Abstract
Successful consolidation of associative memories relies on the coordinated interplay of slow oscillations and sleep spindles during non-rapid eye movement (NREM) sleep. This enables the transfer of labile information from the hippocampus to permanent memory stores in the neocortex. During senescence, the decline of the structural and functional integrity of the hippocampus and neocortical regions is paralleled by changes of the physiological events that stabilize and enhance associative memories during NREM sleep. However, the currently available evidence is inconclusive as to whether and under which circumstances memory consolidation is impacted during aging. To approach this question, 30 younger adults (19–28 years) and 36 older adults (63–74 years) completed a memory task based on scene–word associations. By tracing the encoding quality of participants’ individual memory associations, we demonstrate that previous learning determines the extent of age-related impairments in memory consolidation. Specifically, the detrimental effects of aging on memory maintenance were greatest for mnemonic contents of intermediate encoding quality, whereas memory gain of poorly encoded memories did not differ by age. Ambulatory polysomnography (PSG) and structural magnetic resonance imaging (MRI) data were acquired to extract potential predictors of memory consolidation from each participant’s NREM sleep physiology and brain structure. Partial Least Squares Correlation was used to identify profiles of interdependent alterations in sleep physiology and brain structure that are characteristic for increasing age. Across age groups, both the ‘aged’ sleep profile, defined by decreased slow-wave activity (0.5–4.5 Hz), and a reduced presence of slow oscillations (0.5–1 Hz), slow, and fast spindles (9–12.5 Hz; 12.5–16 Hz), as well as the ‘aged’ brain structure profile, characterized by gray matter reductions in the medial prefrontal cortex, thalamus, entorhinal cortex, and hippocampus, were associated with reduced memory maintenance. However, inter-individual differences in neither sleep nor structural brain integrity alone qualified as the driving force behind age differences in sleep-dependent consolidation in the present study. Our results underscore the need for novel and age-fair analytic tools to provide a mechanistic understanding of age differences in memory consolidation.
Keywords: Aging, Brain structure, Episodic memory, NREM sleep, Memory consolidation, Memory quality, PLSC
Highlights
-
•
Encoding quality modulates age-related impairments in memory consolidation.
-
•
Age differences are maximal for the maintenance of medium-quality memories.
-
•
Partial Least Squares Correlation can integrate sleep and brain structure profiles.
-
•
Aged NREM sleep and brain structure profiles relate to worse memory maintenance.
-
•
Within age groups, sleep–memory and brain–memory associations were not reliable.
1. Introduction
Non-rapid eye movement (NREM) sleep is critical for the long-term retention of memories (Rasch and Born, 2013). Sleep facilitates memory consolidation by the interplay of slow oscillations and sleep spindles that guide the transformation of labile hippocampus-dependent memories into durable neocortical representations (Born and Wilhelm, 2012; Diekelmann and Born, 2010; Gais and Born, 2004; Walker, 2009). In contrast to compelling evidence on the beneficial effect of sleep on memory consolidation in younger adults (Diekelmann and Born, 2010; Jenkins and Dallenbach, 1924; Ngo et al., 2013a, Ngo et al., 2013b; Rasch et al., 2007; Walker et al., 2002), corresponding research in older adults has produced inconsistent findings (for reviews, see Muehlroth et al., submitted; Scullin and Bliwise, 2015). To date, the available evidence is inconclusive as to whether and under which circumstances memory consolidation is affected during advancing age.
By now, several studies along with meta-analytic evidence suggest that the beneficial effect of sleep on episodic memory is reduced in old age (e.g., Baran et al., 2016; Cherdieu et al., 2014; Gui et al., 2017; Mander et al., 2013; Scullin, 2012; Scullin et al., 2017; Varga et al., 2016). For instance, Cordi et al. (2018) recently demonstrated that the targeted reactivation of previously learned word pairs during sleep was only beneficial in younger but not in older adults (e.g., Cairney et al., 2018; Rasch et al., 2007; Schouten et al., 2017; Schreiner and Rasch, 2015, for evidence in younger adults). In contrast, Wilson et al. (2012) reported that sleep, compared to a wake interval, similarly benefitted word pair memory in younger, middle-aged, and older adults (Wilson et al., 2012). Along similar lines, Sonni and Spencer (2015) showed a similar protection of visuospatial memories against memory interference in both younger and older adults when participants received the opportunity to sleep after learning (Sonni and Spencer, 2015). Notably, in both of these studies, the protective effect of sleep was reduced (Wilson et al., 2012) or even absent (Sonni and Spencer, 2015) in low-performing older adults. Hence, the pre-sleep level of memory performance has an influence on whether age-related impairments in memory consolidation during sleep become evident (also Tucker et al., 2011).
The described inconsistency might, in part, arise from unknown variations in the quality of individual memories. The depth of encoding (Craik and Lockhart, 1972) and degree of learning (Tulving, 1967) discriminate memories with regard to their memory strength or memory quality. In general, insufficient and shallow processing of new information appears to hamper learning success in old age (Craik et al., 2010; Naveh-Benjamin et al., 2007; Shing et al., 2008). Further, as older adults have deficits in binding items into cohesive and distinct memory representations (Naveh-Benjamin, 2000; Old and Naveh-Benjamin, 2008; Shing et al., 2008; St-Laurent et al., 2014), the quality of newly formed memories might be critically reduced. Crucially, it is likely that sleep-dependent memory consolidation does not affect all encoded memories in the same way (Conte and Ficca, 2013; Diekelmann et al., 2009; Schoch et al., 2017; Stickgold and Walker, 2013). Several studies imply that memories of intermediate quality are prioritized during sleep, resulting in greater benefits for weakly encoded memories (Diekelmann et al., 2009; Drosopoulos et al., 2007; Kuriyama et al., 2004; Schapiro et al., 2018; Stickgold, 2010; but see Tucker and Fishbein, 2008). Hence, proper assessment of age differences in sleep-dependent memory consolidation requires consideration of the quality of encoded memories.
Already 50 years ago, Tulving called for an improvement of behavioral memory measures (Tulving, 1964, 1967) arguing that “the ‘average’ item is a highly abstract and elusive entity having no readily identifiable counterparts in the empirical realm” (Tulving, 1967, p. 183). Still, research has only recently started to address this problem with regard to memory consolidation (cf. Conte and Ficca, 2013). By looking at each memory’s individual fate it becomes possible to identify whether and how the success of memory encoding influences later consolidation processes (Dumay, 2016; Fenn and Hambrick, 2013): Sleep potentially stabilizes previously successfully encoded memories but may also enhance the availability of initially poor memories above a pre-sleep learning level (Ellenbogen et al., 2006; Nettersheim et al., 2015). So far, prevailing evidence speaks for a primary role of sleep in memory maintenance: By passively protecting memories against interference (Wixted, 2004) and actively reactivating and stabilizing memory engrams (Rasch et al., 2007), memories are maintained across periods of sleep (Fenn and Hambrick, 2013; Schreiner and Rasch, 2018). Behaviorally observed memory gains that reflect the availability of initially poor memories during later memory retrieval appear to rely less on sleep (Dumay, 2018; Fenn and Hambrick, 2013; Schreiner and Rasch, 2018).
Successful consolidation during sleep not only requires intact memory encoding. Brain atrophy in old age directly affects memory-relevant regions in the medial temporal lobe (MTL), especially the hippocampus (Fjell and Walhovd, 2010; Persson et al., 2012; Raz et al., 2005; Raz and Rodrigue, 2006; Walhovd et al., 2010). This potentially leads to impaired encoding, retrieval, and importantly, to deficient consolidation of memories (Buckner, 2004; Craik and Rose, 2012; Pudas et al., 2017; Shing et al., 2011, 2008; Ward et al., 1999; Werkle-Bergner et al., 2006). Moreover, age-related structural changes in brain regions involved in slow oscillation and spindle generation have frequently been related to altered sleep physiology in old age (Dubé et al., 2015; Fogel et al., 2017; Landolt and Borbély, 2001; Mander et al., 2013; Varga et al., 2016). For instance, the medial prefrontal cortex (mPFC), which is particularly vulnerable during aging (Giorgio et al., 2010; Raz and Rodrigue, 2006; Ziegler et al., 2012), has repeatedly been linked to deficient generation and coordination of low-frequency oscillatory activity and concomitant deficits in memory consolidation (Helfrich et al., 2018; Mander et al., 2013; Muehlroth et al., 2019). By impairing the neurophysiological underpinnings of successful consolidation, that is, the generation of slow oscillations, slow waves, and spindles, age-related brain atrophy may lead to worse memory consolidation in old age.
Indeed, aging involves substantial changes in sleep physiology that affect NREM sleep in particular (Mander et al., 2017a). Overall, aging is characterized by increasingly fragmented nighttime sleep, while daytime fatigue and the tendency to nap during the day become more frequent (Carskadon et al., 1982; Vitiello, 2006). Moreover, in old age, the deepest NREM sleep stage, so-called slow-wave sleep (SWS), is strikingly reduced compared to younger adults (Carrier et al., 2011; Mander et al., 2017a, Mander et al., 2017b; Ohayon et al., 2004). Slow oscillations and sleep spindles characteristic of NREM sleep are less frequently observed, appear with changed amplitude, frequency, and topography, and are less precisely coordinated (Carrier et al., 2011; Crowley et al., 2002; Dubé et al., 2015; Fogel et al., 2012; Helfrich et al., 2018; Landolt and Borbély, 2001; Muehlroth et al., 2019). These age differences in NREM sleep may affect memory consolidation and account for dysfunctional protection of memories against forgetting in old age (Baran et al., 2016; Buckley and Schatzberg, 2005; Cherdieu et al., 2014; Harand et al., 2012; Helfrich et al., 2018; Hornung et al., 2005; Mander et al., 2013; Muehlroth et al., 2019; Varga et al., 2016; Westerberg et al., 2012). However, the age differences in NREM sleep are manifold and most likely interdependent (Carrier et al., 2011; Helfrich et al., 2018; Mander et al., 2017a, Mander et al., 2017b; Muehlroth et al., 2019; Ohayon et al., 2004). Hence, the causes driving alterations in memory consolidation during sleep still remain elusive.
Within this article, we relied on a data-driven solution to extract the commonalities of age-related changes in multiple highly correlated indicators of NREM sleep. We used Partial Least Squares Correlation (PLSC) as a multivariate statistical approach to capture the association between each participant’s chronological age and multiple indicators of memory consolidation during NREM sleep (Haenlein and Kaplan, 2004; Krishnan et al., 2011; Lobaugh et al., 2001; McIntosh and Bookstein, 1996; McIntosh et al., 2004; McIntosh and Lobaugh, 2004; McIntosh and Mišić, 2013). As a dimensionality reduction technique, PLSC is ideally suited to derive underlying latent patterns in highly correlated dependent measures. It has thus already been widely adapted and applied in neuroimaging studies (e.g., Düzel et al., 2003; Keresztes et al., 2017; Lobaugh et al., 2001; McIntosh and Bookstein, 1996; McIntosh et al., 2004; McIntosh and Mišić, 2013). Here, we illustrate its applicability to sleep electroencephalography (EEG) data. Moreover, we demonstrate its utility in the context of aging research where it allows us to identify profiles of sleep physiology and brain structure associated with advancing age.
In the present study we tracked the learning history of single scene–word associations within individual younger and older adults (e.g., Dumay, 2018; Fenn and Hambrick, 2013; Schreiner and Rasch, 2018). We hypothesize that age differences in memory consolidation are most pronounced for memories of intermediate encoding quality, as these memories appear to be preferentially consolidated during sleep (e.g., Drosopoulos et al., 2007; Kuriyama et al., 2004; Schapiro et al., 2018). In addition, we expect that pronounced age differences in NREM sleep physiology may – at least partially – account for dysfunctional protection of memories against forgetting in old age (e.g., Baran et al., 2016; Cherdieu et al., 2014; Helfrich et al., 2018; Mander et al., 2013; Muehlroth et al., 2019; Varga et al., 2016; Westerberg et al., 2012). Finally, we assume that brain atrophy in memory- and sleep-related brain regions, as indicated by reduced gray matter volume, may be associated with worse memory consolidation in old age (Helfrich et al., 2018; Mander et al., 2013; Muehlroth et al., 2019). We tackled the latter two questions by using a multivariate analysis approach. With PLSC we aimed at identifying a profile of age-related differences in sleep physiology and brain structure, respectively, that is indicative of advanced chronological age and checked for its relation to age differences in overnight memory consolidation. Thus, using the same multivariate approach, we addressed how age-differences in sleep physiology and brain structure may contribute to alterations in overnight changes in memory performance.
