulating agent (Chen et al., 2017b). Therefore, in the present study, L. plantarum was encapsulated using alginate-based material combined with different biopolymers, including skim milk, dextrin, denatured whey protein or coating with chitosan, undergoing extrusion technique. The survivability of the encapsulated cells was evaluated under different stress conditions that may be encountered in real food processing and after ingestion. Moreover, they were evaluated for production of different bioactive compounds like exopolysaccharides and antimicrobial substances.
Encapsulation efficiency is one of the most important parameters for determining the efficacy of the encapsulation process and the selected encapsulating agent (Çabuk and Tellioğlu Harsa, 2015). Our findings demonstrate that the encapsulation efficiency of the capsules loaded with L. plantarum was ranged between 98.11% and 94.94% with no significant difference upon using the four encapsulating agents. Similar results in different alginate concentration blended with psyllium and fenugreek were reported by Haghshenas et al. (2015). The high encapsulation efficiency (>92%) can be explained by the fact that cell culture from stationary phase exhibits better survivability than that from log phase (Ilha et al., 2015). The mild encapsulation operation could also explain the high encapsulation efficiency (Ding and Shah, 2009). Other studies reported that using extrusion technique is very gentle on probiotic encapsulation and produce high encapsulation efficiency. For instance, Shi et al. (2013) found that the encapsulation efficiency of Alg-milk microsphere loaded with L. bulgaricus was around 100%. Furthermore, the encapsulation efficiency of alginate-human-like collagen microspheres loaded with Bifidobacterium longum BIOMA 5920 ranged between 92–99.2% (Su et al., 2011). It is axiomatic to indicate that high encapsulation efficiency was correlated with a good entrapment of the bacteria into the microcapsules (Burgain et al., 2014).
All the produced capsules in this study were observed irregular in shape with rough surface under the SEM. This could be attributed to an artifact resulting from sample preparation process or the high vacuum applied during SEM analysis. In addition, the capsules appear as drop-like shape with small tail. This could be a consequence of the high surface tension of the used crosslinking solution (CaCl2), resulting in formation of imperfect spherical capsule (Maresca et al., 2016). The shape of the microcapsules formed in this study is coincidence to other studies (Muthukumarasamy et al., 2006; Su et al., 2011). The particle size of the four formulated capsules (Alg-Sm, Alg-Dex, Alg-Ch, and Alg-DWP) loaded with L. plantarum in the dry state ranged between 501.54 and 800.739 μm while in the wet state it ranged between 1492 and 1635 μm. These results come in agreement with Albadran et al. (2015) who found that the Alg-Ch capsules had a diameter of around 2 mm before drying and 0.98 and 1.34 mm after drying and Fareez et al. (2015) who prepared capsules ranged in size between 1312.4 and 1335.7 μm. Also, Muthukumarasamy et al. (2006) prepared alginate and alginate + starch capsules with size ranged between 2-3 mm. Su et al. (2011) found that the particle size of alginate-human-like collagen microspheres ranged between 300–600 μm. On the other hand, Haghshenas et al. (2015) prepared alginate and alginate-psyllium capsules with size ranged between 80-300 μm using extrusion technique. Based on the above mentioned, there was a high variation in bead sizes and this variation could be due to the different polymer concentration and composition (Haghshenas et al., 2015). Application of encapsulating probiotics in food industry is a challenge mainly due to the large size of bacterial cells (typically 1–4 μm) that subsequently lead to production of large capsules, which may negatively affect the sensory qualities of foods (Chen et al., 2017b; Kavitake et al., 2018). In fact, extrusion technique produce capsules with size not smaller than 300 μm (Burgain et al., 2011). The optimum size of the microcapsules until now remains debatable as it varies according to the applications (Rosas-Flores et al., 2013). Chandramouli et al. (2004) and Fareez et al. (2015) found that larger microcapsules provide better protection to the entrapped cells against harsh environment. On the other hand, Martin et al. (2013) reported that the acceptable capsule size for food application should not be up to about 80 μm in order to avoid a negative sensory effect on the product. In the present study, the prepared capsules seem to be sufficiently large for achieving good cell loading and protection of cells against detrimental environmental conditions as will be verified from the encapsulation efficiency and tolerance to different stress conditions which will be discussed later.
Upon studying the survivability of the microencapsulated cells under different heat treatments, it was demonstrated that the temperatures 40 and 45 °C seem to be not lethal for the encapsulated L. plantarum. This observation may be regarded to the fact that the maximum growth temperature of L. plantarum is 45 °C (Wheater, 1955). Similarly, Ouled-Haddar et al. (2016) reported that L. plantarum encapsulated with sodium alginate survived by 100% and 90% after heat treatment at 40 and 50 °C, respectively for 20 min. On the other hand, after exposure to the pasteurization temperature (65 °C for 30 min), Alg-Sm was the only encapsulating agent that secured 106 cfu/g for L. plantarum, which is the recommended dose level of probiotics in the product to be effective and perform their function (Teoh et al., 2011; Bilenler et al., 2017). Other studies also showed that using skim milk in the encapsulation system enhanced the heat tolerance of probiotic bacteria, Wang et al. (2015) confirmed that the incorporation of gellan gum and skim milk powder with the alginate leads to the formation of a strong structure that protect L. kefiranofaciens M1 against heat treatment reached 70 °C. In addition, encapsulation using skim milk and cheese whey had a protective effect for L. paracasei FNU against heating at 65 °C for 30 min in comparison with unencapsulated cells, viable cell count of the encapsulated cells was 6.06 ± 0.14 log cfu/ml, while the free cells was 5.91 ± 0.16 log cfu/ml (Ilha et al., 2015).
