Abstract
Objective:
Accelerated brain aging has been proposed to explain cancer-related cognitive impairment, but empirical evidence for this relationship is lacking. The purpose of this study was to evaluate amyloid beta (Aβ) and tau, biomarkers of neurodegeneration, in relation to cognition in breast cancer survivors (BCSs). We explored relationships among peripheral concentrations of Aβ42, Aβ-40, tau, and cytokines; cognitive function; and psychosomatic symptoms in a cohort of BCSs post-chemotherapy.
Methods:
This secondary analysis of a cross-sectional study was conducted with 65 BCSs. Serum total Aβ-42, Aβ-40, and tau levels were measured with single molecule array technology. Cytokines (interleukin [IL]-6, tumor necrosis factor [TNF]-α, granulocyte-macrophage colony-stimulating factor [GM-CSF], interferon [IFN]-g, IL-10, IL-12, IL-13, IL1-b, IL-2, IL-4, IL-5, IL-7, and IL-8) were simultaneously measured in serum using multiplex assays. Cognitive function was measured with five standardized neuropsychological tests and psychosomatic symptoms (stress, loneliness, anxiety, depressive symptoms, fatigue, sleep quality, and daytime sleepiness) with self-report questionnaires. Data analyses included correlations and random forest regression (RFR).
Results:
Significant correlations were identified among hip-to-waste ratio, number of treatment modalities, Aβ-42, Aβ-40, and tau levels (rs = .27–.35, ps < .05). RFR modeling including Aβ-42, Aβ-40, tau, and cytokines as features explained significant variance in cognitive function (R 2 = .71, F = 9.01, p < .0001) and psychosomatic symptoms (R 2 = .74, F = 10.22, p < .0001).
Conclusions:
This study suggests that neurodegenerative biomarkers interact with cytokines to influence cognitive functioning and psychosomatic symptoms in BCSs following chemotherapy, but additional research is needed.
Keywords: amyloid beta, tau, cancer-related cognitive impairment, psychosomatic symptoms, cytokines, breast cancer survivors
Cancer and its treatment are associated with cognitive dysfunction that can last months to years after treatment ends (Amidi et al., 2015; Janelsins, Kesler, Ahles, & Morrow, 2014; Koppelmans et al., 2012; Wefel, Kesler, Noll, & Schagen, 2015). Researchers have proposed several mechanisms to explain cancer-related cognitive impairment (CRCI), including both the direct and indirect effects of chemotherapy (Ahles, Root, & Ryan, 2012; Ahles & Saykin, 2007), but the exact mechanisms are unknown. Some researchers have proposed that cancer pathology and cancer treatment may accelerate natural aging processes, including brain aging, which may result in neurodegenerative processes and subsequent CRCI (Mandelblatt et al., 2013).
Older cancer patients tend to have poorer cognitive outcomes following chemotherapy than younger patients (Ahles et al., 2010), and gray-matter atrophy following chemotherapy is analogous to approximately 4 years of aging in the brain (Koppelmans et al., 2012). Breast cancer (BC) patients treated with chemotherapy have exhibited decreased resilience to simulated neurodegeneration in comparison with controls (S. R. Kesler, Watson, & Blayney, 2015), and cancer survivors with cognitive complaints have shown slower electroencephalogram patterns than patients without these complaints, a characteristic of advancing age (Hunter et al., 2014). A large epidemiological study found an increased likelihood for a dementia diagnosis in breast cancer survivors (BCSs) treated with chemotherapy compared to those who were chemotherapy naive (Heck, Albert, Franco, & Gorin, 2008). However, questions remain regarding the overlap between mechanisms of CRCI and the neurodegenerative processes that occur in dementia.
Researchers have also made connections between inflammatory markers as proxies of chronic inflammation and cellular aging (Cheung et al., 2015; Ganz et al., 2013; Janelsins et al., 2012; S. Kesler et al., 2013; Patel et al., 2015; Pomykala et al., 2013; Vardy et al., 2017; Williams et al., 2018) and APOE e4 genotypes (Ahles et al., 2003), reduced telomerase activity and DNA damage (Carroll et al., 2019), and CRCI in BCSs. Together, these studies suggest that cancer and/or its treatment may cause systemic cellular damage related to inflammation that interferes with cognitive processing. Research has also implicated the inflammatory response in the development of dementia (Lai et al., 2017). For example, the level of peripheral interleukin (IL)-6 has been associated with amyloid precursor protein (APP) metabolism and has been reported to correlate with cerebrospinal fluid (CSF) concentrations (Lai et al., 2017) in persons with dementia and cognitive impairment. CRCI may be at the intersection of cancer and neurodegeneration or accelerated brain aging. However, mechanistic research on specific neurodegenerative processes underlying CRCI is needed.
Amyloid beta (Aβ) peptides are a main component of plaque structures, and tau is found in the neurofibrillary tangles in the brains of persons with Alzheimer’s disease (AD). Although Aβ and tau are typically studied in relation to neurodegenerative diseases, they are not specific to neurodegeneration (Olsson et al., 2016). Researchers now recognize them as biomarkers of brain injury, and they should be considered within the context of cancer pathology and the long-term effects of cancer and its treatment. Although investigators have studied altered Aβ expression more often in relation to neurodegenerative disease, they have also reported it across many cancer types including BC (Pandey et al., 2016). APPs are overexpressed in many cancers and have been linked to tumor characteristics such as the abnormal growth, invasion, and migration of cells. In preclinical models, researchers have found increased expression of APP in BC cell lines, especially in those that possess higher metastatic potential, reporting significant correlations between the level of APP and tumor development in BC tissues (Lim et al., 2014). Studies have linked tau to metastatic BC as well (Darlix et al., 2019).
Because tau and Aβ are both expressed by BC cells (Bonneau, Gurard-Levin, Andre, Pusztai, & Rouzier, 2015; Lim et al., 2014), it is possible that Aβ and tau may contribute to the underlying mechanisms of CRCI, but empirical evidence for these relationships is lacking.
Furthermore, activated immune pathways and chronic inflammation can promote prion replication, including Aβ (Eisele et al., 2010), suggesting a potential bidirectional relationship between inflammation and Aβ. It is also possible that BC treatments cause damage in the periphery, resulting in cellular production or release of these peptides. Taken together, BC and its treatments may increase risk for Aβ- and tau-related pathologies either in the periphery or central nervous system, thereby contributing to the underlying mechanisms of CRCI, but empirical evidence is lacking. Aβ-42, Aβ-40, and tau have also been identified as correlates of the severity of psychological symptoms including anxiety and depression (Pattinson et al., 2019) and symptoms of posttraumatic stress disorder (Clouston et al., 2019) within clinical populations. Given the high prevalence of CRCI in BCSs and given its unclear mechanisms, it is a logical next step to evaluate Aβ and tau in relation to cognitive functioning in BC patients.
Until recently, researchers could measure Aβ peptides and tau only in brain tissue or CSF. Aβ-40 is the most prominent Aβ peptide; Aβ-42 is the neurotoxic form (Jagtap, Gawande, & Sharma, 2015). Aβ-42 can cross the blood–brain barrier bidirectionally (Blasko et al., 2008). Aβ-42 and Aβ-40 are both found in peripheral blood (Janelidze et al., 2016), but it has been unclear how peripheral levels of Aβ are related to concentrations of these proteins in CSF and the brain. Today, ultrasensitive immunoassay techniques show promise for measuring minute amounts of Aβ and tau in peripheral blood samples (Jack et al., 2018; Rissin et al., 2010; Zetterberg et al., 2013). Researchers have reported small-to-medium relationships between peripheral Aβ-40 and Aβ-42 and brain Aβ levels (Fandos et al., 2017; Janelidze et al., 2016). Investigators have observed elevated levels of plasma Aβ-42 among presymptomatic individuals who are genetically predisposed to neurologic disease (Park et al., 2017) and among women with mild cognitive impairment (MCI) in comparison with both controls and patients with AD (Assini et al., 2004; Sobow, Flirski, Kloszewska, & Liberski, 2005). Similarly, research has revealed associations between peripheral tau levels and neurodegeneration and cognitive decline in cognitively unimpaired and impaired older adults (Dage et al., 2016; Mielke et al., 2017).
It is currently unknown whether circulating Aβ found in peripheral blood (plasma or serum) originates from the brain or from peripheral tissues (or both; Blasko et al., 2008) or how peripheral Aβ-42, Aβ-40, and tau relate to the central nervous system. Some authors have suggested that these proteins move across the blood–brain barrier bidirectionally (Blasko et al., 2008; Frankfort et al., 2008) and others that peripheral Aβ and tau represent systemic pathology (Olsson et al., 2016). Peripheral expression of Aβ likely comes from platelets, which have the highest expression of APP among peripheral tissues (Frankfort et al., 2008). Peripheral measurement of Aβ and tau is not as sensitive as CSF measurements, but blood-based assays are much less invasive and more feasible to assess. Therefore, moving the science forward on peripheral measurement of these proteins could result in increased accessibility and widespread utility.
