Abstract
Rationale: We have a limited understanding of the molecular underpinnings of early adenocarcinoma (ADC) progression. We hypothesized that the behavior of early ADC can be predicted based on genomic determinants.
Objectives: To identify genomic alterations associated with resected indolent and aggressive early lung ADCs.
Methods: DNA was extracted from 21 ADCs in situ (AISs), 27 minimally invasive ADCs (MIAs), and 54 fully invasive ADCs. This DNA was subjected to deep next-generation sequencing and tested against a custom panel of 347 cancer genes.
Measurements and Main Results: Sequencing data was analyzed for associations among tumor mutation burden, frequency of mutations or copy number alterations, mutation signatures, intratumor heterogeneity, pathway alterations, histology, and overall survival. We found that deleterious mutation burden was significantly greater in invasive ADC, whereas more copy number loss was observed in AIS and MIA. Intratumor heterogeneity establishes early, as in AIS. Twenty-one significantly mutated genes were shared among the groups. Mutation signature profiling did not vary significantly, although the APOBEC signature was associated with ADC and poor survival. Subclonal KRAS mutations and a gene signature consisting of PIK3CG, ATM, EPPK1, EP300, or KMT2C mutations were also associated with poor survival. Mutations of KRAS, TP53, and NF1 were found to increase in frequency from AIS and MIA to ADC. A cancer progression model revealed selective early and late drivers.
Conclusions: Our results reveal several genetic driver events, clonality, and mutational signatures associated with poor outcome in early lung ADC, with potential future implications for the detection and management of ADC.
Keywords: overdiagnosis, interception, adenocarcinoma, mutations
At a Glance Commentary
Scientific Knowledge on the Subject
Lung adenocarcinoma has a widely variable clinical course that is difficult to predict based on imaging or pathologic grounds only. Tools are needed to help us predict the behavior of these tumors.
What This Study Adds to the Field
Our study uses a large cohort of early detected preinvasive and invasive lung adenocarcinomas to identify genomic determinants predictive of tumor behavior. Our study reveals differences in deleterious tumor mutation burden, copy number alterations, gene signatures, and driver gene clonality that include association with clinical outcome. Once validated in new cohorts, these results may have an impact on clinical practice.
Lung adenocarcinoma (ADC) is a heterogeneous disease with poor survival in advanced stages. This heterogeneity includes differences in epidemiology, clinical presentation, natural history, rate of progression (1, 2), histology (3), response to therapy (4, 5), and survival (6). When diagnosed and treated early, indolent cancers are less deadly than aggressive ones (70% vs. 30% survival, indolent vs. aggressive) (7). Given the rising frequency of early diagnoses (8), examining the molecular determinants of disease progression is paramount. Recent investigations have explored ways of predicting the aggressiveness of tumors (9, 10). However, molecular explanations for the difference in tumor behavior remain largely unclear, although they are directly relevant to the efficacy and cost-effectiveness of lung cancer screening, especially when considering the risks of overdiagnosis and overtreatment (11, 12).
Many predictive models of lung cancer behavior exist, including histology, structural imaging, and molecular signatures. Pathologic reclassification of lung ADCs (3) has brought new biological insights (13). ADC in situ (AIS), for example, behaves differently than minimally invasive ADC (MIA) or invasive ADC (14, 15). These subtypes have demonstrated differing behaviors, but the study of their cancer-specific outcome remains limited given the surgical treatment indicated. Volume doubling time (16) and structural imaging features (17) have been used to characterize tumor behavior. Molecular approaches have so far led to inconclusive reports. Although previous studies have suggested that the molecular features of advanced lung ADC are established early (9, 18), it is unclear how early they appear and whether tumor behavior is dependent on mechanisms of progression. A few studies have demonstrated mutational landscapes in preinvasive lesions but with small sample sizes that limit statistical significance (19–22).
This background motivated us to investigate the molecular basis of tumor behavior in early cancer using a larger population. To gain insights into the molecular pathogenesis and underlying molecular determinants of clinical behavior in early ADC, we investigated genetic alterations in 102 surgically resected early ADCs. We also investigated whether mutational signatures, tumor heterogeneity, and specific altered signaling pathways might uncover determinants of tumor behavior. Finally, we proposed a new early ADC evolutionary model based on available cross-sectional samples. Some results of this study have been reported previously in the form of an abstract (23).
Methods
Study Population and Collection of Tumor Specimens
In total, 102 formalin-fixed paraffin-embedded tumor samples were retrieved from the archives of the Department of Pathology at Columbia University following an institutional review board–approved protocol (IRB-AAAA3987) in 2002. All specimens were obtained at the time of surgery and before medical treatment. A lung cancer pathologist (A.C.B.) confirmed the diagnosis based on the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society 2011 classification criteria (3). Clinical characteristics of these patients are described in Table 1 and Table E1 in the online supplement. These specimens represent tumors detected without the use of screening. The AISs studied are not synchronous to ADCs.
Table 1.