Interestingly, on studying the survivability of the encapsulated cells during the refrigerated storage for one month, the viability of the encapsulated L. plantarum increased over the storage period. This could be due to the fact that L. plantarum was capable of growing at 8 °C in UHT milk (Matejčeková et al., 2016). In addition, the encapsulated cells were stored in skim milk that could be a nutritional factor to enhance the growth of the encapsulated cells, which was not adopted by the other relevant studies. All the encapsulating agents that are used in this study were efficient in enhancing the viability of L. plantarum during the refrigerated storage at 6 ± 2 ᵒC up to one month. However, Alg-Ch was significantly (P < 0.05) the most appropriate one. Trabelsi et al. (2013) found that Alg-Ch was effective in maintaining the stability of L. plantarum TN8 (approximately 9.34 cfu/ml) under refrigerated storage for 30 days and showed a viability loss from 9.34 to 7 log cfu/ml at the end of the storage period (60 days) in comparison with the viability of the unencapsulated cells that was just 101 cfu/ml after 30 days, that's due to the protection thicker membranes with chitosan. Also, Brinques and Ayub (2011) reported that using pectin and Alg-Ch as encapsulating agents showed little loss of L. plantarum BL011 survival after 38 days under refrigerated storage at 4 °C by 1.95 ± 0.08 and 2.17 ± 0.49 log, respectively as compared with the other tested materials. Coating alginate with chitosan could probably serve as a superior barrier as it strengthen the capsule structure (Fareez et al., 2015). It appears that milk proteins are the most efficient in protecting L. plantarum during freezing storage up to 3 months. This could be due to the adsorption of milk proteins on the cell surface that leads to a partial efflux of water from the cell, thus inhibiting the growth of ice crystals inside the cell, which subsequently reduce cell injury and cell loss (Wang et al., 2015).
The finding that the survivability of the encapsulated cells decreased by increasing salt concentration upon incubation at 37 °C for 24 h or during refrigerated storage for one month, could be attributed to the ion exchange between sodium and calcium ions in the alginate matrix, leading to swelling and destabilization of calcium alginate capsules and affecting the viability of entrapped cells (Gåserød et al., 1999). Despite that the cell reduction was still in the acceptable limit which is 106 cfu/g product. Few reports investigated the tolerance of microencapsulated bacteria to high salt concentration. Bosnea et al. (2014) previously reported that microencapsulation using whey protein isolate and gum Arabic undergoing coacervation technique enhanced the survival of L. paraplantarum B1 and L. paracasei E6 by 4 and 3 logs, respectively after exposure to 9% NaCl for 3 h in comparison with the unencapsulated cells. In addition, Mosilhey (2003) found that microencapsulation using soy milk and gum Arabic undergoing spray drying technique showed better stability to L. acidophilus during storage in (1–5%) NaCl at 5 °C when compared with unencapsulated cells, suggesting that microencapsulation could be an innovative delivery system for probiotics in salted products. However, Castro-Cislaghi et al., (2012) noted no growth for both the unencapsulated and the encapsulated cells after exposure to 5% NaCl and proved that microencapsulation by spray drying with whey did not alter the sensitivity of Bifidobacterium Bb-12 to 5% NaCl. The authors claimed that the reason for that may regarded to the sublethal injuries in cells due to spray drying and the high susceptibility of the culture in its own free form to NaCl, indicating that the nature of the bacterial strain, the encapsulation technique and the materials that used for the encapsulation play a role in the successful of the encapsulation process.
