Abstract
The scaffolding protein Grb2-associated binding protein 3 (Gab3) is a member of the Gab family, whose functions have remained elusive. Here we identify Gab3 as a key determinant of peripheral NK cell expansion. Loss of Gab3 results in impaired IL-2/IL-15-induced NK cell priming and expansion, due to a selective impairment in MAPK- but not STAT5-signaling. In vivo, we show that Gab3 is required for recognition and elimination of “missing-self” and tumor targets. Unexpectedly, our studies reveal that Gab3 plays an important role during pregnancy. Gab3-deficient mice exhibit impaired uterine NK cell expansion that is associated with abnormal spiral artery remodeling and increased trophoblast invasion in the decidua basalis. This coincides with stillbirth, retained placenta, maternal hemorrhage and undelivered fetoplacental units at term. Thus, Gab3 is a key component required for cytokine-mediated NK cell priming and expansion that is essential for anti-tumor responses and limits trophoblast cell invasion during pregnancy.
One Sentence Summary
Our studies identify a key role for the Grb2-associated binding protein 3 (Gab3) in peripheral NK cell expansion and show that it is essential for efficient tumor cell eradication and limits trophoblast invasion during pregnancy.
Introduction
The Grb2-associated binding (Gab) family proteins support assembly of activated signaling complexes through scaffolding and docking functions. Gab family members, comprising Gab1–3, contain a highly conserved N-terminal pleckstrin homology (PH) domain as well as various phosphorylation sites specific for tyrosine kinases(1–3). The PH-domain mediates plasma membrane recruitment by interacting with lipids such as phosphatidyl inositol-3,4,5-triphosphate (PIP3) while the phosphorylated tyrosines are recruitment sites for binding to SH2 domain-containing proteins(1, 2, 4). Gab3, unlike Gab1 and Gab2, is expressed exclusively in cells of the hematopoietic lineage, with the highest expression occurring in natural killer cells, mast cells, and memory CD8+ T cells (http://biogps.org, https://www.immgen.org)(5). Importantly, while the role of Gab1 and Gab2 has been relatively defined, the function of Gab3 has remained elusive.
NK cells play an important role in the recognition and elimination of tumor cells, allogeneic cells, and pathogen-infected cells(6). Activation of NK cells can occur via different receptor pathways that integrate signals derived from inhibitory and activating surface receptors(7). Moreover, NK cells can be activated by cytokines such as IL-2 and IL-15, cytokines that share common receptor signaling components, including the common gamma chain and the IL-2 receptor beta chain (CD122)(8). Both IL-2 and IL-15 initiate NK cell priming; boosting NK cell effector function, but also drive peripheral NK cell expansion(9). NK cells are found in circulation and lymphoid organs but can also reside in various tissues. Uterine NK (uNK) cells residing in the maternal tissues (decidua and MLAP) of the maternal fetal interface are required for the early development and vascular remodeling of the decidua basalis (DB) during pregnancy(10–12). This unique subset of NK cells is highly proliferative and represents the main leukocyte subset within the early mouse and human DB, expanding to peak levels at gestational day 5.5–9.5 in mice, while in humans their numbers increase in the first trimester and decline as pregnancy progresses(11). UNK cells in the mouse can defined by expression of a glycan recognized by Dolichos biflorus agglutinin (DBA) lectin,(13, 14) or expression of CD122 and NK1.1. Moreover, based on their expression of CD49a and EOMES they can be differentiated into ILC1, tissue resident NK cells (trNK cells) and conventional NK cells (cNK cells)(15–17). Besides vascular remodeling, uNK cells play an essential role in regulating fetal trophoblast invasion during DB development and pregnancy(18). While the importance of uNK cells for a successful pregnancy is well appreciated, the pathways that drive their expansion and activation remain poorly defined.
Our current studies identify a key role for Gab3 in NK cells. Specifically, we show that Gab3 is essential for MAPK signaling downstream of the IL-2/IL-15-receptor in NK cells. Gab3-deficiency is associated with a markedly impaired ability to clear tumor cells, while IL-2- and IL-15-induced priming and expansion of NK cells in vitro is abrogated. Moreover, we show a key role for Gab3 in the expansion of uNK cells that is associated with abnormal vascular remodeling and increased frequency of failed pregnancies characterized by stillbirth, retained placenta, maternal hemorrhage and undelivered fetoplacental units at term.
Results
Identification of Gab3 as a critical protein required for NK cell function
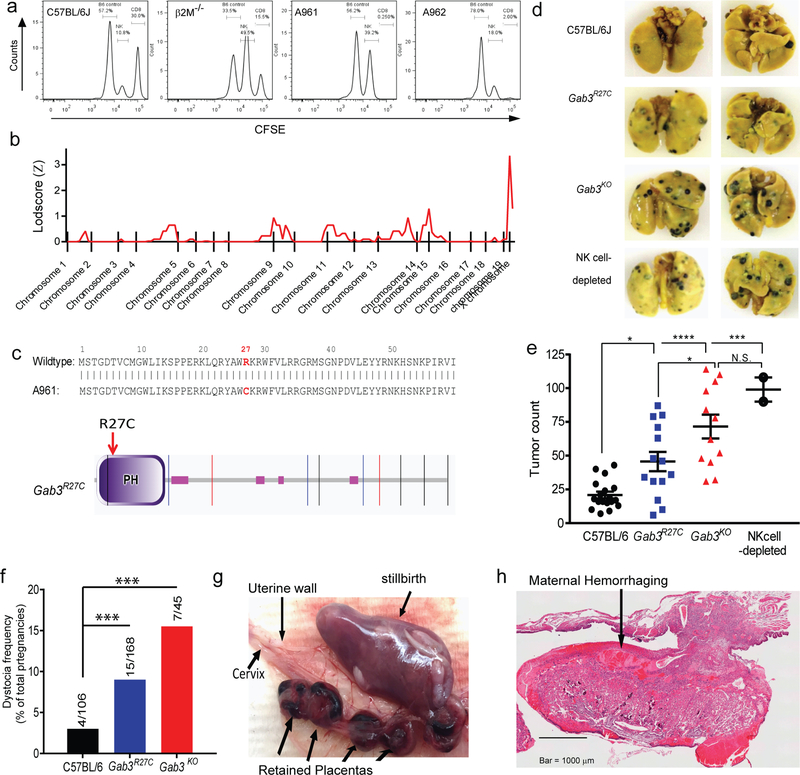
As part of our ENU mutagenesis approach to identify genes involved in NK cell-mediated missing-self recognition(19), we identified a G3 germline mutant (A961) that failed to clear β2M-deficient targets cells, while exhibiting normal antigen-specific CD8+ T cell responses following immunization (Fig.1a). Linkage analysis (19) established the causative mutation to reside on chromosome X with the critical region between 0–93.6Mb (Fig.1b). Subsequent whole exome sequencing (WES) revealed a nucleotide change (C to T at X:722788707bp) in Gab3 that was predicted to be damaging by SIFT and Polyphen analysis. Specifically, the C→T missense mutation causes a single amino-acid change (Arg→Cys or R27C) in the PH-domain of Gab3 (Fig.1c, Fig.S1a). Importantly, alignment studies show that the Arg residue is highly conserved in PH-domains, however a (rare) human missense variant exists for the same Gab3 residue (Arg→His: http://exac.broadinstitute.org/). We hypothesized the Gab3R27C mutation might be causal for the observed NK cell deficiency and to confirm the role of Gab3 in NK cell function, we generated Gab3 knockout mice using CRISPR-Cas9. Specifically, guide RNAs were developed targeting exon 2, causing a single nucleotide insertion, resulting in a frameshift, alternative translation and a premature stop (Fig.S1b–d). Gab3KO mice were viable and confirmed the importance of Gab3 in recognition of β2M-deficient targets in vivo (Fig.S1e). Characterization of other lymphocyte subsets from Gab3R27C and Gab3KO mice, including CD8+, CD4+ and B cells, suggests no differences in the frequency/development of these subsets and normal CD8+ T cell effector function as assessed by immunization with 5E.1 TAKO cells (Fig.1a). Further characterization of peripheral NK cell populations in homozygous Gab3R27C or Gab3KO mice revealed no change in the frequency of NK cells in the spleen (Fig.S2a). Additionally, NK cells from Gab3R27C and Gab3KO mice showed normal NK cell maturation (Fig.S2b), expression of activating receptors Ly49D and Ly49H (Fig.S2c) or expression of inhibitory Ly49 receptors, although the frequency of Ly49G2+ NK cells was reduced in Gab3R27C and Gab3KO mice (Fig.S2d). Nonetheless, stimulation of the NK cells ex vivo using a variety of activating stimuli, e.g. NK1.1, YAC-1 tumor target cells or IL12/IL-18 activating cytokines, revealed similar IFNγ production (Fig.S2e). Finally, we assessed development of NK cells in the bone marrow by flow cytometry as previously described(20). Consistent with the normal peripheral NK cell numbers, no changes were observed in the frequency of NK-committed progenitors or NK1.1+ NK cells in the bone marrow (Fig.S3a,b).
