Summary
We conducted a critical appraisal of published Phase 2 and 3 efficacy trials in relation to the prevention of cervical cancer in women. Our analysis shows the trials themselves generated significant uncertainties undermining claims of efficacy in these data. There were 12 randomised control trials (RCTs) of Cervarix and Gardasil. The trial populations did not reflect vaccination target groups due to differences in age and restrictive trial inclusion criteria. The use of composite and distant surrogate outcomes makes it impossible to determine effects on clinically significant outcomes. It is still uncertain whether human papillomavirus (HPV) vaccination prevents cervical cancer as trials were not designed to detect this outcome, which takes decades to develop. Although there is evidence that vaccination prevents cervical intraepithelial neoplasia grade 1 (CIN1) this is not a clinically important outcome (no treatment is given). Trials used composite surrogate outcomes which included CIN1. High efficacy against CIN1+ (CIN1, 2, 3 and adenocarcinoma in situ (AIS)) does not necessarily mean high efficacy against CIN3+ (CIN3 and AIS), which occurs much less frequently. There are too few data to clearly conclude that HPV vaccine prevents CIN3+. CIN in general is likely to have been overdiagnosed in the trials because cervical cytology was conducted at intervals of 6–12 months rather than at the normal screening interval of 36 months. This means that the trials may have overestimated the efficacy of the vaccine as some of the lesions would have regressed spontaneously. Many trials diagnosed persistent infection on the basis of frequent testing at short intervals, i.e. less than six months. There is uncertainty as to whether detected infections would clear or persist and lead to cervical changes.
Keywords: Vaccination programmes, cervical cancer
The human papillomavirus (HPV) vaccination programme aims to prevent cervical cancer. Globally around 13.1/100,000 women are diagnosed with cervical cancer each year.2 Typically, vaccination is offered to girls aged 9–13 years before sexual debut and naïve to HPV infection. Box 1 gives an overview of licensing and indications in Europe and the US.
Box 1.
Licensing and guidelines.
| Licensing |
| • Gardasil, Gardasil-9 and Cervarix vaccines have been approved for marketing and used in females and males from the age of 9 years throughout the world to prevent cervical cancer. |
| • The European Medicines Agency (EMA) and US Food and Drug Administration (FDA) granted marketing approval for Gardasil in 2006, and for Cervarix in 2007 and 2009, respectively. |
| • Gardasil-9 was approved in 2014 by the FDA and in 2015 by the EMA, but it is not currently used in the UK. |
| • The EMA has licensed all three vaccines for females and males with no upper age limit. The FDA has licensed Gardasil up to age 26 and Gardasil-9 up to age 45 for females and males, and Cervarix for females only up to age 25. |
| Guidelines |
| • The US Centers for Disease Control and Prevention recommends ‘routine vaccination at age 11 or 12 years. (Vaccination can be started at age 9.) The Advisory Committee on Immunization Practices also recommends vaccination for females aged 13 through 26 years not adequately vaccinated previously’.3 |
| • The UK uses Gardasil. Public Health England advises girls to be vaccinated from age 12–18 years. Immunisation Scotland offer the vaccine for girls aged 11–13 years. There is a planned roll-out to boys aged 12–13 in England and Scotland. |
Public health agencies promote the position that the vaccine has been shown to prevent cervical cancer (see Supplement 1). Not all routinely emphasise the limitations of the evidence or the uncertainties which we will discuss.
Background
A key issue for the design of trials and studies of efficacy is the complexity of the epidemiology of the HPV subtypes and the lesions used as surrogate endpoints for cervical cancer, each with their own different natural histories, prevalence and incidence and strength of association with cancer. These measures, especially if combined as composite surrogate endpoints in trials, generate new uncertainties.
i) HPV infection
There are 100+ types of the HPV: 12 of which are carcinogenic to humans, according to the International Association of Cancer Research (IARC).4 Types vary in prevalence, as does their association with cervical cancer. HPV vaccines are licensed for use against oncogenic HPV types 16 and 18 and now 31, 33, 45, 52, 58 in Gardasil-9. Gardasil and Gardasil-9 are also licensed against non-oncogenic types 6 and 11 linked to genital warts.
The lifetime risk of an incident of HPV infection is 79%;5 the majority of HPV infections are transient and 67% clear within one year.6 Around 10% of women without CIN have HPV infection at any one time.7 The mechanism of progression from HPV infection to cervical cancer and its precursors is not well understood.4,8–11
ii) Cervical cancer and pre-cancerous lesions as surrogate endpoints
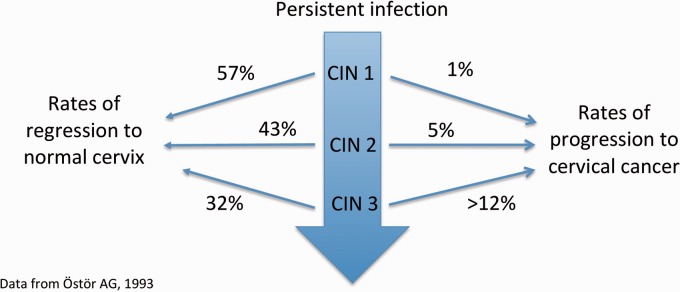
Estimated rates of regression and progression for CIN1, 2 and 3 are presented in Figure 1.12 However, there remain uncertainties due to methodological issues in the epidemiological studies from which these findings originate.12
Figure 1.
CIN natural history.
The IARC has acknowledged that composite endpoints in intervention studies involving CIN2 are sub-optimal13 as CIN2 is often misclassified due to its diagnosis having lower reproducibility and validity.14 Women with CIN2 are currently offered treatment which complicates research into progression to CIN3.
CIN3 can develop via progression of CIN1 and CIN2 or directly as a result of HPV infection, so CIN1 and CIN2 may not be good predictors of progression. Rate of progression from CIN3 to invasive cancer is likely to be higher than Ostor’s estimate of >12%.12 Lifetime risk may be up to 40% without cervical screening and treatment.15
Aim
To describe the uncertainties generated by the design of Phase 2 and 3 efficacy trials for prevention of cervical cancer and its precursors and how they affect the interpretation of efficacy data.