Some food and beverages require the addition of some organic acids for imparting organoleptic characteristics, preservation or increasing the nutritional value. Among the common organic acids used for that purpose are citric, lactic and ascorbic acids. Therefore, it was obvious to test the survivability of the microencapsulated cells in such potential matrix. Our results demonstrated that Alg-Dex showed higher relative cell viability for L. plantarum in 1% and 2% citric acid than the other encapsulating agents. That is due to the capability of L. plantarum to utilize citric acid and grew faster in presence of glucose in the medium (Kennes et al., 1991). Therefore, L. plantarum could use the glucose molecules that are available in the dextrin structure. Also, Alg-Sm was the more appropriate encapsulating agent in protection of L. plantarum, from lactic acid containing environment up to 2%. These results could be explained by the denser hydrogel network formed by alginate (3%) and the supporting materials, milk proteins or chitosan. That strengthen the alginate structure and subsequently could reduce the permeability of the acidic materials into the encapsulated capsules (Shi et al., 2013; Haghshenas et al., 2015). Similarly, Mosilhey (2003) found that protein- and soy-milk containing capsules provide better protection to L. acidophilus from the low pH of lactic, citric and acetic acids. Ascorbic acid (Vitamin C) is used as food additive in food products e.g., fruit juices. It has antioxidant activity and acts as oxygen scavenger. Therefore, all the encapsulated L. plantarum showed better growth in skim milk containing (1–2%) ascorbic acid than citric and lactic acids. This result could be due to the oxygen scavenger activity of ascorbic acid that reduced the oxygen content in the growth medium and subsequently enhanced its growth (Shah, 2000). Based on our results, the growth rate is dependent on the identity and the concentration of the organic acid.
Combining starter culture and probiotics during manufacturing of a fermented product may result in positive or negative interaction depending on the bacterial strain used (Li et al., 2012). All the encapsulating agents secured 108 cfu/g sample; however, L. plantarum encapsulated with Alg-Dex significantly (P < 0.05) showed greater growth in presence of the metabolites of L. rhamnosus, S. thermophilus, Leu. mesenteroides, L. bulgaricus and L. acidophilus than that entrapped within the other encapsulating agents. In fact, the calcium alginate matrix destabilized in presence of chelating agents like lactate, citrate and phosphate, which are commonly excreted during the LAB fermentation (Haghshenas et al., 2015). In addition, L. plantarum grows more optimal on glucose as a carbon source (Sieuwerts et al., 2018). Therefore, L. plantarum could ferment glucose that present in the dextrin structure. Also, the growth of L. plantarum could be stimulated by the metabolites of the other LAB in presence of glucose molecules like that happened with Sieuwerts et al. (2018) who found that L. plantarum was stimulated by S. cerevisiae in presence of glucose concentration above 2 g/l. There is little information available regarding the possible interactions among lactic acid starter and probiotic bacteria. El-Shafei et al. (2004) found that microencapsulation using sodium alginate provided better protection for L. acidophilus from the harmful effect of Lactococcus lactis and vice versa during the refrigerated storage of Talaga cheese for 30 days.
One of the main objectives of microencapsulation is providing protection of probiotic cells during exposure to low pH gastric environment (Çabuk and Tellioğlu Harsa, 2015). For probiotics to perform their function in human (prevention of gastrointestinal diseases), they have to reach the small intestine and colonize the host in appropriate number, which is 106-107 cfu/g (Shori, 2015). Microencapsulation appeared to be a promising technique for improving the survivability of probiotics under gastrointestinal tract conditions (Shori, 2017). According to our results, Alg-Ch and Alg-Sm were the most appropriate encapsulating agents for maintaining the survivability of L. plantarum under simulated gastric condition. Therefore, chitosan and milk proteins played the key role in maintaining the viability of the cells under gastric condition. The cationic nature of chitosan and its ability to buffer acid (Cook et al., 2012) may limit the interaction between the cells and the acidic environment (Chávarri et al., 2010). Fareez et al. (2015) reported that coating alginate microcapsules with chitosan resulted in a great survival of L. plantarum LAB12 that came in accordance with our finding. Likewise, Nualkaekul et al. (2012) found that microencapsulation in alginate coated chitosan improved the survivability of L. plantarum by about 0.5–1 log cycle compared with alginate alone. This result comes from the electrostatic interactions between chitosan and alginate that leads to the formation of a strong membrane on the surface of the capsule, which subsequently reduces the likelihood of leakage of the entrapped cells. Also, milk proteins can improve the survival of bacteria in simulated gastric juice due to the dense protein matrix that may reduce the diffusion rate of the acid into the microcapsules (Shi et al., 2013; Shori, 2017). Alg-Sm microcapsules appeared to be significantly (P < 0.05) the most resistant encapsulating agent to maintain the survivability of L. plantarum under simulated intestinal condition. This result came in accordance with Shi et al. (2013) who found that Alg-Milk capsules tolerated the high bile salt solution and improve the survival of L. bulgaricus after exposure to (1–2%) bile salt solution. The result also revealed that the protection provided using the four encapsulating agents secured 107 cfu/g sample, this viable cell count is within the recommended probiotic dose level to be effective as indicated before. Therefore, all capsules will take more time to be disintegrated and controlled the release of the entrapped cells, thus subsequently shorten the exposure time to the detrimental effect of the intestinal fluid (Fareez et al., 2015).
