This secondary analysis of a randomized clinical trial assesses treatment outcomes in women with different biological types of breast cancer according to germline variant status of BRCA1/2 and non-BRCA1/2 breast cancer predisposition genes.
Key Points
Question
Is germline variant status of BRCA1/2 and non-BRCA1/2 breast cancer predisposition genes associated with higher response rates in patients enrolled in the GeparOcto trial?
Findings
In this secondary analysis of 914 patients included in a randomized clinical trial, women with triple-negative breast cancer with BRCA1/2 variants benefited most from both treatment regimens (paclitaxel and nonpegylated liposomal doxorubicin plus carboplatin, 74.3%; epirubicin, paclitaxel, and cyclophosphamide, 64.7%). A positive BRCA1/2 variant status also was associated with higher response rates in ERBB2-negative, hormone receptor–positive breast cancer.
Meaning
Effective chemotherapy for BRCA1/2-mutated triple-negative breast cancer is commonly suggested to be platinum based; sequential intense dose-dense epirubicin, paclitaxel, and cyclophosphamide appears to also be effective in these patients, though with a lower point estimate. Patients with ERBB2-negative, hormone receptor–positive breast cancer may benefit from BRCA1/2 testing prior to treatment.
Abstract
Importance
The GeparOcto randomized clinical trial compared the efficacy of 2 neoadjuvant breast cancer (BC) treatment regimens: sequential intense dose-dense epirubicin, paclitaxel, and cyclophosphamide (iddEPC) vs weekly paclitaxel and nonpegylated liposomal doxorubicin (PM) in patients with different biological BC subtypes. Patients with triple-negative BC (TNBC) randomized to the PM arm received additional carboplatin (PMCb). Overall, no difference in pathologic complete response (pCR) rates was observed between study arms. It remained elusive whether the germline variant status of BRCA1/2 and further BC predisposition genes are associated with treatment outcome.
Objective
To determine treatment outcome for BC according to germline variant status.
Design, Setting, and Participants
This retrospective biomarker study is a secondary analysis of the GeparOcto multicenter prospective randomized clinical trial conducted between December 2014 and June 2016. Genetic analyses assessing for variants in BRCA1/2 and 16 other BC predisposition genes in 914 of 945 women were performed at the Center for Familial Breast and Ovarian Cancer, Cologne, Germany, from August 2017 through December 2018.
Main Outcomes and Measures
Proportion of patients who achieved pCR (ypT0/is ypN0 definition) after neoadjuvant treatment according to germline variant status.
Results
In the study sample of 914 women with different BC subtypes with a mean (range) age at BC diagnosis of 48 (21-76) years, overall higher pCR rates were observed in patients with BRCA1/2 variants than in patients without (60.4% vs 46.7%; odds ratio [OR], 1.74; 95% CI, 1.13-2.68; P = .01); variants in non-BRCA1/2 BC predisposition genes were not associated with therapy response. Patients with TNBC with BRCA1/2 variants achieved highest pCR rates. In the TNBC subgroup, a positive BRCA1/2 variant status was associated with therapy response in both the PMCb arm (74.3% vs 47.0% without BRCA1/2 variant; OR, 3.26; 95% CI, 1.44-7.39; P = .005) and the iddEPC arm (64.7% vs 45.0%; OR, 2.24; 95% CI, 1.04-4.84; P = .04). A positive BRCA1/2 variant status was also associated with elevated pCR rates in patients with ERBB2-negative, hormone receptor–positive BC (31.8% vs 11.9%; OR, 3.44; 95% CI, 1.22-9.72; P = .02).
Conclusions and Relevance
Effective chemotherapy for BRCA1/2-mutated TNBC is commonly suggested to be platinum based. With a pCR rate of 64.7%, iddEPC may also be effective in these patients, though further prospective studies are needed. The elevated pCR rate in BRCA1/2-mutated ERBB2-negative, hormone receptor–positive BC suggests that germline BRCA1/2 testing should be considered prior to treatment start.