It is possible that complex interactions between circulating Aβ and/or tau and cytokines are related to cognitive performance and/or psychosomatic symptoms in BCSs. To our knowledge, no one has evaluated peripheral Aβ-42, Aβ-40, or tau in BCSs with a history of chemotherapy treatment. Therefore, the purpose of the present study was to explore relationships among concentrations of Aβ-42, Aβ-40, tau, and cytokines and cognitive function and psychosomatic symptoms in a cohort of 65 BCSs at 6 months to 10 years post-chemotherapy.
Materials and Methods
Study Design and Participants
The present study is a secondary analysis of data from an original cross-sectional study conducted from 2016 to 2017. We recruited female BCSs between the ages of 21 and 65 years from community oncology centers, BC patient navigators, the local chapter of the Oncology Nursing Society, and the Army of Women (Dr. Susan Love Research Foundation). All participants had completed chemotherapy treatment from 6 months to 10 years prior to enrollment and had a history of Stage I–IIIc BC. We included women of multiple races and ethnicities along with those being treated with endocrine therapy. In addition, we included women with a range of cognitive abilities to ensure variability in cognitive performance. We excluded participants if they were on systemic steroids or medications for autoimmune disease, if a health-care provider had diagnosed them with inflammatory comorbidities, if they had a precancer history of sleep disorders, or if they had psychiatric disorders that would interfere with their ability to complete surveys and cognitive testing.
Data Collection and Measurements
All data were collected for each participant during a single visit (A. M. Henneghan, Carter, Stuifbergan, Parmelee, & Kesler, 2018; A. M. Henneghan, Palesh, Harrison, & Kesler, 2018; A. Henneghan, Stuifbergen, Becker, Kesler, & King, 2018). The purpose of the parent study was to identify modifiable predictors of inflammation and cognitive function in BCSs. Study procedures included determining eligibility through prescreening and obtaining verbal consent from participants, then scheduling participants for in-person data collection. Prior to their data collection appointments, participants completed demographic and clinical history and self-report questionnaires. In-person appointments were scheduled within 4 hr of participants’ waking in the morning. Participants completed cognitive tests, blood draws, and anthropomorphic measurements (height, weight, hip, and waist measurements) with trained research personnel, according to standardized procedures. The Institutional Review Board at the University of Texas at Austin approved the study (Protocol # 2015-10-0039), and we conducted it in accordance with the Declaration of Helsinki. All participants provided written consent.
Demographic and clinical variables
We used a self-report questionnaire to collect demographic (e.g., age, education, race, ethnicity) and clinical information (e.g., disease characteristics, treatment history, menopausal status). We measured height to the nearest 0.5 cm and weighed all participants with the same digital scale (Tanita Model WB-300 Plus Arlington Heights, IL) to the nearest 100 g. From weight in kilogram and height in centimeter (cm), we calculated body mass index (BMI). We measured waist circumference (cm) between the 12th rib and the iliac crest and hip circumference (cm) around the buttocks at the maximum extension and calculated the hip-to-waist ratio (HWR).
Aβ-42, Aβ-40, tau, and cytokines
Nonfasting blood was collected into serum separator tubes (BD, Franklin Lakes, NJ), 1–4 hr after participants woke in the morning. Blood was allowed to clot for 30–120 min and then centrifuged at 3,330 rpm for 15 min. Serum was aliquoted with filtered pipettes into 1-ml polypropylene tubes and stored at −80°C. Serum total Aβ-42, Aβ-40, and tau levels were measured with a single molecule array (Simoa) HD-1 Analyzer (Instrument ID: 2710010048 STD RUO), using Simoa Human Neurology 3-Plex A ([N3PA], Quanterix, Product Number 101995, Lot 501427) two-step digital immunoassays. This technology measures the quantity of each analyte, as described elsewhere (Andreasson, Blennow, & Zetterberg, 2016). Samples were thawed at room temperature, mixed thoroughly until visibly homogenous via brief vortexing, and centrifuged at 14,000 g for 3 min and diluted according to manufacturer’s recommendations. Calibrators were assayed in triplicate; controls, in duplicate. The Simoa N3PA kit uses four-parameter logistic curve-fit data reduction (with 1/y 2 weighting) to generate calibration curves. Two reference controls were tested to verify the validity of the calibration curve for Aβ-42, Aβ-40, and tau, and two replicates were performed for each control. Controls were within the expected range for the performed run, and coefficients of variation (CVs) were less than 20% for the three analytes.
Human high-sensitivity T cell magnetic, premixed, multiplex assays from EMD Millipore (Darmstadt, Germany) were used for 13 analytes involved in the inflammatory process: interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFN-γ), IL-10, IL-12, IL-13, IL-1β, IL-2, IL4, IL-5, IL-7, and IL-8. These cytokines, which are a mix of pro-inflammatory (TNF-α, GM-CSF, INF-γ, IL-2, IL-1β, IL-5, IL-7, IL-8), anti-inflammatory (IL-10, IL-13), and both pro/anti-inflammatory (IL-6, IL-2, IL-4; Yoshimoto & Yoshimoto, 2014), were chosen based on the original study aims that evaluated IL-6 and TNF-α based on prior CRCI research (S. Kesler et al., 2013; Pomykala et al., 2013). We chose to use a commercially available multiplex assay that included the other 11 cytokines and decided to include all cytokines in these exploratory analyses because cytokines are part of a complex inflammatory network (Yoshimoto & Yoshimoto, 2014). Standards, controls, and samples were added to a 96-well plate and run in duplicate according to the manufacturer’s recommendations. Plates were read using a Luminex 200 (Luminex Corp., Austin, TX) to determine mean fluorescent intensity for each well. Using five-parameter logistic regression, we constructed a standard curve, which we used to calculate control and sample unknown concentrations.
Cognitive outcomes
The following well-established, valid, reliable cognitive measures were used in accordance with the International Cancer and Cognition Task Force Recommendations (Wefel, Vardy, Ahles, & Schagen, 2011): the Hopkins Verbal Learning Test, Revised, a measure of immediate (HVLT-I) and delayed (HVLT-D) verbal memory (Benedict, Schretlen, Groninger, & Brandt, 1998); Trail Making Test A (Trails A) and Trail Making Test B (Trails B), measures of processing speed, executive function, attention, and cognitive flexibility (Tombaugh, 2004); and the Controlled Oral Word Association Test (COWA), a measure of verbal fluency and word finding (Benton, deS Hamsher, Varney, & Spreen, 1983), were all administered per standardized protocols and scored by two trained research staff members. Scores, adjusted for age and education based on established norms, with standard deviations (SDs), percentiles, or T-scores, described the sample. Self-reported cognitive impairment was also measured, but we have not reported it here.
Psychosomatic variables
Patient-Reported Outcomes Measurement Information System (PROMIS; Cella et al., 2010) short forms were used to measure anxiety, depression, and fatigue (Emotional Distress–Anxiety–Short Form 8a, Emotional Distress–Depression–Short Form 8a, and Fatigue–Short Form 8a [PROMIS, 2017]). The UCLA Loneliness Scale, Revised, Version 3 (UCLA-R; Russell, 1996) was used to measure loneliness. The Perceived Stress Scale was used to measure stress (Cohen, Kamarck, & Mermelstein, 1983; Statistics, 2009); the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) was used to measured sleep quality; and the Epworth Sleepiness Scale, an 8-item instrument, was used to measure daytime sleepiness (Enderlin et al., 2011). Higher scores on these scales indicated greater symptom severity, greater feelings of loneliness and stress, greater daytime sleepiness, and worse sleep quality.
Data Analysis
Descriptive statistics were calculated to describe clinical, cognitive, psychosomatic, and biological variables. Almost all of the distributions for the biological variables (Aβ-42, Aβ-40, tau, and cytokines) were skewed per significant Shapiro–Wilk tests, so these variables were natural log transformed (ln) prior to bivariate analyses. Aβ-42/Aβ-40 ratios were calculated by dividing lnAβ-42 pg/ml by lnAβ-40 pg/ml. We used composite scores to evaluate neuropsychological (NP) function (NP composite) and psychosomatic symptoms (Psych composite) because all psychosomatic symptoms and all NP test scores were highly correlated with each other. This strategy also decreased the number of variables and subsequent statistical tests in our analyses, limiting Type I error. Raw scores on individual tests were converted to z-scores (so that higher scores indicated better performance) to create the NP composite (mean of z-scores for all five tests). The Psych composite was created from the three PROMIS scales, UCLA-R, PSS, PSQI, and ESS using principal component analysis (Henderson, Fisher, Cohen, Waltzman, & Weber, 1990). The first component score from the within-class pooled variance–covariance matrix was chosen to establish the coefficients for the composite scores. This component accounted for 54% of the total variance of the measures.
