Abstract
Objectives:
To evaluate the effects of left ventricular support with the microaxial left ventricular pump using the Impella device on the renal resistive index assessed by Doppler ultrasonography in haemodynamically stable patients with cardiogenic shock following myocardial infarction.
Methods:
A non-randomised interventional single-centre study. Consecutive patients with cardiogenic shock supported with an Impella were included during May 2018 and October 2018. The renal resistive index determined as a quotient of (peak systolic velocity – end diastolic velocity)/ peak systolic velocity was obtained using Doppler ultrasound; invasive blood pressure was determined in radial artery simultaneously for safety reasons.
Results:
A total of 15 patients were measured. The renal resistive index was determined in both kidneys in 13 patients and for one kidney in two patients, respectively. The mean difference between right and left renal resistive index was 0.026 ± 0.023 (P=0.72). When increasing the Impella microaxillar mechanical support by a mean of 0.44 L/min (±0.2 L/min), the renal resistive index decreased significantly from 0.66 ± 0.08 to 0.62 ± 0.06 (P<0.001) consistently in all patients, whereas systolic or diastolic blood pressure remained unchanged.
Conclusions:
Microaxillar mechanical support by the Impella device in haemodynamically stable patients with cardiogenic shock led to a significant reduction of the renal resistive index without affecting systolic or diastolic blood pressure. This observation is consistent with the notion that Impella support may promote renal organ protection by enhancing renal perfusion.
Keywords: Impella, renal resistive index, Doppler ultrasonography, cardiogenic shock
Introduction
Acute kidney injury (AKI) is a frequent complication of cardiogenic shock, which is promoted by low cardiac output (CO) and subsequent organ hypoperfusion, i.e. kidney, liver and brain leading to sustained high morbidity and mortality.1–3 Thus the administration of fluids and inotropes to maintain CO and organ perfusion is the hallmark of cardiogenic shock therapy. However, increasing doses of vasopressors develop deleterious effects on organ perfusion, i.e. promote acute renal failure by increasing renal vascular resistance. Therefore, we used a microaxial mechanical circulatory support (MCS) device to maintain haemodynamic stability by augmenting CO and reducing vasoconstrictor demand hoping to improve organ perfusion.4–6 Monitoring of critical organ dysfunction, i.e. AKI, includes urine production as an index of renal perfusion and creatinine clearance as an index of glomerular filtration. However, these parameters do not reflect acute renal haemodynamic changes in the renal vasculature and are useless for the short-term management of mechanical circulatory devices, i.e. the Impella microaxial pump.7,8 Therefore, we here evaluated as an indicator for renal haemodynamics the renal resistive index (RRI) determined by intrarenal artery Doppler measurements. Even though there are controversial data on the pathophysiological relevance of RRI, it is significantly influenced by systemic haemodynamic parameters (i.e. in cardiogenic shock patients), it correlates with renal vascular resistance depicting changes in renal blood flow,7,9,10 while several data indicate that it can predict the occurrence and reversibility of kidney failure in critically ill patients.7,9–11 Thus the aim of this study was to investigate the effect of left ventricular mechanical support using the Impella microaxial pump on the RRI in otherwise stable patients with cardiogenic shock.
Materials and methods
The study was conducted during a 6-month period (May 2018 to October 2018). We included consecutive patients with cardiogenic shock supported with MCS by the Impella microaxial pump in this single-centre study. Cardiogenic shock was defined as systolic blood pressure less than 90 mmHg for more than 30 minutes or catecholamines required to maintain systolic blood pressure at more than 90 mmHg plus clinical signs of pulmonary congestion and impaired end-organ perfusion (at least one of the following: altered mental status, cold and clammy skin, oliguria with urine output <30 ml/hour or serum lactate >2.0 mmol/L). The RRI was obtained in every haemodynamically stable patient using Doppler ultrasound. The two measurements were performed within 6 hours of admission and within the time frame of one hour. The first measurement was obtained when haemodynamic stability of the patient with Impella support was achieved. Haemodynamic stability was defined as mean arterial pressure of 60 mmHg or greater for more than one hour with no changes of Impella MCS level, catecholamine doses or fluid administration rates. After an additional 30 minutes of MCS support the RRI was measured at a different support level. Between the two RRI measurements only the Impella MCS level was changed, whereas all other therapeutic interventions, especially fluid management and doses of catecholamines, remained unchanged.
