Short abstract
The amygdala is important in regulation of emotion-associated behavioral responses both to positive reinforcing stimuli such as addicting opioids and to negative aversive stimuli such as fear and pain. Glutamatergic neurotransmission in amygdala plays a predominant role in amygdala neuronal circuits involved in these emotional responses. However, how specific glutamate receptors act to mediate these amygdala functions remains poorly understood. In this study, we investigated the role of GluA1 subunits of glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in central amygdala in modulating behavioral response to aversive stimuli by pain and by opioid withdrawal. We found that the protein level of GluA1 in the central nucleus of amygdala (CeA) was significantly increased in rats under persistent pain and viral upregulation of CeA GluA1 increased pain responses of both hyperalgesia and allodynia in rats. In contrast, the viral upregulation of CeA GluA1 inhibited, while knockdown of CeA GluA1 enhanced, place aversion induced by naloxone-precipitated morphine withdrawal. These results reveal a differential action of CeA GluA1 on the aversive event of sensory pain and opioid withdrawal, likely reflecting two distinct synaptic circuits of GluA1-predominant AMPA receptors within CeA for regulation of pain sensitivity and emotional response to opioid withdrawal.
Keywords: Morphine, conditioned place aversion, AMPA receptors, pain
Introduction
The amygdala complex, particularly the central nucleus of amygdala (CeA) and the basolateral amygdala (BLA), plays a critical role in mediating emotional responses to both negative events (such as pain and fear) and positive events such as drug reward.1–3 CeA, as the major output of the amygdala complex, receives predominant glutamatergic inputs both from peripheral sites that transmit pain signal and from BLA that functions as a hub conveying processed commanding signal from higher corticolimbic structures.4–8 As such, CeA has been shown to modulate behavioral responses to negative emotion-associated sensory pain and to positive emotion-associated opioid reward.8–10 Amygdala mediates these behavioral responses largely by associative leaning and consolidation of the emotional events.11,12
Central glutamate receptors, particularly glutamate AMPA receptors (AMPARs), are essential in all neuroplasticity involved in normal brain functions such as learning and memory and in the development of neurological diseases including opioid addiction and chronic pain.2,8,13,14 AMPARs are especially crucial for learning and memory of emotional events through activity-dependent synaptic strengthening via recomposition of GluA1 and GluA2 subunits.2,15–21 This AMPAR strengthening is achieved by switching from low conductance, GluA2-containing AMPARs to high conductance, homomeric GluA1 AMPARs.15,21–25 Thus, increased expression of GluA1 subunits enhances AMPAR signaling and strengthened AMPARs increase synaptic response and neuronal excitability, promoting related behaviors.2,17,18,26,27
Pain as an aversive experience is often associated with negative emotion such as aversion and anxiety.28–31 While opioid as a rewarding drug produces positive emotion of euphoria, opioid withdrawal induces a series of negative emotional events including dysphoria and aversion.32–34 Within CeA, two distinct inputs have been identified: while the input from the parabrachial nucleus (PBN) relays peripheral pain signals,8,35,36 the input from BLA carries modulatory signals from corticolimbic structures after evaluating and decision-making processes of emotional events.9,11 Importantly, both the PBN-CeA input and the BLA-CeA input are glutamatergic,6,19,37,38 and glutamate neurotransmission in CeA has been implicated in both opioid addiction and pain.8,13,14 However, it is still largely unknown how activity of the AMPARs in the two CeA glutamate inputs affects pain sensitivity and behavioral responses to addicting opioids.
In this study, we focused on two different aversive events, pain and opioid withdrawal-induced aversion, and determined how activity in GluA1 subunits of AMPARs impacted and altered these two behavioral responses of aversion in rats under persistent pain or after morphine withdrawal.
Materials and methods
Animals
Male Wistar rats (250–300 g) were used in this study. The rats were housed in groups of three with food and water available ad libitum and in a 12 h light/dark cycle. All behavioral experiments and tests were performed between 8:00 a.m. and 18:00 p.m. To induce a persistent pain condition, a rat received a single intraplantar injection of complete Freund’s adjuvant (CFA, 50 μl) in a hind paw, and experiments were conducted at least one day after the CFA injection. All procedures involving the use of animals conformed to the guidelines set by the Institutional Animal Care and Use Committee of MD Anderson Cancer Center.
