Abstract
Immune escape plays a vital role in the development of liver cancer. The interaction between programmed death-ligand 1 (PD-L1) and programmed cell death-1 is a key mediator of cancer immune escape, which leads to the suppression of anticancer immunity and promotion of tumor progression. Hypoxia is a common phenomenon in the tumor microenvironment. Under hypoxic conditions, suppressive immune cells, such as regulatory T cells, myeloid-derived suppressor cells and M2 macrophages, are frequently recruited to tumor tissues to form the immunosuppressive microenvironment in liver cancer. These cells secrete cancer-promoting inflammatory cytokines, which activate the STAT3 and NF-κB signaling pathways. Recent studies have shown that STAT3 is associated with NF-κB and that these transcription factors are often co-activated to regulate tumor proliferation, survival, angiogenesis and invasion. The activation of STAT3 and NF-κB signaling pathways can directly and indirectly induce PD-L1 expression. Therefore, further understanding of the association between hypoxia and PD-L1 may help in the future treatment of liver cancer. The present review summarizes the recent progresses on PD-L1-mediated regulation and facilitation of liver cancer cell immune escape in response to hypoxia.
Keywords: liver cancer, hypoxia, immune escape, programmed cell death 1 ligand 1, STAT3/NF-κB
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most malignant tumors that pose a severe threat to human health. The latest cancer statistics showed that the number of new liver cancer cases and liver cancer-associated deaths worldwide in 2018 was 841,000 and 782,000, respectively (1). Liver cancer ranked sixth in terms of new cancer cases and fourth in terms of cancer-associated deaths worldwide in 2018 (1). The available evidence indicates that immune escape of liver cancer cells plays a vital role in the development of this malignancy (2,3) and impairs the effectiveness of antitumor treatment (4). Therefore, effective blockage of the occurrence of immune escape has become the focus of attention in the prevention and treatment of HCC.
It is now known that the activation or inhibition of immune cells in the body is regulated by positive and negative signals (5,6). Among them, the interaction between programmed cell death-1 (PD-1, also termed CD279) and programmed death-ligand 1 (PD-L1, also termed CD274 and B7-H1) is the primary negative immune regulatory signal, which inhibits the antitumor immune activity of effector cells and mediates tumor immune escape (7–10). Furthermore, immune checkpoint blockers have recently emerged as a mainstream strategy for the treatment of multiple solid tumors, including liver cancer (11–14).
Hypoxia, a common phenomenon in the tumor microenvironment, induces the expression of PD-L1 to promote immune escape (15–17). A number of immune cells with immunosuppressive activities, including tumor-associated regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), are recruited to the tumor tissue to form an immunosuppressive microenvironment (18–20). Moreover, under hypoxic conditions, the expression of PD-L1 is rapidly upregulated in these immunosuppressive cells in a hypoxia-inducible factor 1α (HIF-1α)-dependent manner (21,22). In this regard, the comprehensive analysis of the role and mechanism of PD-L1 in hypoxia-induced immune escape is essential for the improved treatment of liver cancer. The present review summarizes the recent findings regarding the regulation of PD-L1-mediated hypoxia-induced immune escape in HCC cells and discusses the underlying mechanisms.
2. PD-L1/PD-1 interaction in the immune escape of liver cancer
The compromised immune status of the body is associated with the occurrence of liver cancer. When the immune function is weakened or suppressed, the incidence of liver cancer will increase significantly. Normally, once liver cancer cells are formed in the body, the immune system can inhibit or kill these cells in a variety of ways (23–25). However, despite the immune surveillance and scavenger receptors, it remains challenging to curb the occurrence and development of liver cancer (26). The main reason is that liver cancer cells may escape from the immune system attack through various mechanisms. The PD-L1/PD-1 pathway, which promotes cancer cell survival and proliferation, is a key mediator of the immune escape of HCC cells (27–29).
Previous studies have shown that T cell-mediated cellular immunity plays a pivotal role in the recognition and killing of tumor cells (30,31). T cells recognize major histocompatibility complexes that bear antigens derived from the surface of cancer cells, which allows subsequent tumor recognition and targeted killing (32). Recently, it has been demonstrated that various mechanisms play a role in increasing the expression of PD-L1 in tumor cells and in the tumor microenvironment. PD-L1 is a transmembrane glycoprotein composed of 290 amino acids, which belongs to the B7 family of immune-regulatory ligands. The binding of PD-L1 to its PD-1 receptor suppresses T-cell migration, proliferation and secretion of cytotoxic mediators, and restricts tumor cell killing, leading to the occurrence of tumor cell immune escape (33). In the healthy immune system, the PD-L1/PD-1 pathway plays a critical role in maintaining the balance between protective immunity and immune tolerance. However, aberrant activation of the PD-L1/PD-1 signaling pathway in the tumor microenvironment is associated with the development of liver cancer. A multivariate analysis showed that PD-L1 expression is an independent predictor of postoperative recurrence of HCC (7,34).