We first examined two-tailed correlations between (a) Aβ-42, Aβ-40, tau, and Aβ-42:40 ratios and (b) demographic and clinical variables known to be risk factors for cognitive impairment (e.g., BMI, age) and the cytokine panel (IL-6, TNF-α, GM-CSF, IFN-g, IL-10, IL-12, IL-13, IL1-b, IL-2, IL-4, IL-5, IL-7, IL-8) simultaneously. Because our study sample included women with a wide range of ages and time since chemotherapy, both of which could influence NP or Psych composites, we examined Pearson’s correlations among all the key variables (Aβ-42, Aβ-40, tau, NP composite, Psych composite, IL-6, TNF-α, GM-CSF, IFN-g, IL-10, IL-12, IL-13, IL1b, IL-2, IL-4, IL-5, IL-7, IL-8), age, and time since chemotherapy. We also examined Pearson’s correlations between years of education and the key variables because we used raw NP scores in our analyses. The significance level was set at .05 for these exploratory analyses.
Our previous work has demonstrated significant nonlinear biomarker–behavioral variable relationships in cancer survivors (A. M. Henneghan, Palesh, Harrison, & Kesler, 2018; S. R. Kesler, Gugel, Huston-Warren, & Watson, 2016). Machine-learning approaches, which include nonparametric modeling options and the ability to learn complex interactions among predictors with nonlinear relationships (Jordan & Mitchell, 2015), tend to perform better for nonlinear relationships, in addition to analyses involving a small number of cases with a large number of predictors (Witten & Tibshirani, 2010). Since we were interested in evaluating a large number of predictors (21 biological and clinical predictors) in a relatively small samples size (N = 65), we supplemented our correlation analyses with random forest regression (RFR). RFR involves an ensemble of decision trees grown from random subsets of features in order to predict a continuous dependent variable (Breiman, 2001; Breiman, Friedman, Olshen, & Stone, 1984). RFR evaluates the relative importance of many predictors in a data set without data reduction or model overfitting. RFR inherently accounts for interactions (linear and nonlinear) of predictors when modeling the dependent variables (Breiman, 1996) and provides protection against the impact of predictor collinearity, which was present in this study (Matsuki, Kuperman, & Van Dyke, 2016).
We conducted two separate RFR models with NP Psych composite scores as dependent variables. The predictors or “features” for the model included Aβ40, Aβ-42, tau, IL-7, IL-2, IL-10, IL-1β, TNF-α, IL-8, GM-CSF, age, education, HWR, BMI, and time since chemotherapy. We included both HWR and BMI in the model because they were not collinear. RFR model parameters included 1,000 trees and “mtry” (number of possible directions available for splitting at each node of tree) equal to the square root of the number of features and out-of-bag error estimation, which provides intrinsic cross-validation (Breiman, 1996). Increase in node purity (Epifanio, 2017) was calculated to determine feature importance. Higher values indicate greater importance of a variable to the model. We used a threshold of 1 SD above the mean for determining the most important model features. It is important to note that RFR estimates of variable importance can be interpreted only in relation to the variables in the model, not as absolute values (Matsuki et al., 2016; Strobl, Malley, & Tutz, 2009). RFR was conducted in R Studio (RStudio, Inc. Version 1.2.1335) using the “randomForest,” “caret,” “Hmisc,” and “miscTools” libraries.
Results
Sample Description
Descriptive statistics for the demographic and clinical variables are displayed in Table 1. Participants ranged in age from 27 to 65 years, and the majority were White (89.3%), reported 16 or more years of education, had a history of Stage II BC (61.7%), had received four to five different treatment modalities (68.3%; chemotherapy, surgery, radiation, hormonal therapy, HER2 therapy) for their BC, and were on endocrine therapy at the time of the study (66.7%).
Table 1.
Demographic and Clinical Characteristics.
| Characteristic | n (%) | Mean (SD) |
|---|---|---|
| Age (years) | 48.45 (8.8) | |
| 1st quartile (21–41.5) | 16 (24.6) | |
| 2nd quartile (41.6–48.0) | 18 (27.7) | |
| 3rd quartile (48.1–56.0) | 16 (24.6) | |
| 4th quartile (56.1–65.0) | 15 (23.1) | |
| BMI (kg/m2) | 27.25 (5.73) | |
| HWR | 0.81 (0.18) | |
| Education (years) | 16.73 (2.23) | |
| Non-White | 7 (10.7) | |
| Pre/perimenopausal | 18 (27.7) | |
| Postmenopausal (medical or natural) | 47 (72.3) | |
| BC stage | ||
| I | 12 (18.5) | |
| II | 40 (61.5) | |
| III | 13 (20) | |
| Anthracycline chemotherapy | 32 (53.3) | |
| No. of treatment modalities | ||
| 2 | 1 (1.5) | |
| 3 | 19 (29.2) | |
| 4 | 33 (50.8) | |
| 5 | 12 (18.5) | |
| Currently on endocrine therapy | ||
| Selective estrogen receptor modulators | 23 (52.3) | |
| Aromatase inhibitors | 21 (47.7) | |
| Time since chemotherapy (months) | 35.96 (26.23) | |
Note. N = 65. BC = breast cancer; BMI = body mass index; HWR = hip-to-waist ratio.
Cognitive and Biological Variables
We adjusted cognitive test scores for age, gender, and race according to published norms and display them in Table 2. Overall, the participants exhibited average to above average cognitive performance. Only 14 (21.5%) women exhibited at least one cognitive impairment on the neurocognitive tests compared to 51 (78.5%) who scored in the normal range on the tests. For Aβ-40 and Aβ-42, the 65 samples (100%) were within the calibration range of the assay, with a mean concentration CV of 2.5%. For tau, 59 samples (91%) were within the calibration range of the assay, with a mean concentration CV of 13%. A total of nine samples displayed higher % CVs (> 20%). Table 3 displays mean, minimum, and maximum serum dilution–corrected concentrations for Aβ-42, Aβ-40, and tau. For the enzyme-linked immunoassay cytokine assay, R 2s for the standard curves ranged from 0.998 to 1. Table 3 lists the cytokine concentrations as well. All controls were within the range supplied by the manufacturer, and CVs between sample duplicates averaged 6%.
Table 2.
Scores on Cognitive and Psychosomatic Measures.
| Measure | Mean (SD) | Minimum | Maximum |
|---|---|---|---|
| HVLT-immediatea | 0.35 (0.92) | −2.05 | 1.98 |
| HVLT-delayeda | 0.28 (0.81) | −2.11 | 1.22 |
| COWAb | 59.24 (28.00) | 3 | 99 |
| Trails Ac | 50.26 (10.20) | 28 | 76 |
| Trails Bc | 51.83 (9.94) | 23 | 73 |
| NP composited | 0.00 (0.72) | −2.62 | 1.12 |
| PROMIS anxiety | 16.54 (7.91) | 8 | 40 |
| PROMIS depression | 13.28 (6.16) | 8 | 32 |
| PROMIS fatigue | 21.23 (8.11) | 8 | 39 |
| UCLA-R | 37.83 (11.19) | 20 | 60 |
| PSS | 14.15 (8.07) | 0 | 31 |
| PSQI | 7.57 (4.37) | 0 | 17 |
| ESS | 7.05 (4.59) | 0 | 18 |
| Psych compositee | 0.01 (1.96) | −4.8 | 3.39 |
Note. N = 65. COWA = Controlled Oral Word Association Test; ESS = Epworth Sleepiness Scale; HVLT = Hopkins Verbal Learning Test Revised; NP = neuropsychological test; PROMIS = Patient-Reported Outcomes Measurement Information System; PSS = Perceived Stress Scale; Psych = psychosomatic; PSQI = Pittsburgh Sleep Quality Index; Trails = Trail Making Test; UCLA-R = UCLA Loneliness Scale Revised Version 3.
a Adjusted for age and education, standard deviation. bAdjusted for age and education, percentiles. cAdjusted for age and education, T-scores. dMean z-score of five tests. eFirst component score in principal component analysis.
Table 3.
