Abstract
Exogenous gene expression is a fundamental and indispensable technique for testing gene function in neurons. Several ways to express exogenous genes in neurons are available, but each method has pros and cons. The lentivirus vector is useful for high efficiency gene transfer to neurons and stabilizes gene expression via genome integration, but this integration may destroy the host genome. The Epstein-Barr virus (EBV)-derived vector (EB vector) is an accessible and useful vector in human cell lines because the vector is not integrated into the host genome but stays in the nucleus as an episome. However, there has been no report on this process in rodent neurons.
We examined the usefulness of the EB vector for testing gene function in neurons. We found that EB vector-derived exogenous proteins such as green fluorescent protein (GFP) and GFP-tagged actin were easily detectable even after three weeks of transfection. Second, a tetracycline-induced gene expression system in the EB vector was active after three weeks of transfection, indicating that plasmids were retained in neurons for up to three weeks. Third, we determined that only Family of repeat element of the plasmid vector is essential for its long-term presence in neurons. These results show that the modified EB vector is a useful tool for examining gene function in neurons.
Keywords: Cell biology, Neuroscience, Neurology, Nervous system, Neuronal death, Electrophysiology, Plasmid vector, Episome, Postmitotic cell, Long-term exogenous gene expression, Epstein-barr virus based vector
Cell biology; Neuroscience; Neurology; Nervous system; Neuronal death; Electrophysiology; Plasmid vector; Episome; Postmitotic cell; Long-term exogenous gene expression; Epstein-barr virus based vector.
1. Introduction
Exogenous gene expression is a fundamental method for investigating not only the function of genes in the nervous systems but also the underlying mechanisms of neuronal disease, such as dementia, schizophrenia and major depression. Plasmid- and virus-based gene delivery vectors are popular tools to introduce exogenous genes or knock down endogenous genes to reveal their functions in neurons [1, 2, 3]. Plasmid-based gene delivery is the most widely used technique because plasmids are easy to make. However, the expression of these plasmids tends to be temporal, probably due to the breakdown of the exogenous plasmid DNA within the cell.
Additionally, delivering plasmids to neurons is quite inefficient. In contrast, a virus-based method has several advantages for delivering foreign genes to neurons. For example, lentivirus vector, a well-known virus-based vector, is highly efficient in its delivery of genes to neurons [2], and the introduced foreign genes are stable in the neuron via genomic integration. Although the integrated gene is stable in the neurons, it may concurrently obstruct endogenous genes because the genomic integration of a foreign gene is not controlled and may unexpectedly cause gene destruction in host neurons. As a vessel for gene delivery, virus-based vectors have a size limitation for the carriages. In contrast, plasmid-based vectors theoretically have no restrictions in the size of their carriages.
The EB vector is derived from Epstein-Barr virus and consists of two DNA fragments: the DNA sequence OriP (the origin of plasmid replication) and the Epstein-Barr virus-encoded nuclear antigen-1 (EBNA1) gene. EBNA1 proteins bind to the OriP sequence in the vector and attach the vector DNA to nuclear proteins in the host cell, anchoring OriP-containing plasmids in the nucleus as an episome in dividing cells [4, 5, 6, 7, 8, 9].
We utilized this EB vector to improve exogenous gene stability in neurons, and the EB vector was found to be effective in neurons. We observed stable gene expression from the EB vectors more than one month after transfection and confirmed that the exogenous genes were stably retained in neurons for more than three weeks as confirmed by using a derivative vector carrying DOX-responsive element. We could not exclude the possibility that the EB vector enhanced a genomic integration of itself. Using this approach, however, we could manipulate synaptic transmission by inducing the gene expression of a mutated glutamate receptor at three weeks post-transfection.