Methods and analysis
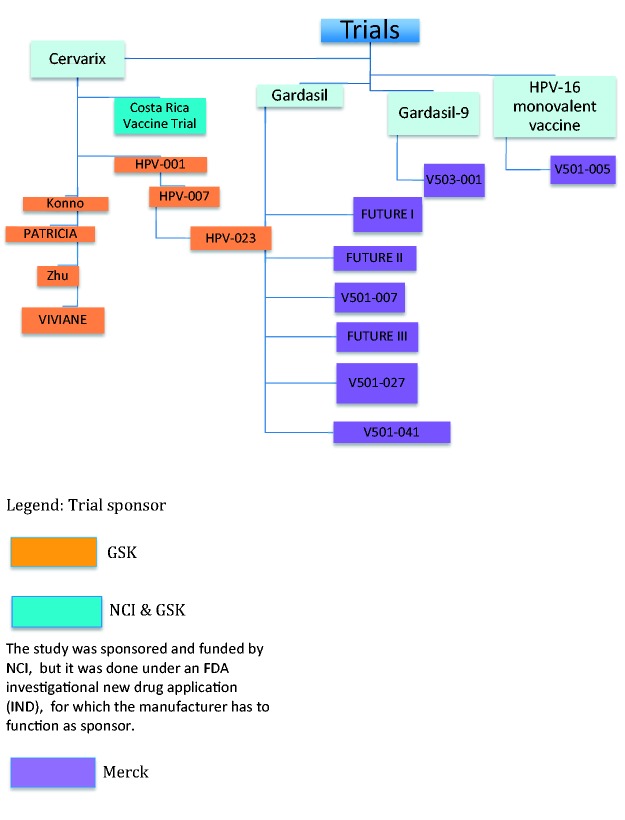
We searched Embase and Medline for papers relating to blinded controlled trials of HPV vaccination in females considering efficacy against cervical cancer and its precursors. See Supplement 2 for search strategy. No trial has tested Gardasil and Cervarix vaccines head to head using clinical outcomes (rather than immunological outcomes). We reviewed 35 published papers relating to 12 published randomised blinded non-HPV vaccine-controlled Phase 2 and 3 trials of Gardasil and Cervarix conducted from 2001 to 2016 assessing efficacy against cervical cancer and its precursors (Table 1 and Figure 2). Throughout this article, we refer to trials by their protocol name as presented in bold in Table 1. But for trials 104798 and 107638, we use the name of their first authors, Konno and Zhu, respectively.
Key Messages.
| • We do not know how well HPV vaccination will protect against cervical cancer. Trials have not focused on the outcome of cervical cancer because they had too few participants and did not follow them up for long enough: cervical cancer may take decades to develop. |
| • Published numbers from randomised controlled trials may overstate efficacy because: (a) testing occurred too frequently in the trials when, in real-world settings, lesions may regress spontaneously; (b) trials used composite surrogate outcomes, some of which, such as HPV-infection and CIN1, occur more frequently than others and are very unlikely to progress to cancer; and (c) subgroups were over-analysed. |
| • The trial populations have limited relevance and validity for real world settings: for example, women in the trials were older than the target population; we do not have enough data on the benefits in women who may have been exposed to HPV before they were vaccinated and who do not know their HPV status. |
| • We do not have enough data on the impact of the vaccine on CIN3, which is more likely than CIN1 and 2 to progress to cervical cancer. We also have less data on the impact on cervical disease due to any HPV type rather than just lesions due to HPV 16 and 18. |
| • Women should still attend regular cervical screening because efficacy in preventing cervical precursors is <100% and there are more oncogenic types than those covered by the vaccines. We have good evidence that cervical screening significantly reduces the risk of cervical cancer in women regardless of whether they have been vaccinated. The number of new cancers and deaths has decreased markedly such that cervical cancer now accounts for only 1% of cancer deaths in women in the UK (854 deaths in 2016).1 |
| • Information from the trials can tell us what happens between five and nine years after vaccination, but we do not know if protection wanes after this time. |
| • A recent observational study provides some evidence of efficacy against CIN3+ in girls vaccinated before sexual debut. Ongoing observational studies may tell us about the long-term effect on rates of cervical cancer, but it will take many years before we have the evidence. |
Table 1.
All Phase 2 and 3 trials by vaccine and control, sponsor, country, start and end dates, number and age of participants and length of follow-up.
| Vaccine and control | Trial name, NCT number (and sponsor) | Research papers | Phase | Country | Start date | End date | Number of participants | Age at enrolment | Average (mean) length of follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Cervarix versus aluminium-hydroxide containing control | HPV-001/580299 NCT00689741 (GSK) | Harper et al.20 | 2 | USA (incl. Puerto Rico), Brazil, Canada | Jan-01 | Apr-03 | 1113 | 15–25 | Up to 27 months (average not given) |
| Cervarix versus placebo aluminium-hydroxide containing control | HPV-007; follow-on study from HPV-001 NCT00120848 (GSK) | Harper et al.,21 Romanowski et al.22 | 2 | USA, Brazil, Canada | Nov-03 | Aug-07 | 776 | 15–25 | 5.9 years from first vaccination |
| Cervarix versus aluminium-hydroxide containing control | HPV-023/109616; follow-on study from HPV-001 and HPV-007 NCT00518336 (GSK) | De Carvalho et al.,23 Roteli-Martins et al.,24 Naud et al.25 | 2 | Brazil | Nov-07 | Jul-08 | 437 | 15–25 | 8.9 years from first vaccination |
| Cervarix versus Hepatitis A vaccine | 104798 (Konno) NCT00316693 (GSK) | Konno et al.26,27 | 2 | Japan | Apr-06 | Feb-09 | 1040 | 20–25 | 24 month after first vaccination |
| Cervarix versus Hepatitis A vaccine | PATRICIA/HPV-008 NCT00122681 (GSK) | Paavonen et al.,28,29 Lehtinen et al.,30 Wheeler et al.,31 Palmroth et al.,32 Szarewski et al.,33 Apter et al.,34 Struyf et al.35 | 3 | USA, Australia, Belgium, Brazil, Canada, Finland, Germany, Italy, Mexico, Philippines, Spain, Taiwan, Thailand, UK | May-04 | Nov-09 | 18,644 | 15–25 | Mean 43.7 months (median 47.4) |
| Cervarix versus Hepatitis A vaccine | Costa Rica Vaccine Trial/CVT/ HPV-009 NCT00128661 (NCI & GSK) | Herrero et al.,36 Kreimer et al.,37 Rodriguez et al.,38 Hildesheim et al.,39,40 Beachler et al.41 | 3 | Costa Rica | Jun-04 (initiation into trial) | Dec-10 (final data collection for primary outcome) | 7465 | 18–25 | 53.8 months |
| Cervarix versus aluminium-hydroxide containing control | VIVIANE/HPV-015/104820 NCT00294047 (GSK) | Skinner et al.,42 Wheeler et al.43 | 3 | Australia, Canada, Mexico, Netherlands, Peru, Philippines, Portugal, Russia, Singapore, Thailand, UK, USA | Feb-06 | Jan-14 | 5747 | 26+ | 5.9 years TVC from first vaccination, 5.7 years in ATP-E group from third vaccination |