Trial Registration
ClinicalTrials.gov Identifier: NCT02125344
Introduction
The GeparOcto randomized clinical trial1 compared the efficacy of treatment with a sequential intense dose-dense epirubicin, paclitaxel, and cyclophosphamide–based chemotherapy regimen (iddEPC) vs a weekly paclitaxel plus nonpegylated liposomal doxorubicin regimen (PM) in 945 patients with different biological subtypes of breast cancer (BC). The study cohort included 403 patients with triple-negative BC (TNBC), 382 patients with ERBB2-positive BC, and 160 patients with ERBB2-negative, estrogen receptor–positive or progesterone receptor–positive (hormone receptor [HR]–positive) BC and histologically verified lymph node involvement. Patients with TNBC randomized to the PM arm received additional carboplatin (PMCb) based on previous results.2 Patients with ERBB2-positive BC received additional trastuzumab and pertuzumab in both study arms (trial protocol in Supplement 1; eFigure in Supplement 2). No overall difference in pathologic complete response (pCR) rates was observed between study arms (iddEPC arm, 48.3%; PM[Cb] arm, 48.0%; ypT0/is ypN0 definition).1 It was concluded that sequential iddEPC appeared more feasible than PM(Cb) owing to elevated nonhematologic adverse effects and lower adherence to treatment with PM(Cb).1
In particular, TNBC is associated with a hereditary cause, and germline variant screening of unselected patients with TNBC revealed a high BRCA1/2 variant prevalence.2,3 BRCA1/2 genes are critical in the homologous recombination repair of DNA double-strand breaks. Many of the other genes involved in homologous recombination repair are now recognized to also contribute to hereditary BC risk. Heterozygous germline inactivation of these genes may be accompanied by a somatic inactivation of the second allele, resulting in a homologous recombination deficiency and limited DNA repair capacity of the tumor cell.4 This functional role in DNA repair may be exploited in the treatment of homologous recombination–deficient cancers with drugs that challenge the DNA repair machinery, such as epirubicin, cyclophosphamide, doxorubicin, and carboplatin, which were used in the GeparOcto trial.1 These data prompted us to conduct this retrospective biomarker study of patients enrolled in the GeparOcto trial to determine treatment efficacies according to germline variant status overall, by treatment arm, and by tumor subtype.
Methods
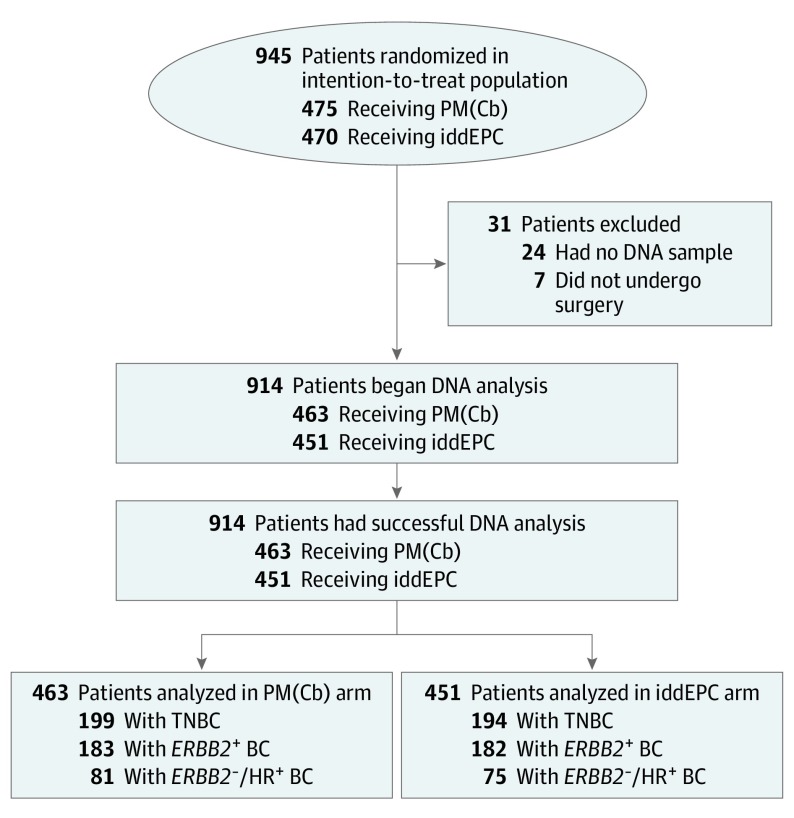
Of the 945 patients enrolled in the original GeparOcto investigation, 914 (96.7%) were successfully analyzed for germline (g) variants in BRCA1/2 and 16 further BC predisposition genes (Figure 1; eMethods in Supplement 2). All patients provided written informed consent for trial participation and translational research. The Ethics Committee of the University of Cologne (07-048) granted ethical approval for genetic analyses. Pearson χ2 test was used to compare pCR rates between groups. Univariate logistic regression analyses were performed to estimate odds ratio (OR) and 95% CIs. Interaction was assessed using the Breslow-Day test; P values less than .05 were considered statistically significant. Analyses were performed with SPSS statistical software version 25 (IBM Corp). This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Figure 1. CONSORT Flow Diagram.
