Abstract
Introduction/Background:
Chronic diseases, like diabetes and heart disease, are considered inflammatory conditions with elevated levels of the proinflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) and the anti-inflammatory cytokine interleukin-10 (IL-10). Disease progression is not consistent from person to person. Psychosocial factors are hypothesized to play a modifying role. Self-efficacy, the confidence in one’s ability to perform well in a specific life domain or at a specific task, is associated with better health outcomes. Coping self-efficacy is confidence in one’s ability to handle life’s problems through emotional regulation, problem-solving, and social support. Little is known about associations between coping self-efficacy and inflammation.
Aim:
The purpose of this pilot study was to examine associations between coping self-efficacy and IL-6, IL-10, and TNF-α levels.
Method:
This was a cross-sectional study conducted over two visits. Sociodemographic variables, chronic disease count, body mass index (BMI), and coping self-efficacy were collected. Inflammatory markers were collected via sweat using the sweat patch, a noninvasive collection device.
Results:
Higher TNF-α and IL-10 levels were significantly associated with low coping self-efficacy (β = −.03, p = .028; β = −.017, p = .007, respectively) after adjustment for age, sex, race, BMI, and chronic disease count. IL-6 trended toward significance after adjustment as well (β = −.22, p = .054).
Conclusions:
This pilot study showed that high coping self-efficacy was associated with lower IL-6, IL-10, and TNF-α levels, indicating a potential buffering effect of high coping self-efficacy. Further longitudinal research with larger sample sizes is needed.
Keywords: coping self-efficacy, chronic disease, sweat patch, interleukin-6, interleukin-10, tumor necrosis factor-α, cytokines
Half of all adults in the United States have a chronic condition (Ward, Schiller, & Goodman, 2014). This number is growing as the population ages and as younger cohorts become more obese (Gerteis et al., 2014). Many of these chronic conditions, like diabetes and heart disease, are also inflammatory conditions (Everett et al., 2013; Lukic et al., 2014). In these conditions, the body exhibits elevated levels of proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α). Recently, investigators identified IL-6 as one of the single best cytokine predictors of all-cause mortality in a large cohort study (Varadhan et al., 2014). Interleukin-10 (IL-10) is an anti-inflammatory cytokine that researcher has regularly evaluated in chronic disease, especially as part of a ratio with IL-6 in order to look at pro- versus anti-inflammatory activity (Taniguchi et al., 1999). Each of these cytokines is a part of the nuclear factor kappa B inflammatory system. TNF-α helps to initiate the system, IL-6 is a downstream proinflammatory product, and IL-10 is potentiated through the system as part of the system’s homeostatic mechanism for downregulation (Moynagh, 2005; Saraiva & O’Garra, 2010).
Not all persons experience the same rate of disease and inflammatory progression. Psychosocial factors play key roles in disease progression. One such factor is self-efficacy, which is defined as confidence in one’s ability to perform a specific task well or function well within a particular life domain (Bandura, 1997). Self-efficacy may be developed in four distinct ways: through personal mastery, verbal persuasion, vicarious experience, and physiological feedback. The concept of self-efficacy is the centerpiece of Bandura’s social cognitive theory, which addresses how people obtain behavioral, emotional, cognitive, and social ability and how they motivate and regulate their behavior, generating social systems that organize and structure their lives (Bandura, 1993, 1997). Research has shown that increases in self-efficacy are associated with positive health outcomes such as decreases in blood glucose in diabetic patients (Zulman, Rosland, Choi, Langa, & Heisler, 2012) and increased cardiac function in patients with heart disease (Sarkar, Ali, & Whooley, 2009). There are also successful self-management programs (such as the Chronic Disease Self-Management Program) based on self-efficacy theory, which aim to improve self-efficacy as a way to improve the effects of chronic disease (Lorig, Ritter, Villa, & Armas, 2009). One particular form of self-efficacy, known as coping self-efficacy, involves a person’s confidence in their ability to cope with challenging situations using problem-solving, emotional regulation, and social support (Chesney, Neilands, Chambers, Taylor, & Folkman, 2006).