Patient Characteristics
| AIS (n = 21) | MIA (n = 27) | ADC (n = 54) | P Value | |
|---|---|---|---|---|
| Age, yr, mean ± SD | 63 ± 15 | 67 ± 9 | 70 ± 9 | 0.24* |
| Sex, n | 0.92† | |||
| F | 15 | 18 | 36 | |
| M | 6 | 9 | 18 | |
| Tumor size, mm, mean ± SD | 13.5 ± 5.6 | 20.6 ± 10.3 | 24 ± 14.6 | 0.001* |
| Smoker, n‡ | 12 | 19 | 36 | 0.62† |
| Stage, n | 0.002† | |||
| IA | 20 | 23 | 27 | |
| IB | 1 | 4 | 14 | |
| IIA | 0 | 0 | 5 | |
| IIIA | 0 | 0 | 8 | |
| OS, mo, mean ± SD | 89.6 ± 32.4 | 77.4 ± 46.8 | 820.9 ± 51.2 | 0.06§ |
| LVI, n | <0.0001† | |||
| Negative | 21 | 27 | 34 | |
| Positive | 0 | 0 | 20 |
Definition of abbreviations: ADC = adenocarcinoma; AIS = adenocarcinoma in situ; LVI = lymphovascular invasion; MIA = minimally invasive adenocarcinoma; OS = overall survival.
P values were calculated using Kruskal-Wallis test.
P values were calculated using chi-square test.
41 patients had true smoking status, whereas smoking status of 61 patients was predicted using a logistic regression model (see Methods in the online supplement).
P values were calculated using log-rank test among three histology groups.
Targeted DNA Sequencing and Analysis
Tumor DNA was extracted using the Qiagen FFPE Tissue Kit from microscope-guided dissection of 5–10 sections (8 μm each) and purified as previously reported (24). Overall, 500 ng of DNA samples were submitted for targeted next-generation sequencing at Vanderbilt Technologies for Advanced Genomics (VANTAGE). A panel of 347 common cancer genes was used in this study (see Table E2). Sequencing data from 102 samples passed quality control and were screened for somatic mutations and copy number variants (CNVs). A detailed description is provided in Figure E1 in the online supplement. Because we lacked paired normal tissue for these tumors, DNA from 10 normal HapMap cell-line samples (25) were extracted, prepared, and sequenced using the same method and platform. In addition, we performed analysis for mutational signatures (26, 27), tumor heterogeneity (28), pathway analysis (29, 30), and cancer evolution analysis (31, 32), as described in the Methods section of the online supplement. Additional details on the methods and statistical analyses are provided in the online supplement.
Data Availability
The raw sequencing data of 102 tumors were deposited into dbGAP (phs001811.v1.p1). The processed and annotated sequencing data of 1,203 nonsynonymous variants in 102 samples are provided in Table E23.
Results
Somatic Gene Mutations and CNVs
We identified 1,203 nonsynonymous somatic mutations in 102 tumors, including 1,096 point mutations, 22 small insertions, and 85 deletions (see Figure E2A). The point mutations include 994 missense and 102 nonsense mutations. In total, we identified 158 mutations in AISs (n = 21), 276 mutations in MIAs (n = 27), and 769 mutations in ADCs (n = 54). A higher mutational burden was significantly associated with sex (male), smoking status, and ADC histology (highest in ADC vs. lowest in AIS) in a univariable negative binomial regression model (see Table E3). In a multivariable model, mutational burden and smoking status were significantly elevated in ADC (P = 0.002 and P = 0.001, respectively; see Table E4). Similarly, patients with ADC carried more deleterious nonsynonymous mutations than patients with AIS (P = 0.01; Table E5) or AIS/MIA combined (P = 0.01; Figure 1A), when adjusted for sex and smoking status.
Figure 1.
Somatic mutations and copy number alterations in adenocarcinoma (ADC) in situ (AIS), minimally invasive ADC, and ADC. (A) Burden of deleterious nonsynonymous mutation is associated with histology, highest in ADC. The red dot in the violin plot represents the mean. The P value was calculated and adjusted using multivariable negative binomial regression models. (B) Overlap of 59 significantly mutated genes among three groups. (C) Frequency of 21 significantly mutated genes in all histologic groups. (D) Greater proportion of copy number loss occurs in AIS and greater proportion of copy number gain occurs in ADC (P = 0.03, Wald test, corrected for sample cluster effect). (E) Significantly amplified and deleted regions identified by Recurrent Unidirectional Break Identification by Clustering approach. MIA = minimally invasive adenocarcinoma.
To identify recurrent mutated genes associated with the progression of lung ADC, we applied both OncodriveFM (33) and OncodriveCLUST algorithms (34) and found 59 significantly mutated genes (Tables E6 and E7 and Figure E2B). Twenty-one genes were significantly mutated in all histologies. We considered these to be putative oncogenic drivers (Figures 1B and 1C and Table E8). Somatic mutations in KRAS occurred with increasing frequency in 10% of AIS, 26% of MIA, and 31% of ADC (P = 0.06, Cochran– Armitage test for trend). Similarly, NF1 and TP53 mutation rates were lower in AIS (5% and 5%) or MIA (4% and 4%) and relatively higher in ADC (15% and 17%; P = 0.11 and P = 0.07, respectively, based on a Cochran-Armitage test for trend). EGFR was mutated in 24% of AIS, 33% of MIA and 30% of ADC (P = 0.72, Cochran-Armitage test for trend; Table E8). Compared with the TCGA LUAD (The Cancer Genome Atlas Lung Adenocarcinoma) cohort (35), mutation frequency of KRAS (31% vs. 32%) was similar, EGFR (29% vs. 14%) was higher (P = 0.002, Fisher exact test), and TP53 (11% vs. 45%) and KEAP1 (6% vs. 16%) were lower (P = 0.0001 and P = 0.02, Fisher exact test) in our ADC cohort. Other significant mutated genes or known drivers including ERBB2, BRAF, RB1, SKT11, and ALK showed no significant correlation with histology. The frequency of these gene mutations was similar when compared with the TCGA cohort (Table E9). Together, these data suggest that mutations of these 21 genes are critical in early tumorigenesis and that some drivers may have a stepwise role in transformation or progression.
