Key Points
Question
Is treatment with carboplatin plus pemetrexed followed by pemetrexed maintenance noninferior compared with docetaxel monotherapy with regard to overall survival for elderly patients with advanced nonsquamous non–small cell lung cancer (NSCLC)?
Findings
This phase 3 randomized clinical trial including 433 elderly patients with a median age of 78 years met its primary end point and demonstrated noninferiority of carboplatin-pemetrexed treatment followed by pemetrexed maintenance compared with docetaxel monotherapy in terms of overall survival for chemotherapy-naive elderly patients with advanced nonsquamous NSCLC.
Meaning
The combination of carboplatin and pemetrexed followed by pemetrexed maintenance is a valid option for first-line treatment of elderly patients with advanced nonsquamous NSCLC.
This phase 3 randomized clinical trial compares treatment with carboplatin plus pemetrexed followed by pemetrexed maintenance with docetaxel monotherapy for overall survival for elderly Japanese patients with advanced nonsquamous non–small cell lung cancer (NSCLC).
Abstract
Importance
Few clinical trials have been specifically designed for elderly patients with advanced non–small cell lung cancer (NSCLC), and the anticipated increase in the number of such patients has prompted a search for new treatment options that provide a greater palliative benefit.
Objective
To determine whether treatment with carboplatin plus pemetrexed followed by pemetrexed maintenance is noninferior compared with docetaxel monotherapy with regard to overall survival (OS) for elderly patients with advanced nonsquamous NSCLC.
Design, Setting, and Participants
This open-label, multicenter, noninferiority phase 3 randomized clinical trial was conducted at 79 institutions in Japan. Cytotoxic chemotherapy–naive patients with advanced nonsquamous NSCLC, an Eastern Cooperative Oncology Group performance status of 0 or 1, and age of 75 years or older were enrolled between August 2013 and February 2017. Data were analyzed from November 2018 to February 2019.
Interventions
Patients were randomized to receive either docetaxel monotherapy (60 mg/m2) every 3 weeks or 4 cycles of carboplatin (area under the curve of 5) plus pemetrexed (500 mg/m2) administered every 3 weeks followed by maintenance therapy with the same dose of pemetrexed for 3 weeks.
Main Outcomes and Measures
The primary end point was OS analyzed on an intention-to-treat basis with a noninferiority margin of 1.154 for the upper limit of the 95% CI of the hazard ratio (HR) estimated with a stratified Cox regression model.
Results
Of the 433 enrolled patients, 250 (57.7%) were male, and the median (range) age was 78 (75-88) years. The median OS was 15.5 months (95% CI, 13.6-18.4) in the docetaxel group (n = 217) and 18.7 months (95% CI, 16.0-21.9) in the carboplatin-pemetrexed group (n = 216), with a stratified HR for OS of 0.850 (95% CI, 0.684-1.056; P for noninferiority = .003). Progression-free survival was also longer in the carboplatin-pemetrexed group (unstratified HR, 0.739; 95% CI, 0.609-0.896). Compared with those in the docetaxel group, those in the carboplatin-pemetrexed had lower rates of leukopenia (60 of 214 [28.0%] vs 147 of 214 [68.7%]) and neutropenia (99 of 214 [46.3%] vs 184 of 214 [86.0%]) of grade 3 or 4 and of febrile neutropenia (9 of 214 [4.2%] vs 38 of 214 [17.8%]) and higher rates of thrombocytopenia (55 of 214 [25.7%] vs 3 of 214 [1.4%]) and anemia (63 of 214 [29.4%] vs 4 of 214 [1.9%]) of grade 3 or 4. Dose reductions were less frequent with carboplatin-pemetrexed.
Conclusion and Relevance
Carboplatin-pemetrexed treatment followed by pemetrexed maintenance is a valid option for first-line treatment of elderly patients with advanced nonsquamous NSCLC.
Trial Registration
University Hospital Medical Information Network Clinical Trials Registry Identifier: UMIN000011460
Introduction
The number of elderly individuals with cancer has increased in recent years, with more than one-third of new lung cancer cases now being diagnosed in patients 75 years or older.1 Although the emergence of molecularly targeted agents for oncogene-driven tumors as well as of immune checkpoint inhibitors has expanded the opportunity for treatment of advanced non–small cell lung cancer (NSCLC) in the elderly, cytotoxic chemotherapy remains the mainstay for the treatment of such patients whose tumors are not driven by known oncogenes or who develop resistance to targeted or immunotherapeutic drugs. In anticipation of a further increase in the number of elderly individuals with advanced NSCLC, it will be important to develop more optimal chemotherapeutic regimens for this patient group.