Although we know that inflammation and chronic disease are highly associated and that self-efficacy and chronic disease are also highly associated, there is little known about the association between self-efficacy and inflammation. In the 1980s, Bandura and colleagues conducted a series of experiments to evaluate the hypothesis that self-efficacy mediates the effects of environmental factors on our physiology. This hypothesis stems from Bandura’s understanding that individuals obtain self-efficacy partially via physiological feedback. This work showed clear attenuation of catecholamine secretion and heart rate, representing the sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal axis, in the presence of high coping self-efficacy. The data around immune system responses and inflammation, however, were more mixed (Bandura, Cioffi, Taylor, & Brouillard, 1988; Bandura, Taylor, Williams, Mefford, & Barchas, 1985). In one experiment (N = 20), subjects with low coping self-efficacy were administered an intervention to aid in the attainment of greater coping self-efficacy. During the self-efficacy acquisition period, investigators observed increases in immune markers like T-cell and B-cell quantities and IL-2, a proinflammatory cytokine. Once subjects attained mastery (and subsequent higher coping self-efficacy), the immune system and inflammatory responses started to attenuate but did not return to baseline (Wiedenfeld et al., 1990).
In contrast, Mausbach and colleagues (2011) more recently found a cross-sectional association between high coping self-efficacy and lower serum IL-6 levels in older adult caregivers with high levels of role stress. These findings are somewhat incongruent with those of Bandura, and they are difficult to compare to each other given the differing study designs, outcomes variables, and patient populations.
Therefore, the purpose of the present pilot study was to examine the association between coping self-efficacy and levels of the inflammatory cytokines IL-6, IL-10, and TNF-α in adults over a range of ages. We hypothesized that higher coping self-efficacy would be associated with lower levels of IL-6, IL-10, and TNF-α.
We opted to collect the biospecimens using sweat for the present study. Sweat is a rich source of physiologic data and allows for biospecimen collection that is noninvasive, avoids diurnal rhythms, and is well tolerated (Hladek et al., 2018). Consequently, there is growing interest in sweat as a biospecimen source in biologic and metabolomic research (Mena-Bravo & Luque de Castro, 2014). Prior researchers have quantified cytokines, cortisol, and electrolytes in sweat (Wilke, Martin, Terstegen, & Biel, 2007). Of particular relevance to this study, Cizza and colleagues (2008) and Marques-Deak and colleagues (2006) examined correlations between sweat and plasma concentrations of nine analytes (including TNF-α and IL-6) and found a high level of correlation between the two sources (Cizza et al., 2008; Marques-Deak et al., 2006).
Method
Design and Participants
This study was cross sectional and conducted over two visits separated by 72 hr. During the first visit, we determined eligibility, obtained consent, and collected survey data. We recruited participants from two sites in May and June 2016: a senior independent-living facility in Maryland and the Johns Hopkins School of Nursing. We excluded potential participants who were undergoing active cancer treatment, had been diagnosed with a terminal illness, or were cognitively impaired. The Johns Hopkins Internal Review Board approved this study (IRB00095668). Participants received a US$25 gift card for their participation.