Figure 1. Identification of Gab3 as a critical determinant of NK cell function, anti-tumor responses and pregnancy.
a) Identification of an ENU germline mutant (A961) exhibiting impaired NK cell function compared to a litter mate control (A962). Control and ENU mice were immunized with 5E.1 TAKO cells. Seven days p.i., mice were injected i.v. with control splenocytes (CFSE-low), NK targets (β2M−/− splenocytes; CFSE-medium) and CD8+ targets (EBI192–200-loaded splenocytes; CFSE-high). After 2 days, the frequency of target populations in blood was determined by flow cytometry. b) Coarse mapping on 34 mice (15 control and 19 mutant mice) using 150 genome-wide SNPs, identified the causal mutation to reside on chromosome X. c) Whole exome sequencing identified a C→T nucleotide change at position X:722788707bp causing a single residue change (Arg27→Cys27) in the pleckstrin-homology (PH) domain of Gab3. d,e) C57BL/6 (●),Gab3R27C ( ), Gab3KO (
), Gab3KO ( ) and NK cell-depleted (
) and NK cell-depleted ( ) mice were injected with 1×105 B16-F10 melanoma cells i.v.. After three weeks, mice were sacrificed, and tumor burden was determined (bars represent mean ± s.e.m). f) Frequency of dystocia observed in WT, Gab3R27C and Gab3KO mice. g,h) Representative images of stillborn pups, retained placentas (g) and maternal hemorrhaging (h) of a Gab3KO female with dystocia. Statistical analysis was performed using one-way ANOVA with Tukey post-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
) mice were injected with 1×105 B16-F10 melanoma cells i.v.. After three weeks, mice were sacrificed, and tumor burden was determined (bars represent mean ± s.e.m). f) Frequency of dystocia observed in WT, Gab3R27C and Gab3KO mice. g,h) Representative images of stillborn pups, retained placentas (g) and maternal hemorrhaging (h) of a Gab3KO female with dystocia. Statistical analysis was performed using one-way ANOVA with Tukey post-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
In vivo, functional Gab3 is essential for tumor cell clearance and successful pregnancy
Missing-self recognition is an important mechanism by which NK cells recognize tumor cells, and we therefore investigated whether Gab3R27C and Gab3KO mice susceptible to a melanoma tumor challenge in vivo. Specifically, we injected WT, NK cell-depleted mice, Gab3R27C and Gab3KO mice with 1×105 B16-F10 melanoma tumor cells i.v.. After 3 weeks, pulmonary tumor nodules were quantified, revealing both Gab3R27C and Gab3KO mice had significantly higher tumor burden in the lungs compared to wildtype control mice (Fig.1d,e). Interestingly, the tumor burden was highest in the Gab3KO mice reaching tumor counts similar to NK cell-depleted mice, while Gab3R27C showed an intermediate phenotype (Fig.1d,e).
Importantly, while maintaining a homozygous colony of the Gab3R27C and Gab3KO mice we noticed significant impediments in their ability to complete successful pregnancies (Fig.1f–h). Specifically, we observed a drastic increase in the rate of dystocia for both Gab3R27C and Gab3KO pregnant females (i.e. initiated birth but a failure to complete delivery over the next 24 hours, coinciding with undelivered fetoplacental units, stillbirth, hemorrhage and retained placentas, Fig.1g,h). Interestingly, the relative frequency of failed pregnancies was highest in Gab3KO females compared to WT females, with Gab3R27C females exhibiting an intermediate rate of failed pregnancies.
Taken together, these findings point to a key role for Gab3 in mature NK cell-mediated recognition of metastatic tumor cells, while Gab3-deficiency is associated with an increased failure to complete delivery during pregnancy.
Gab3 is required for IL-2- and IL-15-induced NK cell priming and expansion
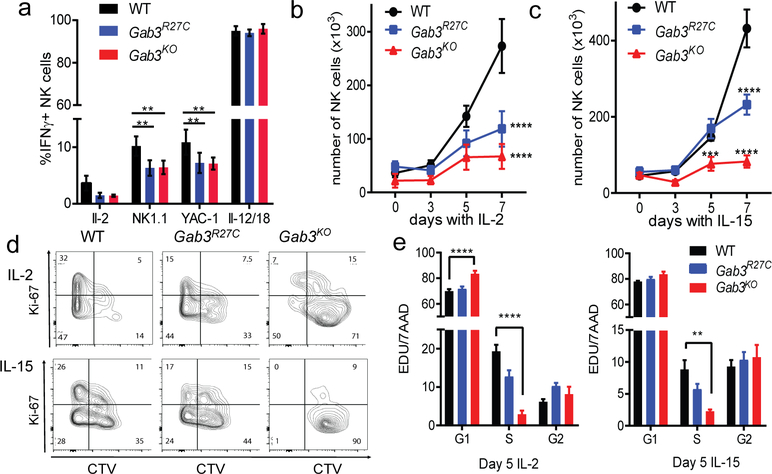
In the context of anti-tumor responses and pregnancy, IL-2 and IL-15 are key cytokines promoting NK cell priming and expansion(21–26). To investigate if NK cells from Gab3R27C and Gab3KO mice have an altered IL-2 or IL-15 response, we assessed NK cell priming and proliferation following IL-2 or IL-15 stimulation. Compared to WT NK cells, Gab3R27C and Gab3KO NK cells showed a significant reduction in IFNγ production when stimulated with NK1.1 or YAC-1 in the presence of IL-2 (Fig.2a), suggesting that IL-2-priming was impaired.
Figure 2. Gab3 is required for IL-2 and IL-15 induced priming and expansion of NK cells.