2. Materials and methods
2.1. Participants and procedure
2.1.1. Participants
Overall, 34 healthy younger adults (19–28 years) and 41 healthy older adults (63–74 years) took part in the experiment. Data from one older adult were excluded due to an incidental finding in the structural magnetic resonance imaging (MRI) data. Four younger and four older adults had to be excluded due to technical failures during data collection, resulting in a final sample of 30 younger (Mage = 23.7 years, SDage = 2.6; 17 females) and 36 older adults (Mage = 68.92 years, SDage = 3.04; 16 females) for the behavioral analyses. Since parts of some participants’ neural data (polysomnography [PSG] or MRI) were missing or of bad quality, final PSG analyses were conducted with 24 younger (Mage = 23.61 years, SDage = 2.55; 13 females) and 31 older adults (Mage = 68.63 years, SDage = 3.10; 15 females). From this sample, two older adults had to be excluded for structural MRI analyses resulting in a sample of 29 older adults for the respective analyses (Mage = 68.64 years, SDage = 3.10; 14 females).
All participants were right-handed native German speakers with no reported history of psychiatric or neurological disease, or any use of psychoactive medication. All older adults completed the Mini-Mental State Examination (MMSE; M = 29.24, SD = 1.12, range: 26–30; Folstein et al., 1975) and passed a brief memory screening before inclusion in the final experiment. General subjective sleep quality was controlled by assessing the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989). The study was approved by the Ethics Committee of the Deutsche Gesellschaft für Psychologie (DGPs) and conducted at the Max Planck Institute for Human Development in Berlin. All participants gave written consent to their participation in the experiment after being informed about the complete study procedure.
2.1.2. General procedure
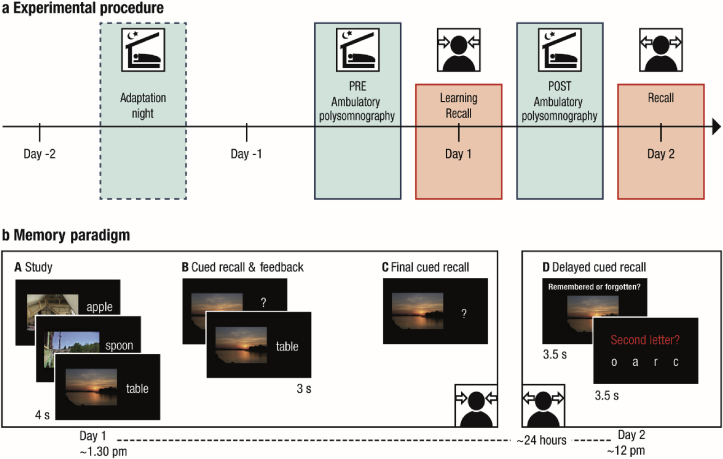
At the core of the experimental design was an age-adapted associative memory paradigm. It consisted of a learning session on the first day (Day 1, starting approximately at 1.30 p.m.) as well as a delayed cued recall task taking place approximately 24 h later (Day 2, starting approximately at 12 p.m.) (see Fig. 1 for illustration of the study procedure). During the nights before and after learning (experimental nights PRE and POST), sleep was monitored at the participants’ homes using ambulatory PSG. Prior to the first experimental night an adaptation night familiarized the participants with the PSG procedure. Structural MRI data were collected on Day 2. Furthermore, EEG was recorded during learning on Day 1. Additionally, functional MRI data were collected during delayed recall on Day 2. Neither the EEG nor the fMRI data are included in the present report (but see Sommer et al., 2019 and Sander et al., in press on oscillatory mechanisms and neural pattern similarity during memory encoding that allow for successful memory formation).
Fig. 1.
a. Experimental procedure (adapted from Muehlroth et al., 2019). The memory task at the core of the experiment consisted of a learning phase and an immediate recall on Day 1 as well as a delayed recall approximately 24 h later (red boxes). Sleep was monitored in the nights before (PRE) and after (POST) learning using ambulatory polysomnography (blue boxes). A prior adaptation night familiarized the participants with the sleep recordings. b. Memory paradigm. (A) During study, participants were instructed to remember 440 (younger adults) or 280 scene–word pairs (older adults). (B) During the cued recall and feedback phase the scene was presented as a cue to recall the corresponding word. Irrespective of recall accuracy, the original pair was presented again to allow for re-studying. The whole cued recall and feedback cycle was performed once in younger and twice in older adults. (C) During final recall, scenes again served as cues to recall the corresponding word, but no feedback was provided. (D) Delayed cued recall took place approximately 24 h later. Participants were presented with the scenes only and had to indicate if they still remembered the associated word. Afterwards they had to select the corresponding second letter of the word to verify their true memory of the associate.
2.2. Memory paradigm
The memory task and the stimulus set are described by Fandakova et al. (2018) and Muehlroth et al. (2019) in detail. During initial study, randomized combinations of scenes and concrete nouns were presented on a black screen for 4000 ms. Participants were instructed to remember these scene–word pairs using a previously practiced learning strategy. This strategy prompted participants to generate integrated and vivid mental images of the respective scene–word association. The strategy was implemented by the participants in a number of practice trials in which examples were discussed in detail until the strategy was well understood and confidently applied. During the ensuing cued recall phase, scenes served as cues for participants to verbally recall the associated word. Independent of recall accuracy, the correct scene–word pair was presented again for 3 s and participants were encouraged to restudy the combinations. At the end of Day 1, participants completed a final cued recall test without feedback.
To answer the question whether memory consolidation is affected during aging, foremost, fair learning conditions for both younger and older adults are required (Conte and Ficca, 2013). Hence, the encoding task was adjusted between the age groups to achieve comparable task difficulty: First, younger adults learned 440 pairs, whereas older adults learned 280 pairs on Day 1. Second, younger adults completed one cued recall block with feedback, whereas older adults completed an additional cued recall block with feedback that was excluded from further analyses (Mdperformance = 3.93% [1.70; 6.25]). Finally, explicit strategy instruction before encoding was used to gain control over study processing and to minimize the frequently reported age-related confound of differential strategy use (Conte and Ficca, 2013; Naveh-Benjamin, 2000; Naveh-Benjamin et al., 2007; Shing et al., 2008). The experimental procedure was established as part of a previous study (cf. Fandakova et al., 2018; Sander et al., in press; Sommer et al., 2019), where it has been proven to result in comparable task difficulty and a pre-sleep recall success of approximately 50% in each age group. Ceiling and floor effects were avoided in this way, and the detection of both memory maintenance and gain was facilitated. Meanwhile, the learning procedure of the current memory task has been tested in a third independent sample and the basic behavioral pattern was successfully replicated (Fandakova et al., 2019).
Delayed cued recall of the scene–word pairs took place approximately 24 h later. Both younger and older adults were presented with 280 scenes for 3500 ms. Via keypress, participants had to indicate whether they still remembered the respective word (“remembered” vs. “forgotten”). Afterwards they were given a 3500 ms-opportunity to select the corresponding second letter of the word out of four letter options to verify their true memory of the associate. Participants were instructed to answer as accurately and quickly as possible. Importantly, for the older age group, all of the 280 studied pairs were presented. For younger adults, items were chosen with regard to their learning history. If permitted by the individual learning performance on Day 1 (cf. Supplementary Fig. 1a), this resulted in a selection of pairs, half of which had been recalled in the criterion cued recall the day before (Supplementary Fig. 1b for inter-individual differences in trial composition during delayed recall on Day 2). All behavioral measures were adjusted for inter-individual differences in learning trajectories on Day 1 (see below). An exact overview of trial numbers on Day 1 and Day 2 is provided in Supplementary Table 6 and Supplementary Fig. 1.
2.3. Behavioral analyses
For each recall phase during the learning task on Day 1 the number of correctly recalled items was calculated and divided by the corresponding number of trials. Answers during delayed recall on Day 2 were only classified as being correct if participants both responded to remember the word and if they indicated the true letter afterwards. Reaction times for giving the “remember vs. forgotten” judgement were extracted for all correct trials.
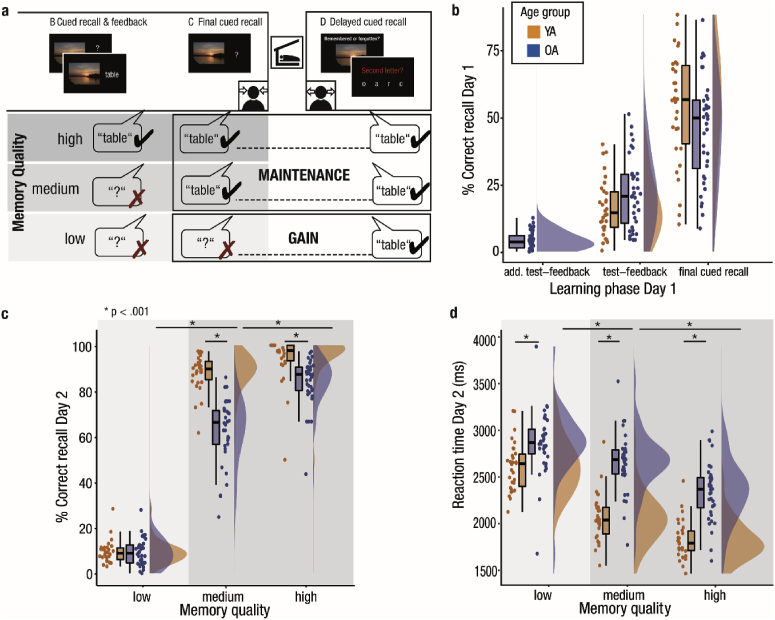
Given the nature of the memory task with several recall phases, we were able to analyze recall success on Day 2 as conditional on the recall success on Day 1 (Fig. 2a, Supplementary Fig. 1, Supplementary Table 6, cf. Dumay, 2016). We thus distinguished two categories of items: (a) maintained items (items recalled both during final cued recall on Day 1 and during delayed recall on Day 2), and (b) gained items (items recalled during delayed recall on Day 2, but not during final cued recall on Day 1). Maintained items were further divided into those items that were recalled during both recall phases on Day 1 (= high memory quality) and into those that were only successfully recalled during final cued recall (= medium memory quality). Gained items that were not at all recalled on Day 1 were considered having a low memory quality with respect to encoding on Day 1 (Fig. 2a). Items that were not remembered in the final cued recall but remembered in the preceding recall round, i.e., forgotten, were excluded from the analyses (cf. Supplementary Table 6). This only applied to a very small number of items (MdYA = 3.00 [1.00; 4.00]; MdOA = 4.00 [2.25; 6.00]). Within each of the resulting categories we determined the probability of recalling an item during delayed recall on Day 2. All analyses focused on successful memory recall on Day 2. Hence, the complementary categories of items not recalled on Day 2 were not included in further analyses.
Fig. 2.
Memory quality limits the effects of aging on memory consolidation. (a) Identification of memory quality. The recall success of items during delayed recall on Day 2 was analyzed based on learning success on Day 1. Recall success during final cued recall on Day 1 determined whether an item was gained (shaded in light gray) or maintained across sleep (shaded in darker gray). Recall success of the test–feedback phase conditioned memory quality. (b) Learning performance on Day 1. The learning performance of both younger (orange) and older adults (blue) increases across repeated learning cycles and is comparable in both age groups. In older adults, an additional test-feedback cycle with low initial recall success was performed. (c) Recall success on Day 2 increases with memory quality (from left to right on the x-axis). Younger and older adults show a similar memory gain across sleep (shaded in light gray), but memory maintenance is reduced in older compared to younger adults (shaded in darker gray). (d) Similar to memory performance, reaction times during delayed recall on Day 2 are conditioned on memory quality. YA: younger adults; OA: older adults.
Mixed factorial ANOVAs with the between factor Age Group and the within factor Memory Quality were calculated for both recall success and reaction times on Day 2 to test for differences in memory retrieval. Post-hoc testing was conducted calculating non-parametric Mann-Whitney U tests for independent samples and Wilcoxon signed-rank tests for matched pairs, as not all behavioral measures met the assumptions for normality (cf. Fig. 2). Although the ANOVA is a standard parametric statistical test, it is considered robust against violations of the normal distribution in case of equally sized samples (e.g., Glass et al., 1972; Harwell et al., 1992). Identical analyses on the logit-transformed recall data did not change reported results qualitatively.
2.4. Sleep EEG data acquisition and analyses
2.4.1. Data acquisition
During the two experimental nights before (PRE) and after learning (POST), sleep was recorded using an ambulatory PSG device (SOMNOscreen plus; SOMNOmedics, Germany). Eight scalp electrodes (AFz, F3, F4, C3, C4, Cz, Pz, Oz) were attached according to the international 10–20 system for electrode positioning (Jasper, 1958; Oostenveld and Praamstra, 2001) along with two electrodes on the mastoids A1 and A2 that later served as the offline reference. All impedances were kept below 6 kΩ. Data were recorded using Cz as the online reference for all EEG derivations and AFz as ground. EEG channels were recorded between 0.2 and 75 Hz with a sampling rate of 128 Hz. Additionally, a bilateral electrooculogram (EOG) was assessed. Two submental electromyogram channels (EMG) were positioned left and right inferior of the labial angle and referenced against one chin electrode. Electrical activity of the heart was recorded using two electrocardiogram channels (ECG).
