Summary
Cancerous Inhibitor of Protein Phosphatase 2A (CIP2A) is an oncogene and a potential cancer therapy target protein. Accordingly, a better understanding of the physiological function of CIP2A, especially in the context of immune cells, is a prerequisite for its exploitation in cancer therapy. Here, we report that CIP2A negatively regulates interleukin (IL)-17 production by Th17 cells in human and mouse. Interestingly, concomitant with increased IL-17 production, CIP2A-deficient Th17 cells had increased strength and duration of STAT3 phosphorylation. We analyzed the interactome of phosphorylated STAT3 in CIP2A-deficient and CIP2A-sufficient Th17 cells and indicated together with genome-wide gene expression profiling, a role of Acylglycerol Kinase (AGK) in the regulation of Th17 differentiation by CIP2A. We demonstrated that CIP2A regulates the strength of the interaction between AGK and STAT3, and thereby modulates STAT3 phosphorylation and expression of IL-17 in Th17 cells.
Subject Areas: Molecular Interaction, Immunology, Systems Biology, Omics
Graphical Abstract

Highlights
-
•
CIP2A deficiency leads to increased IL-17 production by Th17 cells
-
•
STAT3 phosphorylation is increased and prolonged in CIP2A-deficient Th17 cells
-
•
Mass spectrometry-based analysis of pSTAT3 interactome
-
•
CIP2A regulates interaction between acylglycerol kinase (AGK) and pSTAT3
Molecular Interaction; Immunology; Systems Biology; Omics
Introduction
Cancerous Inhibitor of Protein Phosphatase 2A (CIP2A) was first characterized as a modulator of activity of Protein Phosphatase 2A (PP2A) toward serine-62 phosphorylated MYC, leading to stabilization of the MYC protein (Junttila et al., 2007). The oncogenic nature of CIP2A was later confirmed in various human malignancies (Kauko and Westermarck, 2018) making it a promising target for cancer therapy (Junttila et al., 2007, Janghorban et al., 2014, Lucas et al., 2016). Mechanistically, the oncogenic activity of CIP2A can be explained by its activity toward the tumor suppressive PP2A B-subunit B56 and presumed effects in selectively inhibiting PP2A/B56 substrate recognition (Wang et al., 2017). In addition to cancer, increased CIP2A levels have also been detected in the neurons of patients with Alzheimer disease (AD) leading to increased phosphorylation of Tau protein, suggesting CIP2A also to be a potential therapeutic target for AD (Shentu et al., 2018). Although CIP2A is associated with these disease states, there is a need for a better understanding of its role in normal cellular physiology. Its expression has also been associated with autophagy and increased cell proliferation (Yu et al., 2013). It promotes cell cycle progression, premature chromosome segregation, and aneuploidy (Pallai et al., 2015). We have previously demonstrated that CIP2A deficiency results in defects in T cell activation (Côme et al., 2016); however, nothing is known with respect to the function of CIP2A in the differentiation of different T helper (Th) cell subsets.
Interleukin 17 (IL-17)-producing Th17 cells protect the mucosal surfaces and play a crucial role in host defense against pathogens, such as fungi and extracellular bacteria (Gaffen et al., 2011, Romani, 2011). Dysregulated Th17 differentiation leads to several autoimmune and inflammatory pathologies, including psoriasis, rheumatoid arthritis (RA), multiple sclerosis, and inflammatory bowel disease (Kleinewietfeld and Hafler, 2013, Kleinewietfeld et al., 2013, Wu et al., 2013, Yosef et al., 2013, Lee et al., 2014, Meyer Zu Horste et al., 2016). Strategies to limit excessive Th17 response are therefore an attractive target to prevent Th17-mediated pathologies. Notably, the suppression of the activity of transcription factors (TF) driving Th17 differentiation, such as RORγt, has demonstrated impressive efficacy in preclinical disease models (Huh et al., 2011, Xu et al., 2011, Xiao et al., 2014). Moreover, inhibition of IL-17 by neutralizing antibodies has shown remarkable effectiveness in clinical trials in the pathology of several autoimmune diseases, such as psoriasis, ankylosing spondylitis, and multiple sclerosis (Robinson et al., 2013, Lønnberg et al., 2014). A better understanding of how Th17 cells function and their differentiation is regulated would facilitate the development of novel approaches to treat autoimmune diseases and other Th17 cell-mediated disorders.
In the present study, we demonstrated that CIP2A silencing results in a significant increase in IL-17 production in human and mouse Th17 cells. Genome-wide profiling of gene expression in human CIP2A-silenced Th17 cells confirmed the upregulation of many Th17 cell-specific genes, including RORC and MAF. Concomitant with increased IL-17 production, we observed enhanced STAT3 (Y705) phosphorylation in CIP2A-deficient Th17 cells. To identify candidates responsible for enhanced STAT3 phosphorylation, we used a mass spectrometry (MS) (liquid chromatography [LC]-tandem MS [MS/MS])-based proteomics approach to study the interactome of phosphorylated (pSTAT3) in CIP2A-silenced and control Th17 cells. We demonstrated significantly increased interaction between acylglycerol kinase (AGK) and pSTAT3 under CIP2A-deficient condition that in turn may lead to enhanced STAT3 phosphorylation and IL-17 secretion in CIP2A-silenced Th17 cells. Notably, both inhibition of CIP2A and direct inhibition of PP2A catalytic subunit led to enhanced IL-17 expression in Th17 cells. This suggests that CIP2A negatively regulates human Th17 cell differentiation without inhibiting catalytic activity of PP2A complex.
Results
CIP2A Is Downregulated in TH17 Cells
CIP2A is induced upon T cell activation, and its mRNA expression is reduced in Th17 cells (Figures 1A and 1B). Interestingly, in other CD4+ T cell subsets, there was no difference in the expression of CIP2A (Figures S1A and S1B) (Kanduri et al., 2015, Ubaid Ullah et al., 2018). We further confirmed reduced CIP2A expression in Th17 cells at the protein level (72 h) using confocal microscopy (Figures 1C and 1D). CIP2A localization by confocal microscopy suggested similar CIP2A expression in the nuclear and cytoplasmic fractions of Th0 and Th17 cells. Thus, reduced expression of CIP2A is not due to its altered localization in Th17 cells (Khan et al., 2020). On account of the early expression of IL2 in response to T cell receptor (TCR) triggering/activation and its role in immune system, we sought to investigate whether the expression of CIP2A was regulated directly by TCR or through activation-induced autocrine/paracrine IL-2. Naive human CD4+ T cells were stimulated with either TCR or IL-2 alone or in combination for 72 h followed by measuring CIP2A expression by TaqMan qRT-PCR. Although TCR by itself was sufficient to induce the expression, stimulation with IL-2 alone did not result in any detectable expression of CIP2A (Figure 1E). Together, these results indicate that CIP2A is induced in T cells upon TCR triggering and is downregulated in Th17 cells.
Figure 1.
CIP2A Is Downregulated in Th17 Cells
(A) Expression profiles of CIP2A from human activated T cells (Th0) and Th17 cells at the indicated time points post cell activation (RNA-seq data from Tuomela et al. (2016)).
(B) Pairwise TaqMan qRT-PCR analysis of CIP2A expression at 24 h in Th0 and Th17 cells. The significance was determined using unpaired two-tailed t test; ∗p < 0.05.
(C) Confocal microscopic images of CIP2A staining in Th0 (top) and Th17 (bottom) cells (72 h). Scale bar, 7 μm.
(D) Statistical analysis of the confocal microscopy (C). The dot plot shows average corrected total cell fluorescence displayed as arbitrary unit (AU); each dot represents an independent experiment where 50–60 cells were analyzed. The analyses were performed using GraphPad Prism version 7.0d for Mac OS X (GraphPad Software), and the significance was determined using unpaired two-tailed t test; ∗p < 0.05.