Sociodemographic Data, Chronic Disease, and Self-Efficacy Variables
The sociodemographic data we collected included age, sex, race, and ethnicity. We also collected height and weight by self-report and used those data to calculate body mass index (BMI). We measured chronic disease using a simple numerical count of the number of self-reported medical problems, which has been shown to be an effective and efficient measure of chronic disease (de Groot, Beckerman, Lankhorst, & Bouter, 2003; Huntley, Johnson, Purdy, Valderas, & Salisbury, 2012). To measure coping self-efficacy, we used the Coping Self-Efficacy Scale, a 13-item scale with a 10-point Likert-type response option with 1 representing not at all confident, 5 representing moderately confident, and 10 representing totally confident (Chesney et al., 2006). The scale contains three subdomains for problem-solving, emotional regulation, and social support, with Cronbach’s internal consistency coefficient αs of .91, .91, and .80 for these subdomains, respectively. One example item is, “When things aren’t going well for you, or when you’re having problems, how confident or certain are you that you can break an upsetting problem into smaller parts?” The coping self-efficacy score is calculated as the mean score of the 13 items. For the present analysis, we dichotomized the coping self-efficacy mean into high and low coping self-efficacy (< 7.0 vs. ≥7.0) to look at group effects consistent with past self-efficacy-based research (Mausbach et al., 2011). This cutoff value of 7.0 was chosen based on clinical use in self-management programs.
Materials and Physiological Biomarkers
To collect sweat, we used a sweat patch (PharmChem, Fort Worth, TX), a nonocclusive, hypoallergenic, Band-Aid-like device with a semipermeable membrane that allows liquid to evaporate and traps proteins, metabolites, and other biospecimen data in its white absorption pad. The sweat patch is US Food and Drug Administration (FDA) approved and has been used in addiction medicine for the collection of drug metabolites since 1990 (Concheiro, Shakleya, & Huestis, 2011).
Sweat patch placement, removal, and transport
During the first visit, after screening for eligibility, obtaining consent, and completing survey data, we applied the sweat patch to cleaned skin on the abdomen or flank according to participant preference (the two sites having similar quantities of eccrine sweat glands). We chose the abdomen due to its low level of SNS innervation to avoid sweat capture due to SNS activity (Sonner et al., 2015). Participants could shower and perform their daily activities, but we asked them to avoid vigorous activity so that we captured only insensible sweat loss, not exercise-induced sweat loss. During the second visit, 72 hr later (with a ±1-hr window), we removed the patch and immediately placed it on dry ice. We then transported it to a −80 oC freezer until extraction.
Sweat patch extraction techniques
For this study, we used the Gill lab sweat patch extraction protocol (Hladek et al., 2018). The sweat patches were placed in 5-mL conical tubes with 3 mL of buffer comprised of phosphate-buffered saline with 0.1% Tween 20 and 0.2% bovine serum albumin. The buffer and patch were rotated on ice for 20 min and then centrifuged at 3,000 g for 3 min at 4 °C. The samples were then divided into 250-μL aliquots and stored in a −80 °C freezer.
Laboratory studies
IL-6, IL-10, and TNF-α sweat extractions were measured with an ultrasensitive single-molecule enzyme-linked immunoarray (Simoa™, Quanterix Corporation, Lexington, MA) using a method previously described (Rissin et al., 2011). The Cytokine 3-Plex A digital immunoassay kit was used to detect the presence of otherwise scant levels of the three proteins. The samples were all run in duplicate. The minimum limit of detection was .008 pg/mL for IL-6, .003 pg/mL for IL-10, and .015 pg/mL for TNF-α. For confidentiality, the data remained deidentified, and the laboratory staff who executed the experiment were blinded to any participant sociodemographic data.
Data Analyses
Model building was guided by current literature and the best subsets method of minimum Akaike information criterion calculation for model parsimony. Model checking showed that the main dependent variables of IL-6, IL-10, and TNF-α were all approaching normal distribution; therefore, linear regression was used for analysis. Collinearity was checked for all covariates with variance inflation factors all less than 1.5. The final multiple linear regression model included age, race, sex, BMI, and a comprehensive count of medical problems. As this was a pilot study, no power analysis or sample size calculation was done.
Results
We recruited 49 adults with a mean age of 50.9 ± 25.9 years, including 9 men and 40 women. A majority (69.3%) of the sample identified as White. Their mean BMI was 26.4 ± 7.4 kg/m2, and the average number of chronic conditions was 2.4 ± 2.9. We compared sociodemographic data between participants with low and high self-efficacy scores, as shown in Table 1, and found that chronic disease count was the only covariate significantly associated with the main independent variable of coping self-efficacy (p = .023). Cronbach’s α for the Coping Self-Efficacy Scale for this sample was .90.