2.4.2. EEG pre-processing
Data was preprocessed using BrainVision Analyzer 2.1 (Brain Products, Germany), Matlab R2014b (Mathworks Inc., Sherborn, MA), and the open-source toolbox Fieldtrip (Oostenveld et al., 2011). All EEG channels were re-referenced against the average of A1 and A2 and filtered according to the settings recommended by the American Academy for Sleep Medicine (AASM; Iber et al., 2007) (EEG: 0.3–35 Hz; EOG: 0.3–35 Hz; EMG: 10–100 Hz). Additionally, a Notch (50 Hz) filter was applied to the EMG derivations to remove external electrical interference. Afterwards, sleep was visually scored on 30-s epochs according to the standard criteria suggested by the AASM (Iber et al., 2007). Strong body movements were marked and bad EEG channels were visually rejected. For the remaining channels and time points, automatic artefact detection was implemented on 1-s long segments (see Muehlroth et al., 2019, for more details). Global sleep parameters were estimated based on the visually scored sleep data (SchlafAus 1.4; Lübeck, Germany). Total sleep time (TST) was calculated as time spent in stage 1, 2, SWS, and rapid eye movement (REM) sleep. Wake after sleep onset (WASO) was defined as the proportion of time awake between sleep onset and final morning awakening. Sleep latency described the timespan from lights off, as documented by participants, to the first occurrence of stage 2, SWS, or REM sleep in minutes. Finally, sleep efficiency was estimated by dividing TST by the time between sleep onset and final morning awakening.
2.4.3. Power spectral analysis
To get more fine-grained indicators of neural processes during deep NREM sleep, power spectral analyses of the sleep EEG data were conducted. Fast Fourier Transformation (FFT) with frequency limits between 0.5 and 30 Hz was applied on 5-s intervals using a Hanning function with no overlap. Slow-wave activity (SWA), defined as spectral power in the delta frequency range (0.5–4.5 Hz), was calculated for NREM epochs (i.e., stage 2 sleep and SWS). To minimize possible confounds like skull thickness (e.g., Dannhauer et al., 2011; Frodl et al., 2001; Leissner et al., 1970), SWA was normalized to the total power of the whole spectrum. As in previous studies frontal SWA was found to be particularly significant for cognitive functions of sleep (e.g., Mander et al., 2013), we focused our analyses on frontal SWA as the average between the two frontal derivations F3 and F4. No hemispheric differences in frontal SWA were detected (Z = 0.81, p = .417).
2.4.4. Spindle and slow oscillation detection
Slow oscillations and spindles were detected during NREM epochs (i.e., stage 2, and SWS) using established algorithms (spindles: Klinzing et al., 2016, 2018; Mölle et al., 2011; slow oscillations: Mölle et al., 2002; Ngo et al., 2013a, Ngo et al., 2013b). The algorithms are described in detail in Muehlroth et al. (2019). In short, spindle detection was based on the smoothed root-mean-square (RMS) representation of the band-pass filtered EEG time series (6th-order Butterworth filter; slow spindles: 9–12.5 Hz; fast spindles: 12.5–16 Hz; cf. Cox, Schapiro, Manoach and Stickgold, 2017; Ujma et al., 2015 for the definition of spindle frequency bands). To account for individual differences in EEG amplitude, we anchored spindle identification on individually determined amplitude thresholds (Coppieters ’t Wallant, Maquet, & Phillips, 2016). Spindles were tagged if the amplitude of the smoothed RMS signal exceeded its mean by 1.5 SD of the filtered signal for 0.5–3 s. Spindles were merged if their boundaries were closer than 0.25 s and if the resulting spindle event remained within the time limit of 3 s.
For the detection of slow oscillations, the signal was band-pass filtered between 0.2 and 4 Hz at frontal electrodes (6th-order Butterworth filter). Putative slow oscillations were succeeding negative and positive half waves, separated by a zero-crossing, with a frequency between 0.5 and 1 Hz. Adaptive amplitude thresholds were defined separately for each participant: Slow oscillations had to exceed a trough potential of 1.25 times the mean trough potential of all putative slow oscillations and an amplitude of 1.25 times the average amplitude of all potential slow oscillations.
In line with the previously reported topography of slow oscillations, slow, and fast spindles (Klinzing et al., 2016; Mander et al., 2017a, Mander et al., 2017b), we focused our analyses on frontal SWA, slow oscillations, and slow spindles, as well as central fast spindles (detected at electrode Cz). As no hemispheric differences in SWA, slow oscillation, and slow spindle density were detected (SWA: Z = 0.81, p = .417; slow oscillation density: Z = 0.28, p = .781; slow spindle density: Z = 0.72, p = .470), estimates were averaged between the frontal channels F3 and F4. Only artefact-free slow oscillations and spindles were considered in the reported analyses. The density of slow oscillations and spindles was estimated by dividing the number of detected artefact-free events by the analyzed NREM sleep time (in minutes).
2.4.5. Statistical analyses
Sleep was assessed on two occasions (before and after learning). To investigate the variability of sleep architecture and physiology across age groups and experimental nights, we used mixed factorial ANOVAs with the within factor Time and the between factor Age Group. To account for non-normality of the data, post-hoc testing was conducted by calculating non-parametric Mann-Whitney U tests for independent samples and Wilcoxon signed-rank tests for matched pairs. Median and quartile values of the variables were reported. Significance levels were set to α = .05 and tested two-sided. If applicable, statistical significance was controlled for multiple comparisons by Bonferroni-correcting the α-value and dividing it by the number of performed comparisons.
2.4.6. Using PLSC to extract an age-specific latent sleep profile
Slow oscillations and sleep spindles are not discrete entities but interact to enable memory consolidation during sleep (Helfrich et al., 2018; Maingret et al., 2016; Marshall and Born, 2007; Muehlroth et al., 2019). At the same time, aging is characterized by global sleep alterations, affecting slow oscillations and spindles among other neurophysiological indicators, concurrently (Carrier et al., 2011; Mander et al., 2017a, Mander et al., 2017b; Ohayon et al., 2004). PLSC is ideally suited to account for the interdependency of NREM sleep processes and their joint age-related alterations. As a data reduction technique, PLSC allows for the extraction of a latent variable (LV) that captures the maximal correlation of each participant’s chronological age with various neurophysiological indicators of memory consolidation during sleep (Haenlein and Kaplan, 2004; Krishnan et al., 2011; Lobaugh et al., 2001; McIntosh and Bookstein, 1996; McIntosh et al., 2004; McIntosh and Lobaugh, 2004; McIntosh and Mišić, 2013). Here, we applied PLSC to identify a latent NREM sleep profile that captures an expression of frontal SWA, frontal slow oscillation density, frontal slow spindle density, and central fast spindle density that is typical for increasing age. To do so, first, a correlation matrix between the Z-standardized sleep variables (an n×4 matrix) and chronological AGE (an n×1 vector) was computed across all participants. Using singular value decompositions (SVD), the resulting correlation matrix was then decomposed into three matrices (UΔVT) based on which one LV was extracted in a least-squares sense. The resulting LV reflects the specific pattern of inter-individual differences in sleep measures that shares the largest amount of variance with inter-individual differences in the participants’ chronological age (here latent sleep profile). Statistical significance of the extracted LV was determined by 5000 permutations tests of its singular values. LV weights (or saliences) for all included sleep indicators express the degree to which each indicator contributes to the pattern specified by the LV. Measures with a high LV weight signify a strong contribution to the LV as they are strongly correlated with participants’ chronological age. Measures with a low LV weight are less associated with age and, hence, add less to the extracted LV. The reliability of the calculated LV weights of each sleep parameter was tested using 5000 bootstrap samples. Dividing the weights by the bootstrap standard error provides bootstrap ratios (BSR) comparable to Z-scores. Hence, we considered an absolute BSR of 1.96 or higher as reliable, which approximately corresponds to a 95% confidence interval. Finally, by projecting the original matrix of sleep indicators back onto the respective LV weights, brain scores (here latent sleep profile score [LSPS]) that reflect the degree to which each participant expressed the estimated robust latent sleep profile were extracted for each participant. An individual with a higher LSPS is predicted to express the constellation of sleep measures that is typical for advancing age more strongly, whereas an individual with a lower score is assumed to exhibit the identified latent sleep profile to a lesser extent.
To relate the latent sleep profile scores to the behavioral measures of memory gain and maintenance, Spearman’s rank-order correlation coefficients were calculated. Correlation coefficients were computed across the whole sample as well as separately within each age group. Fisher’s Z-transformation was used to test the null hypothesis of equal correlation coefficients in both the younger and the older sample.
All statistical analyses reported in this paper were conducted using Matlab R2014b (Mathworks Inc., Sherborn, MA), the open-source toolbox Fieldtrip (Oostenveld et al., 2011), and RStudio 1.0.53 (RStudio, Inc., Boston, MA). The custom code and data necessary to reproduce all statistical results, figures, and supplementary material of this article are available on the Open Science Framework repository (https://osf.io/w76f3/).
2.5. MRI data acquisition and structural MRI analyses
2.5.1. Data acquisition and extraction
Whole-brain MRI data were acquired with a Siemens Magnetom 3T TimTrio machine (high-resolution T1-weighted MPRAGE sequence: TR = 2500 ms, TE = 4.77 ms, FOV = 256 mm, voxel size = 1×1×1 mm3). Estimates of brain volume in regions of interest (ROI) were derived by means of voxel-based morphometry (VBM) using statistical parametric mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm), the Computational Anatomy Toolbox (CAT 12, http://www.neuro.uni-jena.de/cat), and the REX toolbox (http://web.mit.edu/swg/rex/rex.pdf) (see Muehlroth et al., 2019 for a detailed description of the analysis pipeline). All measures were adjusted for differences in total intracranial volume (cf. Raz et al., 2005). Bilateral mPFC, thalamus, entorhinal cortex, and hippocampus were selected as ROIs due to their involvement in memory consolidation and in the generation of spindles and slow oscillations (Gais and Born, 2004; Nir et al., 2011; Steriade, 2006). The mPFC mask was kindly provided by Bryce A. Mander (cf. Mander et al., 2013). All other ROIs were defined using the WFU PickAtlas toolbox (http://fmri.wfubmc.edu/software/pickatlas).
2.5.2. Using PLSC to extract an age-specific brain structure profile
Comparable to the extraction of a latent sleep profile, PLSC was applied to define a LV of brain structure, reflecting age-related gray matter decline in brain regions relevant for both sleep and memory (Gais and Born, 2004; Nir et al., 2011; Steriade, 2006). We computed PLSC between an n×4 matrix containing ROI volumes (i.e., mPFC, thalamus, entorhinal cortex, and hippocampus) and an n×1 vector containing each participant’s chronological age. By this, we aimed to extract a LV that captures the pattern of gray matter loss typical for advancing age (here, latent brain structure profile). Subsequently, analogously to the methods described above, a latent brain structure score (LBSS) was extracted for each participant that predicts each individual’s manifestation of age-dependent loss in gray matter volume across the four ROIs.
3. Results
Data analyses followed a multi-stage approach. First, to pinpoint how consolidation is influenced by a memory’s encoding quality, recall success was analyzed conditional on memory quality. In search for predictors of age-related consolidation impairments, we then identified how sleep architecture and NREM sleep physiology differ with advancing age. PLSC was used to derive an aging-specific NREM sleep profile. Inter-individual differences in the LSPS were then related to inter-individual differences in overnight memory consolidation. Finally, latent brain structure profiles of gray matter loss typical for advancing age were identified using PLSC. The resulting LBSS were related to the tendency to exhibit a senescent sleep profile and the ability to consolidate memories across sleep.
3.1. The effect of aging on memory consolidation is modulated by memory quality
The depth and success of memory encoding may discriminate memories with regard to their quality (Craik and Lockhart, 1972; Tulving, 1967). We first investigated how variation in memory quality, as defined by differences in pre-sleep recall success, relate to age differences in memory consolidation across sleep. We thus analyzed delayed recall success on Day 2 and corresponding reaction times conditional on item-specific learning trajectories on Day 1 (cf. Dumay, 2016; Fenn and Hambrick, 2013; Schreiner and Rasch, 2018; see Supplementary Fig. 1 and Supplementary Table 6 for trial numbers in each memory-quality category on Day 1 and Day 2).
In line with our attempt to adjust the encoding difficulty of the memory task between age groups (cf. Materials and methods), the percentage of correctly recalled scene–word associations did not differ systematically between age groups in the final cued recall on Day 1 and the preceding test-feedback phase (test feedback: Z = −1.67, p = .096; MdYA = 14.77% [9.38; 22.56]; MdOA = 20.89% [10.9; 29.02]; final cued recall: Z = 1.71, p = .087; MdYA = 56.82% [40.46; 69.55]; MdOA = 50.00% [31.16; 56.61]; Fig. 2b). However, each item’s learning success on Day 1 (cf. Dumay, 2016; Fig. 2a) and the participants’ age systematically modulated recall success of items during delayed recall on Day 2 (Fig. 2c). A mixed measures ANOVA with the between factor Age Group and the within factor Memory Quality yielded significant main effects for both factors (Memory Quality: F (2, 128) = 2224.12, p < .001; Age Group: F (1, 64) = 41.38, p < .001). Overall, successful recall was more likely when memory quality was high and, in general, younger adults had a higher probability of success during delayed recall. Post-hoc tests revealed that both age groups showed an equal rate of gained low-quality memories between Day 1 and Day 2 (Z = −0.08, p = .933; MdYA = 9.12%; MdOA = 9.16%; see Table 1). In contrast, older adults maintained a lower percentage of medium and high-quality memories than younger adults did (medium quality: Z = 6.40, p < .001; MdYA = 89.64%; MdOA = 66.35%; high quality: Z = 5.15, p < .001; MdYA = 97.69%; MdOA = 87.28%). The Age Group × Memory Quality interaction was statistically relevant (F (2, 128) = 50.11, p < .001). Whereas both younger and older adults recalled less medium-quality than high-quality memories (Z = 6.54, p < .001), in comparison, older adults showed greater overnight loss for medium-quality memories (Z = −9.75, p < .001).