a) WT, Gab3R27C and Gab3KO NK cells stimulated with anti-NK1.1 antibody, YAC-1 cells or IL-12/IL-18 in the presence of IL-2. NK cell priming was measured by intracellular IFNγ staining (n=4 mice, ± s.e.m.). b-c) IL-2- or IL-15-induced NK cell expansion in vitro using WT, Gab3R27C or Gab3KO splenocytes (n=6, mean ± s.e.m.). Cells were gated on live NKp46+/NK1.1+ cells. d) Representative plots showing live WT, Gab3R27C and Gab3KO cell-trace violet-labeled splenocytes expanded with exogenous IL-2 or IL-15 in vitro for 5 days and stained for Ki67 (n=4, mean ± s.e.m.). e) Cell cycle analysis using EDU incorporation and 7AAD (DNA content) on IL-2- or IL-15-induced WT, Gab3R27C and Gab3KO NK cells (n=4, mean ± s.e.m.). Statistical analysis was done using a two-way ANOVA with Tukey post-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
We next measured IL-2- or IL-15-induced expansion of NK cells in vitro. Interestingly, both Gab3R27C and Gab3KO NK cell expansion was significantly decreased after 1 week, with the number of Gab3KO and Gab3R27C NK cells reduced to ~20% and ~50% of WT NK cell numbers, respectively (Fig.2b,c). Since reduced expansion could be due to either decreased survival or impaired proliferation, we next assessed proliferation/viability and cell cycle progression. Importantly, NK cells from both Gab3R27C and Gab3KO mice showed markedly reduced proliferation as assessed by Cell Trace Violet dilution and Ki67 expression (Fig.2d) while no apparent loss of NK cell viability was observed. Consistent with the survival, we observed a normal induction of Mcl-1, a critical survival factor induced by IL-2 or IL-15(27), in GabR27C and Gab3KO NK cells compared to WT (Fig.S3c). Finally, we investigated cell cycle progression using EDU incorporation in conjunction with 7-AAD staining during IL-2 and IL-15 expansion. Interestingly, we observed significantly fewer Gab3KO NK cells in S phase, while more NK cells resided in G1 compared to WT NK cells. Similar trends were observed in the Gab3R27C NK cells, although differences did not reach significance (Fig.2e). The latter again suggesting the Gab3R27C mutation to behave as a hypomorphic allele. These data identify a critical role for Gab3 in IL-2- and IL-15-driven priming and expansion of NK cells.
Gab3 is selectively required for MAPK-signaling downstream of IL-2/IL-15-receptors
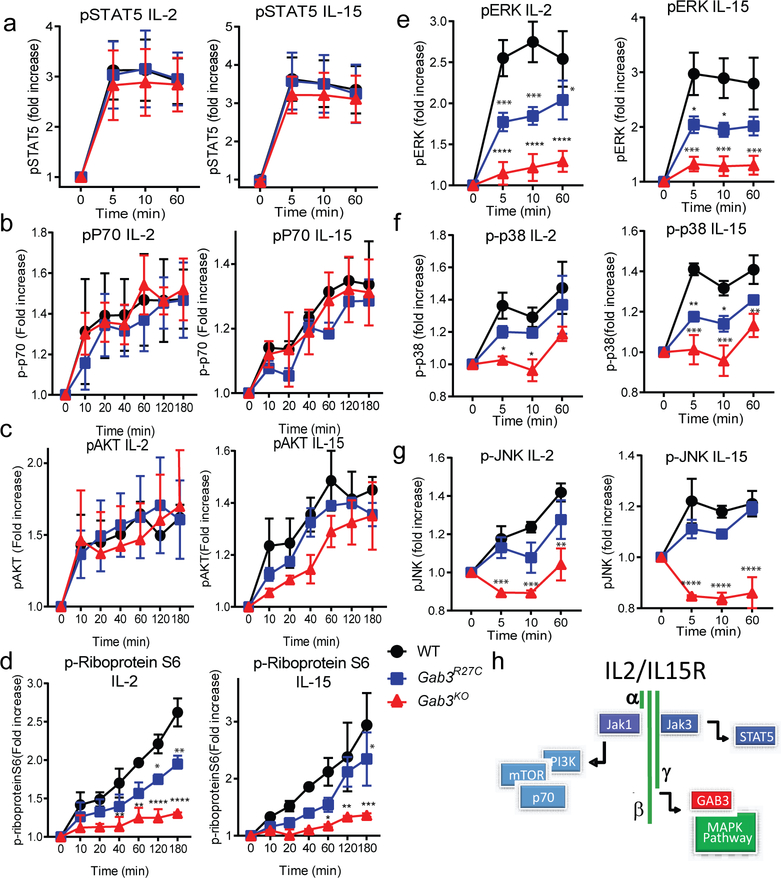
Interestingly, IL-15−/− or IL-15Rα−/− mice display a marked reduction in peripheral NK cell numbers(28–31). Whereas our studies establish a role for Gab3 in IL-15-induced expansion of NK cells, peripheral NK cell numbers remain unaffected in Gab3-deficient mice. Thus, we hypothesized that Gab3 is required for a selective signaling pathway downstream of the IL-2/IL-15 receptor. Activation of the IL-2/IL15-receptor triggers three main signaling pathways including the JAK/STAT5 pathway, the phosphoinositide 3-kinase (PI3K) pathway, and MAP Kinase (MAPK) pathway(32, 33). We investigated downstream signaling in WT, Gab3R27C and Gab3KO NK cells with IL-2 or IL-15 by assessing phosphorylation of STAT5 (JAK/STAT pathway), Akt, p70 S6K, mTORc1, S6 ribosomal protein, and ERK, JNK and p38 (MAPK pathway) at various time points. Both IL-2 and IL-15 induced robust and normal activation of STAT5, Akt and p70 S6 kinase in WT, Gab3R27C and Gab3KO NK cells (Fig.3a–c). In contrast, both Gab3R27C and Gab3KO NK cells showed a profound defect in activation of the MAPK pathways i.e. pERK, pJNK and P-p38 (Fig.3e–g). Importantly, the Gab3R27C displayed a partial reduction while the Gab3KO exhibited a near complete lack of MAPK activation, confirming the Gab3R27C allele to behave as a hypomorphic allele. Interestingly, these studies also revealed a partial yet significant reduction in the phosphorylation of the Ribosomal protein S6 (RpS6) (Fig.3d) in Gab3R27C and Gab3KO NK cells. RpS6 is a key component of the 40S ribosomal subunit required for RNA translation and cell growth and previous studies revealed that phosphorylation of RpS6 is can be mediated by both the PI3K and ERK pathway(34, 35). Thus, the partial reduction in RpS6 phosphorylation is likely derived from the selective loss of ERK, but not PI3K, signaling in Gab3-deficient NK cells.
Figure 3. Gab3 is selectively required for MAPK activation but not AKT or STAT5 signaling downstream of IL-2 or IL-15 activation.
a-g) WT, Gab3R27C and Gab3KO NK cells stimulated directly ex vivo with IL-2 or IL-15 at various time points. a) Phosphorylation of STAT5. b,c) Phosphorylation of downstream targets of PI3K including b) p70 kinase and c) AKT, and d) RpS6. e-g) Phosphorylation of MAPK signaling including e) ERK, f) p38, and g) JNK. h) Proposed working model of where Gab3 functions downstream of the IL-2/IL-15 receptor signaling complex. Statistical analysis was done using a two-way ANOVA with Tukey post-test (experiments were performed in duplicate with at least n≥2 mice). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
These studies thus identify a critical and selective role for Gab3 in the activation of the MAPK pathway downstream of the IL-2/IL-15R, while Gab3 is dispensable for the JAK/STAT- and PI3K- pathways (Fig.3h).