Tests for thresholds of thermal pain and mechanical pain
All rats were extensively handled and habituated to the test environment and test apparatus for three days before the pain test. In the tail–flick test, latency to a radiant heat stimulus applied to the tail was measured every 10 min. To elicit tail–flick responses within a reliable range without a floor effect or skin damage, the heat intensity was adjusted to obtain stable baseline latencies between 6.5 and 7 s for hyperalgesic response and between 4 and 4.5 s for analgesic response. The cutoff time was 12 s. In the paw-withdrawal test for thermal hyperalgesia, a rat was placed in a Plantar Test Instrument (Model 37370, Ugo Basile, Italy), and paw-withdrawal response to an infrared heat stimulus was measured with a Hargreaves apparatus. Latency in seconds from the onset of the heat stimulus to the paw withdrawal was recorded automatically by the apparatus as threshold and was measured twice with a 5-min interval. In the test for mechanical allodynia, the rat was placed in a plastic box with mesh floor and allowed to acclimate for 20 min. A series of calibrated von Frey filaments were applied perpendicularly to the plantar surface of a hind paw with sufficient force to bend the filament. A brisk movement of the hind paw (withdrawal or flinching) was considered as a positive response. The threshold (g) of the tactile stimulus producing a 50% likelihood of withdrawal was determined by the “up-down” calculating method.39 The paw-withdrawal response was measured twice with a 5-min interval.
Cannula implantation and intracranial microinjection
General methods for site-specific microinjection were similar to those used in our previous studies.35,40–42 Briefly, a rat was anesthetized with isoflurane and restrained in a stereotaxic apparatus. A 26-gauge, single guide cannula (Plastic One, Roanoke, VA) was inserted on each side of the brain, aiming at CeA (anteroposterior, −2.3 mm from the Bregma; lateral, ±4.0 mm; ventral, −8.0 mm from dura).43 The guide cannula was then cemented in place to the skull and capped after placement of a solid dummy cannula with the same length as the guide cannula. The implanted rat was housed individually and allowed to recover from the surgery for at least 1 week before experiments. Bilateral microinjection of an agent or viral vector (0.5 or 1 μl each side) into CeA was made through a 33-gauge injector with an infusion pump at a rate of 0.1 μl/min. All cannula placements for bilateral CeA injections were histologically verified afterward.
Adeno-associated virus vectors
The construction and their functional validation of adeno-associated virus (AAV) vectors for GluA1 overexpression (AAV-GluA1) and GluA1 knockdown (AAV-GluA1-shRNA) have been described in our previous studies.35,41,44 The vector AAV-green fluorescent protein (GFP) was used as control. An animal was injected with 1 μl (∼5 × 109 genome copy (GC)/μl) AAV-GluA1 virus, or 1 μl (∼2 × 109 GC/μl) AAV-GluA1-shRNA virus into CeA on each side of the brain. All vector effects on GluA1 proteins were confirmed by Western blots. Behavioral experiments were performed 10 days after the virus injection.
Western blot analysis
In separate rat groups one day and three days after the CFA injection, the rat was deeply anesthetized with isoflurane and decapitated. The brain was cut in a vibratome in cold (4°C) artificial cerebrospinal fluid to obtain brain slices (0.5 mm thick). Both sides of CeA were punched out from the slices with a blunt-end syringe needle (0.8 mm inner diameter), frozen in liquid nitrogen, and stored in a −80°C freezer. CeA tissues were gently homogenized in sucrose buffer and centrifuged at 1000 × g. The supernatant was centrifuged at 10,000 g × 20 min, and the synaptosomal pellet was resuspended in 80 μl radioimmunoprecipitation assay (RIPA) lysis buffer. Protein concentration of each sample was determined by the detergent compatible protein assay from Bio-Rad. Equal amounts of protein (25 μg for total protein, 7.5 μg for crude synaptosome) were loaded per lane and separated on an 8% SDS-PAGE gel. The polyvinylidene difluoride membranes with transferred protein were incubated overnight at 4°C with the primary antibody against GluA1 (1:1000, Millipore, Cat. # 05-855 R) and β-actin (1:1000, Santa Cruz Biotechnology, sc-81178). After washes, the blots were incubated with horseradish peroxidase-conjugated secondary antibody (1:10,000; Jackson ImmunoResearch) for 1 h. The blots were developed with ECL plus reagent (GE Healthcare). The densitometric quantification of immunoreactive bands was performed with the AlphaView software (Alpha Innotech Corp.).