Although the number of clinical trials designed specifically for elderly patients with advanced NSCLC is limited, the Elderly Lung Cancer Vinorelbine Italian Study (ELVIS) trial2 demonstrated the benefits of chemotherapy with vinorelbine in terms of overall survival (OS) and quality of life (QOL) compared with best supportive care in patients with advanced NSCLC 70 years or older with an Eastern Cooperative Oncology Group performance status (PS) of 0 to 2. We subsequently performed a phase 3 randomized clinical trial (West Japan Thoracic Oncology Group Trial [WJTOG] 99043) of chemotherapy with docetaxel compared with vinorelbine in such patients and found that progression-free survival (PFS) and response rate were better in the docetaxel group. The median OS was 14.3 months in the docetaxel group compared with 9.9 months in the vinorelbine group. After single agents became widely accepted for palliative chemotherapy in elderly patients,4 the question of whether such individuals might benefit from platinum-based combinations compared with single agents became of increasing clinical interest. With respect to cisplatin-based combinations, modification of cisplatin dose or schedule was intensively explored. However, to our knowledge, clinically meaningful benefits of such cisplatin-based combinations have not been demonstrated in prospective phase 3 trials designed specifically for elderly patients with advanced NSCLC.5,6,7,8 The Intergroupe Francophone de Cancérologie Thoracique 0501 phase 3 randomized clinical trial9 compared treatment with monthly carboplatin plus weekly paclitaxel with gemcitabine or vinorelbine monotherapy in patients with advanced NSCLC 70 years or older with a PS of 0 to 2. This trial demonstrated a superior outcome for the carboplatin-based doublet in terms of OS, PFS, and response rate, although its effectiveness was associated with a high incidence of toxic effects, with treatment-related death occurring in 4.4% of patients.9
With the emerging role of pemetrexed in the treatment of nonsquamous NSCLC and the good tolerability of this drug, there was interest in evaluation of pemetrexed in combination with carboplatin for elderly patients with advanced nonsquamous NSCLC.10,11,12,13 A single-arm phase 2 study14 demonstrated efficacy (median OS, 20.5 months; median PFS, 5.7 months) and acceptable safety for pemetrexed (500 mg/m2) plus carboplatin (area under the curve of 5) followed by pemetrexed maintenance therapy in patients with advanced nonsquamous NSCLC 75 years or older.13 We therefore undertook and now report the results of JCOG1210/WJOG7813L, a multicenter, noninferiority phase 3 randomized clinical trial of treatment with carboplatin plus pemetrexed followed by pemetrexed maintenance therapy compared with docetaxel monotherapy in cytotoxic chemotherapy–naive patients 75 years or older with advanced nonsquamous NSCLC.
Methods
Study Design and Patients
The present trial, JCOG1210/WJOG7813L, was a collaborative effort of the Japan Clinical Oncology Group (JCOG) and West Japan Oncology Group (WJOG) and was designed as an open-label phase 3 randomized clinical trial to be performed at 79 institutions in Japan. The criteria for patient eligibility included age of 75 years or older; a diagnosis of nonsquamous NSCLC confirmed either histologically or cytologically; disease that was of clinical stage III not amenable to curative radiotherapy, of clinical stage IV, or recurrent disease; no prior cytotoxic chemotherapy; and an Eastern Cooperative Oncology Group PS of 0 or 1. Patients also had to have adequate bone marrow reserve and organ function, including a calculated creatinine clearance of 45 mL/min/1.73 m2 (to convert to milliliters per second per meter squared, multiply by 0.0167) or greater based on the standard Cockcroft and Gault formula. Prior treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) was permitted for patients harboring activating variations of EGFR. Written informed consent was obtained from all patients, and the study protocol was approved by the institutional ethics committees of each participating institution. The study protocol can be found in Supplement 1.
Treatment and Trial Procedures
Patients were randomly assigned (1:1) to receive either docetaxel monotherapy or carboplatin plus pemetrexed treatment followed by pemetrexed maintenance therapy with a minimization method. Patients were stratified by disease clinical stage (III vs IV vs recurrence), sex, EGFR variation status (wild-type vs activating variation [exon 19 deletion or L858R variation of exon 21] vs unknown), and investigator center. Patients assigned to the docetaxel group received an infusion of docetaxel at 60 mg/m2 for 60 minutes on day 1 every 3 weeks until the detection of disease progression or appearance of unacceptable toxic effects. Those assigned to the carboplatin-pemetrexed group received an infusion of pemetrexed at 500 mg/m2 for 10 minutes followed by an infusion of carboplatin at an area under the curve of 5 for 30 minutes on day 1 every 3 weeks, with the combination therapy being repeated for up to 4 courses and followed by 3-week courses of maintenance therapy with the same dose of pemetrexed alone until the detection of disease progression or appearance of unacceptable toxic effects.
Statistical Analysis
The primary end point of the study was OS. Secondary end points included PFS, response rate in patients with measurable lesions, symptom score, and toxic effects. On the basis of our previous data,3,7,12 we hypothesized that median OS for the docetaxel and carboplatin-pemetrexed groups would be 15 and 18 months, respectively. Noninferiority would not be demonstrated if the median OS for the carboplatin-pemetrexed group was 2 or more months shorter than that for the docetaxel group, which corresponds to a noninferiority margin of 1.154 on a hazard ratio (HR) scale. A total of 320 patients (233 events) was required for a power of 80% at a 1-sided significance level of 5%, with an expected accrual period of 4 years and follow-up period of 1.5 years. Given that accrual was faster than expected, the protocol was amended on August 14, 2015, to allow a more stringent 1-sided α level of 2.5% with a power of 85%. A total of 430 patients (339 events) was thus required for the study. As the primary analysis, the P value for noninferiority hypothesis was calculated from a Wald test statistic using a Cox regression model stratified by stage (recurrence or not) and EGFR variation status (variant or not). Outcomes assessment and statistical analysis plans are described in the eMethods in Supplement 2. All analyses were conducted using SAS version 9.4 (SAS Institute).