Mean (SD), Minimum, and Maximum Amyloid Beta 40 and 42, Tau, and Cytokines for Participating Breast Cancer Survivors.
| Measure | Mean (SD) | Minimum | Maximum |
|---|---|---|---|
| Serum Aβ-40a (pg/mL) | 234.35 (67.20) | 70.21 | 597.66 |
| Serum Aβ-42a (pg/mL) | 10.70 (3.91) | 4.05 | 34.73 |
| Serum taua (pg/mL) | 0.71 (0.61) | 0.22 | 4.17 |
| Aβ-42/Aβ-40a ratio | 0.05 (0.01) | 0.03 | .06 |
| IL-6 (pg/mL) | 2.25 (1.81) | 0.05 | 7.62 |
| TNF-α (pg/mL) | 5.85 (1.31) | 2.98 | 8.92 |
| GM-CSF (pg/mL) | 130.91 (184.05) | 4.13 | 1,000 |
| IFN-γ (pg/mL) | 7.88 (6.23) | 0.49 | 28.23 |
| IL-10 (pg/mL) | 10.28 (9.35) | 1.22 | 48.40 |
| IL-12 (pg/mL) | 2.77 (1.79) | 0.21 | 9 |
| IL-13 (pg/mL) | 11.54 (29.93) | 0.11 | 214.63 |
| IL-1β (pg/mL) | 1.02 (0.61) | 0.24 | 3.84 |
| IL-2 (pg/mL) | 1.22 (0.88) | 0.21 | 4.52 |
| IL-4 (pg/mL) | 21.22 (14.02) | 7.32 | 81.51 |
| IL-5 (pg/ml) | 2.96 (7.45) | 0.10 | 55.36 |
| IL-7 (pg/ml) | 7.70 (2.72) | 2.85 | 14.39 |
| IL-8 (pg/mL) | 5.84 (6.52) | 2.18 | 48.70 |
Note. N = 65. Aβ = amyloid beta; GM-CSF = granulocyte-macrophage colony-stimulating factor; IFN = interferon; IL = interleukin; TNF = tumor necrosis factor.
aDilution-corrected concentrations.
Correlations Between Aβ-42, Aβ-40, Tau, and Cytokine Concentrations and Demographic and Clinical Variables; Cognitive Performance; and Psychosomatic Symptoms
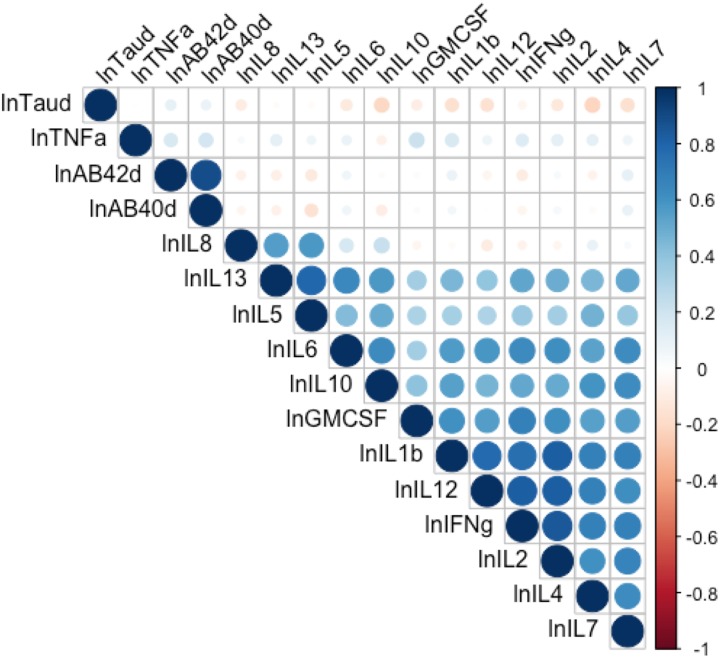
We found significant relationships between participants’ HWRs and lnAβ-42 (r = .35, p < .01), lnAβ-40 (r = .29, p < .05), and lntau (r = .29, p < .05) and between number of treatment modalities and lnAβ-42 (ρ = 0.29, p < .05) and the lnAβ-42:lnAβ-40 ratio (ρ = 0.27, p < .05). We did not find significant relationships between any of the cytokines and Aβ-42, Aβ-40, or tau (all ps > .05, Figure 1). Table 4 displays correlations among demographic and clinical variables and concentrations of Aβ-42, Aβ-40, and tau. Age, time since chemotherapy, and years of education were not significantly related to the variables of interest in this study, except for age and GM-CSF concentration (r = .35, p < .01; see Supplementary Table 1).
Figure 1.
Pearson’s correlation matrix of log-transformed concentrations of cytokines, amyloid beta 42 and 40, and tau. Circle size illustrates strength of the bivariate correlation. Blue illustrates positive and red illustrates negative correlations. The d at the end of lnTaud, lnAβ42d, and lnAβ40d indicates dilution corrected. AB = amyloid beta; GMCSF = granulocyte-macrophage colony-stimulating factor; IFNg = interferon gamma; IL = interleukin; ln = natural log; TNFa = tumor necrosis factor-alpha.
Table 4.
Correlations Between Demographic and Clinical Variables and Concentrations of Amyloid Beta and Tau.
| Measure | Agea | BMIa | HWRa | Time Chemoa | No. Txb | Cancer Stageb |
|---|---|---|---|---|---|---|
| lnAβ-42 | −.00 | .02 | .35** | −.22 | .29* | −.07 |
| lnAβ-40 | .08 | .12 | .29* | −.14 | .23 | −.04 |
| lnTau | .09 | .16 | .29* | −.02 | .18 | .09 |
| lnAβ-42/40 ratio | −.04 | −.03 | .33** | −.23 | .27* | −.11 |
Note. N = 65. Aβ = amyloid beta; BMI = body mass index; HWR = hip-to-waist ratio; No. Tx = number of treatment modalities (surgery, chemotherapy, radiation, endocrine); Time chemo = time since chemotherapy ended in months.
a Pearson’s r. bSpearman’s ρ.
*p < .05. **p < .01.
RFR
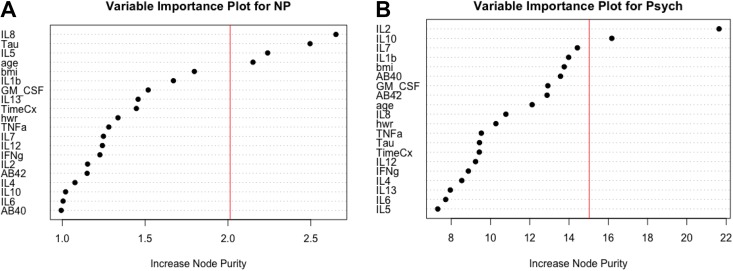
The adjusted R 2 for the NP composite score was .71 (F = 9.01, p < .0001). Increase in node purity indicated that the most important features in the random forest model were IL-8, tau, IL-5, and age (see Figure 2A.) The adjusted R 2 for the Psych composite score was .74 (F = 10.22, p < .0001). Increase in node purity indicated that the most important features in the random forest model were IL-2 and IL-10 (see Figure 2B).
Figure 2.
(A) Variable importance plot of features in the random forest regression model with the neuropsychological (NP) composite score as the dependent variable. All the variables on the y-axis were included as features in the model. IL-8 (interleukin 8), tau, IL-5 (interleukin 5), and age were identified as the most important features, or predictors, in the model per the increase in node purity index. The vertical line represents the a priori threshold (1 SD above the mean) for determining the most important model features. Together, these features explain 71% of the variance in NP composite scores. (B) Variable importance plot of features in the random forest regression model with the psychosomatic (Psych) composite score as the dependent variable. All the variables on the y-axis were included as features in the model. IL-2 (interleukin 8) and IL-10 (interleukin 10) were identified as the most important features, or predictors, in the model per the increase in node purity index. The vertical line represents the a priori threshold (1 SD above the mean) for determining the most important model features. Together, these features explain 74% of the variance in Psych composite scores. AB = amyloid beta; BMI = body mass index; GM-CSF = granulocyte-macrophage colony-stimulating factor; HWR = hip-to-waist ratio; IFNg = interferon gamma; IL = interleukin; IL12 = interleukin-12; TimeCx = time since chemotherapy; TNFa = tumor necrosis factor-alpha.
Discussion
Over the past decade, researchers have studied peripheral Aβ and tau mostly within the context of neurodegeneration, brain injury, and cognitive impairment. Recently, however, oncology research has begun to consider these factors in relation to breast tumor characteristics and brain metastases (Darlix et al., 2019). In the present study, we explored peripheral biomarkers of neurodegeneration (Aβ-42, Aβ-40, tau) in relation to cognitive outcomes in a sample of BCSs within the context of cytokines and psychosomatic variables. We also employed machine learning to simultaneously evaluate the ability of Aβ-42, Aβ-40, tau, and 13 common cytokines to predict objective cognitive performance and psychosomatic symptoms within the BCS population. This study provides preliminary hypothesis-generating data that can be used for future preclinical research investigating the underlying neurobiological mechanisms of CRCI and psychosomatic sequela of BCSs.