RRI was determined using Doppler ultrasound at the patient’s bedside according to standard procedures (Figure 1).12,13 A transparietal 2–6 MHz pulsed-wave Doppler probe (Philips Sparq) was used. Kidneys and interlobar arteries were localised using sonography and colour Doppler. Pulse-wave Doppler measurements in the interlobar arteries were then obtained. On each kidney three pulse-wave measurements were performed and RRI values were averaged to obtain mean values. RRI was defined as (peak systolic velocity – end diastolic velocity)/ peak systolic velocity. All RRI measurements were performed by one investigator experienced in kidney Doppler ultrasonography and certified in echocardiography. Normal values for native kidneys are reported between 0.6 and 0.7. In order to assess the intraobserver variability, the RRI was measured previously in a separate cohort of 10 healthy volunteers by the same investigator. The intraclass correlation coefficient (ICC) was then calculated and had a value of 0.997 (95% confidence interval (CI) 0.991–0.999) with a variance of 0.008.
Figure 1.
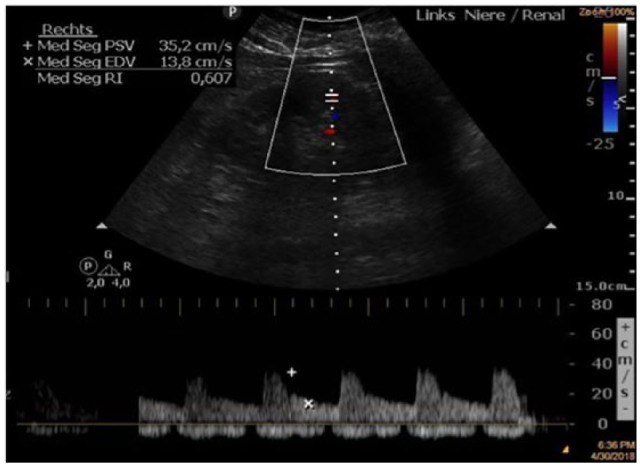
Renal Doppler ultrasound with renal resistive index (RRI) measurement.
The RRI is calculated from the peak systolic and end-diastolic velocities of arterial blood flow in the renal cortex (RRI = peak systolic velocity – end diastolic velocity/ peak systolic velocity).
The study was approved by the local ethics committee of the Philipps University of Marburg, which waived the need for written informed consent, as renal Doppler ultrasonography is an existing feature of our clinical practice and the augmentation of Impella flow level was performed in stable patients without any alterations in the systematic haemodynamic parameters.
Statistical analysis
Data are presented as absolute variables and percentages (%) for categorical variables and either median with interquartile range (IQR: 25th–75th percentile) or mean with standard deviation according to the distribution of the variables. We assessed normality using the Shapiro–Wilk test as well as Pearson tests. After testing for normal distribution, Student’s t-test or Mann–Whitney test was implemented to test for differences between the various characteristics. Intraobserver variability was calculated based on the ICC and its 95% CI.
Results
The study included 15 patients with infarct-related cardiogenic shock supported with an Impella. The demographics and baseline characteristics of these patients are reported in Table 1. Mean age was 66.7 ± 14 years and 73% were men. Mean vasopressor and inotropes doses were 8.9 ± 14.7 µg/min noradrenaline and 233 ± 200 µg/min dobutamine. The systolic left ventricular ejection fraction was 31 ± 7%. Doppler ultrasonography was performed within 6 hours after admission on the intensive care unit. The RRI could be calculated for both kidneys in 13 patients and for one kidney in two patients. The mean difference between right and left RRI was 0.026 ± 0.023, P=0.72. No patient had a difference greater than 0.05. The RRI decreased significantly from 0.66 ± 0.08 to 0.62 ± 0.06 (P<0.001), when increasing the Impella support by a mean of 0.44 L/min (±0.2 L/min) (Table 2), while both systolic and diastolic blood pressure remained unchanged. The decreasing tendency in RRI was consistent in each individual patient (Figure 2). Moreover, intra-renal peak systolic or peak diastolic velocity (Figures 3 and 4) remained unchanged.
