STRUCTURED ABSTRACT
Objective
Determine preoperative patient characteristics associated with postoperative outpatient opioid use and assess the frequency of postoperative opioid overprescribing.
Summary Background Data
Although characteristics associated with inpatient opioid use have been described, the patient factors associated with opioid use after discharge are unknown but could inform the development of individualized approaches to postoperative prescribing.
Methods
We included opioid-naïve patients undergoing hysterectomy, thoracic surgery, and total knee and hip arthroplasty in a single-center prospective observational cohort study. Preoperative phenotyping included self-report measures to assess pain severity, fibromyalgia survey criteria score, pain catastrophizing, depression, anxiety, functional status, fatigue, and sleep disturbance. Our primary outcome measure was self-reported total opioid use in oral morphine equivalents (OMEs). We constructed multivariable linear regression models predicting opioids consumed in the first postoperative month.
Results
We enrolled 1,181 patients; 1,001 had complete primary outcome data and 913 had complete phenotype data. Younger age, non-Caucasian race, lack of a college degree, higher anxiety, greater sleep disturbance, heavy alcohol use, current tobacco use, and larger initial opioid prescription size were associated with increased opioid consumption. Median total OMEs prescribed was 600 (equivalent to 120 5-mg hydrocodone pills), while median opioid consumption was 188 OMEs (38 pills).
Conclusions
In this prospective cohort of opioid-naïve patients undergoing major surgery, we found a number of characteristics associated with greater opioid use in the first month after surgery. Future studies should address the use of non-opioid medications and behavioral therapies in the perioperative period for these higher risk patients.
MINI-ABSTRACT
We assessed the preoperative patient characteristics independently associated with postoperative outpatient opioid use during the first month after four common major surgeries. We found that younger age, non-Caucasian race, absence of a college degree, higher anxiety, greater sleep disturbance, heavy alcohol use, current tobacco use, and larger initial opioid prescription size were significantly associated with increased opioid consumption. Furthermore, we observed a marked discrepancy between prescribed and consumed opioids.
INTRODUCTION
The rise of opioid use disorders in the United States has been termed an epidemic, with 134 Americans dying on average daily.1 While prescribing has decreased in recent years,2 more than 193 billion oral morphine equivalents (OMEs) were prescribed in 2016.3 Most public health and research efforts have focused on the appropriate use of opioids in chronic pain conditions and on medication-assisted treatment for opioid abuse. However, many patients’ initial opioid prescriptions come from surgical care.4 New chronic opioid use may represent the most common complication after a wide variety of elective surgeries.5-10 Additionally, approximately 70% of opioid pills prescribed after surgery go unused and become a source for misuse, abuse, and diversion.11, 12
Recently, a number of states have implemented supply limits of 7 days or fewer for initial opioid prescriptions, including surgical prescribing.13 Although prescribing limits have some potential benefits, such policy efforts are not necessarily patient-centered and may undertreat pain for some patients.14 Moreover, blunt policies that are not data-driven may not resonate with surgeons (who may be fearful of poor patient satisfaction or the need for refills), and limits can also still lead to overprescribing. A more individualized approach based on preoperative patient risk factors to postoperative opioid prescribing may minimize unused opioids and the incidence of prolonged postoperative opioid use while ensuring adequate analgesia.
Prior studies have associated a number of factors with opioid use during the inpatient period, particularly psychological characteristics such as distress, anxiety, and centralized pain.15-17 However, limited data exist regarding patient phenotypes associated with post-discharge opioid use. Furthermore, most known risk factors for opioid consumption are based on studies of single conditions or insurance claims data. Using data from a prospective registry, we designed the present study to fill this knowledge gap by assessing patient factors associated with opioid consumption in the first month following major surgery and to examine the prevalence of opioid overprescribing at a major academic medical center.
MATERIALS AND METHODS
Study Design and Recruitment
The Analgesic Outcome Study is a prospective observational cohort study of acute and chronic postoperative pain. The study was conducted at the University of Michigan Medical School (Ann Arbor, MI) with pre-initiation approval from the Institutional Review Board. Study reporting conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement.18
Between December 2015 and June 2018, research assistants prospectively recruited adult patients (≥18 years old) scheduled for elective hysterectomy, thoracic surgery, and primary unilateral total knee and total hip arthroplasty (TKA and THA, respectively) prior to surgery. Recruitment was carried out at either the preoperative clinic visit (approximately 2 weeks prior to surgery) or on the date of surgery in the preoperative holding area. Some TKA and THA patients were also recruited during a preoperative educational workshop visit.
Prisoners and subjects who were unable to provide written informed consent or did not speak English were excluded. We also implemented several procedure-specific exclusions: bilateral arthroplasty and revision arthroplasty for the TKA and THA cohorts; gynecologic malignancy or additional major surgical procedure in the hysterectomy cohort (patients undergoing oophorectomy, prolapse, or incontinence procedures were not excluded); and metastatic cancer in the thoracic surgery cohort. Each patient’s “current medications” list in the electronic medical record (updated at the preoperative clinic visit to include medications prescribed both by our center as well as outside hospitals) was examined by research assistants on the day of surgery for opioids. Patients were also asked directly if they were currently taking an opioid medication. Subjects who reported using opioids at the time of surgery were excluded from analysis, as were those who reported taking opioids until instructed to stop for surgery by their surgeon. All patients provided written informed consent prior to study participation.
Phenotyping
Prior to surgery, patients completed a battery of validated self-report measures, including several measures from the National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS)19:
Brief Pain Inventory (BPI) Severity Subscale
A 4-item measure assessing severity of overall body and surgical site pain. Each item is measured on an 11-point Likert-type scale ranging from 0 (no pain) to 10 (pain “as bad as you can imagine”).20 We used 2 of the 4 BPI items: average pain and worst pain.
2011 Fibromyalgia Survey Criteria
The Fibromyalgia Survey Criteria score was used as a surrogate of centralized pain.21, 22 This measure has been independently associated with postoperative in-hospital opioid consumption.15, 16 It is a composite score ranging from 0 to 31 composed of two components. The first is a count of pain sites present among 19 possible body areas assessed using the Michigan Body Map (0 to 19 points).23 The second evaluates comorbid symptoms using the Symptom Severity Index (SSI): a 6-item scale that assesses the presence and/or severity of headache, fatigue, difficulty thinking, abdominal cramps, non-refreshing sleep, and depression. It ranges from 0 to 12 points.21, 22
Coping Strategies Questionnaire (CSQ) Catastrophizing Subscale
This subscale consists of six items measured using a numerical rating scale ranging from 0 to 6 that indicate how frequently catastrophizing is used to cope with pain.24
PROMIS 4a Depression Scale
A validated 4-item measure assessing how much depression, helplessness, and hopelessness patients experience. Each item is measured on a 5-point scale ranging from 1 to 5; total raw scores range from 4 to 20.25
PROMIS 4a Anxiety Scale
A validated 4-item measure assessing how much general fear and worry patients experience. Each item is measured on a 5-point scale ranging from 1 to 5. Total raw scores range from 4 to 20.25
PROMIS 4a Physical Function Scale
A validated 4-item measure assessing one’s ability to do daily activities. Each item is measured on a 5-point scale ranging from 1 to 5; total raw scores range from 4 to 20.25
PROMIS 4a Fatigue Scale
A validated 4-item measure assessing how much fatigue people experience and how much their fatigue affects them. Each item is measured on a 5-point scale ranging from 1 to 5. Total raw scores range from 4 to 20.
