Abstract
A standard treatment for patients with early-stage non-small cell lung cancer (NSCLC) who undergo surgery, and subsequently develop local failure or intrathoracic oligo-recurrence, has not yet been established. The present study aimed to assess the feasibility of stereotactic body radiotherapy (SBRT) for this subgroup of patients. Consequently, a retrospective analysis was conducted of patients with NSCLC recurrence who were treated with SBRT, and previously underwent curative surgical resection between October 2011 and October 2016. Post-SBRT survival [overall survival (OS); progression-free survival (PFS); and local control (LC)] and toxicity were analyzed. Prognostic factors for OS were identified using univariate and multivariate analysis. A total of 52 patients and 59 tumors were analyzed. The median follow-up time was 25 months (35 months for surviving patients), and median OS following salvage SBRT was 32 months. The 1- and 3-year OS rates were 84.4 and 67.8%, respectively. 1- and 3-year PFS rates were 80.8 and 58.7%, respectively. Only 4 patients (7.7%) developed local failure. Median LC was 71 months and 1- and 3-year LC rate were 97.9 and 94.9%, respectively. A total of 4 patients experienced grade 3 or higher adverse events (AEs) and two experienced grade 5 AEs (pneumonitis and hemoptysis). Central tumor location and the possibility of re-operation were independent prognostic factors for OS. The present study indicated that post-operative salvage SBRT is a promising therapeutic option for patients with NSCLC with locoregional or intrathoracic oligo-recurrence. We regard toxicity was also acceptable. However, further research is required on the appropriate selection of subjects, and stratification of the analysis by certain risk factors would increase the accuracy of the conclusions.
Keywords: salvage, stereotactic body radiotherapy, post-operative recurrence, oligo-recurrence, oligometastases, non-small cell lung cancer
Introduction
Surgery is considered the primary therapeutic option for the treatment of patients with early stage non-small cell lung cancer (NSCLC) (1–3). Although reported recurrence rates after definitive surgery vary between 28 and 60%, poor post-recurrence survival rates remain a challenge to the long-term survival of patients with NSCLC (4–7).
Standard treatment the for post-operative recurrence of NSCLC remains controversial. It is commonly systemic therapy with cytotoxic agents and/or molecular targeted agents as for metastatic stage IV disease (8,9). However, certain patients with loco-regional (only) recurrence or oligo-recurrences, that is, the state with a limited number of recurrent lesions and controlled primary lesions, a condition termed oligo-recurrence (10–13), are expected to achieve long-term survival and even cure with intensive local therapy alone (14–19).
Loco-regional recurrence of NSCLC is said to occur in 20–45% of patients during follow-up (5,14,20). If oligo-recurrence is included, >50% of patients with recurrence may be suitable for localized curative therapy (21,22). Although salvage surgery is considered to be the most promising current treatment, the majority of candidate patients do not undergo surgery because of post-operative comorbidities or poor baseline pulmonary function. Additionally, most of these patients are unable to tolerate chemotherapy, highlighting the importance of radiotherapy (23–25).
Stereotactic body radiotherapy (SBRT) is an important therapeutic option for patients with medically inoperable early-stage NSCLC or oligometastatic lung tumors (26–28). Even for patients in which operative treatment would be viable, SBRT has previously achieved results similar to those of surgery (29–31), and an increasing number of studies have reported the expansion of factors that may indicate the selection of SBRT, including large tumors and advanced-stage NSCLC (32,33). Nevertheless, few large-scale studies have reported the effect of SBRT on post-operative oligo-recurrence of NSCLC (34,35).
Therefore, the present study aimed to retrospectively assess the efficacy and safety of salvage SBRT for post-operative oligo-recurrence following primary curative lung resection in patients with NSCLC.
Materials and methods
Case eligibility
Following Institutional Review Board approval from the Ethics Committee of the University of Tokyo (Tokyo, Japan), a retrospective review was conducted of patients treated with SBRT, admitted to University of Tokyo Hospital between September 2010 and November 2016. The patients selected had previously received pulmonary resection for a primary NSCLC, and later developed nodular lesions in the thorax, which were determined to be post-operative oligo-recurrences. The median age of patients was 74 years, ranging from 50 years to 86 years. There were 38 males and 14 females. Seven patients rejected surgery at their own discretion. Written informed consent was obtained from all patients prior to treatment initiation.
Inclusion and exclusion criteria
The present study included patients who met the following inclusion criteria: i) Initial resection of NSCLC with curative intent; ii) clinical diagnosis of post-operative recurrence (approved by the Tumor Board for Lung Cancer of University of Tokyo Hospital based on biopsy or image findings and clinical data); iii) recurrent disease within the thorax, including mediastinal and hilar lymph nodes; iv) absence of metastases to solid organs, or pleural seeding; and v) <10 years between initial surgery and SBRT.
The exclusion criteria were as follow: i) <6 months of follow-up completed (excluding mortality as the reason); ii) no CT confirmation of recurrence after completion of SBRT; and iii) history of adjuvant radiotherapy. SBRT was not conducted in patients with apparent interstitial pneumonitis or pulmonary fibrosis on the chest film, due to the absence of established policy regarding performance status or respiratory function in these patients.
Clinical data collected from each patient included age, sex, interval between surgery and recurrence, recurrence sites and smoking index. Staging of the primary tumor was conducted in accordance with the 7th edition of the Union for International Cancer Control staging system for lung cancer (36).
Procedure
The decision to conduct appropriated salvage therapies was approved by the Tumor Board for Lung Cancer when post-operative oligo-recurrence was suspected. SBRT was often selected as the therapeutic approach for locoregional or intrathoracic oligometastases, where it could safely be applied. In other cases, chemoradiotherapy, chemotherapy, immunotherapy, palliative therapy and best supportive care were considered. Although cases that administered chemotherapy before and/or after SBRT were not excluded, there were no cases included in which chemotherapy was performed synchronously with SBRT.
Prior to the initiation of treatment, patients were immobilized in a stereotactic body frame and underwent a four-dimensional (4D) CT scan (2-mm slice thickness). Scans were performed using an external respiratory monitoring system (AZ-733 V®; Anzai Medical Co, Ltd.) under free breathing or with abdominal compression in cases where tumor excursion was >1 cm.
Mechanistically, 4D-CT planning divides the respiratory cycle into 10 sections. Respiratory phase data were transferred to a treatment planning system (TPS; Pinnacle3® version 9.1; Philips Healthcare). Gross tumor volume (GTV) was delineated in each respiratory phase using the lung window (window, 1,600 HU; level, −300 HU). The 10 GTVs were fused to form the internal target volume. A uniform 5-mm margin was then added to create the planning target volume (PTV) (37–39). The main organs at risk (OARs), namely the heart, lungs, esophagus, spinal cord, proximal tracheobronchial tree and brachial plexus, were contoured according to the guidelines outlined in the Radiation Therapy Oncology Group (RTOG) 0236 trial (40).