Table 1.
Demographics and baseline characteristics.
| Age (years) | 67.81 ± 14.18 |
|---|---|
| ΒΜΙ (kg/m²) | 26.4 ± 2.6 |
| LVEF (%) | 31 ± 7 |
| Male/female | 11/4 |
| Cause of CS | |
| AMI | 14 |
| Acute myocarditis | 1 |
| Creatinine (mg/dl) | 1.286 ± 0.684 |
| Heart rate (bpm) | 102 ± 21 |
| SAP (mmHg) | 109.3 ± 17.19 |
| DAP (mmHg) | 60 ± 10 |
| MAP (mmHg) | 85.9 ± 13.2 |
| Noradrenaline (µg/min) | 8.9 ± 14.7 |
| Dobutamine (µg/min) | 233 ± 200 |
| Renal longitudinal length (cm) | 9.58 ± 0.9 |
| Renal parenchymal thickness (cm) | 2 ± 0.3 |
BMI: body mass index; LVEF: left ventricular ejection fraction; CS: cardiogenic shock; AMI: acute myocardial infarction; SAP: systolic arterial pressure; DAP: diastolic arterial pressure; MAP: mean arterial pressure.
Table 2.
RRI values at different Impella support levels.
| Patient | Impella flow (L/min) | RRI |
|---|---|---|
| 1 | 1.4 | 0.76 |
| 1.9 | 0.71 | |
| 2 | 1.4 | 0.73 |
| 2.4 | 0.62 | |
| 3 | 1.3 | 0.76 |
| 2 | 0.69 | |
| 4 | 1.6 | 0.55 |
| 1.9 | 0.52 | |
| 5 | 2 | 0.56 |
| 2.2 | 0.54 | |
| 6 | 3 | 0.63 |
| 3.4 | 0.59 | |
| 7 | 2.1 | 0.74 |
| 2.5 | 0.65 | |
| 8 | 2.2 | 0.78 |
| 2,6 | 0.74 | |
| 9 | 1.5 | 0.63 |
| 2 | 0.58 | |
| 10 | 2.2 | 0.74 |
| 2.5 | 0.69 | |
| 11 | 2 | 0.56 |
| 2.3 | 0.55 | |
| 12 | 1.6 | 0.64 |
| 2 | 0.63 | |
| 13 | 1.6 | 0.62 |
| 1.9 | 0.61 | |
| 14 | 2.8 | 0.62 |
| 3.4 | 0.58 | |
| 15 | 1.8 | 0.64 |
| 2.1 | 0.59 |
RRI: renal resistive index.
Figure 2.
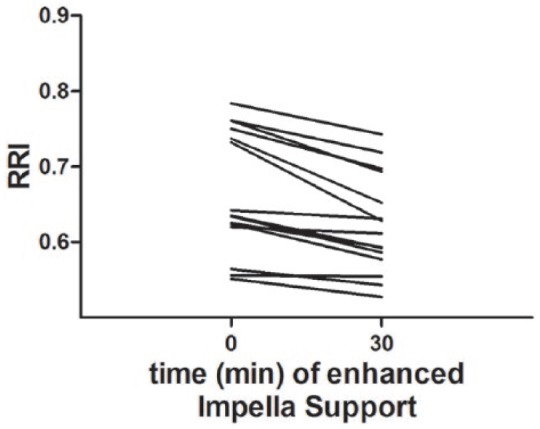
Individual RRI profiles in relation to Impella support.
In every patient a reduction of the RRI was observed after increasing Impella support.
RRI: renal resistive index.
Figure 3.