When considering CNVs, 204 with high level of amplification and 329 deletion events were identified. When combined with 1,321 low-level gain and 1,745 loss events, more copy number losses were seen in AIS and more copy number gains were seen in ADC compared with other histologic subtypes (P = 0.03, Wald test, corrected for cluster effect; Figure 1D). We used Recurrent Unidirectional Break Identification by Clustering (36) to identify recurrent aberrations, an approach that detects recurrent copy number breaks rather than recurrently amplified or deleted regions. In total, we found 7 significantly altered amplification peaks and 13 deletion peaks (Figure 1E and Tables E10 and E11), among which are well-known recurrent altered regions in lung ADCs, such as amplification of 10q21 and 12q15 and deletion of 3p21 and 19q13 (35). The most frequent altered regions or genes are shown in Figure E2C. Together, these results demonstrate that AIS, although preinvasive, has the full genomic alteration profile displayed in invasive cancer (see Figure E2D).
Mutational Signatures
We used nonnegative matrix factorization mutation signature analysis (26) to uncover five main mutational signatures in our cohort (see Figures E3A and E3B), similar to previously reported Catalogue of Somatic Mutations in Cancer mutational signatures 2, 6, 4, 1, and 18, with maximal cosine similarities of 0.71, 0.71, 0.59, 0.80, and 0.28, respectively. The largest contribution of signatures was derived from Signature 6 (33%), attributed to defective DNA mismatch repair (MMR). Signature 1 (aging) was attributed to spontaneous deamination of 5-methylcytosine, signature 4 to smoking, and signature 2 to APOBEC. These signatures comprised 23%, 26%, and 12% of the mutational process, respectively (Figure 2A). The biological implication of signature 18 is unknown and contributed to 6% of all somatic mutations. Mutational burden was significantly higher in APOBEC-enriched tumors compared with MMR-enriched tumors (see Figure E3C). Tumors enriched for the APOBEC signature had worse 10-year overall survival (OS) compared with tumors enriched with the MMR signature (Figure 2B; P = 0.02, log-rank test), independent of age and sex but not mutational burden or histology (Table E12). Although 9 of 12 APOBEC-enriched tumors were ADC, the overall proportion of tumors enriched in each known mutational signature was not associated with histology (Figure 2C; P = 0.3, Cochran-Armitage test for trend).
Figure 2.
Mutational signature and tumor heterogeneity analysis. (A) Five mutation signatures identified by a nonnegative matrix factorization approach and APOBEC enrichment analysis in all tumors. (B) The tumors enriched for signatures of APOBEC were significantly associated with worse 10-year overall survival compared with MMR-enriched tumors (P = 0.02). The P value was calculated using a log-rank test. (C) The proportion of tumors enriched in each mutational signature was not significantly different among the three histologic groups. Adenocarcinoma (ADC) tended to have more APOBEC- enriched tumors (9/12; P = 0.07, chi-square test for trend). (D) The density plot of variant allele frequency of all deleterious mutations in each histology and mutant-allele tumor heterogeneity scores were indicated respectively. The P value was calculated using the Kruskal-Wallis test. (E) ADC tended to have more multiclonal tumors (P = 0.07). The P value was calculated using a chi-square test for trend. (F) Subclonal KRAS codon 12 (G12C, G12D, and G12V) mutations were significantly associated with worse 10-year overall survival. The P value was calculated using a log-rank test. AIS = adenocarcinoma in situ; MIA = minimally invasive adenocarcinoma; MMR = mismatch repair.
Tumor Heterogeneity Analysis
Clonal expansion is a common mechanism of cancer progression. We evaluated clonal architecture by exploring mutant-allele tumor heterogeneity (MATH) as the ratio of the width to the center of the distribution of mutant-allele fractions among tumor-specific mutated loci (28). Although MATH score increased from AIS (MATH = 81.26) or MIA (MATH = 65.95) to ADC (MATH = 108.12) as a group (Figure 2D), it was not significantly associated with histology (P = 0.82, Kruskal–Wallis test), smoking status (P = 0.21; see Figure E3D), age (P = 0.79), stage (P = 0.98), sex (P = 0.45), tumor size (P = 0.14), or survival (P = 0.61). MATH score tended to be associated with mutational burden (r = 0.19, P = 0.06). We further estimated the number and size of clonal populations in 70 tumors based on cancer cell fraction distribution using a Bayesian mixture model (37) and identified 31 multiclonal tumors and 39 monoclonal tumors. The clonality of 32 tumors was not determined because of insufficient number of mutations or insufficient sequencing depth (see Table E13). Although the proportion of multiclonal tumors was highest in ADC (54%, P = 0.07, chi-square test for trend; Figure 2E), the proportion of subclonal mutations was not significantly different for AIS (40%), MIA (30%), and ADC (38%, P = 0.45, chi-square test for trend; Figure E3F), indicating that such complex clonal branching not only occurs before the diagnosis of AIS or MIA but also might be associated with disease progression. No significant association was found between mutational frequency and the clonality of tumors. Nonetheless, subclonal KRAS G12C, G12D, and G12V mutations were significantly associated with worse OS after adjusting for age, sex, and histology (P = 0.03; hazard ratio = 2.35; Figure 2F and Table E14). In contrast, subclonal EGFR mutations were not significantly associated with OS (see Figure E3G). The majority of these were subclonal L858R mutations (see Figure E3H). Together, these findings support the hypothesis that clonality of driver genes and tumor heterogeneity may assist in the design of combinatorial therapy (38).