2. Materials and methods
2.1. Plasmid construction
pEB6CAG has two major components, one of which is for exogenous gene expression and the other a plasmid retention system. The former consists of the ubiquitous promoter CAG and a multicloning site, and the latter consists of two major components for replication and the retention of the plasmid in the nucleus called OriP and EBNA1. GFP or GFP-actin were cloned into pEB6CAG vector (Figure 1). TetOn3G (Clontech, Takara K.K. Shiga, Japan), doxycycline (DOX) responsive transactivator gene, was also amplified by PCR and inserted pEB6CAG vector (Figure 2). A vector named pOriP_3GBi vector (Figure 2) was also constructed by inserting PCR amplified OriP fragment into pTre3G-Bi (Clontech). The partial deletion of OriP and other elements was carried out by standard inverse PCR method. Primers for deletions are as follows. delta DS: 5′-GTTGGTGTAGCCTCCCGTAG -3′, 5′-CCCTGCGGTTTTGGACTGTA-3'. deltaDS-L: 5′- AAAGCCACTCAGTGTTGGCA -3', 5′-GAGCACGGTGGGCTAATGTT-3′, deltaFR:5′-TGAGGACCAACAACCCTGTG-3′, 5′-CCAAGGGGGCGTGAATTTTC-3' (Figure 3). A DS deletion clone is named pFR_Tre3GBi.
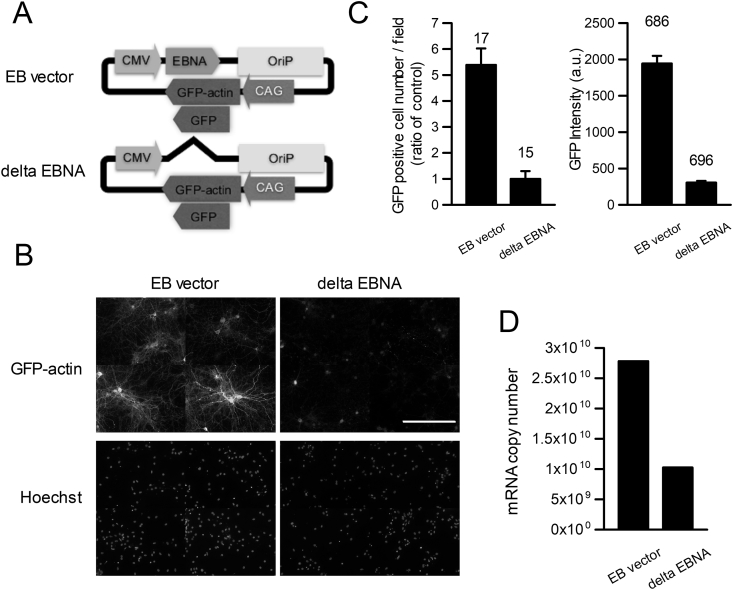
Figure 1.
EBNA1 dependent long-term gene expression in neurons. A. Plasmid maps of vectors, EB vector and EBNA lacking EB vector (delta EBNA). B. A typical fluorescent images of GFP-actin transfected neurons cultured for 23 days (23DIV) with EB or delta EBNA vectors (Upper: GFP, Lower: Hoechst staining, scale bar 100μm). Transfections were done at 0 DIV and neurons were fix at 23DIV and stained. C. The number of GFP signal positive neurons and the intensity of the GFP fluorescence were significantly higher in case of EB vector than delta EBNA vector (GFP signal positive neurons; EB vector: 11.90 ± 1.39, n = 15, delta EBNA: 2.20 ± 0.66, n = 15, p < 0.005, GFP signal intensity; EB vector: 1943.7 ± 105.6, n = 689, delta EBNA: 306.1 ± 23.8, n = 696, p < 0.005). The number of fields and cells for quantification are indicated above each bar. D. mRNA comparison, Quantitative PCR shows high number of mRNA in EB vector transfected cultured neurons that delta EBNA vector transfected one.
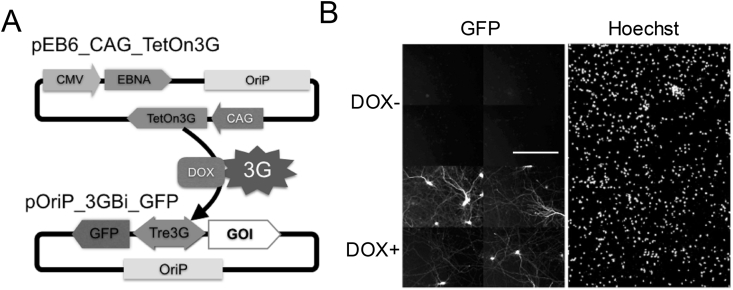
Figure 2.