Table 1.
Sociodemographic Characteristics of Participants by Coping Self-Efficacy Group.
| Characteristic | Low Self-Efficacya, n = 12 | High Self-Efficacy, n = 37 | Total, N = 49 |
|---|---|---|---|
| Age (years), mean (SD) | 59.2 (23.3) | 48.3 (25.8) | 50.9 (25.4) |
| Sex, male:female (% female) | 2:10 (83.3) | 7:30 (81.1) | 9:40 (81.6) |
| Race, % White | 66.7 | 70.3 | 69.3 |
| BMIb (kg/m2), mean (SD) | 26.6 (8.1) | 26.4 (7.3) | 26.4 (7.4) |
| Chronic disease count, mean (SD) | 3.8 (3.9) | 1.92 (2.3) | 2.4* (2.9) |
Note. BMI = body mass index.
aLow self-efficacy refers to an average score of 7.0 or lower on the Coping Self-Efficacy Scale. High self-efficacy indicates a score of 7.1 or higher on the scale.
bBased on 43 observations, six persons did not provide their weight.
*p = .023, indicating that chronic disease count was significantly associated with coping self-efficacy group.
We included only samples with a coefficient of variance (CV) less than 20% in the final cytokine analyses, which amounted to 88%, 92%, and 73.5% of the samples for IL-6, IL-10, and TNF-α, respectively. Average CVs for IL-6, IL-10, and TNF-α were 6.7%, 6.3%, and 6.7%, respectively. We found no differences between participants (in sex, race, age, BMI, chronic-disease count, and coping self-efficacy) based on whether they were included or omitted because of CV in any of the three cytokine analyses. Intercorrelations between the cytokines were as follows: (1) between TNF-α and IL-6: .9450, (2) between TNF-α and IL-10: .9745, and (3) between IL-6 and IL-10: .9476.
We evaluated the associations of all covariates with the cytokine outcome variables, as shown in Table 2. As expected, age and chronic-disease counts were both positively associated with each of the cytokines. The variables of sex, race, and BMI were not associated with any of the cytokines.
Table 2.
Unadjusted Linear Regression Analyses Between Cytokines and Covariates.
| Covariate | IL-6, β (p value) | IL-10, β (p value) | TNF-α, β (p value) |
|---|---|---|---|
| Age | .001 (<.001) | .0007 (<.001) | .001 (<.001) |
| Sex (0 = male, 1 = female) | .005 (.772) | .003 (.799) | .005 (.809) |
| Race (0 = White, 1 = non-White) | −.010 (.428) | −.008 (.354) | −.013 (.345) |
| BMI | .001 (.091) | .0005 (.325) | .001 (.238) |
| Chronic disease count | .008 (<.001) | .005 (<.001) | .007 (<.001) |
Note. BMI = body mass index; IL = interleukin; TNF-α = tumor necrosis factor alpha.
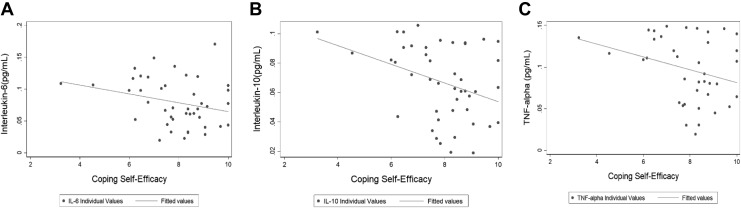
High self-efficacy was associated with lower mean levels of sweat IL-6 (.070 pg/mL ± .04), IL-10 (.061 ± .02), and TNF-α (.087 ± .04) compared to low self-efficacy (.107 ± .02, .087 ± .02, .131 ± .02, respectively). Each of these unadjusted regressions was statistically significant, indicating that those with higher cytokine values were less likely to report high coping self-efficacy (see Figure 1). These relationships remained significant after adjustment for age, sex, race, BMI, and chronic-disease count for IL-10 and TNF-α (β = −.017, p = .007; β = −.03, p = .028, respectively). For IL-6, the relationship with coping self-efficacy approached significance (β = −.22, p = .054) following adjustment, and it remained inversely associated with high coping self-efficacy. The ratios of pro- versus anti-inflammatory cytokines were not significantly different based on coping self-efficacy group (see Table 3).
