Abstract
BACKGROUND:
Schizophrenia and bipolar disorder share genetic liability, and some structural brain abnormalities are common to both conditions. First-degree relatives of patients with schizophrenia (FDRs-SZ) show similar brain abnormalities to patients, albeit with smaller effect sizes. Imaging findings in first-degree relatives of patients with bipolar disorder (FDRs-BD) have been inconsistent in the past, but recent studies report regionally greater volumes compared with control subjects.
METHODS:
We performed a meta-analysis of global and subcortical brain measures of 6008 individuals (1228 FDRs-SZ, 852 FDRs-BD, 2246 control subjects, 1016 patients with schizophrenia, 666 patients with bipolar disorder) from 34 schizophrenia and/or bipolar disorder family cohorts with standardized methods. Analyses were repeated with a correction for intracranial volume (ICV) and for the presence of any psychopathology in the relatives and control subjects.
RESULTS:
FDRs-BD had significantly larger ICV (d = +0.16, q < .05 corrected), whereas FDRs-SZ showed smaller thalamic volumes than control subjects (d = −0.12, q < .05 corrected). ICV explained the enlargements in the brain measures in FDRs-BD. In FDRs-SZ, after correction for ICV, total brain, cortical gray matter, cerebral white matter, cerebellar gray and white matter, and thalamus volumes were significantly smaller; the cortex was thinner (d < −0.09, q < .05 corrected); and third ventricle was larger (d = +0.15, q < .05 corrected). The findings were not explained by psychopathology in the relatives or control subjects.
CONCLUSIONS:
Despite shared genetic liability, FDRs-SZ and FDRs-BD show a differential pattern of structural brain abnormalities, specifically a divergent effect in ICV. This may imply that the neurodevelopmental trajectories leading to brain anomalies in schizophrenia or bipolar disorder are distinct.
Keywords: Bipolar disorder, Familial risk, Imaging, Meta-analysis, Neurodevelopment, Schizophrenia
Schizophrenia and bipolar disorder are highly heritable disorders with partially overlapping symptoms and a genetic correlation (rg) of 0.60–0.68 (1-3). Both disorders are characterized by structural brain abnormalities, with smaller total brain and hippocampal volumes, on average, and larger ventricular volumes. These are among the most consistent and robust structural findings, albeit with smaller effect sizes in patients with bipolar disorder (4-12). On one hand, the shared genetic liability between schizophrenia and bipolar disorder (1-3) is partly reflected in the brain by overlapping findings of smaller white matter volumes and common areas of thinner cortex, suggesting that the disorders share genetic (possibly neurodevelopmental) roots (13). On the other hand, disease-specific brain abnormalities were also reported in the same twin study; genetic liability for schizophrenia was associated with thicker right parietal cortex, whereas genetic liability for bipolar disorder was associated with larger intracranial volume (ICV) (13).
Family members of patients can represent individuals at familial risk for the disorder who do not themselves have confounds, such as medication or illness duration, and can therefore provide unique insight into the effect of familial risk for the disorder on the brain. Multiple imaging studies have investigated individuals at high familial risk for schizophrenia and/or bipolar disorder, but results of these often small studies have been variable. First-degree relatives of patients with schizophrenia (FDRs-SZ) tend to show smaller brain volumes and larger ventricle volumes compared with control subjects (14,15). In contrast, first-degree relatives of patients with bipolar disorder (FDRs-BD) show regionally larger volumes (16-26). Many of these schizophrenia and bipolar disorder family studies grouped all FDRs together regardless of kinship. It remains unclear whether structural brain abnormalities in high-risk individuals are consistent across FDRs, or whether they vary depending on the generational relationship with the proband. In addition, a few studies compared brain structure between FDRs-BD and FDRs-SZ directly, usually in cohorts of modest sample sizes (9,13,27-30). These studies showed brain abnormalities both specific and overlapping for FDRs-SZ and FDRs-BD; if anything, findings were more pronounced in FDRs-SZ than FDRs-BD.
Large-scale multicenter studies offer increased power and generalizability to evaluate the pattern and extent of brain variation in FDRs-BD and FDRs-SZ. Through the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA)-Relatives Working Group, we have performed meta-analyses of magnetic resonance imaging data sets consisting of FDRs-SZ and/or FDRs-BD, probands, and matched control participants on harmonized global and subcortical brain measures. For each disorder, relatives were analyzed as a group as well as per relative type, i.e., monozygotic co-twins, dizygotic co-twins, offspring, siblings, and parents. To investigate potential confounders, analyses were performed both with and without correction for ICV and with and without a correction for having a psychiatric diagnosis in the relatives and control subjects. The latter correction was performed by 1) adding a single dummy variable coding for the presence of any psychiatric diagnosis and 2) by comparing only the healthy relatives with the healthy control subjects. We hypothesized that FDRs-SZ (as a group) would exhibit a pattern of brain volume abnormalities similar to patterns observed in patients, but with smaller effect sizes. Based on dissimilarities in the literature between FDRs-SZ and FDRs-BD, we expected divergent effect sizes. Furthermore, we explored the pattern and extent of brain volume abnormalities per relative type.
METHODS AND MATERIALS
Study Samples
This study included 6008 participants from 34 family cohorts. In total, 1228 FDRs-SZ (49 monozygotic co-twins, 62 dizygotic co-twins, 171 offspring, 842 siblings, 104 parents), 852 FDRs-BD (41 monozygotic co-twins, 48 dizygotic co-twins, 443 offspring, 302 siblings, 18 parents), 2246 control subjects, 1016 patients with schizophrenia, and 666 patients with bipolar disorder were included (Tables 1 and 2). All cohorts included their own control participants. Control subjects did not have a family history of schizophrenia or bipolar disorder. FDRs-SZ or FDRs-BD are defined by having a first-degree family member with schizophrenia or bipolar disorder, respectively, and not having experienced (hypo)mania and/or psychosis themselves. Several cohorts allowed FDRs-SZ, FDRs-BD, or control subjects to have psychiatric diagnoses other than schizophrenia or bipolar disorder (Tables 1 and 2). Demographic characteristics for each cohort and their inclusion criteria are summarized in Tables 1 and 2 and Supplemental Table S1. All study centers obtained approval from their respective medical ethics committee for research following the Declaration of Helsinki. Informed consent was obtained from all participants (and/or parent guardians in the case of minors).
Table 1.
Sample Demographics Bipolar Disorder Family Cohorts
| Relatives |
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls |
Cases |
Total |
MZ Co-twins |
DZ Co-twins |
Offspring |
Siblings |
Parents |
|||||||||||||||||||||
| Sample | n | M/F | Age | Other Diagnoses (Y/N) |
n | M/F | Age | Total N |
n | M/F | Age | Other Diagnoses (Y/N) |
n | M/F | Age | Other Diagnoses (Y/N) |
n | M/F | Age | Other Diagnoses (Y/N) |
n | M/F | Age | Other Diagnoses (Y/N) |
n | M/F | Age | Other Diagnoses (Y/N) |
| BPO_FLB | 7 | 3/4 | 12.9 | 0/7 | 9 | 5/4 | 13.3 | 22 | – | – | 22 | 10/12 | 10.0 | 0/22 | – | – | ||||||||||||
| Cardiff | 79 | 28/51 | 39.8 | 0/79 | 120 | 42/78 | 41.9 | 33 | – | – | – | 33 | 13/20 | 45.9 | 2/31 | – | ||||||||||||
| CliNG-BDa | 19 | 6/13 | 30.9 | 0/19 | – | 19 | – | – | 11 | 4/7 | 23.4 | 0/11 | 8 | 2/6 | 43.8 | 0/8 | – | |||||||||||
| DEU | 29 | 11/18 | 33.1 | 0/29 | 27 | 10/17 | 36.3 | 23 | – | – | 6 | 2/4 | 21.7 | 0/6 | 17 | 9/8 | 34.8 | 0/17 | – | |||||||||
| EGEU | 33 | 13/20 | 33.6 | 0/33 | 27 | 16/11 | 36.7 | 27 | – | – | – | 27 | 10/17 | 34.5 | 0/27 | – | ||||||||||||
| ENBD_UT | 36 | 13/23 | 34.8 | 0/36 | 72 | 23/49 | 36.9 | 52 | – | – | – | 52 | 10/42 | 44.3 | 17/35 | – | ||||||||||||
| HHR | 42 | 17/25 | 21.9 | 0/42 | 8 | 2/6 | 23.3 | 52 | – | – | 52 | 18/34 | 19.5 | 14/38 | – | – | ||||||||||||
| IDIBAPSa | 53 | 21/32 | 12.3 | 12/41 | – | 61 | – | – | 61 | 31/30 | 12.3 | 27/34 | – | – | ||||||||||||||
| IoP-BD | 39 | 9/30 | 35.4 | 9/30 | 34 | 15/19 | 40.6 | 17 | 11 | 2/9 | 43.5 | 6/5 | 6 | 2/4 | 42.4 | 0/6 | – | – | – | |||||||||
| MFS-BDa | 54 | 25/29 | 40.2 | 0/54 | 38 | 15/23 | 41.0 | 41 | – | – | – | 23 | 11/12 | 42.9 | 0/23 | 18 | 6/12 | 57.6 | 0/18 | |||||||||
| MooDS-BDa | 63 | 25/38 | 30.3 | 0/63 | – | 63 | – | – | 53 | 18/35 | 29.2 | 0/53 | 10 | 7/3 | 36.6 | 0/10 | – | |||||||||||
| MSSM | 52 | 25/27 | 35.2 | 0/52 | 41 | 21/20 | 44.3 | 50 | – | – | 27 | 14/13 | 24.9 | 15/12 | 23 | 12/11 | 44.2 | 8/15 | – | |||||||||
| Olin | 68 | 25/43 | 32.2 | 7/61 | 108 | 34/74 | 34.5 | 78 | – | – | – | 78 | 30/48 | 32.0 | 21/57 | – | ||||||||||||
| PHHR | 18 | 7/11 | 23.0 | 0/18 | 8 | 3/5 | 24.0 | 26 | – | – | 26 | 10/16 | 19.9 | 6/20 | – | – | ||||||||||||
| STAR-BDa | 114 | 55/59 | 48.8 | 42/72 | 53 | 19/34 | 49.2 | 38 | 16 | 6/10 | 49.2 | 3/13 | 22 | 10/12 | 50.8 | 6/16 | – | – | – | |||||||||
| SydneyBipolarGroup | 117 | 54/63 | 22.2 | 30/87 | 59 | 17/42 | 25.1 | 150 | – | – | 119 | 53/66 | 19.2 | 58/61 | 31 | 12/19 | 22.6 | 21/10 | – | |||||||||
| UMCU-BD Twinsa | 129 | 55/74 | 39.2 | 4/125 | 62 | 19/43 | 40.3 | 34 | 14 | 4/10 | 38.2 | 6/8 | 20 | 8/12 | 44.3 | 4/16 | – | – | – | |||||||||
| UMCU-DBSOSa | 40 | 21/19 | 12.7 | 7/33 | – | 66 | – | – | 66 | 37/29 | 14.7 | 31/35 | – | – | ||||||||||||||
DZ, dizygotic; F, female; M, male; MZ, monozygotic; N, no; Y, yes.