Long term plasmid retention in neurons. A. Cartoon of DOX induction plasmids system. EBNA1 and TetOn3G were expressed by pEB6_CAG_Teton3G vector (upper) and the complex of expressed 3G and DOX binds to pOrip_3GBi_GFP vector (lower) to activate expression of GFP and Gene of Interest (GOI) expression. B. Induction of GFP expression by DOX addition. There were no GFP positive neurons before DOX induction (upper 4 panels), but adding DOX for 24h induced GFP expression in a number of neurons (lower 4 panels).
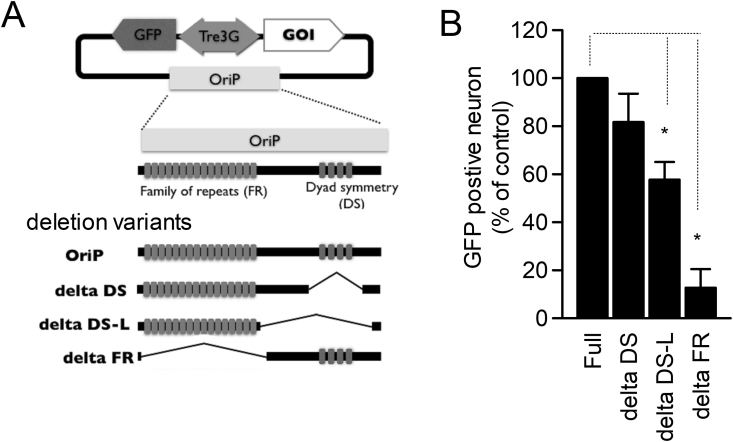
Figure 3.
Identification of critical component of OriP sequence for retention in neurons. A. Cartoon of the pOriPdelta_3GBi_GFP plasmid vector and major components of OriP fragment. Deletion variants are indicated (lower). B. GFP expression from full length vector and deletion clones were compared. Inductions of GFP expression were done 3 weeks after transfection. Deleting FR component from OriP fragment are dramatically reduced the number of GFP positive neurons (delta DS: 81.71 ± 11.83% of full length, n = 5 trial, delta DS-L: 57.71 ± 7.35%, n = 4 trial, delta FR: 12.72 ± 7.74%, n = 5 trial. Full vs. delta DS: p = 0.335, Full vs. delta DS-L, p = 0.037, Full vs. delta FR, p = 0.00273, steel-Dwass test).
2.2. Cell cultures and transfection
Primary cultures of hippocampal and cortical neurons were prepared from embryonic 18-19-day-old fetal rats as described previously [10] with minor modifications. The rats were maintained in the animal house of the AIST animal facilities at AIST Osaka or obtained from a laboratory animal production and supply company (Japan SLC Inc, Tokyo, Japan). The animals were housed in individual cages under a controlled environment (23 °C±2 °C, 40–70% humidity, a 12 h/12 h light/dark cycle) with free access to food and water. All of the procedures were performed in accordance with the animal health care guideline of Japan and approved by the ethics committee of AIST (permission no. 231).
Neurons were plated on 0.1% poly-L-lysine-coated cover glass in DMEM medium supplemented with 10% fetal calf serum (FCS), 2 mM GlutaMAX (GIBCO, Thermo Fisher Scientific K.K., Tokyo, Japan), 30 units/ml penicillin and 60 mg/ml streptomycin. One hour after plating, the culture medium was fully changed to Neurobasal medium (GIBCO) supplemented with B27 (GIBCO). The overproliferation of glial cells was inhibited with 0.1–1 μM AraC treatment for 1–3 days. The neurons were cultured for 2–10 weeks before use. One-half of the culture medium was replaced twice a week. In some cultures, glial cell-conditioned DMEM with 2.5% horse serum was used instead of Neurobasal medium.
Transfections were done with an Amaxa Nucleofector (Lonza Japan, Tokyo, Japan) with electroporation solution (BTX, MS, USA). Electroporation was performed just before plating the neurons according to the manufacturer's instruction.
2.3. Quantification of transgene expression stability
Transfected neurons were fixed and stained with Hoechst to count the cell number. Fluorescent images were captured with a cooled CCD camera (CoolSNAP HQ) controlled by Micromanager [11, 12]. Quantification was done by ImageJ software with a constant threshold through a series of tests. Some experiments were done by manually counting. Statistical tests were done by Kyplot software.