After we adjusted scores on cognitive test for age, gender, and race, we found that approximately 21% of the study sample exhibited an MCI on at least one of the cognitive tests, which is consistent with other studies of CRCI (Ahles et al., 2012; Wefel & Schagen, 2012). Post hoc independent t-test analyses did not reveal any significant differences in biomarkers or demographic factors between those who were impaired and those without impairment. Although we found measurable amounts of Aβ-42, Aβ-40, and tau in the present sample of BCSs, it is difficult to compare the concentrations of these analytes in the sample with any published “norms” because participants were, on average, 48 years old, while prior research involving quantification of peripheral Aβ-42, Aβ-40, and tau concentrations has been primarily in the fields of AD and MCI, usually in older adults, and in traumatic brain injury and concussion diagnostics and/or prognostics, typically in persons prone to concussions, for example, athletes who tend to be less than 30 years old. Furthermore, the assays available to quantify these analytes are designed for research rather than clinical practice, so there are no clinical cutoffs available for comparison (Olsson et al., 2016).
We found significant positive relationships among HWR, number of treatment modalities, and concentrations of Aβ-40, Aβ-42, and tau, suggesting that those with greater HWR, a proxy for abdominal obesity, and a greater number of cancer treatments have higher concentrations of these analytes. This finding is consistent with prior reports of an association between higher obesity indices and levels of Aβ (Craft, 2005; Frisardi et al., 2010; Tucsek et al., 2014). It also supports findings that more aggressive and heterogenous BC tumors express APPs and, consequently, require more complex treatment regimens (Ames et al., 2014; Darlix et al., 2019; Pandey et al., 2016).
We found no correlations between concentrations of Aβ-42, Aβ-40, and tau and those of the 13 cytokines. This finding is surprising because increasing evidence suggests that high levels of cytokines and inflammation are involved in the pathology of AD (Lai et al., 2017). It is possible that the correlations we examined in our analyses, which assumed linearity, failed to capture the complexity of interactions between the cytokines and neurodegenerative biomarkers that the RFR findings suggest.
The RFR, which captures nonlinear relationships and complex predictor interactions, revealed a highly significant model involving Aβ-42, Aβ-40, tau, and cytokines that explained 71% of the variance in NP scores. These findings support those of prior studies that have reported detecting peripheral Aβ concentrations in persons with AD or progressive MCI (Frankfort et al., 2008), but little is known about how Aβ-42, Aβ-40, and tau are linked to normal cognitive functioning that progresses to MCI. CRCI falls into the MCI range and meets the clinical criteria for mild cognitive disorder in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (Sachs-Ericsson & Blazer, 2015). Also, in a recent meta-analysis, Olsson et al. (2016) reported that only tau, not Aβ, could significantly distinguish persons with AD from persons with MCI. It is possible that patterns of Aβ-42, Aβ-40, and tau are different for persons with mild cognitive disorder than for persons with AD or MCI, but additional research is needed.
Along with other reports of significant relationships between cytokines and cognitive performance in BC patients and survivors (Cheung et al., 2015; Ganz et al., 2013; S. Kesler et al., 2013), our findings in the present study support the hypothesis that cytokines contribute to the underlying mechanisms of persistent CRCI in cancer survivors (Ahles et al., 2012; Janelsins et al., 2014). Specifically, our findings suggest that tau and amyloid may also interact with cytokines to impact cognitive performance. It is possible that cytokines stimulate the production or metabolism of APP or tau (Lai et al., 2017); however, the exact mechanisms need to be further investigated. Our RFR results suggest that IL-8, IL-5, tau, and age are important to the model. Authors have characterized IL-8 and IL-5 as pro-inflammatory (Yoshimoto & Yoshimoto, 2014), and the level of IL-8 correlates with the degree of neuroinflammation in persons who are cognitively impaired (Zhu et al., 2017). Research has consistently linked tau to neurodegeneration, brain injury, and cell damage, and it is possible that peripheral tau is a reflection of central nervous system tauopathy or systemic tau production (Olsson et al., 2016). Age is a well-established risk factor for decline in cognitive performance. In a post hoc Pearson’s correlation analysis, age was significantly related to scores on Trails A and B but not on HVLT or COWA. Despite age being a known risk factor for cognitive decline, CRCI occurs in women of all age (Janelsins et al., 2014). Determining feature importance in RFR models is controversial and results need to be interpreted with caution and within the context of all the features of the model.
When we entered all 13 cytokines, Aβ-42, Aβ-40, and tau into the RFR model of psychosomatic symptoms, the model was also highly predictive. This finding suggests complex, nonlinear associations among the cytokines, Aβ-42, Aβ-40, and tau, and psychosomatic symptoms and/or interaction among these biomarkers that influence psychosomatic symptoms. These cytokines and proteins may move bidirectionally across the blood–brain barrier to interfere with psychological, emotional, and mood-related processes in the brain. Our findings build on other reports of relationships between social support and loneliness and markers of inflammation (Muscatell, Eisenberger, Dutcher, Cole, & Bower, 2016; Nausheen et al., 2010; Yang, Li, & Frenk, 2014) and between psychological stressors and inflammatory markers in BC patients (Han et al., 2016; Wenzel et al., 2012; Witek-Janusek, Gabram, & Mathews, 2007) as well as survivors (Bower et al., 2007; Carlson, Speca, Faris, & Patel, 2007; Crosswell, Bower, & Ganz, 2014). The present study thus provides additional insights on how Aβ-42, Aβ-40, and tau may interact with pro- and anti-inflammatory cytokines to influence these psychosomatic outcomes. Our findings are also consistent with research in other populations that has found higher concentrations of tau and Aβ-42/Aβ-40 ratios in persons with posttraumatic stress disorder compared to those without (Clouston et al., 2019). The RFR for psychosomatic symptoms suggests that IL-2 and IL-10 are especially important to the model. Authors typically characterized IL-2 as pro-inflammatory, with the function of stimulating the production of leukocytes, lymphocytes, and macrophages; however, several studies have reported an anti-inflammatory function of IL-2 (Boerrigter et al., 2017; Lan, Selmi, & Gershwin, 2008; Osburn, Levine, Chattergoon, Thomas, & Cox, 2013), resulting in a categorization of IL-2 as both anti- and pro-inflammatory (Bessoles et al., 2008). On the other hand, researchers have consistently characterized IL-10 as anti-inflammatory or an immunosuppressive cytokine (Yoshimoto & Yoshimoto, 2014). Again, determining feature importance in RFR models is controversial and these results need to be interpreted with caution and within the context of all the features of the model.
As mentioned above in the Methods section, our past work has demonstrated nonlinear relationships between biomarkers and behavioral and cognitive outcomes within oncology populations (A. M. Henneghan, Palesh, Harrison, & Kesler, 2018; S. R. Kesler et al., 2016). For this reason, we used RFR for multivariate modeling of the outcome variables (cognitive performance and psychosomatic symptoms) rather than traditional linear methods (e.g., multiple linear regression). There are no standards regarding minimal sample size for RFR, which is true for all statistical methods. RFR does assume that the sample is representative of the population (Breiman, 2001), which in the present study was BCSs who were in the survivorship phase of the cancer trajectory and had a history of chemotherapy treatment. RFR is a nonparametric statistical method and is one of the most popular and easy to use machine-learning methods, largely, because of its accuracy and ability to deal with small sample sizes and large numbers of features (Biau & Scornet, 2016). The major limitation of RFR is the “black box” nature of interpreting the model and its features. While RFR provides more interpretability than other machine-learning methods (e.g., support vector machine learning) through feature importance, results on feature importance can only be interpreted within the context of all features in the model, not as absolute values (Matsuki et al., 2016; Strobl et al., 2009). Even though RFR is robust in terms of model overfitting when small samples and/or a large number of predictors are involved, it is possible that the model overfit the data. These models thus need to be validated in larger samples (Strobl et al., 2009).