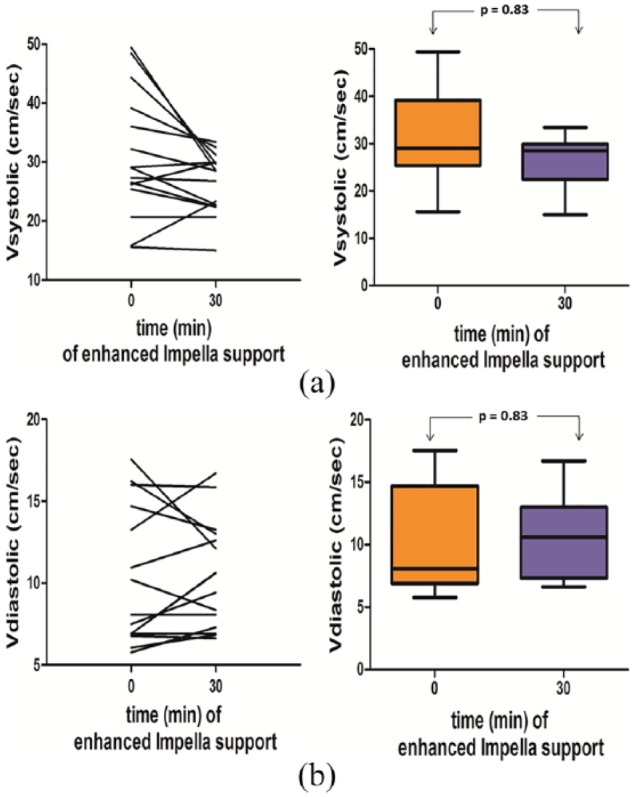
(a) Impact of the augmentation of the Impella flow level on the peak systolic velocity in each patient and between the two time points.
The peak systolic velocity was increased only in three patients, there was no significant difference in the levels of the peak systolic velocity between baseline and after augmentation of the Impella flow level.
(b) Impact of the augmentation of the Impella flow level on the peak diastolic velocity in each patient and between the two time points.
The peak diastolic velocity was increased only in five patients, there was no significant difference in the levels of the peak diastolic velocity between baseline and after augmentation of the Impella flow level.
Figure 4.
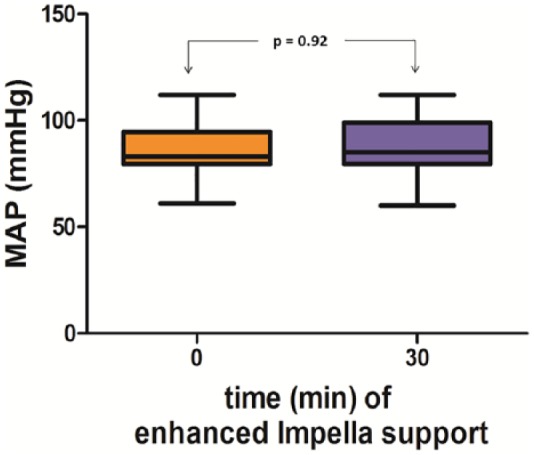
Impact of the augmentation of the Impella flow level on mean arterial pressure. The mean arterial pressure did not change after the increase in the Impella flow level compared to baseline.
Discussion
The RRI has been studied intensively not only to gain diagnostic and prognostic insights into a variety of renal pathologies (such as the progression of chronic kidney disease and renal allograft rejection), but also for the prediction of renal outcomes in critically ill patients.7,11 Darmon and colleagues found that RRI values greater than 0.75 predicted persistent AKI with a good sensitivity and specificity in critically ill patients with mechanical ventilation.11 In this study, the performance of the RRI was better than urinary indices for predicting AKI.11 Moreover, the RRI has been shown to predict AKI with high sensitivity and specificity in the immediate postoperative period after cardiac surgery.14
Here we investigated for the first time the effects of left ventricular mechanical support (MCS) using the Impella microaxial pump on the RRI in patients with cardiogenic shock. The present study shows that a significant decrease in RRI can be observed when increasing CO by Impella MCS without any changes in systolic or diastolic blood pressure (Figure 2).