Figure 1.
Scatter plots of each cytokine by coping self-efficacy score. Cutoff score between low and high coping self-efficacy groups was 7.0. (A) Interleukin (IL)-6. (B) IL-10. (C) Tumor necrosis factor alpha. All cytokine measures are provided in pg/mL.
Table 3.
Cytokine Values (Measured in Sweat) by Low and High Coping Self-Efficacy Groups and With Regression Models.
| Low Coping Self-Efficacy | High Coping Self-Efficacy | Unadjusted Model | Adjusted Modelb | |||||
|---|---|---|---|---|---|---|---|---|
| Sweat Biomarker | N | Mean (SD) | N | Mean (SD) | βa [CI] | p | β [CI] | p |
| IL-6, pg/mL | 11 | 0.107 (.02) | 32 | 0.070 (.04) | −.04 [−.06, −.01] | .003 | −.22 [−.04, .00] | .054 |
| IL-10, pg/mL | 11 | 0.087 (.02) | 34 | 0.061 (.02) | −.03 [−.04, −.01] | .002 | −.017 [−.03, −.01] | .007 |
| TNF-α, pg/mL | 9 | 0.131 (.02) | 29 | 0.087 (.04) | −.04 [−.07, −.02] | .002 | −.03 [−.05, −.003] | .028 |
| IL-6/IL-10 | 11 | 1.23 (.13) | 30 | 1.21 (.16) | −.02 [−.13, .09] | .765 | .007 [−0.11, .13] | .910 |
| TNF-α/IL-10 | 9 | 1.40 (.06) | 28 | 1.43 (.20) | .04 [−.10, .17] | .586 | .024 [−.13, .18] | .752 |
Note. CI = confidence interval; IL = interleukin; SD = standard deviation; TNF-α = tumor necrosis factor alpha.
aβ represents the difference in means between the low and high coping self-efficacy groups.
b Adjusted model includes the following covariates: age, sex, race, body mass index, and chronic disease count.
Discussion
In the present pilot study, we found an inverse association between high coping self-efficacy and levels of three inflammatory cytokines: IL-6, IL-10, and TNF-α. These findings support the work of Mausbach et al. (2011) showing that high coping self-efficacy buffered the effects of high role stress on IL-6 in an older caregiver population. We expanded on that previous work, however, by examining two pro- and one anti-inflammatory cytokine, each of which showed significant associations with coping self-efficacy. Further, the present study directly evaluates the association between coping self-efficacy and each of the cytokines rather than treating it as an interaction term involving stress burden. It also expanded the scope of the findings to include a larger age range.
The present study potentially aids in the interpretation of Bandura’s early work showing concomitant increases in B cells, T cells, and IL-2 as coping self-efficacy increased followed by decreases in each as coping self-efficacy stabilized at a higher level. It may be that the acquisition period for higher coping self-efficacy is itself a stressor that causes subsequent immune system changes. Once that acquisition work is completed, the immune system response decreases. And with subsequent uses of that developed high coping self-efficacy in the presence of an environmental stressor, it acts as a buffer to the stressor on immune-system response, which is consistent with the findings from both the present study and that of Mausbach et al.