Accumulating evidence has revealed that the elevated level of PD-L1 in the tumor microenvironment constrains antitumor immunity via the inhibition of antitumor effector cell function and enhancement of the inhibitory activity of immunosuppressive cells (12,16,35,36). Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are the main local antitumor immune effector cells. Activated CTLs are marked by granzyme B, which is the primary molecular mediator of apoptosis (37). It has been shown that the activation of the PD-1/PD-L1 signaling pathway restrains CTL function by inducing apoptosis, anergy and exhaustion, and promoting the secretion of immunosuppressive factors, leading to the immune escape of tumor cells (38). Hepatoma tumor-infiltrating CTLs express PD-1 molecules, which bind to PD-L1 that are expressed on the surface of tumor cells, resulting in the depletion and apoptosis of CTLs (39).
There are a large number of active immunosuppressive cells in the tumor microenvironment, including Tregs, MDSCs and TAMs (40). These immune cells form a complex multi-cell population, which is an important part of the tumor microenvironment. Indeed, various molecular interactions between immune and cancer cells are considered a crucial step in the direct or indirect induction of the occurrence and development of tumors. These immunosuppressive cells also express a large number of PD-L1 molecules, which induce apoptosis in CTLs by binding to PD-1 (41). Tregs, characterized by the expression of CD4, CD25 and Forkhead box protein P3 (FOXP3), are the most characteristic immunosuppressive cells. The inhibition of the immune response by Tregs is also mediated by cell contact or the secretion of inhibitory cytokines, such as interleukin (IL)-10 and transforming growth factor-β (TGF-β) (42). A previous study found that PD-L1 promoted Treg differentiation by converting CD4+CD25+FOXP3− T cells to CD4+CD25+FOXP3+ Tregs. Furthermore, higher expression levels of PD-L1 on hepatic dendritic cells were associated with an increased Treg cell induction (43). Specific blocking of PD-L1 by small interfering (si)RNA or monoclonal antibodies decreased the production of CD4+CD25+FOXP3+ Tregs and induced Treg apoptosis (44). In a pig xenograft model, PD-L1 was found to enhance Treg function and stimulate IL-10 production, thereby further promoting the immune inhibitory function (45). Clinical data also showed that PD-L1 effectively stimulated the secretion of IL-10 in patients with liver cancer, thereby further enhancing the immunosuppressive effect of Tregs (46). Collectively, these studies have shown that the PD-L1/PD-1 pathway inhibits the antitumor function of CTLs, enhances the immunosuppressive activity of Tregs, and promotes the secretion of immunosuppressive factors by transmitting inhibitory signals, leading to the occurrence of tumor immune escape.
3. Hypoxia-induced recruitment of immunosuppressive cells and regulation of PD-L1 expression
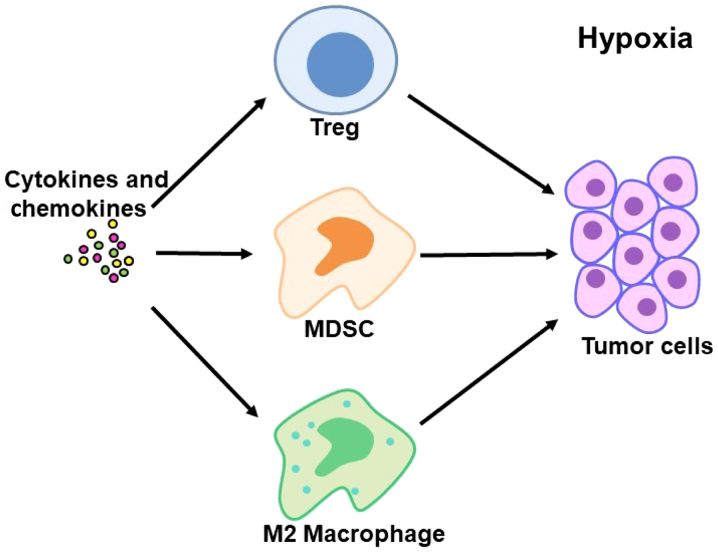
Hypoxia is a common phenomenon in the tumor microenvironment (47–49). Previous studies have revealed that tumor hypoxia alters the composition and activity of tumor-associated immune cells, and that numerous immune cells with immunosuppressive activities are recruited to tumor tissues to form the immunosuppressive microenvironment (18,50,51). Under hypoxic conditions, tumor cells and macrophages secrete a variety of cytokines and chemokines, including C-C motif chemokine (CCL)22, CCL28 and IL-10, which results in the recruitment of CD4+CD25+FOXP3+ Tregs from peripheral blood to inhibit T cell-mediated antitumor responses (18,52,53). Hypoxia also promotes the recruitment of MDSCs (19). MDSCs are a group of undifferentiated, immunosuppressive, bone marrow-derived heterogeneous cell populations, which have a strong immunosuppressive function (54). MDSCs expressing arginase-1, which mediate the depletion of L-arginine, impede T cell proliferation, and are associated with the downregulation of T cell receptor (TCR) subunit CD3ζ, resulting in decreased TCR response (55–57). The occurrence of a tumor in the liver results in increased levels of MDSCs at the tumor site, and the activation of the MyD88-NF-κB pathway stimulates the secretion of IL-10 to