PROMIS 8a Sleep Disturbance Scale
A validated 8-item measure assessing sleep difficulties, trouble falling asleep, and trouble staying asleep. Each item is measured on a 5-point scale ranging from 1 to 5; total raw scores range from 8 to 40.26
Additional Data
We extracted additional data on demographics, medical history, surgery type, and amount of opioid prescribed in OMEs from the preoperative anesthesia history and physical and the University of Michigan Research Data Warehouse.
Outcomes
Research assistants contacted all subjects by telephone at 1 month postoperatively to assess opioid consumption. Assistants reviewed all prescriptions filled since surgery with patients in order to determine accurate counts of bottles, dosages, and number of pills prescribed. Next, patients were guided through individual bottle-by-bottle formal pill counts while the assistant remained on the telephone. If patients were unable to do a formal count, they were instructed to estimate the number of pills remaining. The primary outcome was the amount of opioid used in the first month after surgery following hospital discharge, including refills. We excluded subjects with postoperative falls, emergency department visits, hospital admissions, or additional operations within the 1-month follow-up period. All opioid use was converted to OMEs using conversions from the Centers for Disease Control and Prevention.27
Statistical Analysis
We calculated descriptive statistics including median and interquartile range (IQR) for OMEs of initial opioid prescriptions following surgery, total OMEs prescribed in the first month following surgery, and total OMEs consumed in the first month following surgery. We determined frequencies and percentages for patients who did not consume any opioids following surgery and for those who stopped using opioids within one month following surgery. We computed these descriptive statistics for the overall sample and each surgical group. Next, we constructed univariable linear mixed models predicting total OMEs consumed in the first month following surgery for the overall sample and each surgical group, including year of surgery as a random intercept. This outcome was defined a priori. For our combined analysis across all 4 surgical groups, we standardized OMEs between groups. We used complete case analysis for each univariable model. Finally, we constructed multivariable linear mixed regression models predicting total standardized OMEs consumed in the first month following surgery for the overall sample, as well as total OMEs consumed within each individual surgical group, including year of surgery as a random intercept. All analyses were confirmed by two statisticians working independently. We used complete case analysis for each multivariable model. Stata version 14 (StataCorp, College Station, TX) was used for all analyses; we considered p<0.05 to be statistically significant.
RESULTS
Out of 1,976 patients approached to participate in the study, we enrolled 1,181 patients from December 2015 to May 2018. Compared to patients who declined to participate, enrolled patients did not differ significantly on age or sex. However, enrolled participants were more likely to be Caucasian (Table S1, Supplemental Digital Content). 1,001 (84.8%) remained after exclusion of 158 subjects without complete 1-month opioid consumption data and 22 subjects with excluded postoperative events (Figure 1). We divided these 1,001 participants into 4 cohorts by surgery: TKA (358 subjects), hysterectomy (275), THA (233), and thoracic (135) (Table 1). The hysterectomy and thoracic cohorts were further subdivided by surgical approach. Of the former group, a vaginal approach was used in 105 cases, laparoscopic in 117, robotic in 32, and laparotomy in 21. Among thoracic surgical patients, a laparoscopic/video-assisted thoracoscopic (VATS) approach was used in 75, a robotic approach in 40, and open thoracotomy in 20.
Figure 1.
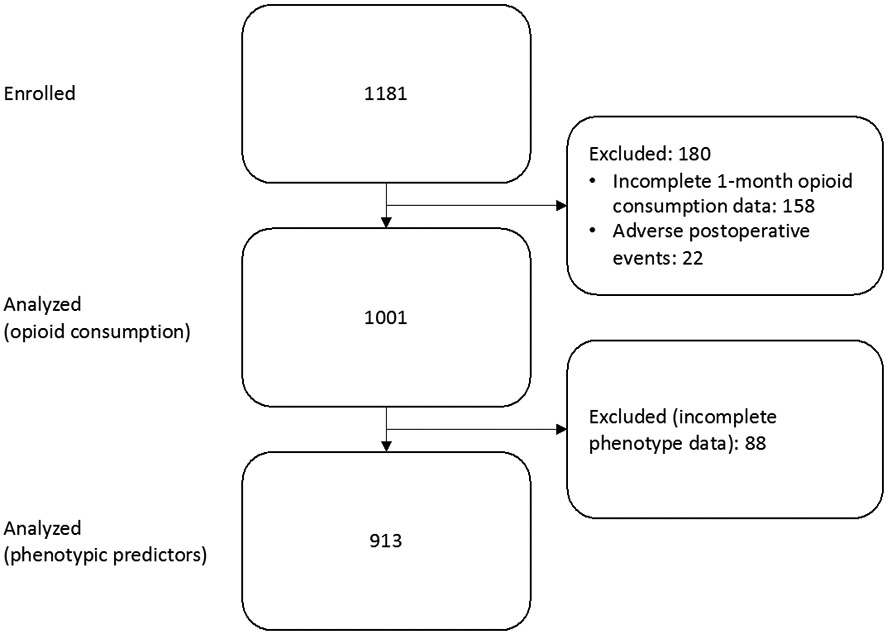
Participant flow diagram.
Table 1.