In addition to the potential that the model overfit the data, this exploratory analysis had several notable limitations. First, we lacked a control group, limiting our ability to interpret our findings regarding the biomarkers to conclusions we could draw based on comparisons with what has been published in the literature. Our study was cross-sectional, but other research groups have recommended longitudinal assessment (change in concentrations) to determine the usefulness of Aβ-42, Aβ-40, and tau in assessing risk for developing cognitive impairment post treatment or AD or MCI later in life (Fandos et al., 2017). The wide range in time since chemotherapy across the sample is another study limitation since cognitive impairment and concentrations of cytokines and neurodegenerative biomarkers could all vary as a function of time; however, our correlation analyses did not reflect this relationship with time since treatment. We used composite scores for NP performance and psychosomatic symptoms, which enhanced the statistical power of the analyses but limited our ability to determine whether relationships reflected a mix of different domains or were dominated by one domain. Similarly, using a composite score for psychosomatic symptom quantification makes it difficult to interpret symptom profiles. Future studies with larger samples should evaluate cognitive and psychosomatic domains separately.
Because this was a secondary analysis, it might have been underpowered and the findings might represent Type II error. On the other hand, in this exploratory analysis, we did not adjust the p value to account for multiple comparisons, so findings could be influenced by Type I error. We were limited in our ability to comprehensively evaluate neurodegenerative biomarkers, including genetic factors like APOEe4, which is considered a risk factor for the development of AD and MCI and has been associated with CRCI (Ahles et al., 2014), so there could be associations or interactions with neurodegenerative biomarkers not accounted for in the present study.
Conclusion
Nurses are often in a position to support and educate BCSs after treatment is over. Most often, these patients want to know what causes CRCI. It is essential that nurses and other providers acknowledge that CRCI may be influenced by many factors that extend far beyond simply receiving chemotherapy or other treatments. Our findings in the present secondary analysis suggest that neurodegenerative biomarkers may interact with cytokines to influence cognitive functioning and psychosomatic symptoms in BCSs following the completion of chemotherapy. Health behaviors that support healthy aging and functionality along with reducing the likelihood of cancer recurrence could be beneficial to survivors experiencing CRCI or psychosomatic symptoms. Future research should evaluate biomarkers longitudinally in combination with neuroimaging to comprehensively evaluate these mechanisms along with the potential for AD syndrome-like presentation in chemotherapy-treated BC patients.
Supplemental Material
Supplemental Material, Henneghan_19060083_toSage_supplTbl for Exploring Relationships Among Peripheral Amyloid Beta, Tau, Cytokines, Cognitive Function, and Psychosomatic Symptoms in Breast Cancer Survivors by Ashley Henneghan, Andreana P. Haley and Shelli Kesler in Biological Research For Nursing
Acknowledgments
The authors would like to acknowledge Michelle Harrison, PhD, and the Health & Integrative Physiology Lab at the University of Texas at Austin for their assistance with the cytokine assay procedures.
Authors’ Note: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: Ashley Henneghan contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Andreana P. Haley contributed to analysis and interpretation; drafted manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Shelli Kesler contributed to acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under award number F31NR015707 and by the National Cancer Institute under award number DP2OD004445. Ashley M. Henneghan was supported by the Doctoral Degree Scholarship in Cancer Nursing, DSCN-15-072, from the American Cancer Society.
ORCID iD: Ashley Henneghan  https://orcid.org/0000-0002-6733-1926
https://orcid.org/0000-0002-6733-1926
Supplemental Material: Supplemental material for this article is available online.
References
- Ahles T. A., Li Y., McDonald B. C., Schwartz G. N., Kaufman P. A., Tsongalis G. J.…Saykin A. J. (2014). Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: The impact of APOE and smoking. Psychooncology, 23, 1382–1390. doi:10.1002/pon.3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles T. A., Root J. C., Ryan E. L. (2012). Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. Journal of Clinical Oncology, 30, 3675–3686. doi:10.1200/JCO.2012.43.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles T. A., Saykin A. J. (2007). Candidate mechanisms for chemotherapy-induced cognitive changes. Nature Reviews Cancer, 7, 192–201. doi:10.1038/nrc2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles T. A., Saykin A. J., McDonald B. C., Li Y., Furstenberg C. T., Hanscom B. S.…Kaufman P. A. (2010). Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. Journal of Clinical Oncology, 28, 4434–4440. doi:10.1200/jco.2009.27.0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles T. A., Saykin A. J., Noll W. W., Furstenberg C. T., Guerin S., Cole B., Mott L. A. (2003). The relationship of APOE genotype to neuropsychological performance in longterm cancer survivors treated with standard dose chemotherapy. Psychooncology, 12, 612–619. [DOI] [PubMed] [Google Scholar]
- Ames S. L., Kisbu-Sakarya Y., Reynolds K. D., Boyle S., Cappelli C., Cox M. G.…Stacy A. W. (2014). Inhibitory control effects in adolescent binge eating and consumption of sugar-sweetened beverages and snacks. Appetite, 81, 180–192. doi:10.1016/j.appet.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amidi A., Christensen S., Mehlsen M., Jensen A. B., Pedersen A. D., Zachariae R. (2015). Long-term subjective cognitive functioning following adjuvant systemic treatment: 7-9 years follow-up of a nationwide cohort of women treated for primary breast cancer. British Journal of Cancer, 113, 794–801. doi:10.1038/bjc.2015.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson U., Blennow K., Zetterberg H. (2016). Update on ultrasensitive technologies to facilitate research on blood biomarkers for central nervous system disorders. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 3, 98–102. doi:10.1016/j.dadm.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assini A., Cammarata S., Vitali A., Colucci M., Giliberto L., Borghi R.…Tabaton M. (2004). Plasma levels of amyloid beta-protein 42 are increased in women with mild cognitive impairment. Neurology, 63, 828–831. [DOI] [PubMed] [Google Scholar]
- Benedict R. H. B., Schretlen D., Groninger L., Brandt J. (1998). Hopkins verbal learning test – revised: Normative data and analysis of inter-form and test-retest reliability. Clinical Neuropsychologist, 12, 43–55. doi:10.1076/clin.12.1.43.1726 [Google Scholar]
- Benton A., deS Hamsher K., Varney N., Spreen O. (1983). Contributions to neuropsychological assessment: A clinical manual. Oxford, England: Oxford University Press. [Google Scholar]
- Bessoles S., Fouret F., Dudal S., Besra G. S., Sanchez F., Lafont V. (2008). IL-2 triggers specific signaling pathways in human NKT cells leading to the production of pro- and anti-inflammatory cytokines. Journal of Leukocyte Biology, 84, 224–233. doi:10.1189/jlb.1007669 [DOI] [PubMed] [Google Scholar]
- Biau G., Scornet E. (2016). A random forest guided tour. Test, 25, 197–227. doi:10.1007/s11749-016-0481-7 [Google Scholar]
- Blasko I., Jellinger K., Kemmler G., Krampla W., Jungwirth S., Wichart I.…Fischer P. (2008). Conversion from cognitive health to mild cognitive impairment and Alzheimer’s disease: Prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiology of Aging, 29, 1–11. doi:10.1016/j.neurobiolaging.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Boerrigter D., Weickert T. W., Lenroot R., O’Donnell M., Galletly C., Liu D.…Weickert C. S. (2017). Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. Journal of Neuroinflammation, 14, 188 doi:10.1186/s12974-017-0962-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau C., Gurard-Levin Z., Andre F., Pusztai L., Rouzier R. (2015). Predictive and prognostic value of the tauprotein in breast cancer. Anticancer Research, 35(10), 5179–5184. [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Aziz N., Olmstead R., Irwin M. R., Cole S. W. (2007). Inflammatory responses to psychological stress in fatigued breast cancer survivors: Relationship to glucocorticoids. Brain, Behavior, and Immunity, 21, 251–258. doi:10.1016/j.bbi.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Breiman L. (1996). Out-of-bag estimation. Berkley, CA: University of California Berkley. [Google Scholar]
- Breiman L. (2001). Random forests. Machine Learning, 45, 5–32. doi:10.1023/A:1010933404324 [Google Scholar]
- Breiman L., Friedman J., Olshen R., Stone C. (1984). Classification and regression trees. New York, NY: Chapman & Hall. [Google Scholar]
