Abstract
Transcranial direct current stimulation (tDCS) has demonstrated effectiveness in reducing clinical and experimental measures of pain in patients with chronic pain; however, research examining the mechanisms of action for the effects of tDCS has been lacking. The present study investigated the effect of active tDCS on measures of inflammation and stress. Older adults (aged 50–70 years) with knee osteoarthritis (OA) were randomly assigned to receive daily 20-min sessions of either tDCS (n = 20) or sham tDCS (n = 20) for 5 consecutive days. Participants provided blood samples at baseline and the end of treatment. The following measures of immune function and stress were collected: interleukin (IL)-6 and 10, tumor necrosis factor-α (TNF-α), C-reactive protein, cortisol, and β-endorphin. Generalized linear modeling evaluated each posttreatment measure as a function of tDCS group, controlling for baseline (measuring residual change, analogous to analysis of covariance). Bayesian statistical inference was used to directly quantify the probability of the effect of active tDCS. IL-6, IL-10, TNF-α, and β-endorphin demonstrated lower levels of stress and inflammation in the active tDCS group. These findings provide preliminary evidence that active (relative to sham) tDCS is associated with reduced levels of inflammation.
Keywords: transcranial direct current stimulation, knee osteoarthritis, inflammation, Bayesian statistical inference
Osteoarthritis (OA) is the most common type of arthritis and a leading cause of pain and disability among older adults (Loeser, Goldring, Scanzello, & Goldring, 2012). OA is progressive and leads to functional decline as well as loss in quality of life, with important health-related and societal costs (Pereira, Ramos, & Branco, 2015).
OA typically affects weight-bearing joints, particularly the knee (Jamshidi, Pelletier, & Martel-Pelletier, 2019). Approximately 10% of people age 60 years or older experience significant pain, physical dysfunction, and reduced quality of life as a result of knee OA (Vos et al., 2012). In addition to pain, individuals with chronic knee OA pain may have sleep disorders, depression, and other issues, with implications for public health costs (Tavares et al., 2018). Age is the main risk factor for knee OA, the prevalence of which is expected to rise due to the increase in population life expectancy. Other common risk factors include female sex, prior joint injury, obesity, and mechanical factors such as malalignment and abnormal joint shape (Loeser et al., 2012).
At present, the treatment of knee OA is effective only at relieving symptoms and/or pain (Jamshidi et al., 2019). This treatment usually consists of a combination of pharmacological and nonpharmacological therapies (Hochberg et al., 2012) that, while they may alleviate pain, can also cause adverse effects (Tavares et al., 2018). Additionally, treatment efficacy and compliance may decrease over time (Reinecke et al., 2015). Currently, there are no disease-modifying therapies for knee OA. The development of such therapies is hampered by (1) the late disease diagnosis, which typically occurs during moderate-to-severe stages, when the joint tissue has already become irreversibly damaged and (2) the uncertainty about the complex disease pathophysiology (Jamshidi et al., 2019). The etiology and pathological mechanisms involved in knee OA are still a matter of debate; however, some research has shown that inflammation contributes to the progression of OA (Berenbaum, 2013; Goldring & Otero, 2011; Zhu et al., 2018) and is a main factor linking cartilage loss and disease symptoms including joint pain, swelling, and stiffness (Sellam & Berenbaum, 2010).
Recently, there has been growing interest in using transcranial direct current stimulation (tDCS) to reduce knee OA–associated pain. tDCS is a noninvasive brain stimulation technique that consists of applying direct current over the scalp using two electrodes: the anode, which increases cortical excitability locally, and the cathode, which has opposite effects (Dasilva et al., 2012; Lefaucheur et al., 2017). tDCS has promise for treating chronic pain due to the involvement of multiple neurotransmitter functions (Medeiros et al., 2012). However, the neurobiological effects involved in tDCS are not well understood: Few studies have investigated the effects of tDCS on immune/inflammatory markers, and the mechanisms of action are not clear (Leffa et al., 2018). In the current secondary analysis, we sought to explore the effects of active tDCS (vs. sham) on measures of inflammation and stress in patients with knee OA.
Method
Participants
Detailed selection criteria and enrollment procedures were published and described previously (Ahn et al., 2017). Briefly, 40 participants between the ages of 50 and 70 with symptomatic knee OA were recruited in North Central Florida. Participants were excluded if they had any concurrent medical conditions that could confound interpretation of outcome measures, pose a safety risk for any of the assessment or intervention, or preclude successful completion of the protocol, including history of stroke, seizure, brain tumor, brain surgery, or intracranial metal implantation.