Day-of-surgery characteristics for overall sample and by surgical group
| Overall (n = 913) |
Hysterectom y (n = 246) |
Knee (n = 325) |
Hip (n = 221) |
Thoracic (n = 121) |
|
|---|---|---|---|---|---|
| Age | 59.7 (12.8) | 49.6 (11.8) | 65.3 (9.4) | 62.4 (10.9) | 60.4 (13.8) |
| Male | 305 (33.4%) | 2 (0.8%) | 149 (45.9%) | 110 (49.8%) | 44 (36.4%) |
| Caucasian | 838 (91.8%) | 210 (85.4%) | 303 (93.2%) | 207 (93.7%) | 118 (97.5%) |
| Married | 671 (73.5%) | 187 (76.0%) | 239 (73.5%) | 163 (73.8%) | 82 (67.8%) |
| College graduate | 479 (52.5%) | 126 (51.2%) | 171 (52.6%) | 138 (62.4%) | 44 (36.4%) |
| Alcohol use | |||||
| None | 457 (50.1%) | 144 (58.5%) | 144 (44.3%) | 98 (44.3%) | 71 (58.7%) |
| Low to moderate | 430 (47.1%) | 99 (40.2%) | 172 (52.9%) | 112 (50.7%) | 47 (38.8%) |
| Heavy/former abuse | 26 (2.9%) | 3 (1.2%) | 9 (2.8) | 11 (5.0%) | 3 (2.5%) |
| Tobacco | |||||
| None | 571 (62.5%) | 184 (74.8%) | 196 (60.3%) | 130 (58.8%) | 61 (50.4%) |
| Former tobacco | 286 (31.3%) | 45 (18.3%) | 115 (35.4%) | 76 (34.4%) | 50 (41.3%) |
| Current tobacco | 56 (6.1%) | 17 (6.9%) | 14 (4.3%) | 15 (6.8%) | 10 (8.3%) |
| Fibromyalgia Survey Criteria Score | 4.8 (3.8) | 5.6 (4.3) | 3.8 (3.2) | 5.6 (3.7) | 4.3 (3.5) |
| Function | 14.1 (4.5) | 18.0 (3.2) | 11.8 (3.4) | 11.3 (3.4) | 17.5 (3.3) |
| Fatigue | 8.8 (4.0) | 9.1 (4.3) | 8.4 (3.8) | 9.3 (4.2) | 8.3 (3.7) |
| Sleep disturbance | 22.9 (7.1) | 22.9 (7.2) | 21.8 (7.1) | 24.4 (7.3) | 22.8 (6.3) |
| Anxiety | 6.2 (2.8) | 6.5 (3.3) | 5.7 (2.4) | 6.4 (2.8) | 6.7 (2.8) |
| Depression | 5.4 (2.5) | 5.4 (2.5) | 5.2 (2.3) | 5.6 (2.6) | 5.5 (2.4) |
| Catastrophizing (n = 908) | 3.2 (4.9) | 3.4 (5.3) | 2.7 (4.4) | 3.5 (5.2) | 3.1 (4.5) |
| BPI overall pain severity | 3.2 (2.7) | 2.6 (2.5) | 3.4 (2.7) | 3.9 (2.9) | 2.7 (2.5) |
| BPI site-specific pain severity | 4.2 (3.2) | 1.7 (2.6) | 6.1 (2) | 6.0 (2.1) | 0.6 (1.9) |
| OME of initial prescription | 458.9 (260.6) | 176.8 (93.5) | 632.0 (195.9) | 591.0 (177.1) | 326.0 (207.3) |
| Hysterectomy surgical approach | |||||
| Laparotomy | 19 (7.7%) | ||||
| Laparoscopy | 101 (41.1%) | ||||
| Vaginal | 97 (39.4%) | ||||
| Robotic | 29 (11.8%) | ||||
| Thoracic surgical approach | |||||
| Thoracotomy | 18 (14.9%) | ||||
| VATS | 67 (55.4%) | ||||
| Robotic | 36 (29.8%) |
Data are presented as mean (standard deviation) or as number (percentage) as appropriate. Two hysterectomy subjects were undergoing female-to-male gender transition and are listed as male.
Univariable Associations with Opioid Use Over the 1-Month Follow-Up
Complete data on all preoperative predictors was available for 913 of our 1,001 patients. Among all subjects, younger age, non-Caucasian race, lack of a college degree, current tobacco use, decreased functional status, and higher fibromyalgia, fatigue, sleep disturbance, anxiety, depression, catastrophizing, surgical site pain severity scores, overall body pain severity scores, and OME of initial prescription were significantly associated with increased opioid consumption in univariable analysis (Table 2). Surgical approach was also included as a categorical predictor of opioid consumption in thoracic and hysterectomy cases. Open surgical approach (thoracotomy or laparotomy, respectively) was used as the reference category. We did not observe any statistically significant differences in opioid consumption between VATS or robotic approaches compared to thoracotomy in the thoracic procedures. Hysterectomy patients undergoing the laparotomy approach used significantly more opioids than those with vaginal approaches. However, in a post hoc analysis of all pairwise comparisons of surgical approaches with a Bonferroni correction, none of the observed differences were statistically significant (Tables S2-S3, Supplemental Digital Content); the number of open cases was relatively small in both groups.
Table 2.
Univariable linear mixed regression models predicting OME consumption during the first month following surgery with year of surgery included as a random intercept.
| Overall (n = 913) (predicting OME standardized by surgery type) |
Hysterectomy (n = 246) |
Knee (n = 325) | Hip (n = 221) | Thoracic (n = 121) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Age | −0.02 | 0.003 | <0.001 | −2.9 | 0.5 | <0.001 | −8.5 | 2.9 | 0.003 | −13.0 | 2.3 | <0.001 | −3.2 | 1.4 | 0.018 |
| Male | 0.09 | 0.07 | 0.221 | -- | -- | -- | 44.2 | 54.4 | 0.416 | 48.8 | 53.7 | 0.364 | 8.7 | 39.5 | 0.825 |
| Caucasian (= 1) | −0.34 | 0.12 | 0.005 | −28.6 | 18.3 | 0.118 | −40.9 | 108.0 | 0.705 | −254.1 | 109.1 | 0.020 | −249.3 | 120.2 | 0.038 |
| Married | 0.04 | 0.07 | 0.586 | 6.9 | 15.1 | 0.646 | 55.5 | 61.5 | 0.367 | −65.6 | 61.0 | 0.282 | 24.0 | 40.6 | 0.554 |