| Cervarix versus aluminium-hydroxide containing control | 107638/Zhu NCT00779766 (GSK) | Zhu et al.44,45 | 3 | China | Oct-08 | Oct-14 | 6051 | 18–25 | Mean 57 months TVC-E from first vaccination, 52 months ATP-E group from third vaccination |
| Gardasil versus aluminium hydroxyphosphate sulphate adjuvant containing control | V501-007 NCT00365716 (Merck) | Villa et al.46,47 | 2 | Brazil, Finland, Sweden, Norway, USA | May-00 | May-04 | 552 (initial study up to three years), 241 (extension study up to five years) | 16–23 | Initial study up to 36 months (average not given). Extension study up to five years (results given for 226 women who completed study to 60 months, average not given) |
| Gardasil versus aluminium hydroxyphosphate sulphate adjuvant containing control | FUTURE I/V501-013 NCT00092521 (Merck) | Garland et al.48 | 3 | Australia, Austria, Brazil, Canada, Colombia, Czech Republic, Germany, Hong Kong, Italy, Mexico, New Zealand, Russia, Thailand, UK, USA (incl. Puerto Rico) | Dec-01 | Jul-07 | 5455 | 16–24 | Average three years from first vaccination |
| Gardasil versus aluminium hydroxyphosphate sulphate adjuvant containing control | FUTURE II/ V501-015 NCT00092534 (Merck) | Future II Study Group49 | 3 | Brazil, Colombia, Denmark, Finland, Iceland, Mexico, Norway, Peru, Poland, Singapore, Sweden, UK, USA (incl. Puerto Rico) | Jun-02 | Jul-07 | 12,167 | 15–26 | Average three years from first vaccination |
| Gardasil versus aluminium hydroxyphosphate sulphate adjuvant containing control | FUTURE III/V501-019 NCT00090220 (Merck) | Munoz et al.50 Castellsague et al.51 | 3 | Colombia, France, Germany, Philippines, Thailand, USA, Spain | Jun-04 | May-09 | 3819 | 24–45 | Median 4 years (mean 3.8 years) |
| Gardasil versus aluminium hydroxyphosphate sulphate adjuvant containing control | V501-027 NCT00378560 (Merck) | Yoshikawa et al.52 | 2 | Japan | Jun-06 | Sep-09 | 1021 | 18–26 | 30 months (23 months after month 7) |
| Gardasil versus aluminium hydroxyphosphate sulphate adjuvant containing control | V501-041 NCT00834106 (Merck) | Wei et al.53 | 3 | China | Jan-09 | Sep-16 | 3006 | 20–45 | Mean 6.07 years after first vaccination |
Figure 2.
Phase 2 and 3 randomised controlled trials on HPV vaccination efficacy.
We excluded trials of the HPV 16 monovalent vaccine (as it was not licensed and data suggest it had different pharmacodynamics to Gardasil and Cervarix16) and the one efficacy trial of Gardasil-9 (as the control was Gardasil, not a placebo).17 All the efficacy studies used an active vaccine (Hepatitis A) as the control, or a control containing an aluminium-adjuvant, rather than a true placebo; this in itself has raised concerns.11 We chose to focus on randomised controlled trials as this offers the highest level of evidence and this is the evidence used for decisions by regulatory bodies and decisions on initiating vaccination programmes.
We also found 39 meta-analyses and systematic reviews of HPV vaccine efficacy; of them many are restricted to post-hoc analyses of subgroups and have inappropriately combined trials in the same analysis, e.g. for different vaccines (see Supplement 3). The 2018 Cochrane review18 has been criticised for failing to include all relevant trials, ignoring evidence of harms and using composite endpoints with different natural histories.19
We compared the eligibility criteria, testing methods for HPV and cervical lesions, outcomes measures, length of follow-up, target group and subgroup definitions used in the different trials. We focused on the evidence for efficacy for CIN3+ and 12-month persistent infection which are deemed the more stringent outcome measures.
Does HPV vaccine prevent cervical cancer?
None of the trials were designed to determine efficacy or effectiveness against cervical cancer. There were no reported cases of cervical cancer in any trials; one case of vulval carcinoma was reported in the vaccinated group of FUTURE I.48
The time between first exposure to HPV and peak development of CIN3 is 7–10 years.13 It takes a further 10 years or so for cervical cancer to develop according to natural history studies.13 All trials had a mean length of follow-up of six or fewer years, apart from the HPV-023 extension with a mean follow-up of 8.9 years.
Does HPV vaccine prevent pre-cancerous lesions?
There were eight powered outcomes relating to cervical disease used across the trials, all of which were surrogates or composite surrogate outcomes (Table 2). Surrogate outcomes are biomarkers used as substitutes for clinical endpoints and used to predict an intervention’s benefits. Key pre-marketing trials evaluated the effect of HPV vaccines against pre-cancerous lesions, endpoints that were accepted by authorities as a surrogate for cervical cancer. There are limitations to surrogate outcomes in general,54 and for HPV and cervical cancer. Using composite surrogate outcomes (combining two or more surrogate outcomes together) is problematic because of differences in epidemiology and their natural history and management – see ‘Background’ section and Figure 1. Five of the 12 trials (VIVIANE, V01-007, FUTURE III, V501-027, V501-041) were powered for composite outcomes that combined cervical disease and persistent HPV infection.43,47,51–53 Four trials combined cervical disease and genital warts in the same primary outcome (FUTURE III, V501-007 and V501-027, V501-041) thereby inflating efficacy measures.47,51–53
Table 2.