BPO_FLB, Bipolar Offspring - Fronto-Limbic; Cardiff, Cardiff University; CliNG-BD, Clinical Neuroscience Goettingen- Bipolar Disorder; DEU, Dokuz Eylul University; EGEU, Ege University; ENBD_UT, Endophenotypes of Bipolar Disorder - University of Texas; HHR, Halifax High Risk Study; IDIBAPS, August Pi i Sunyer Biomedical Research Institute; IoP-BD, Institute of Psychiatry - Bipolar Disorder Twin Study; MFS-BD, Maudsley Family Study - Bipolar Disorder; MooDS-BD, Systematic Investigation of the Molecular Causes of Major Mood Disorders and Schizophrenia - Bipolar Disorder; MSSM, Mount Sinai School of Medicine; Olin, Olin Neuropsychiatry Research Center; PHHR, Prague High Risk Study; STAR-BD, Schizophrenia and Bipolar Twin Study in Sweden - Bipolar Disorder; SydneyBipolarGroup, The Sydney Bipolar Kids and Sibs Study; UMCU-BD Twins, University Medical Center Utrecht - Bipolar Disorder Twin Study; UMCU-DBSOS, University Medical Center Utrecht - Dutch Bipolar and Schizophrenia Offspring Study.
Overlapping control subjects with schizophrenia sample from the same site, i.e., with CliNG-SZ (n = 10), IDIBAPS (n = 53), MFS-SZ (n = 54), MooDS-SZ (n = 36), STAR-SZ (n = 100), UMCU-UTWINS (n = 27), UMCU-DBSOS (n = 40).
Table 2.
Sample Demographics Schizophrenia Family Cohorts
| Relatives |
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls |
Cases |
Total |
MZ Co-twins |
DZ Co-twins |
Offspring |
Siblings |
Parents |
|||||||||||||||||||||
| Sample | n | M/F | Age | Other Diagnoses (Y/N) |
n | M/F | Age | Total N |
n | M/F | Age | Other Diagnoses (Y/N) |
n | M/F | Age | Other Diagnoses (Y/N) |
n | M/F | Age | Other Diagnoses (Y/N) |
n | M/F | Age | Other Diagnoses (Y/N) |
n | M/F | Age | Other Diagnoses (Y/N) |
| C_SFS | 23 | 11/12 | 40.2 | 7/16 | 25 | 13/12 | 40.8 | 23 | – | – | – | 13 | 7/6 | 32.5 | 4/9 | 10 | 1/9 | 54.6 | 3/7 | |||||||||
| CliNG-SZa | 20 | 11/9 | 35.7 | 0/20 | – | 20 | – | – | 6 | 3/3 | 26.7 | 0/6 | 7 | 5/2 | 28.4 | 0/7 | 7 | 3/4 | 51.9 | 0/7 | ||||||||
| EHRS | 89 | 44/45 | 21.0 | 0/89 | 31 | 19/12 | 21.8 | 90 | – | – | 57 | 26/31 | 20.9 | 0/57 | 33 | 18/15 | 21.8 | 0/33 | – | |||||||||
| HUBIN | 102 | 69/33 | 41.9 | 29/73 | 103 | 77/26 | 41.2 | 33 | – | – | – | 33 | 23/10 | 39.4 | 8/25 | – | ||||||||||||
| IDIBAPSa | 53 | 21/32 | 12.3 | 12/41 | – | 37 | – | – | 37 | 22/15 | 11.0 | 18/19 | – | – | ||||||||||||||
| IoP-SZ | 67 | 35/32 | 40.9 | 7/60 | 54 | 39/15 | 34.7 | 18 | 14 | 7/7 | 31.0 | 6/8 | 4 | 1/3 | 40.0 | 1/3 | – | – | – | |||||||||
| LIBD | 364 | 163/201 | 32.4 | 3/361 | 215 | 164/51 | 35.3 | 242 | – | – | – | 242 | 100/142 | 36.2 | 83/159 | – | ||||||||||||
| Maastricht- GROUP |
87 | 33/54 | 30.8 | 14/73 | 87 | 59/28 | 28.2 | 95 | – | – | – | 95 | 49/46 | 29.5 | 19/76 | – | ||||||||||||
| MFS-SZa | 54 | 25/29 | 40.2 | 0/54 | 42 | 31/11 | 36.4 | 56 | – | – | – | 20 | 10/10 | 36.4 | 0/20 | 36 | 11/25 | 56.6 | 0/36 | |||||||||
| MooDS-SZa | 65 | 26/39 | 30.6 | 0/65 | – | 63 | – | – | 31 | 10/21 | 26.5 | 0/31 | 25 | 12/13 | 30.2 | 0/25 | 7 | 2/5 | 49.7 | 0/7 | ||||||||
| NU | 92 | 51/41 | 31.9 | 7/85 | 108 | 74/34 | 34.2 | 83 | – | – | – | 83 | 29/54 | 21.1 | 45/38 | – | ||||||||||||
| STAR-SZa | 104 | 49/55 | 48.9 | 22/82 | 49 | 28/21 | 49.5 | 48 | 15 | 9/6 | 41.9 | 0/15 | 33 | 17/16 | 52.3 | 0/33 | – | – | – | |||||||||
| UMCG-GROUP | 37 | 16/21 | 34.0 | 0/37 | – | 45 | – | – | – | 45 | 22/23 | 30.9 | 0/45 | – | ||||||||||||||
| UMCU-DBSOSa | 40 | 21/19 | 12.7 | 7/33 | – | 40 | – | – | 40 | 12/28 | 13.7 | 24/16 | – | – | ||||||||||||||
| UMCU-GROUP | 167 | 83/84 | 27.7 | 13/154 | 162 | 130/32 | 27.0 | 201 | – | – | – | 201 | 95/106 | 27.7 | 52/149 | – | ||||||||||||
| UMCU-Parents | 41 | 14/27 | 52.8 | 0/41 | – | 44 | – | – | – | – | 44 | 13/31 | 52.9 | 11/33 | ||||||||||||||
| UMCU-UTWINSa | 184 | 84/100 | 31.8 | 17/167 | 56 | 33/23 | 35.6 | 45 | 20 | 12/8 | 36.0 | 11/9 | 25 | 17/8 | 37.8 | 5/20 | – | – | – | |||||||||
| UNIBA | 78 | 52/26 | 31.4 | 0/78 | 84 | 58/26 | 33.3 | 45 | – | – | – | 45 | 23/22 | 33.4 | 4/41 | – | ||||||||||||
DZ, dizygotic; F, female; M, male; MZ, monozygotic; N, no; Y, yes.