Between December 2014 and June 2016, 1204 patients were screened for eligibility, 961 patients were randomized at 57 sites, 945 started treatment (intention-to-treat population), and 938 underwent surgery.1 Of these 938 patients, 24 patients were not included in this secondary analysis because no DNA sample was available. BC indicates breast cancer; CONSORT, Consolidated Standards of Reporting Trials; HR, hormone receptor; iddEPC, intense dose-dense epirubicin, paclitaxel, and cyclophosphamide; PM(Cb), paclitaxel and nonpegylated liposomal doxorubicin with or without carboplatin; TNBC, triple-negative BC.
Results
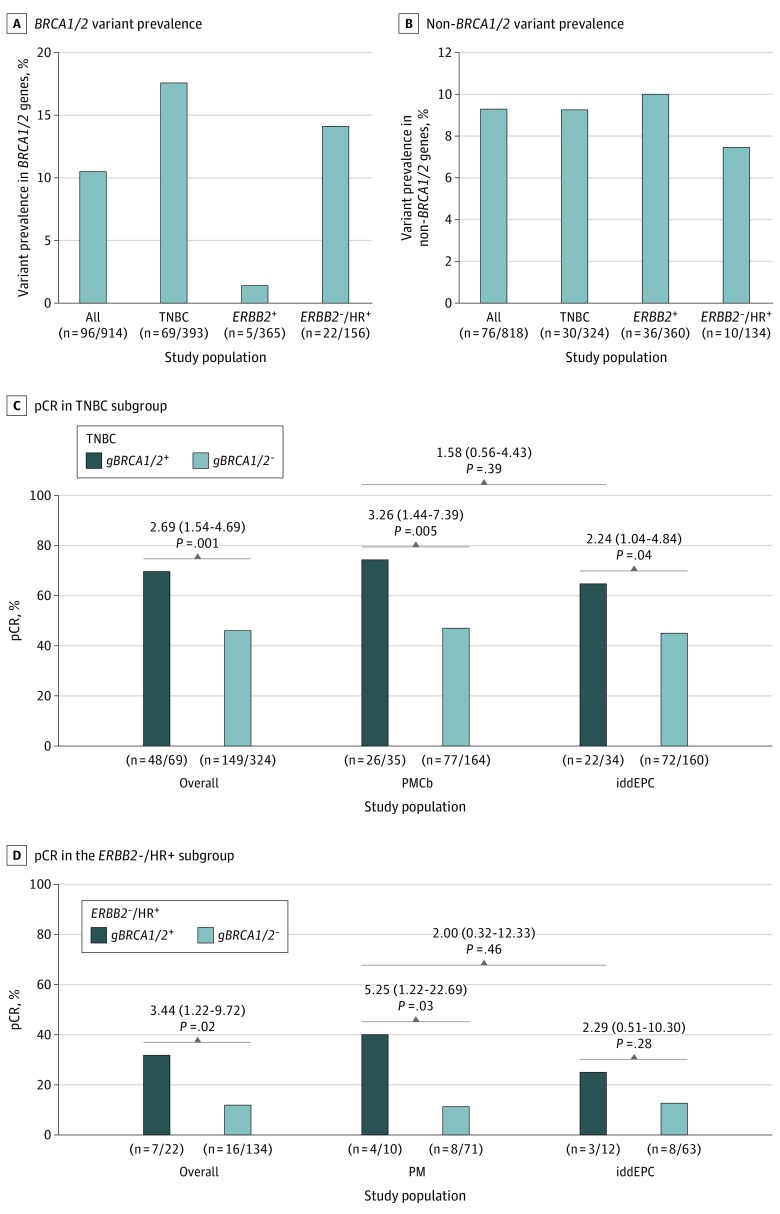
In the subgroup of 914 patients included in this investigation, the mean (range) age at BC diagnosis was 48 (21-76) years. Overall pCR rates were similar to those observed in the original investigation (iddEPC arm: 48.3%; PM[Cb] arm: 47.9%; OR, 0.99; 95% CI, 0.76-1.28; P = .91; ypT0/is ypN0 definition; eTable 1 in Supplement 2). Of the 914 patients, 96 (10.5%) carried pathogenic gBRCA1/2 variants (Figure 2A; eTable 2 in Supplement 2). Irrespective of the treatment arm and subtype, patients with gBRCA1/2 variants had higher pCR rates compared with patients without gBRCA1/2 variants (60.4% vs 46.7%; OR, 1.74; 95% CI, 1.13-2.68; P = .01; eTable 1 in Supplement 2); interaction between gBRCA1/2 variant and study arm was not significant (P = .11).