(E) CIP2A expression analysis by TaqMan qRT-PCR in human T cells at 72 h after stimulation by TCR, IL-2, or both. Data were calculated as dCT values normalized with the housekeeping gene (EF1-alpha) and plotted as 2−dCt. ∗∗∗∗p < 0.0001 (Student's two-tailed unpaired t test). In all figures, the error bars represent the standard error of the mean. RE and AU stand for relative expression and arbitrary unit, respectively.
CIP2A Negatively Regulates TH17 Differentiation
To determine the functional role of CIP2A in human CD4+ T cells, CIP2A was silenced using three different small interfering RNAs (siRNAs), each targeting different regions of the transcript. CIP2A expression was efficiently silenced both at protein and RNA levels by these three (siCIP2A1, siCIP2A4, and siCIP2A5) siRNAs (Figures 2A and 2B). In mouse, CIP2A was depleted using gene trap technology, as previously described (Ventelä et al., 2012). CIP2A homozygous mice (CIP2AHOZ) were viable with normal lifespan and had strong depletion (more than 90%) of CIP2A expression when compared with CIP2A wild-type (CIP2AWT) animals. In our earlier study, we reported reduced expression of CD69, a marker for activated T cells, in CIP2A-deficient human and mouse T cells in response to TCR activation (Côme et al., 2016). In the current study, we tested the effect of CIP2A silencing on IL2RA (CD25) expression, as IL2RA is another key receptor induced upon T cell activation. Like CD69, CD25 expression was also significantly downregulated in cells deficient in CIP2A (Figures S2A and S2B), further supporting the role of CIP2A in T cell activation.
Figure 2.
CIP2A Negatively Regulates Th17 Cell Differentiation
(A) Western blot (WB) analysis of CIP2A silencing by five different siRNAs. NT denotes non-targeting siRNA. Quantification was performed using ImageJ software and plotted above the representative WB where each dot represents an independent experiment. The significance was determined using unpaired two-tailed t test; ∗∗∗∗p < 0.0001.
(B) TaqMan qRT-PCR analysis demonstrating the efficiency of CIP2A silencing by the three functional CIP2A siRNAs shown in (A). The significance was determined using unpaired two-tailed t test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
(C) A flow cytometry dot blot analysis of Th17 cell-specific CCR6 receptor expression in CIP2A-silenced and control 72 h polarized human Th17 cells. A representative example of three biological replicates. The number on the plot shows the percentage of positive cells.
(D) TaqMan qRT-PCR analysis of IL17A expression in CIP2A-silenced human Th17 cells at 72 h. Asterisks denotes significance obtained in Student's t test (two-tailed paired) with ∗p < 0.05.
(E) Luminex analysis of secreted IL-17A in CIP2A-silenced human Th17 cells. The values were normalized by the number of living cells, determined on the basis of cell size and granularity detected by flow cytometry. The significance obtained by Student's t test (two-tailed paired) with ∗∗p < 0.01.
(F) Flow cytometry analysis of IL-17A expression in mouse Th17 cells from CIP2A knockout (KO) and control (WT) animals shown as contour plot of one replicate (left) and the mean of three replicates (right). ∗p < 0.05.
(G) Luminex analysis of IL-17A secretion from Th17 cells generated from CIP2A KO and WT mice cells. In TaqMan qRT-PCR analysis unless otherwise stated, data were calculated as dCT values normalized with the housekeeping gene (EF1-alpha) and plotted as 2−dCt. Asterisks denotes significance obtained in Student's t test (two-tailed paired) with ∗p < 0.05.
CIP2A siRNA (siCIP2A) and non-targeting control siRNA (siNT) transfected human naive CD4+ T cells were differentiated toward the Th17 direction, and CCR6 surface expression and IL-17 levels were measured at 72 h. A significant increase in CCR6 expression (Figure 2C) and enhanced IL-17A expression, both at RNA (Figure 2D) and protein levels (Figure 2E), were observed in the CIP2A-silenced Th17 cells. To study if the effect of CIP2A on IL-17 was true also for mouse, naive CD4+CD62L+ splenic T cells from CIP2A WT and knockout (KO) animals were differentiated to the Th17 direction and IL-17 levels were measured. Similar to human Th17 cells, a consistent increase in IL-17A expression in the CIP2A-deficient mouse Th17 cells was detected at 72 h (Figures 2F and 2G).
To assess whether the increase in IL-17 expression in CIP2A-silenced cells was due to regulation of IL17A gene expression, increased proliferation, or increased cell survival, naive CD4+ T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and the cells were activated under Th17-promoting conditions. The extent of CFSE dilution was monitored 96 h post cell activation, in both CIP2A-silenced and control cells. The rate of proliferation in CIP2A-silenced cells was slower than in the control cells (Figure S2D). Similar results were also obtained in mouse cells deficient in CIP2A (Figure S2E), and there were no significant differences in the cell viability between the cell populations (Figure S2F). These results confirmed that increase in IL-17 upon CIP2A silencing is not due to increased proliferation of these cells and is consistent with several other reports that have found reduced proliferation of CIP2A-deficient cells in other cell types (Junttila et al., 2007, Ventelä et al., 2012, Yang et al., 2016).
To explore the mechanisms underlying the increased Th17 differentiation of CIP2A-deficient cells, we carried out RNA sequencing (RNA-seq) analysis of CIP2A-silenced cells cultured under Th17 condition for 24 h. This time point was chosen to ensure that CIP2A expression is induced and that the siRNA-mediated silencing of CIP2A remains efficient. CIP2A silencing resulted in differential expression of 136 genes (false discovery rate [FDR] <0.05) in cells differentiated to the Th17 direction for 24 h (Figure 3A and Table S1). Fifty of these genes have been shown to be differentially expressed (DE) during Th17 differentiation (Tuomela et al., 2016) (Table S2). Consistent with the increase in IL17 expression, many genes encoding Th17-related TFs, e.g., RORA, RORC, and MAF, were upregulated in the CIP2A-deficient Th17 cells. In contrast, several genes that repress Th17 cell differentiation (e.g., interferon-γ, IL-2, and IRF8) were downregulated in CIP2A-deficient conditions suggesting CIP2A negatively regulates Th17 differentiation.