inhibit the expression of IL-12 in dendritic cells and the activation of T cells (58). MDSCs also induce NK cell inactivation through TGF-β and the NK receptor p30 on the cell surface (59). Furthermore, a previous study indicated that MDSCs inhibit immune response and promote the development of liver cancer by inducing the generation of CD4+CD25+FOXP3+ Tregs (60). In addition, the tumor hypoxia microenvironment directly induces macrophage M2 polarization, angiogenesis, and tumor growth and metastasis (20). M2 type macrophages inhibit the antitumor immune response by producing TGF-β and IL-10, and their numbers in the tumor microenvironment are negatively correlated with the prognosis of liver cancer patients (61,62). M2 microphages also secrete a range of specific chemokines, including CCL17, CCL22 and CCL24, which recruit regulatory T cells to tumor sites (62). As a result, Tregs, MDSCs and M2 macrophages have potent immunosuppressive activities and together promote the occurrence of tumor immune escape (Fig. 1).
Figure 1.
Hypoxia induces the recruitment of immunosuppressive cells to the tumor tissue to promote the immune escape of hepatoma cells. Under hypoxic conditions, Tregs, MDSCs and M2 macrophages are recruited to tumor tissues to form an immunosuppressive microenvironment. These cells exhibit potent immunosuppressive activity and foster the occurrence of tumor immune escape. Treg, regulatory T cell; MDSC, myeloid-derived suppressor cell.
Under hypoxic conditions, HIF-1α is a crucial transcription factor that mediates the effect of hypoxia on the adaptive regulation of tumor cells and the tumor microenvironment (63–65). Under normoxic conditions, HIF-1α is hydroxylated by prolyl hydroxylase (PHD) and ubiquitinated/degraded by the von Hippel-Lindau E3 ubiquitin ligase complex. Under hypoxic conditions, PHD activity is inhibited, and HIF-1α ubiquitination and degradation are decreased, thereby stabilizing HIF-1α (66). Previous studies have shown that HIF-1α is associated with PD-L1 expression (15,22). Under hypoxic conditions, tumor cells, myeloid suppressor cells, macrophages and dendritic cells all undergo rapid upregulation of PD-L1 in a HIF-1α-dependent manner. Chromatin immunoprecipitation and luciferase reporter assays showed that HIF-1α induced the expression of PD-L1 by directly binding to the hypoxia response element region of the PD-L1 promoter. Furthermore, the inhibition of PD-L1 expression significantly decreased the secretion of IL-6 and IL-10 by MDSC, leading to the activation of T cells (22). Another in vitro study also revealed that hypoxia stimulated the expression of PD-L1 in a variety of human and murine tumor cells through HIF-1α (15). These studies demonstrate that hypoxia induces PD-L1 expression by activating the HIF-1α cascade.
4. Involvement of STAT3 and NF-κB in the regulation of PD-L1 expression in liver cancer
Accumulating evidence has indicated that the essential mechanism underlying tumor immune escape is associated with the presence of a large number of cytokines and growth factors with immunosuppressive activities in the tumor microenvironment, such as IL-6, vascular endothelial growth factor, TGF-β, IL-10, IL-13, macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (67–69). These cytokines stimulate immune inhibitory cells, including Tregs, TAMs and MDSCs, and mediate the expression of a series of genes by activating various signaling pathways. Among them, the STAT3 and NF-κB pathways are essential hubs linking these cytokines to cellular responses (70–73).
STAT3 is a member of the STAT family of transcription factors. When cytokines in the tumor microenvironment bind to their receptors, the Janus kinase and/or proto-oncogene tyrosine-protein kinase Src will be activated and able to phosphorylate STAT3. Following dimerization and nuclear translocation, STAT3 will initiate the transcription of downstream genes. A previous study found that STAT3 activation in tumor cells induces the secretion of IL-6 and IL-10 cytokines, which results in Treg proliferation. Moreover, STAT3 is also activated in Tregs and further stimulates the expression of FOXP3, TGF-β and IL-10, which inhibits CTLs and promotes the formation of an immunosuppressive environment (70,74,75).