The RRI is used for assessing instant renal perfusion7 and is one of the most sensitive parameters of renal vascular resistance, which in turn depicts alterations of renal blood flow.10 Therefore, analysing the intrarenal arterial waveforms obtained by Doppler ultrasonography might be useful in patients with cardiogenic shock for the detection of renal hypoperfusion. Prompting then adequate treatment decisions in order to improve renal perfusion may prevent or attenuate persistent AKI.15 Such a prompt response would not be possible if therapeutic manoeuvres are based on delayed criteria of AKI such as serum creatinine or low urine output.11,14 AKI, which often develops in critically ill patients such as in cardiogenic shock, is associated with increased morbidity and mortality.1,2,16,17 Therefore, monitoring kidney function and the early detection of renal hypoperfusion in patients with cardiogenic shock is crucial for implementation of therapeutic measures and adjusting haemodynamic strategies in cardiogenic shock.
Decreased renal blood flow and renal venous congestion are independent determinants of worsening renal function in patients with heart failure in addition to neurohormonal activation, including activation of the sympathetic nervous system.18 A decrease in CO will cause the autoregulatory mechanisms of renal perfusion to reduce renal vascular resistance in order to maintain renal perfusion.19 In heart failure and cardiogenic shock the hyperactivation of the sympathetic nervous system increases vascular resistance and may lead to a decrease in renal perfusion, especially in the presence of reduced CO.19 Moreover, vasopressors, which are often used in cardiogenic shock, may further reduce renal perfusion and increase RRI by direct vasoconstriction.20 In particular, vasopressors, such as norepinephrine, may have vasoconstrictive effects on renal vessels as doses increase, inducing an increase in vascular resistance and thereby reducing renal blood flow.
On the other hand, the maintenance of continuous flow during Impella support in cardiogenic shock may increase CO, reduce vasopressor doses4–6,21 and thereby improve renal perfusion and decrease RRI. In patients undergoing high-risk percutaneous coronary intervention, including patients with severely depressed systolic left ventricular function and cardiogenic shock, Impella support significantly reduced the risk of AKI.22 The repetitive finding of RRI decrease after augmentation of the support through the microaxial Impella pump underlines a causality, which may suggest the importance of Impella support as part of a renal protective strategy.
In conclusion, increasing Impella support in patients with cardiogenic shock led to a significant reduction of the RRI, suggesting improved renal perfusion. Determining the optimal haemodynamic support in patients with cardiogenic shock not only on systemic haemodynamic parameters but also on regional perfusion indices such as the RRI may be beneficial in optimising end-organ perfusion. Whether RRI may in future be a relevant endpoint to titrate Impella support in patients with cardiogenic shock or not remains to be answered in future studies.
Limitations
Our observations are obviously limited by the retrospective and non-randomised and open-label design of our study. However, this is the first study to investigate the effects of Impella support on the RRI in patients with cardiogenic shock. Detailed right heart catheter haemodynamic data before implantation of the Impella device were not available for all patients, but in emergency situations extensive invasive haemodynamic measurements are often not routinely performed. Another limitation of our study is the small number of patients included. However, the purpose of our investigation was to assess the effects of Impella support on the RRI and was not powered to evaluate renal outcomes. Larger studies with longer periods of assessment are needed to determine the effect of titrating Impella support using the RRI on the prevention of acute renal injury.