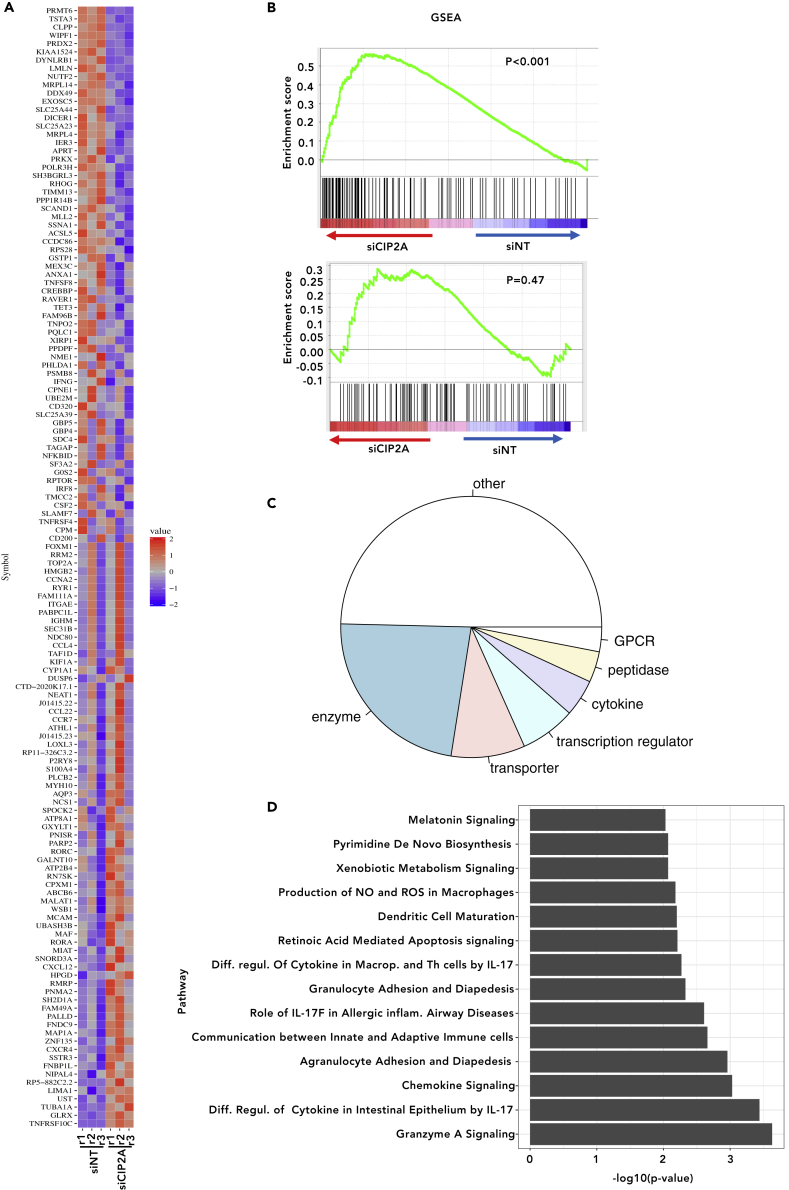
Figure 3.
Several Genes Are Affected upon CIP2A Silencing in Human Th17 Cells
(A) Genes DE between CIP2A-silenced (siCIP2A1) or control (siNT) Th17 cells (24 h) shown as heatmap. The plot is of Z score calculated from RPKM (Reads Per Kilobase of transcript, per Million mapped reads) values. First three columns represent data from siNT, and the last three columns are from siCIP2A-treated cells. “r1,” “r2,” and “r3” denote three replicates.
(B) Enrichment of Th17 signature genes (24 h log2FC > 2) (Tuomela et al., 2016) is shown in the top panel. Panel on the bottom shows lack of enrichment of iTreg genes. Each vertical line in the plot shows one gene. Lines toward the red indicate genes that are enriched in siCIP2A samples, and those toward the blue indicate enrichment in siNT samples. For details, see Transparent Methods section.
(C) Pie chart showing the distribution of different RNA species among DE genes in the siCIP2A1-treated Th17 cells.
(D) IPA analysis showing enriched pathways among the genes DE in CIP2A-deficient human Th17 cells.
Gene set enrichment analysis (Subramanian et al., 2005) was performed to investigate whether there was a global increase in the expression of Th17 cell signature genes upon CIP2A silencing. The Th17 cell signature genes were defined from our earlier study as the top upregulated (24 h, FDR <0.05, log2[FC] > 2) genes in Th17 conditions (Tuomela et al., 2016). The majority of Th17 cell signature genes were more abundant in the CIP2A-deficient samples than in the control samples (Figure 3B upper panel), indicating a general upregulation of Th17 cell signature genes in CIP2A-deficient Th17 cells. In comparison, the corresponding induced regulatory T cell (iTreg) gene set (24 h, FDR <0.05, log2[FC] > 2) was not enriched (Figure 3B lower panel).
Ingenuity pathway analysis (IPA) of the CIP2A-silenced Th17 cell gene expression data was used to gain an overview of the pathways enriched among the DE genes observed in CIP2A-deficient human Th17 cells and the cellular location of different RNA species among the DE genes. Enzymes were the most enriched class among the DE genes, followed by transporters and transcriptional regulators (Figure 3C). Interestingly, among the most enriched pathways were differential regulation of cytokines by IL-17 in epithelia, macrophages, and Th cells, as well as IL-17 regulation in inflammatory diseases, supporting a role for CIP2A in the regulation of signaling involved in IL-17 expression (Figure 3D).
CIP2A Controls TH17 Differentiation by Regulating STAT3 Phosphorylation
To identify the key upstream regulators of the observed RNA-seq transcriptional signature in CIP2A-deficient Th17 cells, we used “upstream regulators” predictor tool from IPA. The tool predicts the key upstream TF or cytokines that may not be upregulated at the RNA level but may have increased activity because of posttranslational modification, e.g., phosphorylation. Interestingly, STAT3, a known positive regulator of Th17 cell differentiation (Chen et al., 2006, Durant et al., 2010, Tripathi et al., 2017), was one of the key upstream regulators predicted to be activated (Z score > 2) in CIP2A-deficient cells (Figure 4A). Furthermore, Transcription Factor Binding Sites enrichment analysis on the promoters of DE genes revealed enrichment of several Th17-related factors including STAT3 (Table S3). Based on these results, we hypothesized that enhanced Th17 cell differentiation upon CIP2A deficiency could be due to increased STAT3 activity.
Figure 4.
CIP2A Regulates Th17 Differentiation by Modulating STAT3 Phosphorylation
(A) Upstream regulators identified using IPA analysis of human DE genes. Z score < −2 or >2 indicate predicted negative and positive upstream regulators, respectively.
(B) Experimental design for studying STAT3 phosphorylation (Y705) in CIP2A-silenced human Th17 cells. Human CD4+ T cells were first activated under Th17 culturing conditions for 48 h. Cells were then harvested and nucleofected with NT or CIP2A siRNA followed by a 48-h rest and then reactivated in the presence of Th17-polarizing cytokines for 15–60 min.
(C) Analysis of STAT3 phosphorylation (Y705) by flow cytometry in cells treated with NT or CIP2A siRNA using culturing conditions as in Figure 4B. Acquisition of the stained cells was made with an LSRII flow cytometer, and data analysis was performed by using either Flowing or FlowJo software (tree star).
(D) Boxplot to represent median fluorescence intensity (MFI) quantification of STAT3 phosphorylation (Y705) in three independent experiments by flow cytometry as shown in Figure 4C.
(E) Analysis of STAT3 phosphorylation (Y705 and S727) by WB. Total STAT3, CIP2A, and beta-actin were detected on the same blot. Quantification was performed using ImageJ software and shown in the form of boxplot for Y705 pSTAT3 above the representative WB. (D and E) Significance obtained in Student's t test (two-tailed unpaired); ∗p < 0.05, ∗∗p < 0.01.
To test whether CIP2A silencing leads to changes in STAT3 activity, levels of STAT3 phosphorylation were determined. Naive CD4+ T cells were activated for 48 h in Th17-polarizing conditions to ensure the expression of CIP2A, and siRNA was then used to silence CIP2A expression. As STAT3 phosphorylation occurs early during Th17 differentiation, cells were re-activated for 15 min under Th17 condition and STAT3 phosphorylation was monitored using intracellular flow cytometry staining and western blotting (WB) (Figure 4B). STAT3 phosphorylation (Y705) was indeed consistently higher in cells depleted of CIP2A by all the three CIP2A siRNAs when compared with controls both in terms of percentage (Figure 4C) and median florescence intensity (Figure 4D). Higher phosphorylation of STAT3 was also observed at serine-727 (Figure S3A). To test whether the increase in pSTAT3 is also sustained for longer duration, we performed similar experiments to those described in Figure 4B, except that the additional time points at 30 and 360 min were included. Interestingly, not only was there an increase in STAT3 phosphorylation but also the phosphorylation was sustained longer in CIP2A-silenced samples (Figure 4E). Besides Tyrosine-705 phosphorylation, serine-727 phosphorylation was also sustained in the CIP2A-deficient Th17 cells (Figure 4E). To test whether the increase in the phosphorylation in CIP2A-deficient cells was specific to STAT3, we measured the phosphorylation of STAT5. As STAT5 is phosphorylated in response to IL-2 stimulation, we first cultured the naive cells as shown in Figure 4B, except that the cells were differentiated under iTreg conditions wherein the cells were activated in the presence of IL-2, all-trans retinoic acid, and transforming growth factor-β. No differences in STAT5 phosphorylation were observed, indicating that the changes were specific to STAT3 (Figures S3B and S3C). Taken together, these results suggest that CIP2A limits Th17 differentiation by modulating STAT3 phosphorylation.
