Abstract
Cryptococcosis is an important opportunistic infection. It is the third most common invasive fungal infection in solid organ transplant recipients. The primary organ affected is the lungs, but infection of the central nervous system and other organ systems are also seen. Here we reported a case of disseminated cryptococcosis in a healthy patient who presented with severe pneumonia, a left upper lobe nodule and enlarged mediastinal lymphadenopathy on a chest computed tomography scan.
Keywords: Yeast, Cryptococcus neoformans, VITEK
Highlights
-
•
Cryptococcosis is an important opportunistic infection.
-
•
A case of disseminated cryptococcosis in a healthy patient was reported.
-
•
Chest CT revealed a left upper lobe nodule and enlarged mediastinal lymphadenopathy.
1. Introduction
Cryptococcosis is an invasive fungal disease caused by pathogenic encapsulated yeasts in the genus Cryptococcus. The main human pathogens are C. neoformans and C. gattii. They are often found in soil in areas frequented by birds, especially pigeons and chickens [1,2]. They have a worldwide distribution, and account for most cases of cryptococcosis in humans. Most patients with cryptococcosis are immunocompromised. The reactivation of cryptococcal infection commonly presents as meningoencephalitis and/or pneumonia [3,4]. In this study, we presented a case of disseminated cryptococcosis in an immunocompetent individual.
1.1. Case presentation
A 30-year-old male presented to the emergency department with night sweats, fever, chills, sharp chest pain associated with productive cough for three weeks. The patient has no significant past medical, surgical or travel history. He is currently not on medication and denies a family history of malignancy or autoimmune diseases. He smokes approximately 5–8 cigarettes per day. He denies alcohol abuse, illicit drug use and high-risk sexual activities. The patient was febrile and tachycardic on presentation. He had equal air entry in both lung fields with normal breath sounds. No lymphadenopathy was found. His oral mucosa showed no signs of pathology. There were no focal neurological deficits. He was tested negative for HIV, hepatitis B and C. Laboratory studies revealed mild leukocytosis and thrombocytosis.
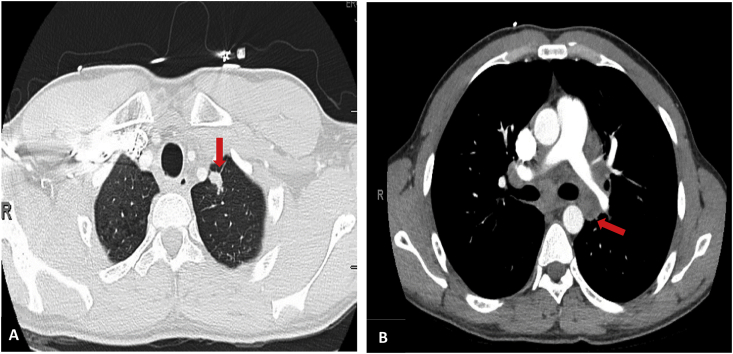
Patient was initially started on cefepime and vancomycin with concerns for infection. Chest X-ray was unremarkable for any parenchymal changes, but questionably superior mediastinal widening. Chest CT discovered a 1.4 cm left upper lobe nodule (Fig. 1A) and enlarged mediastinal lymphadenopathy (Fig. 1B). Bronchoscopy showed left upper lobe mass, extrinsic compression in the apical posterior segment of left upper lobe and mucous plug. Bronchoalveolar lavage (BAL) was collected and sent out for bacterial and fungal cultures. The patient symptomatically improved and was discharged home with a plan to do an endobronchial ultrasound plus biopsy in four days to rule out malignancy. Endobronchial ultrasound was then performed, and a biopsy sample was taken from a lymph node. The lymph node biopsy sample showed granulomatous inflammation with multinucleate cells, and it was negative for malignant cells.
Fig. 1.
Chest CT showing a left upper lobe nodule (A) and enlarged mediastinal lymphadenopathy (B).