2.4. Quantification of gene expression by quantitative PCR
All procedures were carried out according to the manufacturer's instructions. Total RNA was extracted from cultured neurons by ISOGEN reagent (Nippon Gene, Tokyo, Japan) and then purified using an RNeasy Mini kit (Qiagen, Chatsworth, CA) after treatment with DNase I (Qiagen) to remove contaminating genomic and plasmid DNA. The total RNA was then reverse-transcribed into cDNA using Superscript III with a 20 base oligo (dT) primer.
The qualification of mRNA transcribed from residual plasmid DNA in neurons was performed by quantitative real-time PCR (7300 Real-Time PCR system, Applied Biosystems) with Power SYBR Green PCR Master Mix (Applied Biosystems). The GFP-specific primers used were as follows:
GFP417-440: gcacaagctggagtacaactacaa and GFP516-491: gatgttgtggcggatcttgaagttca.
Duplicate standards were included in each experiment, and each sample was measured in triplicate.
2.6. Electrophysiological experiments
Whole cell recordings were obtained from cultured neurons using glass electrodes (3–5 MΩ) filled with a solution containing (in mM) 115 Cs-methanesulfonate, 20 CsCl, 10 HEPES, 2 MgCl2, 4 Na2-ATP, 0.2 Na3-GTP, 0.1 spermine, 10 Na-phosphocreatine and 0.3 EGTA in a HEPES-buffered artificial cerebrospinal fluid solution (ACSF-H) containing (in mM) 130 NaCl, 3 KCl, 2 CaCl2, 10 glucose, 0.1 picrotoxin and 10 HEPES-Na.
To examine the synaptic integration of the induced exogenous GluA2Q receptor, we compared synaptic currents recorded with depolarized and hyperpolarized membrane potentials under the presence of APV and picrotoxin or NMDA receptor and chloride channel-meditated inhibitory synaptic currents, respectively. Rectification index is calculated as EPSC amplitude ratio of recorded at hyperpolarized and depolarized membrane potentials. Smaller score means that synaptic currents are inward rectified.
3. Results
We first examined the long-term expression of GFP using the EB vector. Transfections were carried out on the day of plating, and comparisons were made after the indicated days in culture. Many neurons emitting strong GFP signals were observed after 3 weeks of transfection. If the EB vector worked in neurons as well as in other human cell lines, the EBNA1 protein would anchor the plasmids to nucleus of the neurons. When the vectors with and without EBNA1 were compared, the number of GFP-expressing neurons following transfection with the EB vector with EBNA1 was significantly larger compared to that observed with the EB vector without EBNA1 (data not shown). The expression of GFP-tagged actin was further examined, because GFP-tagged proteins are less stable than GFP itself. Use of the EB vector containing EBNA1 dramatically increased the number of GFP-positive neurons compared to that observed with the plasmid lacking EBNA1 (Figure 1C; without EBNA1: 2.20 ± 0.66, n = 15 fields with 10× objective, with EBNA1: 11.86 ± 1.39, n = 15 fields with 10× objective, p < 0.05, Mann-Whitney U-test), and the GFP fluorescent intensity was also higher following transfection with the plasmid containing EBNA1 than that following transfection with plasmid lacking EBNA1 (Figure 1C; with EBNA1: 1943.8 ± 105.6, n = 686 cells, without EBNA1: 306.1 ± 23.8, n = 969 cells, p < 0.001, t-test). We further examined the amount of transcript mRNA three weeks after transfection; comparison of the mRNA level by quantitative PCR showed that GFP mRNA from the EB vector-transfected sample was approximately 2.5 times greater than that from the sample transfected with EB vector lacking EBNA1 (Figure 1D).
The above data were not enough to prove that the EB vector was retained long-term in the neurons. To confirm this more directly, we utilized a doxycycline (DOX)-induced expression system [13]. This system has an inducible promoter element that is activated by the complex between the 3G protein and DOX. Therefore, gene expression from this system indicates that the plasmids are present in the neurons at the point of DOX addition. We constructed two EB vectors with the 3G Tet-On system (Figure 2A) and examined the response to DOX 3 weeks posttransfection. Before DOX addition, no GFP signals were observed, but 24 h after the addition of DOX, a significant number of GFP-positive neurons were observed (Figure 2B), indicating that the EB vectors were present in neurons for three weeks and had potential for protein expression.