Table 1.
Behavioral results: Delayed recall on Day 2.
| % Correct recall |
Reaction time (ms) |
|||||
|---|---|---|---|---|---|---|
| low quality | medium quality | high quality | low quality | medium quality | high quality | |
| YA – Median [1st quartile; 3rd quartile] |
9.12 [6.42; 11.5] | 89.64 [84.92; 93.00] | 97.69 [93.21; 100.00] | 2642.80 [2398.01; 2745.22] | 2039.48 [1890.29; 2175.32] | 1790.54 [1712.72; 1921.1] |
| OA – Median [1st quartile; 3rd quartile] |
9.16 [4.95; 12.77] | 66.35 [56.71; 71.47] | 87.28 [80.27; 90.41] | 2868.10 [2748.99; 3011.09] | 2687.80 [2531.99; 790.53] | 2369.64 [2170.39; 2492.55] |
Note. % Correct recall is defined for each memory-quality category as the probability of recalling an item during delayed recall on Day 2. Reaction times are reported for giving the “remember vs. forgotten” judgement for all correctly recalled trials. YA: younger adults; OA: older adults.
The effect of memory quality on delayed recall was also reflected in the corresponding reaction times during the delayed recall task on Day 2 (Fig. 2d, Table 1). Overall, older adults were slower in indicating that an item was remembered (F (1,64) = 49.03, p < .001) and in both age groups reaction times improved with higher memory quality (F (2,128) = 315.26, p < .001). Again, we observed a significant Age Group × Memory Quality interaction (F (2,128) = 22.61, p < .001) with smaller, though significant (Z = −3.67, p < .001), age differences in reaction times for memories of low quality compared to both medium- and high-quality pairs. In line with the pronounced loss of medium-quality memories in old age, compared to younger adults, older adults displayed a more pronounced slowing in reaction times for correctly recalled medium-quality in contrast to high-quality memories (Z = 4.10, p < .001).
During delayed recall, after indicating that a word was remembered, participants had to choose the second letter of the associated word out of four letter options to ensure correct word retrieval. Correct answers were thus possible by random selection of the correct letter option (25% hits in case of random answers). To rule out that the comparably small effect of gaining an item was due to random guessing, we computed the probability of a correct letter selection after indicating that a word was remembered for all low-quality items. In both age groups this probability was significantly higher than 25% (younger adults: t (29) = 9.86, p < .001, MYA = 52.24%, 95% confidence interval: [46.52; 57.48]; older adults: t (35) = 5.75, p < .001, MOA = 36.27%, 95% confidence interval: [0.32; 40.09]). Hence, we conclude that correct responses to low-quality items indicated an actual memory gain.
To sum up, we found comparable memory gains for both age groups, whereas memory maintenance was reduced in older adults. This reduction was most pronounced for memories of medium quality. Given that our analysis procedure was based on item-specific learning trajectories within single individuals, our procedure effectively controlled for inter-individual and age differences in memory encoding on Day 1. Hence, we suggest that the overnight changes in memory maintenance reflect age differences in sleep-dependent memory consolidation that vary with initial encoding strength.
3.2. NREM sleep physiology is distinctly altered in older adults
Sleep architecture varies across the adult lifespan (Ohayon et al., 2004; Scullin and Gao, 2018). However, in the short term, it is to some extent also variable within individuals (Gais et al., 2002; Mölle et al., 2011). For instance, it can be shaped by learning experiences (Huber et al., 2004; Mölle et al., 2009). We thus examined intra-as well as inter-individual variation in global sleep parameters.
All sleep measures, except for sleep latency (i.e., the time needed to fall asleep) and the relative amount of REM sleep (both p ≥ .053; both F ≤ 3.92), showed significant main effects of Age Group (see Table 2). More precisely, we found the expected decreased proportion of SWS in older adults compensated for by an increase in the lighter NREM sleep stages 1, and 2. Neither the main effect of Time nor the Age-by-Time interaction were significant for any of the variables, indicating that overall sleep architecture did not change as a result of the intense learning session on Day 1.
Table 2.
Age-related changes in sleep variables.
| YA – Median [1st quartile; 3rd quartile] | OA – Median [1st quartile; 3rd quartile] | F (df1,df2) | p | |
|---|---|---|---|---|
| TST (min) | 459.00 [428.0; 494.88] | 416.25 [368.88; 459.75] | F (1,52) = 7.61 | .008 |
| Sleep latency (min) | 12.0 [6.5; 19.5] | 12.00 [6.50; 182.00] | F (1,48) = 0.1 | .756 |
| Sleep efficiency (%) | 99.11 [97.64; 99.59] | 95.71 [93.03; 98.15] | F (1,52) = 12.23 | .001 |
| Stage 1 (%) | 3.77 [2.50; 6.29] | 7.08 [4.78; 9.74] | F (1,52) = 11.5 | .001 |
| Stage 2 (%) | 52.66 [46.80; 56.92] | 66.05 [61.52; 69.83] | F (1,52) = 64.84 | .001 |
| SWS (%) | 18.9 [15.74; 25.23] | 1.39 [0.00; 11.30] | F (1,52) = 53.37 | .001 |
| REM (%) | 23.58 [19.69; 26.62] | 21.58 [16.70; 24.59] | F (1,52) = 3.92 | .053 |
| WASO (%) | 2.77 [1.12; 5.5] | 8.56 [4.90; 15.19] | F (1,52) = 12.38 | .001 |
| Relative SWA | 0.79 [0.75; 0.85] | 0.67 [0.62; 0.73] | F (1,48) = 23.08 | .001 |
| SO density | 3.71 [3.29; 4.15] | 3.36 [2.88; 3.80] | F (1,48) = 4.76 | .034 |
| Slow SP density | 0.85 [0.65; 1.05] | 0.50 [0.31; 0.72] | F (1,48) = 9.87 | .003 |
| Fast SP density | 1.63 [1.38; 1.77] | 0.57 [0.37; 0.88] | F (1,52) = 67.39 | .001 |
Note. The reported F- and p-values reflect the main effect of Age Group from mixed factorial ANOVAs with the within factor Time (Pre vs. Post) and the between factor Age Group (younger adults vs. older adults). Reported descriptive measures refer to average of the PRE and POST measurement. Slow oscillation, slow, and fast spindle density are defined as the number of identified events per minute of NREM sleep. YA: younger adults; OA: older adults; TST: total sleep time; SWS: slow-wave sleep; REM: rapid eye movement sleep; WASO: wake after sleep onset; SWA: slow-wave activity; SO: slow oscillation; SP: spindle.
Visual scoring of sleep stages is difficult in age-comparative studies (cf. Muehlroth and Werkle-Bergner, 2019). The fixed amplitude criteria for scoring SWS typically penalize older adults, as they generally show lower EEG amplitudes (Carrier et al., 2011; Mander et al., 2017a, Mander et al., 2017b). Accordingly, almost one third of our older sample failed to meet the amplitude criteria of SWS when visually scoring the PSG data (n = 9). Hence, we combined NREM sleep stages 2 and SWS to focus on more fine-grained physiological measures of NREM sleep. SWA, defined as relative power in the delta frequency range (0.5–4.5 Hz), slow oscillations (0.5–1 Hz), slow spindles (9–12.5 Hz), and fast spindles (12.5–16 Hz) were significantly reduced in older compared to younger adults (all F ≥ 4.76, all p ≤ .034). SWA was the only variable showing a significant main effect of Time (F (1,48) = 7.20, p < .001; MdPRE = 0.7 [0.64; 0.79], MdPOST = 0.74 [0.68; 0.8]). Intense learning distinctly boosted SWA. The overnight change in SWA, though, did not relate to any measure of memory consolidation (all |r| ≤ 0.28, p ≥ .051). As neither the main effect of Time nor the Age-by-Time interaction were significant for any other sleep measure, we restricted our analysis to sleep after learning only in the following.
3.3. Senescent sleep physiology profiles alone do not drive inter-individual differences in memory consolidation
Broad alterations in sleep architecture and physiology are observed during aging (Mander et al., 2017a, Mander et al., 2017b; Ohayon et al., 2004). These sleep processes are interdependent and jointly contribute to memory consolidation (Buzsáki, 1998; Steriade, 2003). Hence, we chose PLSC as multivariate approach to examine individual differences in aging as reflected in multiple physiological indicators of sleep. We then asked how these age-related differences in NREM sleep relate to memory consolidation as captured by overnight memory maintenance and gain (Materials and methods; see Supplementary Tables 1–3 for the bivariate sleep–memory associations).
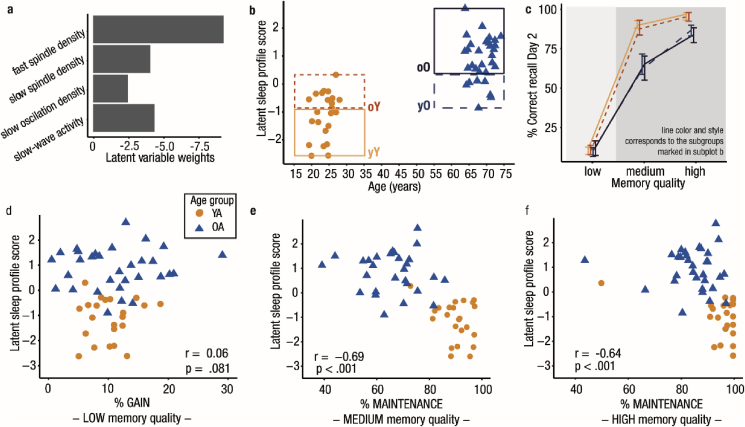
Using PLSC (Haenlein and Kaplan, 2004; Krishnan et al., 2011), we identified a significant latent variable (LV) capturing a pattern of reduced SWA, slow oscillation, slow spindle, and fast spindle density that shares maximal variance with age (rAGE = 0.73, p < .001). Bootstrap ratios (BSR) indicated that all included NREM sleep indicators reliably contributed to the LV (all BSR ≤ −2.27; Fig. 3a). Negative LV weights implied that advancing age is accompanied by simultaneous reductions in multiple neurophysiological markers of memory consolidation during NREM sleep.
Fig. 3.
Sleep–memory associations in younger and older adults. (a) All sleep variables contribute to the latent variable capturing the common variance between participants’ age and sleep. Latent variable weights (in Z-scores) demonstrate that all sleep variables have a stable negative relation to age. (b) Each participant’s expression of the latent variable is plotted against age. Overlap between the age groups is marked by dashed boxes. Sleep in younger and older adults is not completely distinct. (c) Median behavioral performance for all subgroups (grouping and line color and style corresponding to b) is displayed. The first and third quartile is depicted as an error bar. Memory gain (shaded in light gray) is similar in all subgroups. Memory maintenance (shaded in darker gray) is modulated by the sleep profile but differs between younger and older adults, even when they have the same sleep profile. (d–f) Each participant’s latent sleep profile score is plotted against the behavioral measures. Spearman’s rank-order correlation coefficients for the whole sample are displayed. Maintenance of both medium- and high-quality memories relates to the latent sleep profile score across age groups. Note the ceiling performance in younger adults, in particular for high-quality memories. YA: younger adults, OA: older adults, yY: young–Young (= younger adults showing a clearly distinct profile from older adults), oY: old–Young (= younger adults exhibiting a sleep profile comparable to older adults), yO: young–Old (= older adults with a ‘youth-like’ sleep profile), oO: old–Old (= older adults with a sleep profile clearly distinct from younger adults).
Using Spearman’s rank-order correlation coefficient, we did not find a significant correlation between the LSPS and memory gain across both age groups (r = 0.06, p = .681, Fig. 3d). In contrast, maintenance of medium- and high-quality memories was negatively related to the LSPS (rmedium = −0.66, p < .001; rhigh = −0.64, p < .001, Fig. 3e and f). The more participants displayed a senescent sleep profile (i.e., reduced SWA, slow oscillations, slow and fast spindles), the worse their memory maintenance was. When conducted separately within each age group, none of the correlations reached significance (all |r| ≤ 0.13, all p ≥ .548, Supplementary Tables 2 and 3). Moreover, none of the single sleep measures was reliably associated with memory maintenance and gain in younger and older adults (all |r| ≤ .41, all p ≥ .049, α-level adjusted to 0.00028, Supplementary Tables 2 and 3).
