Abstract
Rationale: Identification of younger adults at high risk of developing chronic obstructive pulmonary disease (COPD) could lead to implementation of preventive measures before disease onset and halt progression.
Objectives: To investigate the prevalence, characteristics, and prognosis of individuals with early COPD in the general population.
Methods: We investigated 105,630 randomly chosen adults from a Danish contemporary population-based cohort. Early COPD was defined as FEV1/FVC less than the lower limit of normal in individuals under 50 years of age with 10 pack-years or greater of tobacco consumption.
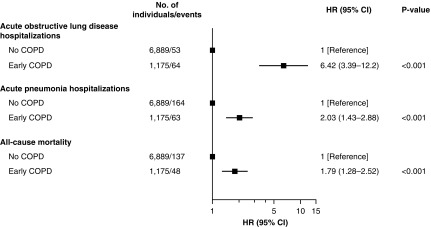
Measurements and Main Results: Among 8,064 individuals under 50 years of age with 10 pack-years or greater of tobacco consumption, 1,175 (15%) had early COPD, of whom 58% were current smokers. Individuals with early COPD more often had chronic respiratory symptoms, severe lung function impairment, asthma, and a history with bronchitis/pneumonia. During the 14.4-year follow-up, we observed 117 acute hospitalizations with obstructive lung disease, 227 acute hospitalizations with pneumonia, and 185 deaths among the 8,064 younger adults. Compared with individuals without COPD, those with early COPD had multivariable adjusted hazard ratios of 6.42 (95% confidence interval, 3.39–12.2) for acute obstructive lung disease hospitalizations, 2.03 (1.43–2.88) for acute pneumonia hospitalizations, and 1.79 (1.28–2.52) for all-cause mortality.
Conclusions: Among individuals under 50 years of age and 10 pack-years or greater of tobacco consumption from the general population, 15% fulfill criteria of early COPD. Individuals with early COPD more often have chronic respiratory symptoms and severe lung function impairment, and an increased risk of acute respiratory hospitalizations and early death.
Keywords: airway obstruction, diagnosis, FEV, chronic bronchitis, emphysema
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic obstructive pulmonary disease (COPD) develops gradually over many years. Identification of younger adults at high risk of developing COPD could lead to implementation of preventive measures before disease onset and halt progression. Recently, an operational definition of early COPD has been proposed by an international group of experts in individuals under 50 years of age with 10 pack-years or greater of tobacco consumption. This is the first study to investigate early COPD in the general population using the proposed operational definition.
What This Study Adds to the Field
Using a Danish contemporary population-based cohort with 105,630 randomly selected individuals and a follow-up time of up to 14.4 years, we investigated the prevalence, characteristics, and prognosis of individuals with early COPD in the general population. We estimated the prevalence of early COPD to be 15%, defined as FEV1/FVC less than the lower limit of normal in individuals under 50 years of age with 10 pack-years or greater of tobacco consumption. More than one-half of individuals with early COPD were active smokers. Already at baseline examination, these individuals more often reported chronic respiratory symptoms and had significant lung function impairment. During follow-up, individuals with early COPD displayed an increased risk of acute respiratory hospitalizations and early death.
Worldwide, chronic obstructive pulmonary disease (COPD) is a prevalent disease and one of the leading causes of morbidity and mortality, a scenario likely to persist for many years (1). One possible explanation for the poor prognosis may be that most patients with COPD are diagnosed and only start treatment late in the disease course, when a significant degree of airflow limitation is already present. Because COPD develops gradually over many years, identification of younger adults at high risk of developing COPD could lead to implementation of preventive measures before disease onset, and thereby halt progression to improve long-term prognosis (2–5).
Recently, an operational definition of early COPD has been proposed by an international group of experts (6). Accordingly, early COPD should be defined in individuals under 50 years of age with 10 pack-years or greater of tobacco consumption with one or more of the following: 1) FEV1/FVC less than the lower limit of normal (LLN); 2) compatible computed tomography (CT) abnormalities (i.e., visual emphysema, air trapping, or bronchial thickening graded mild or worse); and/or 3) evidence of accelerated FEV1 decline of 60 ml/yr or greater. No information is available on the impact of early COPD, and knowledge has been extrapolated from what is known as mild COPD (4).
In the present study, we investigated the prevalence, characteristics, and prognosis of individuals with early COPD in the general population. For this purpose, we used a Danish contemporary population-based cohort with 105,630 randomly selected individuals and a follow-up time of up to 14.4 years.