The potential that self-efficacy could directly affect physiology or act as a buffer to environmental stressors on physiology is compelling. Self-efficacy is a malleable state, meaning that it can be increased and influenced throughout the life span. It may be that increasing self-efficacy could lead to greater health outcomes not only through its effect on health behavior but also through its direct effect on physiology. Unlike taking medications to dampen inflammation, improving self-efficacy has no deleterious side effects and may help in other realms of health and wellness. All of these factors make self-efficacy a notable potential treatment target for chronic diseases associated with a high inflammatory burden that can be monitored with physiologic data such as inflammatory biomarkers.
There are several limitations to this study. First, it is cross sectional, so we cannot establish causation or rule out the possibility that higher inflammation levels led to reports of lower self-efficacy. Although understanding causal pathways between inflammation and self-efficacy is important, it may be more critical to determine whether an intervention that leads people with low self-efficacy to obtain higher self-efficacy is associated with subsequent buffering from inflammation or decreased inflammatory biomarkers.
Second, the collection of cytokines in sweat is a novel method for which there are limited data. Small studies have shown some parallels between sweat and serum biomarker findings with a select number of stress-related proteins including cortisol, neuropeptide Y, and IL-6 (Cizza et al., 2008; Marques-Deak et al., 2006; Wilke et al., 2007). It is also unclear how the processes of sweat excretion, sweat rate, and water loss through the skin itself are influenced by other biological and environmental factors (Heikenfeld, 2016; Mena-Bravo & Luque de Castro, 2014). This dearth of knowledge makes the clinical interpretation of these data challenging. There is, however, a growing interest in the field of sweat science, with a number of research groups exploring the use of wearable sweat sensors, eccrine gland physiology, and protein partitioning into sweat (Mena-Bravo & Luque de Castro, 2014; Sonner et al., 2015).
Third, as a pilot study, this study included only 49 participants and involved no power analysis or sample-size calculations. These facts contribute to the need to repeat this study with a larger sample size. Lastly, dichotomization of the coping self-efficacy variable, while helpful with this small sample size in looking at group effects, creates a loss of information about individual differences and can make data interpretation more difficult (MacCallum, Zhang, Preacher, & Rucker, 2002).
Despite these limitations, the fact that we found a relationship between coping self-efficacy and levels of IL-6, IL-10, and TNF-α measured in sweat patches in a small sample of adults is intriguing, both for self-efficacy as an intervention target and for the emerging field of sweat science (Cizza et al., 2008; Heikenfeld, 2016; Hladek et al., 2017; Marques-Deak et al., 2006). Future studies with longitudinal designs and larger samples sizes will be helpful for this line of research.
Acknowledgment
The authors would like to thank those who helped with the collection and analysis of these data including Laken Roberts, Carolyn Sacko, and Dr. Young-Eun Cho.
Authors’ Note: The sponsors were not involved in the design, methods, subject recruitment, data collection, or analysis for the study or preparation of this article.
Author Contributions: Melissa Hladek contributed to conception and design, acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Jessica Gill contributed to conception and design, acquisition and interpretation; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. Chen Lai contributed to analysis and interpretation, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Kate Lorig contributed to conception, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Sarah Szanton contributed to conception and design, acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this research was provided in part by NIH/NCATS Pre-doctoral Fellowship, Clinical and Translational Science Award (4TL1TR001078-04); Dr. Scholl Foundation Fellowship; NIH/NINR Pre-doctoral Fellowship, Interdisciplinary Cardiovascular Health Research (T32NR012704); NIH/NINR Intramural Research Program; and Johns Hopkins University Provost’s Post-Doctoral Fellowship.
Supplemental Material: Supplemental material for this article is available upon request.