C_SFS, Calgary Schizophrenia Family Study; CliNG-SZ, Clinical Neuroscience Goettingen - Schizophrenia; EHRS, Edinburgh High Risk Study; HUBIN, Human Brain Informatics; IDIBAPS, August Pi i Sunyer Biomedical Research Institute; IoP-SZ, Institute of Psychiatry - Schizophrenia Twin Study; LIBD, Lieber Institute for Brain Development; Maastricht-GROUP, Maastricht - Genetic Risk and Outcome of Psychosis; MFS-SZ, Maudsley Family Study - Schizophrenia; MooDS-SZ, Systematic Investigation of the Molecular Causes of Major Mood Disorders and Schizophrenia - Schizophrenia; NU, Northwestern University; STAR-SZ, Schizophrenia and Bipolar Twin Study in Sweden - Schizophrenia; UMCG-GROUP, University Medical Center Groningen - Genetic Risk and Outcome of Psychosis; UMCU-DBSOS, University Medical Center Utrecht - Dutch Bipolar and Schizophrenia Offspring Study; UMCU-GROUP, University Medical Center Utrecht - Genetic Risk and Outcome of Psychosis; UMCU-Parents, University Medical Center Utrecht - Parents Study; UMCU-UTWINS, University Medical Center Utrecht - Utrecht Twin Schizophrenia Studies; UNIBA, University of Bari “Aldo Moro.”
Overlapping control subjects with bipolar sample from the same site, i.e., with CliNG-BD (n = 10), IDIBAPS (n = 53), MFS-BD (n = 54), MooDS-BD (n = 36), STAR-BD (n = 100), UMCU-BD twins (n = 27), UMCU-DBSOS (n = 40).
Image Acquisition and Processing
Structural T1-weighted brain magnetic resonance imaging scans were acquired at each research center (see Supplemental Table S2 for acquisition parameters of each cohort). Cortical and subcortical reconstruction and volumetric segmentations were performed with the FreeSurfer pipeline (see Table S2 for FreeSurfer version and operating system used in each cohort) (http://surfer.nmr.mgh.harvard.edu/fswiki/recon-all/) (31). The resulting segmentations were quality checked according to the ENIGMA quality control protocol for subcortical volumes (http://enigma.ini.usc.edu/protocols/imaging-protocols/). Global brain measures (i.e., ICV [estimated Total Intracranial Volume from FreeSurfer], total brain [including cerebellum, excluding brainstem], cortical gray matter, cerebral white matter, cerebellar gray and white matter, third and lateral ventricle volume, surface area, and mean cortical thickness) and subcortical volumes (i.e., thalamus, caudate, putamen, pallidum, hippocampus, amygdala, and accumbens) were extracted from individual images (32,33).
Statistical Meta-analyses
All statistical analyses were performed using R (http://www.r-project.org). Linear mixed model analyses were performed within each cohort for bipolar disorder and schizophrenia separately, comparing relatives (per relative type) with control subjects and, if present, patients with control subjects, while taking family relatedness into account (http://CRAN.R-project.org/package=nlme) (34). Given known age and sex effects on brain measures, we included centered age, age squared, and sex as covariates. Brain measures were corrected for lithium use at time of scan (yes/no) in patients with bipolar disorder only. Analysis of multiscanner studies included binary dummy covariates for n–1 scanners. Cohen’s d effect sizes and 95% confidence intervals were calculated within each cohort separately and pooled per disorder for each relative type, for all relatives, and for patients as a group, using an inverse variance-weighted random-effects meta-analysis. All random-effects models were fitted using the restricted maximum likelihood method. False discovery rate (q < .05) thresholding across all phenotypes was used to control for multiple comparisons for each pairwise analysis between relatives, patients, and control subjects or between the different relative types (35). Analyses were performed locally by the research center that contributed the cohort, using codes created within the ENIGMA-Relatives Working Group (scripts available on request). The focus of this study is on first-degree relatives, but patient effects were also computed to show that the effects in patients are in line with earlier work (4-12). Effect sizes were statistically compared between FDRs-BD and FDRs-SZ, FDRs-BD and patients with bipolar disorder, and FDRs-SZ and patients with schizophrenia, and between the different relative types within one disorder (Supplemental Methods). The latter analysis was performed only when more than one cohort was included per relative type.
The regional specificity of the findings was examined by repeating the analyses of the global brain measures and subcortical volumes with ICV added as a covariate. In addition, we repeated the analyses to investigate the effect of psychopathology in the relatives and control subjects using two different approaches. First, we added a single dummy variable for relatives and control subjects with a DSM “No diagnosis” or ICD-9 code V71.09 (other diagnosis = 1, V71.09 = 0). Second, we compared healthy relatives with healthy control subjects. Finally, effects of age were examined using meta-regressions.
RESULTS
Patients
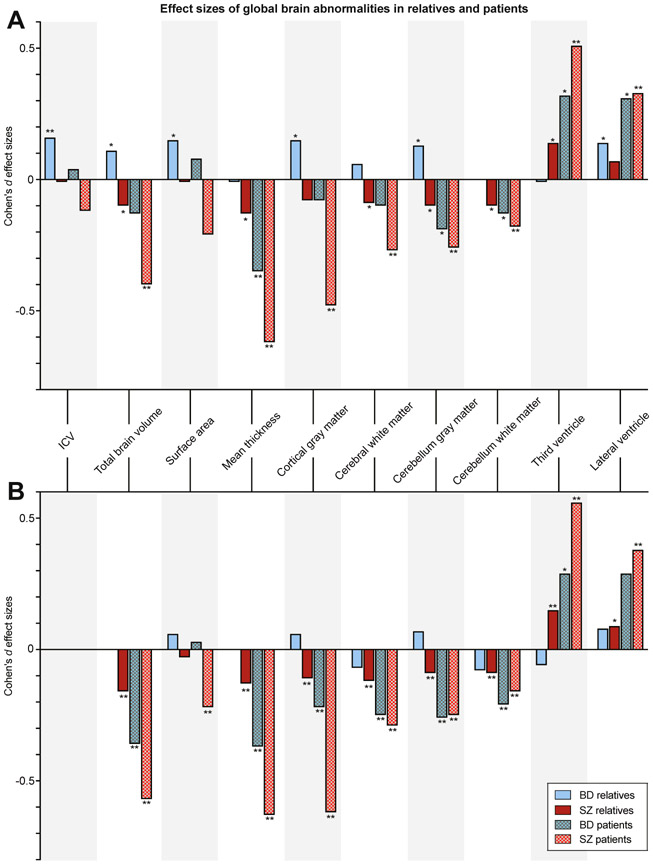
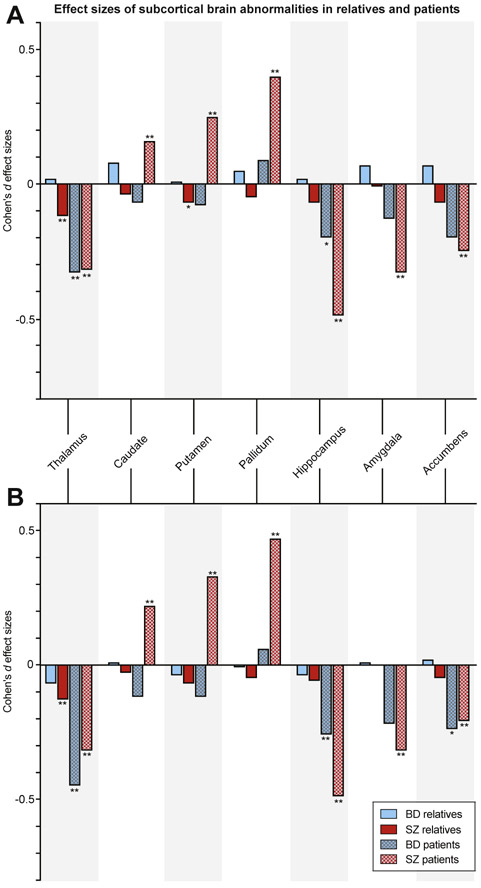
Effects in patients with schizophrenia and bipolar disorder were not the main focus of this study. In short, a thinner cortex and smaller thalamus volume were found in patients with bipolar disorder (d < −0.33, q < .05 corrected); in patients with schizophrenia, smaller volumes of total brain, cortical gray matter, cerebral white matter, cerebellar gray and white matter, thalamus, hippocampus, amygdala, and accumbens, thinner cortex (d < −0.18, q < .05 corrected), and larger volumes of the lateral ventricles, third ventricle, caudate, pallidum, and putamen (d > +0.16, q < .05 corrected) were found. The findings are summarized in Figures 1 and 2, Supplemental Figure S1i-xvii, and Supplemental Tables S3 and S4.
Figure 1.
(A) Cohen’s d effect sizes comparing relatives and patients with bipolar disorder (BD) (blue) and relatives and patients with schizophrenia (SZ) (red) with control subjects for global brain measures, (B) controlled for intracranial volume (ICV). *Nominally significant effect sizes (p < .05, uncorrected); **q < .05, corrected.
Figure 2.
(A) Cohen’s d effect sizes comparing relatives and patients with bipolar disorder (BD) (blue) and relatives and patients with schizophrenia (SZ) (red) with controls for subcortical volumes, (B) controlled for intracranial volume. *Nominally significant effect sizes (p < .05, uncorrected); **q < .05, corrected.