The Institutional Review Board of the University of Florida approved the study prior to commencement. Investigators provided all participants with detailed information about the protocol and obtained written informed consent from all participants.
tDCS Intervention
tDCS (intensity of 2 mA) was applied for 20 min/1 day for 5 consecutive days using a pair of saline-saturated rectangular sponge electrodes (35 cm2). The anode was placed over C3 or C4 (10–20 systems for electrode placement) contralateral to the affected knee, and the cathode was placed over the SO contralateral to the anode (M1-SO montage). Stimulation was given to the resting cortex and not in association with any moment or other intervention. For sham tDCS, electrodes were placed in an identical configuration but only included ramp-up/ramp-down periods (30 s each) at the beginning and end of treatment to mimic perception of active tDCS.
Outcome Measures
Researchers collected seven measures of inflammation and stress as part of data gathering for the present study: β-endorphin, C-reactive protein (CRP), cortisol, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and IL-10. These measures comprised the exhaustive list of inflammation and stress measures evaluated. Due to excessive missingness (∼80%), we excluded IL-1β from the present analysis. We addressed all missingness in the remaining outcomes (approximately split evenly between groups) via listwise deletion for complete case analyses: β-endorphin (n = 3 cases missing: 1 from the active tDCS group, 2 from sham), CRP (n = 4 cases missing: 2 from the active tDCS group, 2 from sham), and IL-6 (n = 18 cases missing: 11 from the active group, 7 from sham). Analyses for cortisol, TNF-α, and IL-10 had no missing data.
Blood draws occurred before commencing treatment on Day 1 and after the fifth treatment was completed on Day 5. Blood was drawn into ethylenediaminetetraacetic acid plasma tubes. Samples were inverted 5 times and kept on ice until further processing. Within 30 min of collection, samples were centrifuged at 1,600 × g for 15 min at 4 ºC, aliquoted, and immediately stored in a −80 ºC freezer. For β-endorphin measurements, prior to centrifugation, aprotinin was added (0.6 trypsin inhibitory units/ml of blood) and the tubes gently mixed several times to inhibit activity of proteinases.
Solid-phase extraction of plasma samples was performed using an Oasis Hydrophilic-Lipophilic-Balanced (HLB) (30 mg) 96-well plate along with a vacuum manifold (Waters Corp., Milford, MA, USA), following manufacturer’s recommendations. Briefly, the plate was conditioned with acetonitrile and equilibrated 2 times with 0.1% trifluoroacetic acid (TFA) in high-performance liquid chromatography (HPLC)-grade water. Samples were acidified with 1% TFA (1:1) and loaded onto the plate. The plate was washed 3 times with 0.1% TFA in HPLC-grade water. Samples were eluted in 60% acetonitrile/40% HPLC-grade water/0.1% TFA and dried in Savant AES1010 Automatic Environmental SpeedVAC w/VaporNet Radiant Cover. Samples were reconstituted using the original sample volume in assay buffer.
β-endorphin was measured in duplicate in the extracted plasma samples with an enzyme immunoassay method using a commercial kit (cat# EK-022-14; Phoenix Pharmaceutical Inc., Belmont, CA). The intra- and interassay coefficients of variation (CVs) were <6.5% and <10%, respectively.
Plasma levels of CRP and cortisol were measured in duplicate using enzyme-linked immunosorbent assays according to the instructions of the manufacturers (cat# DCRP00, R&D Systems, Minneapolis, MN; cat# ADI-900-071, Enzo Life Sciences, Inc., Farmingdale, NY, respectively). The average intra-assay CV was <10% for CRP and <5% for cortisol. Interassay CV was <7% for CRP and <10% for cortisol.
Plasma levels of TNF-α, IL-1β, IL-6, and IL-10 were measured in triplicate using a commercial multiplex immunoassay kit (cat# HCYTMAG-60Kl, MilliporeSigma, Burlington, MA) and analyzed on a MILLIPLEX® Analyzer 3.1 xPONENT system. Data acquisition was accomplished with the same system and data analysis via MILLIPLEX Analyst software. The intra- and interassay CVs were <19% for all of the biomarkers.