This study demonstrated that L. plantarum produce extracellular polysaccharides (approximately 0.44 g/100 ml skim milk), also Zhang et al. (2016) reported that L. plantarum ZDY2013 produce exopolysaccharide and the yield was 0.4294 ± 30.3 g/l MRS. Fascinatingly, there was no significant difference in the production of EPS between the unencapsulated and the encapsulated cells. In addition, according to the data obtained from studying the ability of the encapsulated cell to produce antimicrobial compounds, the cell free supernatant of L. plantarum grown in MRS broth medium at 37 °C for 72 h was active against B. cereus, Staph. aureus and E. coli O157: H7. However, the CFS of L. plantarum grown in MRS at 37 °C after 18 and 48 h and in fresh skim milk under the same condition were ineffective against the tested food spoilage strains. These findings infer that the antimicrobial effect against B. cereus, Staph. aureus and E. coli O157: H7 were regarded to the accumulation of the organic acids in the medium during the incubation period (72 h at 37 °C) not to the bacteriocins. This observation come in agreement with Arena et al. (2016) who suggested that the antimicrobial activity of L. plantarum depends on a pH reduction effect of supernatants and/or on the presence of organic acids. The antimicrobial effect of the organic acids result from their ability to penetrate the cell cytoplasmic membrane in the undissociated form leading to reduction in the intracellular pH due to accumulation of the acids in the cell cytoplasm and ultimately cell death (Mosilhey, 2003). Li et al. (2016) found that the supernatant (pH 3.6) of L. plantarum LZ206 possess antimicrobial activity against various food borne pathogens including Gram-positive bacteria (Staphylococcus aureus and Listeria monocytogenes) with inhibition zone 10–15 and 15–20 mm, respectively, Gram-negative bacteria (Escherichia coli and Salmonella enterica) with inhibition zone 15–20 mm and 20–25 mm, respectively, that is approximately similar to what we have found with the tested pathogens. Furthermore, there was no significant difference between the unencapsulated and the encapsulated cells in production of antimicrobial substances. This indicates that microencapsulation does not affect the functionality and the metabolic activity of the entrapped cells. Our findings come in agreement with Wang et al. (2015) who found that the encapsulation technique does not affect the anti-colitis activity of the entrapped cells. Finally, our results showed a differential behavior among the different encapsulating agents toward the different stress conditions. This means that each encapsulating agent can secure protection to the encapsulated probiotic in a specific stress evaluation. However, based on our findings, Alg-Sm could be the promising capsule for enhancing the stress tolerance of L. plantarum during storage, food processing and transition through gastrointestinal tract conditions. Overall, the type of final application and the potential stress are the factors that can determine the appropriate type of the encapsulating agent. This comprehensive study provides useful guidelines for the application of probiotic encapsulation in the food industry, of course, the simulation studies add value during development of new technology; however, it is not enough and further studies need to be performed to monitor the efficacy of the studied encapsulated materials in real food system and in vivo, using animal models. The organoleptic characteristics and the shelf life of the encapsulated products should also be tested since the sensory evaluation of products with microencapsulated probiotic bacteria will reveal the consumer response to the texture and the changes in organoleptic characteristics of the product.
Declarations
Author contribution statement
Nagwa A. Abdallah, Nabil F. Tawfik: Analyzed and interpreted the data; Wrote the paper.
Kawther El-Shafei, Hoda S. El-Sayed: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mona Mahmoud: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abd El-Salam M.H., El-Shibiny S. Preparation and properties of milk proteins-based encapsulated probiotics: a review. Dairy Sci. Technol. 2015;95:393–412. [Google Scholar]
- Albadran H.A., Chatzifragkou A., Khutoryanskiy V.V., Charalampopoulos D. Stability of probiotic Lactobacillus plantarum in dry microcapsules under accelerated storage conditions. Food Res. Int. 2015;74:208–216. doi: 10.1016/j.foodres.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Arena M.P., Silvain A., Normanno G., Grieco F., Drider D., Spano G., Fiocco D. Use of Lactobacillus plantarum strains as a bio-control strategy against food-borne pathogenic microorganisms. Front. Microbiol. 2016;7:1–10. doi: 10.3389/fmicb.2016.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek Y.J., Lee B.H. Probiotics and prebiotics as bioactive components in dairy products. In: Park Y.W., editor. Bioactive Components of Milk and Dairy Products. 2009. pp. 287–310. Wiley-Black well. [Google Scholar]