FDRs-BD and FDRs-SZ vs. Control Subjects
FDRs-BD had significantly larger ICVs than control subjects (d = +0.16, q < .05 corrected) (Figures 1A and 2A, Supplemental Figure S1i-xvii, and Supplemental Table S3). FDRs-SZ had significantly smaller thalamic volume than control subjects (d = −0.12, q < .05 corrected) (Figures 1A and 2A, Supplemental Figure S1i-xvii, and Supplemental Table S3). When comparing the effect sizes of FDRs-BD and FDRs-SZ directly, FDRs-BD had significantly larger ICV, surface area, total brain, cortical gray matter, cerebral white matter, cerebellar gray matter, thalamus, and accumbens volumes and smaller third ventricle volumes than FDRs-SZ (q < .05 corrected) (Supplemental Table S3). For all nominally significant effect sizes (p < .05 uncorrected, 2-tailed) and comparisons, see Supplemental Table S3.
Regional Specificity of Findings: Correction for ICV
When controlling for ICV, there were no significant differences in brain measures between FDRs-BD and control subjects (Figures 1B and 2B and Supplemental Table S4). In contrast, in FDRs-SZ, total brain, cortical gray matter, cerebral white matter, cerebellar gray and white matter, and thalamus volumes were significantly smaller, cortex was thinner (d < −0.09, q < .05 corrected), and third ventricle was larger (d = +0.15, q < .05 corrected) than in control subjects (Figures 1B and 2B and Supplemental Table S4). FDRs-BD had significantly larger total brain, cortical, and cerebellar gray matter volumes and smaller third ventricle volumes than FDRs-SZ (q < .05 corrected) (Supplemental Table S4).
First-Degree Relatives Subtype Analyses
None of the effect sizes comparing FDRs-BD and FDRs-SZ subtypes with control subjects survived correction for multiple comparisons. Direct comparison between the different relative subtypes showed some significant differences between groups; see Supplemental Tables S7 and S8, Supplemental Figure S1i-xvii and Supplemental Results.
Psychopathology in Relatives
Psychiatric diagnoses other than bipolar disorder or a psychotic disorder were present in 40.4% of FDRs-BD, 31.5% of FDRs-SZ, 12.6% of control subjects in the bipolar sample, and 9.0% of control subjects in the schizophrenia sample (Tables 1 and 2). Controlling for any diagnosis by adding affected status (1 = yes/0 = no) as a covariate in the analysis did not change the pattern of findings in either FDRs-BD or FDRs-SZ (Supplemental Tables S9 and S10). Also, when comparing only healthy relatives with healthy control subjects, the pattern was similar (Supplemental Tables S11 and S12).
Effect of Age
Meta-regression analyses showed no relationship between age and FDRs-BD effect sizes (Supplemental Table S13 and Figure S2i-xvii). A positive relationship between age and FDRs-SZ effect sizes reached nominal significance only in the amygdala (p = .008, which did not survive false discovery rate correction for multiple comparisons) (Supplemental Table S13 and Figure S2i-xvii).
DISCUSSION
This ENIGMA-Relatives initiative allowed for the largest examination to date of FDRs-BD and FDRs-SZ. Through meta-analysis, we investigated whether harmonized subcortical and global brain measures differed between FDRs-BD and FDRs-SZ and control subjects and whether these brain measures differed between the different relative types. The main findings were that 1) FDRs-BD had larger ICVs, whereas FDRs-SZ showed smaller thalamic volumes compared with control subjects; 2) in FDRs-BD, ICV explained enlargements in other brain measures, whereas in FDRs-SZ, brain volumes and thickness became significantly smaller than in control subjects after correction for ICV; 3) abnormalities differed between the relative types, but no clear pattern was detected; and 4) the findings were not confounded by other psychiatric diagnoses in the relatives and control subjects.
Effects in patients with schizophrenia and bipolar disorder were in line with prior studies (4-12). In contrast to smaller brain volumes in patients with bipolar disorder (7,8), we found larger brain volumes in their relatives. This is in keeping with other studies, which have reported larger regional gray matter volumes in participants at genetic risk (16-26). As expected, FDRs-SZ had smaller brain volumes, similar to findings in patients with schizophrenia (6,10-12), but with smaller effect sizes, in line with a previous retrospective meta-analysis and a review (14,36). Effect sizes in both FDRs-SZ and FDRs-BD are small (∣d∣ ≤ 0.16), suggesting that the brain abnormalities in individuals at familial risk are subtle and can be detected only with large sample sizes. These small effect sizes and potential subtle differences could still be meaningful, as they may give information on the familial background of brain deficits in disease. That said, it remains unclear whether brain deficits with these small effect sizes have functional or clinical relevance for FDRs-BD and FDRs-SZ.
Bipolar disorder and schizophrenia have a partially overlapping genetic etiology, with a genetic correlation of rg = 0.60–0.68 based on population and genome-wide association studies (1-3), suggesting that they share to some extent the same risk genes. However, combined large genome-wide association studies of schizophrenia and bipolar disorder have also identified unique risk factors associated with each of these disorders (37). That FDRs-BD and FDRs-SZ show different global brain volume effects compared with control subjects implies that these brain abnormalities are associated with genetic variants unique to each disorder.
Twin studies have shown that schizophrenia (38-41) and bipolar disorder (42,43) have a shared genetic origin for brain volume, and overlapping brain abnormalities have been reported between the two patient groups (4,5,9,13). However, the available evidence for an association between common variants in both schizophrenia and bipolar disorder and brain volume is inconsistent (37,44-46). For example, Smeland et al. (45) used novel conditional false discovery rate methodology and identified 6 shared loci between intracranial, hippocampus, and putamen volumes and schizophrenia, whereas no significant genetic correlation was reported in another study that applied standard statistical tools (44). Genetic risk for bipolar disorder was unrelated to the genetic variants associated with brain measures (37,46). This could suggest either that rare genetic variants, such as copy number variants that are shared between relatives and probands, lead to brain abnormalities or that nongenetic overlap, i.e., shared environmental factors, leads to brain abnormalities in the family members.
The enlargement in several brain measures in FDRs-BD was driven by a larger ICV, whereas the decrements in brain measures in FDRs-SZ were more pronounced when controlling for ICV. This suggests that in contrast to the global ICV finding in FDRs-BD, brain abnormalities in FDRs-SZ not only are a global effect but also represent more regional differences in individuals at familial risk for schizophrenia. ICV reaches its maximum size between the ages of 10 and 15 (47,48); therefore, ICV may be interpreted as a direct marker for neurodevelopment. Indeed, both schizophrenia and bipolar disorder have been characterized as neurodevelopmental disorders (49-51); abnormal neurodevelopment may play a larger role in the onset of schizophrenia than bipolar disorder (52-54). This is in line with differential trajectories of IQ development and school performance found in relation to risk for schizophrenia and bipolar disorder, showing respectively poorer cognitive performance or even decreases over time years before schizophrenia onset and a U-shaped relationship between IQ and later development of bipolar disorder (53). This is also in keeping with a previous study, which found advanced brain age relative to chronological age in participants in early stages of schizophrenia, but not in participants in early stages of bipolar disorder (55). Given the discrepancy in ICV findings between FDRs-BD and FDRs-SZ, individuals at familial risk for either bipolar disorder or schizophrenia may deviate during early neurodevelopment in a disease-specific manner.
Interestingly, in contrast to FDRs-BD, patients with bipolar disorder did not show an ICV enlargement, confirming previous findings in a large meta-analysis (7). In the early stages of the disease, however, regional increases have been reported (21,22,24,26,56,57). Given the positive relationship between genetic risk for bipolar disorder and ICV reported in twins (13), one could argue that the genetic liability for bipolar disorder leads to a larger ICV as represented in our findings of larger ICV in FDRs-BD. That combination of a genetic predisposition for increased ICV and an ICV that is similar between patients with bipolar disorder and control subjects may imply that patient ICV is decreased owing to illness-related factors. Therefore, the discrepancy in ICV findings between patients with bipolar disorder and their relatives might suggest that smaller ICV in patients compared with their relatives can be regarded as a (possibly prodromal) disease effect, similar to what has been reported in schizophrenia. Alternatively, larger ICV in FDRs-BD could represent a relative resilience to developing bipolar disorder, as was suggested in a prior report on hippocampal shape abnormalities in co-twins without bipolar disorder (58).
The pattern and extent of brain abnormalities varied with respect to the type of relationship to the proband. This again suggests a role for environmental influences, as all FDRs share approximately 50% of their common genetic variants with the affected proband (except for monozygotic co-twins). Given that many environmental risk factors, e.g., age, childhood trauma, physical inactivity, and famine, are associated with brain structure (59-61), environmental risk and/or gene-byenvironment interplay are likely also associated with differences in brain abnormalities in individuals at familial risk for schizophrenia or bipolar disorder. However, despite the large sample size, we did not find a consistent pattern of abnormalities among different relative types. Power may still not be sufficient to detect these subtle differences. Alternatively, there are many environmental factors that are unique for an individual—and thus not specific to the relative type—and these could have influenced brain structure.
Psychopathology is more prevalent in individuals at familial risk for either bipolar disorder or schizophrenia than in the general population; for example, offspring studies have shown that 55% to 72% of individuals with a parent with bipolar disorder or schizophrenia developed a lifetime mental disorder (62,63). We showed that the presence of a psychiatric diagnosis in relatives and control subjects did not influence our findings. This suggests that brain abnormalities seen in the relatives represent the familial liability for the disorder and not the presence of psychopathology.