Data Analytic Strategy
Sample characteristics were evaluated via descriptive statistics including measures of frequency and central tendency (i.e., mean and standard deviation). Demographic variables including age, race, sex, income, education, employment status, marital status, and OA severity were screened as potential confounders of the relationship between treatment condition and inflammatory measures following established procedures (Pocock, Assmann, Enos, & Kasten, 2002). None of these potential confounders demonstrated a relationship with treatment condition; we therefore excluded them from subsequent models. Preliminary correlational analyses explored the interrelatedness of the six outcome measures by testing the pairwise relationship between difference scores. Only 1 of 15 pairwise tests demonstrated statistical significance (change scores between CRP and IL-10 were positively related: Spearman’s ρ = .35). Further exploration of this relationship, however, was considered beyond the scope of the present article.
Generalized linear modeling (GLM) was used to evaluate differences in poststudy levels of inflammatory/stress markers between active and sham tDCS treatment groups, adjusted for baseline levels using established recommendations for examining pre- versus posttest data (Vickers & Altman, 2001). In this approach, GLM was used to model each of the six markers at poststudy as a function of treatment while covarying for measurement at baseline (i.e., residual change modeling, akin to analysis of covariance). This manner of statistical modeling has demonstrated unbiased estimates of treatment effects even in the presence of nonequality at baseline (Senn, 2006).
Bayesian statistical inference was utilized to quantify the probability that an effect of active tDCS treatment exists given the present data and weakly informative priors (∼Normal [µ = 0, σ2 = 100]) to maximize the influence of the present data on the posterior distribution. Parameter estimates in this context are derived from the posterior distribution that captures the uncertainty surrounding the magnitude of an effect (Gelman & Hill, 2007). Graphical analysis of histograms indicated skewed distributions for each of the outcome measures. This skew was addressed by modeling each outcome using the lognormal distribution. Suitability of the lognormal distribution to model each outcome was assessed and confirmed via visual inspection of posterior predictive checking graphical plots. Posterior predictive checking proceeded by visually confirming that the observed distribution of each outcome followed (and was entirely subsumed by) the range of distributions produced by 1,000 replications of the outcome from the posterior predictive distribution. Analyses were performed in the R statistical computing environment (R Core Team, 2018) using the packages Rstan Version 2.18.2 (Stan Development Team, 2018) and brms Version 2.6.0 (Bürkner, 2017).
We chose Bayesian statistical inference for the present analysis for three primary reasons. First, the Bayesian posterior probability (PP) provides a straightforward interpretation of the alternative hypothesis that an effect exists. Rather than relying on the traditional frequentist p value, which relates the probability that a value is equal to (or more extreme than) an observed value given that the null hypothesis is true, Bayesian inference directly quantifies the probability that a parameter is nonzero. Second, Bayesian inference may be better equipped for examining small samples with appropriately informative priors (Gelman, 2006; McNeish, 2016). Third, the current research favors Bayesian inference for the manner in which it purposefully deviates from traditionally problematic null hypothesis significance testing in favor of incremental knowledge gain. With the present research, we aim to provide exhaustive results with minimal bias and maximum transparency while also allowing other researchers the opportunity to consider their own subjective threshold that effects are nonzero. Estimates taken from the posterior distribution may be directly applied as iteratively more informative priors in future research. A detailed description of the posterior distribution and its interpretation is provided in the Results section.
In the present study, we considered a PP ≥ 75% that an effect of treatment exists worthy of further investigation; disparate researchers can and should consider their own subjective probability threshold that an effect exists. We chose this value for its (a) consistency with previous work using these data (Ahn et al., 2018), (b) similarity to thresholds researchers chose in other recent trials investigating treatment effects (Bauer et al., 2018; Cazala et al., 2018; Schmitz et al., 2017), and (c) correspondence with the scientific and clinical opinion of the present research team that the current research would be worthy of further investigation if there was at least a 3 in 4 chance that the alternative hypothesis was correct. For further justification of the use of Bayesian inference in the present study (including a brief discussion of frequentist vs. Bayesian inference), see a previous inspection of these data regarding the effects of tDCS on experimental pain measures (Ahn et al., 2018).
Results
Sample Characteristics
Participants (N = 40) were randomly assigned to active tDCS (n = 20) or sham tDCS (n = 20). None of the measured demographic variables were significantly different as a function of treatment group. Participants were split almost evenly by sex (52.5% female), evenly split by race (50% Asian, 50% Caucasian), with mean age = 59.9 years (SD = 9.13 years). A majority were married (75%), had more than a high school education (85%), and were either working (52.5%) or retired (27.5%). Demographic characteristics of the sample by tDCS group are provided in Table 1.
Table 1.