Given the nature of the extracted LV that captures the maximal correlation between a set of sleep variables and age, older adults showed higher individual LSPS. This means that they displayed a greater manifestation of the latent sleep profile that reflects a reduction in several physiological parameters of NREM sleep. Notably, the LSPS considerably overlapped between younger and older adults (Fig. 3b). Hence, although a constellation of NREM sleep indicators typical for advancing age exists, younger and older adults were not completely distinct in their sleep profiles. If age-related changes in NREM sleep, as previously suggested, are the major driving force behind differences in memory maintenance, irrespective of their age, participants with similar sleep profiles should be comparable in their ability to maintain memories across a night of sleep. Based on their LSPS, we identified those younger and older participants revealing a similar expression of the identified latent sleep profile (outlined boxes in Fig. 3b). Based on this grouping we obtained four subgroups (Supplementary Table 4; young–Young [yY] = younger adults showing a clearly distinct sleep profile from older adults [n = 12]; old–Young [oY] = younger adults exhibiting a sleep profile comparable to older adults [n = 11]; young–Old [yO] = older adults with a ‘youth-like’ sleep profile [n = 7]; old–Old [oO] = older adults with a sleep profile clearly distinct from younger adults [n = 24]). Using a mixed factorial ANOVA with the between-subjects factor Sleep Profile Subgroup and the within-subjects factor Memory Quality, we found significant main effects for both factors (Memory Quality: F (2, 100) = 1657.47, p < .001; Sleep Profile Subgroup: F (3, 50) = 12.17, p < .001) along with a significant interaction (F (6,100) = 18.75, p < .001; Fig. 3c). Post-hoc tests indicated that within the two age groups, the classification according to sleep profile did not result in any significant behavioral differences (all |Z| ≤ 1.28, all p ≥ .202). Descriptively, memory maintenance of younger adults with a latent sleep profile similar to older adults (oY) was reduced compared to younger adults with a sleep profile clearly distinct from older adults (yY) (Fig. 3c; Supplementary Table 4). For memories of medium quality, in spite of a comparable sleep profile, maintenance still differed significantly between younger and older adults (Z = 3.31, p < .001). For high-quality memories, though, this age effect disappeared when controlling for multiple testing (Z = 2.37, p = .018, α-level adjusted to 0.006).
To summarize, we found evidence that, in contrast to memory gain, concomitant age-related differences in NREM sleep coincided with worse maintenance of medium- and high-quality memories. Memory maintenance might rely on processes during NREM sleep that are impaired in aged individuals. However, we demonstrate that, within each age group, inter-individual differences in the NREM sleep profile did not reliably account for inter-individual differences in memory consolidation. Moreover, the similarity of the NREM sleep profile between younger and older adults did not reverse the effect of age on the consolidation of medium-quality memories. We thus conclude that a senescent sleep profile alone (characterized by reduced SWA, slow oscillations, slow, and fast spindles) does not account for all observed age-related reductions in memory maintenance.
3.4. Age differences in brain structure coincide with sleep and memory impairments
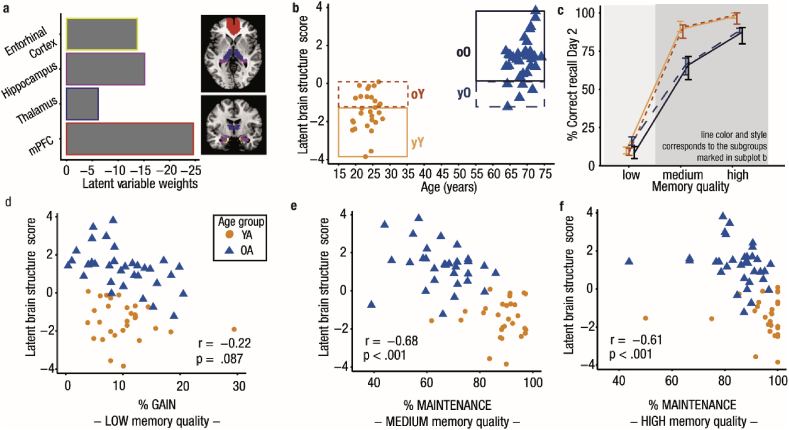
Recently, a more comprehensive view on age-related changes in memory consolidation emerged by including measures of structural brain integrity (Helfrich et al., 2018; Mander et al., 2013; Muehlroth et al., 2019; Varga et al., 2016). Age-related gray matter loss has been linked to impaired slow oscillation and spindle generation and coordination, and to dysfunctional memory consolidation (Fogel et al., 2017; Mander et al., 2013; Muehlroth et al., 2019; Varga et al., 2016). Again, we used PLSC to examine how age-related atrophy in various brain regions relates to the tendency to exhibit a senescent sleep profile and the ability to consolidate memories (cf. Materials and methods; see Supplementary Tables 1–3 for the bivariate brain–memory associations). In analogy to the analysis described above, we used PLSC to extract a LV reflecting a constellation of gray matter volume (defined using VBM) in the mPFC, thalamus, entorhinal cortex, and the hippocampus typical for increasing age (rAGE = 0.78, p < .001). In all selected ROIs, gray matter volume was significantly reduced in older compared to younger adults (mPFC: Z = 6.54, p < .001, MdYA = 0.44 [0.42; 0.46], MdOA = 0.36 [0.33; 0.37]; thalamus: Z = 4.40, p < .001, MdYA = 0.41 [0.37; 0.44], MdOA = 0.32 [0.3; 0.37]; entorhinal cortex: Z = 5.60, p < .001, MdYA = 0.50 [0.49; 0.53], MdOA = 0.45 [0.42; 0.47]; hippocampus: Z = 5.96, p < .001, MdYA = 0.47 [0.44; 0.48], MdOA = 0.4 [0.37; 0.42]; cf. Supplementary Fig. 2). All ROIs reliably contributed to the LV and showed simultaneous gray matter loss (all BSR ≤ −6.12; Fig. 4a). The score quantifying each participant’s expression of the LV, further referred to as LBSS, was positively related to the LSPS (r = 0.65, p < .001; Supplementary Fig. 3). Individuals who more strongly expressed the pattern of gray matter reduction typical for advancing age, were also more likely to reveal the constellation of sleep measures characterizing increasing age. Within each age group separately, however, this association was not significant (all |r| ≤ .13, all p ≥ .500; Supplementary Tables 2 and 3).
Fig. 4.
Brain–memory associations in younger and older adults. (a) Brain regions involved in slow wave and spindle generation and memory processing contribute to the latent variable capturing the common variance between participants’ age and brain structure (quantified using voxel-based morphometry). Latent variable weights (in Z-scores) demonstrate that all regions have a stable negative relation with age (all BSR ≤ −6.12). Bar colors correspond to the colors of the masked regions. (b) Each participant’s expression of the latent variable is plotted against age. Overlap between the age groups is indicated by dashed boxes. Younger and older participant groups with clearly differing brain structure are outlined by a solid line. (c) Median behavioral performance for all subgroups is shown. The first and third quartile is depicted as an error bar. Memory gain (shaded in light gray) is similar in all subgroups. Memory maintenance (shaded in darker gray) differs between younger and older adults, even when they express the same structural brain integrity. Brain structure itself does not modulate behavior within age groups. (d–f) Each participant’s latent brain structure score plotted against the behavioral measures. Spearman’s rank-order correlation coefficients for the whole sample are displayed. Maintenance of both medium- and high-quality memories relates to the latent brain structure score across age groups. BSR: bootstrap ratio, YA: younger adults, OA: older adults, yY: young–Young (= younger adults showing a clearly distinct brain structure profile from older adults), oY: old–Young (= younger adults exhibiting a brain structure profile comparable to older adults), yO: young–Old (= older adults with a ‘youth-like’ brain structure profile), oO: old–Old (=older adults with a brain structure profile clearly distinct from younger adults).
Maintenance of medium- and high-quality memories was negatively related to the LBSS (rmedium = −0.68, p < .001; rhigh = −0.61, p < .001; Fig. 4e and f). Nevertheless, these associations were only stable across age groups (within age groups all |r| ≤ 0.31, all p ≥ .08; Supplementary Tables 2 and 3). Although only on a trend level in the whole sample (r = −0.22, p = .087), the correlation between the LBSS and memory gain reached significance in older, but not younger adults (rOA = −0.47, pOA = .007; rYA = −0.2, pYA = .302). Correlation coefficients, however, did not differ significantly between younger and older adults (Z = −1.13, p = .26).
Only a small proportion of older adults (n = 5) showed a latent brain structure profile comparable to the one expressed by younger adults (Fig. 4b). Accordingly, we could not follow the same rationale as described above to examine whether subjects in different age groups with similar structural brain integrity comparably maintained memories across sleep (but see Fig. 4c and Supplementary Table 5, for an illustration of this effect). Compared to physiological markers of NREM sleep, structural brain integrity separated the two age groups more distinctly (Fig. 4b) but did not seem to differentiate behavior within age groups.
4. Discussion
The present study asked whether variation in the encoding quality of individual associative memories determines the degree of age-related impairments in memory consolidation. We demonstrate that age differences in memory consolidation during sleep were maximized for the maintenance of mnemonic contents of medium encoding quality. By contrast, we did not find age differences in the proportion of associations gained overnight. It appears that age differences in sleep-dependent memory consolidation are confined to consolidation mechanisms that rely on intact NREM sleep. Using PLSC, we successfully integrated patterns of sleep physiology and brain structure typical for advancing age and, thus, overcame the typical use of multiple bivariate correlations. However, in neither NREM sleep physiology nor in brain structure inter-individual differences could fully account for the observed age-related reductions in overnight protection against forgetting.
4.1. Encoding quality determines the extent of age differences in memory consolidation
Meeting the long demand to go beyond average net measures of memory (Tulving, 1964, 1967), we separated different consolidation mechanisms based on the encoding quality of the respective memory (Dumay, 2016; Fenn and Hambrick, 2013). Our results are in line with ongoing discussions in the field, suggesting that the major role of sleep can be seen in the maintenance rather than the gain of memories (Fenn and Hambrick, 2013; Nettersheim et al., 2015; Schreiner and Rasch, 2018).
By demonstrating that memory maintenance is differentially affected by aging, with most age-related deficits for memories of medium encoding quality, we underpin the notion of an active consolidation process that stabilizes mnemonic contents selectively (Diekelmann et al., 2009; Drosopoulos et al., 2007; Kuriyama et al., 2004; Schapiro et al., 2018; Stickgold, 2010; Stickgold and Walker, 2013). Establishing strong robust memory representations already during encoding might render subsequent consolidation processes redundant (Schoch et al., 2017). Memories of intermediate quality, in contrast, might have the necessary but not yet sufficient strength that prioritizes them for subsequent active consolidation mechanisms that include active processes like the reactivation and redistribution of memory traces (Schapiro et al., 2018; Stickgold, 2010). Since these processes are particularly disrupted in old age (Cordi et al., 2018; Gerrard et al., 2008; Helfrich et al., 2018), pronounced age-related deficits in memory, as observed here, might be the consequence.
Memory gain, in contrast, was comparable across age groups. The fact that memories that could not be recalled on Day 1 were readily available on Day 2 may point to an active consolidation mechanism that raises memory representations above a pre-sleep learning threshold. However, although successful recall of a memory indicates the existence of a reliable memory representation, unsuccessful recall does not necessarily indicate the opposite. Reduced attentional resources (Anderson et al., 2000; Craik et al., 2010) and incomplete retrieval search (Grady and Craik, 2000; Raaijmakers and Shiffrin, 1981), which are both known to negatively affect retrieval in older adults, might have resulted in failed pre-sleep memory retrieval on Day 1 despite the availability of the respective memory trace (Habib and Nyberg, 2007). Moreover, retrieval itself can result in memory suppression, a phenomenon called ‘retrieval-induced forgetting’ (Bäuml and Kliegl, 2017; MacLeod and Macrae, 2001). Importantly, several studies suggest that retrieval-induced forgetting is temporary and recovers over an interval of 24 h (Abel and Bäuml, 2014; MacLeod and Macrae, 2001; Bäuml and Kliegl, 2017 for a review). This is equivalent to the interval used in this study. In line with the similar memory gain in younger and older adults observed here, retrieval-induced forgetting has also been shown to be independent of aging (Hogge et al., 2008). We thus speculate that the effect of memory gain in our analyses reflects the inaccessibility of specific memories in the final recall on Day 1 (despite general availability of the respective trace) rather than a memory improvement across sleep. However, as our study design did not involve a wake control group, the interpretation that memory maintenance and gain differentially rely on sleep and wakefulness remains tentative.
To conclude, our findings demonstrate that by taking into account unknown variation in the encoding quality of individual memories, differential effects of aging on memory consolidation can be explained. These results align with the notion that the pre-sleep level of memory performance determines whether age-related deficits in memory consolidation are observed (Sonni and Spencer, 2015; Wilson et al., 2012). Both Sonni and Spencer (2015) and Wilson et al. (2012) found memory consolidation to be unaffected in high-performing older adults. When studying age differences in sleep-dependent memory consolidation, a tight control of learning success is thus required (cf. Conte and Ficca, 2013).