References
- Bandura A. (1993). Perceived self-efficacy in cognitive development and functioning. Educational Psychologist, 28, 117–148. doi:10.1207/s15326985ep2802_3 [Google Scholar]
- Bandura A. (1997). Self-Efficacy: The exercise of control. New York, NY: W. H. Freeman. [Google Scholar]
- Bandura A., Cioffi D., Taylor C. B., Brouillard M. E. (1988). Perceived self-efficacy in coping with cognitive stressors and opioid activation. Journal of Personality and Social Psychology, 55, 479–488. Retrieved from https://www.uky.edu/∼eushe2/Bandura/Bandura1988JPSP.pdf [DOI] [PubMed] [Google Scholar]
- Bandura A., Taylor C. B., Williams S. L., Mefford I. N., Barchas J. D. (1985). Catecholamine secretion as a function of perceived coping self-efficacy. Journal of Consulting and Clinical Psychology, 53, 406–414. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4008724 [DOI] [PubMed] [Google Scholar]
- Chesney M. A., Neilands T. B., Chambers D. B., Taylor J. M., Folkman S. (2006). A validity and reliability study of the coping self-efficacy scale. British Journal of Health Psychology, 11, 421–437. doi:10.1348/135910705X53155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizza G., Marques A. H., Eskandari F., Christie I. C., Torvik S., Silverman M. N.…Sternberg E. M. (2008). Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: The POWER study. Biological Psychiatry, 64, 907–911. doi:10.1016/j.biopsych.2008.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concheiro M., Shakleya D. M., Huestis M. A. (2011). Simultaneous analysis of buprenorphine, methadone, cocaine, opiates and nicotine metabolites in sweat by liquid chromatography tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 400, 69–78. doi:10.1007/s00216-010-4392-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot V., Beckerman H., Lankhorst G. J., Bouter L. M. (2003). How to measure comorbidity: A critical review of available methods. Journal of Clinical Epidemiology, 56, 221–229. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12725876 [DOI] [PubMed] [Google Scholar]
- Everett B. M., Pradhan A. D., Solomon D. H., Paynter N., Macfadyen J., Zaharris E.…Ridker P. M. (2013). Rationale and design of the cardiovascular inflammation reduction trial: A test of the inflammatory hypothesis of atherothrombosis. American Heart Journal, 166, 199–207. doi:10.1016/j.ahj.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerteis J., Izrael D., Deitz D., LeRoy L., Ricciardi R., Miller T., Basu J. (2014). Multiple chronic conditions chartbook [AHRQ Publications No. Q14-0038]. Rockville, MD: Agency for Healthcare Research and Quality; Retrieved from http://www.ahrq.gov/professionals/prevention-chronic-care/decision/mcc/mccchartbook.pdf [Google Scholar]
- Heikenfeld J. (2016). Non-invasive analyte access and sensing through eccrine sweat: Challenges and outlook circa 2016. Electroanalysis, 28, 1242–1249. doi:10.1002/elan.201600018 [Google Scholar]
- Hladek M. D., Szanton S. L., Cho Y.-E., Lai C., Sacko C., Roberts L., Gill J. (2017). Using sweat to measure cytokines in older adults compared to younger adults: A pilot study. Journal of Immunological Methods. doi:10.1016/j.jim.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladek M. D., Szanton S. L., Cho Y.-E., Lai C., Sacko C., Roberts L., Gill J. (2018). Using sweat to measure cytokines in older adults compared to younger adults: A pilot study. Journal of Immunological Methods, 454, 1–5. doi:10.1016/j.jim.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley A. L., Johnson R., Purdy S., Valderas J. M., Salisbury C. (2012). Measures of multimorbidity and morbidity burden for use in primary care and community settings: A systematic review and guide. Annals of Family Medicine, 10, 134–141. doi:10.1370/afm.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig K., Ritter P. L., Villa F. J., Armas J. (2009). Community-based peer-led diabetes self-management: A randomized trial. The Diabetes Educator, 35, 641–651. doi:10.1177/0145721709335006 [DOI] [PubMed] [Google Scholar]