Methods
Study Design and Population
We recruited individuals aged 20–100 years from the Copenhagen General Population Study, a Danish contemporary population-based cohort initiated in November 26, 2003 with ongoing enrolment (7, 8). In the present study, we included 105,630 individuals with complete information on smoking and lung function recruited up to April 28, 2015. In Denmark, all individuals are assigned a unique identification number at birth or immigration in the national Danish Civil Registration System. Individuals living in the Capital Region of Denmark were randomly invited from the national Danish Civil Registration System to reflect the adult Danish population (response rate, 43%). All participants completed a questionnaire, underwent a physical examination, and provided blood for biochemical analyses. Questionnaires were reviewed at the day of attendance by a healthcare professional together with the participant. To avoid potential recall bias, questionnaires were completed and retrieved before the physical examination and blood analyses, and the participants were unaware of specific future studies and clinical outcomes. The study was approved by Herlev and Gentofte Hospital and the regional ethics committee (approval no. H-KF-01-144/01), and was conducted according to the Declaration of Helsinki. All participants provided written informed consent.
Early COPD
Date of birth and sex were obtained from the national Danish Civil Registration System. Information on smoking status and tobacco consumption was obtained from the questionnaire. Smoking status was defined as never, former, or current smoking. Cumulative tobacco consumption was calculated in pack-years based on information on age at smoking initiation and cessation (or, for current smokers, until age at baseline examination), duration of tobacco consumption, and amount of consumed tobacco in form of number of daily consumed cigarettes, cheroots, and cigars, and grams of weekly consumed pipe tobacco (one cheroot = 3 g of tobacco; one cigar = 5 g of tobacco; and one cigarette = 1 g of tobacco): a pack-year was defined as 20 cigarettes or equivalent smoked daily for 1 year. Thus, individuals not likely to have early COPD were excluded (i.e., aged ≥50 yr and tobacco consumption <10 pack-years).
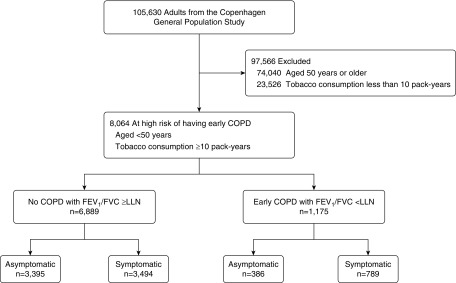
Prebronchodilator FEV1 and FVC were measured at the baseline examination. Spirometry use in the Copenhagen General Population Study has undergone a rigorous validation process (9). Predicted values were calculated according to national Danish lung function reference equations, which are based on 11,288 healthy, asymptomatic, never-smoking individuals, with age and height as covariates separately for men and women (9). The LLN, defined as the bottom fifth percentile of the predicted value, was calculated as the mean value minus 1.645 SD. Early COPD was defined as an FEV1/FVC less than LLN in individuals under 50 years of age with 10 pack-years or greater of tobacco consumption (Figure 1). Furthermore, individuals were subsequently stratified according to presence of at least one of the following chronic respiratory symptoms: chronic mucus hypersecretion; dyspnea; wheezing; and/or cough; Detailed descriptions of lung function procedures, chronic respiratory symptoms, and other characteristics are provided in the online supplement.
Figure 1.
Study population. Symptoms included chronic mucus hypersecretion, dyspnea, wheezing, and/or cough. COPD = chronic obstructive pulmonary disease; LLN = lower limit of normal.
Outcomes
Information on outcomes was obtained from Danish nationwide health registries. Main outcomes included acute hospitalizations due to obstructive lung disease (i.e., COPD and asthma) and pneumonia and all-cause mortality. Acute hospitalizations included all emergency department visits and hospital admissions with the primary discharge diagnosis of International Classification of Diseases (ICD)-10:J41-J46 for obstructive lung disease and ICD-10:J12-J18 for pneumonia. The information was available from the national Danish Patient Registry, which covers all public and private hospital contacts in Denmark, recorded from baseline until April 10, 2018. Information on vital status was available from the national Danish Civil Registration System, which contains date of death and emigration for all residents in Denmark, recorded from baseline until April 19, 2018.
Secondary outcomes included cause-specific mortality and comorbidities. A detailed description of secondary outcomes is provided in the online supplement.
As follow-up was done by combining the nationwide health registries with the national Danish Civil Registration System through the unique identification number provided to everyone at birth or immigration, no person was lost to follow-up, and individuals who emigrated were censored at the date of emigration (n = 452). All diagnoses recorded in the registries are made by a medical doctor according to national Danish laws using the World Health Organizations ICD codes.