- Buysse D. J., Reynolds C. F., 3rd, Monk T. H., Berman S. R., Kupfer D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Carlson L. E., Speca M., Faris P., Patel K. D. (2007). One year pre-post intervention followup of psychological, immune, endocrine and blood pressure outcomes of mindfulnessbased stress reduction (MBSR) in breast and prostate cancer outpatients. Brain, Behavior, and Immunity, 21, 1038–1049. doi:10.1016/j.bbi.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Carroll J. E., Van Dyk K., Bower J. E., Scuric Z., Petersen L., Schiestl R.…Ganz P. A. (2019). Cognitive performance in survivors of breast cancer and markers of biological aging. Cancer, 125, 298–306. doi:10.1002/cncr.31777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S.,…PROMIS Cooperative Group. (2010). The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. Journal of Clinical Epidemiology, 63, 1179–1194. doi:10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Y. T., Ng T., Shwe M., Ho H. K., Foo K. M., Cham M. T.…Chan A. (2015). Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: A multi-centered, prospective, cohort study. Annals of Oncology, 26, 1446–1451. doi:10.1093/annonc/mdv206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouston S. A. P., Deri Y., Diminich E., Kew R., Kotov R., Stewart C.…Luft B. J. (2019). Posttraumatic stress disorder and total amyloid burden and amyloid-beta 42/40 ratios in plasma: Results from a pilot study of world trade center responders. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 11, 216–220. doi:10.1016/j.dadm.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Craft S. (2005). Insulin resistance syndrome and Alzheimer’s disease: Age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiology of Aging, 26, 65–69. doi:10.1016/j.neurobiolaging.2005.08.021 [DOI] [PubMed] [Google Scholar]
- Crosswell A. D., Bower J. E., Ganz P. A. (2014). Childhood adversity and inflammation in breast cancer survivors. Psychosomatic Medicine, 76, 208–214. doi:10.1097/PSY.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dage J. L., Wennberg A. M., Airey D. C., Hagen C. E., Knopman D. S., Machulda M. M.…Mielke M. M. (2016). Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12, 1226–1234. doi:10.1016/j.jalz.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix A., Hirtz C., Thezenas S., Maceski A., Gabelle A., Lopez-Crapez E.…Lehmann S. (2019). The prognostic value of the Tau protein serum level in metastatic breast cancer patients and its correlation with brain metastases. BMC Cancer, 19, 110 doi:10.1186/s12885-019-5287-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele Y. S., Obermuller U., Heilbronner G., Baumann F., Kaeser S. A., Wolburg H.…Jucker M. (2010). Peripherally applied Abeta-containing inoculates induce cerebral betaamyloidosis. Science, 330, 980–982. doi:10.1126/science.1194516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderlin C. A., Coleman E. A., Cole C., Richards K. C., Kennedy R. L., Goodwin J. A.…Mack K. (2011). Subjective sleep quality, objective sleep characteristics, insomnia symptom severity, and daytime sleepiness in women aged 50 and older with nonmetastatic breast cancer. Oncology Nursing Forum, 38, E314–325. doi:10.1188/11.ONF.E314-E325 [DOI] [PubMed] [Google Scholar]
- Epifanio I. (2017). Intervention in prediction measure: A new approach to assessing variable importance for random forests. BMC Bioinformatics, 18, 230 doi:10.1186/s12859017-1650-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandos N., Perez-Grijalba V., Pesini P., Olmos S., Bossa M., Villemagne V. L.,…AIBL Research Group. (2017). Plasma amyloid beta 42/40 ratios as biomarkers for amyloid beta cerebral deposition in cognitively normal individuals. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 8, 179–187. doi:10.1016/j.dadm.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfort S. V., Tulner L. R., van Campen J. P., Verbeek M. M., Jansen R. W., Beijnen J. H. (2008). Amyloid beta protein and tau in cerebrospinal fluid and plasma as biomarkers for dementia: A review of recent literature. Current Clinical Pharmacology, 3, 123–131. [DOI] [PubMed] [Google Scholar]
- Frisardi V., Solfrizzi V., Seripa D., Capurso C., Santamato A., Sancarlo D.…Panza F. (2010). Metabolic-cognitive syndrome: A cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Research Reviews, 9, 399–417. doi:10.1016/j.arr.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Ganz P. A., Bower J. E., Kwan L., Castellon S. A., Silverman D. H., Geist C.…Cole S. W. (2013). Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain, Behavior, and Immunity, 30, S99–108. doi:10.1016/j.bbi.2012.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T. J., Felger J. C., Lee A., Mister D., Miller A. H., Torres M. A. (2016). Association of childhood trauma with fatigue, depression, stress, and inflammation in breast cancer patients undergoing radiotherapy. Psychooncology, 25, 187–193. doi:10.1002/pon.3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck J. E., Albert S. M., Franco R., Gorin S. S. (2008). Patterns of dementia diagnosis in surveillance, epidemiology, and end results breast cancer survivors who use chemotherapy. Journal of the American Geriatrics Society, 56, 1687–1692. doi:10.1111/j.1532-5415.2008.01848.x [DOI] [PubMed] [Google Scholar]
- Henderson W. G., Fisher S. G., Cohen N., Waltzman S., Weber L. (1990). Use of principal components analysis to develop a composite score as a primary outcome variable in a clinical trial. The VA cooperative study group on cochlear implantation. Controlled Clinical Trials, 11, 199–214. [DOI] [PubMed] [Google Scholar]
- Henneghan A. M., Carter P., Stuifbergan A., Parmelee B., Kesler S. (2018). Relationships between self-reported sleep quality components and cognitive functioning in breast cancer survivors up to 10 years following chemotherapy. Psychooncology. doi:10.1002/pon.4745 [DOI] [PubMed] [Google Scholar]
- Henneghan A. M., Palesh O., Harrison M., Kesler S. (2018). Identifying cytokine predictors of cognitive functioning in breast cancer survivors up to 10 years post chemotherapy using machine learning. Journal of Neuroimmunology, 320, 38–47. doi:10.1016/j.jneuroim.2018.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneghan A., Stuifbergen A., Becker H., Kesler S., King E. (2018). Modifiable correlates of perceived cognitive function in breast cancer survivors up to 10 years after chemotherapy completion. Journal of Cancer Survivorship, 12, 224–233. doi:10.1007/s11764-0170661-9 [DOI] [PubMed] [Google Scholar]
- Hunter A. M., Kwan L., Ercoli L. M., Mills B. K., Cook I. A., Ganz P. A., Leuchter A. F. (2014). Quantitative electroencephalography biomarkers of cognitive complaints after adjuvant therapy in breast cancer survivors: A pilot study. Psychooncology, 23, 713–715. doi:10.1002/pon.3487 [DOI] [PubMed] [Google Scholar]
- Jack C. R., Jr, Bennett D. A., Blennow K., Carrillo M. C., Dunn B., Haeberlein S. B.…Contributors. (2018). NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 14, 535–562. doi:10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap A., Gawande S., Sharma S. (2015). Biomarkers in vascular dementia: A recent update. Biomarkers and Genomic Medicine, 7, 43–56. [Google Scholar]
- Janelidze S., Stomrud E., Palmqvist S., Zetterberg H., van Westen D., Jeromin A.…Hansson O. (2016). Plasma beta-amyloid in Alzheimer’s disease and vascular disease. Scientific Reports, 6, 26801 doi:10.1038/srep26801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins M. C., Kesler S. R., Ahles T. A., Morrow G. R. (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry, 26, 102–113. doi:10.3109/09540261.2013.864260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins M. C., Mustian K. M., Palesh O. G., Mohile S. G., Peppone L. J., Sprod L. K.…Morrow G. R. (2012). Differential expression of cytokines in breast cancer patients receiving different chemotherapies: Implications for cognitive impairment research. Supportive Care in Cancer, 20, 831–839. doi:10.1007/s00520-011-1158-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. I., Mitchell T. M. (2015). Machine learning: Trends, perspectives, and prospects. Science, 349, 255–260. doi:10.1126/science.aaa8415 [DOI] [PubMed] [Google Scholar]
- Kesler S., Janelsins M., Koovakkattu D., Palesh O., Mustian K., Morrow G., Dhabhar F. S. (2013). Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain, Behavior, and Immunity, 30, S109–S116. doi:10.1016/j.bbi.2012.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S. R., Gugel M., Huston-Warren E., Watson C. (2016). Atypical structural connectome organization and cognitive impairment in young survivors of acute Lymphoblastic Leukemia. Brain Connectivity, 6, 273–282. doi:10.1089/brain.2015.0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S. R., Watson C. L., Blayney D. W. (2015). Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiology of Aging, 36, 2429–2442. doi:10.1016/j.neurobiolaging.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V., Breteler M. M., Boogerd W., Seynaeve C., Gundy C., Schagen S. B. (2012). Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. Journal of Clinical Oncology, 30, 1080–1086. doi:10.1200/JCO.2011.37.0189 [DOI] [PubMed] [Google Scholar]