Mutations Correlated with OS
Kaplan-Meier survival analysis showed that histology was significantly associated with 10-year OS when comparing ADC with AIS/MIA combined (P = 0.03, log-rank test; Figure 3A). A univariable Cox regression analysis revealed that age, sex, lymphovascular invasion status (LVI), and stage were also significantly associated with OS (see Table E15). In a multivariable model, only age and LVI status remained significant (see Table E16). When examining the association between mutations and OS using an elastic-net penalized regression model (39), eight genes with nonzero contribution to the overall risk of death were selected for further Kaplan-Meier analysis (Table E17 and Figure E4A). Five gene mutations (Gene5) including PIK3CG, ATM, EPPK1, EP300, and KMT2C were associated with worse OS, and three gene mutations (Gene3) including ATR, KDM6A, and POLQ were associated with better OS. Patients with the Gene5 mutation set (n = 28) had significantly worse survival compared with the rest of the population (P = 0.02; Figures 3B and E4D). This association with worse survival was independent of the three known driver mutations: EGFR, KRAS, and TP53 (P = 0.001; Figure E4B). In a multivariable Cox proportional hazards model, patients with only Gene5 mutations (n = 28) had the poorest OS after adjusting for age, sex, LVI, and histology (P = 8.58 × 105; Figure E4C and Table E18). When excluding patients with TP53, KRAS, or EGFR mutations (n = 18), the combined effect of the Gene5 mutation set on worse OS (n = 10) remained significant in multivariable Cox proportional hazards models (Figure E4D and Table E19). Among those 10 patients, 4 had AIS (40%), 2 had MIA (20%), and 4 had ADC (40%). These results suggest that the Gene5 mutation set might represent critical early driver events promoting tumor progression independent of both histology and the known drivers TP53, KRAS, and EGFR. We also examined the association of significantly altered pathways with OS. A total of 10 pathways were found to be progressively altered from AIS/MIA to ADC (Figure 3C and Table E20), including several well-known oncogenic pathways such as p53 (hsa04115), NF-κβ (hsa04064), cell cycle (hsa04310), and Jak-Stat pathways, four of which were also associated with worse OS in univariable Cox proportional hazards models (see Table E21). These results further underscored pivotal roles for these pathways in lung cancer progression (30).
Figure 3.
Somatic mutations correlated with overall survival. (A) Patients with adenocarcinoma (ADC) had worse outcome compared with ADC in situ/minimally invasive ADC combined (P = 0.03). P values were calculated using a log-rank test. (B) Tumors harboring mutations in any of the Gene5 set (PIK3CG, ATM, EPPK1, EP300, and KMT2C) but not the Gene3 set (ATR, KDM6A, and POLQ) (n = 28) were associated with worse 10-year overall survival compared with the rest of the population (P = 0.02) (n = 74). The P value was calculated using a log-rank test. (C) Among 10 progressively altered pathways in all tumors, 4 pathways including TP53, NF-κβ, cell cycle, and Jak-Stat (denoted by an asterisk) were significantly altered and associated with worse survival (see Table E21). False discovery rates shown were calculated using a binomial test. AIS = adenocarcinoma in situ; FDR = false discovery rate; MIA = minimally invasive adenocarcinoma.
Cancer Evolution Model in Early ADC
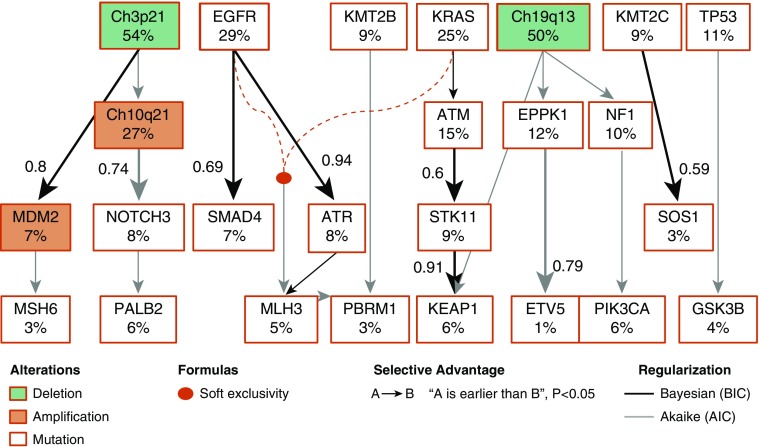
To gain further insights into the temporal heterogeneity of somatic alterations in early ADC evolution, we applied the cancer inference (PiCnIc) approach (40) to our 102 tumors. We selected 59 significant mutations present in all histologic subtypes and four significant CNVs associated with inferior OS, including Chr3p21, Chr10q21, Chr19q13, and MDM2 (see Table E22), for analysis. This approach combines techniques for sample stratification, driver selection, and identification of fitness-equivalent exclusive alterations to exploit an algorithm based on Suppes’s probabilistic causation (41). Our model captured well-known early drivers including EGFR, KRAS, and chromosomal 3p21 and 19q13 deletions. We confirmed newly reported co-occurring mutations between STK11 and KEAP1 (42, 43) (Figure E5D) that occur in a specific order following KRAS (nonparametric bootstrap score [NPB] 60%) and ATM (NPB 42%) mutations (Figures 4 and E5A) with an NPB (91%). The selection of ATR mutation by EGFR (NPB 94%) was also confirmed. Both ATM and ATR are attractive therapeutic targets in cancer therapy, and such co-occurring genomic alterations may identify subgroups of EGFR/KRAS-mutant lung tumors with distinct biology and therapeutic vulnerabilities (43). Our evolutionary trajectories also revealed possible EPPK1, KMT2C, KMT2D, NOTCH3, and NF1 mutations as early events in the progression of lung ADC, two of which, together with ATM mutation, were associated with OS in our cohort, pointing to potential novel therapeutic targets. Furthermore, when we tested this model in AIS/MIA and ADC separately (see Figures E5B and E5C), we found that the likelihood of KEAP1 selection by STK11 (NPB 93%) and ATM selection by KRAS (NPB 89%) were enhanced in ADC. Similarly, the selection of SMAD4 mutation by EGFR more likely occurs in ADC (NPB 95%; see Figure E5C) as opposed to AIS/MIA (NPB 31% via KMT2C; see Figure E5B), suggesting a functional association between SMAD4 and EGFR in ADC progression (44, 45).