Patients admitted between September 2010 and March 2013 were treated using a conventional SBRT plan using 6–12 beams, whereas patients admitted from April 2013 onwards were treated using volumetric modulated arc therapy (VMAT)-SBRT with 6 or 10 MV beams using an Elekta-synergy system (Elekta Instrument AB). There was no significant difference in treatment outcome between the two methods (41). VMAT plans were designed using a single partial arc with angle ranges of −40° to 180° (left lung) or −180° to 40° (right lung), as previously detailed (37,38,41,42). Dosimetric planning and plan analysis were performed using Pinnacle3. The collapsed cone convolution method (comparable to the superposition method) in the TPS was used (42,43). All final calculations were performed using a grid size of 2.0 mm. Dose distributions were calculated using CT data obtained at peak exhalation. Treatment was performed with 48–55 Gy in 4 fractions for peripheral tumors (39 lesions) or 56 Gy in 7 fractions for central tumors (17 cases) to cover 95% of the PTV (D95%).
The central lesion was defined as a tumor <2 cm from the proximal bronchial tree, according to the RTOG 0236 guidelines, or <2 cm in any direction from a critical mediastinal structure, including the bronchial tree, esophagus, heart, brachial plexus, major vessels, spinal cord, phrenic nerve and recurrent laryngeal nerve, as in most recent studies (44,45). For certain hilar or mediastinal tumors, or tumors invading the trachea or bronchi, a regimen with increased number of fractions (such as 50 Gy in 10 fractions) was used (46,47). The dose constraints of OARs were defined based on the protocol of the Japan Clinical Oncology Group (JCOG) 0403 trial (48). Regarding the pulmonary dose, adding to the constraints described in JCOG 0403 (V20, V15, mean lung dose, V10 (<40%) and V5 (<70%) were also restricted to reduce the volume of low dose area, which tends to be large in VMAT plans (49,50) (Table I). Furthermore, dosage deviations to organs other than those listed in Table I were permitted according to clinical benefit (51).
Table I.
Dose constraints of organs at risk.
| Organ at risk | Dose constraints | Dose effort targets |
|---|---|---|
| Lung | ||
| MLD <18.0 Gy | MLD <18.0 Gy | |
| V20 <20% | V20 <20% | |
| V15 <25% | V15 <25% | |
| V10 <40% | V10 <40% | |
| V5 <70% | V5 <70% | |
| Spinal cord | 25 Gy/4–10 fractions (0 cm3) | 40 Gy/4–10 fractions (0cm3) |
| Heart (mean) | 25 Gy/4–10 fractions (0 cm3) | |
| Heart (max) | 55 Gy/4–10 fractions (0 cm3) | |
| Liver | 30 Gy/4–10 fractions (<10 cm3) | |
| Esophagus | 40 Gy/4–10 fractions (0 cm3) | |
| pulmonary artery | 40 Gy/4–10 fractions (0 cm3) | |
| Trachea/bronchus | 40 Gy/4–10 fractions (0 cm3) | |
| rib, chest wall | 40 Gy/4–10 fractions (0 cm3) |
MLD, mean lung dose; V20/15/10/5, percentage of the volume of an organ receiving 20, 15, 10 and 5 Gy. No constraints: Esophagus, pulmonary artery, trachea/bronchus, rib, chest wall; aimed for less than 40 Gy for these organs.
Follow-up and chart review
Follow-up commenced on the first day of radiotherapy initiation and ended on May 1, 2018. Patients underwent a CT scan of the chest and abdomen every 3 months for 2 years, and received ≥1 scan every 6 months thereafter.
Tumor recurrence was diagnosed as progressive and increasing by CT scan abnormalities, and confirmed by progressive and incremental increases in the standardized uptake value of a lesion, following serial PET imaging (with or without biopsy). Furthermore, locoregional recurrence was defined as disease recurrence at the surgical margin, ipsilateral hemi-thorax or regional lymph nodes. Distant metastasis was defined as metastasis to the contralateral lung and to outside of the hemi-thorax or mediastinum.
Overall survival (OS) was defined as the period from the first day of SBRT initiation to the date of mortality from any cause, or to the last follow-up visit or telephone contact prior to May 1, 2018. Progression-free survival (PFS) and local control (LC) were defined as the interval from the first day of SBRT to documented disease progression and locoregional recurrence (or mortality/follow-out), respectively. If SBRT was performed twice after surgery to treat recurrence, PFS for the first SBRT was defined as the interval between the two SBRTs.
Evaluation of toxicity
Toxicity was evaluated and graded according to the Common Terminology Criteria for Adverse Events v4.0 (52). Radiation pneumonitis (RP) was diagnosed according to clinical symptoms, including cough, shortness of breath, fever and radiologic findings in the absence of any other likely cause. All uncertain cases were discussed by the tumor board and either verified via biopsy or by consensus of the board. All hospital records, follow-up notes and images were reviewed, including all patient data regarding tumor and treatment characteristics.
Statistical analysis
The 1- and 3-year OS, RFS and LC rate were calculated using the Kaplan-Meier method; the log rank test was used for group comparisons. Survival was calculated from the end of SBRT. Cox proportional hazards models were used to assess factors associated with survival. All statistical analyses were performed using EZR version 1.36 which is a graphical interface for R (The R Foundation for Statistical Computing) (53), and the significance of univariate and multivariate analyses was set at P<0.05. All statistical tests were two-sided. The biologically effective dose (BED) was calculated using the BED10 linear-quadratic equation with an α/β value of 10 for tumors (54).
Results
Patient and treatment characteristics
A total of 61 patients (with 70 lesions among them) received chest SBRT for post-operative oligo-recurrence of NSCLC. Following exclusion of 9 patients in whom the time from surgery to SBRT was >10 years, a total of 52 patients, with 59 lesions among them, were evaluated. Patient characteristics are summarized in Table II.
Table II.
Clinicopathological characteristics.
| Characteristics | Value |
|---|---|
| Patient characteristics (n=52) | |
| Median age at recurrence, years (range) | 74 (50–86) |
| Sex (male:female), n | 38:14 |
| KPS (≥90:<90), n | 47:5 |
| Smoking history (yes:no), n | 30:22 |
| Median Brinkman index, n (range) | 580 (0–3,000) |
| Operability of recurrent tumor (operable:inoperable) | 7:45 |
| Tumor characteristics (n=59) | |
| Median SUVmax, n (range) | 4.65 (0.87–19.5) |
| Histological type at primary surgery (Ad:Sq), n | 45:14 |
| pT classification (pT1:pT≥2) | 27:32 |
| pN classification (pN0:pN≥1) | 50:9 |
| pM classification (pM0:pM≥1) | 59:0 |
| Type of initial surgery (lobectomy or pneumonectomy:sublobular resection), n | 41:18 |
| Median disease-free interval prior to SBRT, months | 30.5 |
| Disease-free interval prior to SBRT (<1:≥1 years), n | 12:47 |
| Disease-free interval prior to SBRT (<5:≥5 years), n | 47:12 |
| SBRT for the recurrence tumor (n=59) | Value |
| Median tumor size, cm (range) | 1.7 (0.1–5.6) |
| Tumor size (≤2:>2 cm), n | 34:25 |
| Recurrent site (central:peripheral), n | 20:39 |
| Dose prescription | |
| Median BED10, Gy (range) | 112.5 (75–130.6) |
| 55 Gy in 4 fractions (BED10 130.6 Gy)), n (%) | 21 (35.6%) |
| 50 Gy in 4 fractions (BED10 112.5 Gy), n (%) | 18 (30.5%) |
| 56 Gy in 7 fractions (BED10 100.8 Gy), n (%) | 18 (30.5%) |
| 50 Gy in 10 fractions (BED10 75 Gy), n (%) | 2 (3.4%) |
KPS, Karnofsky performance status; Brinkman index, cigarettes/day × years; SUVmax, maximum standardized uptake value; Ad, adenocarcinoma; Sq, squamous cell carcinoma; SBRT, stereotactic body radiotherapy; BED10, biologically effective dose (using the LQ model with the α/β=10 Gy); pT, pathological T classification; pN, pathological N classification; pM, pathological M classification.