| College graduate | −0.23 | 0.07 | <0.001 | −28.5 | 12.8 | 0.026 | −38.1 | 54.3 | 0.483 | −206.9 | 53.8 | <0.001 | −28.2 | 39.5 | 0.475 |
| Alcohol use | |||||||||||||||
| None (reference) | |||||||||||||||
| Low to moderate | 0.08 | 0.07 | 0.250 | 7.4 | 13.2 | 0.575 | 103.7 | 55.0 | 0.060 | −86.7 | 54.4 | 0.111 | 44.6 | 38.0 | 0.241 |
| Heavy/former abuse | 0.38 | 0.20 | 0.057 | 72.2 | 59.7 | 0.226 | −172.1 | 166.9 | 0.302 | 211.2 | 125.2 | 0.091 | 337.5 | 119.1 | 0.005 |
| Tobacco | |||||||||||||||
| None (reference) | |||||||||||||||
| Former tobacco | 0.05 | 0.07 | 0.464 | 4.6 | 16.8 | 0.786 | 121.7 | 56.6 | 0.025 | −24.1 | 56.3 | 0.669 | −45.8 | 39.7 | 0.248 |
| Current tobacco | 0.49 | 0.14 | 0.001 | 25.7 | 25.8 | 0.319 | 329.4 | 133.8 | 0.014 | 335.2 | 106.4 | 0.002 | 3.7 | 71.0 | 0.958 |
| Fibromyalgia Survey Criteria Score | 0.04 | 0.01 | <0.001 | 4.2 | 1.5 | 0.005 | 16.8 | 8.3 | 0.044 | 18.6 | 7.2 | 0.009 | 8.7 | 5.5 | 0.112 |
| Function | −0.02 | 0.01 | 0.004 | −4.2 | 2.0 | 0.034 | −4.0 | 7.9 | 0.608 | −24.6 | 7.8 | 0.001 | −6.1 | 5.8 | 0.294 |
| Fatigue | 0.04 | 0.01 | <0.001 | 3.7 | 1.5 | 0.011 | 9.8 | 7.2 | 0.175 | 20.9 | 6.2 | 0.001 | 7.8 | 5.2 | 0.131 |
| Sleep disturbance | 0.03 | 0.01 | <0.001 | 2.8 | 0.9 | 0.002 | 9.4 | 3.8 | 0.012 | 12.8 | 3.6 | <0.001 | 5.8 | 3.0 | 0.049 |
| Anxiety | 0.06 | 0.01 | <0.001 | 8.1 | 1.9 | <0.001 | 14.3 | 11.3 | 0.207 | 36.7 | 9.2 | <0.001 | 7.3 | 7.0 | 0.277 |
| Depression | 0.04 | 0.01 | 0.001 | 6.1 | 2.5 | 0.017 | 0.3 | 11.8 | 0.981 | 43.3 | 9.8 | <0.001 | −4.1 | 8.1 | 0.609 |
| Catastrophizing (n = 906) | 0.03 | 0.01 | <0.001 | 1.8 | 1.2 | 0.136 | 6.8 | 6.2 | 0.278 | 23.0 | 5.0 | <0.001 | 9.3 | 4.2 | 0.028 |
| Pain severity - Overall body pain (BPI) | 0.02 | 0.01 | 0.047 | 2.0 | 2.6 | 0.431 | −4.0 | 10.2 | 0.699 | 25.2 | 9.3 | 0.006 | 7.1 | 7.7 | 0.349 |
| Pain severity – Surgery-site-specific pain (BPI) | 0.03 | 0.01 | 0.001 | 5.5 | 2.5 | 0.024 | 17.8 | 13.6 | 0.191 | 29.2 | 12.4 | 0.019 | 23.7 | 10.1 | 0.019 |
| Initial prescription size (OMEs) | 0.001 | 0.0001 | <0.001 | 0.41 | 0.07 | <0.001 | 0.87 | 0.13 | <0.001 | 0.69 | 0.15 | <0.001 | 0.52 | 0.08 | <0.001 |
| Hysterectomy approach | |||||||||||||||
| Laparotomy (reference) | |||||||||||||||
| Laparoscopy | −44.8 | 25.0 | 0.074 | ||||||||||||
| Vaginal | −60.5 | 25.0 | 0.016 | ||||||||||||
| Robotic | −28.2 | 29.4 | 0.338 | ||||||||||||
| Thoracic surgical approach | |||||||||||||||
| Thoracotomy (reference) | |||||||||||||||
| VATS | −83.4 | 55.5 | 0.130 | ||||||||||||
| Robotic | −74.3 | 59.8 | 0.214 | ||||||||||||
Coefficients for opioid consumption in the “Overall” category refer to standardized OMEs across all 4 surgical groups. Coefficients for the individual surgical groups refer to non-standardized OMEs (in mg). OME = oral morphine equivalent.
Multivariable Models of Opioid Use Over the 1-Month Follow-Up
We then developed multivariable linear mixed regression models predicting OME consumption during the first month post-surgery (Table 3). Among all participants, we found that younger age, non-Caucasian race, non-college-graduate status, current tobacco use, and heavy alcohol use were significantly associated with increased opioid consumption (age: B coefficient −0.02 standardized OME units (−8.0 unstandardized OME units), SE 0.003, p<0.001; Caucasian: B coefficient −0.23 standardized OME units (−99.6 unstandardized OME units), SE 0.11, p=0.037; college graduate status: B coefficient −0.17 standardized OME units (−71.7 unstandardized OME units), SE 0.06, p=0.007; tobacco use: B coefficient 0.35 standardized OME units (150.2 unstandardized OME units), SE 0.13, p=0.007: heavy alcohol use: B coefficient 0.38 standardized OME units (164.2 unstandardized OME units), SE 0.18, p=0.039). We also found that higher anxiety and sleep disturbance scores were significantly associated with increased opioid consumption (anxiety: B coefficient 0.04 standardized OME units (17.5 unstandardized OME units), SE 0.01, p=0.003; sleep disturbance: B coefficient 0.01 standardized OME units (5.2 unstandardized OME units), SE 0.01, p=0.019). Finally, OME of initial prescription was associated with increased opioid consumption following surgery (B coefficient 0.001 standardized OME units (0.58 unstandardized OME units), SE 0.0001, p<0.001). Catastrophizing score was removed from the model due to it being highly correlated with depression (r=0.582, p<0.001), which created a suppression effect making the relationship between depression and opioid consumption negative despite a positive univariable correlation.
Table 3.
Multivariable linear mixed regression models predicting OME consumption during the first month following surgery with year of surgery included as a random intercept.