The OriP fragment in the EB vector is responsible for plasmid retention as episomes in dividing cells. In which process, the duplication of the plasmids and their tethering to the the host genome are dependent on the dyad symmetry element (DS) and the Family of repeats (FR) of the OriP fragment, respectively [8]. Due to the non-dividing nature of neurons, the long-term retention of plasmids in neurons would only depend on the FR region. We determined which part of OriP fragment was critical for the long-term presence of plasmids in neurons by using a series of partially deleted plasmids. The number of GFP-positive neurons that responded to DOX addition was drastically decreased in the vector lacking the FR region compared to that in the full vector (Figure 3B, delta FR: 12.7 ± 7.7% of control vector, average of 5 experiments, Steel-Dwass Test p < 0.05). This result is consistent with previous reports [8]. On the other hand, deleting DS region did not cause significant decrease in the number of DOX-induced GFP expression (Figure 3B, delta DS: 91.7 ± 11.8% of control vector, average of 5 experiments). Further deleting DS around region (DS-L, Figure 3) caused mild but significant decrease the number (Figure 3B, delta DS-L: 57.7 ± 7.4% of control vector, average of 4 experiments, Steel-Dwass Test p < 0.05), indicating that intermediate region between FR and DS would also have a function for plasmid retention. We thereafter, utilized DS lacking vector as a minimum EB vector for neuron and named pFR_Tre3GBi.
Next, we examined the usefulness of the pFR_Tre3GBi vector to manipulate synaptic function in mature neurons for disease-related gene analysis. Glutamate receptors are major synaptic transmitter receptors in the CNS, and the GluA2 subunit defines the calcium ion permeability of AMPA receptors. This permeability is a consequence of the editing of GluA2 mRNA, which changes glutamine to arginine amino acid 602, resulting in reduced calcium permeability in GluR2(R)-containing AMPA receptors. However, malfunction of this RNA editing causes GluA2(Q)-containing AMPA receptors to have higher calcium permeability than properly edited AMPA receptors. This kind of abnormality in RNA editing has been reported in Amyotrophic Lateral Sclerosis (ALS) patients, in which GluA2Q is expressed [14, 15].
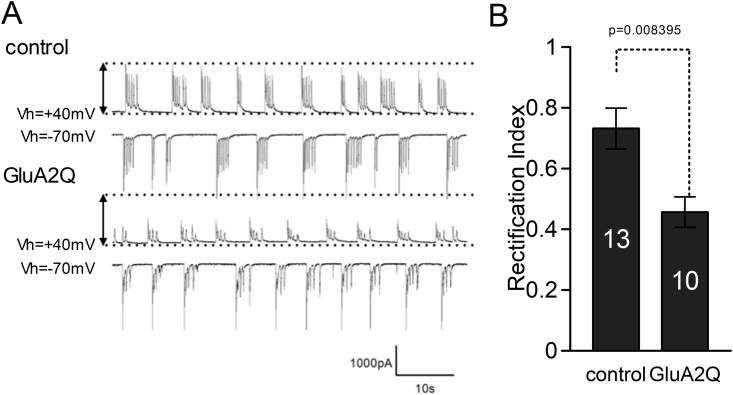
To mimic synaptic property as an ALS model, we used this pFR_Tre3GBi vector with a gene encoding the calcium-permeable GluA2Q subunit and GFP (pFR_Tre3GBi_GFP_GluA2Q), and cotransfected cells with pEB6_CAG_TetOn3G vector at 0 DIV. Gene expression was induced at 20 DIV and examined at 21–23 DIV. If expressed GluA2Q subunits were incorporated into the synapses, the rectification of synaptic currents would be altered when whole-cell recording of the transfected neurons was conducted [16, 17]. As expected, inward rectified synaptic currents were recorded in the transfected neurons (Figure 4A, B, control: 0.731 ± 0.067, n = 13 cells, GluA2Q: 0.456 ± 0.05, n = 10 cells). This result strongly suggested that the combination of the pEB6CAG_3G and pFR_Tre3GBi vectors was useful to examine exogenous genes not only for their long-term effects but also for their timely manipulation of neuronal function.