AGK Potentiates STAT3 Phosphorylation in the Absence OF CIP2A
We hypothesized that the increase in pSTAT3 in CIP2A-deficient cells is due to changes in the interacting partners of STAT3 in the presence and the absence of CIP2A. To test this hypothesis, we performed immunoprecipitation (IP) of pSTAT3 (Y705) followed by LC-MS/MS of whole-cell lysates to identify proteins that interact with pSTAT3 in CIP2A-sufficient or CIP2A-deficient conditions in human Th17 cells. Cells were first activated for 48 h under Th17 condition. The cells were then nucleofected, rested, and re-activated for 15 min in Th17-polarizing condition, and pSTAT3 was measured by WB (Figure S3D). STAT3 was enriched many folds in IP samples when compared with control (IgG) samples (Figure 5A). Interestingly, pSTAT3 was higher in siCIP2A when compared with siNT, confirming the results shown in Figures 4C and 4E. Proteins interacting with pSTAT3 were pulled down together with the pSTAT3 and identified by MS analysis. STAT3 was the most enriched protein in MS analysis of both CIP2A-sufficient and CIP2A-deficient conditions, confirming that the IP and MS were successful. Following statistical analysis of the MS data from the IP samples, 335 of the identified proteins were discerned to interact with pSTAT3 in the CIP2A-sufficient or CIP2A-deficient conditions and were distinct from the IgG-only baits (Table S4). The detected interacting proteins were filtered to include those differentially abundant between prey and bait (p < 0.05, paired t test) and passing a Significance Analysis of Interactome (SAINT) probability score of >0.7 (Emani et al., 2015). Among these, 217 proteins interacted with pSTAT3 both in CIP2A-sufficient and CIP2A-deficient conditions (Figures 5B and 5C), whereas the interaction of 69 and 49 proteins were distinguished in the CIP2A-sufficient and CIP2A-deficient conditions, respectively (Table S4).
Figure 5.
Phospho-STAT3 (Y705) Interactome in CIP2A-Silenced Human Th17 Cells
(A) Western blot (WB) analysis of pSTAT3 (Y705) immunoprecipitation (IP) in CIP2A-silenced and control Th17 cells (cultured as indicated in Figure 4B). Representative IP WB, input, flow-through (FT), IgG control IP, and pSTAT3 IP lanes are shown.
(B) A Venn diagram of the proteins interacting with pSTAT3 (Y705) under CIP2A-deficient (siCIP2A) or CIP2A-sufficient (siNT) Th17 cells.
(C) Heatmap showing top 50 proteins interacting with pSTAT3 (Y705) in control and CIP2A-silenced Th17 cells in four replicate experiments. Log2-transformed intensity values are plotted.
(D) Network STAT3-interacting proteins generated using STRING database and visualized using the Cytoscape software. The nodes in the network were clustered using the Markov clustering algorithm. The most enriched GO biological process term is shown for each cluster with four or more members. The strength of interaction with STAT3 in the different conditions is indicated by the coloring of the nodes. Blue color corresponds to interactions with STAT3 only in the siNT condition, red color indicates interactions with STAT3 only in the siCIP2A condition, and white corresponds to interactions of equal strength in both conditions.
The STAT3 interactome data were analyzed with Gene Ontology (GO) and network analysis tools to gain an overview of the biological processes associated with the proteins through which STAT3 mediates its function. STAT3-interacting proteins associated with both the CIP2A-sufficient and CIP2A-deficient conditions were selected for the network analysis. A network was constructed using the STRING database (Szklarczyk et al., 2017) to gather the known interactions between the proteins. The resulting network was further visualized with Cytoscape (Shannon et al., 2003) and enriched biological processes were identified using DAVID (Huang et al., 2009a, Huang et al., 2009b) and PANTHER (Mi et al., 2013, Mi et al., 2017), revealing RNA processing, immune response, and cell adhesion as the processes most frequently linked with the STAT3-associated proteins (Figure 5D). Collectively, these results suggest that STAT3 is involved in multiple steps, ranging from RNA production to the production of a functional protein and in cell signaling.
Further analysis of pSTAT3 interactors in IPA revealed enrichment of CD28 signaling in Th cells in CIP2A-sufficient condition (Figures S4A and S4B). Conversely, protein kinase A signaling was enriched among pSTAT3 interactors only under CIP2A-deficient conditions. In terms of their organellar associations, the largest proportion of the pSTAT3 interactors were cytoplasmic, followed by nuclear and plasma membrane, both under CIP2A-sufficient and CIP2A-deficient conditions (Figure S4C). Functionally, the largest proportion of pSTAT3 interactors belonged to “enzymes,” followed by transcription regulators and transporters (Figure S4D).
To identify STAT3-interacting partners associated with the increase in pSTAT3 observed in CIP2A-deficient cells, we searched for proteins preferentially interacting with STAT3 either in CIP2A-silenced or control Th17 cells. Interestingly, a significantly enhanced interaction was detected between a lipid kinase AGK and STAT3 in CIP2A-silenced cells (Table S4) when compared with control cells. These results were validated in independent co-IP experiments (Figure 6A). Furthermore, using confocal microscopy, we detected a significantly higher co-localization of AGK and pSTAT3 in CIP2A-deficient human Th17 cells than in control cells (Figure 6B). Notably, AGK was also identified as a CIP2A-interacting protein in a reciprocal CIP2A IP experiment (Figure 6C left). The CIP2A-AGK interaction was further confirmed by targeted selected reaction monitoring-based MS analysis of CIP2A IP samples (Figure 6C right). It has been reported that AGK directly interacts with STAT3 in cancer cells and promotes its phosphorylation by JAKs (Chen et al., 2013). Silencing of AGK decreased pSTAT3 (Y705) levels, whereas its overexpression led to an increase in pSTAT3 (Y705) levels (Chen et al., 2013). We confirmed that indeed AGK-pSTAT3 interaction is enhanced in the absence of CIP2A. We tested if AGK silencing will bring back the increased phosphorylation of STAT3 in CIP2A-deficient Th17 cells. First, we efficiently silenced AGK using three different siRNAs (Figure 6D). STAT3 levels were unaffected, as reported earlier in epithelial cells (Chen et al., 2013), whereas AGK silencing significantly reduced pSTAT3 in Th17 cells (Figure 6D). Furthermore, depletion of AGK in CIP2A-silenced cells was able to neutralize the enhanced pSTAT3 levels as measured both by flow cytometry (Figures 6E and 6F) and WB analysis (Figure 6G), as well as enhanced IL-17 production (Figure 6H). Taken together these data suggest that CIP2A interacts with AGK, regulates the interaction between AGK and STAT3, and thereby controls the phosphorylation of STAT3 in Th17 cells.
Figure 6.
AGK Potentiates STAT3 Phosphorylation in the Absence of CIP2A
(A) pSTAT3 IP experiment showing its interaction with AGK in CIP2A-silenced and control Th17 cells. Quantification performed by ImageJ and shown in the form of bar graph of the WB from four replicate experiments. Statistics by Student's t test, two-tailed paired, ∗p < 0.05. Error bars represent SEM in the figure.