Some limitations should be considered in interpreting the results. This study is a meta-analysis of multiple cohorts from research centers around the world, with heterogeneity across samples (among others, acquisition protocols, field strength, FreeSurfer version, inclusion and exclusion criteria). Meta-analysis will find consistent effects despite this variance but cannot remove all sources of heterogeneity. However, clinical heterogeneity within and across sites is representative of the broad, clinically varied, and ecologically valid nature of bipolar disorder and schizophrenia and allows generalizable alterations to be detected. One source of heterogeneity in the offspring in particular might also be the substantial age differences between the different offspring cohorts. Both adult and children/adolescent offspring cohorts were included in the analyses, and the fact that the brains of the child and adolescent offspring have not reached adult size might have influenced the findings of the overall offspring effects. In addition, inclusion criteria varied with respect to psychopathology in FDRs or control subjects at the different research centers. For example, some cohorts included only healthy relatives, yet others included relatives with other psychiatric diagnosis (except for having the disorder itself). We accounted for this with additional analyses covarying for any diagnosis or assessing only the healthy relatives. These approaches might not be sufficient. In addition, the composition of the FDRs-SZ and FDRs-BD groups differed. FDRs-SZ had a greater sample size and consisted in particular of more siblings, whereas there were more offspring in the FDRs-BD group. Finally, the discrepancy in ICV between FDRs-BD and FDRs-SZ may be associated with current IQ or parental socioeconomic status (SES). Both IQ and parental SES have been associated with brain structure (64-67). This might suggest that the larger ICV found in FDRs-BD is related to higher IQ or parental SES. Lower IQ has been reported in FDRs-SZ (68). However, the literature regarding current IQ in individuals at familial risk for bipolar disorder is less clear. Cognitive deficits have been associated with genetic risk for bipolar disorder (69,70). One study showed that siblings of patients with bipolar disorder had lower IQ but that they did not differ on educational level compared with control subjects (71). In contrast, a bipolar twin study showed that both the proband and the co-twin without bipolar disorder completed significantly fewer years of education than control twins (72). Furthermore, population studies show that premorbid IQ or educational attainment are often not affected or are even higher in individuals who later develop bipolar disorder (73-76), whereas IQ during childhood and adolescence is lower in individuals who develop schizophrenia later in life (77-82). The question remains how these measures interact with brain development in individuals at familial risk. As recently reported in a study that included only FDRs-SZ from one site (Utrecht, The Netherlands), current IQ was intertwined with most of the brain abnormalities (15). However, in FDRs-BD, it still remains unclear how IQ and risk for bipolar disorder act on the brain. In the current study, few cohorts had information available on parental SES or subjects’ IQ, thereby excluding the possibility to address these variables as potential confounders. Investigating the influence of current IQ on the difference in brain measures between relatives and control subjects was outside the scope of this study, and we are collecting and harmonizing these data from the cohorts for future analysis.
In conclusion, FDRs of patients with schizophrenia or bipolar disorder represent a group of individuals who can provide insight into the effect of familial risk on the brain. Although liability for schizophrenia and bipolar disorder overlap in the general populations, individuals at familial risk assessed here showed a differential pattern of structural brain abnormalities. This study found differences in brain abnormalities between FDRs-SZ and FDRs-BD, in particular, a divergent effect in ICV. This converse effect on ICV suggests that there may be different neurodevelopmental trajectories for each disorder early in life. Taken together, our findings may imply that brain abnormalities in schizophrenia and bipolar disorder are due to genetic variants or gene-by-environment interplay specific to each disorder.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
The researchers and studies included in this article were supported by the Research Council of Norway (Grant No. 223273), National Institutes of Health (NIH) (Grant No. R01 MH117601 [to NJ], Grant Nos. R01 MH116147, R01 MH111671, and P41 EB015922 [to PMT], Grant Nos. 5T32MH073526 and U54EB020403 [to CRKC], and Grant No. R03 MH105808 [to CEB and SCF]) and National Institute on Aging (NIA) (Grant No. T32AG058507 [to CRKC]).
C-SFS: This work was supported by Canadian Institutes of Health Research.
Cardiff: This work was supported by the National Centre for Mental Health, Bipolar Disorder Research Network, 2010 National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (Grant No. 17319).
DEU: This work was supported by Dokuz Eylul University Department of Scientific Research Projects Funding (Grant No. 2012.KB.SAG.062). This report represents independent research funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, or Department of Health.
EGEU: This work was supported by the Ege University School of Medicine Research Foundation (Grant No. 2009-D-00017).
EHRS: The Edinburgh High Risk Study was supported by the Medical Research Council.
GROUP: The infrastructure for the GROUP study was supported by the Geestkracht program of the Netherlands Organisation for Health Research and Development (Grant No. 10-000-1002).
ENBD_UT/BPO_FLB: This work was supported by the National Institute of Mental Health (Grant No. R01 MH 085667).
HHR/PHHR: This work was supported by the Canadian Institutes of Health Research (Grant Nos. 103703, 106469, and 341717), Nova Scotia Health Research Foundation, Dalhousie Clinical Research Scholarship (to TH), 2007 Brain and Behavior Research Foundation Young Investigator Award (to TH), and Ministry of Health of the Czech Republic (Grant Nos. NR8786 and NT13891).
HUBIN: This work was supported by the Swedish Research Council (Grant Nos. K2007-62X-15077-04-1, K2008-62P-20597-01-3, K2010-62X-15078-07-2, K2012-61X-15078-09-3), regional agreement on medical training and clinical research between Stockholm County Council and the Karolinska Institutet, Knut and Alice Wallenberg Foundation, and HUBIN project.
IDIBAPS: This work was supported by the Spanish Ministry of Economy and Competitiveness/Instituto de Salud Carlos III (Grant Nos. PI070066, PI1100683, and PI1500467) and Fundacio Marato TV3 (Grant No. 091630), co-financed by ERDF Funds from the European Commission (“A Way of Making Europe”), Brain and Behaviour Research Foundation (NARSAD Young Investigator Award), and Alicia Koplowitz Foundation.
IoP-BD: The Maudsley Bipolar Twin Study was supported by the Stanley Medical Research Institute and NARSAD.
IoP-SZ: This work was supported by a Wellcome Trust Research Training Fellowship (Grant No. 064971 to MMP), NARSAD Young Investigator Award (to TT), and European Community’s Sixth Framework Programme through a Marie Curie Training Network called the European Twin Study Network on Schizophrenia.
Lieber Institute for Brain Development (LIBD): This work was supported by the NIMH Intramural Research Program (to DRW’s laboratory). LIBD is a nonprofit research institute located in Baltimore, MD. The work performed at LIBD was performed in accordance with an NIMH material transfer agreement with LIBD.
MFS: The Maudsley Family Study cohort collection was supported by the Wellcome Trust (Grant Nos. 085475/B/08/Z and 085475/Z/08/Z), NIHR Biomedical Research Centre at University College London Hospital, Medical Research Council (Grant No. G0901310), and British Medical Association Margaret Temple Fellowship 2016.
MooDS: This work was supported by the German Federal Ministry for Education and Research grants NGFNplus MooDS (Systematic Investigation of the Molecular Causes of Major Mood Disorders and Schizophrenia) and Integrated Network IntegraMent (Integrated Understanding of Causes and Mechanisms in Mental Disorders) under the auspices of the e:Med program (Grant Nos. O1ZX1314B and O1ZX1314G) and Deutsche Forschungsgemeinschaft (Grant No. 1617 [to AH]).
MSSM: This work was supported by NIMH (Grant Nos. R01 MH116147 and R01 MH113619).
NU: This work was supported by NIH (Grant Nos. U01 MH097435, R01 MH084803, and R01 EB020062) and National Science Foundation (Grant Nos. 1636893 and 1734853).
OLIN: This work was supported by NIH (Grant No. R01 MH080912).
STAR: This work was supported by NIH (Grant No. R01 MH052857).
SydneyBipolarGroup: The Australian cohort collection was supported by the Australian National Health and Medical Research Council Program Grants (Grant No. 510135 [to PBM] and Grant No. 1037196 [to PBM and PRS]) and Project Grants (Grant No. 1063960 [to JMF and PRS] and Grant No. 1066177 [to JMF]).
UMCU: This work was supported by NARSAD (Grant No. 20244 [to MHJH]), ZonMw (Grant No. 908-02-123 [to HEHP]), VIDI (Grant No. 452-11-014 [to NEMvH] and Grant No. 917-46-370 [to HEHP]), and Stanley Medical Research Institute.
CliNG: We thank Anna Fanelli, Kathrin Jakob, and Maria Keil for help with data acquisition.
All authors have contributed to and approved the contents of this manuscript.
GS has received research and travel support from Janssen Pharmaceutica and Otsuka Pharmaceutical and honoraria from Adamed Pharma. NY has been an investigator in clinical studies conducted together with Janssen-Cilag, Corcept Therapeutics, and COMPASS Pathways in the last 3 years. AM-L has received consultant fees from Boehringer Ingelheim, BrainsWay, Elsevier, Lundbeck International Neuroscience Foundation, and Science Advances. CRKC has received partial research support from Biogen, Inc. (Boston, MA) for work unrelated to the topic of this manuscript. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2019.03.985.