In addition, STAT3 and NF-κB are often coactivated in tumor cells and play a vital role in the regulation of the expression of cancer-promoting inflammatory genes (76). The coordination between STAT3 and NF-κB is mainly manifested in the following aspects: i) Multiple inflammatory factors, especially IL-6, induced by NF-κB are essential activators of STAT3; ii) STAT3 directly interacts with the NF-κB family member transcription factor p65 (RelA), leading to its acetylation and inhibition of nuclear export, and constitutive activation of NF-κB; iii) STAT3 and NF-κB co-regulate the expression of a number of oncogenes and inflammatory genes; and iv) the inflammatory factors induced by NF-κB and STAT3 form a positive feedback loop to further activate NF-κB and STAT3 (77,78).
Notably, it has been shown that the expression of HIF-1α is regulated by both NF-κB and STAT3. Under hypoxic conditions, STAT3 is activated by phosphorylation, which not only blocks HIF-1α degradation but also increases the synthesis of HIF-1α (79). In human breast cancer MCF-7 cells, the depletion of STAT3 by siRNA inhibited CoCl2-induced HIF-1α nuclear accumulation (80). The NF-κB signaling pathway is also activated under hypoxic conditions (81). Gel shift assay and chromatin immunoprecipitation experiments confirmed that the NF-κB subunits p50 and RelA bind to the promoter of HIF-1α and activate its transcription (82). Since HIF-1α transcriptionally induces PD-L1, these studies indicate that the activation of the STAT3 and NF-κB pathways may indirectly stimulate PD-L1 expression under hypoxic conditions.
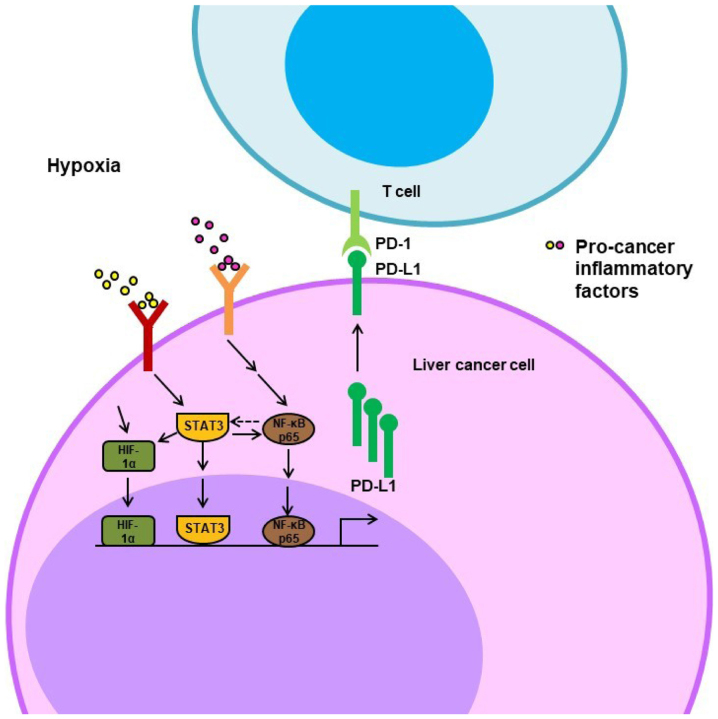
Furthermore, several studies have shown that the STAT3 and NF-κB signaling pathways are also involved in the direct regulation of PD-L1 at the transcriptional level (83–86). It has been demonstrated that the co-culture of liver cancer cells (BEL-7402 and SMMC-7721) with macrophages resulted in increased PD-L1 mRNA and protein levels and that blocking either the NF-κB or the STAT3 signaling pathway inhibited this co-culture effect on PD-L1 expression (83). Another study showed that EB virus latent membrane protein 1 (LMP1) induced the expression of PD-L1 by the activation of NF-κB or STAT3; the inhibition of one of these pathways notably decreased LMP1-stimulated PD-L1 expression (84). Chromatin immunoprecipitation and reporter assays revealed direct binding of STAT-3 and NF-κB to the PD-L1 promoter, triggering PD-L1 transcription (85,86). These studies indicate that the STAT3/NF-κB pathways directly and indirectly regulate PD-L1 expression in the hypoxic microenvironment (Fig. 2).
Figure 2.
Schematic representation of hypoxia-induced activation of STAT3, NF-κB and HIF-1α pathways resulting in increased PD-L1 expression. Under hypoxic conditions, the expression of PD-L1 is upregulated in a HIF-1α-dependent manner. Furthermore, immunosuppressive cells secrete inflammatory cytokines to activate the STAT3 and NF-κB signaling pathways, which are often coactivated to induce the expression of PD-L1 directly, by binding to and stimulating its promoter, or indirectly, by increasing the expression level of HIF-1α. HIF-1α, hypoxia inducible factor-1α; PD-L1, programmed death-ligand 1; PD-1, programmed cell death-1.