The patient was seen in the outpatient setting one week after discharge and endorsed a constant headache associated with blurry vision and sleep disturbances. Patient was subsequently admitted with concerns for cryptococcal meningitis. Lumbar puncture showed normal glucose and protein with no white blood cells making CNS infection unlikely. Cryptococcal antigen was negative from both serum and cerebrospinal fluid. Head CT showed no intracranial pathology. CT head/neck angiography showed lymph nodes encasing left common carotid without significant stenosis. The specimens from both the BAL and lymph node biopsy eventually grew Cryptococcus neoformans. Patient started on oral fluconazole 400 mg OD for six months and advised to follow-up outpatient for further care.
2. Discussion
Cryptococcus is a genus of basidiomycetous fungi with more than 30 species ubiquitously distributed in the environment. C. neoformans and C. gattii are commonly known to cause cryptococcosis in humans [[3], [4], [5]]. The clinical presentation of cryptococcosis due to the two species is generally indistinguishable [3,6]. Although cryptococcosis is rare in immunocompetent individuals, there was a recent outbreak of cryptococcosis in healthy people in North America and Canada [7]. The percentage of cryptococcal infections due to C. gattii in healthy people is significantly higher than for C. neoformans [3,8]. Smoking is an important risk factor for C. gattii infection [9]. In addition, C. gattii is commonly associated with eucalyptus trees in tropical and subtropical regions [2,3].
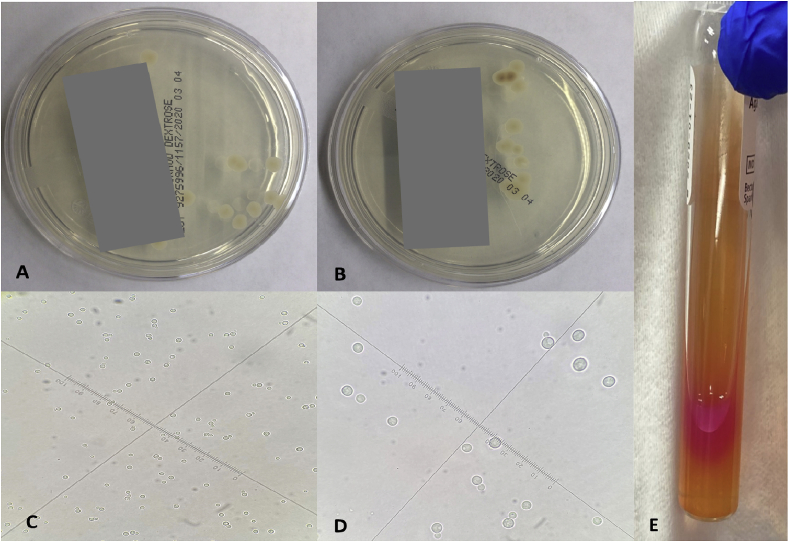
The bronchoalveolar lavage and lymph node biopsy samples from this patient were submitted to the clinical microbiology laboratory for bacterial and fungal cultures. Sabouraud agar plates produced white, mucoid colonies after 48 hours of incubation at 30 °C (Fig. 2A and B). The colonies were identified as C. neoformans by the VITEK 2 system. Microscopically, C. neoformans is a spherical, budding, encapsulated yeast, which can vary in size on the wet mount slide (Fig. 2B and C). It was found that C. neoformans produced urease on Christensen's urea agar slant (Fig. 2D). No other pathogenic microorganisms were identified on other solid media, including sheep blood, chocolate, MacConkey, and Columbia CNA agars.
Fig. 2.
Sabouraud agar plates produced white, mucoid colonies of Cryptococcus neoformans in (A) the bronchoalveolar lavage and (B) the lymph node biopsy. Microscopic image of Cryptococcus neoformans on the wet mount slide captured with (C) 40x objective lens and (D) 100x objective lens. (E) Positive reaction on Christensen's urea agar slant.