Seven patients rejected surgery at their own discretion. Decreased respiratory function was the most common parameter used for selection of SBRT instead of surgery (55,56). The initial pulmonary resection was standard surgery (lobectomy with mediastinal lymph node dissection) or more in 35/52 patients (67.3%) with 41/59 lesions (69.5%), one case of which was pneumonectomy, whereas limited resection was performed for the remaining 17/52 cases (32.7%) with 18/59 lesions (30.5%). The majority of patients were unsuitable for chemotherapy, and only 8/52 (15.4%) patients received chemotherapy each before and after SBRT.
Pathological diagnosis at primary surgery was adenocarcinoma in 39/52 patients (75.0%) and squamous carcinoma in 13/52 (25.0%). Further, 9/52 patients (17.3%) exhibited pathological lymph node metastases. The epidermal growth factor receptor mutation status was detected in 4/11 of the patients in which it was tested for.
The median interval between initial resection and salvage SBRT for recurrence was 30.5 months (range, 2.0–99.0 months). A total of 20/59 lesions (33.9%) were located in the central area, which is a greater proportion than that in the whole SBRT cohort of University of Tokyo Hospital during the same period (41).
The most common fractionation schemes were 55 Gy in 4 fractions (BED10=130.6 Gy; 21 lesions, 35.6%), followed by 50 Gy in 4 fractions (BED10=112.5 Gy; 18 lesions, 30.5%), and 56 Gy in 7 fractions (BED10=100.8 Gy; 18 lesions, 30.5%), resulting in a median BED10 of 112.5 Gy (Table II).
Treatment outcomes and failure patterns
The median follow-up time for all patients was 25 months (range, 3–63 months), and for surviving patients it was 35 months (range, 10–71 months). During follow-up, 18 (34.6%) patients experienced disease progression. Among these, locoregional recurrence was observed in only 4 (7.7%) patients, all of whom suffered from intrapulmonary metastasis and/or pleural dissemination, other than local recurrence. In 8 (44.4%) patients with relapse, ≥2 lesions were found at first recurrence. The most common site of recurrent lesions was the contralateral lung (8 cases; 44.4%), followed by lymph node (6 cases; 33.3%), local and bone (4 cases; 22.2%) and brain and pleural dissemination (3 cases; 16.7%).
Survival and prognostic factors
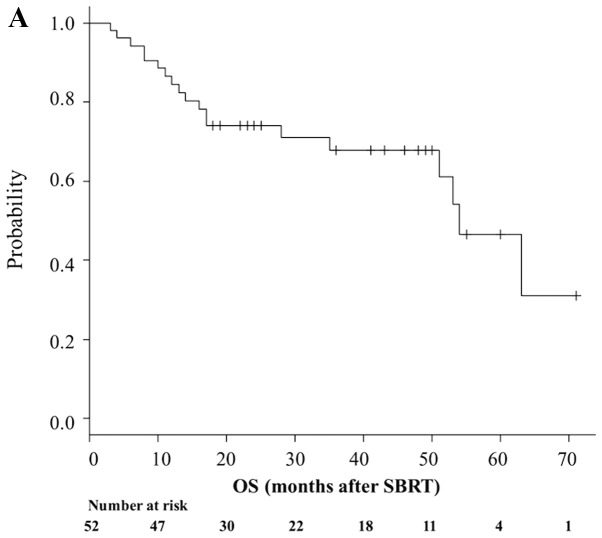
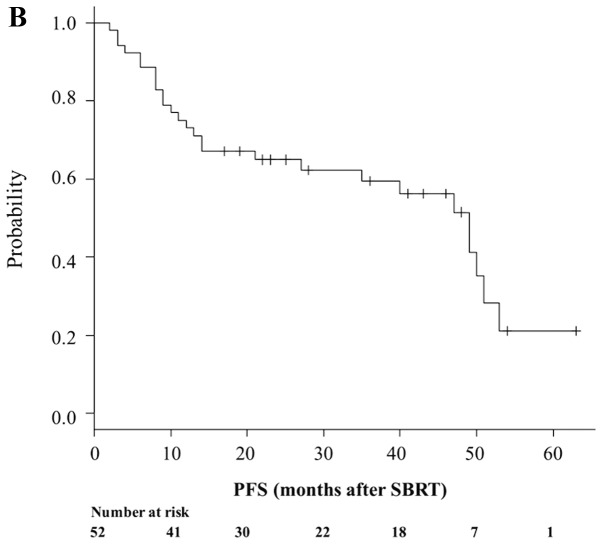
A total of 19 patients (36.5%) died during follow-up. Median OS time was 54 months (95% CI, 51-NA; Fig. 1A), while 1- and 3-year OS rates were 84.4% (95% CI, 71.3–91.9%) and 67.8% (95% CI, 51.8–79.5%), respectively (Fig. 1A). A total of 33 patients (63.5%) remained alive at the last follow-up, while 25 (48.1%) were both alive and progression-free. Median PFS time was 51 months (95% CI, 28–60), while 1- and 3-year PFS rates were 80.8% (95% CI, 67.2–89.2) and 58.7% (95% CI, 43.2–71.3), respectively (Fig. 1B). A total of 4 patients (7.7%) developed local failure. Median LC was 71 months (range, 60 months-NA). The 1- and 3-year Kaplan-Meier-estimated LC rate was 97.9% (95% CI, 85.8–99.7) and 94.9% (95% CI, 80.8–98.7), respectively (Fig. 1C).
Figure 1.

Prognosis of patients who underwent SBRT. Kaplan-Meier estimates of (A) OS, (B) PFS and (C) LC for patients who underwent SBRT. OS, overall survival; PFS, progression-free survival; LC, local control; SBRT, stereotactic body radiotherapy.