Demographic Characteristics of the Sample.
| Sham tDCS (n = 20) | Active tDCS (n = 20) | |
|---|---|---|
| Measure | ||
| Sex, female | 11 (55) | 10 (50) |
| Race | ||
| Asian | 10 (50) | 10 (50) |
| Caucasian | 10 (50) | 10 (50) |
| Education | ||
| High school | 3 (15) | 3 (15) |
| 2 years college | 5 (25) | 2 (10) |
| College | 5 (25) | 7 (35) |
| Master’s | 3 (15) | 3 (15) |
| Doctoral | 4 (20) | 5 (25) |
| Employment | ||
| Working | 11 (55) | 10 (50) |
| Retired | 5 (25) | 6 (30) |
| Unemployed/other | 4 (20) | 4 (20) |
| Marital status | ||
| Married | 15 (75) | 15 (75) |
| Other | 5 (25) | 5 (25) |
| Age (years), mean (SD) | 59.3 (8.6) | 60.6 (9.8) |
| BMI (kg/m2), mean (SD) | 26.0 (4.1) | 27.0 (3.3) |
Note. Data are presented as frequencies (n), unless otherwise noted. There were no significant differences between groups on any of these characteristics. tDCS = transcranial direct current stimulation.
Modeling Overview
Table 2 presents means and standard deviations for each of the six primary outcome measures for each treatment group. In Table 3, we present a point estimate, 95% credible interval (CrI), and PP for the effect of active tDCS on each of the outcome measures, with probabilities ≥ 75% noted in bold.
Table 2.
Mean (SD) Levels of Inflammatory Markers by Group and Time.
| Sham tDCS (n = 20) | Active tDCS (n = 20) | |||
|---|---|---|---|---|
| Measure | Baseline | Posttreatment | Baseline | Posttreatment |
| IL-10 (pg/ml) | 8.91 (5.36) | 8.17 (4.56) | 6.25 (3.2) | 5.68 (2.85) |
| IL-6 (pg/ml) | 3.57 (3.65) | 3.37 (2.62) | 2.12 (1.78) | 2.4 (1.43) |
| TNF-α (pg/ml) | 10.2 (5.78) | 10.36 (5.98) | 7.63 (3.42) | 7.41 (3.27) |
| CRP (ng/nl) | 1,758.26 (2,833.17) | 3,372.98 (5,490.5) | 1,394.33 (1,278.41) | 1,496.74 (1,469.79) |
| Cortisol (pg/ml) | 39,358.75 (29,387.62) | 38,853.23 (24,343.37) | 47,085.48 (36,657.95) | 46,588.5 (32,249.18) |
| β-endorphin (ng/nl) | 0.04 (0.02) | 0.05 (0.02) | 0.04 (0.01) | 0.04 (0.02) |
Note. CRP = C-reactive protein; IL = interleukin; tDCS = transcranial direct current stimulation; TNF-α = tumor necrosis factor-α.
Table 3.
Effect of Active tDCS on Inflammatory Markers.
| Measure | Point Estimate | 95% Credible Interval | Posterior Probability |
|---|---|---|---|
| IL-10 | −.1156 | [−.3849, .1569] | 80.36% |
| IL-6 | −.2406 | [−.7754, .2849] | 82.14% |
| TNF-α | −.0729 | [−.2612, .1184] | 77.86% |
| CRP | −.1266 | [−.6866, .4358] | 67.48% |
| Cortisol | −.0320 | [−.6398, .5717] | 54.26% |
| β-endorphin | −.2693 | [−.6600, .1234] | 91.42% |
Note. Summary of outcome measures from Bayesian generalized linear modeling analyses for each outcome, describing the Bayesian estimate of the effect, the lower and upper 95% credible intervals, and the maximum nonzero posterior probability, with ≥ 75% as the criterion for a group effect shown in bold typeface. CRP = C-reactive protein; IL = interleukin; tDCS = transcranial direct current stimulation; TNF-α = tumor necrosis factor-α.
Outcome measures, with probabilities ≥ 75% noted in bold.