4.2. NREM sleep physiology and brain structure alone do not account for age differences in memory maintenance
Prominent age-related changes in sleep physiology that particularly affect NREM sleep (Carrier et al., 2011; Mander et al., 2017a, Mander et al., 2017b; Ohayon et al., 2004), are assumed to constitute one of the key mechanisms causing consolidation deficits in old age. Indeed, older adults in our sample exhibited reductions in SWA as well as in slow oscillation, slow, and fast spindle density. Moreover, reductions in the expression of rhythmic neural activity during NREM sleep typical for advancing age coincided with worse maintenance of medium- and high-quality memories (but not overnight memory gain). The reduced presence of slow waves, slow oscillations, and spindles may be indicative for an impaired coordinated dialogue between the hippocampus and neocortex and hinder the transfer of labile memory representations to permanent stores in the neocortex (Helfrich et al., 2018; Mander et al., 2013; Muehlroth et al., 2019; Varga et al., 2016). However, equating younger and older adults with regard to their NREM sleep profiles did not entirely remove age differences in memory maintenance. Also within age groups, inter-individual differences in the extent of the expression of an ‘aged’ sleep profile did not account for inter-individual differences in either memory gain or maintenance. Hence, we suggest that inter-individual differences in NREM sleep physiology may not qualify as the sole source of age differences in memory maintenance observed in the present study.
Age-related changes in sleep physiology may be driven by senescent changes in the structural integrity of brain areas involved in slow wave and spindle generation (Saletin et al., 2013). Indeed, previous reports suggested age-related gray matter atrophy as the main cause of changes in sleep with advancing age (Dubé et al., 2015; Fogel et al., 2017; Landolt and Borbély, 2001; Mander et al., 2013; Varga et al., 2016) – the consequence of which is a dysfunctional protection of memories against forgetting in old age (Cordi et al., 2018; Gerrard et al., 2008; Helfrich et al., 2018; Mander et al., 2013; Muehlroth et al., 2019). In line with these findings, in general, we observed that participants with reduced structural brain integrity exhibit a more ‘aged’ sleep profile and worse maintenance of both medium- and high-quality memories. However, correlations between brain structure, sleep, and memory only held across, but not within age groups. Similar to the results of age-related differences in sleep physiology and their relation to overnight memory maintenance, we do not find strong support for the assumption that changes in structural brain integrity are the major predictor for inter-individual variation in sleep and memory consolidation.
4.3. Towards a mechanistic understanding of age-related alterations in memory consolidation during sleep
The present study provides cross-sectional evidence that age differences in NREM sleep physiology and structural brain integrity relate to age differences in sleep-dependent memory consolidation (cf., Helfrich et al., 2018; Mander et al., 2013; Varga et al., 2016). However, our results raise doubts that inter-individual differences in sleep physiology can sufficiently predict the success of memory consolidation during sleep. Thus, with the currently used methodology, it might be too early to consider sleep a possible biomarker of age-related pathological and non-pathological memory decline (e.g., Mander et al., 2016; Muehlroth and Werkle-Bergner, 2019).
The importance of slow oscillations and spindles for memory consolidation has been underscored by a variety of studies experimentally manipulating sleep physiology (e.g., Marshall et al., 2004; Ngo et al., 2013a, Ngo et al., 2013b; van der Werf et al., 2009; for reviews and meta-analytic evidence: Barham et al., 2016; Bellesi et al., 2014; Marshall and Campos-Beltrán, 2017; Wilckens et al., 2018; Zhang and Gruber, 2019) and by studies showing the benefit of targeted memory reactivation during periods of NREM sleep (e.g., Cairney et al., 2018; Rasch et al., 2007; Schreiner et al., 2015; for reviews: Oudiette and Paller, 2013; Schouten et al., 2017). Nevertheless, correlational studies sometimes fail to detect similar relationships (e.g., Ackermann and Rasch, 2014; Lo et al., 2014; Pardilla-Delgado and Payne, 2017; Payne et al., 2009; Piosczyk et al., 2013; for recent discussions, see Conte and Ficca, 2013; Mantua, 2018; Muehlroth and Werkle-Bergner, 2019). Crucially, this does not contradict the important role of NREM sleep physiology for memory consolidation.
To date, it has frequently been proposed that intra-individual variation in sleep might be more predictive of an individual’s memory consolidation than the inter-individual differences in sleep physiology that were in the focus of the present examination (Ackermann et al., 2015; Schabus et al., 2004, 2008; Schabus, 2009; Spiegel et al., 1986). Rather than being stable, memory consolidation could represent a variable process that is modulated by intra-individual fluctuations in sleep physiology. In combination with structural and functional brain prerequisites, this variation might shape the success of memory consolidation within each individual. Yet, our results do not provide much evidence for changes in sleep physiology across two experimental nights (besides an increase in SWA). Hence, short-term variation in sleep over a single night might be relatively small in the present study (Buckelmüller et al., 2006; Schabus et al., 2008; Tucker et al., 2007). By comparing different experimental conditions (e.g., learning vs. non-learning) or manipulating specific oscillatory components during sleep, intra-individual variation in sleep could be induced and, potentially, corresponding alterations in memory consolidation revealed (Barham et al., 2016; Bellesi et al., 2014; Marshall and Campos-Beltrán, 2017; Wilckens et al., 2018; Zhang and Gruber, 2019).
Finally, in line with theoretical models of memory consolidation (e.g., Diekelmann and Born, 2010; Marshall and Born, 2007), the reported lack of within-agegroup sleep–memory associations points to the fact that inter-individual differences in the mere occurrence of slow oscillations and sleep spindles might be a weak predictor of between-person differences in memory consolidation. This view is supported by recent reports suggesting that success of memory consolidation relies on the fine-tuned coordination of rhythmic neural events during NREM sleep rather than their simple presence (Latchoumane et al., 2017; Maingret et al., 2016). Along these lines, dispersed slow oscillation–spindle coupling could predict consolidation impairments in older adults (Helfrich et al., 2018; Muehlroth et al., 2019). Hence, we suggest that a mechanistic understanding of the causes and consequences of age differences in memory consolidation ultimately requires novel analytic tools that disclose the fine-tuned interplay between rhythmic neural events during NREM sleep, the interaction of different brain structures or the interplay of brain structure, and sleep oscillations.
5. Conclusions
In this study we demonstrate that variation in the quality of individual memories must be taken into account when studying memory consolidation. Moreover, we show that research can profit from the application of multivariate analytic tools that can deal with the interdependency of sleep processes that concomitantly age and jointly affect memory consolidation. By transcending net measures of memory consolidation, we successfully disentangled age effects on the consolidation of associative memories of different encoding quality. Whereas the maintenance of successfully encoded memories was impaired in older adults, overnight memory gain was not affected by aging. Age-related consolidation impairments were particularly pronounced for mnemonic contents of intermediate encoding quality. These deficits in memory maintenance were related to profiles of NREM sleep physiology and brain structure typical for advancing age. Hence, we suggest that maintenance of associative memories relies on NREM sleep-specific consolidation mechanisms that are impacted during aging. How these findings relate to other memory domains, like procedural memory, remains to be clarified (e.g., Fogel et al., 2014; Gudberg et al., 2014; Mander et al., 2017a, Mander et al., 2017b). Finally, within age groups, inter-individual differences in the presence of slow oscillations and sleep spindles were not sufficient to predict memory consolidation. Consequently, we stress the need for novel and age-fair analytic tools to provide a mechanistic understanding of age differences in memory consolidation (cf. Muehlroth and Werkle-Bergner, 2019). This will hopefully pave the road for novel interventions that can reveal their full therapeutic capability to reduce or delay cognitive decline in old age.
Author contributions
BEM, MCS, YF, THG, BR, YLS, and MWB designed research. BEM performed research. BEM and MWB analyzed data. BEM, MCS, YF, THG, BR, YLS, and MWB wrote the paper.
Funding
This work was partially financed by the Max Planck Society and the German Research Foundation (DFG, WE 4269/3-1). Beate E. Muehlroth was supported by the Max Planck International Research Network on Aging, and the Max Planck School of Cognition. Markus Werkle-Bergner’s and Yee Lee Shing’s work were both supported by the Jacobs Foundation (Early Career Research Fellowships). Yee Lee Shing and Myriam C. Sander were each supported via Minerva Research Groups awarded by the Max Planck Society. Yee Lee Shing is moreover funded by the European Research Council (ERC-2018-StG-PIVOTAL-758898).
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
This study was conducted within the ‘Cognitive and Neural Dynamics of Memory across the Lifespan (ConMem)’ project at the Center for Lifespan Psychology, Max Planck Institute for Human Development. We thank Maren J. Cordi for helping us to set up the technical equipment, Xenia Grande for organizing data collection, Kristina Günther for help in participant recruitment, Julia Delius for editorial assistance, and all student assistants of the ConMem project collecting the data. We are grateful to all members of the ConMem project for helpful feedback on the analysis. Finally, we thank all study participants for their time.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2019.116490.
Contributor Information
Beate E. Muehlroth, Email: muehlroth@mpib-berlin.mpg.de.