5. Relevance for clinical practice
Immunotherapy is emerging as an appealing and attractive strategy for the treatment of HCC. Novel immune checkpoint inhibitors have revolutionized pharmacological treatment options for cancer with remarkable clinical outcomes in a number of human malignancies, including advanced HCC. It has been shown that the inhibition of PD-L1 improves overall survival rates in patients with HCC (87). Moreover, since HIF-1α plays a vital role in regulating immune escape in the hypoxic tumor microenvironment, a HIF-1α inhibitor is being investigated for the treatment of HCC (88–91). Several inhibitors of STAT3 and/or NF-κB are undergoing clinical trials for HCC (92,93). In addition, due to the upregulation of PD-L1 by STAT3, NF-κB and HIF-1α, a combination of a PD-L1 antibody with small molecule inhibitors of STAT3, NF-κB or HIF-1α could be a more effective therapeutic strategy in advanced liver cancer.
6. Conclusions
Immune escape is a key cause of tumor development. Enhancing antitumor immunity of the body, as the core treatment strategy, is being extensively studied in cancer care and research. In the tumor hypoxic microenvironment, PD-L1 overexpression is a crucial factor contributing to liver cancer immune escape and is associated with the activation of the STAT3/NF-κB pathway and HIF-1α. Therefore, the inhibition of STAT3 and NF-κB pathways or HIF-1α should decrease PD-L1 expression and reverse immune escape. Agents blocking STAT3, NF-κB or HIF-1α have great potential for cancer immunotherapy, particularly in patients developing resistance to PD-L1 and PD1 inhibitors.
Acknowledgements
Not applicable.
Funding
This work was supported in part by the National Natural Science Foundation of China (grant no. 81873249), the Young Taishan Scholars Program of Shandong Province (grant no. tsqn201909200) and the Natural Science Foundation of Shandong Province (grant. no. ZR2019MH058).
Availability of data and materials
Not applicable.
Authors' contributions
SJ contributed to the conception of the study. QW wrote the manuscript with support from SJ, TH and ZW. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Han C, Jiang Y, Wang Z, Wang H. Natural killer cells involved in tumour immune escape of hepatocellular carcinomar. Int Immunopharmacol. 2019;73:10–16. doi: 10.1016/j.intimp.2019.04.057. [DOI] [PubMed] [Google Scholar]
- 3.Xie Y, Xiang Y, Sheng J, Zhang D, Yao X, Yang Y, Zhang X. Immunotherapy for hepatocellular carcinoma: Current advances and future expectations. J Immunol Res. 2018;2018:8740976. doi: 10.1155/2018/8740976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding JJ, El Dika I, Abou-Alfa GK. Immunotherapy in hepatocellular carcinoma: Primed to make a difference? Cancer. 2016;122:367–377. doi: 10.1002/cncr.29769. [DOI] [PubMed] [Google Scholar]
- 5.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Najafi M, Farhood B, Mortezaee K. Contribution of regulatory T cells to cancer: A review. J Cell Physiol. 2019;234:7983–7993. doi: 10.1002/jcp.27553. [DOI] [PubMed] [Google Scholar]
- 7.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shergold AL, Millar R, Nibbs RJB. Understanding and overcoming the resistance of cancer to PD-1/PD-L1 blockade. Pharmacol Res. 2019;145:104258. doi: 10.1016/j.phrs.2019.104258. [DOI] [PubMed] [Google Scholar]
- 9.Pio R, Ajona D, Ortiz-Espinosa S, Mantovani A, Lambris JD. Complementing the cancer-immunity cycle. Front Immunol. 2019;10:774. doi: 10.3389/fimmu.2019.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prestipino A, Zeiser R. Clinical implications of tumor-intrinsic mechanisms regulating PD-L1. Sci Transl Med. 2019;11(pii):eaav4810. doi: 10.1126/scitranslmed.aav4810. [DOI] [PubMed] [Google Scholar]
- 11.Iñarrairaegui M, Melero I, Sangro B. Immunotherapy of hepatocellular carcinoma: Facts and hopes. Clin Cancer Res. 2018;24:1518–1524. doi: 10.1158/1078-0432.CCR-17-0289. [DOI] [PubMed] [Google Scholar]
- 12.Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: Perspectives and issues. Int J Clin Oncol. 2016;21:462–473. doi: 10.1007/s10147-016-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mocan T, Sparchez Z, Craciun R, Bora CN, Leucuta DC. Programmed cell death protein-1 (PD-1)/programmed death-ligand-1 (PD-L1) axis in hepatocellular carcinoma: Prognostic and therapeutic perspectives. Clin Transl Oncol. 2019;21:702–712. doi: 10.1007/s12094-018-1975-4. [DOI] [PubMed] [Google Scholar]