Powered endpoints across trials.
| Powered endpoint | Number of trials using the endpoint | Vaccine: Trials using the endpoint |
|---|---|---|
| HPV 16/18 incident infection | 1 | Cervarix: HPV-001/007/023 |
| HPV 16/18 12-month persistent infection | 1 | Cervarix: CVT |
| HPV 16/18 6-month persistent infection | 1 | Cervarix: Konno |
| HPV 6/11/16/18 CIN1+ | 1 | Gardasil: FUTURE I |
| HPV 16/18 CIN2+ | 5 | Cervarix: CVT, PATRICIA, Zhu Gardasil: FUTURE II, V501-041 |
| HPV 16/18 6-month persistent infection or CIN1+ | 2 | Cervarix: VIVIANE, Zhu |
| HPV 16/18 6-month persistent infection or external genital lesions or CIN1+ | 1 | Gardasil: FUTURE III |
| HPV 6/11/16/18 6-month persistent infection or external genital lesions or CIN1+ | 4 | Gardasil: V501-007, FUTURE III, V501-027, V501-041 |
CIN1+
The trial outcomes included surrogates CIN1 and CIN2, which are more common than CIN3/AIS and cervical cancer, but which often regress and are of limited clinical concern (see ‘Background’ section and Figure 1). For example, intervention is not recommended for CIN1. Seven trials (FUTURE I, VIVIANE, V01-007, FUTURE III, V501-027, Zhu, V501-041) included CIN1 with CIN2, CIN3 and AIS in the same primary outcome (making a composite outcome), potentially inflating vaccine efficacy as there are many more CIN1 cases than CIN2+.43,45,47,48,51–53
CIN2+
The incidence (rate of detection) of CIN2, CIN3 and AIS in the trials was low so although many trials showed high efficacy for the vaccine, this was in the context of very few cases of CIN2+. For example, the HPV-023 trial showed high vaccine efficacy (100% against CIN2 and CIN3 over nine years follow-up) with very low incidence (only three cases, all in the control group, out of 212 participants).25 The trials were powered for the minimum number of events needed to obtain a statistically significant result, and many trials were designed to stop once this number had been achieved. But the powered outcomes often included CIN1, which means those trials were not powered to reach a minimum number of higher-grade CIN cases. Instead of multiple short duration trials, this problem of power could have been overcome by having one large trial of longer duration in each country.
CIN3+
CIN3 is generally agreed to be the best marker for risk of cervical cancer, with rates of progression of at least 12%.12 New evidence suggests that clinical intervention following detection on screening may be best reserved for women with CIN3.55 Only three of the 12 trials (FUTURE I, FUTURE II, PATRICIA) reported CIN3+ or AIS in subgroups that represented the target population of women naïve to HPV (see Supplement 4).30,49,56 The incidence of AIS in the trials is very low and only three trials (FUTURE I, FUTURE II, PATRICIA) published results for AIS alone.30,49,56
In these three trials, vaccine efficacy against CIN3 and AIS due to HPV 16/18 was 100% (see Supplement 4) but there were small numbers and wide confidence intervals, sometimes showing non-significance (where the confidence interval crosses zero).49,56 Vaccine efficacy against CIN3 and AIS due to any HPV type varied substantially between the vaccines.30,56
What is the evidence that vaccination prevents clinically meaningful HPV infection?
It is possible to diagnose new HPV infections (incident) and ongoing infection (persistent). Studies have shown median length of HPV 16 infection to be 8.5–19.4 months and HPV 18, 7.8–12 months.13
The HPV001/007/023 trial used incident infection of HPV 16/18 as the primary outcome.20,22,25 The results are not relevant to policy decision making as the current consensus reported by the WHO is that incident HPV infection is not an adequate surrogate outcome because it rarely progresses to cervical disease.57
There is a lack of agreement on what time period defines persistent infection,13 and the trials may have overestimated vaccine efficacy by picking time periods that are shorter than the duration of most self-limiting infections, for example six months. In some trials, the testing interval for diagnosing six-month persistent infection was four months36,47,52 or five months.44,51,53
Only one Gardasil trial, V501-041, used 12-month persistent infection as an outcome; however, the study authors only presented data for combined HPV 6/11/16/18, not for 16/18 or any oncogenic type.53 In the Cervarix trials, 12-month HPV 16/18 persistent infection vaccine efficacy varied from 85.3 to 100% (see Supplement 5).25,27,29,33,45 Vaccine efficacy for 12-month persistent infection by any oncogenic HPV type ranged from 10.4% to 50.1% across trials with wide confidence intervals for most trials.25,27,29,33,45 The results were not statistically significant for the HPV-023 trial and Zhu (see Supplement 5).25,45
Not all trials analysed HPV types 16 and 18 separately. The incidence of HPV infection varies by HPV type.30 HPV 18 was much less common than HPV 16. Combining their results makes the efficacy against HPV 18 appear more solid. In some trials the results for HPV 18 on its own were not statistically significant and were only significant when combined with results for HPV 16. For example, in the per-protocol population subgroup of V501-027, the six-month persistent infection or genital disease (the trial primary outcome) vaccine efficacy was 100% (59.7,100) for HPV 16, 86.0% (−8.9, 99.7) for HPV 18 and 94.5% (65.2, 99.9) for HPV 16/18.52 In the according-to-protocol cohort for efficacy (ATP-E) subgroup of the PATRICIA trial, CIN3+ vaccine efficacy for HPV 16 was 90.2% (59.7, 98.9), HPV 18: 100% (−8.2, 100) and HPV 16/18: 91.7% (66.6, 99.1), respectively.30 This means the vaccine may not protect as well against cervical cancer related to HPV 18. The proportion of cervical cancers related to HPV 18 ranges from 13% in South/Central America to 22% in North America.58
How much information is there on long-term outcomes and how long does protection last?
All trials were six or fewer years in length, apart from the extension study HPV-023 with mean follow-up of 8.9 years, (which maintained blinding and kept a control group) it only included 437 of the original 1113 participants in HPV-001.25 The longest study of Gardasil was V501-041, which was extended from 30 to 78 months with 2601 out of the initial 3006 participants.53
Features of the trials may bias the findings in overestimating long-term efficacy. For example, HPV 16/18 related CIN3 presents earlier than non-vaccine type CIN3 so shorter efficacy trials will be biased in favour of finding HPV 16/18 related CIN3.59
Although incidence and progression of disease differ over time and by age, V501-007 combined the results of participants from the original trial with those who completed an extra two-year extension.47 In HPV-007 and HPV-023, results for participants from the preceding trials were considered together.22,25
How similar were the females in the trials to the target vaccination groups?
Females in the trials are typically older than those in real-life vaccine programmes, and it is unclear whether their outcomes are similar. We do not know efficacy rates in girls aged between 9 and 13 years.