Conditioned place aversion
The conditioned place aversion (CPA) procedure for naloxone-precipitated morphine withdrawal was modified from previous reports45,46 and has been described in our previous studies.35,41 CPA tests were conducted in a standard three-chamber apparatus (MED Associates, St. Albans, VT). The CPA procedure included three phases: (1) pretest (day 1) for baseline of preference/aversion behavior, (2) CPA training (days 2–5), and (3) posttest (day 6) for CPA measurement. In the pretest, after habituation to the test chambers, a rat received an injection of saline (1 ml/kg, s.c.), was placed in the center chamber and was allowed to move freely among the chambers for 15 min. The time the rat spent in each of the two conditioning chambers was recorded automatically. After the pretest, animals were randomly divided into four groups: three control groups and one CPA group. In daily injection procedure, a rat received a first injection in its home cage in the morning and 4 h later in the afternoon, it received a second injection and was immediately confined to a chamber for 40 min. On the first conditioning day (day 2), rats of all groups received an injection of saline for both the first and second injections. On the second conditioning day (day 3), the following agents were given for the first and second injections: for the three control groups: saline and saline, morphine (5.6 mg/kg, s.c.) and saline, and saline and naloxone (0.3 mg/kg, s.c.); for the CPA group: morphine and naloxone. The injection/conditioning procedures for days 2 and 3 were repeated on day 4 and 5, respectively. On day 6, all rats underwent a posttest for 15 min. The CPA was expressed as percentage of the time a rat spent in the naloxone-paired chamber versus the total test time (15 min) during the pretest and posttest. AMPA (100 ng in 0.5 μl each side) was bilaterally infused into CeA 15 min before the posttest.
Statistical analysis
Comparisons of averages of two groups were performed with the two-tailed, unpaired Student’s t test. One-way and two-way analysis of variance for repeated measures with post hoc analysis of the Bonferroni method were used to determine statistical significance in behavioral experiments for effects of treatment and between-group interactions at each time point. A p value of <0.05 was considered statistically significant. All statistical analyses were performed with the Prism software version 7.0 (GraphPad Software). Data are presented as mean ± standard error of the mean.
Results
AMPARs in CeA potentiate baseline pain response
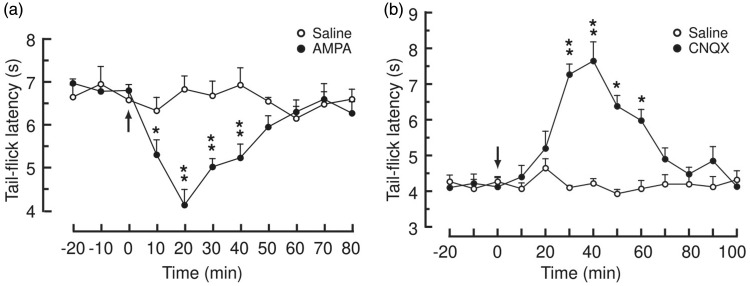
We first examined how glutamate AMPARs in CeA modulated baseline pain behavior. We found that bilateral microinjection of the AMPAR agonist AMPA (100 ng in 0.5 μl each side) into CeA induced a significant decrease in basal pain threshold measured by the tail–flick test in rats when compared to similar CeA microinjection of saline (Figure 1(a)), suggesting that general activation of AMPARs in CeA is pronociceptive, increasing basal sensitivity of pain response. To confirm this pain-enhancing role of CeA AMPARs, we then determined the effect of blocking CeA AMPARs on the pain behavior. In contrast to the effect of AMPA, bilateral CeA microinjection of the AMPAR antagonist cyanquixaline (6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 1.5 μg in 0.5 μl each side) produced a significant antinociceptive effect, increasing the pain threshold (Figure 1(b)). These findings indicate that activity of AMPARs in CeA promotes pain behavior in normal conditions and the pronociceptive AMPAR activity is tonically active, as its removal by AMPAR antagonist decreases basal pain responses.
Figure 1.
Activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in central amygdala increases basal pain response. Basal pain thresholds measured by the tail–flick test before and after bilateral microinjection (arrows) of saline or AMPA (a, 0.1 μg in 0.5 μl each side), or the AMPA receptor antagonist cyanquixaline (6-cyano-7-nitroquinoxaline-2,3-dione (CNQX)) (b, 1.5 μg in 0.5 μl each side) into the central nucleus of amygdala. N = 6 rats in each group. *p < 0.05. **p < 0.01 (two-way analysis of variance with Bonferroni post hoc analysis).