Predictive factors for OS
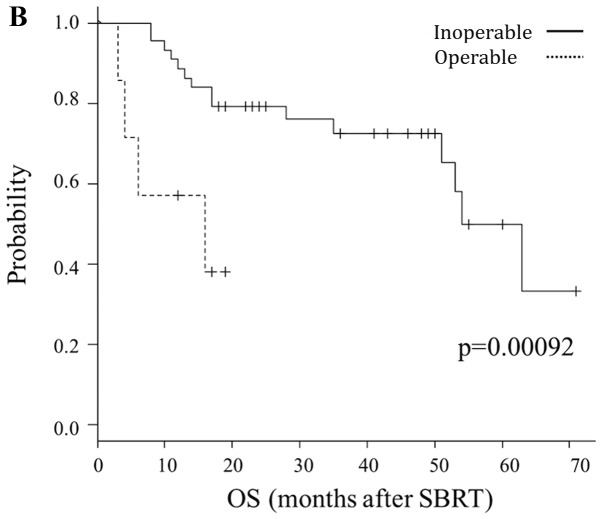
Table III provides the results of univariate and multivariate analyses for OS. Sex, possibility of re-operation, disease-free interval between initial surgery and local recurrence (≥1 vs. <1 years) and location (central or peripheral) and dose prescription were revealed to be significant prognostic factors for OS, following univariate analysis. By contrast, multivariate analysis indicated that location (central vs. peripheral; P=0.0012) and possibility of re-operation (impossible vs. possible; P=0.00092) were significant prognostic factors for OS. Fig. 2 illustrates the Kaplan-Meier curves according to these factors.
Table III.
Analysis of clinical and dosimetric variables associated with OS (patients, n=52; tumors, n=59).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Patient | ||||
| Age at recurrences, years (≤75 vs. >75) | 1.81 (0.22–1.48) | 0.74 | ||
| Sex (male vs. female) | 1.72 (4.31–6.90) | 0.0062 | 3.94 (0.80–19.37) | 0.091 |
| Smoking history (yes vs. no) | 1.11 (0.01–1.05) | 0.55 | ||
| Initial surgery for primary NSCLC | ||||
| Histology (adenocarcinoma vs. alternative subtypes) | 1.19 (0.0038–3.73) | 0.23 | ||
| Extent of pulmonary resection (sublobular resection vs. lobectomy or pneumonectomy) | 4.16 (0.048–3.61) | 0.43 | ||
| T status (pT2 vs. pT1) | 6.38 (0.10–3.93) | 0.63 | ||
| Lymphatic invasion (present vs. absent) | 3.51 (0.023–5.32) | 0.45 | ||
| Lymph node metastasis (pN≥1 vs. pN0) | 1.37 (0.73–2.56) | 0.080 | ||
| Disease-free interval, years (≥1 vs. <1) | 4.76 (1.99–1.14) | 0.017 | 0.92 (4.26–19.52) | 0.062 |
| Disease-free interval, years (≥5 vs. <5) | 2.50 (0.24–2.55) | 0.44 | ||
| SBRT for recurrent tumors | ||||
| Possibility of re-operation (impossible vs. possible) | 2.46 (29.07–2.08) | <0.0010 | 9.53 (2.51–36.15) | <0.001 |
| Tumor size (≤2 cm vs. >2 cm) | 6.05 (0.043–8.52) | 0.71 | ||
| Tumor size (≤3 cm vs. >3 cm) | 2.38 (0.0058–9.77) | 0.45 | ||
| SUVmax (≥5.0 vs. <5.0) | 1.57 (0.17–1.43) | 0.69 | ||
| Location (lower or mediastinum vs. upper or middle) | 1.18 (0.29–4.86) | 0.82 | ||
| Central lesion (central vs. peripheral) | 1.22 (0.37–4.050) | 0.011 | 5.51 (1.96–15.49) | 0.0012 |
| Dose prescription (55 Gy 4 Fr vs. the others (50 Gy, 4 Fr; | 2.52 (2.10–3.00) | 0.011 | 0.03 (0.27–2.31) | 0.23 |
| 56 Gy, 7 Fr; 50 Gy, 10 Fr) | ||||
| Chemotherapy after SBRT (present vs. absent) | 7.97 (0.035–1.82) | 0.89 | ||
P-values were calculated using Cox regression analysis for univariate and multivariate analysis. OS, overall survival; HR, hazard ratio; SBRT, stereotactic body radiotherapy; SUVmax, maximum standardized uptake value; Fr, fraction.
Figure 2.
Comparison of OS by prognostic factors. OS according to (A) tumor location (peripheral vs. central) and (B) possibility of re-operation (operable vs. inoperable). OS, overall survival; SBRT, stereotactic body radiotherapy.
Regarding PFS, in addition to these factors, age (≥75 years; P=0.037), type of prior surgery (limited surgery; P=0.0046), diameter of the primary tumor (pT ≥2; P=0.014) and recurrent lesion (≥2 cm; P=0.039) were also significant prognostic factors.
Toxicity
In total, 9 patients (17.3%) developed grade 2 adverse events (AEs), and 4 patients (7.7%) developed grade 3 or greater AEs. Of these, two patients (3.8%) developed grade 5 AEs, meaning mortality due to toxicity. One grade 5 case was radiation pneumonitis and the second was hemoptysis. The prescribed dose for both patients was 56 Gy in 7 fractions. Further details of the two aforementioned cases have been reported by the present authors in a previous study (33).
The doses for OARs in these two cases were summarized in Table IV. In both cases, dose-restricted organs were not irradiated above the limit, but some unrestricted OARs were being given higher doses than the effort target (in other words, the indicators to achieve if possible). The other common features of the two cases were having recurrence following lung lobectomy, central lesions and presence of a smoking history.
Table IV.
Irradiated dose for organs at risk of two patients who exhibited grade 5 AEs.
| OAR | Patient 1 | Patient 2 |
|---|---|---|
| ITV, cm2 | 5.0 | 9.6 |
| PTV, cm2 | 19.4 | 33.0 |
| Lung | 5.0 | 9.6 |
| V5 (%) | 16.6 | 31.2 |
| V10 (%) | 6.1 | 22.8 |
| V20 (%) | 3.8 | 11.3 |
| Mean (cGy) | 355.8 | 703.9 |
| Trachea | ||
| Max (cGy; point) | 628.7 | 162.4 |
| Max (cGy; 5cc) | 226.1 | 122.3 |
| Carina | ||
| Max (cGy; point) | 6,109.3 | 4,644.6 |
| Max (cGy; 5cc) | – | 489.3 |
| Esophagus | ||
| Max (cGy; point) | 3,402.0 | 5,364.6 |
| Max (cGy; 5cc) | 1,809.0 | 1,223.2 |
| Pulmonary artery | ||
| Max (cGy; point) | 5,583.2 | 4,654.9 |
| Max (cGy; 5cc) | 226.1 | 1,467.8 |
| Pulmonary veins | ||
| Max (cGy; point) | 2,412.2 | 5,610.4 |
| Max (cGy; 5cc) | – | – |
| Aorta | ||
| Max (cGy; point) | 2,818.5 | 3,834.4 |
| Max (cGy; 5cc) | 2,366.8 | 2477 |
| Superior vena cava | ||
| Max (cGy; point) | 3,641.2 | 5,770.8 |
| Max (cGy; 5cc) | 2,788.9 | 3,914.3 |
| Heart | ||
| Mean (cGy) | 274.3 | 879 |
| V30 (%) | 1.3 | 5.7 |
| Spine | ||
| Max (cGy; point) | 1,387.5 | 2,034.8 |
| Chest wall | ||
| Max (cGy; point) | – | 4,084.6 |
Dose prescription; 56 Gy in 7 fractions, Patient1; hemoptysis, Patient 2; pneumonitis. AE, adverse events; OAR, organ at risk; ITV, internal target volume; PTV, planning target volume; V5/10/20/30, percentage of the volume of an organ receiving 5, 10, 20 and 30 Gy; 5cc, cubic centimeter; cGy, centi Gy.