The youngest trial participants were aged 15 years and trials did not restrict recruitment to girls before sexual debut. Therefore, previous exposure to HPV is likely for some girls. Per-protocol subgroups with much fewer participants were used to analyse those with no evidence of previous HPV exposure but as shown earlier, most trials did not present data for CIN3+ outcomes in these subgroups.
Efficacy in girls aged 9–13 years has been estimated using immunobridging trials (where immune response levels are measured) rather than using clinical outcomes.60 We do not know what level of antibody titres define a surrogate level of protection against cervical cancer or its precursors and how long protection will last (Gardasil anti-HPV 18 titres are not different from natural infection as early as 24 months after vaccination).60 Therefore, it is possible that protection will wane by time of peak exposure when vaccinated at an earlier age.
Three trials recruited older women (FUTURE III (aged 24–45), VIVIANE (aged 26+) and V501-041 (aged 20–45)).42,50,53 In VIVIANE a subset of up to 15% of women with a history of HPV-associated infection or disease were included (defined as two or more abnormal smears in sequence, abnormal colposcopy, or biopsy or treatment of the cervix after abnormal smear or colposcopy findings) but this means that the Total Vaccine Cohort may not reflect the proportion of women with a history of HPV-associated disease in the wider population.42 There were then restrictions based on HPV DNA and serostatus for inclusion in the according to protocol for efficacy and total vaccine cohort for efficacy subgroups. In FUTURE III, women with a history of past or present genital warts or cervical disease were excluded; the primary tests of efficacy were in the HPV type-specific per-protocol efficacy analyses (PPE), which required women to be seronegative to relevant type on day 1 and PCR negative to that type in cervicovaginal swabs or biopsy samples, or both, from day 1 until month 7.50 V501-041 excluded women with more than four previous sexual partners and those with a history of genital warts or ‘significant cervical disease’ – the study authors did not specify what this meant.53
Seven trials excluded women with more than four to six previous sexual partners.20,28,46,48,52,53,61 Five excluded women with previous abnormal cervical smears20,46,48,52,61 and an additional three excluded women with a history of previous colposcopy.26,28,44 CVT was the only trial with no restrictions based on genital warts or cervical or sexual history.36 These restrictions may make the vaccine appear more efficacious in the intention-to-treat (ITT) population than in the general population of women of the same age. In CVT, the efficacy in the ITT group for 12-month persistent infection with any oncogenic HPV type was 11% (95% CI 2.2, 19.5); they did not give efficacy against CIN3+ in the ITT group.36
There is also global variation in the epidemiology of HPV which means that the trial findings may be poorly generalisable to some settings, including Africa, so it is important to know if results differ by study region. None of the trials considering efficacy outcomes were conducted in Africa (we are only aware of a safety and immunogenicity trial in Africa62) despite this being the world region with by far the highest incidence of cervical cancer.2 The Cochrane review acknowledges that ‘differences in the population HPV prevalence in the trial sites, or differences in study protocols and assays used, may explain the contrast in efficacy’ between Cervarix and Gardasil.18
What is the risk of oncogenic HPV-type substitution?
Vaccines may protect against HPV types, which are not included in the vaccine. There was some evidence of cross-protection against three high-risk HPV types (31, 33 and 45) for Cervarix (see Supplement 6).31,36,43,45 There was cross-protection against one non-vaccine HPV type by Gardasil31 (see Supplement 6).63 But there was evidence of a statistically significant increased risk of HPV type 51 and 58 in the Cervarix trials, compared with the control vaccine.31,36 It is unknown whether vaccine targeting will lead to substitution by other oncogenic types, as with pneumococcal vaccination.64
Methodological factors from the trials which may affect interpretation of the results
Multiple underpowered analyses
All trials undertook multiple subgroup analyses, which increase the likelihood of positive statistical findings in the absence of true effect. The subgroup definitions varied across trials, so that results cannot be compared across trials. Results were not given for all subgroups, and were not broken down by country, by study site or for each outcome. This is important given different epidemiology of HPV in different areas of the world. It may have been reported this way because incidence was low. We have included a table giving the different subgroup definitions in Supplement 7.
Problems with reporting of trial results
The trials report vaccine efficacy as the primary outcome, which shows relative risk reduction. This can over-emphasise efficacy compared with absolute risk reduction such as numbers needed to vaccinate, which is more useful for clinicians, patients and policy makers. None of the trials gave numbers needed to vaccinate. CVT is the only trial that presented results in terms of absolute risk reduction.36,38,39 The absolute risk reduction for the PATRICIA trial for CIN3+ due to any oncogenic HPV type (see Supplement 4) (our calculation) is 0.75%, giving a number needed to vaccinate of 133.
Frequency of cervical screening
All the trials did Pap cytology at 6–12 monthly intervals. Cervical cancer screening is recommended in England every three years, between the age of 25 and 49 years.65 Increased frequency of screening can lead to over-diagnosis and overtreatment of cytological abnormalities that would normally resolve and not be detected.66 Increasing the frequency of testing suits early trial completion but may overestimate vaccine efficacy.
Testing methods for HPV
The tests for DNA positivity to a particular HPV type (indicating ongoing infection) and seropositivity (indicating previous infection) have limited specificity and sensitivity.67 This is another reason HPV infection has limitations as a surrogate of cervical cancer. Only 50–70% of HPV infections result in detectable anti-HPV responses,51 and initial seropositive status may revert to negative.68 So subgroups of women considered naïve to HPV may have had previous exposure. Also, latent infection may be undetectable on current tests. The IARC has noted that ‘it is not known how frequently this [latent infection] occurs in immunocompetent individuals, how long it lasts, what causes re-emergence into a detectable state or what fraction of cancers arises after a period of latency’.13 This also raises the question of whether subgroups naïve to HPV can reliably represent girls before sexual debut, and whether HPV infection is a valid surrogate outcome.
Meta-analysis of limited value due to trial heterogeneity
Differences in trial endpoints and subgroups limit the ability to compare and aggregate data from trials. This is compounded by lack of standardisation across studies for a range of measures: tests of previous HPV exposure, serological assays to detect HPV infection and sampling methods including frequency of testing.67
There are no agreed criteria for defining the causal HPV type for clinical lesions, and different trials used different criteria (see Supplement 8). There was no standard approach to assess efficacy against disease and infection due to HPV types not found in the vaccine, for example whether they considered non-vaccine oncogenic types or all non-vaccine HPV types (see Supplement 9). Given that Merck and GSK were involved in all the trials it is unclear why there was no consistency in methods and tests across trials.
Is ongoing research likely to resolve the uncertainties?