Figure 4.
Exogenous gene products, GluR2Q, alters synaptic property. A. Typical spontaneous synaptic currents recorded from transfected neurons with holding potentials at -70 and +40mV presented in lower and upper waveform, respectively. Synaptic currents at +40mV from GluA2 expressing neuron are greatly reduced compared to the control one. This inward rectification of synaptic currents means GluA2Q subunit is incorporated into synaptic glutamate receptors. B. Summary data of rectification index are plotted (control: 0.731 ± 0.067, n = 13 cells, GluA2Q: 0.456 ± 0.05, n = 10 cells).
4. Discussion
To stabilize exogenous plasmids in neurons without distractions of the host genome is an essential issue and indispensable technique for investigating gene function in neurons. To this end, we showed that EB vectors are very useful for exploring gene function not only in the short term but also in the long term for at least 3 weeks.
The EB vector widely used for human cell lines needs the OriP sequence to exist as an episome for a long time. However, we also showed there is no need for the full length OriP sequence in neuronal cells. The FR region of OriP was major critical part for plasmid long term existence. This was putatively because neurons are postmitotic cells that do not divide, and the DS region of OriP that is involved in the replication of the plasmids would be dispensable [4, 5, 18]. Additionally, our data shows that intermediated region between DS and FR have some function to plasmid retain because the plasmid lacking DS and the intermediated region showed mild but significant reduction of gene expression from retained plasmid. It might be due to undefined function from the intermediate region and/or DS region.
Do transfected plasmids localize in the nucleus as episomes? We tried to confirm localization directly in the neuron using fluorescent dye-labeled plasmids. However, we did not detect enough signal to indicate that the transfected plasmids were incorporated into and localized in the nucleus as reported [19]. This may be due to the low number of plasmids used and transferred. We also try to examine a possibility that the EB vector increases genomic integration of the vector resulting long term exogenous gene expression in neurons. Southern blot experiments did not show any signals not only from integrated plasmid DNA but also transfected plasmid DNA purified from a set of electroporated neurons. It also may be due to the low number of plasmids.
We also examined EBNA1 protein localization, because EBNA1 nucleus localization would stabilize plasmid retention in the nucleus. Using GFP-tagged EBNA1 imaging, EBNA1-GFP was distributed in the cell soma, including the nucleus, but not in axon, indicating that EBNA1 proteins functioned in neurons. The addition of strong nuclear localization signals to EBNA1 may artificially promote plasmid nuclear localization and its long-term retention in the nucleus.
Usage of adeno-associated virus (AAV) mediated gene delivery has been robustly increased in the neuroscience research field because this virus vector doesn't integrate into host genome and is non-pathogenic. The latter point is especially critical for gene therapy and animal experiments. Adenovirus is also an episomal vector, but it has toxicity and may cause immunoreaction in host animals. The maximal cargo capacity of AAV is about 5kbp. This is much smaller than lentiviral and adenoviral vectors. And also, like adenovirus, the serotype of AAV shows the selectivity of infection to neurons [2]. This can be a disadvantage in the universality of use [20].
AAV vectors are generally superior to EB vectors in transgenic efficiency, especially in neurons. But EB vectors have a larger cargo capacity. A combined gene delivery method is developed that EBNA and Orip fragment with recombination sites are incorporated into the baculoviral vector, and this fragment is recombined in a host cell to create episome vector [21]. This combined method would be useful in neurons to improve transgenic efficiency.
5. Conclusions
We found that EB vector provided not only long-term but also timely controlled exogenous gene expression in a neuron. This finding would be useful in basic neuroscience studies that employ ectopic gene expression. It may also be valuable for the development of medical gene therapy applications for brain diseases.
Declarations
Author contribution statement
Kazuyuki Kiyosue: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yoshihiro Miwa: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by JSPS KAKENHI Grant Number JP24500400, 15H04281, 16H01630, 16K14589 and internal grant of AIST, Japan.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Megumi ONISHI for helping experiments, especially in constructing plasmid vector and cell culture.