Figure 4.
Evolution model of early adenocarcinoma. A simplified version of the model identified by the CAPRI algorithm with its default parameters is shown. Both Akaike and Bayesian information criteria were used for regularization to prevent overfitting. The complete input data set shown in Figure E5A contains all tumors harboring 59 significantly mutated genes plus 4 significantly altered copy number variants. Deletion events are marked by green rectangles. Amplification events are marked by red circles. The rest is shown using mutation events. The frequencies of mutations are also shown. Mutually exclusive events (EGFR and KRAS mutations) are displayed in expanded form; their events are connected by dashed lines, with colors representing the type of exclusivity. The nonparametric bootstrap scores >0.5 are shown. This model captures both the current knowledge about adenocarcinoma progression (i.e., association of ATR and EGFR, KEAP1 with STK11) and novel testable hypotheses (association of SOS1 with KMT2C mutation or SMAD4 with EGFR mutation). AIC = Akaike information criterion; BIC = Bayesian information criterion.
Discussion
The number of lung cancers that are detected early, most of which are ADC, is increasing following the successful detection by chest computed tomography–based lung cancer screening in high-risk populations (8, 46). Early lung ADC poses a difficult clinical challenge because we cannot easily distinguish indolent from aggressive cancers (6). This study represents the largest analysis so far of in situ and early ADC lesions using targeted sequencing. We identified common patterns of genomic alterations to AIS, MIA, and ADC, including a tendency for amplifications to increase with worsening histologic grade. Our work uncovered molecular features associated with aggressive early ADC clinical behavior. We demonstrated several sequences of driver events, driver gene clonality, and gene signatures associated with the progression of lung ADC. Previous studies may not have discovered these features because of smaller sample sizes (20–22). Our work may have future implications for the management of early ADC of the lung, including selection of patients for adjuvant therapy. However, validation of our findings in another independent cohort is required before any clinical applications can be considered.
We observed that most genomic alterations found in ADC are already present in AIS. This work confirms (20–22) and expands upon other studies. Although tumors displayed heterogeneous molecular profiles, an unsupervised cluster analysis of mutations in our assay does not allow differentiation among AIS, MIA, and ADC (see Figure E6). In contrast, we found that tumor mutation burden and proportion of copy number gains increases with increasing histologic grade (Figures 1A and 1D). Interestingly, 21 significantly mutated genes including EGFR, KRAS, and TP53 were shared among three groups, suggesting these mutations were early events and may have a stepwise role in malignant transition. We further speculated that these genes conferred a selective advantage during lung ADC development and demonstrated this in an ensemble-level progression model using phylogenetic analysis. Our model was able to infer the role of many known events in lung ADC progression (e.g., EGFR, KRAS, or TP53) and the selection of KEAP1 by STK11. This model also introduced several new players in early lung ADC development, such as EPPK1, ATM, SMAD4, KMT2C, and KMT2D, that warrant further investigation. In particular, mutations in five genes—PIK3CA, ATM, EPPK1, EP300, and KMT2C—together predicted inferior survival in 10 patients even in the absence of EGFR, KRAS, and TP53 mutations (Figure E3B). In contrast, none of the 10 patients having ATR, KDM6A or POLQ mutations without concurrent Gene5 mutations died. Predicting clinical behavior is a rich area of investigation; although many molecular candidates have been proposed, none have proven clinical utility (47). Because these mutations were not associated with histology in 20 of the patients, our findings suggest that careful molecular stratification of disease may provide better insights into disease management and needs further validation in a large cohort.