| Overall (n = 913) (predicting OME standardized by surgery type) |
Hysterectomy (n = 246) |
Knee (n = 325) |
Hip (n = 221) |
Thoracic (n = 121) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coeff. | SE | p | Coeff. | SE | p | Coeff. | SE | p | Coeff. | SE | p | Coeff. | SE | p | |
| Age | −0.02 | 0.003 | <0.001 | −2.5 | 0.6 | <0.001 | −4.7 | 2.8 | 0.090 | −8.09 | 2.3 | <0.001 | −1.5 | 1.3 | 0.227 |
| Male | −0.03 | 0.07 | 0.663 | -- | -- | -- | −0.01 | 53.5 | 1.000 | 54.7 | 47.7 | 0.251 | 1.2 | 37.4 | 0.975 |
| Caucasian (= 1) | −0.23 | 0.11 | 0.037 | −20.8 | 16.7 | 0.213 | 40.1 | 103.1 | 0.697 | −186.7 | 92.6 | 0.044 | −86.2 | 113.8 | 0.448 |
| Married | 0.09 | 0.07 | 0.178 | 18.9 | 13.9 | 0.175 | 31.6 | 58.4 | 0.588 | −27.1 | 54.5 | 0.619 | 27.0 | 34.8 | 0.438 |
| College graduate | −0.17 | 0.06 | 0.007 | −17.5 | 11.9 | 0.142 | 12.2 | 51.7 | 0.814 | −106.6 | 49.7 | 0.032 | −8.3 | 33.8 | 0.806 |
| Alcohol use | |||||||||||||||
| None (reference) | |||||||||||||||
| Low to moderate | 0.07 | 0.06 | 0.243 | 20.7 | 11.6 | 0.075 | 57.8 | 51.5 | 0.262 | −25.1 | 47.6 | 0.598 | 59.4 | 33.0 | 0.072 |
| Heavy/former abuse | 0.38 | 0.18 | 0.039 | 102.2 | 52.0 | 0.049 | −151.6 | 158.6 | 0.339 | 136.5 | 110.7 | 0.217 | 314.6 | 108.0 | 0.004 |
| Tobacco | |||||||||||||||
| None (reference) | |||||||||||||||
| Former tobacco | 0.03 | 0.07 | 0.649 | 1.8 | 15.3 | 0.907 | 96.2 | 54.2 | 0.076 | −43.5 | 49.0 | 0.375 | −40.6 | 38.1 | 0.287 |
| Current tobacco | 0.35 | 0.13 | 0.007 | 11.9 | 23.6 | 0.615 | 380.9 | 134.3 | 0.005 | 218.0 | 91.6 | 0.017 | −53.9 | 63.7 | 0.398 |
| Fibromyalgia Survey Criteria Score | 0.01 | 0.01 | 0.620 | 2.8 | 2.3 | 0.235 | 14.1 | 11.9 | 0.235 | −20.6 | 9.7 | 0.035 | −2.3 | 7.1 | 0.72 |
| Function | 0.004 | 0.01 | 0.718 | −2.4 | 2.1 | 0.250 | −4.9 | 8.6 | 0.571 | −3.3 | 8.2 | 0.685 | −7.6 | 6.3 | 0.226 |
| Fatigue | 0.004 | 0.01 | 0.718 | −3.9 | 2.3 | 0.081 | −2.3 | 10.7 | 0.827 | 4.1 | 8.6 | 0.634 | 1.1 | 6.3 | 0.858 |
| Sleep disturbance | 0.01 | 0.01 | 0.019 | 0.93 | 1.0 | 0.339 | 9.1 | 4.4 | 0.040 | 7.1 | 3.9 | 0.073 | 1.2 | 2.9 | 0.676 |
| Anxiety | 0.04 | 0.01 | 0.003 | 4.8 | 2.3 | 0.039 | −0.4 | 13.8 | 0.975 | 26.7 | 10.0 | 0.007 | 10.8 | 7.6 | 0.159 |
| Depression | −0.01 | 0.02 | 0.453 | −0.3 | 2.9 | 0.918 | −20.1 | 14.8 | 0.175 | 12.7 | 10.9 | 0.242 | −13.5 | 9.1 | 0.136 |
| Pain severity - Overall body pain (BPI) | −0.02 | 0.01 | 0.240 | −3.9 | 2.8 | 0.152 | −16.4 | 10.7 | 0.125 | 2.8 | 9.5 | 0.770 | 1.2 | 8.0 | 0.886 |
| Pain severity – Surgery-site-specific pain (BPI) | −0.01 | 0.01 | 0.337 | 1.1 | 2.4 | 0.652 | 9.1 | 14.8 | 0.537 | 10.2 | 12.8 | 0.424 | 9.1 | 9.7 | 0.343 |
| Initial prescription size (OMEs) | 0.001 | 0.0001 | <0.001 | 0.28 | 0.06 | <0.001 | 0.85 | 0.13 | <0.001 | 0.47 | 0.14 | 0.001 | 0.41 | 0.08 | <0.001 |
| Hysterectomy approach | |||||||||||||||
| Laparotomy (reference) | |||||||||||||||
| Laparoscopy | −39.8 | 22.1 | 0.071 | ||||||||||||
| Vaginal | −19.4 | 23.4 | 0.408 | ||||||||||||
| Robotic | −21.7 | 26.8 | 0.417 | ||||||||||||
| Thoracic surgical approach | |||||||||||||||
| Thoracotomy (reference) | |||||||||||||||
| VATS | −4.9 | 50.4 | 0.922 | ||||||||||||
| Robotic | −8.4 | 53.9 | 0.876 | ||||||||||||
| Random intercept | Estimate | SE | 95% CI (lower, upper) |
Estimate | SE | 95% CI (lower, upper) |
Estimate | SE | 95% CI (lower, upper) |
Estimate | SE | 95% CI (lower, upper) |
Estimate | SE | 95% CI (lower, upper) |
| Year | 0.01 | 0.01 | 0.002, 0.08 | 275.8 | 309.3 | 30.6, 2483.5 | 1042.8 | 2155.6 | 18.1, 59942.4 | 456.7 | 1723.3 | 0.3, 744053.4 | 0.00 | 0.00 | 0.00 |
Coefficients for opioid consumption in the “Overall” category refer to standardized OMEs across all 4 surgical groups. Coefficients for the individual surgical groups refer to non-standardized OMEs (in mg). OME = oral morphine equivalent.
Individual models were then created for each of the surgical cohorts. Among hysterectomy patients, younger age, heavy alcohol use, increased anxiety, and larger initial opioid prescription size were significantly associated with increased opioid use. For TKA patients, current tobacco users, those with greater sleep disturbance, and those with larger initial opioid prescriptions consumed significantly more opioids, as did THA patients who were younger, non-college-graduates, current tobacco users, more anxious, and who had larger initial prescriptions. In the THA group, higher fibromyalgia score was associated with less opioid consumption following surgery. However, given the positive relationship between fibromyalgia score and opioid use in the univariable model, this is likely evidence of a suppressor effect. Finally, we found significant associations among thoracic surgery patients between higher opioid consumption and current or prior heavy alcohol abuse, as well as between increased opioid consumption and larger initial prescription size. We did not observe any significant differences in opioid consumption by surgical approach in either the hysterectomy or the thoracic surgery cohorts in either the multivariable model or following pairwise comparison of all groups with Bonferroni correction (Tables S4-S5, Supplemental Digital Content).
Opioid Prescribing and Consumption
Surgeons initially prescribed a median 450 mg OMEs to postoperative patients (IQR 200-675 mg), and a median total 600 mg OMEs (including refills) over the month-long period (IQR 225-792 mg). Subjects used a median 188 mg OMEs over the same period (IQR 40-540 mg). In total, 628,475 mg OMEs were prescribed to our cohort, and 360,650 mg OMEs were consumed (57.4%). The total number of remaining opioid pills was equivalent to 53,565 hydrocodone tablets (hysterectomy: 5,560 pills, TKA 24,407 pills, THA 18,179 pills, thoracic surgery 5,420 pills). 137 patients (13.7%) reported using no opioids at all, while 179 (17.9%) reported continued opioid use 1 month after surgery. Subjects with continued use at 1 month included 7 hysterectomy (2.5%), 127 TKA (35.4%), 34 THA (14.6%), and 11 thoracic surgery (8.2%) patients. We were unable to determine if 13 patients (1.3%) had continued opioid use at 1 month. We report opioid prescribing and consumption stratified by surgical procedure in Table 4.
Table 4.