(B) Representative confocal images of three replicates for studying co-localization of pSTAT3 (Y705) and AGK in CIP2A-silenced (lower panel) and control (upper panel) Th17 cells by Zeiss LSM780 confocal microscope. Cells were stained for endogenous pSTAT3 (green), AGK (magenta), and nuclei (DAPI). Scale bar, 7 μm. Pearson's correlation coefficient (PCC) for data on the right plotted as boxplot and determined by ImageJ software cloloc2 plugin (n > 30 cells). Statistics by Student's t test, two-tailed paired, ∗∗p < 0.01.
(C) The left panel shows the WB analysis of co-IP of AGK and CIP2A by anti-CIP2A pull-down in human Th17 cells 72 h post cell activation. In the representative blot of two experiments, input, IgG control IP, and CIP2A IP lanes are shown. The right panel shows selected reaction monitoring targeted mass spectrometry showing interaction of AGK and CIP2A in 72 h polarized Th17. Averaged results from three replicates are presented in the form of a boxplot. Statistical significance was determined using a two-tailed paired Student's t test; ∗∗∗p < 0.001. The error bars represent 95% confidence interval.
(D) Representative WB of two replicates showing AGK silencing by three individual AGK siRNAs. The effect of siRNA-mediated AGK depletion on pSTAT3 (Y705) expression in 15-min reactivated Th17 cells, prepared as described in Figure 4B, is also shown. Histone 2B was used as a loading control.
(E and F) Flow cytometry analysis of pSTAT3 in control, CIP2A-silenced, and CIP2A/AGK double-silenced human Th17 cells shown as overlapping histograms (E) and bar charts of MFI from four biological replicates (F). Statistics by a two-tailed paired Student's t test, ∗∗p < 0.01. The error bars represent SEM in the figure.
(G) A representative WB analysis of four experiments showing expression of pSTAT3 (Y705) in Th17 cells treated with siNT, siCIP2A, or siCIP2A + siAGK siRNAs. Cells were cultured and nucleofected as described in Figure 4B.
(H) Secretion of IL-17A (pg/mL) measured by Luminex at 72 h following cell activation in Th17 cells treated with siNT, siCIP2A, or siCIP2A + siAGK siRNAs. Statistics by a two-tailed paired Student's t test, ∗∗p < 0.01. The error bars represent SEM in the figure.
Regulation of TH17 Differentiation by CIP2A and by Inhibition of PP2A Catalytic Phosphatase Activity
In mouse T cells, overexpression of PP2A catalytic subunit (PP2Ac) was shown to facilitate the transcription of pro-inflammatory genes including IL17A (Apostolidis et al., 2013). In addition, PP2Ac was recently identified as an essential regulator of Th17 differentiation. T cell-specific deletion of PP2Ac (Ppp2ca) resulted in impaired Th17 differentiation and rendered mice resistance to myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (Xu et al., 2019). Thereby, the results that both CIP2A and PP2A inhibit IL-17 expression in humans could be reconciled by the current model that CIP2A does not regulate catalytic PP2Ac subunit phosphatase activity, but more selectively modulates PP2A/B56 complex substrate recognition (Junttila et al., 2007, Wang et al., 2017).
To further study this, we directly compared the role of CIP2A and PP2Ac catalytic activity in regulation of IL-17 gene expression. First, we silenced the PP2A-A subunit, which is a scaffold for PP2A complex and essential for PP2A activity, using two different concentrations of the siRNA-targeting PP2A-A subunit (Figures 7A and S5A). PP2A-A silencing led to a significant increase in IL-17 expression both at RNA (Figure 7B) and protein (Figure 7C) levels. Furthermore, we modulated the activity of PP2A either by a chemical inhibitor or activator, and measured IL-17 expression at RNA and protein levels. Serine/threonine phosphatase inhibitor okadaic acid (OA) was used to inhibit PP2A in Th17 cells. The applied concentration of 10 nM was selected to achieve relative selectivity toward PP2A over PP1, PP4, and PP6 (Apostolidis et al., 2013). This concentration was well tolerated by T cells (Figure S5B). Similar to siRNA-mediated inhibition of PP2A-A, treatment of cells with OA led to increased production of IL-17 (Figures 7D and 7E). Furthermore, treatment with FTY720 (2-amino-2-[2-(4-octylphenyl) ethyl] propane-1,3-diol; Fingolimod, Novartis), which directly activates PP2Ac via blocking binding of PP2A inhibitor (I2PP2A/SET) (Saddoughi et al., 2013, Switzer et al., 2011), led to reduced IL-17 levels (Figures 7F and 7G). Together, these results demonstrate opposite outcomes by direct modulation of PP2Ac catalytic activity or by B56-selective modulation of PP2A via CIP2A. This in turn provides a unique opportunity for immunomodulation, which cannot be predicted from the effects induced by direct manipulation of PP2A catalytic activity toward presumably all PP2A-regulated targets.
Figure 7.
PP2A Inhibition Enhances IL-17A Production in Human Th17 Cells
(A) Representative WB analysis of PP2A-silenced Th17 cells. Two different concentrations (3 and 6 μg) of PP2A siRNA were used to deplete PP2A in human Th17 cells. Beta-actin was used as loading control.
(B and C) TaqMan PCR analysis of IL-17A expression (B) and Luminex detection of IL-17A protein secretion (C) in PP2A-silenced Th17 cells where each point represents individual replicate. PP2A was silenced as described in (A). Protein measurements of the Luminex analysis was normalized with the sample cell number detected with flow cytometry. Expression at RNA level (dCt) was measured relative to EF1-alpha. Statistics by a two-tailed paired Student's t test; ∗p < 0.05, ∗∗p < 0.01.
(D and E) PP2A inhibition by okadiac acid (OA) followed by TaqMan PCR analysis of IL-17A expression (D) or Luminex analysis for measuring IL-17 secretion from culture supernatant from three replicate experiments (E). OA was added at the start of the culture. Statistics by a two-tailed paired Student's t test; ∗p < 0.05, ∗∗∗p < 0.001.
(F and G) PP2A activation for 72 h in cells polarizing toward Th17 by FTY 720 in three replicates. IL-17A mRNA expression and IL-17A protein secretion were detected by TaqMan PCR (F) and Luminex (G) analysis, respectively. In TaqMan PCR analysis, dCT was calculated relative to EF1-alpha, and in Luminex, OA was used as a control. Statistics by a two-tailed unpaired Student's t test; ∗∗p < 0.01, ∗∗∗p < 0.001.
Discussion
CIP2A is upregulated in a variety of human cancers. Its inhibitory function toward PP2A/B56 thereby stabilizes the oncoprotein MYC in cancer cells (Junttila et al., 2007, Khanna et al., 2013, De et al., 2014). Here, we delineate previously unappreciated immune regulatory role of CIP2A and demonstrate that it acts as a negative regulator of human and mouse Th17 cell differentiation. Our data revealed that CIP2A silencing results in increased expression of proinflammatory cytokine IL-17 at both the protein and RNA levels. Consistent with increased IL17 in CIP2A-deficient cells, IRF8, a negative regulator of Th17 cell differentiation (Ouyang et al., 2011), was downregulated upon CIP2A silencing (Figure 3A). Furthermore, several Th17-related TFs were upregulated including RORC, RORA, and MAF (Figure 3A).