Results
Patients Characteristics
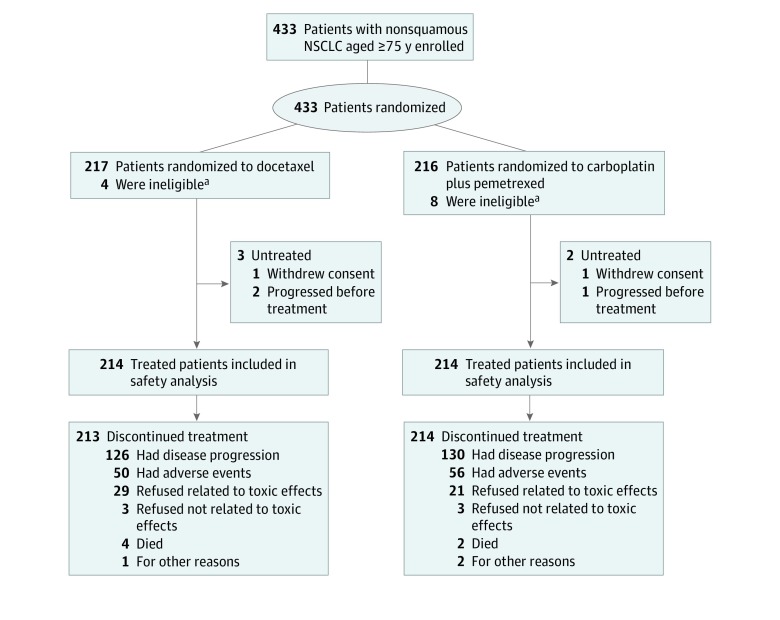
Between August 12, 2013, and February 20, 2017, 433 patients (250 [57.7%] men; median [range] age, 78 [75-88] years) were enrolled at 79 institutions in Japan and were randomly assigned to the docetaxel group (217 patients) or the carboplatin-pemetrexed group (216 patients) (Figure 1). The baseline demographic and disease-related characteristics of the study participants were well balanced between the 2 treatment groups (Table 1). EGFR variation status was evaluated in 420 of 433 patients (97.0%), with 95 individuals (21.9%) harboring an activating EGFR variation, all of whom had been previously treated with at least 1 EGFR TKI. All 433 randomly assigned patients were included in efficacy analysis. Three patients in the docetaxel group and 2 patients in the carboplatin-pemetrexed group did not receive protocol treatment, with the result that safety analysis was performed for 428 patients (Figure 1).
Figure 1. CONSORT Diagram.
NSCLC indicates non–small cell lung cancer.
aThese ineligible patients received study treatment and so were included in the safety analysis.
Table 1. Baseline Patient Demographic and Clinical Characteristics.
| Characteristic | Group, No. (%) | |
|---|---|---|
| Docetaxel (n = 217) | Carboplatin-pemetrexed (n = 216) | |
| Age, median (range), y | 78 (75-88) | 78 (75-88) |
| Age ≥80 y | 56 (25.8) | 51 (23.6) |
| Sex | ||
| Male | 126 (58.1) | 124 (57.4) |
| Female | 91 (41.9) | 92 (42.6) |
| ECOG performance status | ||
| 0 | 85 (39.2) | 91 (42.1) |
| 1 | 132 (60.8) | 125 (57.9) |
| Clinical stage | ||
| III | 16 (7.4) | 14 (6.5) |
| IV | 164 (75.6) | 161 (74.5) |
| Recurrent | 37 (17.1) | 41 (19.0) |
| Smoking status | ||
| Smoker | 128 (59.0) | 130 (60.2) |
| Never | 89 (41.0) | 86 (39.8) |
| EGFR variant status | ||
| Negative | 165 (76.0) | 160 (74.1) |
| Positive | 47 (21.7) | 48 (22.2) |
| Unknown | 5 (2.3) | 8 (3.7) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
Delivered Chemotherapy
The median (range) number of treatment cycles administered was 4 (1-44) in the docetaxel group and 6 (1-59) in the carboplatin-pemetrexed group. Dose reductions occurred in 133 patients (61.3%) in the docetaxel group and in 49 patients (22.7%) in the carboplatin-pemetrexed group. Most docetaxel dose reductions were because of a decline in the neutrophil count, whereas dose reductions for those in the carboplatin-pemetrexed group were most commonly attributable to a drop in the neutrophil or platelet count. Among the 214 treated patients in the docetaxel group and 214 treated patients in the carboplatin-pemetrexed group, the major reasons for treatment discontinuation were disease progression (126 [58.9%] vs 130 [60.7%], respectively), adverse events (50 [23.4%] vs 56 [26.2%]), and patient refusal to continue treatment as a result of toxic effects (29 [13.6%] vs 21 [9.8%]) (Figure 1).