Opioids prescribed and consumed during the first month following surgery
| Overall (n = 1001) | Hysterectomy (n = 275) | Knee (n = 358) | Hip (n = 233) | Thoracic (n = 135) | |
|---|---|---|---|---|---|
| Median initially prescribed OMEs | 450 (200; 675) | 150 (125; 200) | 675 (600; 675) | 675 (600; 675) | 300 (200; 420) |
| Median total prescribed OMEs | 600 (225; 792) | 150 (150; 225) | 825 (675; 1275) | 675 (600; 675) | 350 (200; 488) |
| Median OMEs used | 188 (40; 540) | 70 (15; 145) | 569 (300; 945) | 225 (23; 525) | 100 (0; 250) |
| Total prescribed OMEs | 628,475 | 53,810 | 355,465 | 169,618 | 49,583 |
| Total consumed OMEs (% of prescribed) | 360,650 (57.4%) | 26,011 (48.3%) | 233,432 (65.7%) | 78,723 (46.4%) | 22,485 (45.3%) |
| Total remaining pills (hydrocodone 5 mg) | 53,565 (15; 86) | 5,560 (7; 30) | 24,407 (30; 101) | 18,179 (38; 120) | 5,420 (9; 60) |
| No opioids used | 137 (13.7%) | 43 (15.6%) | 20 (5.6%) | 40 (17.2%) | 34 (25.2%) |
| All opioids used | 90 (9.0%) | 38 (13.8%) | 16 (4.5%) | 14 (6.0%) | 22 (16.3%) |
| Continued opioid use at 1 month | 179 (17.9%) | 7 (2.6%) | 127 (35.5%) | 34 (14.6%) | 11 (8.2%) |
| Missing data on continued opioid use at 1 month | 13 (1.3%) | 6 (2.2%) | 1 (0.3%) | 3 (1.3%) | 3 (2.2%) |
OME data is presented as: mg OMEs (25th percentile; 75th percentile in mg), with the exception of “Total remaining pills.” Pills remaining was converted from OMEs to 5 mg hydrocodone tablets; data is presented as: total remaining pills (25th percentile; 75th percentile of individual patients’ remaining pills). OME = oral morphine equivalent.
Opioid Prescribing and Consumption by Specialty
Orthopedic surgeons prescribed markedly larger initial and total opioid amounts than gynecologists and thoracic surgeons. Both the TKA and THA cohorts received a median 675 mg OMEs initially (IQR 600-675 mg for both groups); TKA patients filled a median total 825 mg OMEs over the 1-month period (IQR 675-1275 mg), while THA patients received a median 675 mg OMEs (IQR 600-675 mg). By comparison, hysterectomy patients received a median 150 mg OMEs initially (IQR 125-200 mg) and median 1-month total 150 mg OMEs (IQR 150-225 mg), and thoracic surgery patients filled a median 300 mg OMEs initially (IQR 200-420 mg) and median 1-month total 350 mg OMEs (IQR 200-488 mg).
Patients undergoing TKA and THA reported using more opioid over the 1-month follow-up period than the other two cohorts. TKA patients used a median 569 mg total OMEs (IQR 300-945 mg), and THA patients a median 225 mg total OMEs (IQR 23-525 mg). Thoracic surgery patients consumed a median 100 mg OMEs (IQR 0-250 mg), and hysterectomy patients 70 mg OMEs (IQR 15-145 mg). TKA patients consumed 65.7% of total prescribed OMEs, hysterectomy patients 48.3%, THA patients 46.4%, and thoracic surgery patients 45.3%. By group, continued opioid use at 1 month was seen in 35.5% of TKA patients, 14.6% of THA patients, 8.2% of thoracic surgery patients, and 2.6% of hysterectomy patients.
DISCUSSION
We examined opioid consumption during the first postoperative month in our prospective cohort of 1,001 patients undergoing 4 common types of major surgery. Multivariable linear regression models showed significantly higher consumption of opioids during the first month after surgery among patients who were younger, non-Caucasian, had not graduated college, were heavy alcohol users, actively used tobacco, had higher anxiety and sleep disturbance scores, and had larger initial opioid prescriptions. Separately, we noted that opioid overprescription was common across all surgical types; overall median opioid consumed was less than half of median opioid prescribed.
Our results inform two related but distinct issues. Physician overprescribing of opioids has received considerable attention in both the lay press28 and scientific literature29, 30 in recent years. In additon, individualizing opioid prescribing for common surgeries, has been relatively unstudied. Although much work remains to stem the iatrogenic contribution to the current opioid epidemic, we must also ensure that patients receive appropriate treatment for post-surgical pain. While legislative,13 regulatory,31 and commercial32 initiatives to restrict opioid prescribing may accomplish the former, they run the risk of preventing the latter.14 We carried out this study envisioning that providing more granular data on which patients consume more or fewer opioids after surgery might enable a more personalized approach to postoperative prescribing, including targeted non-opioid adjunctive medication prescribing and, potentially, behavioral interventions.
Preoperative Age, Race, Education, Tobacco Use, Alcohol Use, Sleep Disturbance, Anxiety, and Initial Opioid Prescription Size Independently Associated with 1-Month Postoperative Opioid Consumption
In our combined cohort of 913 subjects with complete phenotypic data available, we saw that younger patients, non-Caucasians, non-college-graduates, heavy alcohol users, current tobacco users, those with higher levels of anxiety and sleep disturbance, and those with larger initial opioid prescriptions used significantly more opioids. Anxiety and other aspects of negative affect (e.g., depressive symptoms) have been previously associated with opioid use and dependence, albeit in retrospective administrative data or non-surgical populations.5, 33 Similar associations have been reported between opioid dependence and sleep disturbance, again in non-surgical populations.34, 35 Patient-reported outcome measures of negative affect (e.g., depression and anxiety) can validly assess the spectrum of affective symptoms, including subclinical anxiety and undiagnosed or undocumented anxiety. Data on anxiety and sleep disturbance are not routinely or systematically collected prior to surgery but could be of high value to integrate into routine preoperative care to inform opioid prescribing. Smoking status and alcohol consumption—which is included in many electronic medical records—has also been associated with increased levels of opioid use and misuse.36-38 The link between opioid prescription size and opioid consumption has been reported previously.39
There are certainly barriers to identifying anxiety and sleep disturbance in the surgical or preoperative evaluation clinic; however, brief 4-item measures have been validated for this purpose25 and could be incorporated into standard preoperative questionnaires. It would also be feasible for physicians to take into account age, race, tobacco and alcohol use, education level, and the presence of anxiety- or sleep-related disorders noted in the electronic medical record in determining both postoperative prescription size and how closely patients should be observed for adverse effects such as misuse or persistent use. Although patients with anxiety consumed more opioid after surgery in our cohort (perhaps to self-medicate for symptoms of anxiety), prescribing more opioid to patients with higher anxiety symptoms is likely unwarranted and may increase postoperative morbidity. Rather, if physicians identify anxiety preoperatively, they could consider educational interventions to set expectations for normal postoperative pain and to establish that it is acceptable for patients to use alternatives to opioids after surgery. For example, behavioral techniques such as deep breathing exercises and guided imagery have been shown to effectively reduce perioperative anxiety and postoperative pain, length of stay, and overall costs.40 Future studies are needed to evaluate the effect of addressing anxiety preoperatively through such behavioral interventions, as well as the use of non-opioid alternatives such as serotonin-norepinephrine reuptake inhibitors, which can treat both pain and anxiety.41