Moreover, CIP2A deficiency led to enhanced expression of factors important for Th17 differentiation, including phosphorylation of STAT3. Altered signaling of STATs or their negative regulators can lead to pathological conditions such as chronic inflammation, inadequate immune response, or cancer (Grivennikov et al., 2010, Multhoff et al., 2011, Landskron et al., 2014). Enhanced STAT3 phosphorylation in CIP2A-silenced human Th17 cells may explain upregulation of Th17-related genes in human and mouse CIP2A-deficient Th17 cells. STAT3 is a key upstream factor driving Th17 differentiation. It also plays an important role in many other types of cells including cancer cells. In spite of these, STAT3 interactome has not been studied previously in Th17 cells. In this study, we delineated pSTAT3 interactome in CIP2A-silenced and control cells. Our data revealed many new protein interactions with STAT3 including ubiquitin ligase TRIM21 and kinase AGK. The interactome of pSTAT3 identified in this study can be used as a resource for future studies aiming at further understanding of STAT3 functions.
AGK preferentially interacts with pSTAT3 in CIP2A-deficient cells. In cancer cells, AGK facilitates STAT3 phosphorylation by inhibiting autoinhibitory JH2 domain on JAK2 to phosphorylate STAT3 (Chen et al., 2013). Similar to cancer cells, AGK silencing resulted in reduced STAT3 phosphorylation in Th17 cells. We demonstrated that in CIP2A-silenced cells, increased interaction between AGK and STAT3 results in enhanced pSTAT3. Thus, we propose that CIP2A regulates the strength and duration of STAT3 phosphorylation in Th17 cells by regulating AGK-STAT3 interaction in Th17 cells.
TRIM21 deficiency in mice leads to enhanced production of the pro-inflammatory cytokines, IL-6, IL-12, IL-23, and IL-17 (Espinosa et al., 2009) as well as tissue inflammation and systemic autoimmunity through the IL-23-Th17 pathway (Espinosa et al., 2009, Chikuma et al., 2012, Ahn et al., 2017). Interestingly, TRIM21 interacts with IRF8 (Yang et al., 2009, Yoshimi et al., 2012, Lazzari et al., 2014), a negative regulator of Th17 differentiation (Ouyang et al., 2011) and marks it for degradation by proteasomes (Yang et al., 2009, Yoshimi et al., 2012, Lazzari et al., 2014). Further studies are required to clarify if the reduced interaction between STAT3 and TRIM21, as well as reduced IRF8 expression in CIP2A-silenced human Th17 cells, contributes to enhanced Th17 differentiation.
CIP2A was initially identified as a PP2A-interacting protein (Junttila et al., 2007). In agreement with this, in a parallel study, we found CIP2A to interact with PP2A subunits in Th17 cells (Khan et al., 2020). Previously, it was shown that overexpression of PP2A catalytic subunit in mice led to increased IL-17 production (Apostolidis et al., 2013). Recently, impaired Th17 differentiation was demonstrated in mice with PP2A catalytic subunit gene (Ppp2ca) KO specific to T cells (Xu et al., 2019). Contrary to the findings in mouse, we observed increased IL-17 production upon PP2A inhibition by siRNA and OA. Furthermore, treatment with PP2A activator FTY720 led to reduced IL-17 levels in human Th17 cells. Interestingly, FTY720 has also been used as an immunosuppressant drug for the treatment of patients with multiple sclerosis. Oral treatment with FTY720 reduced the number of IL-17-producing Th17 cells in peripheral blood when compared with placebo-treated patients (Mehling et al., 2008, Brinkmann, 2009, Chun and Hartung, 2010). Thus, the effect of PP2A inhibition on Th17 cells appears to be opposite in human and mouse. Other species-specific examples of gene function are known in the literature, such as SATB1. SATB1-deficient human T cells upon Th17 differentiation produced increased IL-17 (Tripathi et al., 2019), whereas in mouse, SATB1 deficiency led to reduced IL-17 levels (Ciofani et al., 2012). In addition, both inhibition of total catalytic activity of PP2A and inhibition of CIP2A resulted in IL-17 induction, highlighting the substrate selectivity of CIP2A toward PP2A/B56 (Wang et al., 2017). Thus, these results emphasize the significance of PP2A-mediated responses in human Th17 cells.
Using mouse cells, in this study we show that the function of CIP2A in Th17 regulation is conserved in mice and the effect on Th17 differentiation is more pronounced when the knockdown of CIP2A is more efficient. Nevertheless, we focused on human cells because the details of CIP2A function in human and mouse system may not be similar. Furthermore, the in vivo results in mouse regarding regulation of Th17 differentiation by PP2A suggest exactly the opposite of what we found in human cells. Thus, a better understanding of the regulation of Th17 cells by CIP2A may provide new approaches for therapeutic intervention in autoimmune and inflammatory diseases as well as in cancer. Overall, our results indicate that CIP2A expression influences several mechanisms important for Th17 response and associated regulation of the immune system that could provide useful insight for the use of CIP2A targeting in cancer therapy.
Limitations of the Study
One of the limitations of the study is the lack of in vivo data. It remains to be seen if the increase in IL17 expression upon CIP2A silencing leads to the increased propensity of IL-17-mediated inflammatory disease. However, there is a growing consensus that human and mouse systems are different at multiple levels. Indeed, we showed earlier that there is only a little overlap between human and mouse Th17 cell differentiation (Tuomela et al., 2016, Tripathi et al., 2019). Therefore, caution is required in interpreting the results from mouse versus human as fundamental regulatory differences may exist between the two species. Another limitation of the study is that the mechanism of AGK action on STAT3 phosphorylation was not completely delineated. As pointed out earlier in the discussion, in cancer cells, it was demonstrated that AGK removes an autoinhibitory domain of JAK2 to facilitate STAT3 phosphorylation (Chen et al., 2013). We showed that (1) AGK interacts with STAT3, (2) silencing of AGK results in reduced STAT3 phosphorylation, (3) AGK interacts with pSTAT3, and (4) STAT3 phosphorylation is reduced in AGK-depleted Th17 cells suggesting that a similar mechanism operates in Th17 cells. However, further experiments are needed to address the detailed mechanism.
Ethical Approval
Ethics Committee of Hospital District of Southwest Finland approved usage of the blood of unknown donors. The animal cells were used according to the university animal welfare guidelines.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the staff of Turku University Hospital, Department of Obstetrics and Gynecology, Maternity Ward, for the cord blood collection. We also thank Marjo Hakkarainen and Sarita Heinonen for excellent technical help. We also duly acknowledge core facilities at the department, namely, the Finnish Functional Genomics Centre (FFGC), the Proteomics Facility, and Cell Imaging Core (CIC) Facility supported by BioCenter Finland. The Finnish Centre for Scientific Computing (CSC) is duly acknowledged for their efficient servers and their resources in data analysis. M.M.K. was supported by University of Turku graduate school on Turku Doctoral Programme of Molecular Medicine (TuDMM) as well as a central grant from Finnish Cultural Foundation. R.L. was supported by the Academy of Finland, AoF, Centre of Excellence in Molecular Systems Immunology and Physiology Research (2012-2017) grant 250114; by the AoF grants 292335, 294337, 292482, and 31444; by grants from the JDRF; the Sigrid Jusélius Foundation; and the Finnish Cancer Foundation. Z.C. was funded by Academy of Finland grant no. 258313, and J.W. was funded by Sigrid Jusélius Foundation.
Author Contributions
M.M.K. designed and performed the experiments, analyzed data, prepared figures, and wrote the manuscript; U.U. designed experiments, analyzed data, prepared figures, and wrote the manuscript; M.H.K. designed and performed the experiments, analyzed data, and prepared figures; L.K. analyzed and prepared figures for RNA-seq data; T.V. performed network and enrichment analysis of STAT interactome and wrote related legends and methods in manuscript; R.M. analyzed the proteomics data and contributed to writing the manuscript; S.D.B. performed the proteomics experiments data analysis; E.K. performed experiments; O.R. designed the experiments, analyzed the data, and wrote the manuscript; Z.C. designed experiments and provided scientific input, expertise, and feedback on the manuscript; L.L.E. supervised T.V. and provided scientific expertise and feedback on the manuscript; J.W. provided scientific input, expertise, mice, and reagents and edited the manuscript; R.L. designed and supervised the study and wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100947.