The focus of this paper is randomised controlled trials, but we have also looked at whether observational studies can answer some of the uncertainties, acknowledging that this is a lower level of evidence but practically the most likely source of future information in the absence of long-term randomised controlled trials. We identified 19 Phase 4, observational and non-blinded follow-up studies (including a meta-analysis of ecological studies) that are potentially relevant to the uncertainties discussed in this paper (see Supplement 10). None of these studies is ideal. Many are small, of short duration or not looking at CIN3+. One observational study (#5) showed a reduction in relative risk of CIN3 amongst those vaccinated of 0.45.69 The PATRICIA trial follow-up (#3), the only trial planned for 20 years post-vaccination, and the Mexican FASTER trial (#18) are likely to provide more long-term efficacy data on the more clinically relevant efficacy endpoints.
The recently published observational study conducted in Scotland by Palmer et al.70 provides new evidence on reduction of CIN3+ regardless of HPV type. The authors note the following limitations which may have inflated measures of efficacy: the study gathered data only on the first round of cervical screening at age 20 years (now changed to age 25 years in line with England) with underrepresentation of the unvaccinated group (23% screening attendance versus 51% in the vaccinated group at aged 20 or 21); and shorter follow-up time for women born in 1995 and 1996 necessarily affects the robustness of the estimate of vaccine effectiveness for younger women. In addition, the basis for the claim of herd protection is not well explained for the unvaccinated women in the 1995–1996 cohort, compared with unvaccinated women in 1988–1990. Nor do the authors consider how changes in sexual activity may have contributed to the observed decrease in CIN prevalence independent of the vaccine: between 2002 and 2014 (the latest period for which there are data) the proportion of 15-year-olds in Scotland who have ever had sex reduced, although socio-economic inequalities persist for sexual initiation and condom use.71 Screening uptake also varies by socio-economic status.72
What should we do in the light of the uncertainty?
Policy
We ask policy makers to:
Establish national baseline epidemiological data on cancer incidence, mortality and HPV subtype prevalence to support evidence based decisions about whether the currently available vaccines are likely to be cost-effective and should be a priority.
Ensure that cancer surveillance and registries are in place before any vaccination programme is implemented so that changes in incidence of cervical cancer and its precursors can be studied.
Initiate national long-term efficacy and effectiveness studies that are free of industry funding, focus on clinically meaningful outcomes, and enrol and analyse the vaccine target populations.
Research
In the UK, cervical screening is estimated to prevent more than 80% of cervical cancers.15 A cost-effectiveness analysis in Australia suggested that immunisation is not cost-effective in settings with established cervical screening.73 We still do not know how many cases of cervical disease prevented by vaccination would have been detected by cervical screening. Further research is needed on whether adding vaccination where screening exists will be cost-effective. Box 2 shows our recommendations for further research to address uncertainties. We also call for more research on HPV to be free from industry funding.
Box 2.
Recommendation for future trials to address the uncertainties.
| • Vaccinate prior to onset of sexual activity and begin assessment of endpoints at age of usual cervical screening once sexually active |
| • Make all clinical study reports including anonymised individual patient data publicly available |
| • Separate trials to assess the benefit in women already exposed to HPV, without restrictions based on risk factors |
| • Analyse data by country and study site |
| • Ensure the testing interval is in line with usual cervical screening protocols |
| • Continue follow-up for minimum 20 years from the time of sexual debut |
| • Power trials for primary composite outcome CIN3/AIS/cervical cancer due to oncogenic HPV types |
| • Define secondary outcome of persistent infection with HPV 16/18 at a minimum of 12 months |
| • Use standardised testing methods for HPV detection. |
| • Undertake a saline placebo-controlled efficacy trial of Gardasil-9 in previously unvaccinated participants, as it is difficult to draw conclusions on efficacy and risk of harms based on the trial comparing Gardasil-9 against Gardasil. |
Conclusion
This review has revealed many methodological problems with the Phase 2 and 3 efficacy trials of HPV vaccination leading to uncertainty regarding understanding its efficacy.
Cervical cancer – It is uncertain whether HPV vaccination prevents cervical cancer. The trials were not designed to detect this outcome, which takes decades to develop. For most outcomes, follow-up data exist for an average of only four or five years.
CIN – There is evidence that vaccination prevents CIN1; however, this is not a clinically important outcome (no treatment is given). Trials used composite surrogate outcomes which included CIN1, but high efficacy against CIN1+ (CIN1, 2, 3 and AIS) does not necessarily mean high efficacy against CIN3+ (CIN3 and AIS), which occurred much less frequently. There are too few data to clearly conclude that HPV vaccine prevents CIN3+. CIN in general is likely to have been overdiagnosed in the trials because most carried out cervical cytology at intervals of 6–12 months rather than at the normal screening interval of 36 months. This means that the trials may have overestimated the efficacy of the vaccine as some of the lesions would have regressed spontaneously.
Persistent HPV infection – The outcomes for HPV infection are difficult to interpret. Many trials diagnosed persistent infection on the basis of frequent testing at short intervals, i.e. less than six months. This leaves uncertainty as to whether detected infections would clear or persist and lead to cervical changes. In the current Public Health England cervical screening programme, patients who are HPV positive but cytology negative are not retested for 12 months.74
Differences between trial and real world populations – Most of the people in the trials were older than the 9- to 13-year-olds who are typically offered vaccination. Efficacy in girls aged 9–13 years has been estimated using immunobridging trials (where immune response levels are measured) rather than using clinical efficacy outcomes.60 We do not know what level of antibody titres protect against cervical cancer and its precursors, or how long protection will last.60 Similarly data on the outcomes for women older than 24 years are limited, and all trials apart from the Costa Rica Vaccine Trial (CVT) had exclusions on eligibility related to sexual history or history of genital warts or cervical disease, limiting the generalisability to catch-up vaccination populations.36 HPV epidemiology varies globally. No efficacy studies were done in Africa.
Cross-protection and HPV-type substitution – There is uncertainty about whether the vaccine will provide cross-protection against oncogenic HPV types not targeted by the vaccines. There is also a risk of substitution where a non-vaccine oncogenic HPV type fills the void left by the reduction of an HPV type targeted by the vaccines.
Methodological considerations – Many trials included multiple underpowered subgroup analyses, which increase the chance of false-positive findings. All trials except CVT reported relative rather than absolute effects, which tend to overstate efficacy, and none provided numbers needed to vaccinate.