Opioids Were Overprescribed After Surgery
Our data show that overprescribing of opioids remains a significant issue at our academic medical center. A total 628,475 mg OMEs were prescribed to our cohort over the first postoperative month, while patients consumed only 360,650 mg OMEs (57.4%). This is equivalent to an overall excess of 53,565 individual 5-mg hydrocodone pills in these 1,001 participants (average 54 pills per patient). By surgery type, thoracic surgery patients received the most excess opioid, consuming only 28.6% of prescribed OMEs by median with a median overprescription of 250 mg OMEs. Even TKA patients, who had the lowest amount of overprescribing by percentage (consuming 68.9% of prescribed opioids by median), were prescribed a median 256 mg OMEs excess opioid (equivalent to 51 5-mg hydrocodone tablets). These data are congruent with previously reported studies, which found post-surgical overprescribing rates ranging from 42-73%.11, 42-44
Our total overprescription amount of 267,825 mg OMEs (from a cohort of 1,001 subjects) emphasizes the sheer number of excess opioid pills potentially being released into communities nationwide through post-surgical prescribing. According to weighted estimates from the National Inpatient Sample of the Agency for Healthcare Research and Quality, 723,086 TKAs, 505,170 THAs, 184,950 hysterectomies, and 69,625 lobectomies or pneumonectomies were performed in the inpatient setting in the United States in 2014.45 If our surgery-specific median overprescription amounts are extrapolated to this national dataset, total 2014 overprescribing for these procedures would be 444,819,538 mg OME (185,290,788 mg OMEs for TKA, 227,326,500 mg OMEs for THA, 14,796,000 mg OMEs for hysterectomy, and 17,406,250 mg OMEs for thoracic surgery). This is equivalent to more than 88 million total excess hydrocodone 5 mg tablets for these four surgical conditions. Overprescribing is not benign: Of the 11.5 million Americans currently misusing prescription opioids and 1.9 million with prescription opioid use disorder, the majority were either directly prescribed these medications or received them from a friend or relative with a prescription.46 Nor is the problem of overprescribing insurmountable: A targeted intervention to reduce opioid prescribing after laparoscopic cholecystectomy has been shown to reduce prescription sizes by 63% without increasing refill frequency or pain scores.47
However, our data also revealed a wide IQR (500 mg) for median overall opioid consumption over the 1-month period. These results, as well as the fact that 13.7% of our cohort took no opioids at all after surgery while 17.9% were still taking opioids a month later, emphasize the heterogeneity of the opioid-prescribed postoperative population. The few existing postoperative prescribing guidelines make recommendations based on either the number of pills taken per day prior to discharge48 or the particular surgery performed.49 We hope that our identification of preoperative phenotypic factors associated with postoperative opioid consumption may eventually enable greater personalization of guidelines, as well as targeted interventions to better care for high-risk individuals.
Limitations
Our study has a number of limitations. First, it is comprised of observational data, with its inherent risk of confounding by unmeasured variables. Differences in preoperative treatment, surgical and anesthesia technique, postoperative complications, insurance coverage, and family or physician support might have introduced error into our results. Second, our cohort was comprised of several different surgeries—2 orthopedic procedures, 1 gynecologic procedure, and a number of thoracic procedures. Although we saw some similarities between separate surgical groups, the heterogeneous nature of our population may have provided generalizability but also hindered our ability to identify characteristics linked with opioid consumption in some surgical cohorts but not others. These procedures also differ in the severity and duration of acute post-surgical pain, which may influence the amount and duration of opioid use. Finally, our institutional review board protocol permitted us to keep only demographic data for patients who elected not to participate in the study. Consequently, we were unable to obtain prescribing data for those who declined participation.
Conclusion
In this prospective cohort of opioid-naïve postoperative patients, we found that patients who were younger, non-Caucasian, non-college-graduates, current tobacco users, heavy alcohol users, had more anxiety and sleep disturbance, and had larger initial opioid prescriptions consumed significantly more opioids during the first month after surgery. Additionally, we observed a marked discrepancy between prescribed and consumed opioids. These data may be used to help personalize opioid prescribing, encourage the use of non-opioid adjunctive medications, and potentially recommend behavioral interventions to better manage acute and subacute postoperative pain.
Supplementary Material
Acknowledgments:
The authors thank the research assistants from the Division of Pain Research of the Department of Anesthesiology and surgical teams for their assistance in the success of this study.
The study was funded by the following NIH grants: NIAMS R01AR060392 (MPI Clauw and Brummett), NIDA R01DA038261 (MPI Clauw and Brummett) and NIDA R01DA042859 (MPI Waljee and Brummett). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Drug Abuse, or the National Institutes of Health. Additional funding was provided by the Department of Anesthesiology, the Medical School Dean’s Office, and the Michigan Genomics Initiative of the University of Michigan (Ann Arbor, MI). The study sponsors had no role in the design, conduct, collection, analysis, or interpretation of this study, or the preparation, review, or approval of the manuscript.
Footnotes
Conflicts of interest and source of funding:
Dr. Brummett has received research funding from Neuros Medical (Willoughby Hills, OH), not related to the present work. He is also a consultant for Heron Therapeutics (San Diego, CA, not related to this work. Dr. Clauw has received research funding from Cerephex (Los Altos, CA), Forest Laboratories (New York, NY), Merck (Kenilworth, NJ), and Pfizer (New York, NY), and serves as a consultant for Tonix Pharmaceuticals (New York, NY), Pfizer, Depomed (Newark, CA), Samumed (San Diego, CA), Aptinyx (Evanston, IL), and Zynerba Pharmaceuticals (Devon, PA); all not related to this work. Dr. Hassett is a consultant for AbbVie (North Chicago, IL); not related to this work. Dr. As-Sanie is a consultant for AbbVie and Myovant Sciences (Brisbane, CA); not related to this work.
No conflicts of interest or sources of funding were declared for the remaining authors.