Persistent pain increases GluA1 in CeA
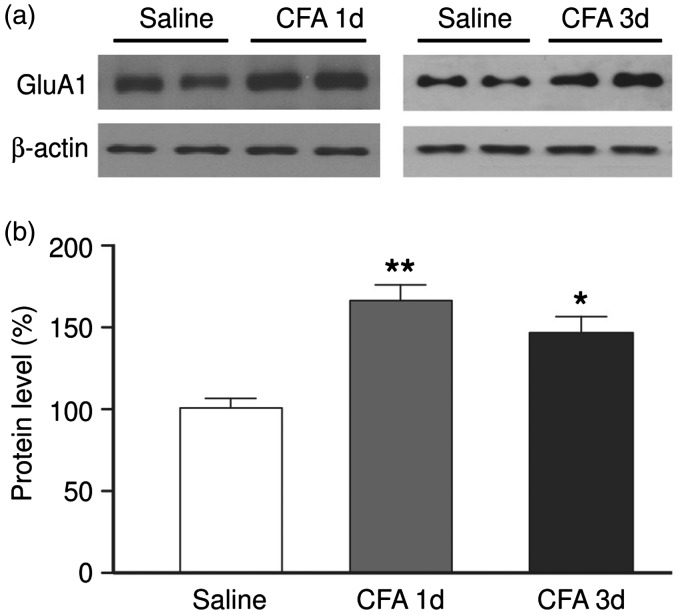
Given the pain-promoting role of CeA AMPARs shown above, we determined whether the AMPAR level changes under pain conditions, measuring the protein level of GluA1, the predominant subunits of AMPARs. In rats with persistent pain induced by an intraplantar injection of CFA (50 μl) as we have shown before,35,41 we found that the protein level of GluA1 was significantly increased one day and three days after CFA injection (Figure 2). This result is consistent with the pain-promoting role of AMPARs and GluA1 subunits in CeA, indicating the possibility that the increase in the activity of CeA AMPARs contributes to the persistent pain conditions.
Figure 2.
Persistent pain increases GluA1 protein in central amygdala. Western blots (a) and normalized group data (b) of GluA1 subunits of AMPAR protein from the CeA of rats on one and three days after an intraplantar injection of saline or CFA (50 μl). N = 5–7 rats per group. *p < 0.05, ** p < 0.01 (one-way analysis of variance).
CFA: complete Freund’s adjuvant.
Overexpression of GluA1 in CeA promotes pain
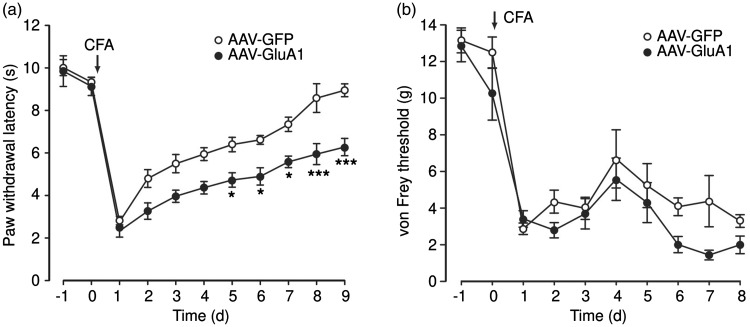
To further validate the causal role of CeA GluA1 in promoting pain response, we used a viral vector AAV-GluA1, which has been constructed and functionally verified in our previous studies,35,41,44 to overexpress the GluA1 subunits of AMPARs locally in CeA, and examined the behavioral effect of this upregulated function of GluA1 and AMPARs in rats under pain conditions. In rats with bilateral infusion of the control vector AAV-GFP into CeA, an intraplantar injection of CFA (50 μl) induced persistent sensitization of inflammatory pain, which lasted more than a week as measured by the paw-withdrawal test for thermal pain of hyperalgesia and by the von Frey test for mechanical pain of allodynia (Figure 3). This CFA-induced pain sensitization is similar to that we reported previously in normal rats.35,41,42 However, in rats with bilateral infusion of the AAV-GluA1 vector for GluA1 overexpression, the CFA-induced hyperalgesia of thermal pain was significantly potentiated throughout the nine-day period tested when compared to the control vector-injected rats (Figure 3). When mechanical pain was measured, the GluA1 vector also significantly increased the CFA-induced pain response of allodynia although to a less extent (p = 0.039, Figure 3). These results provide evidence for a causal role of predominate GluA1-containing AMPARs in CeA in mediating heightened pain response under pain conditions.
Figure 3.
Overexpression of GluA1 in central amygdala increases pain. CFA-induced pain responses for thermal pain of hyperalgesia (a) and mechanical pain of allodynia (b) in rats (n = 6 each group) 10 days after bilateral infusion of control vector AAV-GFP or AAV-GluA1 vector into CeA. *p < 0.05. *** p < 0.001 (two-way analysis of variance with Bonferroni post hoc analysis).
CFA: complete Freund’s adjuvant; AAV: adeno-associated virus; GFP: green fluorescent protein.