Supplemental Material
Supplemental material, JRS899308 Supplemental material for Will HPV vaccination prevent cervical cancer? by Claire P Rees, Petra Brhlikova and Allyson M Pollock in Journal of the Royal Society of Medicine
Declarations
Competing Interests
None declared.
Funding
None declared.
Ethics approval
Not applicable.
Guarantor
AMP.
Contributorship
AMP had the idea for the article; CPR did the literature review and wrote the first drafts of the article; CPR, AMP and PB contributed to the analysis and redrafting the article.
Acknowledgements
We are grateful to Peter Roderick for commenting on drafts and help with editing and to the reviewers and the editorial team at the BMJ. We would also like to thank the Newcastle University PPI team for their helpful comments.
Provenance
Not commissioned; editorial review of peer review comments from another journal and subsequent revision.
ORCID iDs
Claire P Rees https://orcid.org/0000-0001-5649-7835
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Cancer Research UK. Cervical Cancer Mortality Statistics. See https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/mortality (2019, last checked 17 Apr 2019).
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. HPV Vaccine Recommendations. See https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html (2016, last checked 7 Oct 2017).
- 4.IARC. Biological Agents – A Review of Human Carcinogens – Human Papillomaviruses, Lyon: IARC, 2012. [Google Scholar]
- 5.Syrjanen K, Hakama M, Saarikoski S, Vayrynen M, Yliskoski M, Syrjanen S, et al. Prevalence, incidence, and estimated life-time risk of cervical human papillomavirus infections in a nonselected finnish female population. Sex Transm Dis 1990; 17: 15–19. [PubMed] [Google Scholar]
- 6.Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 2008; 100: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO/ICO Information Centre on HPV and Cervical Cancer. HPV and cervical cancer in the 2007 report. Vaccine 2007; 25: C1–C230. [DOI] [PubMed]
- 8.Couto E, Saeterdal I, Juvet LK, Klemp M. HPV catch-up vaccination of young women: a systematic review and meta-analysis. BMC Public Health 2014; 14: 867–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Mario S, Basevi V, Lopalco PL, Balduzzi S, D’Amico R, Magrini N. Are the two human papillomavirus vaccines really similar? A systematic review of available evidence: efficacy of the two vaccines against HPV. J Immunol Res 2015; 2015: 435141–435141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haug CJ. Human papillomavirus vaccination – reasons for caution. N Engl J Med 2008; 359: 861–862. [DOI] [PubMed] [Google Scholar]
- 11.Tomljenovic L, Spinosa JP, Shaw CA. Human papillomavirus (HPV) vaccines as an option for preventing cervical malignancies: (how) effective and safe? Curr Pharm Des 2013; 19: 1466–1487. [PubMed] [Google Scholar]
- 12.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol 1993; 12: 186–192. [PubMed] [Google Scholar]
- 13.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 90. Human Papillomaviruses, Lyon: IARC, 2007. [PMC free article] [PubMed] [Google Scholar]
- 14.Carreon JD, Sherman ME, Guillen D, Solomon D, Herrero R, Jeronimo J, et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: results from a histological review of population-based cervical samples. Int J Gynecol Pathol 2007; 26: 441–446. [DOI] [PubMed] [Google Scholar]
- 15.Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004; 364: 249–256. [DOI] [PubMed] [Google Scholar]
- 16.EMA. Gardasil: EPAR – Scientific Discussion. See http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000703/WC500021140.pdf (2006, last checked 28 Oct 2017).
- 17.Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet 2017; 390(10108): 2143–2159. [DOI] [PubMed] [Google Scholar]
- 18.Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev 2018; 5: CD009069–CD009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorgensen L, Gotzsche PC, Jefferson T. The Cochrane HPV vaccine review was incomplete and ignored important evidence of bias. BMJ Evid Based Med 2018; 23: 165–168. [DOI] [PubMed] [Google Scholar]
- 20.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364: 1757–1765. [DOI] [PubMed] [Google Scholar]
- 21.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 22.Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374: 1975–1985. [DOI] [PubMed] [Google Scholar]
- 23.De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, Zahaf T, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28: 6247–6255. [DOI] [PubMed] [Google Scholar]
- 24.Roteli-Martins CM, Naud P, De Borba P, Teixeira JC, De Carvalho NS, Zahaf T, et al. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother 2012; 8: 390–397. [DOI] [PubMed] [Google Scholar]
- 25.Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, de Borba PC, Sanchez N, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother 2014; 10: 2147–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konno R, Tamura S, Dobbelaere K, Yoshikawa H. Efficacy of human papillomavirus 16/18 AS04-adjuvanted vaccine in Japanese women aged 20 to 25 years: interim analysis of a phase 2 double-blind, randomized, controlled trial. Int J Gynecol Cancer 2010; 20: 404–410. [DOI] [PubMed] [Google Scholar]
- 27.Konno R, Tamura S, Dobbelaere K, Yoshikawa H. Efficacy of human papillomavirus type 16/18 AS04-adjuvanted vaccine in Japanese women aged 20 to 25 years: final analysis of a phase 2 double-blind, randomized controlled trial. Int J Gynecol Cancer 2010; 20: 847–855. [DOI] [PubMed] [Google Scholar]
- 28.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369: 2161–2170. [DOI] [PubMed] [Google Scholar]
- 29.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374: 301–314. [DOI] [PubMed] [Google Scholar]
- 30.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13: 89–99. [DOI] [PubMed] [Google Scholar]
- 31.Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13: 100–110. [DOI] [PubMed] [Google Scholar]
- 32.Palmroth J, Merikukka M, Paavonen J, Apter D, Eriksson T, Natunen K, et al. Occurrence of vaccine and non-vaccine human papillomavirus types in adolescent Finnish females 4 years post-vaccination. Int J Cancer 2012; 131: 2832–2838. [DOI] [PubMed] [Google Scholar]
- 33.Szarewski A, Poppe WAJ, Skinner SR, Wheeler CM, Paavonen J, Naud P, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15–25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer 2012; 131: 106–116. [DOI] [PubMed] [Google Scholar]