Contributor Information
Sonja M.C. de Zwarte, Department of Psychiatry, University Medical Center Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands
Rachel M. Brouwer, Department of Psychiatry, University Medical Center Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands
Ingrid Agartz, Norwegian Centre for Mental Disorders Research (NORMENT), K.G. Jebsen Centre, Institute of Clinical Medicine, University of Oslo, Oslo; Centre for Psychiatric Research, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Department of Psychiatry, Diakonhjemmet Hospital, Oslo, Norway.
Martin Alda, Department of Psychiatry, Dalhousie University, Halifax, Nova Scotia, Canada; National Institute of Mental Health, Third Faculty of Medicine, Charles University, Prague, Czech Republic.
André Aleman, Cognitive Neuroscience Center, Department of Biomedical Sciences of Cells and Systems, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Kathryn I. Alpert, Department of Psychiatry and Behavioral Sciences, Northwestern University Feinberg School of Medicine, Chicago, Illinois
Carrie E. Bearden, Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles, Los Angeles, California; Department of Psychology, University of California, Los Angeles, Los Angeles, California
Alessandro Bertolino, Department of Basic Medical Science, Neuroscience and Sense Organs, University of Bari Aldo Moro, Bari, Italy.
Catherine Bois, Division of Psychiatry, Royal Edinburgh Hospital, University of Edinburgh, Edinburgh.
Aurora Bonvino, Department of Basic Medical Science, Neuroscience and Sense Organs, University of Bari Aldo Moro, Bari, Italy.
Elvira Bramon, Division of Psychiatry, Neuroscience in Mental Health Research Department, University College London, London, United Kingdom.
Elizabeth E.L. Buimer, Department of Psychiatry, University Medical Center Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands
Wiepke Cahn, Department of Psychiatry, University Medical Center Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands.
Dara M. Cannon, Centre for Neuroimaging and Cognitive Genomics and National Centre for Biomedical Engineering (NCBES) Galway Neuroscience Centre, National University of Ireland Galway, Galway, Ireland
Tyrone D. Cannon, Department of Psychology, Yale University, New Haven, Connecticut
Xavier Caseras, MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, United Kingdom.
Josefina Castro-Fornieles, Psychology and Psychology, 2017SGR881, Institute of Neuroscience, Hospital Clínic of Barcelona, Institute d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), University of Barcelona, Spain.
Qiang Chen, Lieber Institute for Brain Development, Baltimore, Maryland.
Yoonho Chung, Department of Psychology, Yale University, New Haven, Connecticut.
Elena De la Serna, Psychology and Psychology, 2017SGR881, Institute of Neuroscience, Hospital Clínic of Barcelona, Institute d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), University of Barcelona, Spain.
Annabella Di Giorgio, Department of Experimental and Clinical Medicine, Università Politecnica delle Marche, Ancona, Italy.
Gaelle E. Doucet, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York
Mehmet Cagdas Eker, SoCAT LAB, Department of Psychiatry, School of Medicine, Ege University, Bornova, Izmir, Turkey; Department of Psychiatry, Renaissance School of Medicine at Stony Brook University, Stony Brook, New York.
Susanne Erk, Research Division of Mind and Brain, Department of Psychiatry and Psychotherapy, Charité Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Scott C. Fears, Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, California; Center for Neurobehavioral Genetics, University of California, Los Angeles, Los Angeles, California
Sonya F. Foley, Cardiff University Brain Research Imaging Centre, Cardiff University, United Kingdom
Sophia Frangou, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York.
Andrew Frankland, School of Psychiatry, University of New South Wales, Sydney, Australia.
Janice M. Fullerton, School of Medical Sciences, University of New South Wales; Neuroscience Research Australia, Sydney, Australia
David C. Glahn, Olin Neuropsychiatry Research Center, Institute of Living, Hartford Hospital, Hartford, Connecticut; Tommy Fuss Center for Neuropsychiatric Disease Research, Boston Children’s Hospital; Harvard Medical School (DCG), Boston, Massachusetts
Vina M. Goghari, Department of Psychology and Graduate Department of Psychological Clinical Science, University of Toronto, Toronto, Ontario, Canada
Aaron L. Goldman, Lieber Institute for Brain Development, Baltimore, Maryland
Ali Saffet Gonul, Department of Psychiatry and Behavioral Sciences, Mercer University School of Medicine, Macon, Georgia.
Oliver Gruber, Experimental Psychopathology and Neuroimaging, Department of General Psychiatry, University of Heidelberg, Heidelberg, Germany.
Lieuwe de Haan, Early Psychosis Unit, Department of Psychiatry, Academic Medical Center, Amsterdam.
Tomas Hajek, Department of Psychiatry, Dalhousie University, Halifax, Nova Scotia, Canada; National Institute of Mental Health, Third Faculty of Medicine, Charles University, Prague, Czech Republic.
Emma L. Hawkins, Division of Psychiatry, Royal Edinburgh Hospital, University of Edinburgh, Edinburgh
Andreas Heinz, Research Division of Mind and Brain, Department of Psychiatry and Psychotherapy, Charité Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Manon H.J. Hillegers, Department of Psychiatry, University Medical Center Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands; Department of Child and Adolescent Psychiatry/Psychology, Erasmus University Medical Center-Sophia Children’s Hospital, Rotterdam, Netherlands
Hilleke E. Hulshoff Pol, Department of Psychiatry, University Medical Center Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands
Christina M. Hultman, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
Martin Ingvar, Centre for Psychiatric Research, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden.
Viktoria Johansson, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Erik G. Jönsson, Norwegian Centre for Mental Disorders Research (NORMENT), K.G. Jebsen Centre, Institute of Clinical Medicine, University of Oslo, Oslo; Centre for Psychiatric Research, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
Fergus Kane, Psychosis Studies, King’s College London, London, United Kingdom.
Matthew J. Kempton, Psychosis Studies, King’s College London, London, United Kingdom
Marinka M.G. Koenis, Olin Neuropsychiatry Research Center, Institute of Living, Hartford Hospital, Hartford, Connecticut; Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut
Miloslav Kopecek, National Institute of Mental Health, Third Faculty of Medicine, Charles University, Prague, Czech Republic; Klecany, and Department of Psychiatry, Third Faculty of Medicine, Charles University, Prague, Czech Republic.
Lydia Krabbendam, Department of Clinical, Neuro and Developmental Psychology, Faculty of Behaviour and Movement Sciences, Vrije Universiteit, Amsterdam, Netherlands.
Bernd Krämer, Experimental Psychopathology and Neuroimaging, Department of General Psychiatry, University of Heidelberg, Heidelberg, Germany.
Stephen M. Lawrie, Division of Psychiatry, Royal Edinburgh Hospital, University of Edinburgh, Edinburgh
Rhoshel K. Lenroot, Neuroscience Research Australia, Sydney, Australia; Department of Psychiatry and Behavioral Sciences, University of New Mexico, Albuquerque, New Mexico
Machteld Marcelis, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University Medical Centre, Maastricht University, Maastricht, Netherlands.
Jan-Bernard C. Marsman, Cognitive Neuroscience Center, Department of Biomedical Sciences of Cells and Systems, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
Venkata S. Mattay, Lieber Institute for Brain Development, Baltimore, Maryland; Department of Radiology, The Johns Hopkins University School of Medicine, Baltimore, Maryland
Colm McDonald, Centre for Neuroimaging and Cognitive Genomics and National Centre for Biomedical Engineering (NCBES) Galway Neuroscience Centre, National University of Ireland Galway, Galway, Ireland.
Andreas Meyer-Lindenberg, Clinical Department of Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany..
Stijn Michielse, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University Medical Centre, Maastricht University, Maastricht, Netherlands.
Philip B. Mitchell, School of Psychiatry, University of New South Wales, Sydney, Australia
Dolores Moreno, Child and Adolescent Psychiatry Department, Hospital General Universitario Gregorio Marañón (IiSGM), School of Medicine, Universidad Complutense, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid, Spain.
Robin M. Murray, Psychosis Studies, King’s College London, London, United Kingdom
Benson Mwangi, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, Texas.
Pablo Najt, Centre for Neuroimaging and Cognitive Genomics and National Centre for Biomedical Engineering (NCBES) Galway Neuroscience Centre, National University of Ireland Galway, Galway, Ireland.
Emma Neilson, Division of Psychiatry, Royal Edinburgh Hospital, University of Edinburgh, Edinburgh.
Jason Newport, Department of Psychiatry, Dalhousie University, Halifax, Nova Scotia, Canada.
Jim van Os, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University Medical Centre, Maastricht University, Maastricht, Netherlands.
Bronwyn Overs, Neuroscience Research Australia, Sydney, Australia.
Aysegul Ozerdem, Department of Psychiatry, Faculty of Medicine, and Department of Neurosciences, Health Sciences Institute, Dokuz Eylül University, Turkey.
Marco M. Picchioni, Department of Forensic and Neurodevelopmental Science, King’s College London, London, United Kingdom
Anja Richter, Experimental Psychopathology and Neuroimaging, Department of General Psychiatry, University of Heidelberg, Heidelberg, Germany.