- Barbosa M.S., Todorov S.D., Ivanova I.V., Belguesmia Y., Choiset Y., Rabesona H., Chobert J.-M., Haertlé T., Franco B.D.G.M. Characterization of a two-peptide plantaricin produced by Lactobacillus plantarum MBSa4 isolated from Brazilian salami. Food Contr. 2016;60:103–112. [Google Scholar]
- Bilenler T., Karabulut I., Candogan K. Effects of encapsulated starter cultures on microbial and physicochemical properties of traditionally produced and heat treated sausages (sucuks) LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;75:425–433. [Google Scholar]
- Bosnea L.A., Moschakis T., Biliaderis C.G. Complex coacervation as a novel microencapsulation technique to improve viability of probiotics under different stresses. Food Bioprocess Technol. 2014;7:2767–2781. [Google Scholar]
- Brinques G.B., Ayub M.A.Z. Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions , refrigeration , and yogurt. J. Food Eng. 2011;103:123–128. [Google Scholar]
- Burgain J., Gaiani C., Linder M., Scher J. Encapsulation of probiotic living cells: from laboratory scale to industrial applications. J. Food Eng. 2011;104:467–483. [Google Scholar]
- Burgain J., Scher J., Lebeer S., Vanderleyden J., Cailliez-Grimal C., Corgneau M., Francius G., Gaiani C. Significance of bacterial surface molecules interactions with milk proteins to enhance microencapsulation of Lactobacillus rhamnosus GG. Food Hydrocolloids. 2014;41:60–70. [Google Scholar]
- Çabuk B., Tellioğlu Harsa Ş. Protection of Lactobacillus acidophilus NRRL-B 4495 under in vitro gastrointestinal conditions with whey protein/pullulan microcapsules. J. Biosci. Bioeng. 2015;120:650–656. doi: 10.1016/j.jbiosc.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Castro J.M., Tornadijo M.E., Fresno J.M., Sandoval H. Biocheese: a food probiotic carrier. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/723056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Cislaghi F.P. De, Silva C.D.R.E., Fritzen-Freire C.B., Lorenz J.G., Sant'Anna E.S. Bifidobacterium Bb-12 microencapsulated by spray drying with whey: survival under simulated gastrointestinal conditions, tolerance to NaCl, and viability during storage. J. Food Eng. 2012;113:186–193. [Google Scholar]
- Chandramouli V., Kailasapathy K., Peiris P., Jones M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J. Microbiol. Methods. 2004;56:27–35. doi: 10.1016/j.mimet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Chávarri M., Marañón I., Ares R., Ibáñez F.C., Marzo F., Villarán C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010;142:185–189. doi: 10.1016/j.ijfoodmicro.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Chen H.Y., Li X.Y., Liu B.J., Meng X.H. Microencapsulation of Lactobacillus bulgaricus and survival assays under simulated gastrointestinal conditions. J. Funct. Foods. 2017;29:248–255. [Google Scholar]
- Chen J., Wang Q., Liu C.M., Gong J. Issues deserve attention in encapsulating probiotics: critical review of existing literature. Crit. Rev. Food Sci. Nutr. 2017;57:1228–1238. doi: 10.1080/10408398.2014.977991. [DOI] [PubMed] [Google Scholar]
- Cook M.T., Tzortzis G., Charalampopoulos D., Khutoryanskiy V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Contr. Release. 2012;162:56–67. doi: 10.1016/j.jconrel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Corona-Hernandez R.I., Álvarez-Parrilla E., Lizardi-Mendoza J., Islas-Rubio A.R., la Rosa L.A. de, Wall-Medrano A. Structural stability and viability of microencapsulated probiotic bacteria: a review. Compr. Rev. Food Sci. Food Saf. 2013;12:614–628. doi: 10.1111/1541-4337.12030. [DOI] [PubMed] [Google Scholar]
- Das D., Rameshbabu A.P., Ghosh P., Patra P., Dhara S., Pal S. Biocompatible nanogel derived from functionalized dextrin for targeted delivery of doxorubicin hydrochloride to MG 63 cancer cells. Carbohydr. Polym. 2017;171:27–38. doi: 10.1016/j.carbpol.2017.04.068. [DOI] [PubMed] [Google Scholar]
- Dehkordi S.S., Alemzadeh I., Vaziri A.S., Vossoughi A. Optimization of alginate-whey protein isolate microcapsules for survivability and release behavior of probiotic bacteria. Appl. Biochem. Biotechnol. 2019:182–196. doi: 10.1007/s12010-019-03071-5. [DOI] [PubMed] [Google Scholar]