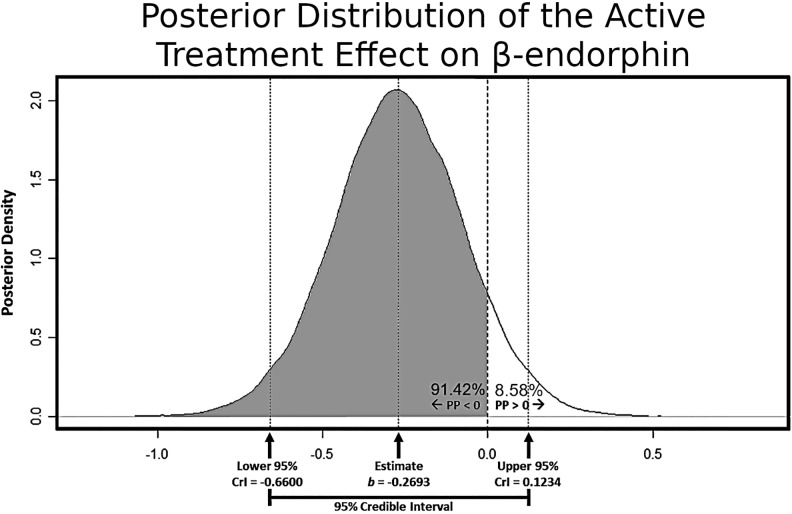
Interpretation of results from Bayesian statistical inference requires an understanding of the posterior distribution, that is, the distribution of values capturing the uncertainty in the magnitude and direction of an effect in a statistical model, given the present data and a set prior distribution. Figure 1 depicts the posterior distribution that captures the uncertainty in estimating the effect of active tDCS (relative to sham) on β-endorphin in this study. The probability that the effect is negative (91.42%) is captured by the gray-shaded region to the left of the value 0 on the x-axis. The corresponding probability that the effect is positive (8.58%) is captured by the nonshaded region to the right of the value 0 on the x-axis. In the present study, a PP > 75% in either direction was taken as noteworthy evidence that an effect was nonzero. The mean of the posterior distribution is captured as the highest probability point estimate of the effect; here, b = −.2693, with a 95% CrI ranging from −.66 to +.12.
Figure 1.
Posterior distribution for the effect of active transcranial direct current simulation (tDCS; relative to sham) on β-endorphin. Arrows along the x-axis highlight the point estimate of the effect as well as its corresponding 95% credible interval. The gray-shaded region depicts the posterior probability that the effect exists in the negative direction. This figure demonstrates that analyses found a 91.42% probability that active tDCS (relative to sham) was related to lower β-endorphin.
We found that the effect of active tDCS was more likely to be negative for each outcome, indicating lower levels of inflammatory cytokines and β-endorphin for the active tDCS group as compared to the sham tDCS group. Controlling for pretreatment levels, analyses of posttreatment levels demonstrated that patients subjected to the active tDCS presented PPs that exceeded the probability threshold for IL-10 (PP = 80%, b = −.12, 95% CrI [−.39, .16]), IL-6 (PP = 82%, b = −.24, 95% CrI [−.78, .29]), and TNF-α (PP = 78%, b = −.07, 95% CrI [−.26, .12]). Analyses of posttreatment levels of CRP and cortisol (controlling for pretreatment levels) did not find evidence for differences between groups (PP = 67% and 54% for CRP and cortisol, respectively). Analysis of posttreatment levels of β-endorphin (controlling for baseline levels) found a 91% PP of lower levels for active tDCS relative to sham (b = −.27, 95% CrI [−.66, .12]).
Discussion
tDCS has shown promising results in patients with knee OA (Ahn et al., 2017; Chang et al., 2017; da Graca-Tarragó et al., 2019). However, there has been a lack of research that defines the mechanisms associated with pain reduction after tDCS. In the present study, we investigated the effects of active, relative to sham, tDCS on inflammatory and stress markers in patients with knee OA. Controlling for baseline levels of inflammation, we demonstrated that patients subjected to active tDCS presented reduced levels of inflammatory cytokines IL-6, IL-10, and TNF-α as well as of β-endorphin. These findings build on previous findings of reduction in pain for older adults with knee OA (Ahn et al., 2018). To the best of our knowledge, this is the first study that evaluated inflammatory markers after tDCS treatment in knee OA.