Gloria Roberts, School of Psychiatry, University of New South Wales; Neuroscience Research Australia, Sydney, Australia.
Aybala Saricicek Aydogan, Faculty of Medicine, and Department of Neurosciences, Health Sciences Institute, Dokuz Eylül University; Department of Psychiatry, Faculty of Medicine, Izmir Katip Çelebi University, Turkey.
Peter R. Schofield, School of Medical Sciences, University of New South Wales, Sydney, Australia
Fatma Simsek, Department of Psychological Medicine, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom; Department of Psychiatry, Cigli State Hospital, Izmir.
Jair C. Soares, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, Texas
Gisela Sugranyes, Psychology and Psychology, 2017SGR881, Institute of Neuroscience, Hospital Clínic of Barcelona, Institute d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), University of Barcelona, Spain.
Timothea Toulopoulou, Department of Basic and Clinical Neuroscience, King’s College London, London, United Kingdom; Department of Psychology, Bilkent University, Ankara, Turkey; Department of Psychology, University of Hong Kong, Hong Kong, China.
Giulia Tronchin, Centre for Neuroimaging and Cognitive Genomics and National Centre for Biomedical Engineering (NCBES) Galway Neuroscience Centre, National University of Ireland Galway, Galway, Ireland.
Henrik Walter, Research Division of Mind and Brain, Department of Psychiatry and Psychotherapy, Charité Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Lei Wang, Department of Psychiatry and Behavioral Sciences, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Daniel R. Weinberger, Lieber Institute for Brain Development, Baltimore, Maryland
Heather C. Whalley, Division of Psychiatry, Royal Edinburgh Hospital, University of Edinburgh, Edinburgh
Nefize Yalin, Centre for Affective Disorders, King’s College London, London, United Kingdom.
Ole A. Andreassen, Norwegian Centre for Mental Disorders Research (NORMENT), K.G. Jebsen Centre, Institute of Clinical Medicine, University of Oslo, Oslo; Division of Mental Health and Addiction, Oslo University Hospital, Oslo, Norway
Christopher R.K. Ching, Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, California; Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles, Los Angeles, California; Imaging Genetics Center, Mark and Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Marina del Rey
Theo G.M. van Erp, Clinical Translational Neuroscience Laboratory, Department of Psychiatry and Human Behavior, and Center for the Neurobiology of Learning and Memory, University of California, Irvine, Irvine, California
Jessica A. Turner, Department of Psychology and Neuroscience Institute, Georgia State University, Atlanta, Georgia
Neda Jahanshad, Imaging Genetics Center, Mark and Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Marina del Rey.
Paul M. Thompson, Imaging Genetics Center, Mark and Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Marina del Rey
René S. Kahn, Department of Psychiatry, University Medical Center Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands; Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York
Neeltje E.M. van Haren, Department of Psychiatry, University Medical Center Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands; Department of Child and Adolescent Psychiatry/Psychology, Erasmus University Medical Center-Sophia Children’s Hospital, Rotterdam, Netherlands
REFERENCES
- 1.Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM (2009): Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. Lancet 373:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, et al. (2013): Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 45:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brainstorm Consortium, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, et al. (2018): Analysis of shared heritability in common disorders of the brain. Science 360:eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM (2009): Magnetic resonance imaging studies in bipolar disorder and schizophrenia: Meta-analysis. Br J Psychiatry 195:194–201. [DOI] [PubMed] [Google Scholar]
- 5.Ellison-Wright I, Bullmore E (2010): Anatomy of bipolar disorder and schizophrenia: A meta-analysis. Schizophr Res 117:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Haijma SV, Van Haren NEM, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS (2013): Brain volumes in schizophrenia: A meta-analysis in over 18 000 subjects. Schizophr Bull 39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hibar DP, Westlye LT, van Erp TG, Rasmussen J, Leonardo CD, Faskowitz J, et al. (2016): Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry 21:1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. (2018): Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry 23:932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, Murray RM (2004): Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry 61:974–984. [DOI] [PubMed] [Google Scholar]
- 10.van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. (2016): Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 21:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Erp TG, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. (2018): Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 controls via the ENIGMA consortium. Biol Psychiatry 84:644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada N, Fukunaga M, Yamashita F, Koshiyama D, Yamamori H, Ohi K, et al. (2016): Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry 21:1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulshoff Pol HE, van Baal GC, Schnack HG, Brans RG, van der Schot AC, Brouwer RM, et al. (2012): Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Arch Gen Psychiatry 69:349–359. [DOI] [PubMed] [Google Scholar]
- 14.Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS (2007): Brain volumes in relatives of patients with schizophrenia: A meta-analysis. Arch Gen Psychiatry 64:297–304. [DOI] [PubMed] [Google Scholar]
- 15.de Zwarte SMC, Brouwer RM, Tsouli A, Cahn W, Hillegers MHJ, Hulshoff Pol HE, et al. (2018): Running in the family? Structural brain abnormalities and IQ in offspring, siblings, parents and co-twins of patients with schizophrenia [published online ahead of print]. Schizophr Bull. [DOI] [PMC free article] [PubMed]
- 16.Nery FG, Monkul ES, Lafer B (2013): Gray matter abnormalities as brain structural vulnerability factors for bipolar disorder: A review of neuroimaging studies of individuals at high genetic risk for bipolar disorder. Aust N Z J Psychiatry 47:1124–1135. [DOI] [PubMed] [Google Scholar]
- 17.Kempton MJ, Haldane M, Jogia J, Grasby PM, Collier D, Frangou S (2009): Dissociable brain structural changes associated with predisposition, resilience, and disease expression in bipolar disorder. J Neurosci 29:10863–10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frangou S (2011): Brain structural and functional correlates of resilience to bipolar disorder. Front Hum Neurosci 5:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer IE, Sanches M, Suchting R, Green CE, El Fangary NM, Zunta-Soares GB, Soares JC (2014): Amygdala enlargement in unaffected offspring of bipolar parents. J Psychiatr Res 59:200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin K, Xu G, Wong NML, Wu H, Li T, Lu W, et al. (2015): A multidimensional and integrative approach to examining the high-risk and ultra-high-risk stages of bipolar disorder. EBioMedicine 2:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajek T, Cullis J, Novak T, Kopecek M, Blagdon R, Propper L, et al. (2013): Brain structural signature of familial predisposition for bipolar disorder: Replicable evidence for involvement of the right inferior frontal gyrus. Biol Psychiatry 73:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sariçiçek A, Yalın N, Hıdıroğlu C, Çavuşoğlu B, Taş C, Ceylan D, et al. (2015): Neuroanatomical correlates of genetic risk for bipolar disorder: A voxel-based morphometry study in bipolar type I patients and healthy first degree relatives. J Affect Disord 186:110–118. [DOI] [PubMed] [Google Scholar]
- 23.Roberts G, Lenroot R, Frankland A, Yeung PK, Gale N, Wright A, et al. (2016): Abnormalities in left inferior frontal gyral thickness and parahippocampal gyral volume in young people at high genetic risk for bipolar disorder. Psychol Med 46:2083–2096. [DOI] [PubMed] [Google Scholar]
- 24.Macoveanu J, Baaré W, Madsen KH, Kessing LV, Siebner HR, Vinberg M (2017): Risk for affective disorders is associated with greater prefrontal gray matter volumes: A prospective longitudinal study. Neuroimage Clin 17:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladouceur CD, Almeida JRC, Birmaher B, Axelson DA, Nau S, Kalas C, et al. (2008): Subcortical gray matter volume abnormalities in healthy bipolar offspring: Potential neuroanatomical risk marker for bipolar disorder? J Am Acad Child Adolesc Psychiatry 47:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drobinin V, Slaney C, Garnham J, Propper L, Uher R, Alda M, Hajek T (2019): Larger right inferior frontal gyrus volume and surface area in participants at genetic risk for bipolar disorders. Psychol Med 49:1308–1315. [DOI] [PubMed] [Google Scholar]
- 27.Sugranyes G, de la Serna E, Romero S, Sanchez-Gistau V, Calvo A, Moreno D, et al. (2015): Grey matter volume decrease distinguishes schizophrenia from bipolar offspring during childhood and adolescence. J Am Acad Child Adolesc Psychiatry 54:677–684. [DOI] [PubMed] [Google Scholar]
- 28.Collin G, Scholtens LH, Kahn RS, Hillegers MHJ, van den Heuvel MP (2017): Affected anatomical rich club and structural-functional coupling in young offspring of schizophrenia and bipolar disorder patients. Biol Psychiatry 82:746–755. [DOI] [PubMed] [Google Scholar]
- 29.McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, et al. (2006): Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry 163:478–487. [DOI] [PubMed] [Google Scholar]
- 30.McIntosh AM, Job DE, Moorhead TWJ, Harrison LK, Forrester K, Lawrie SM, Johnstone EC (2004): Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry 56:544–552. [DOI] [PubMed] [Google Scholar]
- 31.Fischl B (2012): FreeSurfer. Neuroimage 62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischl B, Sereno MI, Dale AM (1999): Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- 33.Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinheiro JC, Bates DM (2000): Mixed-Effects Models in S and S-PLUS. New York: Springer. [Google Scholar]
- 35.Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc 57:289–300. [Google Scholar]
- 36.Moran ME, Hulshoff Pol HE, Gogtay N (2013): A family affair: Brain abnormalities in siblings of patients with schizophrenia. Brain 136:3215–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruderfer DM, Ripke S, McQuillin A, Boocock J, Stahl EA, Pavlides JM, et al. (2018): Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell 173:1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baaré WF, van Oel CJ, Hulshoff Pol HE, Schnack HG, Durston S, Sitskoorn MM, Kahn RS (2001): Volumes of brain structures in twins discordant for schizophrenia. Arch Gen Psychiatry 58:33–40. [DOI] [PubMed] [Google Scholar]
- 39.Cannon TD, Thompson PM, van Erp TGM, Toga AW, Poutanen VP, Huttunen M, et al. (2002): Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci U S A 99:3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rijsdijk FV, van Haren NEM, Picchioni MM, McDonald C, Toulopoulou T, Hulshoff Pol HE, et al. (2005): Brain MRI abnormalities in schizophrenia: Same genes or same environment? Psychol Med 35:1399–1409. [DOI] [PubMed] [Google Scholar]
- 41.van Haren NEM, Rijsdijk F, Schnack HG, Picchioni MM, Toulopoulou T, Weisbrod M, et al. (2012): The genetic and environmental determinants of the association between brain abnormalities and schizophrenia: The Schizophrenia Twins and Relatives Consortium. Biol Psychiatry 71:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kieseppä T, Van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen VP, et al. (2002): The volumetric findings in MRI brain study of bipolar twins and their healthy co-twins. Bipolar Disord 4(Suppl 1): 29–30. [DOI] [PubMed] [Google Scholar]
- 43.van der Schot AC, Vonk R, Brans RG, van Haren NE, Koolschijn PC, Nuboer V, et al. (2009): Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry 66:142–151. [DOI] [PubMed] [Google Scholar]
- 44.Franke B, Stein JL, Ripke S, Anttila V, Hibar DP, van Hulzen KJ, et al. (2016): Genetic influences on schizophrenia and subcortical brain volumes: Large-scale proof of concept. Nat Neurosci 19:420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smeland OB, Wang Y, Frei O, Li W, Hibar DP, Franke B, et al. (2018): Genetic overlap between schizophrenia and volumes of hippocampus, putamen, and intracranial volume indicates shared molecular genetic mechanisms. Schizophr Bull 44:854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, et al. (2018): The genetic architecture of the human cerebral cortex [published online ahead of print Sep 9]. bioRxiv.