Statistical Analyses
Wilcoxon’s rank-sum, Pearson’s χ2, and Fisher’s exact tests were used for group comparison. Cumulative incidences of acute hospitalization due to obstructive lung disease and pneumonia were determined with all-cause mortality and emigration as competing events using the method of Coviello and Boggess (10), and differences were assessed using the method of Pepe and Mori (11). Cumulative incidence for all-cause mortality was determined using the Kaplan-Meier estimator, and difference was assessed using a log-rank test. Cox proportional regression model with age as the underlying timescale (age adjustment) and left truncation (delayed entry) was used to determine risk of acute hospitalizations due to obstructive lung disease and pneumonia and of all-cause mortality. For acute hospitalizations, we performed multiple failure-time analysis using the Andersen-Gill approach (i.e., individuals were at risk of recurrent events) (12). To avoid counting a single event multiple times, we decided that hospitalized individuals during follow-up had to be clinically stable for at least 4 weeks after discharge before they were at risk for a subsequent event, in accordance with previous recommendations (13–16). Single failure-time analysis was used for all-cause and cause-specific mortality and for comorbidities. Analyses were adjusted for potential confounders, including age (as timescale), sex, smoking status, and pack-years of tobacco consumption. Stratified analyses in individuals with early COPD were undertaken to investigate potential prognostic predictors of acute hospitalizations and death; effect modification was assessed by using Wald’s test for interaction. In sensitivity analyses: 1) LLN was defined by calculating predicted values according to the Global Lung Initiative lung function reference equations (17); 2) individuals reporting asthma and/or treatment with airway medication were excluded; and 3) individuals with known interstitial lung disease were excluded. Prognosis of individuals with early COPD was also investigated by adjusting for presence of chronic respiratory symptoms, as chronic respiratory symptoms have been associated with increased risk of respiratory hospitalizations and death in individuals with normal spirometry (18). In addition, to bring a perspective to the clinical significance of the findings in individuals with early COPD, we also determined the prognosis of older individuals with COPD, defined as FEV1/FVC less than LLN in individuals aged 50 years or older with 10 pack-years or greater of tobacco consumption. Analyses were performed using STATA/SE 13.1 for Windows (StataCorp), and a two-sided P value less than 0.05 was considered significant.
Results
Prevalence
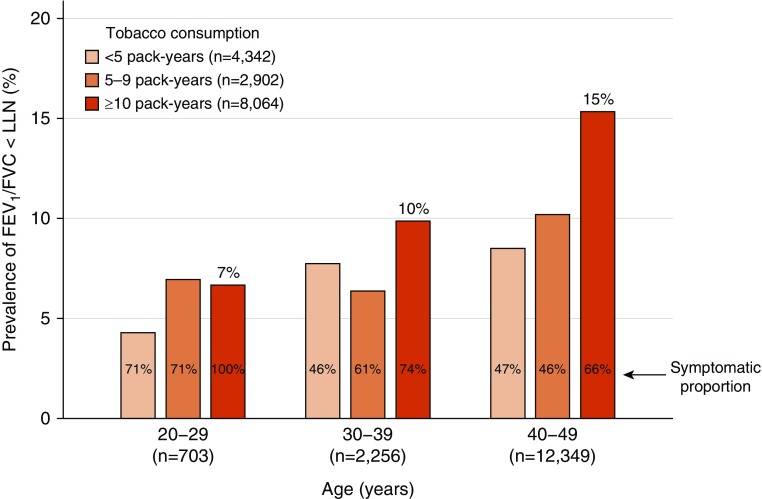
Among 105,630 individuals from the Copenhagen General Population Study, 8,064 (8%) were under 50 years of age with 10 pack-years or greater of tobacco consumption, of whom 1,175 (15%) had early COPD, defined as FEV1/FVC less than LLN (Figure 1). Among these 8,064 individuals at risk, early COPD was prevalent in 7% of those aged 20–29 years, 10% of those aged 30–39 years, and 15% of those aged 40–49 years (Figure 2). Interestingly, more than two-thirds of individuals with early COPD reported at least one chronic respiratory symptom. As expected, the prevalence of FEV1/FVC less than LLN was lower among smokers with fewer than 10 pack-years of tobacco consumption. For comparison, the prevalence of FEV1/FVC less than LLN was 6% in asymptomatic, never-smoking individuals under 50 years of age (722 out of 11,398 individuals).
Figure 2.
Prevalence of FEV1/FVC less than the lower limit of normal (LLN) according to age and amount of tobacco consumption. Included individuals are smokers under 50 years of age. Symptoms included chronic mucus hypersecretion, dyspnea, wheezing, and/or cough.