- 14.Ho CM, Chen HL, Hu RH, Lee PH. Harnessing immunotherapy for liver recipients with hepatocellular carcinoma: A review from a transplant oncology perspective. Ther Adv Med Oncol. 2019;11:1758835919843463. doi: 10.1177/1758835919843463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-14-2598. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409–416. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 17.Guo R, Li Y, Wang Z, Bai H, Duan J, Wang S, Wang L, Wang J. Hypoxia-inducible factor-1α and nuclear factor-κB play important roles in regulating programmed cell death ligand 1 expression by epidermal growth factor receptor mutants in non-small-cell lung cancer cells. Cancer Sci. 2019;110:1665–1675. doi: 10.1111/cas.13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 19.Terry S, Buart S, Chouaib S. Hypoxic stress-induced tumor and immune plasticity, suppression, and impact on tumor heterogeneity. Front Immunol. 2017;8:1625. doi: 10.3389/fimmu.2017.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe E, Elkrim Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- 21.Labiano S, Palazon A, Melero I. Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol. 2015;42:378–386. doi: 10.1053/j.seminoncol.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reig M, Boix L, Mariño Z, Torres F, Forns X, Bruix J. Liver cancer emergence associated with antiviral treatment: An immune surveillance failure? Semin Liver Dis. 2017;37:109–118. doi: 10.1055/s-0037-1601349. [DOI] [PubMed] [Google Scholar]
- 24.Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–1426. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 26.Owusu Sekyere S, Schlevogt B, Mettke F, Kabbani M, Deterding K, Wirth TC, Vogel A, Manns MP, Falk CS, Cornberg M, et al. HCC Immune surveillance and antiviral therapy of hepatitis C virus infection. Liver Cancer. 2019;8:41–65. doi: 10.1159/000490360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudo M. Systemic therapy for hepatocellular carcinoma: Latest advances. Cancers (Basel) 2018;10(pii):E412. doi: 10.3390/cancers10110412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahin B. Enlighting the shadow for advanced hepatocellular carcinoma: Immunotherapy with immune checkpoint inhibitors. J Gastrointest Cancer. 2017;48:288–290. doi: 10.1007/s12029-017-9996-8. [DOI] [PubMed] [Google Scholar]
- 29.Waidmann O. Recent developments with immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther. 2018;18:905–910. doi: 10.1080/14712598.2018.1499722. [DOI] [PubMed] [Google Scholar]
- 30.Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. 2019;234:8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 31.Kosti P, Maher J, Arnold JN. Perspectives on chimeric antigen receptor T-cell immunotherapy for solid tumors. Front Immunol. 2018;9:1104. doi: 10.3389/fimmu.2018.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine. 2011;56:231–238. doi: 10.1016/j.cyto.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 35.Lian S, Xie R, Ye Y, Xie X, Li S, Lu Y, Li B, Cheng Y, Katanaev VL, Jia L. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. EBioMedicine. 2019;42:281–295. doi: 10.1016/j.ebiom.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barry M, Heibein JA, Pinkoski MJ, Lee SF, Moyer RW, Green DR, Bleackley RC. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol Cell Biol. 2000;20:3781–3794. doi: 10.1128/MCB.20.11.3781-3794.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanmamed MF, Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 2014;20:256–261. doi: 10.1097/PPO.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Q, Xiao X, Wu Y, Wei Y, Zhu LY, Zhou J, Kuang DM. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur J Immunol. 2011;41:2314–2322. doi: 10.1002/eji.201041282. [DOI] [PubMed] [Google Scholar]
- 40.Andersen MH. The balance players of the adaptive immune system. Cancer Res. 2018;78:1379–1382. doi: 10.1158/0008-5472.CAN-17-3607. [DOI] [PubMed] [Google Scholar]
- 41.Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228:1404–1412. doi: 10.1002/jcp.24260. [DOI] [PubMed] [Google Scholar]
- 42.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H, Bakthavatsalam R, Meng Z, Li Z, Li W, Perkins JD, Reyes J. PD-L1 signal on liver dendritic cells is critical for Foxp3(+)CD4(+)CD25(+) Treg and liver tolerance induction in mice. Transplant Proc. 2013;45:1853–1855. doi: 10.1016/j.transproceed.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Beswick EJ, Pinchuk IV, Das S, Powell DW, Reyes VE. Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells. Infect Immun. 2007;75:4334–4341. doi: 10.1128/IAI.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding Q, Lu L, Zhou X, Zhou Y, Chou KY. Human PD-L1-overexpressing porcine vascular endothelial cells induce functionally suppressive human CD4+CD25hiFoxp3+ Treg cells. J Leukoc Biol. 2011;90:77–86. doi: 10.1189/jlb.1210691. [DOI] [PubMed] [Google Scholar]