- 47.Sgouros S, Goldin JH, Hockley AD, Wake MJ, Natarajan K (1999): Intracranial volume change in childhood. J Neurosurg 91:610–616. [DOI] [PubMed] [Google Scholar]
- 48.Blakemore SJ (2012): Imaging brain development: The adolescent brain. Neuroimage 61:397–406. [DOI] [PubMed] [Google Scholar]
- 49.Murray RM, Lewis SW (1987): Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 295:681–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nasrallah HA (1991): Neurodevelopmental aspects of bipolar affective disorder. Biol Psychiatry 29:1–2. [DOI] [PubMed] [Google Scholar]
- 51.Weinberger DR (1987): Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44:660–669. [DOI] [PubMed] [Google Scholar]
- 52.Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C (2004): A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res 71:405–416. [DOI] [PubMed] [Google Scholar]
- 53.Parellada M, Gomez-Vallejo S, Burdeus M, Arango C (2017): Developmental differences between schizophrenia and bipolar disorder. Schizophr Bull 43:1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker J, Curtis V, Shaw P, Murray RM (2002): Schizophrenia and bipolar disorder are distinguished mainly by differences in neurodevelopment. Neurotox Res 4:427–436. [DOI] [PubMed] [Google Scholar]
- 55.Hajek T, Franke K, Kolenic M, Capkova J, Matejka M, Propper L, et al. (2019): Brain age in early stages of bipolar disorders or schizophrenia. Schizophr Bull 45:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adleman NE, Fromm SJ, Razdan V, Kayser R, Dickstein DP, Brotman MA, et al. (2012): Cross-sectional and longitudinal abnormalities in brain structure in children with severe mood dysregulation or bipolar disorder. J Child Psychol Psychiatry 53:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adler CM, Levine AD, DelBello MP, Strakowski SM (2005): Changes in gray matter volume in patients with bipolar disorder. Biol Psychiatry 58:151–157. [DOI] [PubMed] [Google Scholar]
- 58.van Erp TG, Thompson PM, Kieseppä T, Bearden CE, Marino AC, Hoftman GD, et al. (2012): Hippocampal morphology in lithium and non-lithium-treated bipolar I disorder patients, non-bipolar co-twins, and control twins. Hum Brain Mapp 33:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. (2012): Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71:286–293. [DOI] [PubMed] [Google Scholar]
- 60.Hulshoff Pol HE, Hoek HW, Susser E, Brown AS, Dingemans A, Schnack HG, et al. (2000): Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry 157:1170–1172. [DOI] [PubMed] [Google Scholar]
- 61.Voelcker-Rehage C, Niemann C (2013): Structural and functional brain changes related to different types of physical activity across the life span. Neurosci Biobehav Rev 37:2268–2295. [DOI] [PubMed] [Google Scholar]
- 62.Rasic D, Hajek T, Alda M, Uher R (2014): Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: A meta-analysis of family high-risk studies. Schizophr Bull 40:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mesman E, Nolen WA, Reichart CG, Wals M, Hillegers MHJ (2013): The Dutch bipolar offspring study: 12-year follow-up. Am J Psychiatry 170:542–549. [DOI] [PubMed] [Google Scholar]
- 64.McDaniel MA (2005): Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence 33:337–346. [Google Scholar]
- 65.Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LF (2012): Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Ann Neurol 71:653–660. [DOI] [PubMed] [Google Scholar]
- 66.Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ (2013): Associations between children’s socioeconomic status and prefrontal cortical thickness. Dev Sci 16:641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, et al. (2015): Family income, parental education and brain structure in children and adolescents. Nat Neurosci 18:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Haren NEM, Van Dam DS, Stellato RK; Genetic Risk and Outcome of Psychosis (GROUP) investigators (2019): Change in IQ in schizophrenia patients and their siblings: A controlled longitudinal study [published online ahead of print Jan 24]. Psychol Med. [DOI] [PubMed]
- 69.Arts B, Jabben N, Krabbendam L, van Os J (2008): Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med 38:771–785. [DOI] [PubMed] [Google Scholar]
- 70.Glahn DC, Almasy L, Barguil M, Hare E, Perlalta JM, Kent JW Jr, et al. (2010): Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Arch Gen Psychiatry 67:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vreeker A, Boks MP, Abramovic L, Verkooijen S, van Bergen AH, Hillegers MH, et al. (2016): High educational performance is a distinctive feature of bipolar disorder: A study on cognition in bipolar disorder, schizophrenia patients, relatives and controls. Psychol Med 46:807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vonk R, van der Schot AC, van Baal GC, van Oel CJ, Nolen WA, Kahn RS (2012): Premorbid school performance in twins concordant and discordant for bipolar disorder. J Affect Disord 136:294–303. [DOI] [PubMed] [Google Scholar]
- 73.Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, Lewis G (2004): A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry 61:354–360. [DOI] [PubMed] [Google Scholar]
- 74.Tiihonen J, Haukka J, Henriksson M, Cannon M, Kieseppä T, Laaksonen I, et al. (2005): Premorbid intellectual functioning in bipolar disorder and schizophrenia: Results from a cohort study of male conscripts. Am J Psychiatry 162:1904–1910. [DOI] [PubMed] [Google Scholar]
- 75.MacCabe JH, Lambe MP, Cnattingius S, Sham PC, David AS, Reichenberg A, et al. (2010): Excellent school performance at age 16 and risk of adult bipolar disorder: National cohort study. Br J Psychiatry 196:109–115. [DOI] [PubMed] [Google Scholar]
- 76.Smith DJ, Anderson J, Zammit S, Meyer TD, Pell JP, Mackay D (2015): Childhood IQ and risk of bipolar disorder in adulthood: Prospective birth cohort study. BJPsych Open 1:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woodberry KA, Giuliano AJ, Seidman LJ (2008): Premorbid IQ in schizophrenia: A meta-analytic review. Am J Psychiatry 165:579–587. [DOI] [PubMed] [Google Scholar]
- 78.Khandaker GM, Barnett JH, White IR, Jones PB (2011): A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr Res 132:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dickson H, Laurens KR, Cullen AE, Hodgins S (2012): Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med 42:743–755. [DOI] [PubMed] [Google Scholar]
- 80.Agnew-Blais J, Seidman LJ (2013): Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: A quantitative and qualitative review. Cogn Neuropsychiatry 18:44–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kendler KS, Ohlsson H, Sundquist J, Sundquist K (2015): IQ and schizophrenia in a Swedish national sample: Their causal relationship and the interaction of IQ with genetic risk. Am J Psychiatry 172:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hochberger WC, Combs T, Reilly JL, Bishop JR, Keefe RSE, Clementz BA, et al. (2018): Deviation from expected cognitive ability across psychotic disorders. Schizophr Res 192:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.