Characteristics
Individuals with early COPD were more often current smokers (58% vs. 46%) and had a higher tobacco consumption (22.5 pack-years vs. 18.8 pack-years), compared with those at risk, but without COPD (Table 1). A substantial proportion had lung function impairment with FEV1 less than 80% of predicted (40% vs. 9%), FEV1 less than 50% of predicted (3% vs. <1%), and FEV1/FVC less than 0.70 (75% vs. <1%); the latter observation being in line with the LLN criterion included in the early COPD definition. Furthermore, individuals with early COPD more often reported chronic respiratory symptoms, asthma (15% vs. 5%), treatment with airway medication (13% vs. 4%), and a history with bronchitis/pneumonia episodes (35% vs. 27%). Among individuals with early COPD, these characteristics were even more noticeable in the subgroup with FEV1 less than 80% of predicted (see Table E1 in the online supplement).
Table 1.
Baseline Characteristics according to Presence of Early COPD among Individuals under 50 Years of Age with 10 Pack-Years or Greater of Tobacco Consumption in the Copenhagen General Population Study
| No COPD (n = 6,889) | Early COPD (n = 1,175) | |
|---|---|---|
| General characteristics | ||
| Age, yr | 45.0 (42.0–47.6) | 45.9 (42.8–48.1)* |
| Sex, M, no. (%) | 3,181 (46) | 490 (42)* |
| BMI, kg/m2 | 25.7 (23.4–28.7) | 24.6 (22.5–27.3)* |
| FEV1 predicted, % | 97 (88–105) | 83 (74–92)* |
| FVC predicted, % | 99 (90–107) | 101 (90–111)* |
| FEV1/FVC | 0.79 (0.76–0.83) | 0.68 (0.65–0.70)* |
| Current smokers, no. (%) | 3,167 (46) | 678 (58)* |
| Tobacco consumption, pack-years | 18.8 (13.5–26.0) | 22.5 (15.0–30.0)* |
| Age at smoking initiation, yr | 15.0 (14.0–17.0) | 15.0 (14.0–17.0)* |
| Poor socioeconomic status, no. (%) | 1,123 (16) | 233 (20)* |
| Clinical characteristics, no. (%) | ||
| FEV1 < LLN of predicted | 638 (9) | 492 (42)* |
| FEV1 < 80% of predicted | 596 (9) | 471 (40)* |
| FEV1 < 50% of predicted | 2 (<1) | 32 (3)* |
| FEV1/FVC < 0.70 | 2 (<1) | 879 (75)* |
| Chronic mucus hypersecretion | 722 (10) | 234 (20)* |
| Dyspnea | 2,307 (33) | 538 (46)* |
| mMRC ≥2 | 454 (7) | 124 (11)* |
| Night-time dyspnea | 293 (4) | 108 (9)* |
| Wheezing | 1,867 (27) | 543 (46)* |
| Cough | 1,339 (19) | 378 (32)* |
| First-degree relative with asthma | 1,347 (20) | 336 (29)* |
| Childhood asthma/allergy | 1,259 (18) | 280 (24)* |
| Asthma | 354 (5) | 180 (15)* |
| Treatment with airway medication | 245 (4) | 155 (13)* |
| Physically inactive | 763 (11) | 162 (14)* |
| Bronchitis/pneumonia episodes in the last 10 yr | 1,868 (27) | 414 (35)* |
| General practitioner visits in the last 12 mo | 5,280 (77) | 947 (81)* |
| Alpha-1 antitrypsin < 1 g/L | 648 (9) | 107 (9) |
| Blood eosinophils ≥ 300 cells/μl | 1,304 (19) | 254 (22)* |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; LLN = lower limit of normal; mMRC = modified Medical Research Council dyspnea scale.
Data presented as median (25th–75th percentiles) or number (%). Early COPD was defined as FEV1/FVC less than LLN.
P < 0.05 for comparison with individuals without COPD, obtained from Wilcoxon’s rank-sum, Pearson’s χ2 tests, or Fischer’s exact test.
When individuals with and without early COPD were stratified according to presence of chronic respiratory symptoms, symptomatic individuals without COPD were comparable to symptomatic individuals with early COPD; however, the latter group had markedly lower lung function and a higher prevalence of asthma and treatment with airway medication (Table E2). Similarly, asymptomatic individuals with and without early COPD were comparable except for lung function parameters.