The pattern of mutational process identified in our cohort was not associated with histology except the APOBEC signature, which seemed to be associated with ADC and worse survival compared with the MMR signature (Figure 2B). This finding suggested that multiple somatic mutational processes could occur simultaneously or early during ADC progression and that the APOBEC signature might represent an important mutational process associated with poor disease progression. This observation parallels the APOBEC mutational signature in myeloma and breast cancer associated with poor prognosis (48). In contrast, the MMR signature might be an early event associated with better survival (26, 49). Clonal diversity was not significantly associated with either histology (Figures 2C and 2D) or mutation signature (Figure E7), even though ADC tended to have more intratumoral heterogeneity (Figure 2E). These findings together further emphasize the hypothesis that substantial heterogeneity could happen before the diagnosis of AIS (21). In addition, we found that subclonal KRAS codon 12 mutation is strongly associated with worse OS (Figure 2F), supporting the results obtained by others (50, 51). Our findings suggested that specific codon 12 mutations are associated with persistent activation of the pathway and predict aggressive tumor behavior. Importantly, the survival difference is greatly pronounced in early stage disease and thus puts further emphasis on its clinical significance.
Our study’s limitations include a small number of ADC patients for subgroup analyses and a single-sample design for the investigation of clonality. Intratumoral heterogeneity calls for caution in developing an evolutionary model from such cross-sectional samples. Unlike previous studies (20), we were not able to study malignant transition because we did not study alveolar atypical hyperplasia, a presumed precursor lesion of AIS. In addition, our cohort did not include multiple specimens acquired from the same tumor to capture spatial heterogeneity. The ideal tissue specimens would require multiple samples from each preinvasive tumor—a difficult task in the clinic. Although our study was conducted in a relatively large sample set of AIS/MIA, a greater number of preinvasive lesions would improve the statistical power to extend our findings. Because our study was exploratory rather than confirmatory, we did not emphasize controlling type I error using multiple comparisons. This may have led to missed clinically significant findings. Nonetheless, the mutations that were associated with OS reported in this article warrant validation. Another shortcoming was that matched normal samples were not available for our targeted sequencing. This tumor-only mutation-detection approach may have led to lower precision but similar sensitivity (52). Matched tumor/normal mutation detection would have been a better way to accurately call novel mutations. The presence of subclonality for major drivers in lung ADC and its clinical significance is a debatable subject (53, 54). Detection of subclonal mutations of cancer-driver genes is common practice but still presents strong statistical challenges (55, 56). Finally, these analyses do not address tumor stromal signaling alterations driven by the microenvironment and the immune system in the unique genetic landscape of these early ADCs.
Conclusions
Our results demonstrate several sequences of genetic driver events, clonality, and mutated gene signatures associated with outcome in early lung ADC, with potential future implications for the management of early ADC. Our study provides new insights into cancer genomic evolution, helps elucidate the interplay of somatic mutation and the adaptation of clones, and brings new insights into the key distinction between indolent and aggressive tumor behavior. The implication of these findings could be applied to targeted therapy using tissue-derived biomarkers. Our work supports the need for detailed genomic analysis of early lung ADC to identify those more likely to progress with aggressive behavior and to avoid overtreatment of indolent tumors.
Supplementary Material
Acknowledgments
Acknowledgment
The Vanderbilt Technologies for Advanced Genomics (VANTAGE) laboratory provided technical assistance for this work. The authors thank Alex Camai for diligent proofreading of the text.
Footnotes
Supported by research funding awards to C.A.P. (CA163772) and P.P.M. (CA196405). The Vanderbilt VANTAGE Core provided technical assistance. VANTAGE is supported in part by CTSA Grant 5 UL1 RR024975–03, the Vanderbilt Ingram Cancer Center (P30 CA68485), the Vanderbilt Vision Center (P30 EY08126), and NIH/NCRR (G20 RR030956).
Author Contributions: C.A.P., A.C.B., and P.P.M. initiated and conceived the study. P.P.M. supervised the study. A.C.B. provided clinical data and pathology review of the samples. J.Q. and S.Z. performed bioinformatics analyses. J.Q. and H.C. performed statistical analyses. Y.Z., S.M.J.R., and M.-F.S. collected samples and extracted DNA. T.S. designed and performed targeted sequencing. J.Q. and P.P.M. wrote the manuscript. J.Q., C.A.P., A.C.B., and P.P.M. proofed the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201902-0294OC on November 20, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Meza R, ten Haaf K, Kong CY, Erdogan A, Black WC, Tammemagi MC, et al. Comparative analysis of 5 lung cancer natural history and screening models that reproduce outcomes of the NLST and PLCO trials. Cancer. 2014;120:1713–1724. doi: 10.1002/cncr.28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spira A, Halmos B, Powell CA. Update in lung cancer 2015. Am J Respir Crit Care Med. 2016;194:661–671. doi: 10.1164/rccm.201604-0898UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J. Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas R, Gao S, Cultraro CM, Maity TK, Venugopalan A, Abdullaev Z, et al. Genomic profiling of multiple sequentially acquired tumor metastatic sites from an “exceptional responder” lung adenocarcinoma patient reveals extensive genomic heterogeneity and novel somatic variants driving treatment response. Cold Spring Harb Mol Case Stud. 2016;2:a001263. doi: 10.1101/mcs.a001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Maldonado F, Duan F, Raghunath SM, Rajagopalan S, Karwoski RA, Garg K, et al. Noninvasive computed tomography-based risk stratification of lung adenocarcinomas in the national lung screening trial. Am J Respir Crit Care Med. 2015;192:737–744. doi: 10.1164/rccm.201503-0443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillerdal G. Indolent lung cancers: time for a paradigm shift. A review. J Thorac Oncol. 2008;3:208–211. doi: 10.1097/JTO.0b013e3181653ce3. [DOI] [PubMed] [Google Scholar]
- 8.Gould MK, Tang T, Liu IL, Lee J, Zheng C, Danforth KN, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192:1208–1214. doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 9.Dama E, Melocchi V, Dezi F, Pirroni S, Carletti RM, Brambilla D, et al. An aggressive subtype of stage I lung adenocarcinoma with molecular and prognostic characteristics typical of advanced lung cancers. Clin Cancer Res. 2017;23:62–72. doi: 10.1158/1078-0432.CCR-15-3005. [DOI] [PubMed] [Google Scholar]
- 10.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369:245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patz EF, Jr, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, et al. NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174:269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thalanayar PM, Altintas N, Weissfeld JL, Fuhrman CR, Wilson DO. Indolent, potentially inconsequential lung cancers in the Pittsburgh Lung Screening Study. Ann Am Thorac Soc. 2015;12:1193–1196. doi: 10.1513/AnnalsATS.201412-577OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanton C, Govindan R. Clinical implications of genomic discoveries in lung cancer. N Engl J Med. 2016;374:1864–1873. doi: 10.1056/NEJMra1504688. [DOI] [PubMed] [Google Scholar]
- 14.Yanagawa N, Shiono S, Abiko M, Ogata SY, Sato T, Tamura G. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg. 2014;98:453–458. doi: 10.1016/j.athoracsur.2014.04.108. [DOI] [PubMed] [Google Scholar]
- 15.Boland JM, Froemming AT, Wampfler JA, Maldonado F, Peikert T, Hyland C, et al. Adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive pulmonary adenocarcinoma: analysis of interobserver agreement, survival, radiographic characteristics, and gross pathology in 296 nodules. Hum Pathol. 2016;51:41–50. doi: 10.1016/j.humpath.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Wilson DO, Ryan A, Fuhrman C, Schuchert M, Shapiro S, Siegfried JM, et al. Doubling times and CT screen–detected lung cancers in the Pittsburgh Lung Screening Study. Am J Respir Crit Care Med. 2012;185:85–89. doi: 10.1164/rccm.201107-1223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [Published erratum appears in Nat Commun 5:4644.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borczuk AC, Sole M, Lu P, Chen J, Wilgus ML, Friedman RA, et al. Progression of human bronchioloalveolar carcinoma to invasive adenocarcinoma is modeled in a transgenic mouse model of K-ras-induced lung cancer by loss of the TGF-β type II receptor. Cancer Res. 2011;71:6665–6675. doi: 10.1158/0008-5472.CAN-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivakumar S, Lucas FAS, McDowell TL, Lang W, Xu L, Fujimoto J, et al. Genomic landscape of atypical adenomatous hyperplasia reveals divergent modes to lung adenocarcinoma. Cancer Res. 2017;77:6119–6130. doi: 10.1158/0008-5472.CAN-17-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izumchenko E, Chang X, Brait M, Fertig E, Kagohara LT, Bedi A, et al. Targeted sequencing reveals clonal genetic changes in the progression of early lung neoplasms and paired circulating DNA. Nat Commun. 2015;6:8258. doi: 10.1038/ncomms9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinayanuwattikun C, Le Calvez-Kelm F, Abedi-Ardekani B, Zaridze D, Mukeria A, Voegele C, et al. Elucidating genomic characteristics of lung cancer progression from in situ to invasive adenocarcinoma. Sci Rep. 2016;6:31628. doi: 10.1038/srep31628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park E, Ahn S, Kim H, Park SY, Lim J, Kwon HJ, et al. Targeted sequencing analysis of pulmonary adenocarcinoma with multiple synchronous ground-glass/lepidic nodules. J Thorac Oncol. 2018;13:1776–1783. doi: 10.1016/j.jtho.2018.07.097. [DOI] [PubMed] [Google Scholar]
- 23.Qian JZS, Zou Y, Rahman SJ, Stricker T, Chen H, Powell CA, et al. Genomic underpinnings of tumor behavior in early lung adenocarcinoma [abstract] Am J Respir Crit Care Med. 2018;197:A7091. doi: 10.1164/rccm.201902-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massion PP, Kuo WL, Stokoe D, Olshen AB, Treseler PA, Chin K, et al. Genomic copy number analysis of non-small cell lung cancer using array comparative genomic hybridization: implications of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2002;62:3636–3640. [PubMed] [Google Scholar]