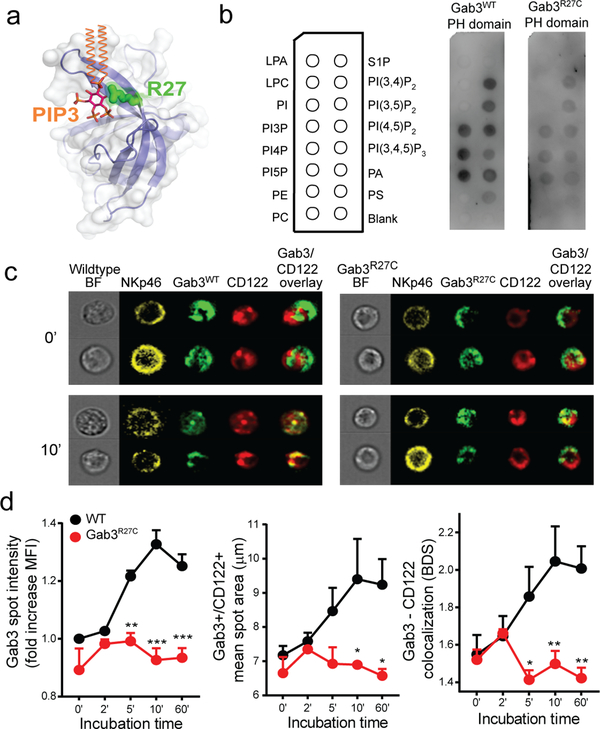
The R27C mutation disrupts Gab3 PH-domain phosphoinositide lipid binding
Based on 3D modeling of the PH-domain in complex with PtdIns(3,4,5)P3, we predicted that the R27C mutation would impact the ability of the PH-domain to recognize and bind lipids (Fig.4a). We thus generated and purified PH-domains from WT Gab3 or R27C Gab3 and assessed their ability to bind to various phosphatidylinositols and membrane lipids using PIP strips incubated with the WT or R27C PH-domain of Gab3 (Fig.4b). WT bound the mono-phosphorylated (PIP), as well the bis-phosphorylated (PIP2), and tri-phosphorylated (PIP3) phosphatidylinositols (Fig.4b). In contrast, lipid binding was significantly disrupted in the presence of the R27C mutation; only minimal binding is observed for lipids that strongly bind WT Gab3 (Fig. 4b). To confirm PIP specificity and quantitatively assess differences between the lipid-binding capacities of WT or R27C Gab3 domains, we repeated the protein-lipid overlay assay using serial dilutions of specific phosphatidylinositols. Importantly, the strongest binding of the Gab3 PH-domain was observed for PIP2 species, with the highest binding observed for PtIns(3,4)P2 and PtIns(3,4)P2, while limited binding for these lipids was observed with the Gab3R27C PH-domain (Fig.S4). These studies suggest that the R27C missense mutation may hamper recruitment of Gab3 to the membrane and/or IL-2/IL-15R complex upon IL-2/IL-15 stimulation. To test this hypothesis, we examined the localization of WT or R27C Gab3 protein in resting and IL-2/IL-15-activated NK cells. Specifically, we generated lentiviral vectors expressing GFP-coupled Gab3WT or Gab3R27C mutant proteins and subsequently transfected primary Gab3KO NK cells to assess protein localization. Following cytokine stimulation, we assessed Gab3 co-localization with the IL2Rβ (CD122) component in time using ImageStream. During resting conditions, WT Gab3-GFP or Gab3R27C -GFP fusion proteins showed similar levels of expression while no significant differences were observed in cellular distribution and limited colocalization with the IL2Rβ component (Fig.4c,d). Importantly, following stimulation with IL-2, the cellular distribution of the Gab3R27C protein is significantly different from WT Gab3. Whereas WT Gab3 is rapidly recruited to CD122+ (IL-2Rβ) locations (Fig.4c,d), this recruitment was largely abrogated in the case of Gab3R27C-GFP. Thus, these findings suggest that the Gab3R27C variant in the PH-domain impairs phosphoinositide lipid binding, thereby abrogating the recruitment of the scaffolding protein Gab3 to the IL-2/IL-15-receptor complex, ultimately resulting in a failure to activate MAPK signaling.
Figure 4. The R27C missense mutation disrupts Gab3 PH-domain binding to PIPs and impairs recruitment to the IL2-receptor complex.
a) 3D model of the PH-domain of WT Gab3 complexed with phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) (orange & magenta). The Arg27 position (green) is predicted to interact with the phosphoinositide headgroup. b) PIP strips incubated with Gab3WT or Gab3R27C PH-domain. c) Representative images of Gab3KO NK cells transfected with Gab3WT-GFP or Gab3R27C-GFP fusion proteins to assess Gab3 colocalization with CD122 upon stimulation with IL-2. d) ImageStream colocalization studies between CD122 and Gab3WT-GFP or Gab3R27C-GFP following various incubation times with IL-2. Colocalization was defined by the intensity of Gab3 in CD122+ vesicles, the diameter and area of Gab3/CD122+ vesicles and colocalization assessed by bright detail similarity. ImageStream data represent mean values ± s.e.m of 3 experiments with >500 NK cells per experiment. A two-way ANOVA with Tukey post-test was performed to assess significance. *P<0.05, **P<0.01, ***P<0.001.
Loss of Gab3 impairs uNK cell function and expansion
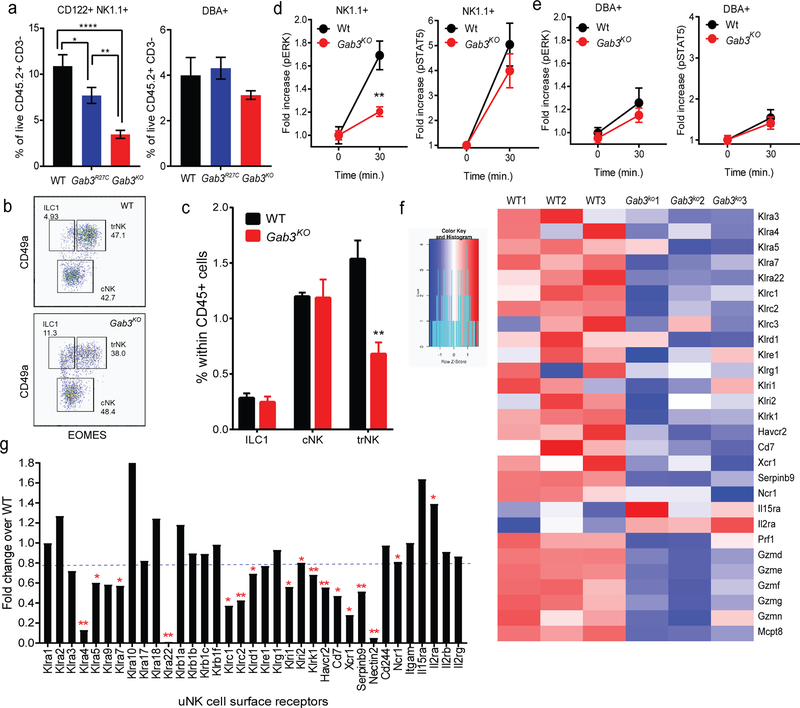
Given the increased frequencies of dystocia and pregnancy-associated abnormalities seen in Gab3R27C and Gab3KO females (Fig.1f–h), we sought to identify the underlying mechanisms for these observations. The uNK population include unique subsets that can be defined by a variety of surface markers, including NK1.1, CD122, Dolichos biflorus agglutinin (DBA) lectin binding, CD49a and EOMES(13, 14, 16, 17). Given the observed pregnancy complications, we posited that uNK cell expansion/function may be impaired during early gestation in Gab3R27C and Gab3KO mice. Flow cytometric analysis of WT and Gab3KO gd8.5 implantation sites revealed a dramatically reduced frequency of CD45+, CD122+ CD3−, DBA− NK1.1+ NK cells (down to ~30% of WT levels) in Gab3KO mice, while the CD45+, CD122+ CD3−, DBA+ NK1.1− population remained relatively unperturbed (Fig.5a), suggesting that the NK1.1+/CD122+ uNK subset specifically requires Gab3 for its expansion early during pregnancy. Further, characterization of the innate lymphoid populations to differentiate ILC1, conventional NK cells (cNK) from tissue resident NK cells (trNK) based on CD49a and EOMES expression (15, 16), revealed a selective reduction in the number of trNK cells in Gab3KO compared to WT implantation sites (Fig.5b,c). The frequency of cNK and ILC1 cells at gd8.5 remained relatively unperturbed compared to WT implantation sites (Fig.5b,c). Interestingly, previous work suggested only trNK cells to show evidence of proliferation (17). Importantly, ex vivo activation of isolated uNK cells with IL-15 further confirmed a selective defect in the NK1.1+/CD122+ NK cells showing reduced ERK (but not STAT5) phosphorylation, while pERK in DBA+NK1.1−/CD122+ NK cells was unaffected (Fig.5d,e).