Previous studies have evaluated the immunomodulatory effects of tDCS in psychiatric conditions. In a recent clinical trial comparing the efficacy of tDCS and escitalopram in major depression, researchers found that plasma levels of cytokines and neurotrophic factors were not associated with tDCS effects (Brunoni et al., 2018). Although the authors observed that the blood levels of several inflammatory molecules (IL-12p70, IL-10, IL1-β, IL-8, and sTNFr1) decreased over time, this effect was independent of treatment. The same research team had previously demonstrated that the acute antidepressant effects of tDCS and sertraline (separately and combined) did not specifically involve normalization of the immune system. They found that cytokine levels (except TNF-α) decreased over time but that this effect was similar across the different intervention groups and in both responders and nonresponders (Brunoni et al., 2013). These results demonstrate that the antidepressant effects of tDCS are not associated with normalization of immunological activity in patients with depression. However, it is worth mentioning that the mechanisms associated with depression are very complex and differ from those involved in pain due to knee OA. Therefore, it is possible that the reduced pain in knee OA after active tDCS (relative to sham) is associated with anti-inflammatory/immunomodulatory activity.
The present findings corroborate previous studies that have demonstrated the effects of tDCS on inflammatory markers in preclinical models of pain. In an animal model of chronic stress-induced pain, treatment with tDCS reduced hippocampal levels of TNF-α (in addition to the analgesic effect; Spezia et al., 2012). tDCS treatment also decreased IL-1β levels in a rat model of neuropathic pain (Cioato et al., 2016). Given the effects of tDCS in pain induced by active peripheral inflammation, it is possible that its modulatory effects on pain sensation involve an anti-inflammatory mechanism (Laste et al., 2012). Leffa et al. (2018) demonstrated the immunomodulatory effects of tDCS in a rat model of attention-deficit/hyperactivity disorder. Besides improving memory, tDCS reduced brain protein levels of TNF-α and IL-1β. However, the treatment did not change the levels of IL-10, an anti-inflammatory cytokine.
In one previous clinical trial, investigators evaluated β-endorphin levels after treatment with tDCS in patients with fibromyalgia (Khedr et al., 2017). Patients with primary fibromyalgia were randomized to receive active or sham tDCS of the left motor cortex (M1) daily for 10 days. After 10 sessions of active tDCS, patients presented relieved pain, improvement in mood, and increased β-endorphin levels. These findings followed from research that increased levels of β-endorphin are associated with pain relief (Chaudhry & Bhimji, 2018). Nevertheless, in the present study, we observed that patients subjected to active tDCS presented lower posttreatment levels of β-endorphin than patients who received sham tDCS. It is possible that this effect resulted from the significant placebo response associated with the sham tDCS. On the other hand, it may be possible that active tDCS mitigates pain to the extent that β-endorphin does not increase in the same way that it otherwise might.
The present study may be limited in that blood sample collection was not limited to a specific time of the day; instead, we accommodated each participant’s varying daily routine to allow flexibility in the scheduling of the blood draw before the first and after the fifth treatment. Further, as there were no follow-up evaluations, we cannot establish the long-term effects of treatment from our findings. Moreover, the present results do not evaluate overall change in inflammation over time; instead, they compare changes in inflammation between groups by measuring differences at posttest while controlling for pretest (so-called residual change). This approach allows direct estimation of the probability that there was an effect of active tDCS relative to sham.
The findings from the present study provide a foundation for future nursing research investigating the mechanisms by which active tDCS influences inflammation. First, conducting a future study with a larger sample and longer follow-up assessment should extend and validate our findings of the effects of tDCS on markers of inflammation in adults with OA pain. Second, future studies that incorporate additional biological and psychological measures could further elucidate underlying mechanisms that contribute to the effects of tDCS. The present study demonstrated that active tDCS (as compared to sham tDCS) is associated with reduced levels of IL-6, IL-10, TNF-α, and β-endorphin. These findings suggest that treatment with active tDCS may have therapeutic benefits over and above sham tDCS for reduction of inflammation in patients with knee OA.
Acknowledgments
The authors wish to thank Brian Bouverat, Jini Curry, and Marvin Dirain for their technical assistance with the extractions and immunoassays.
Footnotes
Author Contributions: Robert Suchting contributed to analysis and interpretation, drafted the article, critically revised the article, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Gabriela D. Colpo contributed to interpretation, drafted the article, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Natalia P. Rocha contributed to interpretation, critically revised the article, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Hyochol Ahn contributed to conception, design, and interpretation; drafted the article; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by the Claude D. Pepper Older Americans Independence Center (P30 AG028740) and the University of Florida Center for Cognitive Aging and Memory. The funding agencies had no role in the study design, methods, or data collection and analysis or in the preparation of the article.