Figure 2. Germline Variant Prevalence and Pathologic Complete Response (pCR) Rates According to Variant Status and Study Arm.
A, Number of patients with germline BRCA1/2 (gBRCA1/2) variants in the overall study sample (n = 914) and according to biological subtype (triple-negative breast cancer [TNBC]; ERBB2-positive breast cancer; ERBB2-negative, hormone receptor [HR]–positive breast cancer). B, Number of patients with germline variants in non-BRCA1/2 genes among the subgroup of patients without gBRCA1/2 variants (n = 818) overall and according to the biological subtype. C and D, Rates of pCR according to gBRCA1/2 variant status in the TNBC subgroup (C) and in the ERBB2-negative, HR-positive subgroup (D), overall and by study arm. For the comparison of pCR rates according to gBRCA1/2 variant status, odds ratios (95% CI) are shown (univariate logistic regression). Abbreviations: iddEPC, intense dose-dense epirubicin, paclitaxel, and cyclophosphamide; PM, paclitaxel and nonpegylated liposomal doxorubicin; PMCb, PM with carboplatin.
The gBRCA1/2 variant prevalence as well as the pCR rates were highly different when analyzed by biological tumor subtypes. As expected from the GeparSixto trial,2 a high gBRCA1/2 variant prevalence was observed in patients with TNBC (69 of 393 [17.6%]; Figure 2A; eTable 2 in Supplement 2). Irrespective of the treatment arm, patients with TNBC and gBRCA1/2 variants achieved higher pCR rates compared with patients with TNBC without gBRCA1/2 variants (69.6% vs 46.0%; OR, 2.69; 95% CI, 1.54-4.69; P = .001; Figure 2C; eTable 1 in Supplement 2). For TNBC, a positive gBRCA1/2 variant status was associated with therapy response in both the PMCb arm (74.3% vs 47.0%; OR, 3.26; 95% CI, 1.44-7.39; P = .005) and the iddEPC arm (64.7% vs 45.0%; OR, 2.24; 95% CI, 1.04-4.84; P = .04; Figure 2C; eTable 1 in Supplement 2). Differences between treatment arms were not significant (74.3% vs 64.7%; OR, 1.58; 95% CI, 0.56-4.43; P = .39; Figure 2C). Interaction between gBRCA1/2 variant and study arm was not significant (P = .51).
In ERBB2-positive BC, a low gBRCA1/2 variant prevalence was suggested.5 Five gBRCA1/2 variant carriers (5 of 365 [1.4%]; Figure 2A; eTable 2 in Supplement 2) were present in this subgroup; therefore, we refrained from calculating ORs according to gBRCA1/2 variant status. Overall, pCR rates of approximately 60% were observed in ERBB2-positive BC (eTable 1 in Supplement 2).
In ERBB2-negative, HR-positive BC, a high gBRCA1/2 variant prevalence was observed (22 of 156 [14.1%]; Figure 2A; eTable 2 in Supplement 2). Irrespective of the treatment arm, a positive gBRCA1/2 variant status was associated with a higher pCR rate (31.8% vs 11.9%; OR, 3.44; 95% CI, 1.22-9.72; P = .02; Figure 2D, eTable 1 in Supplement 2); interaction between gBRCA1/2 variant and study arm was not significant (P = .44).
It is currently unknown whether variants in additional, non-BRCA1/2 BC predisposition genes are associated with chemotherapy response. Among the 818 patients without gBRCA1/2 variants, 76 patients (9.3%) carried at least 1 variant in the 16 non-BRCA1/2 genes (Figure 2B; eTable 2 in Supplement 2). The overall variant prevalence was mainly driven by the ATM, CHEK2, FANCM, and PALB2 BC predisposition genes,6,7 and a high variant prevalence was found in all 3 biological subgroups (Figure 2B). In accordance with previous studies, variants in the FANCM and PALB2 genes are associated with TNBC, while variants in the ATM and CHEK2 genes are associated with ERBB2-positive and ERBB2-negative, HR-positive BC7,8 (eTable 2 in Supplement 2). Patients without pathogenic gBRCA1/2 variants but germline variants in non-BRCA1/2 genes achieved pCR rates comparable to patients without any variant (eTable 1 in Supplement 2); however, gene-specific effects cannot be excluded.