Figure 5. Gab3 is required for expansion of CD122+ NK1.1+ uNK cells during pregnancy.
a) Frequency of CD122+/NK1.1+ and DBA+ uNK cells in gd8.5 implantation sites from Gab3KO or WT control females as quantified by flow cytometry (n=6). b,c) Frequency of ILC1, cNK and trNK cells in WT and Gab3KO implantation sites as defined by CD49a and EOMES expression using flow cytometry. d,e) Phosphorylation of ERK and STAT5 in CD45+/CD122+/CD3−/NK1.1+ uNK (d) and DBA+ uNK cell subsets (e) isolated from gd8.5 implantation sites from Gab3KO females stimulated ex vivo with IL-15 (n=3, mean ± s.e.m.). f) NK cell receptor expression on gd8.5 uNK cells (CD45+/CD3−/CD122+) from WT and Gab3KO pregnant females as assessed by RNAseq analysis. g) Relative mRNA expression of surface and cytotoxicity molecules in Gab3KO uNK cells compared to WT. Heatmap graphs depict the Z-score, representing the number of standard deviations away from the mean expression. Statistical analysis was done using a one-way ANOVA (a) or two-way ANOVA (b,c) with Tukey post-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
We then assessed whether the remaining uNK cells in Gab3KO mice are different in terms of their transcriptional program compared to WT uNK cells. Specifically, we isolated gd8.5 uNK cells from implantation sites from pregnant WT and Gab3KO females based on the expression of CD45.2+/CD3−/CD122+ (Fig.S5a) and performed RNAseq analysis. Differential expression analysis identified a total of 613 genes with a P value of <0.05 that were differentially expressed between Gab3KO and WT uNK cells (249 genes were up and 364 genes were down in Gab3KO uNK cells) (Fig.S5b). Gene enrichment analyses (ToppFun) identified gene sets with reduced expression in Gab3KO uNK cells that were involved in 1) Eukaryotic Translation, 2) Citric Acid and respiratory electron transport, and 3) Natural killer cell mediated cytotoxicity (Fig.S5c–f). The reduced expression of gene sets in translation/elongation and metabolism are consistent with the signaling defects and reduced Rps6 phosphorylation observed in IL-15-stimulated NK cells (Fig.3d), and suggest a reduced uNK cell growth/expansion in the DB. In addition, Gab3KO uNK cells exhibited significant changes in the RNA expression of surface NK cell receptors and non-cytoxic granzymes such as Gzmd, Gzme, GzmF, GzmG, and GzmN (Fig.5f, Fig.S5f). These Granzymes are thought to be involved in tissue remodeling and are highly expressed in trNK cells(15), thus the reduced expression is consistent with the overall reduction in trNK cells observed in Gab3KO implantation sites. Finally, a modest but overall reduced level of chemokine (C-C motif) ligand 1 (Ccl1), migration and invasion inhibitory protein (Miip) and macrophage migration inhibitory factor (Mif) were observed for uNK cells in the DB of Gab3KO females (Fig.S5g). Importantly, no differences were observed in Ifnγ expression levels and angiogenic factors (i.e. Vegfa,b,c) between WT and Gab3KO uNK cells. In contrast, uNK cells from Gab3KO mice exhibited a ~10-fold increase in the expression of gonadotropin releasing hormone 1 (Gnrh1) (Fig.S5g). The latter has previously been shown to be involved in implantation and is suggested to promote the attachment and invasion of trophoblasts into the endometrium(36).
Finally, uNK cells from Gab3KO implantation sites showed an increased cytokine/IFN signature (Fig.S5h,i) that correlated with increased expression of Ifnε measured in total RNA of implantation site (Fig.S5j); however, Ifnε was not produced by uNK cells directly, given that the RNAseq revealed no significant IFNε expression in either WT or Gab3KO uNK cells. Interestingly, a number of these IFN signature genes are part of a highly enriched pathway in ILC1s involving antigen processing and presentation of peptide via MHC class II (i.e. H2-Aa, H2-DMb1, H2-Ab1, and CD74)(15). Thus, the IFN signature observed may in part reflect the relative enrichment of ILC1s in our RNAseq analysis.
Together, these data suggest that loss of Gab3 results in a reduced expansion of trNK cells in the DB. Moreover, the reduction of trNK cells impacts genes implicated with important effector functions in the development of the placenta during pregnancy.
Loss of Gab3 is associated with abnormally invasive trophoblast
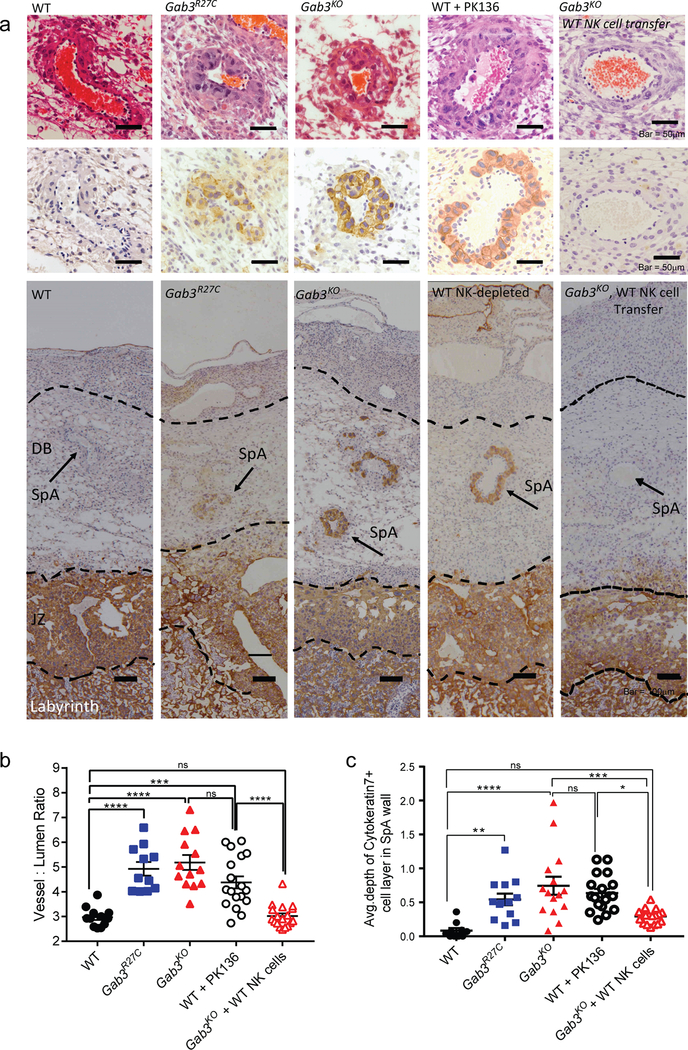
The role of maternally-derived uNK cells during pregnancy is evident at an early stage when they expand rapidly in the DB and initiate spiral artery remodeling as well as controlling the depth and pattern of interstitial and endovascular trophoblast invasion(26, 37–40). Nonetheless, their regulatory function in both successful and unsuccessful pregnancies remains poorly understood(41–44). Given the marked impact on uNK cell expansion in Gab3-deficient mice, we investigated whether this correlated with changes in placental development, spiral artery remodeling and trophoblast giant cell (TGC) infiltration in gd12.5 placentas of Gab3-deficient mice. Histological assessment of the placentas revealed no major differences in the size of the labyrinth and junctional zone, while a trend for reduced decidual depth was observed, particularly in the Gab3KO compared to WT (Fig.S6a–c). Interestingly, the spiral artery walls appeared to be heavily invaded by TGCs, as determined by staining with cytokeratin-7, a pan trophoblast maker(45) (Fig.6a), resulting in significantly smaller lumens and thicker artery walls. We therefore assessed spiral artery remodeling by measuring the vessel to lumen ratio, i.e. the area measurement of the whole vessel divided by the vessel lumen area and assessed the frequency of TGCs in the spiral arteries by cytokeratin-7 staining. Gab3R27C and Gab3KO females exhibited a significantly higher vessel to lumen ratio (Fig.6b) while also showing a significant increase in the number of TGCs within spiral arteries, indicating that the increased vessel to lumen ratio is due to the invading cytokeratin-7+ trophoblast giant cells (Fig.6c).