REFERENCES
- 1.Ahmad F, Rossen L, Spencer M, et al. Provisional drug overdose death counts. National Center for Health Statistics 2018. [Google Scholar]
- 2.Guy GP Jr., Zhang K, Bohm MK, et al. Vital Signs: Changes in Opioid Prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep 2017; 66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill MV, Stucke RS, Billmeier SE, et al. Guideline for Discharge Opioid Prescriptions after Inpatient General Surgical Procedures. J Am Coll Surg 2018; 226(6):996–1003. [DOI] [PubMed] [Google Scholar]
- 4.Larach DB, Waljee JF, Hu HM, et al. Patterns of Initial Opioid Prescribing to Opioid-Naive Patients. Ann Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 2017; 152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke H, Soneji N, Ko DT, et al. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. Bmj 2014; 348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn LK, Yerra S, Fang S, et al. Incidence and Risk Factors for Chronic Postoperative Opioid Use After Major Spine Surgery: A Cross-Sectional Study With Longitudinal Outcome. Anesth Analg 2018; 127(1):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deyo RA, Hallvik SE, Hildebran C, et al. Use of prescription opioids before and after an operation for chronic pain (lumbar fusion surgery). Pain 2018; 159(6):1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JS, Hu HM, Edelman AL, et al. New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. J Clin Oncol 2017; 35(36):4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain 2016; 157(6):1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bicket MC, Long JJ, Pronovost PJ, et al. Prescription Opioid Analgesics Commonly Unused After Surgery: A Systematic Review. JAMA Surg 2017; 152(11):1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill MV, McMahon ML, Stucke RS, et al. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann Surg 2017; 265(4):709–714. [DOI] [PubMed] [Google Scholar]
- 13.Meara E, Horwitz JR, Powell W, et al. State Legal Restrictions and Prescription-Opioid Use among Disabled Adults. N Engl J Med 2016; 375(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua KP, Brummett CM, Waljee JF. Opioid Prescribing Limits for Acute Pain: Potential Problems With Design and Implementation. Jama 2019. [DOI] [PubMed] [Google Scholar]
- 15.Brummett CM, Janda AM, Schueller CM, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology 2013; 119(6):1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janda AM, As-Sanie S, Rajala B, et al. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology 2015; 122(5):1103–11. [DOI] [PubMed] [Google Scholar]
- 17.Ip HY, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology 2009; 111(3):657–77. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014; 12(12):1495–9. [DOI] [PubMed] [Google Scholar]
- 19.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010; 63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan G, Jensen MP, Thornby JI, et al. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain 2004; 5(2):133–7. [DOI] [PubMed] [Google Scholar]
- 21.Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15):1547–55. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011; 38(6):1113–22. [DOI] [PubMed] [Google Scholar]
- 23.Brummett CM, Bakshi RR, Goesling J, et al. Preliminary validation of the Michigan Body Map. Pain 2016; 157(6):1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandroni P, Benrud-Larson LM, McClelland RL, et al. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain 2003; 103(1-2):199–207. [DOI] [PubMed] [Google Scholar]
- 25.Reeve BB, Hays RD, Bjorner JB, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care 2007; 45(5 Suppl 1):S22–31. [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med 2011; 10(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention, “Calculating Total Daily Dose of Opioids for Safer Dosage”; https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf, accessed April 6, 2018.
- 28.Calabresi M Why America can’t kick its painkiller problem. TIME Magazine 2015; 185(22). [Google Scholar]
- 29.Murthy VH. Ending the Opioid Epidemic — A Call to Action. New England Journal of Medicine 2016; 375(25):2413–2415. [DOI] [PubMed] [Google Scholar]
- 30.Beauchamp GA, Winstanley EL, Ryan SA, et al. Moving beyond misuse and diversion: the urgent need to consider the role of iatrogenic addiction in the current opioid epidemic. Am J Public Health 2014; 104(11):2023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soelberg CD, Brown RE Jr., Du Vivier D, et al. The US Opioid Crisis: Current Federal and State Legal Issues. Anesth Analg 2017; 125(5):1675–1681. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention, “Annual Surveillance Report of Drug-Related Risks and Outcomes — United States, 2017. Surveillance Special Report 1”; published August 31, 2017; https://www.cdc.gov/drugoverdose/pdf/pubs/2017-cdc-drug-surveillance-report.pdf, accessed March 27, 2018. [Google Scholar]
- 33.Sullivan MD, Edlund MJ, Zhang L, et al. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med 2006; 166(19):2087–93. [DOI] [PubMed] [Google Scholar]
- 34.Nordmann S, Lions C, Vilotitch A, et al. A prospective, longitudinal study of sleep disturbance and comorbidity in opiate dependence (the ANRS Methaville study). Psychopharmacology (Berl) 2016; 233(7):1203–13. [DOI] [PubMed] [Google Scholar]
- 35.Hartwell EE, Pfeifer JG, McCauley JL, et al. Sleep disturbances and pain among individuals with prescription opioid dependence. Addict Behav 2014; 39(10):1537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zale EL, Dorfman ML, Hooten WM, et al. Tobacco Smoking, Nicotine Dependence, and Patterns of Prescription Opioid Misuse: Results From a Nationally Representative Sample. Nicotine Tob Res 2015; 17(9):1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young-Wolff KC, Klebaner D, Weisner C, et al. Smoking Status and Opioid-related Problems and Concerns Among Men and Women on Chronic Opioid Therapy. Clin J Pain 2017; 33(8):730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciesielski T, Iyengar R, Bothra A, et al. A Tool to Assess Risk of De Novo Opioid Abuse or Dependence. Am J Med 2016; 129(7):699–705.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard R, Fry B, Gunaseelan V, et al. Association of Opioid Prescribing With Opioid Consumption After Surgery in Michigan. JAMA Surg 2018:e184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwab D, Davies D, Bodtker T, et al. A study of efficacy and cost-effectiveness of guided imagery as a portable, self-administered, presurgical intervention delivered by a health plan. Adv Mind Body Med 2007; 22(1):8–14. [PubMed] [Google Scholar]
- 41.YaDeau JT, Brummett CM, Mayman DJ, et al. Duloxetine and Subacute Pain after Knee Arthroplasty when Added to a Multimodal Analgesic Regimen: A Randomized, Placebo-controlled, Triple-blinded Trial. Anesthesiology 2016; 125(3):561–72. [DOI] [PubMed] [Google Scholar]
- 42.As-Sanie S, Till SR, Mowers EL, et al. Opioid Prescribing Patterns, Patient Use, and Postoperative Pain After Hysterectomy for Benign Indications. Obstet Gynecol 2017; 130(6):1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiels CA, Ubl DS, Yost KJ, et al. Results of a Prospective, Multicenter Initiative Aimed at Developing Opioid-prescribing Guidelines After Surgery. Ann Surg 2018; 268(3):457–468. [DOI] [PubMed] [Google Scholar]
- 44.Fujii MH, Hodges AC, Russell RL, et al. Post-Discharge Opioid Prescribing and Use after Common Surgical Procedure. J Am Coll Surg 2018; 226(6):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, “HCUP National Inpatient Sample (NIS)”; 2014; www.hcup-us.ahrq.gov/nisoverview.jsp, accessed April 1, 2018.
- 46.Han B, Compton WM, Blanco C, et al. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 2017; 167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 47.Reduction in Opioid Prescribing Through Evidence-Based Prescribing Guidelines. JAMA Surg 2018; 153(3):285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill MV, Stucke RS, Billmeier SE, et al. Guideline for Discharge Opioid Prescriptions after Inpatient General Surgical Procedures. J Am Coll Surg 2017:ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Hill MV, Stucke RS, McMahon ML, et al. An Educational Intervention Decreases Opioid Prescribing After General Surgical Operations. Ann Surg 2017; 267:468–472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