- 46.Geng L, Deng J, Jiang G, Song P, Wang Z, Jiang Z, Zhang M, Zheng S. B7-H1 up-regulated expression in human hepatocellular carcinoma tissue: Correlation with tumor interleukin-10 levels. Hepatogastroenterology. 2011;58:960–964. [PubMed] [Google Scholar]
- 47.Sormendi S, Wielockx B. Hypoxia pathway proteins as central mediators of metabolism in the tumor cells and their microenvironment. Front Immunol. 2018;9:40. doi: 10.3389/fimmu.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng J, Li J, Sarde A, Lines JL, Lee YC, Qian DC, Pechenick DA, Manivanh R, Le Mercier I, Lowrey CH, et al. Hypoxia-induced VISTA promotes the suppressive function of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Immunol Res. 2019;7:1079–1090. doi: 10.1158/2326-6066.CIR-18-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi Y, Yokota A, Harada H, Huang G. Hypoxia/pseudohypoxia-mediated activation of hypoxia-inducible factor-1α in cancer. Cancer Sci. 2019;110:1510–1517. doi: 10.1111/cas.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chouaib S, Umansky V, Kieda C. The role of hypoxia in shaping the recruitment of proangiogenic and immunosuppressive cells in the tumor microenvironment. Contemp Oncol (Pozn) 2018;22:7–13. doi: 10.5114/wo.2018.73874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo X, Xue H, Shao Q, Wang J, Guo X, Chen X, Zhang J, Xu S, Li T, Zhang P, et al. Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget. 2016;7:80521–80542. doi: 10.18632/oncotarget.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaupel P, Multhoff G. Hypoxia-/HIF-1α-driven factors of the tumor microenvironment impeding antitumor immune responses and promoting malignant progression. Adv Exp Med Biol. 2018;1072:171–175. doi: 10.1007/978-3-319-91287-5_27. [DOI] [PubMed] [Google Scholar]
- 53.Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143:512–519. doi: 10.1111/imm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez PC, Ochoa AC, Al-Khami AA. Arginine metabolism in myeloid cells shapes innate and adaptive immunity. Front Immunol. 2017;8:93. doi: 10.3389/fimmu.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munder M, Choi BS, Rogers M, Kropf P. L-arginine deprivation impairs Leishmania major-specific T-cell responses. Eur J Immunol. 2009;39:2161–2172. doi: 10.1002/eji.200839041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 58.Hu CE, Gan J, Zhang RD, Cheng YR, Huang GJ. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol. 2011;46:156–164. doi: 10.3109/00365521.2010.516450. [DOI] [PubMed] [Google Scholar]
- 59.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 61.Amann T, Bataille F, Spruss T, Mühlbauer M, Gäbele E, Schölmerich J, Kiefer P, Bosserhoff AK, Hellerbrand C. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. 2009;100:646–653. doi: 10.1111/j.1349-7006.2009.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ju MJ, Qiu SJ, Fan J, Xiao YS, Gao Q, Zhou J, Li YW, Tang ZY. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol. 2009;131:498–510. doi: 10.1309/AJCP86PPBNGOHNNL. [DOI] [PubMed] [Google Scholar]
- 63.Soni S, Padwad YS. HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. 2017;56:503–515. doi: 10.1080/0284186X.2017.1301680. [DOI] [PubMed] [Google Scholar]
- 64.Xia Y, Jiang L, Zhong T. The role of HIF-1α in chemo-/radioresistant tumors. Onco Targets Ther. 2018;11:3003–3011. doi: 10.2147/OTT.S158206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. doi: 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Günter J, Ruiz-Serrano A, Pickel C, Wenger RH, Scholz CC. The functional interplay between the HIF pathway and the ubiquitin system-more than a one-way road. Exp Cell Res. 2017;356:152–159. doi: 10.1016/j.yexcr.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 67.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bian X, Xiao YT, Wu T, Yao M, Du L, Ren S, Wang J. Microvesicles and chemokines in tumor microenvironment: Mediators of intercellular communications in tumor progression. Mol Cancer. 2019;18:50. doi: 10.1186/s12943-019-0973-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gun SY, Lee SWL, Sieow JL, Wong SC. Targeting immune cells for cancer therapy. Redox Biol. 2019;25:101174. doi: 10.1016/j.redox.2019.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Shen Y, Wang S, Shen Q, Zhou X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018;415:117–128. doi: 10.1016/j.canlet.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huynh J, Chand A, Gough D, Ernst M. Therapeutically exploiting STAT3 activity in cancer-using tissue repair as a road map. Nat Rev Cancer. 2019;19:82–96. doi: 10.1038/s41568-018-0090-8. [DOI] [PubMed] [Google Scholar]