- Dianawati D., Mishra V., Shah N.P. Stability of microencapsulated Lactobacillus acidophilus and Lactococcus lactis ssp. cremoris during storage at room temperature at low aw. Food Res. Int. 2013;50:259–265. [Google Scholar]
- Ding W.K., Shah N.P. Effect of homogenization techniques on reducing the size of microcapsules and the survival of probiotic bacteria therein. J. Food Sci. 2009;74:231–236. doi: 10.1111/j.1750-3841.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- Divya J.B., Varsha K.K., Nampoothiri K.M., Ismail B., Pandey A. Probiotic fermented foods for health benefits. Eng. Life Sci. 2012;12:377–390. [Google Scholar]
- El-Shafei k., Sadek Z.I., Salem M.M.E. Encapsulation of lactic acid bacteria to avoid the antagonism during the manufacture of probiotic soft cheese. Minufiya J. Agric. Res. 2004;29:1279–1293. [Google Scholar]
- Etchepare M. de A., Barin J.S., Cichoski A.J., Jacob-Lopes E., Wagner R., Fries L.L.M., Menezes C.R. de. Microencapsulation of probiotics using sodium alginate. Ciência Rural. 2015;45:1319–1326. [Google Scholar]
- Fareez I.M., Lim S.M., Mishra R.K., Ramasamy K. Chitosan coated alginate-xanthan gum bead enhanced pH and thermotolerance of Lactobacillus plantarum LAB12. Int. J. Biol. Macromol. 2015;72:1419–1428. doi: 10.1016/j.ijbiomac.2014.10.054. [DOI] [PubMed] [Google Scholar]
- Feucht A., Kwak H.S. Microencapsulation of lactic acid bacteria (LAB) Kor. J. Food Sci. Anim. Res. 2013;33:229–238. [Google Scholar]
- Gåserød O., Sannes A., Skjåk-Bræk G. Microcapsules of alginate-chitosan. II. A study of capsule stability and permeability. Biomaterials. 1999;20:773–783. doi: 10.1016/s0142-9612(98)00230-0. [DOI] [PubMed] [Google Scholar]
- Gbassi G.K., Vandamme T., Ennahar S., Marchioni E. Microencapsulation of Lactobacillus plantarum spp in an alginate matrix coated with whey proteins. Int. J. Food Microbiol. 2009;129:103–105. doi: 10.1016/j.ijfoodmicro.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Gibbs B.F., Kermasha S., Alli I., Mulligan C.N. Encapsulation in the food industry: a review. Int. J. Food Sci. Nutr. 1999;50:213–224. doi: 10.1080/096374899101256. [DOI] [PubMed] [Google Scholar]
- Giraffa G., Chanishvili N., Widyastuti Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 2010;161:480–487. doi: 10.1016/j.resmic.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Haghshenas B., Abdullah N., Nami Y., Radiah D., Rosli R., Yari Khosroushahi A. Microencapsulation of probiotic bacteria Lactobacillus plantarum 15HN using alginate-psyllium-fenugreek polymeric blends. J. Appl. Microbiol. 2015;118:1048–1057. doi: 10.1111/jam.12762. [DOI] [PubMed] [Google Scholar]
- Homayouni A., Azizi A., Ehsani M.R., Yarmand M.S., Razavi S.H. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem. 2008;111:50–55. [Google Scholar]
- Homayouni A., Azizi A., Javadi M., Mahdipour S., Ejtahed H. Factors influencing probiotic survival in ice cream: a review. Int. J. Dairy Sci. 2012;7:1–10. [Google Scholar]
- Huq T., Khan A., Khan R.A., Riedl B., Lacroix M. Encapsulation of probiotic bacteria in biopolymeric system. Crit. Rev. Food Sci. Nutr. 2013;53:909–916. doi: 10.1080/10408398.2011.573152. [DOI] [PubMed] [Google Scholar]
- Ilha E.C., Silva T. da, Lorenz J.G., Oliveira Rocha G. de, Sant’Anna E.S. Lactobacillus paracasei isolated from grape sourdough: acid, bile, salt, and heat tolerance after spray drying with skim milk and cheese whey. Eur. Food Res. Technol. 2015;240:977–984. [Google Scholar]
- Kailasapathy K., Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000;78:80–88. doi: 10.1046/j.1440-1711.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- Kavitake D., Kandasamy S., Devi P.B., Shetty P.H. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods – a review. Food Biosci. 2018;21:34–44. [Google Scholar]
- Kechagia M., Basoulis D., Konstantopoulou S., Dimitriadi D., Gyftopoulou K., Skarmoutsou N., Fakiri E.M. Health benefits of probiotics: a review. ISRN Nutrition. 2013;2013:1–7. doi: 10.5402/2013/481651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennes C., Dubourguler H.C., Albagnac G., Nyns E.-J. Citrate metabolism by Lactobacillus plantarum isolated from orange juice. J. Appl. Bacteriol. 1991;70:380–384. [Google Scholar]
- Kilara A., Panyam D. Peptides from milk proteins and their properties. Crit. Rev. Food Sci. Nutr. 2003;43:607–633. doi: 10.1080/10408690390251138. [DOI] [PubMed] [Google Scholar]
- Krasaekoopt W., Bhandari B., Deeth H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003;13:3–13. [Google Scholar]
- Kühbeck D., Mayr J., Häring M., Hofmann M., Quignard F., Díaz Díaz D. Evaluation of the nitroaldol reaction in the presence of metal ion-crosslinked alginates. New J. Chem. 2015;39:2306–2315. [Google Scholar]