Overexpression of GluA1 in CeA inhibits morphine withdrawal-induced aversion
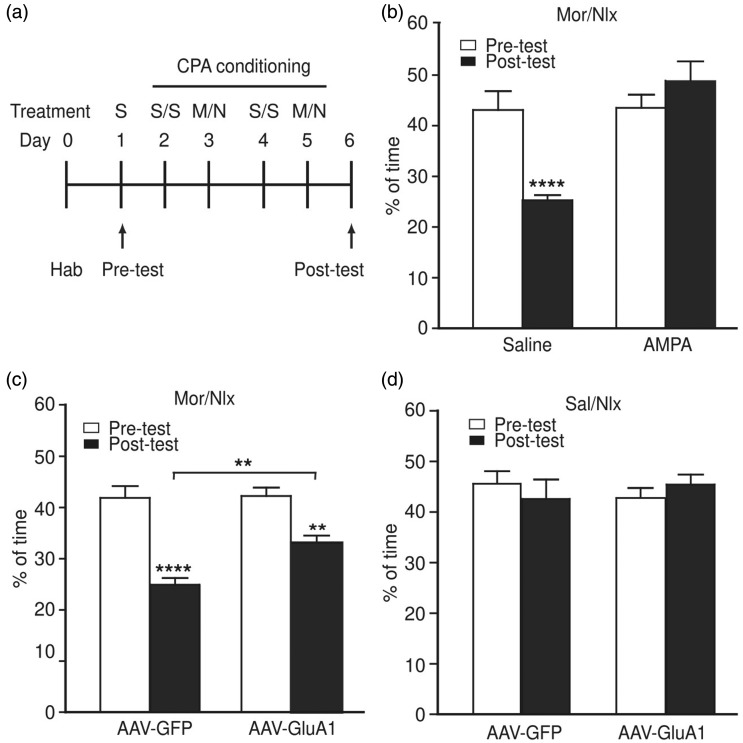
We have shown previously that CeA GluA1 promotes response to opioid reward of positive emotional stimulation and maintain opioid use during opioid withdrawal.35,41 To determine the function of CeA AMPARs in regulation of opioid-related emotional behavior, we then examined the role of CeA GluA1 in response to the negative emotional state induced by opioid withdrawal. Using the paradigm of conditioned place preference (CPP)/CPA in rats with naloxone-precipitated morphine withdrawal (Figure 4(a)), we first conducted the experiments after general activation of CeA AMPARs with CeA-applied AMPA in rats. We found that bilateral microinjection of AMPA (100 ng in 0.5 μl each side) totally blocked the CPA behavior induced by naloxone-precipitated morphine withdrawal (Figure 4(b)), indicating that activation of AMPARs in CeA may inhibit negative aversion behavior under opioid withdrawal.
Figure 4.
Overexpression of GluA1 in central amygdala inhibits morphine withdrawal-induced aversion. (a) Schematic illustration of experimental procedures for induction of CPA by naloxone-precipitated morphine withdrawal. S, saline; M, morphine; N, naloxone; Hab, habituation. (b) CPA behavior in rats with naloxone (nlx)-precipitated morphine (mor) withdrawal after bilateral infusion of saline (n = 6 rats) or AMPA (100 ng each side, n = 8 rats) into CeA. (c, d) CPA behavior in rats with naloxone-precipitated morphine withdrawal (c, n = 9 rats each group) and in rats conditioned with saline (sal) and naloxone (d, n = 6 rats each group) after bilateral infusion of AAV-GFP or AAV-GluA1 into CeA. **p < 0.01, ****p < 0.0001 (two-way analysis of variance).
CPA: conditioned place aversion; AAV: adeno-associated virus; GFP: green fluorescent protein.
To further confirm this role of CeA AMPARs in inhibiting the aversion behavior, we determined the effect of GluA1 overexpression in CeA on morphine withdrawal-induced aversion 10 days after bilateral infusion of AAV-GluA1 or AAV-GFP vector into the CeA of rats. In AAV-GFP-infused control rats, naloxone-precipitated morphine withdrawal induced a significant CPA behavior; however, in AAV-GluA1-infused rats, the aversion behavior was significantly reduced when compared to that in the control rats (Figure 4(c)). In another control experiment, CeA infusion of naloxone alone did not alter the preference/aversion behavior; and interestingly, overexpression of CeA GluA1 itself in the absence of morphine or morphine withdrawal had no effect on the preference/aversion behavior (Figure 4(d)). Thus, it appears that enhanced function of GluA1 AMPARs in CeA inhibits opioid withdrawal-induced negative response of place aversion, but unlike pain response shown above, increasing the function of CeA GluA1 AMPARs per se is ineffective in altering the preference or aversion behavior.