- 72.Loh CY, Arya A, Naema AF, Wong WF, Sethi G, Looi CY. Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: Functions and therapeutic implication. Front Oncol. 2019;9:48. doi: 10.3389/fonc.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Capece D, Verzella D, Tessitore A, Alesse E, Capalbo C, Zazzeroni F. Cancer secretome and inflammation: The bright and the dark sides of NF-κB. Semin Cell Dev Biol. 2018;78:51–61. doi: 10.1016/j.semcdb.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 75.Kitamura H, Ohno Y, Toyoshima Y, Ohtake J, Homma S, Kawamura H, Takahashi N, Taketomi A. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017;108:1947–1952. doi: 10.1111/cas.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kunnumakkara AB, Sailo BL, Banik K, Harsha C, Prasad S, Gupta SC, Bharti AC, Aggarwal BB. Chronic diseases, inflammation, and spices: How are they linked? J Transl Med. 2018;16:14. doi: 10.1186/s12967-018-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao P, Niu N, Wei T, Tozawa H, Chen X, Zhang C, Zhang J, Wada Y, Kapron CM, Liu J. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget. 2017;8:69139–69161. doi: 10.18632/oncotarget.19932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cascio S, D'Andrea A, Ferla R, Surmacz E, Gulotta E, Amodeo V, Bazan V, Gebbia N, Russo A. miR-20b modulates VEGF expression by targeting HIF-1 alpha and STAT3 in MCF-7 breast cancer cells. J Cell Physiol. 2010;224:242–249. doi: 10.1002/jcp.22126. [DOI] [PubMed] [Google Scholar]
- 81.Van Welden S, Selfridge AC, Hindryckx P. Intestinal hypoxia and hypoxia-induced signalling as therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:596–611. doi: 10.1038/nrgastro.2017.101. [DOI] [PubMed] [Google Scholar]
- 82.Belaiba RS, Bonello S, Zahringer C, Schmidt S, Hess J, Kietzmann T, Gorlach A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007;18:4691–4697. doi: 10.1091/mbc.e07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen J, Li G, Meng H, Fan Y, Song Y, Wang S, Zhu F, Guo C, Zhang L, Shi Y. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother. 2012;61:101–108. doi: 10.1007/s00262-011-1094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fang W, Zhang J, Hong S, Zhan J, Chen N, Qin T, Tang Y, Zhang Y, Kang S, Zhou T, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget. 2014;5:12189–12202. doi: 10.18632/oncotarget.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wölfle SJ, Strebovsky J, Bartz H, Sähr A, Arnold C, Kaiser C, Dalpke AH, Heeg K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41:413–424. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- 86.Huang G, Wen Q, Zhao Y, Gao Q, Bai Y. NF-κB plays a key role in inducing CD274 expression in human monocytes after lipopolysaccharide treatment. PLoS One. 2013;8:e61602. doi: 10.1371/journal.pone.0061602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kudo M. Immuno-oncology in hepatocellular carcinoma: 2017 update. Oncology. 2017;93(Suppl 1):S147–S159. doi: 10.1159/000481245. [DOI] [PubMed] [Google Scholar]
- 88.Lin D, Wu J. Hypoxia inducible factor in hepatocellular carcinoma: A therapeutic target. World J Gastroenterol. 2015;21:12171–12178. doi: 10.3748/wjg.v21.i42.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu L, Fu Z, Zhou S, Gong J, Liu CA, Qiao Z, Li S. HIF-1α and HIF-2α: Siblings in promoting angiogenesis of residual hepatocellular carcinoma after high-intensity focused ultrasound ablation. PLoS One. 2014;9:e88913. doi: 10.1371/journal.pone.0088913. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Liang Y, Zheng T, Song R, Wang J, Yin D, Wang L, Liu H, Tian L, Fang X, Meng X, et al. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in hepatocellular carcinoma. Hepatology. 2013;57:1847–1857. doi: 10.1002/hep.26224. [DOI] [PubMed] [Google Scholar]
- 91.Liu F, Wang P, Jiang X, Tan G, Qiao H, Jiang H, Krissansen GW, Sun X. Antisense hypoxia-inducible factor 1alpha gene therapy enhances the therapeutic efficacy of doxorubicin to combat hepatocellular carcinoma. Cancer Sci. 2008;99:2055–2061. doi: 10.1111/j.1349-7006.2008.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brambilla L, Genini D, Laurini E, Merulla J, Perez L, Fermeglia M, Carbone GM, Pricl S, Catapano CV. Hitting the right spot: Mechanism of action of OPB-31121, a novel and potent inhibitor of the signal transducer and activator of transcription 3 (STAT3) Mol Oncol. 2015;9:1194–1206. doi: 10.1016/j.molonc.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ciombor KK, Feng Y, Benson AB, III, Su Y, Horton L, Short SP, Kauh JS, Staley C, Mulcahy M, Powell M, et al. Phase II trial of bortezomib plus doxorubicin in hepatocellular carcinoma (E6202): A trial of the Eastern Cooperative Oncology Group. Invest New Drugs. 2014;32:1017–1027. doi: 10.1007/s10637-014-0111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.