Discussion
Patients with gBRCA1/2 variants benefited most from both treatment regimens, while variants in non-BRCA1/2 BC predisposition genes were not associated with therapy response. Especially following the studies by Byrski et al9 and most recently the Triple Negative Trial by Tutt et al,10 effective chemotherapy of gBRCA1/2-mutated TNBC is suggested to be platinum based, a result that was also confirmed in our investigation. In gBRCA1/2-mutated TNBC, we demonstrate that iddEPC appears to be also effective, though with a pCR rate approximately 10 percentage points lower than that observed in the PMCb arm. It remains elusive whether this difference is associated with survival outcome. However, our study was not powered to compare PM(Cb) vs iddEPC treatment efficacies specifically in patients with gBRCA1/2-mutated BC; larger prospective studies are required to address this question. Of further interest is the high gBRCA1/2 variant prevalence in ERBB2-negative, HR-positive BC along with the elevated pCR rate of gBRCA1/2 variant carriers compared to noncarriers. Although the subgroup of patients with ERBB2-negative, HR-positive BC was small in our study (n = 156), we suggest that patients with ERBB2-negative, HR-positive BC may benefit from BRCA1/2 testing prior to treatment start.
Limitations
The study did have a limitation. It was not powered to compare PM(Cb) vs iddEPC treatment efficacies in patients with gBRCA1/2-mutated BC.
Conclusions
Effective chemotherapy for gBRCA1/2-mutated TNBC is commonly suggested to be platinum based. With a pCR rate of 64.7%, we demonstrate that iddEPC appears to be also effective in these patients and may be considered in future prospective studies. The elevated pCR rate in gBRCA1/2-mutated ERBB2-negative, HR-positive BC suggests that germline BRCA1/2 testing should be considered prior to treatment start.
Trial Protocol
eMethods
eFigure. GeparOcto study design
eTable 1. Pathological complete response (pCR) rates (ypT0/is ypN0 definition) according to germline (g) variant status (BRCA1/2 and non-BRCA1/2 breast cancer predisposition genes), overall and by treatment arm
eTable 2. Prevalence of germline variants in BRCA1/2 and 16 non-BRCA1/2 genes in 914 breast cancer patients
eReferences
Data Sharing Statement
References
- 1.Schneeweiss A, Möbus V, Tesch H, et al. . Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto-GBG 84): a randomised phase III trial. Eur J Cancer. 2019;106:181-192. doi: 10.1016/j.ejca.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 2.Hahnen E, Lederer B, Hauke J, et al. . Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 2017;3(10):1378-1385. doi: 10.1001/jamaoncol.2017.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahnen E, Hauke J, Engel C, Neidhardt G, Rhiem K, Schmutzler RK. Germline mutations in triple-negative breast cancer. Breast Care (Basel). 2017;12(1):15-19. doi: 10.1159/000455999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Severson TM, Peeters J, Majewski I, et al. . BRCA1-like signature in triple negative breast cancer: molecular and clinical characterization reveals subgroups with therapeutic potential. Mol Oncol. 2015;9(8):1528-1538. doi: 10.1016/j.molonc.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans DG, Lalloo F, Howell S, Verhoef S, Woodward ER, Howell A. Low prevalence of HER2 positivity amongst BRCA1 and BRCA2 mutation carriers and in primary BRCA screens. Breast Cancer Res Treat. 2016;155(3):597-601. doi: 10.1007/s10549-016-3697-z [DOI] [PubMed] [Google Scholar]
- 6.Easton DF, Pharoah PD, Antoniou AC, et al. . Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243-2257. doi: 10.1056/NEJMsr1501341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neidhardt G, Hauke J, Ramser J, et al. . Association between loss-of-function mutations within the FANCM gene and early-onset familial breast cancer. JAMA Oncol. 2017;3(9):1245-1248. doi: 10.1001/jamaoncol.2016.5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauke J, Horvath J, Groß E, et al. . Gene panel testing of 5589 BRCA1/2-negative index patients with breast cancer in a routine diagnostic setting: results of the German Consortium for Hereditary Breast and Ovarian Cancer. Cancer Med. 2018;7(4):1349-1358. doi: 10.1002/cam4.1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrski T, Huzarski T, Dent R, et al. . Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147(2):401-405. doi: 10.1007/s10549-014-3100-x [DOI] [PubMed] [Google Scholar]
- 10.Tutt A, Tovey H, Cheang MCU, et al. . Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med. 2018;24(5):628-637. doi: 10.1038/s41591-018-0009-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods
eFigure. GeparOcto study design
eTable 1. Pathological complete response (pCR) rates (ypT0/is ypN0 definition) according to germline (g) variant status (BRCA1/2 and non-BRCA1/2 breast cancer predisposition genes), overall and by treatment arm
eTable 2. Prevalence of germline variants in BRCA1/2 and 16 non-BRCA1/2 genes in 914 breast cancer patients
eReferences
Data Sharing Statement