- 25.International HapMap Consortium. The international HapMap project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 26.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [Published erratum appears in Nature 502:258.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blokzijl F, Janssen R, van Boxtel R, Cuppen E. MutationalPatterns: comprehensive genome-wide analysis of mutational processes. Genome Med. 2018;10:33. doi: 10.1186/s13073-018-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mroz EA, Tward AD, Hammon RJ, Ren Y, Rocco JW. Intra-tumor genetic heterogeneity and mortality in head and neck cancer: analysis of data from the Cancer Genome Atlas. PLoS Med. 2015;12:e1001786. doi: 10.1371/journal.pmed.1001786. [Published erratum appears in PLoS Med 12:e1001818, e1001844.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Sano L, Caravagna G, Ramazzotti D, Graudenzi A, Mauri G, Mishra B, et al. TRONCO: an R package for the inference of cancer progression models from heterogeneous genomic data. Bioinformatics. 2016;32:1911–1913. doi: 10.1093/bioinformatics/btw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramazzotti D, Caravagna G, Olde Loohuis L, Graudenzi A, Korsunsky I, Mauri G, et al. CAPRI: efficient inference of cancer progression models from cross-sectional data. Bioinformatics. 2015;31:3016–3026. doi: 10.1093/bioinformatics/btv296. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Perez A, Lopez-Bigas N. Functional impact bias reveals cancer drivers. Nucleic Acids Res. 2012;40:e169. doi: 10.1093/nar/gks743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamborero D, Gonzalez-Perez A, Lopez-Bigas N. OncodriveCLUST: exploiting the positional clustering of somatic mutations to identify cancer genes. Bioinformatics. 2013;29:2238–2244. doi: 10.1093/bioinformatics/btt395. [DOI] [PubMed] [Google Scholar]
- 35.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [Published erratum appears in Nature 514:262; 559:E12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 37.Miller CA, White BS, Dees ND, Griffith M, Welch JS, Griffith OL, et al. SciClone: inferring clonal architecture and tracking the spatial and temporal patterns of tumor evolution. PLoS Comput Biol. 2014;10:e1003665. doi: 10.1371/journal.pcbi.1003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jamal-Hanjani M, Quezada SA, Larkin J, Swanton C. Translational implications of tumor heterogeneity. Clin Cancer Res. 2015;21:1258–1266. doi: 10.1158/1078-0432.CCR-14-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon N, Friedman J, Hastie T, Tibshirani R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J Stat Softw. 2011;39:1–13. doi: 10.18637/jss.v039.i05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caravagna G, Graudenzi A, Ramazzotti D, Sanz-Pamplona R, De Sano L, Mauri G, et al. Algorithmic methods to infer the evolutionary trajectories in cancer progression. Proc Natl Acad Sci USA. 2016;113:E4025–E4034. doi: 10.1073/pnas.1520213113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suppes P. A probabilistic theory of causality. Amsterdam, the Netherlands: North-Holland; 1970. [Google Scholar]
- 42.Arbour KC, Jordan E, Kim HR, Dienstag J, Yu HA, Sanchez-Vega F, et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res. 2018;24:334–340. doi: 10.1158/1078-0432.CCR-17-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5:860–877. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blakely CM, Watkins TBK, Wu W, Gini B, Chabon JJ, McCoach CE, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet. 2017;49:1693–1704. doi: 10.1038/ng.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozawa H, Ranaweera RS, Izumchenko E, Makarev E, Zhavoronkov A, Fertig EJ, et al. SMAD4 loss is associated with cetuximab resistance and induction of MAPK/JNK activation in head and neck cancer cells. Clin Cancer Res. 2017;23:5162–5175. doi: 10.1158/1078-0432.CCR-16-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wistuba II, Behrens C, Lombardi F, Wagner S, Fujimoto J, Raso MG, et al. Validation of a proliferation-based expression signature as prognostic marker in early stage lung adenocarcinoma. Clin Cancer Res. 2013;19:6261–6271. doi: 10.1158/1078-0432.CCR-13-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker BA, Wardell CP, Murison A, Boyle EM, Begum DB, Dahir NM, et al. APOBEC family mutational signatures are associated with poor prognosis translocations in multiple myeloma. Nat Commun. 2015;6:6997. doi: 10.1038/ncomms7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H, Thienpont B, Yesilyurt BT, Moisse M, Reumers J, Coenegrachts L, et al. ANECS. Mismatch repair deficiency endows tumors with a unique mutation signature and sensitivity to DNA double-strand breaks. eLife. 2014;3:e02725. doi: 10.7554/eLife.02725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ihle NT, Byers LA, Kim ES, Saintigny P, Lee JJ, Blumenschein GR, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. 2012;104:228–239. doi: 10.1093/jnci/djr523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon E, Bick T, Sarji S, Shentzer T, Prinz E, Yehiam L, et al. Clinically significant sub-clonality for common drivers can be detected in 26% of KRAS/EGFR mutated lung adenocarcinomas. Oncotarget. 2017;8:45736–45749. doi: 10.18632/oncotarget.17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teer JK, Zhang Y, Chen L, Welsh EA, Cress WD, Eschrich SA, et al. Evaluating somatic tumor mutation detection without matched normal samples. Hum Genomics. 2017;11:22. doi: 10.1186/s40246-017-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGranahan N, Favero F, de Bruin EC, Birkbak NJ, Szallasi Z, Swanton C. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med. 2015;7:283ra54. doi: 10.1126/scitranslmed.aaa1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lan Y, Zhao E, Luo S, Xiao Y, Li X, Cheng S. Revealing clonality and subclonality of driver genes for clinical survival benefits in breast cancer. Breast Cancer Res Treat. 2019;175:91–104. doi: 10.1007/s10549-019-05153-8. [DOI] [PubMed] [Google Scholar]
- 56.Gerstung M, Papaemmanuil E, Campbell PJ. Subclonal variant calling with multiple samples and prior knowledge. Bioinformatics. 2014;30:1198–1204. doi: 10.1093/bioinformatics/btt750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequencing data of 102 tumors were deposited into dbGAP (phs001811.v1.p1). The processed and annotated sequencing data of 1,203 nonsynonymous variants in 102 samples are provided in Table E23.