- Lukic L., Lalic N. M., Rajkovic N., Jotic A., Lalic K., Milicic T.…Gajovic J. S. (2014). Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and IL-6 cytokine levels: Potential targets for an efficient preventive intervention. International Journal of Environmental Research and Public Health, 11, 3586–3598. doi:10.3390/ijerph110403586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum R. C., Zhang S., Preacher K. J., Rucker D. D. (2002). On the practice of dichotomization of quantitative variables. Psychological Methods, 7, 19–40. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11928888 [DOI] [PubMed] [Google Scholar]
- Marques-Deak A., Cizza G., Eskandari F., Torvik S., Christie I. C., Sternberg E. M., Phillips T. M. (2006). Measurement of cytokines in sweat patches and plasma in healthy women: Validation in a controlled study. Journal of Immunological Methods, 315, 99–109. doi:10.1016/j.jim.2006.07.011 [DOI] [PubMed] [Google Scholar]
- Mausbach B. T., von Kanel R., Roepke S. K., Moore R., Patterson T. L., Mills P. J., Grant I. (2011). Self-efficacy buffers the relationship between dementia caregiving stress and circulating concentrations of the proinflammatory cytokine interleukin-6. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 19, 64–71. doi:10.1097/JGP.0b013e3181df4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Bravo A., Luque de Castro M. D. (2014). Sweat: A sample with limited present applications and promising future in metabolomics. Journal of Pharmaceutical and Biomedical Analysis, 90, 139–147. doi:10.1016/j.jpba.2013.10.048 [DOI] [PubMed] [Google Scholar]
- Moynagh P. N. (2005). The NF-B pathway. Journal of Cell Science, 118, 4589–4592. doi:10.1242/jcs.02579 [DOI] [PubMed] [Google Scholar]
- Rissin D. M., Fournier D. R., Piech T., Kan C. W., Campbell T. G., Song L.…Duffy D. C. (2011). Simultaneous detection of single molecules and singulated ensembles of molecules enables immunoassays with broad dynamic range. Analytical Chemistry, 83, 2279–2285. doi:10.1021/ac103161b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M., O’Garra A. (2010). The regulation of IL-10 production by immune cells. Nature Reviews Immunology, 10, 170–181. doi:10.1038/nri2711 [DOI] [PubMed] [Google Scholar]
- Sarkar U., Ali S., Whooley M. A. (2009). Self-efficacy as a marker of cardiac function and predictor of heart failure hospitalization and mortality in patients with stable coronary heart disease: Findings from the heart and soul study. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 28, 166–173. doi:10.1037/a0013146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner Z., Wilder E., Heikenfeld J., Kasting G., Beyette F., Swaile D.…Naik R. (2015). The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics, 9, 031301 doi:10.1063/1.4921039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Koido Y., Aiboshi J., Yamashita T., Suzaki S., Kurokawa A. (1999). The ratio of interleukin-6 to interleukin-10 correlates with severity in patients with chest and abdominal trauma. The American Journal of Emergency Medicine, 17, 548–551. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10530532 [DOI] [PubMed] [Google Scholar]
- Varadhan R., Yao W., Matteini A., Beamer B. A., Xue Q. L., Yang H.…Walston J. (2014). Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69, 165–173. doi:10.1093/gerona/glt023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. W., Schiller J. S., Goodman R. A. (2014). Multiple chronic conditions among US adults: A 2012 update. Preventing Chronic Disease, 11, E62 doi:10.5888/pcd11.130389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenfeld S. A., O’Leary A., Bandura A., Brown S., Levine S., Raska K. (1990). Impact of perceived self-efficacy in coping with stressors on components of the immune system. Journal of Personality and Social Psychology, 59, 1082–1094. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2148350 [DOI] [PubMed] [Google Scholar]
- Wilke K., Martin A., Terstegen L., Biel S. S. (2007). A short history of sweat gland biology. International Journal of Cosmetic Science, 29, 169–179. doi:10.1111/j.1467-2494.2007.00387.x [DOI] [PubMed] [Google Scholar]
- Zulman D. M., Rosland A. M., Choi H., Langa K. M., Heisler M. (2012). The influence of diabetes psychosocial attributes and self-management practices on change in diabetes status. Patient Education and Counseling, 87, 74–80. doi:10.1016/j.pec.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]