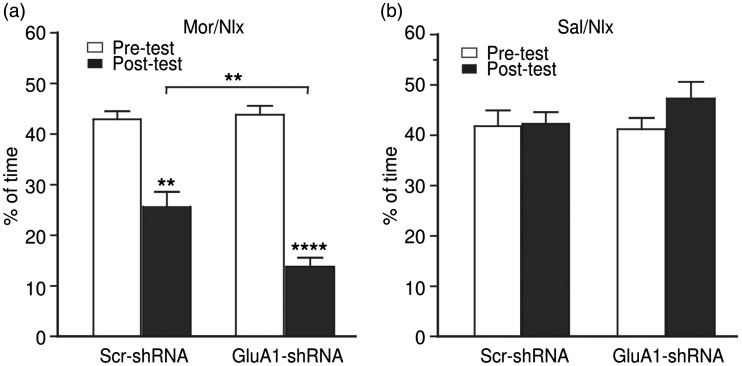
Knockdown of GluA1 in CeA potentiates morphine withdrawal-induced aversion
Finally, we further verified the results of this GluA1 role by knocking down the GluA1 expression in CeA with an AAV vector expressing a short-hairpin interfering RNA against the GluA1-encoding gene Gria1 (AAV-GluA1-shRNA) and a control vector with scrambled shRNA sequence, as we described and validated previously.41,44 We found that CeA infusion of the AAV-GluA1-shRNA vector for GluA1 knockdown failed to change the preference/aversion behavior under normal conditions; however, the GluA1 knockdown significantly increased the withdrawal-induced CPA behavior (Figure 5), an effect opposite to that of GluA1 overexpression shown above. These results further support the notion that the activity of GluA1 AMPARs in CeA reduces the aversive effect of opioid withdrawal.
Figure 5.
Knockdown of GluA1 in central amygdala potentiates morphine withdrawal-induced aversion. CPA behavior in rats with naloxone-precipitated morphine withdrawal (a, n = 8 rats each group) and in rats conditioned with saline and naloxone (b, n = 6 rats each group) after bilateral infusion of AAV-GluA1-shRNA or AAV-GluA1-scrambled (scr) shRNA into CeA. **p < 0.01. ****p < 0.0001 (two-way analysis of variance).
Discussion
In this study, we have shown that GluA1, the predominant subunits of AMPARs, in CeA exerts differential actions on pain and opioid withdrawal-induced aversion, increasing pain response while decreasing aversive effect of opioid withdrawal. These results highlight the nonuniform roles of CeA AMPARs in modulation of emotional events and indicate distinct synaptic circuits of AMPARs in CeA in the modulating process.
CeA modulation of emotional event, including pain, anxiety and drug reward, has been accentuated by recent identification of two functionally distinct glutamatergic inputs onto CeA neurons: the PBN-CeA pathway and BLA-CeA pathway. The PBN-CeA pathway relays pain signals from the spinal cord to CeA8,36,47 and conveys an affective pain signal that induces a threat memory.36 Pain is known as an aversive stimulus and is often associated with negative emotion such as aversion and anxiety.28,30,31 We have shown that CFA-induced persistent pain causes strong and long-lasting (> a month) place aversion in rats.41 Consistent with this function of the PBN-CeA input, we have shown in this study that upregulation of CeA GluA1 directly increases pain sensitivity of thermal hyperalgesia and mechanical allodynia. Thus, it appears that GluA1 in the AMPAR synapses of the PBN-CeA input functions to transmit signals of pain and associated negative emotion of aversion and anxiety. On the other hand, the BLA-CeA pathway carries processed signals from higher corticolimbic structures about evaluation and decision information on emotional events and its activation inhibits negative emotion of anxiety.6 We have shown in this study that upregulation of CeA GluA1 reduces opioid withdrawal-induced aversion. Thus, it is likely that GluA1 in the AMPAR synapses of the BLA-CeA input functions to inhibit negative emotion of anxiety and opioid withdrawal. While the viral upregulation of CeA GluA1 in this study is not pathway-specific, the notion of distinct functions of the PBN-CeA and BLA-CeA glutamate pathways in CeA modulation of emotional events is demonstrated by our recent pathway-specific study in which we show that, while selective optogenetic activation of the PBN-CeA pathway causes behaviors of negative emotion including aversion, anxiety and depression in normal rats, optogenetic activation of the BLA-CeA pathway opposes these behaviors of negative emotion, inhibiting anxiety and depression and promoting positive emotion of opioid reward.35 Therefore, the distinct functions of the two glutamatergic CeA inputs likely underlie the current results of increasing aversive pain but reducing aversive opioid withdrawal by nonselective viral upregulation of GluA1 in CeA.