Efficacy
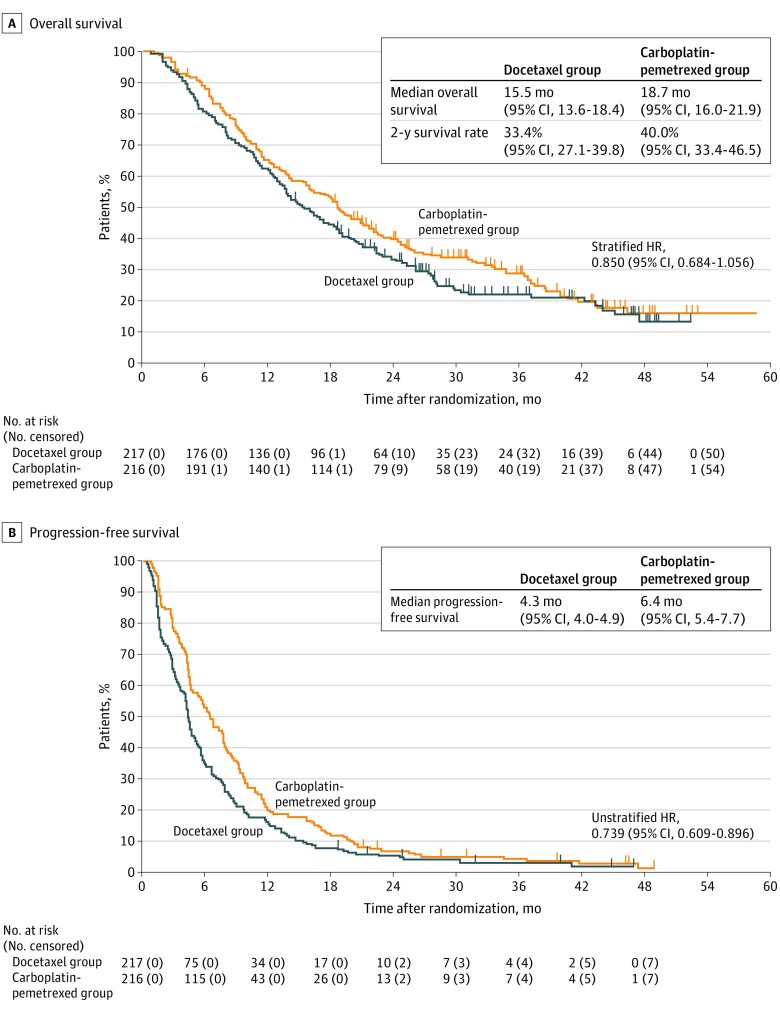
With a median (interquartile range) follow-up time of 17.1 (8.7-27.5) months, 328 patients (167 [77.0%] in the docetaxel group and 161 [74.5%] in the carboplatin-pemetrexed group) had died. The stratified HR for OS in the intention-to-treat population was 0.850 (95% CI, 0.684-1.056; P for noninferiority = .003) (Figure 2A). The upper limit of the 95% CI was lower than the prespecified noninferiority margin of 1.154, thus confirming the noninferiority of treatment with carboplatin-pemetrexed. However, the upper limit exceeded the prespecified superiority margin of 1.000, which failed to show a superiority (1-sided P for superiority = .07). The median OS was 15.5 months (95% CI, 13.6-18.4) in the docetaxel group and 18.7 months (95% CI, 16.0-21.9) in the carboplatin-pemetrexed group, with the 2-year OS rates being 33.4% (95% CI, 27.1-39.8) and 40.0% (95% CI, 33.4-46.5), respectively.
Figure 2. Kaplan-Meier Plots for Overall Survival and Progression-Free Survival in the Intention-to-Treat Population.
Hazard ratios (HRs) were stratified by clinical stage (III/IV vs recurrence) and EGFR variation status (wild type/unknown vs variation).