The primary infection is usually acquired via inhalation of yeast spores, and typically affects the lungs. Many patients present asymptomatically with a primary cryptococcal infection [10,11]. A key feature of cryptococcal pathogenesis involves the exit of this fungus from the lungs into blood, and entry into the central nervous system, and other organs in the body. In this patient, the lymph node involvement in the mediastinum was more advanced than anticipated for similar pulmonary involvement. Disseminated cryptococcosis is less common in immunocompetent individuals. Most cases of disseminated cryptococcosis occur in HIV-infected people [12,13].
3. Conclusion
In the study, we presented a unique case of disseminated cryptococcosis in an immunocompetent patient. Accurate diagnosis and prompt treatment are critical for disseminated cryptococcosis and ultimately lead to better patient outcomes.
CRediT authorship contribution statement
Rahul Bollam: Conceptualization, Writing - original draft. Mohamed Yassin: Conceptualization, Writing - original draft, Resources, Writing - review & editing. Tung Phan: Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgement
We thank the staff of the clinical microbiology laboratory at UPMC Mercy for help with initial isolation and characterization of the isolate.
References
- 1.May R.C., Stone N.R., Wiesner D.L., Bicanic T., Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016;14:106–117. doi: 10.1038/nrmicro.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Poeta M., Casadevall A. Ten challenges on Cryptococcus and cryptococcosis. Mycopathologia. 2012;173:303–310. doi: 10.1007/s11046-011-9473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maziarz E.K., Perfect J.R. Cryptococcosis. Infect. Dis. Clin. North. Am. 2016;30:179–206. doi: 10.1016/j.idc.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gullo F.P., Rossi S.A., Sardi Jde C., Teodoro V.L., Mendes-Giannini M.J., Fusco-Almeida A.M. Cryptococcosis: epidemiology, fungal resistance, and new alternatives for treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:1377–1391. doi: 10.1007/s10096-013-1915-8. [DOI] [PubMed] [Google Scholar]
- 5.Firacative C., Lizarazo J., Illnait-Zaragozí M.T., Castañeda E. Latin American cryptococcal study group. Mem. Inst. Oswaldo Cruz. 2018;113 doi: 10.1590/0074-02760170554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setianingrum F., Rautemaa-Richardson R., Denning D.W. Pulmonary cryptococcosis: a review of pathobiology and clinical aspects. Med. Mycol. 2019;57:133–150. doi: 10.1093/mmy/myy086. [DOI] [PubMed] [Google Scholar]
- 7.Byrnes E.J., Marr K.A. The outbreak of Cryptococcus gattii in Western North America: epidemiology and clinical issues. Curr. Infect. Dis. Rep. 2011;13:256–261. doi: 10.1007/s11908-011-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz J.H. The disease ecology, epidemiology, clinical manifestations, and management of emerging cryptococcus gattii complex infections. Wilderness Environ. Med. 2019:30175–30179. doi: 10.1016/j.wem.2019.10.004. pii: S1080-6032. [DOI] [PubMed] [Google Scholar]
- 9.Chen S.C., Meyer W., Sorrell T.C. Cryptococcus gattii infections. Clin. Microbiol. Rev. 2014;27:980–1024. doi: 10.1128/CMR.00126-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z., Lu G., Meng G. Pathogenic fungal infection in the lung. Front. Immunol. 2019;10:1524. doi: 10.3389/fimmu.2019.01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Setianingrum F., Rautemaa-Richardson R., Denning D.W. Pulmonary cryptococcosis: a review of pathobiology and clinical aspects. Med. Mycol. 2019;57:133–150. doi: 10.1093/mmy/myy086. [DOI] [PubMed] [Google Scholar]
- 12.Park B.J., Wannemuehler K.A., Marston B.J., Govender N., Pappas P.G., Chiller T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2019;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 13.Rajasingham R., Smith R.M., Park B.J., Jarvis J.N., Govender N.P., Chiller T.M., Denning D.W., Loyse A., Boulware D.R. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 2017;17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]