- Lee Y., Ji Y.R., Lee S., Choi M.J., Cho Y. Microencapsulation of probiotic Lactobacillus acidophilus kbl409 by extrusion technology to enhance survival under simulated intestinal and freeze-drying conditions. J. Microbiol. Biotechnol. 2019;29:721–730. doi: 10.4014/jmb.1903.03018. [DOI] [PubMed] [Google Scholar]
- Li S., Walsh H., Gokavi S., Guo M. Interactions between Lactobacillus acidophilus strains and the starter cultures, Lactobacillus bulgaricus and Streptococcus thermophilus during fermentation of goats’ milk. Afr. J. Biotechnol. 2012;11:11271–11279. [Google Scholar]
- Li P., Gu Q., Zhou Q. Complete genome sequence of Lactobacillus plantarum LZ95, a potential probiotic strain producing bacteriocins and B-group vitamin riboflavin. J. Biotechnol. 2016;238:52–55. doi: 10.1016/j.jbiotec.2016.04.048. [DOI] [PubMed] [Google Scholar]
- Liu X., Cao S., Zhang X. Modulation of gut microbiota-brain Axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 2015;63:7885–7895. doi: 10.1021/acs.jafc.5b02404. [DOI] [PubMed] [Google Scholar]
- Liu H., Cui S.W., Chen M., li Y., Liang R., Xu F., Zhong F. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: a review. Crit. Rev. Food Sci. Nutr. 2019;59:2863–2878. doi: 10.1080/10408398.2017.1377684. [DOI] [PubMed] [Google Scholar]
- Mallesha Shylaja R., Selvakumar D., Jagannath J.H. Isolation and identification of lactic acid bacteria from raw and fermented products and their antibacterial activity. J. Recent Res. Sci. Technol. 2010;2:42–46. [Google Scholar]
- Maresca D., Prisco A. De, Storia A., La Cirillo T., Esposito F., Mauriello G. Microencapsulation of nisin in alginate beads by vibrating technology: preliminary investigation. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;66:436–443. [Google Scholar]
- Martin M.J., Lara-Villoslada F., Ruiz M.A., Morales M.E. Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2013;53:480–486. [Google Scholar]
- Matejčeková Z., Liptáková D., Spodniaková S., Valík Ľ. Characterization of the growth of Lactobacillus plantarum in milk in dependence on temperature. Acta Chim. Slovaca. 2016;9:104–108. [Google Scholar]
- Mirzaei H., Pourjafar H., Homayouni A. Effect of calcium alginate and resistant starch microencapsulation on the survival rate of Lactobacillus acidophilus La5 and sensory properties in Iranian white brined cheese. Food Chem. 2012;132:1966–1970. [Google Scholar]
- Mosilhey S.H. Institute of Food Technology, Faculty of Agriculture, University of Bonn; Germany: 2003. Influence of Different Capsule Materials on the Physiological Properties of Microencapsulated Lactobacillus acidophilus.http://hss.ulb.uni-bonn.de/2003/0154/0154.pdf Thesis PhD-Ingeneur. Available from: Accessed 2012 July. [Google Scholar]
- Moumita S., Goderska K., Johnson E.M., Das B., Indira D., Yadav R., Kumari S., Jayabalan R. Evaluation of the viability of free and encapsulated lactic acid bacteria using in-vitro gastro intestinal model and survivability studies of synbiotic microcapsules in dry food matrix during storage. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;77:460–467. [Google Scholar]
- Muthukumarasamy P., Allan-Wojtas Paula, Holley R.a. Stability of Lactobacillus reuteri in different types of microcapsules. J. Food Sci. 2006;71:20–24. [Google Scholar]
- Nie P., Li Z., Wang Y., Zhang Y., Zhao M., Luo J., Du S., Deng Z., Chen J., Wang Y., Chen S., Wang L. Gut microbiome interventions in human health and diseases. Med. Res. Rev. 2019;39:2286–2313. doi: 10.1002/med.21584. [DOI] [PubMed] [Google Scholar]
- Nualkaekul S., Lenton D., Cook M.T., Khutoryanskiy V.V., Charalampopoulos D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr. Polym. 2012;90:1281–1287. doi: 10.1016/j.carbpol.2012.06.073. [DOI] [PubMed] [Google Scholar]
- Ouled-Haddar H., Sifour M., Idoui T., Bouridane H., Arid S. Lactobacillus plantarum G1 microencapsulation enhanced its viability during storage and gastrointestinal transit. Sains Malays. 2016;45:1049–1055. [Google Scholar]
- Patil A., Disouza J., Pawar S. Shelf life stability of encapsulated lactic acid bacteria isolated from sheep milk thrived in different milk as natural media. Small Rumin. Res. 2019;170:19–25. [Google Scholar]
- Quigley E.M.M. Prebiotics and probiotics in digestive health. Clin. Gastroenterol. Hepatol. 2019;17:333–344. doi: 10.1016/j.cgh.2018.09.028. [DOI] [PubMed] [Google Scholar]
- Rajam R., Karthik P., Parthasarathi S., Joseph G.S., Anandharamakrishnan C. Effect of whey protein - alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J. Funct. Foods. 2012;4:891–898. [Google Scholar]
- Rathore S., Desai P.M., Liew C.V., Chan L.W., Heng P.W.S. Microencapsulation of microbial cells. J. Food Eng. 2013;116:369–381. [Google Scholar]