Prognosis
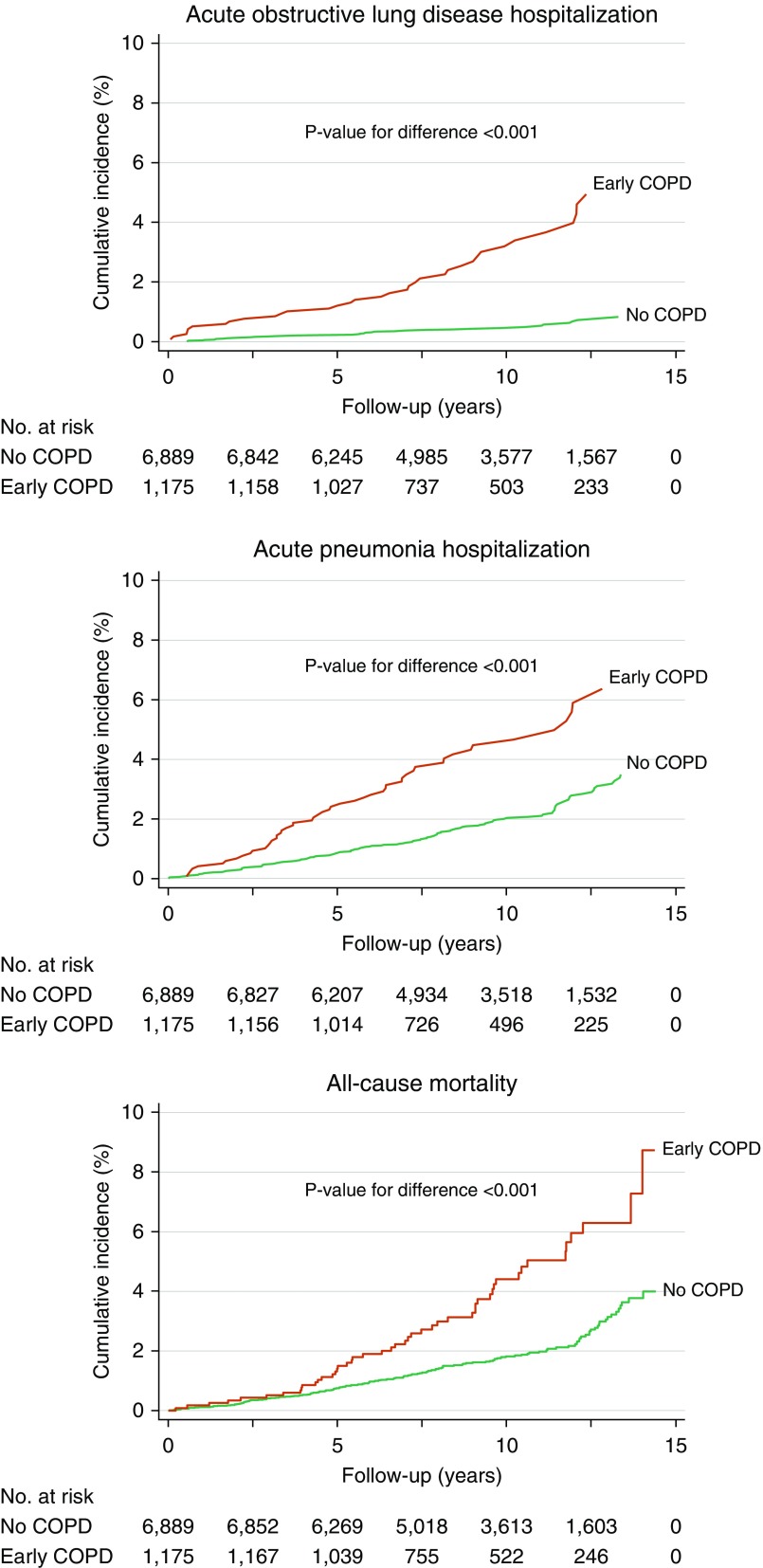
During up to 14.4 years of follow-up (median, 10.1 yr), we observed 117 acute hospitalizations with obstructive lung disease, 227 acute hospitalizations with pneumonia, and 185 deaths among the 8,064 younger adults at risk of early COPD. After a short follow-up time, individuals with early COPD displayed a higher incidence of acute hospitalizations with obstructive lung disease and pneumonia compared with those without COPD (Figure 3). No difference was observed during the first 4 years of follow-up with regard to incidence of all-cause mortality, followed by an increase in individuals with early COPD. Compared with individuals without COPD, individuals with early COPD had multivariable adjusted hazard ratios (HRs) of 6.42 (95% confidence interval [CI], 3.39–12.2) for acute obstructive lung disease hospitalizations, 2.03 (1.43–2.88) for acute pneumonia hospitalizations, and 1.79 (1.28–2.52) for all-cause mortality (Figure 4).
Figure 3.
Cumulative incidence of acute hospitalizations due to obstructive lung disease and pneumonia and of death in individuals with and without early chronic obstructive pulmonary disease (COPD). Included individuals are under 50 years of age with 10 pack-years or greater of tobacco consumption. Early COPD was defined as FEV1/FVC less than the lower limit of normal.
Figure 4.