- 34.Apter D, Wheeler CM, Paavonen J, Castellsague X, Garland SM, Skinner SR, et al. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin Vaccine Immunol 2015; 22: 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Struyf F, Colau B, Wheeler CM, Naud P, Garland S, Quint W, et al. Post hoc analysis of the PATRICIA randomized trial of the efficacy of human papillomavirus type 16 (HPV-16)/HPV-18 AS04-adjuvanted vaccine against incident and persistent infection with nonvaccine oncogenic HPV types using an alternative multiplex type-specific PCR assay for HPV DNA. Clin Vaccine Immunol 2015; 22: 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrero R, Wacholder S, Rodriguez AC, Solomon D, Gonzalez P, Kreimer AR, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov 2011; 1: 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011; 103: 1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez AC, Solomon D, Herrero R, Hildesheim A, Gonzalez P, Wacholder S, et al. Impact of human papillomavirus vaccination on cervical cytology screening, colposcopy, and treatment. Am J Epidemiol 2013; 178: 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hildesheim A, Wacholder S, Catteau G, Struyf F, Dubin G, Herrero R, et al. Efficacy of the HPV-16/18 vaccine: final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine 2014; 32: 5087–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hildesheim A, Gonzalez P, Kreimer AR, Wacholder S, Schussler J, Rodriguez AC, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol 2016; 215: 212.e1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beachler DC, Kreimer AR, Schiffman M, Herrero R, Wacholder S, Rodriguez AC, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV infection. J Natl Cancer Inst 2016; 108: 1: djv302–djv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skinner SR, Szarewski A, Romanowski B, Garland SM, Lazcano-Ponce E, Salmeron J, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet 2014; 384: 2213–2227. [DOI] [PubMed] [Google Scholar]
- 43.Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis 2016; 16: 1154–1168. [DOI] [PubMed] [Google Scholar]
- 44.Zhu F-C, Chen W, Hu Y-M, Hong Y, Li J, Zhang X, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18–25 years: results from a randomized controlled trial. Int J Cancer 2014; 135: 2612–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu FC, Hu SY, Hong Y, Hu YM, Zhang X, Zhang YJ, et al. Efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine in Chinese women aged 18–25 years: event-triggered analysis of a randomized controlled trial. Cancer Med 2017; 6: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villa LL, Costa RLR, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6: 271–278. [DOI] [PubMed] [Google Scholar]
- 47.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95: 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356: 1928–1943. [DOI] [PubMed] [Google Scholar]
- 49.Future II Study Group. Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis 2007; 196: 1438–1446. [DOI] [PubMed] [Google Scholar]
- 50.Munoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet 2009; 373: 1949–1957. [DOI] [PubMed] [Google Scholar]
- 51.Castellsague X, Munoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer 2011; 105: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshikawa H, Ebihara K, Tanaka Y, Noda K. Efficacy of quadrivalent human papillomavirus (types 6, 11, 16 and 18) vaccine (GARDASIL) in Japanese women aged 18–26 years. Cancer Sci 2013; 104: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei L, Xie X, Liu J, Zhao Y, Chen W, Zhao C, et al. Efficacy of quadrivalent human papillomavirus vaccine against persistent infection and genital disease in Chinese women: a randomized, placebo-controlled trial with 78-month follow-up. Vaccine 2019; 37(27): 3617–3624. [DOI] [PubMed] [Google Scholar]
- 54.Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ 2011; 343: d7995–d7995. [DOI] [PubMed] [Google Scholar]
- 55.Tainio K, Athanasiou A, Tikkinen KAO, Aaltonen R, Cardenas J, Hernandes, et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. BMJ 2018; 360: k499–k499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010; 102: 325–339. [DOI] [PubMed] [Google Scholar]
- 57.Pagliusi SR, Aguado MT. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine 2004; 23: 569–578. [DOI] [PubMed] [Google Scholar]
- 58.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121: 621–632. [DOI] [PubMed] [Google Scholar]
- 59.Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102: 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harper DM, DeMars LR. HPV vaccines – a review of the first decade. Gynecol Oncol 2017; 146: 196–204. [DOI] [PubMed] [Google Scholar]
- 61.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356: 1915–1927. [DOI] [PubMed] [Google Scholar]
- 62.Sow PS, Watson-Jones D, Kiviat N, Changalucha J, Mbaye KD, Brown J, et al. Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomized trial in 10–25-year-old HIV-seronegative African girls and young women. J Infect Dis 2013; 207: 1753–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis 2009; 199: 926–935. [DOI] [PubMed] [Google Scholar]
- 64.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378: 1962–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Public Health England. Cervical Screening: Programme Overview. See https://www.gov.uk/guidance/cervical-screening-programme-overview (2015, last checked 28 Oct 2017).
- 66.Force USPST. U.S. Preventive Services Task Force Cervical Cancer Screening. Archived Final Recommendation. See https://www.uspreventiveservicestaskforce.org/Page/Document/Recommendation StatementFinal/cervical-cancer-screening#consider (2013, last checked 26 May 2019).
- 67.Eklund C, Forslund O, Wallin KL, Zhou T, Dillner J. Network WHOHPL. The 2010 global proficiency study of human papillomavirus genotyping in vaccinology. J Clin Microbiol 2012; 50: 2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dillner J. The serological response to papillomaviruses. Semin Cancer Biol 1999; 9: 423–430. [DOI] [PubMed] [Google Scholar]
- 69.Pollock KG, Kavanagh K, Potts A, Love J, Cuschieri K, Cubie H, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer 2014; 111: 1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palmer T, Wallace L, Pollock KG, Cuschieri K, Robertson C, Kavanagh K, et al. Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12–13 in Scotland: retrospective population study. BMJ 2019; 365: l1161–l1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neville FG, McEachran J, Aleman-Diaz A, Whitehead R, Cosma A, Currie D, et al. Trends in the sexual behaviour of 15-year olds in Scotland: 2002–14. Eur J Public Health 2017; 27: 835–839. [DOI] [PubMed] [Google Scholar]
- 72.National Statistics. Scottish Cervical Screening Programme Statistics 2017/18. See https://www.isdscotland.org/Health-Topics/Cancer/Publications/2018-09-04/2018-09-04-Cervical-Screening-Report.pdf (2018, last checked 22 Apr 2019).
- 73.Wilyman J. HPV vaccination programs have not been shown to be cost-effective in countries with comprehensive Pap screening and surgery. Infect Agent Cancer 2013; 8: 21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Public Health England. Cervical Screening: Implementation Guide for Primary HPV screening. See https://www.gov.uk/government/publications/cervical-screening-primary-hpv-screening-implementation/cervical-screening-implementation-guide-for-primary-hpv-screening (2019, last checked 29 Mar 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JRS899308 Supplemental material for Will HPV vaccination prevent cervical cancer? by Claire P Rees, Petra Brhlikova and Allyson M Pollock in Journal of the Royal Society of Medicine