Figure 6. Functional Gab3 is required for controlling trophoblast giant cell invasion.
a) Representative H&E- or cytokeratin-7-stained images of gd12.5 placentas showing decidua basalis (DB) with spiral arteries (SpA), junctional zone (JZ) and labyrinth from WT, Gab3R27C, Gab3KO, NK cell-depleted (PK-136 treatment) WT, or Gab3KO injected with WT NK cells at gd9.5 females. b) Spiral artery remodeling was assessed by measuring the vessel to lumen ratio of spiral arteries on mid sagittal sections of gd12.5 placentas stained with H&E (n=4 mice, each symbol represents an individual placenta, lines represent mean ± s.e.m.). c) Loss of Gab3 is associated with increased TGC invasion within the maternal spiral arteries. The average depth of cytokeratin-7+ cell layer in SpA walls were quantified on mid sagittal sections of gd12.5 placentas stained with cytokeratin-7 antibody and a hematoxylin counterstain (n=4 mice, each symbol represents an individual placenta, lines represent mean ± s.e.m.). Statistical analysis was done using a one-way ANOVA with Tukey post-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
These observations extended to gd18.5 at which point we observed invasion of TGCs into the uterine wall (Fig.S7a). In addition, we observed a significant expansion of trophoblasts in the labyrinth and junctional zone of both Gab3R27C and Gab3KO placentas leading to an overall increase in the depth of labyrinth, junctional zone and overall placenta (Fig.S7b–d). In the decidua basalis, we observed discontinuous trophoblast invasion with select areas showing deep invasion reaching the uterine wall in Gab3R27C and Gab3KO placentas. By analyzing serial sections stained with proliferin we next determined the smallest distance between areas of trophoblast invasion and the uterine wall for WT, Gab3R27C and Gab3KO placentas. Importantly, placentas from Gab3KO mice but also Gab3R27C mutant mice had an average minimal distance of ~130μm between trophoblasts and the uterine wall, while in WT placentas the average minimal distance was ~490μm, more than triple the distance observed in both Gab3R27C and Gab3KO placentas (Fig.S7a,e). Together, these data suggest that loss of Gab3 results in a failure to control trophoblast invasion throughout pregnancy, ultimately leading to continuous trophoblast invasion towards the uterine wall up to term.
To investigate the causal role of uNK cells in abnormal arterial remodeling and invasion of TGCs, we depleted uNK cells at gd0.5, gd3.5 and gd8.5 in pregnant WT females using NK1.1-depleting Abs (Fig.S8). Subsequently, we examined both arterial remodeling and the presence of TGCs in the spiral arteries at gd12.5 using cytokeratin-7 immunohistochemistry Importantly, we observed a significant increase in the frequency TGCs in spiral arteries, while the vessel-lumen ratio was also similar to Gab3KO placentas (Fig.6a–c).
We next tested whether the adoptive transfer of WT NK cells into pregnant Gab3KO females could correct the increased trophoblast invasion in the maternal spiral arteries. Specifically, we isolated splenic NK cells from WT mice that were expanded in vitro with IL-15 for 4 days. Subsequently, 4 million NK cells were injected i.v. into pregnant Gab3KO females at gd9.5. The recipient females were sacrificed 3 days post-injection (gd12.5) and arterial remodeling and trophoblast invasion in the spiral arteries were assessed following H&E and cytokeratin-7 staining. Intriguingly, both the vessel-to-lumen ratio as well as the average depth of cytokeratin-7+ cell layers in the spiral artery walls were reduced to WT levels (Fig.6a–c).
Together, these studies confirm the critical role of uNK cells in limiting trophoblast invasion and promoting spiral artery remodeling in the DB and reveal that Gab3 loss-of-function abrogates uNK cell expansion in the DB resulting in abnormal spiral artery remodeling and trophoblast infiltration that may ultimately impede a successful pregnancy outcome.
Discussion
While the function of Gab family members Gab1 and Gab2 have been defined, the biological function of Gab3 has remained entirely elusive. Here, we identify Gab3 as a critical determinant of IL-2- and IL-15–induced activation of NK cells. Strikingly, consistent with a reduced ability to eradicate missing-self targets, we observed that Gab3 loss-of-function in vivo leads to a markedly impaired recognition and elimination of metastatic tumor cells. Moreover, our studies identify Gab3 to be important during pregnancy, as pregnant Gab3-deficient females exhibited reduced expansion of uNK cells, abnormal arterial remodeling and increased trophoblast invasion into the DB. At the cellular/molecular level, we identified a clear defect in IL-2/IL-15-induced NK cell priming and expansion. Both cytokines share receptor signaling components including the IL2Rβ-chain (CD122) and the common γ-chain (CD132). Our studies reveal that Gab3 in is selectively required for the activation of MAPK signaling pathways downstream of the IL2/IL-15 receptor, while the STAT5 and PI3K signaling pathways were unaffected. As such, Gab3-deficient mice deviate from complete IL-15-deficient mice, retaining a relative normal peripheral development and survival of NK cells(28, 46, 47).
A previous study performed an immune analysis of independently generated Gab3 KO mice and the authors reported no obvious immune phenotype(5). However, the study limited immune analysis to macrophages and T cells, while no assessment of NK cell function was reported. The latter may be challenging when working with a mixed C57BL/6J/129SvJ background causing variable background-specific NK cell receptor expression. Our current studies involve two independent mouse models on the C57BL/6J background, a complete loss-of-function knockout and an ENU germline carrying a hypomorph R27C missense mutation, both corroborating the critical role of Gab3 in NK cells, anti-tumor responses and successful pregnancy.
Previous genome-wide association studies linked single nucleotide polymorphisms (SNPs) in the promoter region of Gab3 with risk for human type I diabetes (T1D)(48, 49), while recent studies link GAB3 overexpression with tumor cell growth(50, 51). Interestingly, the ExAC database (a database containing >80,000 WES from human patients/controls) reveals an absence of human complete loss-of-function mutations, suggesting the latter may be incompatible with successful human survival or reproduction. Nonetheless, missense variants in human GAB3 have been identified, including a rare missense mutation affecting the same Arg27 (Arg→His: http://exac.broadinstitute.org/) that is affected in our ENU mouse model. Importantly, alignment studies show that the Arg residue is highly conserved in PH-domains and our studies suggest this residue to be critical for the interaction of the PH-domain with phosphoinositides. Thus, our ENU mouse model not only presents a unique model to molecularly assess the role of the PH-domain in PIP binding, it may also be predictive of a potential NK cell deficiency in humans carrying this R27→H missense mutation.
The pregnancy complications in Gab3-deficient mice associated with invasive fetal trophoblasts in the uterine wall, resemble the human condition placenta accreta. The latter presents a clinical condition when placental trophoblasts invade deeper into the uterine wall and fail to separate from the uterine wall. The incidence of placenta accreta has increased significantly over the last decades and is currently estimated to be 1 in ~530 births. While this increase may be linked to the increase in cesarean delivery rate, immunological and/or genetic factors are likely to play a role as well. For instance, recent studies link the development of placenta accreta with significantly reduced numbers of uNK, while no significant association with the number of uterine scars was observed(52, 53). Whether the rare R27H missense mutation presents a hypomorph allele in humans and like the mouse model results in impaired uNK cell expansion and/or is associated with the development of placenta accreta in humans remains to be addressed.