ORCID iDs: Robert Suchting  https://orcid.org/0000-0002-2822-3754
https://orcid.org/0000-0002-2822-3754
Hyochol Ahn  https://orcid.org/0000-0002-9998-4876
https://orcid.org/0000-0002-9998-4876
References
- Ahn H., Suchting R., Woods A. J., Miao H., Green C., Cho R. Y.…Fillingim R. B. (2018). Bayesian analysis of the effect of transcranial direct current stimulation on experimental pain sensitivity in older adults with knee osteoarthritis: Randomized sham-controlled pilot clinical study. Journal of Pain Research, 11, 2071–2082. doi:10.2147/JPR.S173080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn H., Woods A., Kunik M., Bhattacharjee A., Chen Z., Choi E., Fillingim R. (2017). Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: An experimenter- and participant-blinded, randomized, sham-controlled pilot clinical study. Brain Stimulation, 10, 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer I. E., Suchting R., Cazala F., Alpak G., Sanches M., Nery F. G.…Soares J. C. (2018). Changes in amygdala, cerebellum, and nucleus accumbens volumes in bipolar patients treated with lamotrigine. Psychiatry Research: Neuroimaging, 278, 13–20. [DOI] [PubMed] [Google Scholar]
- Berenbaum F. (2013). Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis and Cartilage, 21, 16–21. [DOI] [PubMed] [Google Scholar]
- Brunoni A., Machado-Vieira R., Zarate C., Valiengo L., Vieira E., Benseñor I.…Teixeira A. (2013). Cytokines plasma levels during antidepressant treatment with sertraline and transcranial Direct Current Stimulation (tDCS): Results from a factorial, randomized, controlled trial. Psychopharmacology, 231, 1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A., Padberg F., Vieira E. L. M., Teixeira A. L., Carvalho A. F., Lotufo P. A.…Benseñor I. M. (2018). Plasma biomarkers in a placebo-controlled trial comparing tDCS and escitalopram efficacy in major depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 86, 211–217. [DOI] [PubMed] [Google Scholar]
- Bürkner P. (2017). Brms: An R package for bayesian multilevel models using Stan. Journal of Statistical Software, 80, 1–28.30220889 [Google Scholar]
- Cazala F., Suchting R., Zeni C. P., Bauer I. E., Mwangi B., Wu M. J.…Soares J. C. (2018). Effects of valproate on brain volumes in pediatric bipolar disorder: A preliminary study. Psychiatry Research: Neuroimaging, 278, 65–68. [DOI] [PubMed] [Google Scholar]
- Chang W., Bennell K., Hodges P., Hinman R., Young C., Buscemi V.…Schabrun S. (2017). Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: A pilot randomised controlled trial. PLoS One, 12, e0180328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry S. R., Bhimji S. S. (2018). Biochemistry, endorphin In StatPearls. Treasure Island, FL: StatPearls; Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/29262177 [Google Scholar]
- Cioato S., Medeiros L., Marques Filho P., Vercelino R., de Souza A., Scarabelot V.…Cioato L. (2016). Long-lasting effect of transcranial direct current stimulation in the reversal of hyperalgesia and cytokine alterations induced by the neuropathic pain model. Brain Stimulation, 9, 209–217. [DOI] [PubMed] [Google Scholar]
- da Graca-Tarragó M., Lech M., Angoleri L. D. M., Santos D. S., Deitos A., Brietzke A. P.…Caumo W. (2019). Intramuscular electrical stimulus potentiates motor cortex modulation effects on pain and descending inhibitory systems in knee osteoarthritis: A randomized, factorial, sham-controlled study. Journal of Pain Research, 12, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasilva A. F., Mendonca M. E., Zaghi S., Lopes M., Dossantos M. F., Spierings E. L.…Fregni F. (2012). TDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache, 52, 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A. (2006). Prior distribution for variance parameters in hierarchical models. Bayesian Analysis, 1, 515–533. [Google Scholar]
- Gelman A., Hill J. (2007). Data analysis using regression and multilevel/hierarchical models. New York, NY: Cambridge University Press. [Google Scholar]
- Goldring M., Otero M. (2011). Inflammation in osteoarthritis. Curr Opin Rheumatol, 23, 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg M. C., Altman R. D., April K. T., Benkhalti M., Guyatt G., McGowan J.…Tugwell P. (2012). American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care and Research, 64, 465–474. [DOI] [PubMed] [Google Scholar]
- Jamshidi A., Pelletier J. P., Martel-Pelletier J. (2019). Machine-learning-based patient-specific prediction models for knee osteoarthritis. Nature Reviews Rheumatology, 15, 49–60. [DOI] [PubMed] [Google Scholar]