Adaptive change in GluA1 of AMPARs is an integrate part of central synaptic plasticity involved in both normal brain functions such as learning and memory and emotion-related pathological conditions including pain and drug addiction.15 Under those conditions, synaptic signaling of AMPARs is strengthened by adaptive upregulation of GluA1 subunits relative to GluA2 subunits through subunit recomposition of AMPARs in glutamate neurotransmission.22,23 Thus, pain conditions have been shown to elevate the GluA1 level and associated AMPAR function in central pain-processing sites.48–50 Our present results show that upregulation of CeA GluA1 is sufficient to increase pain, suggesting a causal role of CeA GluA1 and AMPARs in promoting pain response. GluA1 expression is also increased by addicting drugs of abuse such as morphine and cocaine in the amygdala and other structures of the brain’s reward circuits.51,52 Upregulation of GluA1 in the ventral tegmental area potentiates the rewarding effect of morphine53 and as we have shown before, CeA-applied AMPA is rewarding by inducing CPP and GluA1 upregulation in CeA facilitates associative learning and acquisition of morphine reward.44 Opposite to opioid reward, opioid withdrawal is associated with aversion and other negative emotions, nevertheless, GluA1 level in CeA, as we reported recently,41 is also increased during morphine withdrawal in rats. While this increase might be related to associative learning of the withdrawal condition, knockdown of CeA GluA1 inhibits morphine-seeking behavior after morphine withdrawal, suggesting that CeA GluA1 maintains morphine-seeking behavior after opioid withdrawal,41 which is consistent with a general, reward-promoting role of CeA GluA1. Thus, it appears that GluA1 in CeA promotes opioid use after opioid withdrawal as reflected in its effects of both maintaining opioid seeking and reducing withdrawal-induced aversion.
A quite interesting question is how the two input-specific circuits of GluA1 AMPARs in CeA that differentially regulate pain and opioid-related emotional responses interact and influence each other. The synaptic connections between the two AMPAR circuits or the two CeA glutamate inputs are still unknown. Nevertheless, we have recently shown that the direct interaction most likely occurs within CeA, as activating the BLA-CeA pathway inhibits various negative emotions induced by activation of the PBN-CeA pathway, suggesting two parallel and interacting AMPAR synaptic circuits and neuronal populations within CeA.35 Previous studies also suggest circuit-specific and neuron-specific encoding of negative and positive behavioral outcomes in amygdala.6,9 Thus, these AMPAR circuits and neurons in CeA, the major output of the amygdala complex, receive and process the signal of peripheral pain and associated emotion, which is then integrated with the modulating signal from higher corticolimbic structures via BLA for the ultimate output signal that regulates both pain sensitivity and emotional responses to emotion-affecting events such as opioid use.
In summary, we have shown in this study that, while GluA1 upregulation in CeA is sufficient to increase the aversive response of pain, it decreases the aversion of opioid withdrawal, revealing two differential modulating effects of CeA GluA1 on pain sensitivity and on emotional aspect of opioid withdrawal. These results highlight the diverse functions of GluA1-dominant AMPARs in CeA in regulation of different aspects of emotion-associated stimuli and behavioral conditions.
Acknowledgments
The authors would like to thank Drs. Zhi Zhang and Wei Wang for their technical support and helpful academic discussions during the course of this study.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (grants DE025943 and NS113256).
ORCID iD
Zhizhong Z Pan https://orcid.org/0000-0002-4022-8750
References
- 1.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci 2002; 3: 563–573. [DOI] [PubMed] [Google Scholar]
- 2.Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron 2005; 47: 783–786. [DOI] [PubMed] [Google Scholar]
- 3.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci 2009; 10: 423–433. [DOI] [PubMed] [Google Scholar]
- 4.Fernando AB, Murray JE, Milton AL. The amygdala: securing pleasure and avoiding pain. Front Behav Neurosci 2013; 7: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 2003; 301: 1104–1107. [DOI] [PubMed] [Google Scholar]
- 6.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 2011; 471: 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 2012; 35: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol 2015; 227: 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature 2015; 517: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci (Regul Ed) 2007; 11: 489–497. [DOI] [PubMed] [Google Scholar]
- 11.Bocchio M, Nabavi S, Capogna M. Synaptic plasticity, engrams, and network oscillations in amygdala circuits for storage and retrieval of emotional memories. Neuron 2017; 94: 731–743. [DOI] [PubMed] [Google Scholar]
- 12.Letzkus JJ, Wolff SB, Luthi A. Disinhibition, a circuit mechanism for associative learning and memory. Neuron 2015; 88: 264–276. [DOI] [PubMed] [Google Scholar]
- 13.Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci 2000; 909: 12–24. [DOI] [PubMed] [Google Scholar]