It is evident that uNK cells play a critical role in the DB development and determine the successful outcome of a pregnancy(11, 26, 37, 39, 40, 54). NK cell deficiencies have previously been linked with impaired regulating of trophoblast invasion in both the mouse and rat abnormal uNK cell numbers have been associated with gestational complications, including recurrent spontaneous abortion and placenta accreta(55–59). Moreover, IL-15-deficient murine models lack peripheral and uNK cells and display a robust invasive trophoblast phenotype and spiral artery remodeling similar to what is observed in Gab3-deficient mice, although significant differences exist between spiral artery remodeling between IL-15-deficient mice and rats(24, 26, 60). Nonetheless, these studies corroborate the importance of IL-15 in placental development and pregnancy. Interestingly, while Gab3-deficient mice exhibit reduced uNK cell numbers, they have relatively normal peripheral NK cell numbers in the blood circulation that have the potential to interact with fetal trophoblasts aligned within the spiral arteries. Given the impaired recognition and clearance of MHC-I-deficient target cells or tumor cells by Gab3-deficient NK cells, the possibility exists that this mechanism may contribute to the invasive trophoblast phenotype. Fetal trophoblasts express a unique pattern of predominantly non-MHC antigens and lack the expression of a number of classical MHC antigens(61–63), thus representing a “missing-self” target. Whether peripheral NK cells within the maternal arteries play a role in containing trophoblast invasion is currently unclear and remains to be investigated.
Gab3 is expressed in a variety of cell types including CD8+ T cells and Mast cells. While we have not observed abnormal CD8+ T cell responses or changes in their development, we cannot exclude a role for these cell types in the abnormal pregnancy phenotype observed. Nonetheless, our studies establish an important role for Gab3 in uNK cell expansion and the control of decidual trophoblast invasion. Importantly, our “add-back” experiments, in which we transplant IL-15-stimulated WT NK cells in Gab3KO-recipients at gd9.5, can largely overcome decidual trophoblast invasion as analyzed by gd12.5. These experiments point to an effective therapeutic approach to limit trophoblast invasion in the maternal spiral arteries that deserves further investigation.
Gab3 expression is predominantly observed in hematopoietic cells and is particularly high in NK cells. Our current study reveals a key role for Gab3 in IL-2/IL-15-induced NK cell expansion, specifically in mediating MAPK activation downstream of the IL-2/IL-15R. This pathway provides a new opportunity to selectively induce or repress NK cell function in settings of autoimmunity, anti-tumor immunity, transplantation, and other therapeutically important settings. Moreover, we posit that Gab3 loss-of-function is associated with increased trophoblast invasion and development of placenta accreta, a significant pregnancy complication in humans.
Material and methods
Study Design
The goal of the studies was to characterize the role of Gab3 in NK cell function. The studies were performed using groups of age and gender matched mice, five weeks or older. Timed pregnancy studies and dystocia assessment were performed using homozygous WT, Gab3R27C and Gab3KO breeding colonies. Power analysis indicated that analyses of 3–4 mice and 3 placentas per mouse would give 80% power (P<0.05, 2-sided) to detect a 50% increase/decrease in vascular trophoblasts numbers. Histological analyses were performed double-blinded where appropriate.
Generation of Gab3KO mice through CRISPR/Cas9 genome editing
Gab3KO mice were generated by CRIPSR-Cas9 genome editing. Gab3-specific shRNA guides were designed using Benchling software and full sequence of Gab3. The following guide sequences were used: Gab3 shRNA guide_1 – 5’ACACTATCTGGAGTCACTTA3’, Gab3 shRNA guide_2 −5’CTACCTAGTAGCCAAGACTG3’, Gab3 shRNA guide_3 5’AGTCATCTGGAAGATGGTGC3’. The guides were designed to target a 201 base pair region of exon 2 [Ensemble Genome Browser, ENSMUSG00000032750, genome assembly version GRCm38.p6]. Micro injections into C57BL/6 ES cells was done by the Transgenic Animal and Genome Editing Core at CCHMC. Founder animals were screened for mutations in Gab3 by PCR/sequencing. A founder animal was selected that carried a frame shift causing a premature stop at residue 104, resulting in expression of a truncated protein presenting the proximal PH-domain. The genome-editing was functionally confirmed at the RNA transcript (cDNA) level.
Statistics
Two-way ANOVA with a Tukey post-test for multiple comparison and one-way ANOVA were used where noted; statistical tests were run using Graph Pad software (v 7.02). Standard error of the mean was reported for all experiments and p<0.05 were taken as significant for all tests.
For extensive further material and methods description see online supplementary file.
Supplementary Material
Supplemental Figure 1. Generation of Gab3-deficient mouse models
Supplemental Figure 2. Loss of Gab3 does not affect peripheral NK cell numbers or their maturation.
Supplemental Figure 3. Loss of Gab3 does not affect NK cell development in the bone marrow.
Supplemental Figure 4. The R27C missense mutation disrupts the PH domain binding to phosphotyidylinositols.
Supplemental Figure 5. Altered gene expression in gd8.5 uNK cells (CD45+/CD3-/CD122+) isolated from Gab3KO compared to WT uNK cells.
Supplemental Figure 6. Loss of Gab3 has limited effect on the decidual depth, labyrinth or junctional zone area at gd12.5
Supplemental Figure 7. Functional Gab3 is required for controlling trophoblast invasion.
Supplemental Figure 8. WT mice treated with anti-NK1.1 antibodies (PK136) have efficient depletion of NK cells in the spleen and placentas at gd12.5
Acknowledgement
Funding: The research was funded by the NIH, PHS Grant P30 DK078392 (Integrative Morphology Core of the Cincinnati Digestive Disease Research Core Center) and the NIAID/NIH R21AI135380 R21 grant and the Maren Foundation.
Footnotes
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Wohrle FU, Daly RJ, Brummer T, Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun Signal 7, 22 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakaoka Y, Komuro I, Gab docking proteins in cardiovascular disease, cancer, and inflammation. Int J Inflam 2013, 141068 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishida K, Hirano T, The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci 94, 1029–1033 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemmon MA, Ferguson KM, Molecular determinants in pleckstrin homology domains that allow specific recognition of phosphoinositides. Biochem Soc Trans 29, 377–384 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Seiffert M, Custodio JM, Wolf I, Harkey M, Liu Y, Blattman JN, Greenberg PD, Rohrschneider LR, Gab3-deficient mice exhibit normal development and hematopoiesis and are immunocompetent. Mol Cell Biol 23, 2415–2424 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S, Functions of natural killer cells. Nat Immunol 9, 503–510 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL, Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 9, 495–502 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA, Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med 180, 1395–1403 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A, Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26, 503–517 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashkar AA, Di Santo JP, Croy BA, Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med 192, 259–270 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratsep MT, Felker AM, Kay VR, Tolusso L, Hofmann AP, Croy BA, Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction 149, R91–102 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Chen Z, Smith GN, Croy BA, Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol 8, 1–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Zhang J, Hatta K, Lima PD, Yadi H, Colucci F, Yamada AT, Croy BA, DBA-lectin reactivity defines mouse uterine natural killer cell subsets with biased gene expression. Biol Reprod 87, 81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JH, Yamada AT, Croy BA, DBA-lectin reactivity defines natural killer cells that have homed to mouse decidua. Placenta 30, 968–973 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Filipovic I, Chiossone L, Vacca P, Hamilton RS, Ingegnere T, Doisne JM, Hawkes DA, Mingari MC, Sharkey AM, Moretta L, Colucci F, Molecular definition of group 1 innate lymphoid cells in the mouse uterus. Nat Commun 9, 4492 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, Riley JK, Zhu J, Tian Z, Yokoyama WM, Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 3, e01659 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sojka DK, Yang L, Plougastel-Douglas B, Higuchi DA, Croy BA, Yokoyama WM, Cutting Edge: Local Proliferation of Uterine Tissue-Resident NK Cells during Decidualization in Mice. J Immunol 201, 2551–2556 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lash GE, Otun HA, Innes BA, Percival K, Searle RF, Robson SC, Bulmer JN, Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod 25, 1137–1145 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Cashman S, Lampe K, Sheridan R, Hoebe K, An ENU mutagenesis approach to dissect “self”-induced immune responses: Unraveling the genetic footprint of immunosurveillance. Oncoimmunology 1, 856–862 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, Weissman IL, Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood 118, 5439–5447 