Discussion
Post-operative recurrence of NSCLC is commonly treated using a multifaceted treatment program, including systemic therapy, as with metastatic stage IV disease (8,9). However, certain recurrent cases with local lesions alone or a limited number of metastatic lesions (termed oligo-recurrence), may occasionally be cured using localized therapy alone (11–14).
A number of studies have evaluated second resection for local recurrence or intrathoracic oligo-recurrence in patients who received surgery as initial treatment (6,57–59). Hung et al (6) reported 1- and 2-year post-recurrence OS rates of 48.7 and 17.6%, respectively, in their study of 74 patients with local recurrence. Notably, Kim et al (57) reported a 5-year OS rate after second resection of 33.4% with an operative mortality for the second resection of 5.8%. Previously, Yukiue et al (58) achieved 2- and 5-year OS rates after second resection of 87.8 and 62.9%, respectively. However, 9 patients (23%) exhibited serious post-operative complications and 1 (2.6%) died during surgery, raising concerns regarding the safety of the operation (58). Alternative reports on post-operative recurrence have also indicated that re-operation may represent an effective treatment for post-operative lung cancer recurrence, in certain patients in which the oncological benefits outweigh the surgical risk (14,59).
By contrast, SBRT has been recently recognized as an alternative therapy for patients with inoperable early-stage NSCLC or those who refuse surgery (26,28,31). Regarding post-operative oligo-recurrence, SBRT is not an established standard therapy and no large prospective clinical trials have been conducted. Takeda et al (60) analyzed the outcomes of SBRT in 23 patients with isolated post-surgical local recurrence. LC and OS rates were revealed to be 94.7 and 86.8% at 1 year and 84.0 and 76.4% at 2 years, respectively. Regarding AEs, 3 patients (13.0%) suffered from RP grade ≥3 and the authors concluded that SBRT, when used to treat isolated postsurgical local recurrence, achieves high LC with limited toxicity (60). Nishiyama et al (61) subsequently investigated 41 patients with medically inoperable diseases who underwent SBRT for second pulmonary nodules arising from different types of cancer and reported that grade 2 RP AEs occurred in five patients and one succumbed to grade 5 RP.
In the present study, the 3-year OS, PFS and LC rates of all included patients were 67.8, 58.7 and 94.9%, respectively, and these results are comparable not only to previous studies on SBRT (4,16,17), but also on re-operation. Considering that More than two thirds of patients (≥67.3%) in the present analysis were regarded as ineligible for surgery or chemotherapy, the survival outcomes for salvage SBRT as a therapeutic technique are promising.
Additionally, it was revealed that the major failure pattern after radical radiotherapy was distant metastasis. This finding is consistent with the results of a study by Kelsey et al (62), in which 50% of patients developed distant metastases following salvage radiotherapy. SBRT, which can achieve good local control, may be expected to improve recurrence-free survival by combination with recently developed immunotherapy.
Several retrospective studies have investigated prognostic factors for OS time in patients with local recurrence (62–64); the reported factors associated with prolonged OS time were female, young age, long disease-free interval between initial surgery and local recurrence (5,28,62–65), early stage of primary tumor (5,66) and high prescribed radiation dose (67,68). In the present study, female and disease-free interval were significantly associated with OS time only in univariate analysis. In multivariate analysis, central lesions and re-operative lesions (operative refusal by patients) were significantly associated with poor survival prognosis.
Similar to previous findings (47–49,69–72), central lesions exhibited a worse prognosis than peripheral lesions in the present study. One possible reason for this may be an increase in the number of AEs, which are associated with central SBRT (44,46). Among the included patients, all 4 cases of AEs graded ≥3 (including a grade 5 case) occurred in patients with central lesions.
Additionally, relatively low doses for central tumors may also contribute to the inferior outcomes; higher radiation doses have been reported to be associated with prolonged OS time, even in patients with post-operative recurrence (67,68), although there was no indication of a survival difference between high and low BED (above and below BED10 ≤130.6), in the present study. It was concluded that the cause was that most patients treated at University of Tokyo Hospital have been treated with BED10 100 Gy or higher. Notably, in a study by Kim et al (4), it was suggested that determining whether increasing radiation alone improves survival may be difficult in a situation where high doses were administered and irradiation technology was developed (4).
In the present study, patients who underwent sublobular resection exhibited an improved prognosis compared with those who received lobectomy or pneumonectomy. The prognosis of initial surgery itself is considered to be improved with lobectomy compared with sublobular resection (73,74), indicating that the results are reversed in cases of post-operative oligo-recurrence. The current findings may be a result of the limited number of cases that were considered as appropriate (based on invasion characteristics) reduce the ablation range, or even the small population size, especially in the operable group.
In the present study, the irradiated dose for the OARs of two patients with grade 5 AEs were reviewed. As described in the results section, the dose delivered to restricted OARs in these two cases did not exceed the constraints, but certain unrestricted OARs were being treated with a higher dosage than the effort target (48). The present results indicated dose restrictions on certain OARs, such as blood vessels and trachea, which have not currently been restricted.
In addition, patient factors, such as smoking history (75) and interstitial lung disease (76,77), have been reported as risk factors too. The occurrence of severe AEs may be associated with the clinicopathological factors of patients and tumors as well as the radiation dose. All these factors act synergistically and it is difficult to accurately quantify the relative contribution of each factor. Although a conclusion was not reached in the present study, risk stratification combining both patient and radiation factors should be performed in future research. Collecting and analyzing data of serious AEs is difficult for a single institution; thus, risk analyses will require multi-center, long-term data accumulation to improve their statistical power.
The present study had several limitations. Primarily, it was conducted at a single institution and using a retrospective design. Therefore, a degree of intrinsic bias may remain, and information regarding clinical examinations (respiratory function, PET and status of gene expression) was insufficient in some cases, so that it was not possible to examine the associations between treatment outcomes. Additionally, the number of patients was low, which may have limited the statistical confidence of the results. Further research is necessary, including prospective studies with a large sample size, in order to support the conclusions of the present study. Finally, it is difficult to distinguish between post-operative recurrence and multiple primary lung cancers, even when pathological examinations are performed.
Furthermore, it is difficult to compare AE risk in cases of different prescriptions, because dose division for each dose restriction has not yet been established. This is an issue to be clarified in future research.
The present study suggested that salvage SBRT represents a promising treatment for patients with NSCLC exhibiting post-operative locoregional or intrathoracic oligo-recurrence, particularly in LC. Independent risk factors associated with a decreased OS were a central lesion and the possibility of re-operation. The AEs were also considered as tolerable. However, further research is required on the selection of subjects and stratification by risk factors.
Acknowledgements
The authors would like to thank Dr Libby Cone for editing the drafts of this manuscript.