Risk of acute hospitalizations due to obstructive lung disease and pneumonia and of death in individuals with and without early chronic obstructive pulmonary disease (COPD). Included individuals are under 50 years of age with 10 pack-years or greater of tobacco consumption. Early COPD was defined as FEV1/FVC less than the lower limit of normal. Analyses were adjusted for age (as timescale), sex, smoking status, and pack-years of tobacco consumption. CI = confidence interval; HR = hazard ratio.
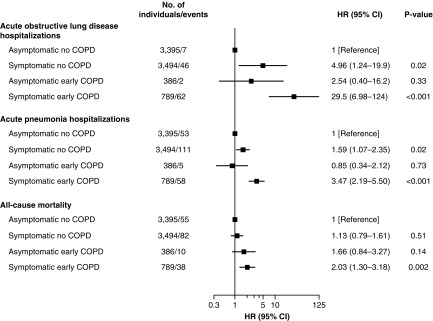
When individuals with and without early COPD were stratified according to presence of chronic respiratory symptoms, symptomatic individuals without COPD had an increased risk of acute hospitalizations, but not of death, whereas symptomatic individuals with early COPD had an increased risk of all outcomes (Figure 5). Asymptomatic individuals with and without early COPD did not differ with regard to prognosis. Compared with asymptomatic individuals without COPD, multivariable adjusted HRs for acute obstructive lung disease hospitalizations were 4.96 (95% CI, 1.24–19.9) in symptomatic individuals without COPD, 2.54 (0.40–16.2) in asymptomatic individuals with early COPD, and 29.5 (6.98–124) in symptomatic individuals with early COPD. Corresponding HRs were 1.59 (1.07–2.35), 0.85 (0.34–2.12), and 3.47 (2.19–5.50) for acute pneumonia hospitalizations and 1.13 (0.79–1.61), 1.66 (0.84–3.27), and 2.03 (1.30–3.18) for all-cause mortality, respectively. However, there was no clear statistical evidence of effect modification between early COPD and chronic respiratory symptoms on risk of acute obstructive lung disease hospitalizations (P value for interaction = 0.39), acute pneumonia hospitalizations (P value for interaction = 0.06), or of all-cause mortality (P value for interaction = 0.84) (Figure 6).
Figure 5.
Risk of acute hospitalizations due to obstructive lung disease and pneumonia and of death in symptomatic and asymptomatic individuals with and without early chronic obstructive pulmonary disease (COPD). Included individuals are under 50 years of age with 10 pack-years or greater of tobacco consumption. Early COPD was defined as FEV1/FVC less than the lower limit of normal. Symptoms included chronic mucus hypersecretion, dyspnea, wheezing, and/or cough. Analyses were adjusted for age (as timescale), sex, smoking status, and pack-years of tobacco consumption. CI = confidence interval; HR = hazard ratio.
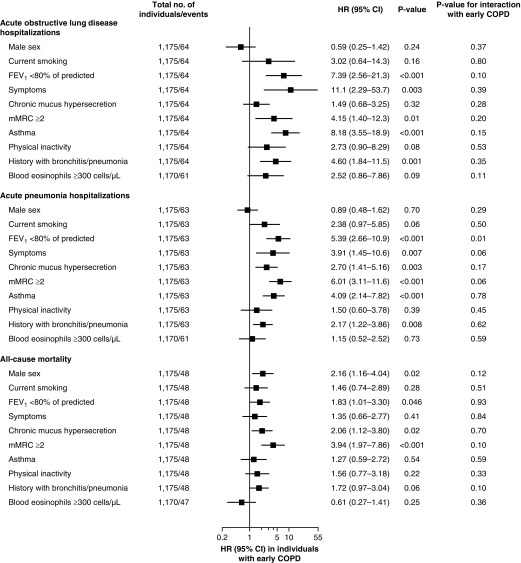
Figure 6.
Risk of acute hospitalizations due to obstructive lung disease and pneumonia and of death in individuals with early chronic obstructive pulmonary disease (COPD). Early COPD was defined as FEV1/FVC less than the lower limit of normal in individuals under 50 years of age with 10 pack-years or greater of tobacco consumption. Analyses were adjusted for age (as timescale), sex, smoking status, and pack-years of tobacco consumption. Symptoms included chronic mucus hypersecretion, dyspnea, wheezing, and/or cough. P value for interaction with covariates was calculated in individuals under 50 years of age with 10 pack-years or greater of tobacco consumption with and without early COPD on risk of acute hospitalizations and death. CI = confidence interval; HR = hazard ratio; mMRC = modified Medical Research Council dyspnea scale.