- Rokka S., Rantamäki P. Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. Eur. Food Res. Technol. 2010;231:1–12. [Google Scholar]
- Rosas-Flores W., Ramos-Ramírez E.G., Salazar-Montoya J.A. Microencapsulation of Lactobacillus helveticus and Lactobacillus delbrueckii using alginate and gellan gum. Carbohydr. Polym. 2013;98:1011–1017. doi: 10.1016/j.carbpol.2013.06.077. [DOI] [PubMed] [Google Scholar]
- Shah N.P. Probiotic bacteria: selective enumeration and survival in dairy foods. J. Dairy Sci. 2000;83:894–907. doi: 10.3168/jds.S0022-0302(00)74953-8. [DOI] [PubMed] [Google Scholar]
- Shi L.E., Li Z.H., Li D.T., Xu M., Chen H.Y., Zhang Z.L., Tang Z.X. Encapsulation of probiotic Lactobacillus bulgaricus in alginate’milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng. 2013;117:99–104. [Google Scholar]
- Shori A.B. The potential applications of probiotics on dairy and non-dairy foods focusing on viability during storage. Biocatal. Agric. Biotechnol. 2015;4:423–431. [Google Scholar]
- Shori A.B. Microencapsulation improved probiotics survival during gastric transit. HAYATI J. Biosci. 2017;24:1–5. [Google Scholar]
- Sieuwerts S., Bron P.A., Smid E.J. Mutually stimulating interactions between lactic acid bacteria and Saccharomyces cerevisiae in sourdough fermentation. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2018;90:201–206. [Google Scholar]
- Su R., Zhu X.L., Fan D.Di, Mi Y., Yang C.Y., Jia X. Encapsulation of probiotic Bifidobacterium longum BIOMA 5920 with alginate-human-like collagen and evaluation of survival in simulated gastrointestinal conditions. Int. J. Biol. Macromol. 2011;49:979–984. doi: 10.1016/j.ijbiomac.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Suez J., Zmora N., Segal E., Elinav E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019;25 doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- Sultana K., Godward G., Reynolds N., Arumugaswamy R., Peiris P., Kailasapathy K. Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int. J. Food Microbiol. 2000;62:47–55. doi: 10.1016/s0168-1605(00)00380-9. [DOI] [PubMed] [Google Scholar]
- Teoh P.L., Mirhosseini H., Mustafa S., Hussin A.S.M., Abdul Manap M.Y. Recent approaches in the development of encapsulated delivery systems for probiotics. Food Biotechnol. 2011;25:77–101. [Google Scholar]
- Trabelsi I., Bejar W., Ayadi D., Chouayekh H., Kammoun R., Bejar S., Salah R. Ben. Encapsulation in alginate and alginate coated-chitosan improved the survival of newly probiotic in oxgall and gastric juice. Int. J. Biol. Macromol. 2013;61:36—42. doi: 10.1016/j.ijbiomac.2013.06.035. [DOI] [PubMed] [Google Scholar]
- Tripathi M.K., Giri S.K. Probiotic functional foods: survival of probiotics during processing and storage. J. Funct. Foods. 2014;9:225–241. [Google Scholar]
- Umeki K., Yamamoto T. Structures of multi-branched dextrins produced by saccharifying α-amylase from starch. J. Biochem. 1975;78:897–903. doi: 10.1093/oxfordjournals.jbchem.a130995. [DOI] [PubMed] [Google Scholar]
- Vinderola C.G., Mocchiutti P., Reinheimer J.A. Interactions among lactic acid starter and probiotic bacteria used for fermented dairy products. J. Dairy Sci. 2002;85:721–729. doi: 10.3168/jds.S0022-0302(02)74129-5. [DOI] [PubMed] [Google Scholar]
- Wang S.Y., Ho Y.F., Chen Y.P., Chen M.J. Effects of a novel encapsulating technique on the temperature tolerance and anti-colitis activity of the probiotic bacterium Lactobacillus kefiranofaciens M1. Food Microbiol. 2015;46:494–500. doi: 10.1016/j.fm.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Wheater D.M. The characteristics of Lactobacillus plantarum, L. helveticus and L. casei. J. Gen. Microbiol. 1955;12:133–139. doi: 10.1099/00221287-12-1-133. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Liu Z., Tao X., Wei H. Characterization and sulfated modification of an exopolysaccharide from Lactobacillus plantarum ZDY2013 and its biological activities. Carbohydr. Polym. 2016;153:25–33. doi: 10.1016/j.carbpol.2016.07.084. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Martins E., Groboillot a., Champagne C., Neufeld R.J. Spectrophotometric quantification of lactic bacteria in alginate and control of cell release with chitosan coating. J. Appl. Microbiol. 1998;84:342–348. [Google Scholar]
- Zhu X.Y., Liu F. 2017. Review Article Probiotics as an Adjuvant Treatment in Helicobacter pylori Eradication Therapy. [DOI] [PubMed] [Google Scholar]
- Zisu B., Shah N.P. Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophilus 1275. J. Dairy Sci. 2003;86:3405–3415. doi: 10.3168/jds.S0022-0302(03)73944-7. [DOI] [PubMed] [Google Scholar]