Funding
The present study was supported by a Grant-in-Aid from Japan Society for the Promotion of Science, KAKENHI JP Scientific Research (C) (grant no. 18K07667).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SA, HY, WT, JNa, MS, OA and KeN participated in research design. Acquisition of the data was performed by SA, TI, SO and TK. Evaluation of the images was conducted by SA, KaN, TO and YN. Interpretation of the data was conducted by SA, MA and JNi. The manuscript was prepared by SA, HY and WT, and written by SA and HY. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee, University of Tokyo Hospital [Tokyo, Japan; 3372-(3)/2016]. Written informed consent for data collection and analysis was obtained from the respective patients.
Patient consent for publication
Patients provided written consent for the publication of their data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et al. A Japanese lung cancer registry study: Prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 2.Goya T, Asamura H, Yoshimura H, Kato H, Shimokata K, Tsuchiya R, Sohara Y, Miya T, Miyaoka E, Japanese Joint Committee of Lung Cancer Registry Prognosis of 6644 resected non-small cell lung cancers in Japan: A Japanese lung cancer registry study. Lung Cancer. 2005;50:227–234. doi: 10.1016/j.lungcan.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, Nomori H, Fujii Y, Okumura M, Yokoi K, Japanese Joint Committee for Lung Cancer Registration Japanese lung cancer registry study of 11,663 surgical cases in 2004: Demographic and prognosis changes over decade. J Thorac Oncol. 2011;6:1229–1235. doi: 10.1097/JTO.0b013e318219aae2. [DOI] [PubMed] [Google Scholar]
- 4.Kim E, Song C, Kim MY, Kim JS. Long-term outcomes after salvage radiotherapy for postoperative locoregionally recurrent non-small-cell lung cancer. Radiat Oncol J. 2017;35:55–64. doi: 10.3857/roj.2016.01928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugimura H, Nichols FC, Yang P, Allen MS, Cassivi SD, Deschamps C, Williams BA, Pairolero PC. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83:409–418. doi: 10.1016/j.athoracsur.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 6.Hung JJ, Hsu WH, Hsieh CC, Huang BS, Huang MH, Liu JS, Wu YC. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax. 2009;64:192–196. doi: 10.1136/thx.2007.094912. [DOI] [PubMed] [Google Scholar]
- 7.Endo C, Sakurada A, Notsuda H, Noda M, Hoshikawa Y, Okada Y, Kondo T. Results of long-term follow-up of patients with completely resected non-small cell lung cancer. Ann Thorac Surg. 2012;93:1061–1068. doi: 10.1016/j.athoracsur.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Socinski MA, Evans T, Gettinger S, Hensing TA, VanDam Sequist L, Ireland B, Stinchcombe TE. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl 5):e341S–e368S. doi: 10.1378/chest.12-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mok TS, Lee K, Leung L. Targeting epidermal growth factor receptor in the management of lung cancer. Semin Oncol. 2014;41:101–109. doi: 10.1053/j.seminoncol.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: The new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107–111. doi: 10.1093/jjco/hyp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niibe Y, Chang JY. Novel insights of oligometastases and oligo-recurrence and review of the literature. Pulm Med. 2012;2012:261096. doi: 10.1155/2012/261096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niibe Y, Chang JY, Onishi H, Salama J, Hiraki T, Yamashita H. Oligometastases/Oligo-recurrence of lung cancer. Pulm Med. 2013;2013:438236. doi: 10.1155/2013/438236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niibe Y, Jingu K, Onishi H. Oligo-recurrence and Sync-oligometastases. J Thorac Oncol. 2018;13:e59–e60. doi: 10.1016/j.jtho.2017.11.115. [DOI] [PubMed] [Google Scholar]
- 14.Hishida T, Yoshida J, Aokage K, Nagai K, Tsuboi M. Postoperative oligo-recurrence of non-small-cell lung cancer: Clinical features and survival†. Eur J Cardiothorac Surg. 2016;49:847–853. doi: 10.1093/ejcts/ezv249. [DOI] [PubMed] [Google Scholar]
- 15.Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer. 2013;82:197–203. doi: 10.1016/j.lungcan.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Niibe Y, Jingu K, Onishi H. Long-term outcome of surgery or stereotactic radiotherapy for lung oligo-recurrence. J Thorac Oncol. 2017;12:e191. doi: 10.1016/j.jtho.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita H, Niibe Y, Yamamoto T, Katsui K, Jingu K, Kanazawa S, Terahara A, Nakagawa K. Lung stereotactic radiotherapy for oligometastases: Comparison of oligo-recurrence and sync-oligometastases. Jpn J Clin Oncol. 2016;46:687–691. doi: 10.1093/jjco/hyw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekihara K, Hishida T, Yoshida J, Oki T, Omori T, Katsumata S, Ueda T, Miyoshi T, Goto M, Nakasone S, et al. Long-term survival outcome after postoperative recurrence of non-small-cell lung cancer: Who is ‘cured’ from postoperative recurrence? Eur J Cardiothorac Surg. 2017;52:522–528. doi: 10.1093/ejcts/ezx127. [DOI] [PubMed] [Google Scholar]
- 19.Brooks ED, Verma V, Senan S, De Baere T, Lu S, Brunelli A, Chang JY, International Association for the Study of Lung Cancer Advanced Radiation Technology Committee Salvage therapy for locoregional recurrence after stereotactic ablative radiotherapy for early-stage NSCLC. J Thorac Onco. 2019 Nov 9; doi: 10.1016/j.jtho.2019.10.016. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor MD, Nagji AS, Bhamidipati CM, Theodosakis N, Kozower BD, Lau CL, Jones DR. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg. 2012;93:1813–1821. doi: 10.1016/j.athoracsur.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Voltolini L, Paladini P, Luzzi L, Ghiribelli C, Di Bisceglie M, Gotti G. Iterative surgical resections for local recurrent and second primary bronchogenic carcinoma. Eur J Cardiothorac Surg. 2000;18:529–534. doi: 10.1016/S1010-7940(00)00572-8. [DOI] [PubMed] [Google Scholar]
- 22.Battafarano RJ, Force SD, Meyers BF, Bell J, Guthrie TJ, Cooper JD, Patterson GA. Benefits of resection for metachronous lung cancer. J Thorac Cardiovasc Surg. 2004;127:836–842. doi: 10.1016/j.jtcvs.2003.08.055. [DOI] [PubMed] [Google Scholar]
- 23.Sun B, Brooks ED, Komaki R, Liao Z, Jeter M, McAleer M, Balter PA, Welsh JD, O'Reilly M, Gomez D, et al. Long-term outcomes of salvage stereotactic ablative radiotherapy for isolated lung recurrence of non-small cell lung cancer: A phase II clinical trial. J Thorac Oncol. 2017;12:983–992. doi: 10.1016/j.jtho.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saisho S, Yasuda K, Maeda A, Yukawa T, Okita R, Hirami Y, Shimizu K, Nakata M. Post-recurrence survival of patients with non-small-cell lung cancer after curative resection with or without induction/adjuvant chemotherapy. Interact Cardiovasc Thorac Surg. 2013;16:166–172. doi: 10.1093/icvts/ivs450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K, Kim HR, Kim DK, Kim YH, Park SI, Choi SH, Han J. Post-recurrence survival analysis of stage I non-small-cell lung cancer. Asian Cardiovasc Thorac Ann. 2017;25:623–629. doi: 10.1177/0218492317737641. [DOI] [PubMed] [Google Scholar]