Stratified analyses in individuals with early COPD revealed that presence of FEV1 less than 80% of predicted, chronic respiratory symptoms, asthma, and a history with bronchitis/pneumonia were mainly associated with increased risk of acute hospitalizations and early death (Figure 6). Effect modification with early COPD was only observed for FEV1 less than 80% of predicted on risk of acute pneumonia hospitalizations (all other P values for interaction ≥ 0.05).
Because we recorded a relatively low number of deaths in this young population, we were limited in investigating cause-specific mortality. We observed that individuals with early COPD had an increased risk of deaths from other causes than respiratory disease, cardiovascular disease, or cancer compared with those without COPD (Figure E1). Nonetheless, symptomatic individuals with early COPD had an increased risk of respiratory mortality compared with asymptomatic individuals without COPD, with a multivariable adjusted HR of 11.4 (95% CI, 1.13–116) (Figure E2). We observed no differences between individuals with versus those without early COPD, regardless of the presence of symptoms, with regard to risk of ischemic heart disease, heart failure, lung cancer, or any cancer (Figures E3 and E4).
Sensitivity Analyses and Comparison with Older Individuals with COPD
Results were similar after redefining LLN by calculating predicted values according to the Global Lung Initiative lung function reference equations (compare Figures 4 and 5 with Figures E5 and E6). Results were also largely similar after exclusion of individuals reporting asthma and/or treatment with airway medication (compare Figures 4 and 5 with Figures E7 and E8); however, results for symptomatic individuals without COPD were attenuated and no longer associated with risk of acute hospitalizations. Results were likewise similar after exclusion of individuals with interstitial lung disease (compare Figures 4 and 5 with Figures E9 and E10). Finally, results were similar after adjusting for presence of chronic respiratory symptoms (compare Figure 4 with Figure E11). Interestingly, after adjusting for FEV1/FVC less than LLN, symptomatic individuals had an increased risk of acute hospitalizations due to obstructive lung disease and pneumonia, but did not differ with regard to all-cause mortality, compared with asymptomatic individuals (Figure E12).
Lastly, prognosis for main outcomes was compared between younger individuals with early COPD and older individuals with COPD. Although the prevalence of early COPD was 15%, the prevalence of COPD was 25%, defined as FEV1/FVC less than LLN in individuals aged 50 years or older with 10 pack-years or greater of tobacco consumption. Younger individuals with early COPD had lower relative risk estimates for acute obstructive lung disease hospitalizations, but higher risk estimates for acute pneumonia hospitalizations and all-cause mortality, compared with older individuals with COPD (Figure E13 and Table E3). These differences were smaller when comparing symptomatic younger individuals with early COPD and symptomatic older individuals with COPD (Figure E14 and Table E4). Asymptomatic older individuals with COPD had poorer prognosis than asymptomatic younger individuals with early COPD, probably due to a more substantial lung function impairment with FEV1 less than 80% predicted (40% in the older vs. 24% in the younger) and FEV1 less than 50% predicted (2% in the older vs. 0% in the younger).
Discussion
By using a Danish contemporary population-based cohort with 105,630 randomly selected individuals, we estimated the prevalence of early COPD to be 15%, defined as FEV1/FVC less than LLN in individuals under 50 years of age with 10 pack-years or greater of tobacco consumption. Individuals with early COPD more often had chronic respiratory symptoms and severe lung function impairment with an increased risk of acute obstructive lung disease– and pneumonia-related hospitalizations and early death. Importantly, the relative risks for these outcomes in younger individuals with early COPD were comparable to those in older individuals with COPD.
More than one-half of individuals with early COPD were active smokers, and thus at high risk of progression of lung function impairment leading to development of clinical disease with accompanying reduced life expectancy. Early detection and intervention in the form of smoking cessation could potentially halt progression and change the disease course accordingly (19). It is important to note that a substantial proportion of individuals with early COPD already had clinical signs of disease onset at baseline examination in the form of chronic respiratory symptoms and lung function impairment with FEV1 less than 80% of predicted. Furthermore, an increased risk of acute hospitalizations due to obstructive lung disease and pneumonia was already observed after a very short follow-up time. These observations suggest that the newly proposed operational definition of early COPD may capture not only mild but also moderate cases of COPD (6). Perhaps we need to refine the definition so the very early phases of disease development can be captured to implement prevention before substantial lung damage has taken place.