Data and Code Availability
The accession number for the RNA-seq data shown in this paper is GSE118094. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Vizcaíno et al., 2016) partner repository with the dataset identifier PXD010612.
Supplemental Information
The proteins associated with the interactome are listed together with the data from the MaxQuant results (unique peptides, sequence coverage, intensity, and Andromeda score), representation in the CRAPome, and SAINT analysis.
For biological process the FAT terms were used and provide an overview of the association of the proteins in Figure 5. These terms are listed together with an indication of their occurrence cluster by cluster in terms of number of proteins and proportion of associated nodes.
The enrichment of biological processes was calculated relative to a background of proteins reported from a proteomic characterization of Th17 cells (Tripathi et al., 2019).
These data are represented in Figure S4.
References
- Ahn Y., Hwang J.H., Zheng Z., Bang D., Kim D.Y. Enhancement of Th1/Th17 inflammation by TRIM21 in Behçet’s disease. Sci. Rep. 2017;7:3018. doi: 10.1038/s41598-017-03251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Rauen T., Hedrich C.M., Tsokos G.C., Crispín J.C. Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling. J. Biol. Chem. 2013;288:26775–26784. doi: 10.1074/jbc.M113.483743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V. FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol. 2009;158:1173. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Laurence A., Kanno Y., Pacher-Zavisin M., Zhu B.M., Tato C., Yoshimura A., Hennighausen L., O'Shea J.J. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Ying Z., Lin X., Lin H., Wu J., Li M., Song L. Acylglycerol kinase augments JAK2/STAT3 signaling in esophageal squamous cells. J. Clin. Invest. 2013;123:2576–2589. doi: 10.1172/JCI68143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikuma S., Suita N., Okazaki I.M., Shibayama S., Honjo T. TRIM28 prevents autoinflammatory T cell development in vivo. Nat. Immunol. 2012;13:596–603. doi: 10.1038/ni.2293. [DOI] [PubMed] [Google Scholar]
- Chun J., Hartung H.-P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 2010;33:91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M., Madar A Galan C., Sellars M., Mace K., Pauli F., Agarwal A., Huang W., Parkhurst C.N., Muratet M., Newberry K.M. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côme C., Cvrljevic A., Khan M.M., Treise I., Adler T., Aguilar-Pimentel J.A., Au-Yeung B., Sittig E., Laajala T.D., Chen Y. CIP2A promotes T-cell activation and immune response to Listeria monocytogenes infection. PLoS One. 2016;11:1–18. doi: 10.1371/journal.pone.0152996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De P., Carlson J., Leyland-Jones B., Dey N. Oncogenic nexus of cancerous inhibitor of protein phosphatase 2A (CIP2A): an oncoprotein with many hands. Oncotarget. 2014;5:4581–4602. doi: 10.18632/oncotarget.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant L., Watford W.T., Ramos H.L., Laurence A., Vahedi G., Wei L., Takahashi H., Sun H.W., Kanno Y., Powrie F., O'Shea J.J. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emani M.R., Närvä E., Stubb A., Chakroborty D., Viitala M., Rokka A., Rahkonen N., Moulder R., Denessiouk K., Trokovic R. The L1TD1 protein interactome reveals the importance of post-transcriptional regulation in human pluripotency. Stem Cell Rep. 2015;4:519–528. doi: 10.1016/j.stemcr.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa A., Dardalhon V., Brauner S., Ambrosi A., Higgs R., Quintana F.J., Sjöstrand M., Eloranta M.L., Ní Gabhann J., Winqvist O. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23–Th17 pathway. J. Exp. Med. 2009;206:1661–1671. doi: 10.1084/jem.20090585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen S.L., Hernández-Santos N., Peterson A.C. IL-17 signaling in host defense against Candida albicans. Immunol. Res. 2011;50:181–187. doi: 10.1007/s12026-011-8226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huh J.R., Leung M.W., Huang P., Ryan D.A., Krout M.R., Malapaka R.R., Chow J., Manel N., Ciofani M., Kim S.V. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janghorban M., Farrell A.S., Allen-Petersen B.L., Pelz C., Daniel C.J., Oddo J., Langer E.M., Christensen D.J., Sears R.C. Targeting c-MYC by antagonizing PP2A inhibitors in breast cancer. Proc. Natl. Acad. Sci. U S A. 2014;111:9157–9162. doi: 10.1073/pnas.1317630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila M.R., Puustinen P., Niemelä M., Ahola R., Arnold H., Böttzauw T., Ala-aho R., Nielsen C., Ivaska J., Taya Y. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Kanduri K., Tripathi S., Larjo A., Mannerström H., Ullah U., Lund R., Hawkins R.D., Ren B., Lähdesmäki H., Lahesmaa R. Identification of global regulators of T-helper cell lineage specification. Genome Med. 2015;7:122. doi: 10.1186/s13073-015-0237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauko O., Westermarck J. Non-genomic mechanisms of protein phosphatase 2A (PP2A) regulation in cancer. Int. J. Biochem. Cell Biol. 2018;96:157–164. doi: 10.1016/j.biocel.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Khan M.M., Välikangas T., Khan M.H., Moulder R., Ullah U., Bhosale S.D., Komsi E., Butt U., Westermarck J., Elo L.L., Lahesmaa R. Protein interactome of the cancerous inhibitor of protein phosphatase 2A (CIP2A) in Th17 cells. Curr. Res. Immunol. 2020 doi: 10.1016/j.crimmu.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A., Pimanda J.E., Westermarck J. Cancerous inhibitor of protein phosphatase 2a, an emerging human oncoprotein and a potential cancer therapy target. Cancer Res. 2013;73:6548–6553. doi: 10.1158/0008-5472.CAN-13-1994. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M., Hafler D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013;25:305–312. doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M., Manzel A., Titze J., Kvakan H., Yosef N., Linker R.A., Muller D.N., Hafler D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron G., De la Fuente M., Thuwajit P., Thuwajit C., Hermoso M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzari E., Korczeniewska J., Ní Gabhann J., Smith S., Barnes B.J., Jefferies C.A. TRIpartite motif 21 (TRIM21) differentially regulates the stability of interferon regulatory factor 5 (IRF5) isoforms. PLoS One. 2014;9:e103609. doi: 10.1371/journal.pone.0103609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Collins M., Kuchroo V.K. Unexpected targets and triggers of autoimmunity. J. Clin. Immunol. 2014;34 Suppl 1(S1):S56–S60. doi: 10.1007/s10875-014-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lønnberg A.S., Zachariae C., Skov L. Targeting of interleukin-17 in the treatment of psoriasis. Clin. Cosmet. Investig. Dermatol. 2014;7:251–259. doi: 10.2147/CCID.S67534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C.M., Milani M., Butterworth M., Carmell N., Scott L.J., Clark R.E., Cohen G.M., Varadarajan S. High CIP2A levels correlate with an antiapoptotic phenotype that can be overcome by targeting BCL-XL in chronic myeloid leukemia. Leukemia. 2016;30:1273–1281. doi: 10.1038/leu.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling M., Brinkmann V., Antel J., Bar-Or A., Goebels N., Vedrine C., Kristofic C., Kuhle J., Lindberg R.L., Kappos L. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71:1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- Meyer Zu Horste G., Wu C., Wang C., Cong L., Pawlak M., Lee Y., Elyaman W., Xiao S., Regev A., Kuchroo V.K. RBPJ controls development of pathogenic Th17 cells by regulating IL-23 receptor expression. Cell Rep. 2016;16:392–404. doi: 10.1016/j.celrep.2016.