Markus Werkle-Bergner, Email: werkle@mpib-berlin.mpg.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abel M., Bäuml K.H.T. The roles of delay and retroactive interference in retrieval-induced forgetting. Mem. Cogn. 2014;42(1):141–150. doi: 10.3758/s13421-013-0347-0. [DOI] [PubMed] [Google Scholar]
- Ackermann S., Rasch B. Differential effects of non-REM and REM sleep on memory consolidation? Curr. Neurol. Neurosci. Rep. 2014;14:430. doi: 10.1007/s11910-013-0430-8. [DOI] [PubMed] [Google Scholar]
- Ackermann S., Hartmann F., Papassotiropoulos A., de Quervain D.J.F., Rasch B. No associations between interindividual differences in sleep parameters and episodic memory consolidation. Sleep. 2015;38(6):951–959. doi: 10.5665/sleep.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M.C., Bjork E.L., Bjork R.A. Retrieval-induced forgetting: evidence for a recall-specific mechanism. Psychon. Bull. Rev. 2000;7(3):522–530. doi: 10.3758/bf03214366. [DOI] [PubMed] [Google Scholar]
- Baran B., Mantua J., Spencer R.M.C. Age-related changes in the sleep-dependent reorganization of declarative memories. J. Cogn. Neurosci. 2016;28(6):792–802. doi: 10.1162/jocn_a_00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham M.P., Enticott P.G., Conduit R., Lum J.A.G. Transcranial electrical stimulation during sleep enhances declarative (but not procedural) memory consolidation: evidence from a meta-analysis. Neurosci. Biobehav. Rev. 2016;63:65–77. doi: 10.1016/j.neubiorev.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Bäuml K.T., Kliegl O. Retrieval-induced remembering and forgetting. In: Byrne J.H., editor. Learning and Memory: A Comprehensive Reference. second ed. Academic Press; Oxford: 2017. pp. 27–51. [Google Scholar]
- Bellesi M., Riedner B.A., Garcia-Molina G.N., Cirelli C., Tononi G., Gerstner J.R. Enhancement of sleep slow waves: underlying mechanisms and practical consequences. Front. Syst. Neurosci. 2014;8:208. doi: 10.3389/fnsys.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J., Wilhelm I. System consolidation of memory during sleep. Psychol. Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckelmüller J., Landolt H.P., Stassen H.H., Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138(1):351–356. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Buckley T.M., Schatzberg A.F. Aging and the role of the HPA axis and rhythm in sleep and memory-consolidation. Am. J. Geriatr. Psychiatry. 2005;13(5):344–352. doi: 10.1176/appi.ajgp.13.5.344. [DOI] [PubMed] [Google Scholar]
- Buckner R.L. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., III, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Memory consolidation during sleep: a neurophysiological perspective. J. Sleep Res. 1998;7(Suppl. 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Cairney S.A., Marj N. El, Staresina B.P. Memory consolidation is linked to spindle-mediated information processing during sleep. Curr. Biol. 2018;28:948–954. doi: 10.1016/j.cub.2018.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier J., Viens I., Poirier G., Robillard R., Lafortune M., Vandewalle G. Sleep slow wave changes during the middle years of life. Eur. J. Neurosci. 2011;33:758–766. doi: 10.1111/j.1460-9568.2010.07543.x. [DOI] [PubMed] [Google Scholar]
- Carskadon M.A., Brown E.D., Dement W.C. Sleep fragmentation in the elderly: relationship to daytime sleep tendency. Neurobiol. Aging. 1982;3:321–327. doi: 10.1016/0197-4580(82)90020-3. [DOI] [PubMed] [Google Scholar]
- Cherdieu M., Reynaud E., Uhlrich J., Versace R., Mazza S. Does age worsen sleep-dependent memory consolidation? J. Sleep Res. 2014;23:53–60. doi: 10.1111/jsr.12100. [DOI] [PubMed] [Google Scholar]
- Conte F., Ficca G. Caveats on psychological models of sleep and memory: a compass in an overgrown scenario. Sleep Med. Rev. 2013;17(2):105–121. doi: 10.1016/j.smrv.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Coppieters ’t Wallant D., Maquet P., Phillips C. Sleep spindles as an electrographic element: description and automatic detection methods. Neural Plast. 2016;2016:6783812. doi: 10.1155/2016/6783812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordi M.J., Schreiner T., Rasch B. No effect of vocabulary reactivation in older adults. Neuropsychologia. 2018;119:253–261. doi: 10.1016/j.neuropsychologia.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Cox R., Schapiro A.C., Manoach D.S., Stickgold R. Individual differences in frequency and topography of slow and fast sleep spindles. Front. Hum. Neurosci. 2017;11:433. doi: 10.3389/fnhum.2017.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik F.I.M., Lockhart R.S. Levels of processing: a framework for memory research. J. Verb. Learn. Verb. Behav. 1972;11:671–684. [Google Scholar]
- Craik F.I.M., Luo L., Sakuta Y. Effects of aging and divided attention on memory for items and their contexts. Psychol. Aging. 2010;25(4):968–979. doi: 10.1037/a0020276. [DOI] [PubMed] [Google Scholar]
- Craik F.I.M., Rose N.S. Memory encoding and aging: a neurocognitive perspective. Neurosci. Biobehav. Rev. 2012;36(7):1729–1739. doi: 10.1016/j.neubiorev.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Crowley K., Trinder J., Kim Y., Carrington M., Colrain I.M. The effects of normal aging on sleep spindle and K-complex production. Clin. Neurophysiol. 2002;113:1615–1622. doi: 10.1016/s1388-2457(02)00237-7. [DOI] [PubMed] [Google Scholar]
- Dannhauer M., Lanfer B., Wolters C.H., Knösche T.R. Modeling of the human skull in EEG source analysis. Hum. Brain Mapp. 2011;32(9):1383–1399. doi: 10.1002/hbm.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S., Born J. The memory function of sleep. Neuroscience. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Diekelmann S., Wilhelm I., Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med. Rev. 2009;13:309–321. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Drosopoulos S., Schulze C., Fischer S., Born J. Sleep’s function in the spontaneous recovery and consolidation of memories. J. Exp. Psychol. Gen. 2007;136(2):169–183. doi: 10.1037/0096-3445.136.2.169. [DOI] [PubMed] [Google Scholar]
- Dubé J., Lafortune M., Bedetti C., Bouchard M., Franc J., Doyon J. Cortical thinning explains changes in sleep slow waves during adulthood. J. Neurosci. 2015;35(20):7795–7807. doi: 10.1523/JNEUROSCI.3956-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumay N. Sleep not just protects memories against forgetting, it also makes them more accessible. Cortex. 2016;74:288–296. doi: 10.1016/j.cortex.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Dumay N. Look more carefully: even your data show sleep makes memories more accessible. A reply to Schreiner and Rasch. Cortex. 2018;101:288–293. doi: 10.1016/j.cortex.2017.12.013. (in press) [DOI] [PubMed] [Google Scholar]
- Düzel E., Habib R., Schott B., Schoenfeld A., Lobaugh N., McIntosh A.R. A multivariate, spatiotemporal analysis of electromagnetic time-frequency data of recognition memory. Neuroimage. 2003;18(2):185–197. doi: 10.1016/s1053-8119(02)00031-9. [DOI] [PubMed] [Google Scholar]
- Ellenbogen J.M., Payne J.D., Stickgold R. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Curr. Opin. Neurobiol. 2006;16:716–722. doi: 10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Fandakova Y., Sander M.C., Grandy T.H., Cabeza R., Werkle-Bergner M., Shing Y.L. Age differences in false memory: the importance of retrieval monitoring processes and their modulation by memory quality. Psychol. Aging. 2018;33(1):119–135. doi: 10.1037/pag0000212. [DOI] [PubMed] [Google Scholar]
- Fandakova Y., Werkle-Bergner M., Sander M.C. (Only) time can tell: age differences in false memory are magnified at longer delays. PsyArXiv. 2019 doi: 10.1037/pag0000465. [DOI] [PubMed] [Google Scholar]
- Fenn K.M., Hambrick D.Z. What drives sleep-dependent memory consolidation: greater gain or less loss? Psychon. Bull. Rev. 2013;20(3):501–506. doi: 10.3758/s13423-012-0366-z. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B. Structural brain changes in aging: courses, causes and cognitive consequences. Rev. Neurosci. 2010;21(3):187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fogel S.M., Martin N., Lafortune M., Barakat M., Debas K., Laventure S. NREM sleep oscillations and brain plasticity in aging. Front. Neurol. 2012;3:176. doi: 10.3389/fneur.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S.M., Albouy G., Vien C., Popovicci R., King B.R., Hoge R. fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum. Brain Mapp. 2014;35(8):3625–3645. doi: 10.1002/hbm.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S.M., Vien C., Karni A., Benali H., Carrier J., Doyon J. Sleep spindles: a physiological marker of age-related changes in gray matter in brain regions supporting motor skill memory consolidation. Neurobiol. Aging. 2017;49:154–164. doi: 10.1016/j.neurobiolaging.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E.M., Müller D., Leinsinger G., Juckel G., Hahn K. The effect of the skull on event-related P300. Clin. Neurophysiol. 2001;112(9):1773–1776. doi: 10.1016/s1388-2457(01)00587-9. [DOI] [PubMed] [Google Scholar]
- Gais S., Born J. Declarative memory consolidation: mechanisms acting during human sleep. Learn. Mem. 2004;11:679–685. doi: 10.1101/lm.80504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S., Mölle M., Helms K., Born J. Learning-dependent increases in sleep spindle density. J. Neurosci. 2002;22(15):6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J.L., Burke S.N., McNaughton B.L., Barnes C.A. Sequence reactivation in the hippocampus is impaired in aged rats. J. Neurosci. 2008;28(31):7883–7890. doi: 10.1523/JNEUROSCI.1265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A., Santelli L., Tomassini V., Bosnell R., Smith S., De Stefano N., Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass G.V., Peckham P.D., Sanders J.R. Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Rev. Educ. Res. 1972;42(3):237–288. [Google Scholar]
- Grady C.L., Craik F.I. Changes in memory processing with age. Curr. Opin. Neurobiol. 2000;10:224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Gudberg C., Wulff K., Johansen-Berg H. Sleep-dependent motor memory consolidation in older adults depends on task demands. Neurobiol. Aging. 2014;36(3):1409–1416. doi: 10.1016/j.neurobiolaging.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui W.-J., Li H.-J., Guo Y.-H., Peng P., Lei X., Yu J. Age-related differences in sleep-based memory consolidation: a meta-analysis. Neuropsychologia. 2017;97(2):46–55. doi: 10.1016/j.neuropsychologia.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Habib R., Nyberg L. Neural correlates of availability and accessibility in memory. Cerebr. Cortex. 2007;18(7):1720–1726. doi: 10.1093/cercor/bhm201. [DOI] [PubMed] [Google Scholar]
- Haenlein M., Kaplan A.M. A beginner’s guide to Partial Least Squares analysis. Underst. Stat. 2004;3(4):283–297. [Google Scholar]
- Harand C., Bertran F., Doidy F., Guénolé F., Desgranges B., Eustache F., Rauchs G. How aging affects sleep-dependent memory consolidation? Front. Neurol. 2012;3:8. doi: 10.3389/fneur.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwell M.R., Rubinstein E.N., Hayes W.S., Olds C.C. Summarizing Monte Carlo results in methodological research: the one- and two-factor fixed effects ANOVA cases. J. Educ. Stat. 1992;17(4):315–339. [Google Scholar]
- Helfrich R.F., Mander B.A., Jagust W.J., Knight R.T., Walker M.P. Old brains come uncoupled in sleep: slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron. 2018;97:221–230. doi: 10.1016/j.neuron.2017.11.020. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogge M., Adam S., Collette F. Retrieval-induced forgetting in normal ageing. J. Neuropsychol. 2008;2:463–476. doi: 10.1348/174866407x268533. [DOI] [PubMed] [Google Scholar]
- Hornung O.P., Danker-Hopfe H., Heuser I. Age-related changes in sleep and memory: commonalities and interrelationships. Exp. Gerontol. 2005;40:279–285. doi: 10.1016/j.exger.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Huber R., Ghilardi M.F., Massimini M., Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Iber C., Ancoli-Israel S., Chesson A.L., Quan S.F. first ed. American Academy of Sleep Medicine; Westchester, IL: 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- Jasper H.H. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Jenkins J.G., Dallenbach K.M. Obliviscence during sleep and waking. Am. J. Psychol. 1924;35(4):605–612. [Google Scholar]
- Keresztes A., Bender A.R., Bodammer N.C., Lindenberger U., Shing Y.L., Werkle-Bergner M. Hippocampal maturity promotes memory distinctiveness in childhood and adolescence. Proc. Natl. Acad. Sci. U. S. A. 2017;114(34):9212–9217. doi: 10.1073/pnas.1710654114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinzing J.G., Kugler S., Soekadar S.R., Rasch B., Born J., Diekelmann S. Odor cueing during slow-wave sleep benefits memory independently of low cholinergic tone. Psychopharmacology. 2018;235:291–299. doi: 10.1007/s00213-017-4768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinzing J.G., Mölle M., Weber F., Supp G., Hipp J.F., Engel A.K., Born J. Spindle activity phase-locked to sleep slow oscillations. Neuroimage. 2016;134:607–616. doi: 10.1016/j.neuroimage.2016.04.031. [DOI] [PubMed] [Google Scholar]
- Krishnan A., Williams L.J., McIntosh A.R., Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56(2):455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Kuriyama K., Stickgold R., Walker M.P. Sleep-dependent learning and motor-skill complexity. Learn. Mem. 2004;11:705–713. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt H.-P., Borbély A.A. Age-dependent changes in sleep EEG topography. Clin. Neurophysiol. 2001;112:369–377. doi: 10.1016/s1388-2457(00)00542-3. [DOI] [PubMed] [Google Scholar]
- Latchoumane C.-F.V., Ngo H.V., Born J. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron. 2017;95(2):424–435. doi: 10.1016/j.neuron.2017.06.025. e6. [DOI] [PubMed] [Google Scholar]
- Leissner P., Lindholm L.-E., Petersen I. Alpha amplitude dependence on skull thickness as measured by ultrasound technique. Electroencephalogr. Clin. Neurophysiol. 1970;29:392–399. doi: 10.1016/0013-4694(70)90047-7. [DOI] [PubMed] [Google Scholar]
- Lo J.C., Dijk D.J., Groeger J.A. Comparing the effects of nocturnal sleep and daytime napping on declarative memory consolidation. PLoS One. 2014;9:9. doi: 10.1371/journal.pone.0108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaugh N.J., West R., McIntosh A.R. Spatiotemporal analysis of experimental differences in event-related potential data with partial least squares. Psychophysiology. 2001;38(3):517–530. doi: 10.1017/s0048577201991681. [DOI] [PubMed] [Google Scholar]
- MacLeod M., Macrae C.N. Gone but not forgotten: the transient nature of retrieval-induced forgetting. Psychol. Sci. 2001;12(2):148–152. doi: 10.1111/1467-9280.00325. [DOI] [PubMed] [Google Scholar]
- Maingret N., Girardeau G., Todorova R., Goutierre M., Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 2016;19(7):959–964. doi: 10.1038/nn.4304. [DOI] [PubMed] [Google Scholar]