- 26.Ricardi U, Badellino S, Filippi AR. Stereotactic radiotherapy for early stage non-small cell lung cancer. Radiat Oncol J. 2015;33:57–65. doi: 10.3857/roj.2015.33.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crabtree TD, Denlinger CE, Meyers BF, El Naqa I, Zoole J, Krupnick AS, Kreisel D, Patterson GA, Bradley JD. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:377–386. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 28.Kimura T, Nagata Y, Eba J, Ozawa S, Ishikura S, Shibata T, Ito Y, Hiraoka M, Nishimura Y, Radiation Oncology Study Group of the Japan Clinical Oncology Group A randomized phase III trial of comparing two dose-fractionations stereotactic body radiotherapy (SBRT) for medically inoperable stage IA non-small cell lung cancer or small lung lesions clinically diagnosed as primary lung cancer: Japan clinical oncology group study JCOG1408 (J-SBRT trial) Jpn J Clin Oncol. 2017;47:277–281. doi: 10.1093/jjco/hyw198. [DOI] [PubMed] [Google Scholar]
- 29.Rosen JE, Salazar MC, Wang Z, Yu JB, Decker RH, Kim AW, Detterbeck FC, Boffa DJ. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg. 2016;152:44–54.e9. doi: 10.1016/j.jtcvs.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 30.Chang JY, Liu YH, Zhu Z, Welsh JW, Gomez DR, Komaki R, Roth JA, Swisher SG. Stereotactic ablative radiotherapy: A potentially curable approach to early stage multiple primary lung cancer. Cancer. 2013;119:3402–3410. doi: 10.1002/cncr.28217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onimaru R, Shirato H, Shibata T, Hiraoka M, Ishikura S, Karasawa K, Matsuo Y, Kokubo M, Shioyama Y, Matsushita H, et al. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer with PTV<100 cc using a continual reassessment method (JCOG0702) Radiother Oncol. 2015;116:276–280. doi: 10.1016/j.radonc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Verma V, Shostrom VK, Zhen W, Zhang M, Braunstein SE, Holland J, Hallemeier CL, Harkenrider MM, Iskhanian A, Jabbour SK, et al. Influence of fractionation scheme and tumor location on toxicities after stereotactic body radiation therapy for large (≥5 cm) non-small cell lung cancer: A multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;97:778–785. doi: 10.1016/j.ijrobp.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 2018;4:e173501. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang HH, Zaorsky NG, Meng MB, Zeng XL, Deng L, Song YC, Zhuang HQ, Li FT, Zhao LJ, Yuan ZY, et al. Stereotactic radiation therapy for oligometastases or oligorecurrence within mediastinal lymph nodes. Oncotarget. 2016;7:18135–18145. doi: 10.18632/oncotarget.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobin LH, Gospodarowicz MK, Wittekind C, editors. 7th. Wiley-Blackwell; Oxford: 2009. International Union Against Cancer (UICC): TNM classification of malignant tumours. [Google Scholar]
- 37.Yamashita H, Takahashi W, Haga A, Kida S, Saotome N, Nakagawa K. Stereotactic body radiotherapy for small lung tumors in the University of Tokyo hospital. Biomed Res Int. 2014;2014:136513. doi: 10.1155/2014/136513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagawa K, Haga A, Kida S, Masutani Y, Yamashita H, Takahashi W, Sakumi A, Saotome N, Shiraki T, Ohtomo K, et al. 4D registration and 4D verification of lung tumor position for stereotactic volumetric modulated arc therapy using respiratory-correlated cone-beam CT. J Radiat Res. 2013;54:152–156. doi: 10.1093/jrr/rrs058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa K, Haga A, Sakumi A, Yamashita H, Igaki H, Shiraki T, Ohtomo K, Iwai Y, Yoda K. Impact of flattening-filter-free techniques on delivery time for lung stereotactic volumetric modulated arc therapy and image quality of concurrent kilovoltage cone-beam computed tomography: A preliminary phantom study. J Radiat Res. 2014;55:200–202. doi: 10.1093/jrr/rrt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, et al. Stereotactic body radiation therapy for medically inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aoki S, Yamashita H, Haga A, Nawa K, Imae T, Takahashi W, Abe O, Nakagawa K. Flattening filter-free technique in volumetric modulated arc therapy for lung stereotactic body radiotherapy: A clinical comparison with the flattening filter technique. Oncol Lett. 2018;15:3928–3936. doi: 10.3892/ol.2018.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aoki S, Yamashita H, Haga A, Ota T, Takahashi W, Ozaki S, Nawa K, Imae T, Abe O, Nakagawa K. Stereotactic body radiotherapy for centrally-located lung tumors with 56 Gy in seven fractions: A retrospective study. Oncol Lett. 2018;16:4498–4506. doi: 10.3892/ol.2018.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahnesjö A. Collapsed cone convolution of radiant energy for photon dose calculation in heterogeneous media. Med Phys. 1989;16:577–592. doi: 10.1118/1.596360. [DOI] [PubMed] [Google Scholar]
- 44.Roesch J, Panje C, Sterzing F, Mantel F, Nestle U, Andratschke N, Guckenberger M. SBRT for centrally localized NSCLC-What is too central? Radiat Oncol. 2016;11:157. doi: 10.1186/s13014-016-0732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng MB, Wang HH, Zaorsky NG, Sun BS, Zhu L, Song YC, Li FT, Dong Y, Wang JS, Chen HM, et al. Risk-adapted stereotactic body radiation therapy for central and ultra-central early-stage inoperable non-small cell lung cancer. Cancer Sci. 2019;110:3553–3564. doi: 10.1111/cas.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang JH, Poon I, Erler D, Zhang L, Cheung P. The safety and effectiveness of stereotactic body radiotherapy for central versus ultracentral lung tumors. Radiother Oncol. 2018;129:277–283. doi: 10.1016/j.radonc.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Chaudhuri AA, Tang C, Binkley MS, Jin M, Wynne JF, von Eyben R, Hara WY, Trakul N, Loo BW, Jr, Diehn M. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer. 2015;89:50–56. doi: 10.1016/j.lungcan.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Nagata Y, Hiraoka M, Shibata T, Onishi H, Kokubo M, Karasawa K, Shioyama Y, Onimaru R, Kozuka T, Kunieda E, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan clinical oncology group study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93:989–996. doi: 10.1016/j.ijrobp.2015.07.2278. [DOI] [PubMed] [Google Scholar]