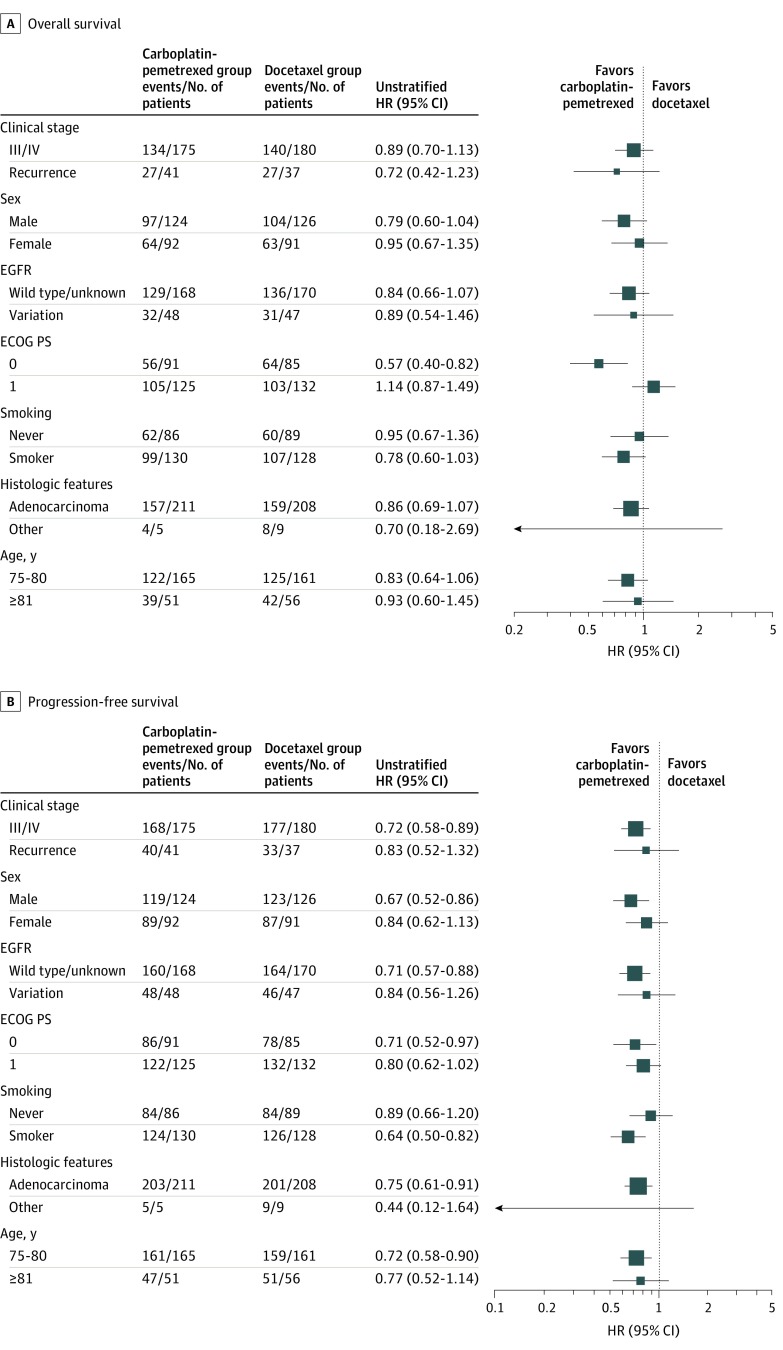
With regard to PFS, 210 patients (96.8%) in the docetaxel group and 208 patients (96.3%) in the carboplatin-pemetrexed group experienced events. Progression-free survival was significantly longer in the carboplatin-pemetrexed group than in the docetaxel group (median, 6.4 months [95% CI, 5.4-7.7] vs 4.3 months [95% CI, 4.0-4.9]; unstratified HR, 0.739; 95% CI, 0.609-0.896; P < .001) (Figure 2B). The results for subgroup analysis of OS and PFS were consistent with those for the intention-to-treat population (Figure 3).
Figure 3. Forest Plots of Hazard Ratios (HRs) for Overall Survival and Progression-Free Survival in the Intention-to-Treat Population by Patient Characteristics at Baseline.
ECOG PS indicates Eastern Cooperative Oncology Group performance status.
Response to treatment was assessed in 209 patients (96.3%) in the docetaxel group and 204 patients (94.4%) in the carboplatin-pemetrexed group who had measurable lesions. The overall response rate was 28.2% (95% CI, 22.2-34.9) in the docetaxel group and 36.8% (95% CI, 30.1-43.8) in the carboplatin-pemetrexed group (P = .07).
Safety
The incidence of a decrease in white blood cell count (147 of 214 patients [68.7%] in the docetaxel group vs 60 of 214 patients [28.0%] in the carboplatin-pemetrexed group) or in neutrophil count (184 patients [86.0%] in the docetaxel group vs 99 patients [46.3%] in the carboplatin-pemetrexed group) of grade 3 or 4 was lower in the carboplatin-pemetrexed group than in the docetaxel group, as was the incidence of febrile neutropenia (38 patients [17.8%] in the docetaxel group vs 9 patients [4.2%] in the carboplatin-pemetrexed group; P < .001) (Table 2). Conversely, treatment with carboplatin plus pemetrexed was associated with a higher incidence of anemia (4 patients [1.9%] in the docetaxel group vs 63 patients [29.4%] in the carboplatin-pemetrexed group) and a decrease in platelet count (3 patients [1.4%] in the docetaxel group vs 55 patients [25.7%] in the carboplatin-pemetrexed group) of grade 3 or 4 (Table 2). Treatment-related death occurred in 2 of 214 patients (0.9%) in the docetaxel group (1 each due to acute respiratory distress syndrome and pneumonitis) and in 2 of 214 patients (0.9%) in the carboplatin-pemetrexed group (1 each due to dyspnea and pneumonitis).