- Khedr E. M., Omran E. A. H., Ismail N. M., El-Hammady D. H., Goma S. H., Kotb H.…Ahmed G. A. (2017). Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimulation, 10, 893–901. doi:10.1016/j.brs.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Laste G., Caumo W., Adachi L. N. S., Rozisky J. R., de Macedo I. C., Filho P. R. M.…Torres I. L. S. (2012). After-effects of consecutive sessions of transcranial direct current stimulation (tDCS) in a rat model of chronic inflammation. Experimental Brain Research, 221, 75–83. [DOI] [PubMed] [Google Scholar]
- Lefaucheur J. P., Antal A., Ayache S. S., Benninger D. H., Brunelin J., Cogiamanian F.…Paulus W. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology, 128, 56–92. [DOI] [PubMed] [Google Scholar]
- Leffa D. T., Bellaver B., Salvi A. A., de Oliveira C., Caumo W., Grevet E. H.…Torres I. L. S. (2018). Transcranial direct current stimulation improves long-term memory deficits in an animal model of attention-deficit/hyperactivity disorder and modulates oxidative and inflammatory parameters. Brain Stimulation, 11, 743–751. [DOI] [PubMed] [Google Scholar]
- Loeser R. F., Goldring S. R., Scanzello C. R., Goldring M. B. (2012). Osteoarthritis: A disease of the joint as an organ. Arthritis and Rheumatism, 64, 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish D. (2016). On using bayesian methods to address small sample problems. Structural Equation Modeling: A Multidisciplinary Journal, 23, 750–773. [Google Scholar]
- Medeiros L., de Souza I., Vidor L., de Souza A., Deitos A., Volz M.…Torres I. (2012). Neurobiological effects of transcranial direct current stimulation: A review. Frontiers in Psychiatry, 3, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira D., Ramos E., Branco J. (2015). Osteoarthritis. Acta Med Port, 28, 99–106. [DOI] [PubMed] [Google Scholar]
- Pocock S. J., Assmann S. E., Enos L. E., Kasten L. E. (2002). Subgroup analysis, covariate adjustment and baseline comparisions in clinical trial reporting: Current practice and problems. Statistics in Medicine, 21, 2917–2930. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Reinecke H., Weber C., Lange K., Simon M., Stein C., Sorgatz H. (2015). Analgesic efficacy of opioids in chronic pain: Recent meta-analyses. British Journal of Pharmacology, 172, 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. M., Green C. E., Hasan K. M., Vincent J., Suchting R., Weaver M. F.…Lane S. D. (2017). PPAR-gamma agonist pioglitazone modifies craving intensity and brain white matter integrity in patients with primary cocaine use disorder: A double-blind randomized controlled pilot trial. Addiction, 112, 1861–1868. doi:10.1111/add.13868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam J., Berenbaum F. (2010). The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature Reviews Rheumatology, 6, 625–635. [DOI] [PubMed] [Google Scholar]
- Senn S. (2006). Change from baseline and analysis of covariance revisited. Statistics in Medicine, 25, 4334–4344. [DOI] [PubMed] [Google Scholar]
- Spezia L., Caumo W., Laste G., Fernandes Medeiros L., Ripoll Rozisky J., de Souza A.…Torres I. L. (2012). Reversal of chronic stress-induced pain by transcranial direct current stimulation (tDCS) in an animal model. Brain Research, 1489, 17–26. [DOI] [PubMed] [Google Scholar]
- Stan Development Team. (2018). RStan: The R interface to Stan. R package version 2.18.2. Retrieved from http://mc-stan.org
- Tavares D., Okazaki J., Rocha A., Santana M., Pinto A., Civile V.…Trevisani V. (2018). Effects of transcranial direct current stimulation on knee osteoarthritis pain in elderly subjects with defective endogenous pain-inhibitory systems: Protocol for a randomized controlled trial. JMIR Research Protocols, 7, e11660 doi:10.2196/11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers A. J., Altman D. G. (2001). Analysing controlled trials with baseline and follow up measurements. British Medical Journal, 323, 1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T., Flaxman A. D., Naghavi M., Lozano R., Michaud C., Ezzati M.…Murray C. J. L. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 380, 2163–2196. doi:10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Weng Z., Shen P., Zhou J., Zeng J., Weng F.…Yang H. (2018). S100B regulates inflammatory response during osteoarthritis via fibroblast growth factor receptor 1 signaling. Molecular Medicine Reports, 18, 4855–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]