- 49.Sapkaroski D, Osborne C, Knight KA. A review of stereotactic body radiotherapy-is volumetric modulated arc therapy the answer? J Med Radiat Sci. 2015;62:142–151. doi: 10.1002/jmrs.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang X, Li T, Liu Y, Zhou L, Xu Y, Zhou X, Gong Y. Planning analysis for locally advanced lung cancer: Dosimetric and efficiency comparisons between intensity-modulated radiotherapy (IMRT), single-arc/partial-arc volumetric modulated arc therapy (SA/PA-VMAT) Radiat Oncol. 2011;6:140. doi: 10.1186/1748-717X-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita H, Haga A, Takahashi W, Takenaka R, Imae T, Takenaka S, Nakagawa K. Volumetric modulated arc therapy for lung stereotactic radiation therapy can achieve high local control rates. Radiat Oncol. 2014;9:243. doi: 10.1186/s13014-014-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 Published, (v4.03, June 14, 2010) 2009 [Google Scholar]
- 53.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol. 1985;58:515–528. doi: 10.1259/0007-1285-58-690-515. [DOI] [PubMed] [Google Scholar]
- 55.Puri V, Crabtree TD, Bell JM, Kreisel D, Krupnick AS, Broderick S, Patterson GA, Meyers BF. National cooperative group trials of ‘high-risk’ patients with lung cancer: Are they truly ‘high-risk’? Ann Thorac Surg. 2014;97:1678–1685. doi: 10.1016/j.athoracsur.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferguson MK, Watson S, Johnson E, Vigneswaran WT. Predicted postoperative lung function is associated with all-cause long-term mortality after major lung resection for cancer. Eur J Cardiothorac Surg. 2014;45:660–664. doi: 10.1093/ejcts/ezt462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim GJ, Koshy M, Hanlon AL, Horiba MN, Edelman MJ, Burrows WM, Battafarano RJ, Suntharalingam M. The benefit of chemotherapy in esophageal cancer patients with residual disease after trimodality therapy. Am J Clin Oncol. 2016;39:136–141. doi: 10.1097/COC.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yukiue H, Tanahashi M, Haneda H, Suzuki E, Yoshii N, Niwa H. Surgical treatment for recurrent and second primary lung cancer. Kyobu Geka. 2010;63:944–949. (In Japanese) [PubMed] [Google Scholar]
- 59.Subotic D, Molins L, Soldatovic I, Moskovljevic D, Collado L, Hernández J. Completion pneumonectomy: A valuable option for lung cancer recurrence or new primaries. World J Surg Oncol. 2018;16:98. doi: 10.1186/s12957-018-1398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeda A, Sanuki N, Eriguchi T, Enomoto T, Yokosuka T, Kaneko T, Handa H, Aoki Y, Oku Y, Kunieda E. Salvage stereotactic ablative irradiation for isolated postsurgical local recurrence of lung cancer. Ann Thorac Surg. 2013;96:1776–1782. doi: 10.1016/j.athoracsur.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Nishiyama K, Kodama K, Teshima T, Tada H. Stereotactic body radiotherapy for second pulmonary nodules after operation for an initial lung cancer. Jpn J Clin Oncol. 2015;45:947–952. doi: 10.1093/jjco/hyv113. [DOI] [PubMed] [Google Scholar]
- 62.Kelsey CR, Clough RW, Marks LB. Local recurrence following initial resection of NSCLC: Salvage is possible with radiation therapy. Cancer J. 2006;12:283–288. doi: 10.1097/00130404-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Gagliasso M, Migliaretti G, Ardissone F. Assessing the prognostic impact of the international association for the study of lung cancer proposed definitions of complete, uncertain, and incomplete resection in non-small cell lung cancer surgery. Lung Cancer. 2017;111:124–130. doi: 10.1016/j.lungcan.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 64.Yoshino I, Yohena T, Kitajima M, Ushijima C, Nishioka K, Ichinose Y, Sugimachi K. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg. 2001;7:204–209. [PubMed] [Google Scholar]
- 65.Sasaki H, Suzuki A, Tatematsu T, Shitara M, Hikosaka Y, Okuda K, Moriyama S, Yano M, Fujii Y. Prognosis of recurrent non-small cell lung cancer following complete resection. Oncol Lett. 2014;7:1300–1304. doi: 10.3892/ol.2014.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walsh GL, O'Connor M, Willis KM, Milas M, Wong RS, Nesbitt JC, Putnam JB, Jr, Lee JJ, Roth JA. Is follow-up of lung cancer patients after resection medically indicated and cost-effective? Ann Thorac Surg. 1995;60:1563–1570. doi: 10.1016/0003-4975(95)00893-4. [DOI] [PubMed] [Google Scholar]
- 67.Ichinose Y, Kato H, Koike T, Tsuchiya R, Fujisawa T, Shimizu N, Watanabe Y, Mitsudomi T, Yoshimura M, Japan Clinical Oncology Group Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: Questionnaire survey of the Japan clinical oncology group to plan for clinical trials. Lung Cancer. 2001;34:29–36. doi: 10.1016/S0169-5002(01)00207-0. [DOI] [PubMed] [Google Scholar]
- 68.Kagami Y, Nishio M, Narimatsu N, Mjoujin M, Sakurai T, Hareyama M, Saito A. Radiotherapy for locoregional recurrent tumors after resection of non-small cell lung cancer. Lung Cancer. 1998;20:31–35. doi: 10.1016/S0169-5002(98)00008-7. [DOI] [PubMed] [Google Scholar]
- 69.Jeremic B, Bamberg M. External beam radiation therapy for bronchial stump recurrence of non-small-cell lung cancer after complete resection. Radiother Oncol. 2002;64:251–257. doi: 10.1016/S0167-8140(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 70.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, Fletcher J. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 71.Oskan F. The quality of toxicity reporting and the story of the lung SBRT ‘No-Fly Zone’. Int J Radiat Oncol Biol Phys. 2015;92:514–515. doi: 10.1016/j.ijrobp.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 72.Timmerman RD. The quality of toxicity reporting and the story of the lung SBRT ‘No-Fly Zone’. In Regard to Oskan. Int J Radiat Oncol Biol Phys. 2015;93:726–727. doi: 10.1016/j.ijrobp.2015.07.2268. [DOI] [PubMed] [Google Scholar]
- 73.Song KJ, Flores RM. Is survival after sublobar resection vs. lobectomy made equivalent by extent of lymphadenectomy? Ann Transl Med. 2019;7(Suppl 6):S191. doi: 10.21037/atm.2019.07.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Locoregional recurrence after segmentectomy for clinical-T1aN0M0 radiologically solid non-small-cell lung carcinoma. Eur J Cardiothorac Surg. 2017;51:518–525. doi: 10.1093/ejcts/ezw336. [DOI] [PubMed] [Google Scholar]
- 75.Kim H, Pyo H, Noh JM, Lee W, Park B, Park HY, Yoo H. Preliminary result of definitive radiotherapy in patients with non-small cell lung cancer who have underlying idiopathic pulmonary fibrosis: Comparison between X-ray and proton therapy. Radiat Oncol. 2019;14:19. doi: 10.1186/s13014-019-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamaguchi S, Ohguri T, Ide S, Aoki T, Imada H, Yahara K, Narisada H, Korogi Y. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: The potential risk of extensive radiation pneumonitis. Lung Cancer. 2013;82:260–265. doi: 10.1016/j.lungcan.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 77.Glick D, Lyen S, Kandel S, Shapera S, Le LW, Lindsay P, Wong O, Bezjak A, Brade A, Cho BCJ, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival in patients treated with lung stereotactic body radiation therapy (SBRT) Clin Lung Cancer. 2018;19:e219–e226. doi: 10.1016/j.cllc.2017.06.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.