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller JS, Therapeutic applications: natural killer cells in the clinic. Hematology Am Soc Hematol Educ Program 2013, 247–253 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Sim GC, Radvanyi L, The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev 25, 377–390 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Lanier LL, Natural killer cells and cancer. Adv Cancer Res 90, 127–156 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA, Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol 171, 2937–2944 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Takashima A, Ishikawa F, Kuwabara T, Tanaka Y, Kinoshita T, Ito M, Kakiuchi T, Uterine natural killer cells severely decrease in number at gestation day 6 in mice. Biol Reprod 89, 101 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Renaud SJ, Scott RL, Chakraborty D, Rumi MA, Soares MJ, Natural killer-cell deficiency alters placental development in rats. Biol Reprod 96, 145–158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sathe P, Delconte RB, Souza-Fonseca-Guimaraes F, Seillet C, Chopin M, Vandenberg CJ, Rankin LC, Mielke LA, Vikstrom I, Kolesnik TB, Nicholson SE, Vivier E, Smyth MJ, Nutt SL, Glaser SP, Strasser A, Belz GT, Carotta S, Huntington ND, Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat Commun 5, 4539 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ, Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 191, 771–780 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohteki T, Ho S, Suzuki H, Mak TW, Ohashi PS, Role for IL-15/IL-15 receptor beta-chain in natural killer 1.1+ T cell receptor-alpha beta+ cell development. J Immunol 159, 5931–5935 (1997). [PubMed] [Google Scholar]
- 30.Kawamura T, Koka R, Ma A, Kumar V, Differential roles for IL-15R alpha-chain in NK cell development and Ly-49 induction. J Immunol 171, 5085–5090 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Gilmour KC, Fujii H, Cranston T, Davies EG, Kinnon C, Gaspar HB, Defective expression of the interleukin-2/interleukin-15 receptor beta subunit leads to a natural killer cell-deficient form of severe combined immunodeficiency. Blood 98, 877–879 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Mishra A, Sullivan L, Caligiuri MA, Molecular pathways: interleukin-15 signaling in health and in cancer. Clin Cancer Res 20, 2044–2050 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA, IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 4, 329–336 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J, RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem 282, 14056–14064 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmond RJ, Emery J, Okkenhaug K, Zamoyska R, MAPK, phosphatidylinositol 3-kinase, and mammalian target of rapamycin pathways converge at the level of ribosomal protein S6 phosphorylation to control metabolic signaling in CD8 T cells. J Immunol 183, 7388–7397 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Atwood CS, Vadakkadath Meethal S, The spatiotemporal hormonal orchestration of human folliculogenesis, early embryogenesis and blastocyst implantation. Mol Cell Endocrinol 430, 33–48 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O, Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12, 1065–1074 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL, Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 198, 1201–1212 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty D, Rumi MA, Konno T, Soares MJ, Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci U S A 108, 16295–16300 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ain R, Canham LN, Soares MJ, Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Developmental biology 260, 176–190 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Clark DA, Popular myths in reproductive immunology. J Reprod Immunol 104–105, 54–62 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Sacks G, Enough! Stop the arguments and get on with the science of natural killer cell testing. Hum Reprod 30, 1526–1531 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Lash GE, Bulmer JN, Do uterine natural killer (uNK) cells contribute to female reproductive disorders? J Reprod Immunol 88, 156–164 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Tang AW, Alfirevic Z, Quenby S, Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic review. Hum Reprod 26, 1971–1980 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Lamm KYB, Johnson ML, Baker Phillips J, Muntifering MB, James JM, Jones HN, Redline RW, Rokas A, Muglia LJ, Inverted formin 2 regulates intracellular trafficking, placentation, and pregnancy outcome. eLife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, Taniguchi M, Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol 10, e1001255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP, Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS, Type C 1 Diabetes Genetics, Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 41, 703–707 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pociot F, Akolkar B, Concannon P, Erlich HA, Julier C, Morahan G, Nierras CR, Todd JA, Rich SS, Nerup J, Genetics of type 1 diabetes: what’s next? Diabetes 59, 1561–1571 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia P, Li F, Gu W, Zhang W, Cai Y, Gab3 overexpression in human glioma mediates Akt activation and tumor cell proliferation. PLoS One 12, e0173473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiang S, Wang N, Hui P, Ma J, Gab3 is required for human colorectal cancer cell proliferation. Biochem Biophys Res Commun 484, 719–725 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Laban M, Ibrahim EA, Elsafty MS, Hassanin AS, Placenta accreta is associated with decreased decidual natural killer (dNK) cells population: a comparative pilot study. Eur J Obstet Gynecol Reprod Biol 181, 284–288 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Wicherek L, Galazka K, The possible correlation between the patient’s immune tolerance level during cesaerean section and the incidence of subsequent emergency peripartum hysterectomy. Clin Dev Immunol 2007, 63596 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bulmer JN, Lash GE, The Role of Uterine NK Cells in Normal Reproduction and Reproductive Disorders. Adv Exp Med Biol 868, 95–126 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Gomaa MF, Serag Eldeen IF, Farid LA, El-Saeed MM, Abas AM, Aawd NM, Uterine natural killer cells dysregulation in idiopathic human preterm birth: a pilot study. J Matern Fetal Neonatal Med, 1–5 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Moffett A, Hiby SE, Sharkey AM, The role of the maternal immune system in the regulation of human birthweight. Philos Trans R Soc Lond B Biol Sci 370, 20140071 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quenby S, Nik H, Innes B, Lash G, Turner M, Drury J, Bulmer J, Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum Reprod 24, 45–54 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Long W, Shi Z, Fan S, Liu L, Lu Y, Guo X, Rong C, Cui X, Ding H, Association of maternal KIR and fetal HLA-C genes with the risk of preeclampsia in the Chinese Han population. Placenta 36, 433–437 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Williams PJ, Bulmer JN, Searle RF, Innes BA, Robson SC, Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: a comparison with late normal pregnancy. Reproduction 138, 177–184 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Barber EM, Pollard JW, The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol 171, 37–46 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Chazara O, Xiong S, Moffett A, Maternal KIR and fetal HLA-C: a fine balance. J Leukoc Biol 90, 703–716 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Sharkey AM, Xiong S, Kennedy PR, Gardner L, Farrell LE, Chazara O, Ivarsson MA, Hiby SE, Colucci F, Moffett A, Tissue-Specific Education of Decidual NK Cells. J Immunol 195, 3026–3032 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trowsdale J, Moffett A, NK receptor interactions with MHC class I molecules in pregnancy. Semin Immunol 20, 317–320 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Georgel P, Du X, Hoebe K, Beutler B, ENU mutagenesis in mice. Methods Mol Biol 415, 1–16 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Lampe K, Endale M, Cashman S, Fang H, Mattner J, Hildeman D, Hoebe K, Slp-76 is a critical determinant of NK-cell mediated recognition of missing-self targets. Eur J Immunol 45, 2072–2083 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ, The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10, 845–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kieckbusch J, Gaynor LM, Colucci F, Assessment of Maternal Vascular Remodeling During Pregnancy in the Mouse Uterus. J Vis Exp, e53534 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Generation of Gab3-deficient mouse models
Supplemental Figure 2. Loss of Gab3 does not affect peripheral NK cell numbers or their maturation.
Supplemental Figure 3. Loss of Gab3 does not affect NK cell development in the bone marrow.
Supplemental Figure 4. The R27C missense mutation disrupts the PH domain binding to phosphotyidylinositols.
Supplemental Figure 5. Altered gene expression in gd8.5 uNK cells (CD45+/CD3-/CD122+) isolated from Gab3KO compared to WT uNK cells.
Supplemental Figure 6. Loss of Gab3 has limited effect on the decidual depth, labyrinth or junctional zone area at gd12.5
Supplemental Figure 7. Functional Gab3 is required for controlling trophoblast invasion.
Supplemental Figure 8. WT mice treated with anti-NK1.1 antibodies (PK136) have efficient depletion of NK cells in the spleen and placentas at gd12.5