- Mander B.A., Rao V., Lu B., Saletin J.M., Lindquist J.R., Ancoli-Israel S. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci. 2013;16(3):357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander B.A., Winer J., Jagust W.J., Walker M.P. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s Disease? Trends Neurosci. 2016;39(8):552–566. doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander B.A., Winer J.R., Walker M.P. Sleep and human aging. Neuron. 2017;94:19–36. doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander B.A., Zhu A.H., Lindquist J.R., Villeneuve S., Rao V., Lu B. White matter structure in older adults moderates the benefit of sleep spindles on motor memory consolidation. J. Neurosci. 2017;37(48):11675–11687. doi: 10.1523/JNEUROSCI.3033-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantua J. Sleep physiology correlations and human memory consolidation: where do we go from here? Sleep. 2018;41(2) doi: 10.1093/sleep/zsx204. [DOI] [PubMed] [Google Scholar]
- Marshall L., Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn. Sci. 2007;11(10):442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Marshall L., Campos-Beltrán D. Electric stimulation to improve memory consolidation during sleep. In: Axmacher N., Rasch B., editors. Cognitive Neuroscience of Memory Consolidation. Springer; 2017. pp. 301–312. [Google Scholar]
- Marshall L., Mölle M., Hallschmid M., Born J. Transcranial direct current stimulation during sleep improves declarative memory. J. Neurosci. 2004;24(44):9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.R., Bookstein F.L. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Chau W.K., Protzner A.B. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23(2):764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Lobaugh N.J. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23:S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Mišić B. Multivariate statistical analyses for neuroimaging data. Annu. Rev. Psychol. 2013;64:499–525. doi: 10.1146/annurev-psych-113011-143804. [DOI] [PubMed] [Google Scholar]
- Mölle M., Bergmann T.O., Marshall L., Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34(10):1411–1421. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M., Eschenko O., Gais S., Sara S.J., Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur. J. Neurosci. 2009;29:1071–1081. doi: 10.1111/j.1460-9568.2009.06654.x. [DOI] [PubMed] [Google Scholar]
- Mölle M., Marshall L., Gais S., Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J. Neurosci. 2002;22(24):10941–10947. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlroth, B. E., Rasch, B., & Werkle-Bergner, M. (submitted). Episodic Memory Consolidation during Sleep in Healthy Aging. [DOI] [PubMed]
- Muehlroth B.E., Sander M.C., Fandakova Y., Grandy T.H., Rasch B., Shing Y.L., Werkle-Bergner M. Precise slow oscillation–spindle coupling promotes memory consolidation in younger and older adults. Sci. Rep. 2019;9:1940. doi: 10.1038/s41598-018-36557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlroth B.E., Werkle-Bergner M. Understanding the interplay of sleep and aging: methodological challenges. Psychophysiology. 2019;0 doi: 10.1111/psyp.13523. (in press) [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: tests of an associative deficit hypothesis. J. Exp. Psychol. Learn. Mem. Cogn. 2000;26(5):1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M., Brav T.K., Levy O. The associative memory deficit of older adults: the role of strategy utilization. Psychol. Aging. 2007;22(1):202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Nettersheim A., Hallschmid M., Born J., Diekelmann S. The role of sleep in motor sequence consolidation: stabilization rather than enhancement. J. Neurosci. 2015;35(17):6696–6702. doi: 10.1523/JNEUROSCI.1236-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo H.-V.V., Claussen J.C., Born J., Mölle M. Induction of slow oscillations by rhythmic acoustic stimulation. J. Sleep Res. 2013;22(1):22–31. doi: 10.1111/j.1365-2869.2012.01039.x. [DOI] [PubMed] [Google Scholar]
- Ngo H.-V.V., Martinetz T., Born J., Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Nir Y., Staba R.J., Andrillon T., Vyazovskiy V.V., Cirelli C., Fried I., Tononi G. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon M.M., Carskadon M.A., Guilleminault C., Vitiello M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Old S.R., Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychol. Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;156869 doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R., Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurement. Clin. Neurophysiol. 2001;112:713–719. doi: 10.1016/s1388-2457(00)00527-7. [DOI] [PubMed] [Google Scholar]
- Oudiette D., Paller K.A. Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn. Sci. 2013;17(3):142–149. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Pardilla-Delgado E., Payne J.D. The impact of sleep on true and false memory across long delays. Neurobiol. Learn. Mem. 2017;137:123–133. doi: 10.1016/j.nlm.2016.11.016. [DOI] [PubMed] [Google Scholar]
- Payne J.D., Schachetr D.L., Propper R., Huang L.-W., Wamsley E., Tucker M.A. The role of sleep in false memory formation. Neurobiol. Learn. Mem. 2009;92(3):327–334. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J., Pudas S., Lind J., Kauppi K., Nilsson L.G., Nyberg L. Longitudinal structure-function correlates in elderly reveal MTL dysfunction with cognitive decline. Cerebr. Cortex. 2012;22(10):2297–2304. doi: 10.1093/cercor/bhr306. [DOI] [PubMed] [Google Scholar]
- Piosczyk H., Holz J., Feige B., Spiegelhalder K., Weber F., Landmann N. The effect of sleep-specific brain activity versus reduced stimulus interference on declarative memory consolidation. J. Sleep Res. 2013;22:406–413. doi: 10.1111/jsr.12033. [DOI] [PubMed] [Google Scholar]
- Pudas S., Josefsson M., Rieckmann A., Nyberg L. Longitudinal evidence for increased functional response in frontal cortex for older adults with hippocampal atrophy and memory decline. Cerebr. Cortex. 2017;28(3):936–948. doi: 10.1093/cercor/bhw418. [DOI] [PubMed] [Google Scholar]
- Raaijmakers J.G., Shiffrin R.M. Search of associative memory. Psychol. Rev. 1981;88(2):93–134. [Google Scholar]
- Rasch B., Born J. About sleep’s role in memory. Physiol. Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B., Büchel C., Gais S., Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315(5817):1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Raz N., Lindenberger U., Rodrigue K.M., Kennedy K.M., Head D., Williamson A. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebr. Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N., Rodrigue K.M. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci. Biobehav. Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletin J.M., Van der Helm E., Walker M.P. Structural brain correlates of human sleep oscillations. Neuroimage. 2013;83:658–668. doi: 10.1016/j.neuroimage.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M., Gruber G., Parapatics S., Sauter C., Klösch G., Anderer P. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- Schabus M., Hoedlmoser K., Pecherstorfer T., Anderer P., Gruber G., Parapatics S. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–135. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, M.C., Fandakova, Y., Grandy, T.H., Shing, Y.L., Werkle-Bergner, M., in press. Oscillatory mechanisms of successful memory formation in younger and older adults are related to structural integrity. Cerebral Cortex 530121. doi: 10.1093/cercor/bhz339 [DOI] [PMC free article] [PubMed]
- Schabus M. Still missing some significant ingredients. Sleep. 2009;32(3):291–293. doi: 10.1093/sleep/32.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro A.C., McDevitt E.A., Rogers T.T., Mednick S.C., Norman K.A. Human hippocampal replay during rest prioritizes weakly learned information and predicts memory performance. Nat. Commun. 2018;9:3920. doi: 10.1038/s41467-018-06213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S.F., Cordi M.J., Rasch B. Modulating influences of memory strength and sensitivity of the retrieval test on the detectability of the sleep consolidation effect. Neurobiol. Learn. Mem. 2017;175:181–189. doi: 10.1016/j.nlm.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Schouten D.I., Pereira S.I.R., Tops M., Louzada F.M. State of the art on targeted memory reactivation: sleep your way to enhanced cognition. Sleep Med. Rev. 2017;32:123–131. doi: 10.1016/j.smrv.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Schreiner T., Göldi M., Rasch B. Cueing vocabulary during sleep increases theta activity during later recognition testing. Psychophysiology. 2015;52(11):1538–1543. doi: 10.1111/psyp.12505. [DOI] [PubMed] [Google Scholar]
- Schreiner T., Rasch B. Boosting vocabulary learning by verbal cueing during sleep. Cerebr. Cortex. 2015;25(11):4169–4179. doi: 10.1093/cercor/bhu139. [DOI] [PubMed] [Google Scholar]
- Schreiner T., Rasch B. To gain or not to gain: the complex role of sleep for memory: comment on Dumay (2016) Cortex. 2018;101:11–16. doi: 10.1016/j.cortex.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Scullin M.K. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol. Aging. 2012;28(1):105–114. doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin M.K., Bliwise D.L. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect. Psychol. Sci. 2015;10(1):97–137. doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin M.K., Fairley J., Decker M.J., Bliwise D.L. The effects of an afternoon nap on episodic memory in young and older adults. Sleep. 2017;40 doi: 10.1093/sleep/zsx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin M.K., Gao C. Dynamic contributions of slow wave sleep and REM sleep to cognitive longevity. Curr. Sleep. Med. Rep. 2018;4(4):284–293. doi: 10.1007/s40675-018-0131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing Y.L., Rodrigue K.M., Kennedy K.M., Fandakova Y., Bodammer N., Werkle-Bergner M. Hippocampal subfield volumes: age, vascular risk, and correlation with associative memory. Front. Aging Neurosci. 2011;3:2. doi: 10.3389/fnagi.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing Y.L., Werkle-Bergner M., Li S.-C., Lindenberger U. Associative and strategic components of episodic memory: a life-span dissociation. J. Exp. Psychol. Gen. 2008;137(3):495–513. doi: 10.1037/0096-3445.137.3.495. [DOI] [PubMed] [Google Scholar]
- Sommer V.R., Fandakova Y., Grandy T.H., Shing Y.L., Werkle-Bergner M., Sander M.C. Neural pattern similarity differentially affects memory performance of younger and older adults. J. Neurosci. 2019;39(41):8089–8099. doi: 10.1523/JNEUROSCI.0197-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonni A., Spencer R.M.C. Sleep protects memories from interference in older adults. Neurobiol. Aging. 2015;36(7):2272–2281. doi: 10.1016/j.neurobiolaging.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel R., Koberle S., Allen S.R. Significance of slow wave sleep: considerations from a clinical viewpoint. Sleep. 1986;9(1):66–79. doi: 10.1093/sleep/9.1.66. [DOI] [PubMed] [Google Scholar]
- St-Laurent M., Abdi H., Bondad A., Buchsbaum B.R. Memory reactivation in healthy aging: evidence of stimulus-specific dedifferentiation. J. Neurosci. 2014;34(12):4175–4186. doi: 10.1523/JNEUROSCI.3054-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. The corticothalamic system in sleep. Front. Biosci. 2003;8:d878–899. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Stickgold R. How do I remember? Let me count the ways. Sleep Med. Rev. 2010;13(5):305–308. doi: 10.1016/j.smrv.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R., Walker M.P. Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci. 2013;16(2):139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A.M., Dinges D.F., van Dongen H.P. Trait interindividual differences in the sleep physiology of healthy young adults. J. Sleep Res. 2007;16(2):170–180. doi: 10.1111/j.1365-2869.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- Tucker M.A., Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31(2):197–203. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M.A., McKinley S., Stickgold R. Sleep optimizes motor skill in older adults. J. Am. Geriatr. Soc. 2011;59:603–609. doi: 10.1111/j.1532-5415.2011.03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Intratrial and intertrial retention: notes towards a theory of free recall verbal learning. Psychol. Rev. 1964;71(3):219–237. doi: 10.1037/h0043186. [DOI] [PubMed] [Google Scholar]
- Tulving E. The effects of presentation and recall of material in free-recall learning. J. Verb. Learn. Verb. Behav. 1967;6(2):175–184. [Google Scholar]
- Ujma P.P., Gombos F., Genzel L., Konrad B.N., Simor P., Steiger A., Dresler M., Bódizs R. A comparison of two sleep spindle detection methods based on all night averages: individually adjusted vs. fixed frequencies. Front. Hum. Neurosci. 2015;9:52. doi: 10.3389/fnhum.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf Y.D., Altena E., Schoonheim M.M., Sanz-Arigita E.J., Vis J.C., De Rijke W., Van Someren E.J.W. Sleep benefits subsequent hippocampal functioning. Nat. Neurosci. 2009;12(2):122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- Varga A.W., Ducca E.L., Kishi A., Fischer E., Parekh A., Koushyk V. Effects of aging on slow wave sleep dynamics and human spatial navigational memory consolidation. Neurobiol. Aging. 2016;42:142–149. doi: 10.1016/j.neurobiolaging.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello M.V. Sleep in normal aging. Sleep. Med. Clin. 2006;1:171–176. doi: 10.1016/j.jsmc.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Dale A.M., Mcevoy L.K., Brewer J., Karow D.S. Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiol. Aging. 2010;31(7):1107–1121. doi: 10.1016/j.neurobiolaging.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M.P. The role of slow wave sleep in memory processing. J. Clin. Sleep. Med. 2009;5(Suppl. 2):S20–S26. [PMC free article] [PubMed] [Google Scholar]
- Walker M.P., Brakefield T., Morgan A., Hobson J.A., Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Ward M.T., Oler J.A., Markus E.J. Hippocampal dysfunction during aging I: deficits in memory consolidation. Neurobiol. Aging. 1999;20:363–372. doi: 10.1016/s0197-4580(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Werkle-Bergner M., Müller V., Li S.-C., Lindenberger U. Cortical EEG correlates of successful memory encoding: implications for lifespan comparisons. Neurosci. Biobehav. Rev. 2006;30:839–854. doi: 10.1016/j.neubiorev.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Westerberg C.E., Mander B.A., Florczak S.M., Weintraub S., Mesulam M., Zee P.C., Paller K.A. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 2012;18:490–500. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens K.A., Ferrarelli F., Walker M.P., Buysse D.J. Slow-wave activity enhancement to improve cognition. Trends Neurosci. 2018;41(7):470–482. doi: 10.1016/j.tins.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.K., Baran B., Pace-Schott E.F., Ivry R.B., Spencer R.M.C. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol. Aging. 2012;33:991–1000. doi: 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted J.T. The psychology and neuroscience of forgetting. Annu. Rev. Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Gruber R. Can slow-wave sleep enhancement improve memory? A review of current approaches and cognitive outcomes. Yale J. Biol. Med. 2019;92:63–80. [PMC free article] [PubMed] [Google Scholar]
- Ziegler G., Dahnke R., Jäncke L., Yotter R.A., May A., Gaser C. Brain structural trajectories over the adult lifespan. Hum. Brain Mapp. 2012;33(10):2377–2389. doi: 10.1002/hbm.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.