Table 2. Treatment-Related Adverse Events.
| Adverse event | Group, No. (%) | |||||
|---|---|---|---|---|---|---|
| Docetaxel (n = 214) | Carboplatin-pemetrexed (n = 214) | |||||
| Grade 1 or 2 | Grade 3 | Grade 4 | Grade 1 or 2 | Grade 3 | Grade 4 | |
| Decrease in white blood cell count | 53 (24.8) | 129 (60.3) | 18 (8.4) | 96 (44.9) | 54 (25.2) | 6 (2.8) |
| Decrease in neutrophil count | 19 (8.9) | 33 (15.4) | 151 (70.6) | 88 (41.1) | 63 (29.4) | 36 (16.8) |
| Anemia | 192 (89.7) | 3 (1.4) | 1 (0.5) | 148 (69.2) | 53 (24.8) | 10 (4.7) |
| Decrease in platelet count | 76 (35.5) | 1 (0.5) | 2 (0.9) | 139 (65.0) | 35 (16.3) | 20 (9.3) |
| Febrile neutropenia | NA | 36 (16.8) | 2 (0.9) | NA | 9 (4.2) | 0 |
| Fatigue | 121 (56.5) | 10 (4.7) | NA | 112 (52.3) | 13 (6.1) | NA |
| Anorexia | 117 (54.8) | 11 (5.1) | 0 | 121 (56.5) | 16 (7.5) | 0 |
| Hyponatremia | 132 (61.7) | 15 (7.0) | 0 | 131 (61.2) | 18 (8.4) | 0 |
| Any infection | 17 (7.9) | 16 (7.5) | 0 | 20 (9.3) | 14 (6.5) | 0 |
Abbreviation: NA, not applicable.
Quality of Life
A total of 432 of 433 patients (99.8%) completed the baseline symptom score questionnaire. The proportions of patients who completed the questionnaire at 6, 12, and 18 weeks after enrollment were 95.8%, 91.0%, and 85.9%, respectively. In the docetaxel and carboplatin-pemetrexed groups, 62 of 216 patients (28.7%) and 63 of 216 patients (29.2%), respectively, had scores that improved from baseline to 18 weeks. Although the least-squares mean of the symptom score remained constant in the carboplatin-pemetrexed group, a reduction from baseline to 6 weeks was not subsequently reversed in the docetaxel group (eFigure 1 in Supplement 2).
Poststudy Treatment
For the intention-to-treat population, 141 of 217 patients (65.0%) in the docetaxel group and 144 of 216 patients (66.7%) in the carboplatin-pemetrexed group received at least 1 subsequent therapy. Pemetrexed was administered in 106 patients (48.8%) in the docetaxel group, whereas docetaxel was administered in 81 patients (37.5%) in the carboplatin-pemetrexed group. Antibodies to programmed cell death 1 (PD-1) or to programmed cell death ligand 1 (PD-L1) were administered in 43 patients (19.8%) in the docetaxel group and 56 patients (25.9%) in the carboplatin-pemetrexed group, and EGFR TKIs were administrated in 33 patients (15.2%) and 38 patients (17.6%), respectively.
Discussion
Whereas no consensus has been achieved on the definition of elderly, previous phase 3 studies in elderly patients with advanced NSCLC have included individuals 70 years or older.2,3,4,5,6,7,8,9 However, platinum-based combination chemotherapy is usually administered as a first-line treatment for patients aged 70 to 74 years with preserved organ function and a PS of 0 or 1. Given that it appears justifiable to consider patients 75 years or older as elderly with reduced spare ability, we selected such individuals as the study population in the present JCOG1210/WJOG7813L trial. To the best of our knowledge, our study is the first phase 3 randomized clinical trial specifically designed for patients 75 years and older with advanced nonsquamous NSCLC. Reflecting the need for chemotherapy in the increasing number of elderly patients with advanced NSCLC, patient enrollment in the present study was steady, resulting in an increase in the sample size during the study period from 320 to 430 and an increased power of detection with the assumed setting. The median age of the enrolled patients was 78 years, which is higher than that for previous phase 3 studies of elderly patients with advanced NSCLC. The median OS in the docetaxel group was 15.5 months, which is slightly better than that for the docetaxel group in our previous phase 3 trials for elderly patients (at least aged 70 years) with advanced NSCLC (14.3 months in WJTOG 99043; 14.8 months in JCOG0803/WJOG4307L7). The primary objective of the present study—determination of the noninferiority of carboplatin-pemetrexed therapy compared with docetaxel monotherapy with respect to OS—was met with the protocol-specified noninferiority margin of 1.154 (HR, 0.850; 95% CI, 0.684-1.056). Although superiority was not demonstrated, the median OS was 3.2 months longer in the carboplatin-pemetrexed group than in the docetaxel group. Progression-free survival was also significantly longer in patients who received the combination of carboplatin and pemetrexed than in those who received docetaxel alone, and the overall response rate and QOL results favored the carboplatin-pemetrexed group. Collectively, our data thus demonstrate robust effectiveness of carboplatin plus pemetrexed followed by pemetrexed maintenance for first-line therapy of patients with advanced nonsquamous NSCLC 75 years and older.