Footnotes
Conflict of interest: BS, KK, BM and UL have received speaker’s honoraria from Abiomed. NP, GC and HA have no conflicts of interest to disclose.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Koreny M, Karth GD, Geppert A, et al. Prognosis of patients who develop acute renal failure during the first 24 hours of cardiogenic shock after myocardial infarction. Am J Med; 2002; 112: 115–119. [DOI] [PubMed] [Google Scholar]
- 2. Marenzi G, Assanelli E, Campodonico J, et al. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med 2010; 38: 438–444. [DOI] [PubMed] [Google Scholar]
- 3. Lauridsen MD, Gammelager H, Schmidt M, et al. Acute kidney injury treated with renal replacement therapy and 5-year mortality after myocardial infarction-related cardiogenic shock: a nationwide population-based cohort study. Crit Care 2015; 19: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schiller P, Vikholm P, Hellgren L. The Impella Recover mechanical assist device in acute cardiogenic shock: a single-centre experience of 66 patients. Interact Cardiovasc Thorac Surg 2016; 22: 452–458. [DOI] [PubMed] [Google Scholar]
- 5. Casassus F, Corre J, Leroux L, et al. The use of Impella 2.5 in severe refractory cardiogenic shock complicating an acute myocardial infarction. J Interv Cardiol 2015; 28: 41–50. [DOI] [PubMed] [Google Scholar]
- 6. Karatolios K, Chatzis G, Markus B, et al. Impella support compared to medical treatment for post-cardiac arrest shock after out of hospital cardiac arrest. Resuscitation 2018; 126: 104–110. [DOI] [PubMed] [Google Scholar]
- 7. Le Dorze M, Bouglé A, Deruddre S, et al. Renal Doppler ultrasound: a new tool to assess renal perfusion in critical illness. Shock 2012; 37: 360–365. [DOI] [PubMed] [Google Scholar]
- 8. Schnell D, Darmon M.Bedside Doppler ultrasound for the assessment of renal perfusion in the ICU: advantages and limitations of the available techniques. Crit Ultrasound J 2015; 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cauwenberghs N, Kuznetsova T. Determinants and prognostic significance of the renal resistive index. Pulse (Basel) 2016; 3: 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Granata A, Zanoli L, Clementi S, et al. Resistive intrarenal index: myth or reality? Br J Radiol 2014; 87: 20140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darmon M, Schortgen F, Vargas F, et al. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med 2011; 37: 68–76. [DOI] [PubMed] [Google Scholar]
- 12. Ponte B, Pruijm M, Ackermann D, et al. Reference values and factors associated with renal resistive index in a family-based population study. Hypertension 2014; 63: 136–142. [DOI] [PubMed] [Google Scholar]
- 13. Pruijm M, Ponte B, Ackermann D, et al. Heritability, determinants and reference values of renal length: a family-based population study. Eur Radiol 2013; 23: 2899–2905. [DOI] [PubMed] [Google Scholar]
- 14. Bossard G, Bourgoin P, Corbeau JJ, et al. Early detection of postoperative acute kidney injury by Doppler renal resistive index in cardiac surgery with cardiopulmonary bypass. Br J Anaesth 2011; 107: 891–898. [DOI] [PubMed] [Google Scholar]
- 15. Esson ML, Schrier RW. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med 2002; 137: 744–752. [DOI] [PubMed] [Google Scholar]
- 16. Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813–818. [DOI] [PubMed] [Google Scholar]
- 17. Brivet FG, Kleinknecht DJ, Loirat P, et al. Acute renal failure in intensive care units – causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med 1996; 24: 192–198. [DOI] [PubMed] [Google Scholar]
- 18. Damman K, Navis G, Smilde TDJ, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 2007; 9: 872–878. [DOI] [PubMed] [Google Scholar]
- 19. Grande D, Terlizzese P, Iacoviello M. Role of imaging in the evaluation of renal dysfunction in heart failure patients. World J Nephrol 2017; 6: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rozemeijer S, Haitsma Mulier JLG, Röttgering JG, et al. Renal Resistive index: response to shock and its determinants in critically ill patients. Shock 2019; 1: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyns B, Stolinski J, Leunens V, et al. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J Am Coll Cardiol 2003; 41: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 22. Flaherty MP, Pant S, Patel SV, et al. Hemodynamic support with a microaxial percutaneous left ventricular assist device (Impella) protects against acute kidney injury in patients undergoing high-risk percutaneous coronary intervention. Circ Res 2017; 120: 692–700. [DOI] [PubMed] [Google Scholar]