References
- 1.Lentz T.B., Gray S.J., Samulski R.J. Viral vectors for gene delivery to the central nervous system. Neurobiol. Dis. 2012;48:179–188. doi: 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karra D., Dahm R. Transfection techniques for neuronal cells. J. Neurosci. 2010;30:6171–6177. doi: 10.1523/JNEUROSCI.0183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson B.L., Breakefield X.O. Viral vectors for gene delivery to the nervous system. Nat. Rev. Neurosci. 2003;4:353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- 4.Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc. Natl. Acad. Sci. U.S.A. 1984;81:3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yates J.L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein–Barr virus in various mammalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 6.Lupton S., Levine A.J. Mapping genetic elements of Epstein-Barr virus that facilitate extrachromosomal persistence of Epstein-Barr virus-derived plasmids in human cells. Mol. Cell Biol. 1985;5:2533–2542. doi: 10.1128/mcb.5.10.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka J., Miwa Y., Miyoshi K., Ueno A., Inoue H. Construction of Epstein-Barr virus-based expression vector containing mini-oriP. Biochem. Biophys. Res. Commun. 1999;264:938–943. doi: 10.1006/bbrc.1999.1617. [DOI] [PubMed] [Google Scholar]
- 8.Lufino M.M., Edser P.A., Wade-Martins R. Advances in high-capacity extrachromosomal vector technology: episomal maintenance, vector delivery, and transgene expression. Mol. Ther. 2008;16:1525–1538. doi: 10.1038/mt.2008.156. [DOI] [PubMed] [Google Scholar]
- 9.Rawlins D.R., Milman G., Hayward S.D., Hayward G.S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985;42:859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama K., Kiyosue K., Taguchi T. Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J. Neurosci. 2005;25:4040–4051. doi: 10.1523/JNEUROSCI.4115-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelstein A.D., Tsuchida M.A., Amodaj N., Pinkard H., Vale R.D., Stuurman N. Advanced methods of microscope control using μManager software. J. Biol. Methods. 2014;1:10. doi: 10.14440/jbm.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes J.A., Papagiakoumou E., Ruffault P.-L., Emiliani V., Fortin G. Computer-aided neurophysiology and imaging with open-source PhysImage. J. Neurophysiol. 2018;120:23–36. doi: 10.1152/jn.00048.2017. [DOI] [PubMed] [Google Scholar]
- 13.Fan X., Petitt M., Gamboa M., Huang M., Dhal S., Druzin M.L. Transient, inducible, placenta-specific gene expression in mice. Endocrinology. 2012;153:5637–5644. doi: 10.1210/en.2012-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akamatsu M., Yamashita T., Hirose N., Teramoto S., Kwak S. The AMPA receptor antagonist perampanel robustly rescues amyotrophic lateral sclerosis (ALS) pathology in sporadic ALS model mice. Sci. Rep. 2016;6:28649. doi: 10.1038/srep28649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tateno M., Tateno M., Sadakata H., Sadakata H., Tanaka M., Tanaka M. Calcium-permeable AMPA receptors promote misfolding of mutant SOD1 protein and development of amyotrophic lateral sclerosis in a transgenic mouse model. Hum. Mol. Genet. 2004;13:2183–2196. doi: 10.1093/hmg/ddh246. [DOI] [PubMed] [Google Scholar]
- 16.Bowie D., Mayer M.L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 17.Shi S., Hayashi Y., Esteban J.A., Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 18.Wysokenski D.A., Yates J.L. Multiple EBNA1-binding sites are required to form an EBNA1-dependent enhancer and to activate a minimal replicative origin within oriP of Epstein-Barr virus. J. Virol. 1989;63:2657–2666. doi: 10.1128/jvi.63.6.2657-2666.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reisinger J., Rumpler S., Lion T., Ambros P.F. Visualization of episomal and integrated Epstein-Barr virus DNA by fiber fluorescence in situ hybridization. Int. J. Canc. 2006;118:1603–1608. doi: 10.1002/ijc.21498. [DOI] [PubMed] [Google Scholar]
- 20.Howard D.B., Powers K., Wang Y., Harvey B.K. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 2008;372:24–34. doi: 10.1016/j.virol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo W.-H., Hwang S.-M., Chuang C.-K., Chen C.-Y., Hu Y.-C. Development of a hybrid baculoviral vector for sustained transgene expression. Mol. Ther. 2009;17:658–666. doi: 10.1038/mt.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]