Marked differences in the incidence of specific adverse events were apparent between the 2 treatment groups. Docetaxel treatment resulted in a typically high incidence of a decrease in neutrophil count of grade 3 or 4 (184 of 214 [86.0%]) as well as of febrile neutropenia (38 of 214 [17.8%]) compared with values of 99 of 214 (46.3%) and 9 of 214 (4.2%), respectively, for carboplatin plus pemetrexed. The incidence of febrile neutropenia in the docetaxel group is slightly higher than that observed in our previous phase 3 trials for elderly patients (70 years and older) with advanced NSCLC,3,7 possibly as a result of the increased age of the patients in the present study. Indeed, febrile neutropenia occurred more frequently in patients 81 years or older in the present study (55 [27.3%] in the docetaxel group and 49 [6.1%] in the carboplatin-pemetrexed group). Febrile neutropenia is a serious adverse event of cytotoxic chemotherapy that negatively affects survival in patients with advanced NSCLC.15,16 Carboplatin-pemetrexed treatment was associated with a higher incidence of anemia and thrombocytopenia, although the frequency of these events was similar to that apparent in a previous study of carboplatin-pemetrexed treatment in Japanese patients 20 years or older.12 Chemotherapy-induced anemia is associated with decreased functional capacity and QOL. With regard to nonhematologic toxic effects, with the exception of febrile neutropenia, no toxic effects of grade 3 or 4 were encountered in more than 10% of patients throughout the study treatment in either group. Despite the advanced age of the patients enrolled in the present study, treatment-related deaths occurred in only 0.9% of patients in each group. Given its efficacy and tolerability, the combination of carboplatin and pemetrexed followed by pemetrexed maintenance will be more readily generalizable to daily clinical practice for the first-line treatment of elderly patients with advanced nonsquamous NSCLC.
In an unplanned exploratory post hoc analysis, the OS benefit for carboplatin-pemetrexed vs docetaxel was notable in patients with a PS of 0 at baseline (eFigure 2 in Supplement 2), and PS showed a weak trend toward qualitative interaction with OS, whereas the beneficial effects of carboplatin-pemetrexed on PFS (eFigure 3 in Supplement 2) and the rate of adverse events (eTable in Supplement 2) were preserved across PS.
The introduction of antibodies to PD-1 and to PD-L1 has greatly changed the therapeutic algorithm for first-line treatment of advanced NSCLC.17 The KEYNOTE-189 phase 3 trial18 demonstrated that addition of pembrolizumab to the combination of cisplatin or carboplatin with pemetrexed significantly prolonged PFS and OS compared with chemotherapy alone in patients with advanced nonsquamous NSCLC without sensitizing EGFR variations or ALK translocations. However, only 9% of patients enrolled in the KEYNOTE-189 trial18 were 75 years and older, and there has been a concern that an age-related decline in the immune system might affect the efficacy of the combination of immunotherapy and platinum-based chemotherapy in elderly patients.
Limitations
Our study had limitations. Our Japan-specific trial may limit the generalizability of the results, since all enrolled participants are Japanese patients with NSCLC who usually show better survival than Western populations, and the use of low-dose 60 mg/m2 docetaxel used in the study is not the standard dose (75 mg/m2) in the rest of the world. A potential weakness of our study is related to the lack of significant differences in OS between the 2 groups, although carboplatin-pemetrexed treatment showed a statistically significant PFS advantage over single-agent docetaxel. A substantial proportion of patients received poststudy systemic treatment. Given that active drugs available for second-line or third-line therapies might confer prolonged survival after progression, an improvement in PFS was not likely to translate into an OS advantage.19,20 Another limitation of our study is the lack of cost-effectiveness analysis, given that medical expenditures put pressure on patient and government finances.
Conclusions
In conclusion, the combination of carboplatin and pemetrexed followed by pemetrexed maintenance in cytotoxic chemotherapy–naive patients 75 years and older with advanced nonsquamous NSCLC provides a clinically significant benefit with regard to its effectiveness and tolerability. This combination should therefore be considered as a standard option for treatment in this setting.
Trial protocol.
eMethods.
eFigure 1. Quality of life assessment according to the 7-item Lung Cancer Subscale of the Functional Assessment of Cancer Therapy–Lung (FACT-LCS) questionnaire.
eFigure 2. Kaplan-Meier plots for overall survival in patients with a performance status of 0 (A) or 1 (B).
eFigure 3. Kaplan-Meier plots for progression-free survival in patients with a performance status of 0 (A) or 1 (B).
eTable. Treatment-related adverse events according to performance status.
Data sharing statement.