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Muruganujan A., Casagrande J.T., Thomas P.D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Huang X., Muruganujan A., Tang H., Mills C., Kang D., Thomas P.D. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017;45:D183–D189. doi: 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G., Molls M., Radons J. Chronic inflammation in cancer development. Front. Immunol. 2011;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X., Zhang R., Yang J., Li Q., Qin L., Zhu C., Liu J., Ning H., Shin M.S., Gupta M. Transcription factor IRF8 directs a silencing programme for TH17 cell differentiation. Nat. Commun. 2011;2:314. doi: 10.1038/ncomms1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallai R., Bhaskar A., Barnett-Bernodat N., Gallo-Ebert C., Nickels J.T., Jr., Rice L.M. Cancerous inhibitor of protein phosphatase 2A promotes premature chromosome segregation and aneuploidy in prostate cancer cells through association with shugoshin. Tumor Biol. 2015;36:6067–6074. doi: 10.1007/s13277-015-3284-7. [DOI] [PubMed] [Google Scholar]
- Robinson K.M., Manni M.L., Biswas P.S., Alcorn J.F. Clinical consequences of targeting IL-17 and TH17 in autoimmune and allergic disorders. Curr. Allergy Asthma Rep. 2013;13:587–595. doi: 10.1007/s11882-013-0361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L. Immunity to fungal infections. Nat. Rev. Immunol. 2011;11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- Saddoughi S.A., Gencer S., Peterson Y.K., Ward K.E., Mukhopadhyay A., Oaks J., Bielawski J., Szulc Z.M., Thomas R.J., Selvam S.P. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol. Med. 2013;5:105–121. doi: 10.1002/emmm.201201283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shentu Y.-P., Huo Y., Feng X.L., Gilbert J., Zhang Q., Liuyang Z.Y., Wang X.L., Wang G., Zhou H., Wang X.C. CIP2A causes Tau/APP phosphorylation, synaptopathy, and memory deficits in alzheimer’s disease. Cell Rep. 2018;24:713–723. doi: 10.1016/j.celrep.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer C.H., Cheng R.Y.S., Vitek T.M., Christensen D.J., Wink D.A., Vitek M.P. Targeting SET/I2PP2A oncoprotein functions as a multi-pathway strategy for cancer therapy. Oncogene. 2011;30:2504–2513. doi: 10.1038/onc.2010.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S.K., Chen Z., Larjo A., Kanduri K., Nousiainen K., Äijo T., Ricaño-Ponce I., Hrdlickova B., Tuomela S., Laajala E. Genome-wide analysis of STAT3-mediated transcription during early human Th17 cell differentiation. Cell Rep. 2017;19:1888–1901. doi: 10.1016/j.celrep.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Tripathi S.K., Välikangas T., Shetty A., Khan M.M., Moulder R., Bhosale S.D., Komsi E., Salo V., De Albuquerque R.S., Rasool O. Quantitative proteomics reveals the dynamic protein landscape during initiation of human Th17 cell polarization. iScience. 2019;11:334–355. doi: 10.1016/j.isci.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomela S., Rautio S., Ahlfors H., Öling V., Salo V., Ullah U., Chen Z., Hämälistö S., Tripathi S.K., Äijö T. Comparative analysis of human and mouse transcriptomes of Th17 cell priming. Oncotarget. 2016;7:13416–13428. doi: 10.18632/oncotarget.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubaid Ullah U., Andrabi S.B.A., Tripathi S.K., Dirasantha O., Kanduri K., Rautio S., Gross C.C., Lehtimäki S., Bala K., Tuomisto J. Transcriptional repressor HIC1 contributes to suppressive function of human induced regulatory T cells. Cell Rep. 2018;22:2094–2106. doi: 10.1016/j.celrep.2018.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventelä S., Côme C., Mäkelä J.A., Hobbs R.M., Mannermaa L., Kallajoki M., Chan E.K., Pandolfi P.P., Toppari J., Westermarck J. CIP2A promotes proliferation of spermatogonial progenitor cells and spermatogenesis in mice. PLoS One. 2012;7:e33209. doi: 10.1371/journal.pone.0033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaíno J.A., Csordas A., Del-Toro N., Dianes J.A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016;44:D447–D456. doi: 10.1093/nar/gkv1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Okkeri J., Pavic K., Wang Z., Kauko O., Halonen T., Sarek G., Ojala P.M., Rao Z., Xu W., Westermarck J. Oncoprotein CIP2A is stabilized via interaction with tumor suppressor PP2A/B56. EMBO Rep. 2017;18:437–450. doi: 10.15252/embr.201642788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Yosef N., Thalhamer T., Zhu C., Xiao S., Kishi Y., Regev A., Kuchroo V.K. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Yosef N., Yang J., Wang Y., Zhou L., Zhu C., Wu C., Baloglu E., Schmidt D., Ramesh R. Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity. 2014;40:477–489. doi: 10.1016/j.immuni.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Wang X., Zhong B., Nurieva R.I., Ding S., Dong C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J. Biol. Chem. 2011;286:22707–22710. doi: 10.1074/jbc.C111.250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Jin X., Zheng M., Rohila D., Fu G., Wen Z., Lou J., Wu S., Sloan R., Wang L. Phosphatase PP2A is essential for TH17 differentiation. Proc. Natl. Acad. Sci. U S A. 2019;116:982–987. doi: 10.1073/pnas.1807484116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Shi H.X., Liu X.Y., Shan Y.F., Wei B., Chen S., Wang C. TRIM21 is essential to sustain IFN regulatory factor 3 activation during antiviral response. J. Immunol. 2009;182:3782–3792. doi: 10.4049/jimmunol.0803126. [DOI] [PubMed] [Google Scholar]
- Yang X., Zhang Y., Liu H., Lin Z. Cancerous inhibitor of PP2A silencing inhibits proliferation and promotes apoptosis in human multiple myeloma cells. Biomed. Res. Int. 2016;2016:6864135. doi: 10.1155/2016/6864135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef N., Shalek A.K., Gaublomme J.T., Jin H., Lee Y., Awasthi A., Wu C., Karwacz K., Xiao S., Jorgolli M. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 2013;496:461–468. doi: 10.1038/nature11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi R., Ishigatsubo Y., Ozato K. Autoantigen TRIM21/Ro52 as a possible target for treatment of systemic lupus erythematosus. Int. J. Rheumatol. 2012;2012:718237. doi: 10.1155/2012/718237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.-C., Hou D.R., Liu C.Y., Lin C.S., Shiau C.W., Cheng A.L., Chen K.F. Cancerous inhibitor of protein phosphatase 2A mediates bortezomib-induced autophagy in hepatocellular carcinoma independent of proteasome. PLoS One. 2013;8:e55705. doi: 10.1371/journal.pone.0055705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The proteins associated with the interactome are listed together with the data from the MaxQuant results (unique peptides, sequence coverage, intensity, and Andromeda score), representation in the CRAPome, and SAINT analysis.
For biological process the FAT terms were used and provide an overview of the association of the proteins in Figure 5. These terms are listed together with an indication of their occurrence cluster by cluster in terms of number of proteins and proportion of associated nodes.
The enrichment of biological processes was calculated relative to a background of proteins reported from a proteomic characterization of Th17 cells (Tripathi et al., 2019).
These data are represented in Figure S4.
Data Availability Statement
The accession number for the RNA-seq data shown in this paper is GSE118094. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Vizcaíno et al., 2016) partner repository with the dataset identifier PXD010612.