- Lai K. S. P., Liu C. S., Rau A., Lanctot K. L., Kohler C. A., Pakosh M.…Herrmann N. (2017). Peripheral inflammatory markers in Alzheimer’s disease: A systematic review and metaanalysis of 175 studies. Journal of Neurology, Neurosurgery, and Psychiatry, 88, 876–882. doi:10.1136/jnnp-2017-316201 [DOI] [PubMed] [Google Scholar]
- Lan R. Y., Selmi C., Gershwin M. E. (2008). The regulatory, inflammatory, and T cell programming roles of interleukin-2 (IL-2). Journal of Autoimmunity, 31, 7–12. doi:10.1016/j.jaut.2008.03.002 [DOI] [PubMed] [Google Scholar]
- Lim S., Yoo B. K., Kim H.-S., Gilmore H. L., Lee Y., Lee H. P.…Lee H. G. (2014). Amyloid-β precursor protein promotes cell proliferation and motility of advanced breast cancer. BMC Cancer, 14, 928 doi:10.1186/1471-2407-14-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt J. S., Hurria A., McDonald B. C., Saykin A. J., Stern R. A., VanMeter J. W.,…Living With Cancer, S (2013). Cognitive effects of cancer and its treatments at the intersection of aging: What do we know; what do we need to know? Seminals in Oncology, 40, 709–725. doi:10.1053/j.seminoncol.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki K., Kuperman V., Van Dyke J. A. (2016). The Random Forests statistical technique: An examination of its value for the study of reading. Scientific Studies of Reading, 20, 20–33. doi:10.1080/10888438.2015.1107073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. M., Hagen C. E., Wennberg A. M. V., Airey D. C., Savica R., Knopman D. S.…Dage J. L. (2017). Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the Mayo clinic study on aging. JAMA Neurology, 74, 1073–1080. doi:10.1001/jamaneurol.2017.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K. A., Eisenberger N. I., Dutcher J. M., Cole S. W., Bower J. E. (2016). Links between inflammation, amygdala reactivity, and social support in breast cancer survivors. Brain, Behavior, and Immunity, 53, 34–38. doi:10.1016/j.bbi.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nausheen B., Carr N. J., Peveler R. C., Moss-Morris R., Verrill C., Robbins E.…Gidron Y. (2010). Relationship between loneliness and proangiogenic cytokines in newly diagnosed tumors of colon and rectum. Psychosomatic Medicine, 72, 912–916. doi:10.1097/PSY.0b013e3181f0bc1c [DOI] [PubMed] [Google Scholar]
- Olsson B., Lautner R., Andreasson U., Ohrfelt A., Portelius E., Bjerke M.…Zetterberg H. (2016). CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurology, 15, 673–684. doi:10.1016/S14744422(16)00070-3 [DOI] [PubMed] [Google Scholar]
- Osburn W. O., Levine J. S., Chattergoon M. A., Thomas D. L., Cox A. L. (2013). Antiinflammatory cytokines, pro-fibrogenic chemokines and persistence of acute HCV infection. Journal of Hepatitis, 20, 404–413. doi:10.1111/jvh.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P., Sliker B., Peters H. L., Tuli A., Herskovitz J., Smits K.…Solheim J. C. (2016). Amyloid precursor protein and amyloid precursor-like protein 2 in cancer. Oncotarget, 7, 19430–19444. doi:10.18632/oncotarget.7103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. C., Han S. H., Cho H. J., Byun M. S., Yi D., Choe Y. M.…Mook-Jung I. (2017). Chemically treated plasma Abeta is a potential blood-based biomarker for screening cerebral amyloid deposition. Alzheimer’s Research & Therapy, 9, 20 doi:10.1186/s13195-0170248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. K., Wong A. L., Wong F. L., Breen E. C., Hurria A., Smith M.…Bhatia S. (2015). Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. Journal of the National Cancer Institute, 107, 1–7. doi:10.1093/jnci/djv131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient-Reported Outcomes Measurement Information System 2017. (2017). Retrieved from http://www.healthmeasures.net/explore-measurement-systems/promis/obtain-administer-measures
- Pattinson C. L., Shahim P., Taylor P., Dunbar K., Guedes V. A., Motamedi V.…Gill J. M. (2019). Elevated Tau in military personnel relates to chronic symptoms following traumatic brain injury. Journal of Head Trauma Rehabilitation. doi:10.1097/HTR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomykala K. L., Ganz P. A., Bower J. E., Kwan L., Castellon S. A., Mallam S.…Silverman D. H. (2013). The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging and Behavior, 7, 511–523. doi:10.1007/s11682-013-9243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissin D. M., Kan C. W., Campbell T. G., Howes S. C., Fournier D. R., Song L.…Duffy D. C. (2010). Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nature Biotechnology, 28, 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W. (1996). UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. Journal of Personality Assessment, 66(1), 20–40. doi:10.1207/s15327752jpa6601_2 [DOI] [PubMed] [Google Scholar]
- Sachs-Ericsson N., Blazer D. G. (2015). The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment. Aging and Mental Health, 19, 2–12. doi:10.1080/13607863.2014.920303 [DOI] [PubMed] [Google Scholar]
- Sobow T., Flirski M., Kloszewska I., Liberski P. P. (2005). Plasma levels of alpha beta peptides are altered in amnestic mild cognitive impairment but not in sporadic Alzheimer’s disease. Acta Neurobiologiae Experimentalis, 65, 117–124. [DOI] [PubMed] [Google Scholar]
- Statistics I. S. (2009). PASW (Version 18.0). Chicago, IL: Retrieved from http://www.spss.com/ [Google Scholar]
- Strobl C., Malley J., Tutz G. (2009). An introduction to recursive partitioning: Rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychological Methods, 14, 323–348. doi:10.1037/a0016973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh T. (2004). Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology, 19, 203–214. doi:10.1016/s08876177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- Tucsek Z., Toth P., Sosnowska D., Gautam T., Mitschelen M., Koller A.…Csiszar A. (2014). Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: Effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. Journals of Gerontology, 69, 1212–1226. doi:10.1093/gerona/glt177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy J. L., Stouten-Kemperman M. M., Pond G., Booth C. M., Rourke S. B., Dhillon H. M.…Tannock I. F. (2017). A mechanistic cohort study evaluating cognitive impairment in women treated for breast cancer. Brain Imaging and Behavior. doi:10.1007/s11682-017-9728-5 [DOI] [PubMed] [Google Scholar]
- Wefel J. S., Kesler S. R., Noll K. R., Schagen S. B. (2015). Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA: A Cancer Journal for Clinicians, 65, 123–138. doi:10.3322/caac.21258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel J. S., Schagen S. B. (2012). Chemotherapy-related cognitive dysfunction. Current Neurology and Neuroscience Reports, 12, 267–275. doi:10.1007/s11910-012-0264-9 [DOI] [PubMed] [Google Scholar]
- Wefel J. S., Vardy J., Ahles T., Schagen S. B. (2011). International cognition and cancer task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncology, 12(7), 703–708. doi:10.1016/S1470-2045(10)70294-1 [DOI] [PubMed] [Google Scholar]
- Wenzel L., Osann K., Lester J., Kurz R., Hsieh S., Nelson E. L., Karlan B. (2012). Biopsychological stress factors in BRCA mutation carriers. Psychosomatics, 53, 582590 doi:10.1016/j.psym.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. M., Shah R., Shayne M., Huston A. J., Krebs M., Murray N.…Janelsins M. C. (2018). Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. Journal of Neuroimmunology, 314, 17–23. doi:10.1016/j.jneuroim.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek-Janusek L., Gabram S., Mathews H. L. (2007). Psychologic stress, reduced NK cell activity, and cytokine dysregulation in women experiencing diagnostic breast biopsy. Psychoneuroendocrinology, 32, 22–35. doi:10.1016/j.psyneuen.2006.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten D. M., Tibshirani R. (2010). Survival analysis with high-dimensional covariates. Statistical Methods in Medical Research, 19, 29–51. doi:10.1177/0962280209105024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Li T., Frenk S. M. (2014). Social network ties and inflammation in U.S. adults with cancer. Biodemography and Social Biology, 60, 21–37. doi:10.1080/19485565.2014.899452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Yoshimoto T. (2014). Cytokine frontiers: Regulation of immune responses. Tokyo, Japan: Springer. [Google Scholar]
- Zetterberg H., Wilson D., Andreasson U., Minthon L., Blennow K., Randall J., Hansson O. (2013). Plasma tau levels in Alzheimer’s disease. Alzheimer’s Research & Therapy, 5, 1–3. doi:10.1186/alzrt163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Chai Y. L., Hilal S., Ikram M. K., Venketasubramanian N., Wong B. S.…Lai M. K. (2017). Serum IL-8 is a marker of white-matter hyperintensities in patients with Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, 41–47. doi:10.1016/j.dadm.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Henneghan_19060083_toSage_supplTbl for Exploring Relationships Among Peripheral Amyloid Beta, Tau, Cytokines, Cognitive Function, and Psychosomatic Symptoms in Breast Cancer Survivors by Ashley Henneghan, Andreana P. Haley and Shelli Kesler in Biological Research For Nursing