References
- 1.Marosi C, Köller M. Challenge of cancer in the elderly. ESMO Open. 2016;1(3):e000020. doi: 10.1136/esmoopen-2015-000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Elderly Lung Cancer Vinorelbine Italian Study Group Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 1999;91(1):66-72. doi: 10.1093/jnci/91.1.66 [DOI] [PubMed] [Google Scholar]
- 3.Kudoh S, Takeda K, Nakagawa K, et al. . Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group trial (WJTOG 9904). J Clin Oncol. 2006;24(22):3657-3663. doi: 10.1200/JCO.2006.06.1044 [DOI] [PubMed] [Google Scholar]
- 4.Gridelli C, Perrone F, Gallo C, et al. ; MILES Investigators . Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003;95(5):362-372. doi: 10.1093/jnci/95.5.362 [DOI] [PubMed] [Google Scholar]
- 5.Ohe Y, Niho S, Kakinuma R, et al. . A phase II study of cisplatin and docetaxel administered as three consecutive weekly infusions for advanced non-small-cell lung cancer in elderly patients. Ann Oncol. 2004;15(1):45-50. doi: 10.1093/annonc/mdh015 [DOI] [PubMed] [Google Scholar]
- 6.Gridelli C, Maione P, Illiano A, et al. . Cisplatin plus gemcitabine or vinorelbine for elderly patients with advanced non small-cell lung cancer: the MILES-2P studies. J Clin Oncol. 2007;25(29):4663-4669. doi: 10.1200/JCO.2007.12.5708 [DOI] [PubMed] [Google Scholar]
- 7.Abe T, Takeda K, Ohe Y, et al. . Randomized phase III trial comparing weekly docetaxel plus cisplatin versus docetaxel monotherapy every 3 weeks in elderly patients with advanced non-small-cell lung cancer: the intergroup trial JCOG0803/WJOG4307L. J Clin Oncol. 2015;33(6):575-581. doi: 10.1200/JCO.2014.55.8627 [DOI] [PubMed] [Google Scholar]
- 8.Gridelli C, Morabito A, Cavanna L, et al. . Cisplatin-based first-line treatment of elderly patients with advanced non-small-cell lung cancer: joint analysis of MILES-3 and MILES-4 phase III trials. J Clin Oncol. 2018;36(25):2585-2592. doi: 10.1200/JCO.2017.76.8390 [DOI] [PubMed] [Google Scholar]
- 9.Quoix E, Zalcman G, Oster JP, et al. ; Intergroupe Francophone de Cancérologie Thoracique . Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378(9796):1079-1088. doi: 10.1016/S0140-6736(11)60780-0 [DOI] [PubMed] [Google Scholar]
- 10.Scagliotti GV, Parikh P, von Pawel J, et al. . Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543-3551. doi: 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 11.Paz-Ares L, de Marinis F, Dediu M, et al. . Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13(3):247-255. doi: 10.1016/S1470-2045(12)70063-3 [DOI] [PubMed] [Google Scholar]
- 12.Okamoto I, Aoe K, Kato T, et al. . Pemetrexed and carboplatin followed by pemetrexed maintenance therapy in chemo-naïve patients with advanced nonsquamous non-small-cell lung cancer. Invest New Drugs. 2013;31(5):1275-1282. doi: 10.1007/s10637-013-9941-z [DOI] [PubMed] [Google Scholar]
- 13.Tamiya A, Tamiya M, Shiroyama T, et al. . Dose escalation study of carboplatin-pemetrexed followed by maintenance pemetrexed for elderly patients with advanced nonsquamous nonsmall-cell lung cancer. Ann Oncol. 2013;24(4):980-985. doi: 10.1093/annonc/mds544 [DOI] [PubMed] [Google Scholar]
- 14.Tamiya M, Tamiya A, Kaneda H, et al. . A phase II study of pemetrexed plus carboplatin followed by maintenance pemetrexed as first-line chemotherapy for elderly patients with advanced non-squamous non-small cell lung cancer. Med Oncol. 2016;33(1):2. doi: 10.1007/s12032-015-0715-7 [DOI] [PubMed] [Google Scholar]
- 15.Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer. 2010;116(23):5555-5563. doi: 10.1002/cncr.25332 [DOI] [PubMed] [Google Scholar]
- 16.Cupp J, Culakova E, Poniewierski MS, Dale DC, Lyman GH, Crawford J. Analysis of factors associated with in-hospital mortality in lung cancer chemotherapy patients with neutropenia. Clin Lung Cancer. 2018;19(2):e163-e169. doi: 10.1016/j.cllc.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 17.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 18.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 19.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101(23):1642-1649. doi: 10.1093/jnci/djp369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi H, Okamoto I, Morita S, Taguri M, Nakagawa K. Postprogression survival for first-line chemotherapy of patients with advanced non-small-cell lung cancer. Ann Oncol. 2012;23(6):1537-1541. doi: 10.1093/annonc/mdr487 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eMethods.
eFigure 1. Quality of life assessment according to the 7-item Lung Cancer Subscale of the Functional Assessment of Cancer Therapy–Lung (FACT-LCS) questionnaire.
eFigure 2. Kaplan-Meier plots for overall survival in patients with a performance status of 0 (A) or 1 (B).
eFigure 3. Kaplan-Meier plots for progression-free survival in patients with a performance status of 0 (A) or 1 (B).
eTable. Treatment-related adverse events according to performance status.
Data sharing statement.