- 14.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016; 3: 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron 2010; 67: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 2002; 25: 103–126. [DOI] [PubMed] [Google Scholar]
- 17.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 2010; 330: 1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole CJ, Mercaldo V, Restivo L, Yiu AP, Sekeres MJ, Han JH, Vetere G, Pekar T, Ross PJ, Neve RL, Frankland PW, Josselyn SA. MEF2 negatively regulates learning-induced structural plasticity and memory formation. Nat Neurosci 2012; 15: 1255–1264. [DOI] [PubMed] [Google Scholar]
- 19.McCool BA, Christian DT, Diaz MR, Lack AK. Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. Int Rev Neurobiol 2010; 91: 205–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat Rev Neurosci 2010; 11: 675–681. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 2007; 23: 613–643. [DOI] [PubMed] [Google Scholar]
- 22.Isaac JTR, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 2007; 54: 859–871. [DOI] [PubMed] [Google Scholar]
- 23.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci 2007; 8: 844–858. [DOI] [PubMed] [Google Scholar]
- 24.Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 1995; 15: 193–204. [DOI] [PubMed] [Google Scholar]
- 25.Polgar E, Watanabe M, Hartmann B, Grant SG, Todd AJ. Expression of AMPA receptor subunits at synapses in laminae I-III of the rodent spinal dorsal horn. Mol Pain 2008; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watabe AM, Ochiai T, Nagase M, Takahashi Y, Sato M, Kato F. Synaptic potentiation in the nociceptive amygdala following fear learning in mice. Mol Brain 2013; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 2011; 13: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci 2004; 5: 565–575. [DOI] [PubMed] [Google Scholar]
- 29.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain 2003; 106: 127–133. [DOI] [PubMed] [Google Scholar]
- 30.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000; 288: 1769–1772. [DOI] [PubMed] [Google Scholar]
- 31.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005; 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuckit MA. Treatment of opioid-use disorders. N Engl J Med 2016; 375: 357–1597. [DOI] [PubMed] [Google Scholar]
- 33.Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 2001; 81: 299–343. [DOI] [PubMed] [Google Scholar]
- 34.Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry 2020; 87: 44–53. [DOI] [PubMed] [Google Scholar]
- 35.Cai YQ, Wang W, Paulucci-Holthauzen A, Pan ZZ. Brain circuits mediating opposing effects on emotion and pain. J Neurosci 2018; 38: 6340–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an affective pain circuit that creates a threat memory. Cell 2015; 162: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 2016; 17: 485–496. [DOI] [PubMed] [Google Scholar]
- 38.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci (Regul Ed) 2011; 15: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, Tao W, Hou YY, Wang W, Kenny PJ, Pan ZZ. MeCP2 repression of G9a in regulation of pain and morphine reward. J Neurosci 2014; 34: 9076–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bie B, Zhang Z, Cai YQ, Zhu W, Zhang Y, Dai J, Lowenstein CJ, Weinman EJ, Pan ZZ. Nerve growth factor-regulated emergence of functional delta-opioid receptors. J Neurosci 2010; 30: 5617–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou YY, Cai YQ, Pan ZZ. Persistent pain maintains morphine-seeking behavior after morphine withdrawal through reduced MeC. P2 repression of GluA1 in rat central amygdala. J Neurosci 2015; 35: 3689–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 2011; 17: 1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed Sydney: Academic Press, 1986. [Google Scholar]
- 44.Cai YQ, Wang W, Hou YY, Zhang Z, Xie J, Pan ZZ. Central amygdala GluA1 facilitates associative learning of opioid reward. J Neurosci 2013; 33: 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003; 170: 42–50. [DOI] [PubMed] [Google Scholar]
- 46.White DA, Hwang ML, Holtzman SG. Naltrexone-induced conditioned place aversion following a single dose of morphine in the rat. Pharmacol Biochem Behav 2005; 81: 451–458. [DOI] [PubMed] [Google Scholar]
- 47.Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci 2001; 2: 83–91. [DOI] [PubMed] [Google Scholar]
- 48.Guan Y, Guo W, Zou SP, Dubner R, Ren K. Inflammation-induced upregulation of AMPA receptor subunit expression in brain stem pain modulatory circuitry. Pain 2003; 104: 401–413. [DOI] [PubMed] [Google Scholar]
- 49.Larsson M, Broman J. Translocation of GluR1-containing AMPA receptors to a spinal nociceptive synapse during acute noxious stimulation. J Neurosci 2008; 28: 7084–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 2008; 28: 7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci 1996; 16: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glass MJ, Kruzich PJ, Colago EE, Kreek MJ, Pickel VM. Increased AMPA GluR1 receptor subunit labeling on the plasma membrane of dendrites in the basolateral amygdala of rats self-administering morphine. Synapse 2005; 58: 1–12. [DOI] [PubMed] [Google Scholar]
- 53.Carlezon WA, Jr, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ. Sensitization to morphine induced by viral-mediated gene transfer. Science 1997; 277: 812–814. [DOI] [PubMed] [Google Scholar]