Because chronic respiratory symptoms may precede the development of COPD (18, 20–24), we chose to stratify individuals with and without early COPD accordingly. Interestingly, one-half of individuals without COPD reported chronic respiratory symptoms. It seemed that symptomatic individuals with and without early COPD had several clinical features in common at baseline examination, and shared a similar poor prognosis besides risk of all-cause mortality. It is possible that some of these symptomatic individuals with FEV1/FVC of LLN or greater may have early COPD despite of a normal spirometry (e.g., evidenced by CT abnormalities and/or increased FEV1 decline). Individuals with normal spirometry and chronic respiratory symptoms have been observed with increased airway wall thickness, pulmonary emphysema, gas trapping, and abnormal diffusing capacity, and a prognosis resembling that of COPD (18, 25, 26). However, it is remarkable that, upon exclusion of individuals with asthma from the analyses, symptomatic individuals without COPD no longer had an increased risk of acute hospitalizations due to obstructive lung disease or pneumonia, suggesting that asthma may have driven the poor prognosis in this group (compare Figure 5 with Figure E8). In contrast, symptomatic individuals with early COPD still had an increased risk of acute hospitalizations and early death. Nonetheless, it is noticeable that, despite only using one of the three criteria of the proposed operational definition (i.e., FEV1/FVC <LLN and not CT abnormalities nor FEV1 decline of ≥60 ml/yr [6]), we still ended up with an early COPD prevalence of 15%, suggesting that this number is an underestimate, and that only a certain proportion of the true high burden of early COPD has been revealed in the present study.
As much as one-third of individuals with early COPD was asymptomatic. Asymptomatic individuals with early COPD had the same risk of investigated outcomes as asymptomatic individuals without COPD. Although this could easily be due to insufficient statistical power, they shared similar characteristics at baseline examination, except for lung function parameters, which were markedly lower in those with early COPD. However, asymptomatic individuals with early COPD differed substantially from symptomatic individuals with early COPD. The presence of chronic respiratory symptoms may be a prognostic marker of disease progression in early COPD, which is also in accordance with previous observations (18, 20–24). An intervention in symptomatic individuals with early COPD would probably have greater impact than in asymptomatic individuals. Despite lung function impairment, asymptomatic individuals with early COPD may not develop clinical disease. Nonetheless, investigating progression of early COPD in those with and without chronic respiratory symptoms seems warranted.
A few previous studies have investigated clinical characteristics and prognosis in young patients with COPD. In a clinical study comprising 1,708 patients with COPD, severity distribution and progression of disease in those aged 55 years or younger were like those aged 65 years or older, suggesting that COPD has its origin of disease components in young age (27). This is in keeping with the present findings of younger individuals with early COPD having similar distribution of clinical characteristics and prognosis like older individuals with COPD. In a prespecified post hoc analysis of the UPLIFT trial comprising 356 patients with moderate to severe COPD aged 40–50 years, those treated with tiotropium had improved quality of life and decreased exacerbation rate compared with those treated with placebo, suggesting that early treatment with a long-acting bronchodilator may benefit long-term prognosis in early COPD (28).
Strengths of the present study include a large contemporary population-based cohort study with randomly selected individuals with a long and complete follow-up, and with information on COPD-related characteristics and outcomes.
A major limitation is that only prebronchodilator, and not post-bronchodilator, spirometric indices were available, precluding characterization of airflow limitation into reversible or irreversible types. Although using prebronchodilator instead of post-bronchodilator values has been shown to overestimate prevalence of COPD (29, 30), there seems to be no difference in diagnostic accuracy for COPD between them (31). Nonetheless, as early COPD was defined in a high-risk population with substantial cumulative tobacco consumption, which is usually an inclusion criterion in clinical trials with COPD and an exclusion criterion in clinical trials with asthma (32), we believe that we identified most cases of early COPD correctly. In accordance with the proposed operational definition of early COPD (6), we deliberately did not exclude individuals with asthma from the present study, as asthma may precede and contribute to the risk of developing COPD later in life (33, 34). Nonetheless, results were similar in sensitivity analyses after exclusion of individuals reporting asthma and/or treatment with airway medication.
Another potential limitation is that we did not have information on lung imaging with CT. Thus, some individuals with normal spirometry may have CT abnormalities, suggesting the presence of early COPD. However, this would bias results toward the null, and therefore cannot explain our findings.
Lastly, although we used the proposed operational definition of early COPD requiring tobacco exposure, we cannot ignore the fact that some never-smokers with relevant risk factors will develop COPD and could also be identified early in the disease course (35).
Clinical implications of the present study relate to early detection and intervention in COPD to influence disease course. Early COPD seems to be prevalent among young and middle-aged adults with tobacco exposure, and these individuals had a relatively poor prognosis similar to older individuals with COPD. Because more than one-half of them were active smokers, smoking cessation is the most obvious intervention. Nonetheless, we need trials investigating whether treatment intervention in these young, susceptible smokers will halt progression and improve long-term prognosis. Our results suggest that especially presence of chronic respiratory symptoms, asthma, and a history of bronchitis/pneumonia were all important characteristics that could be used to identify subgroups with the highest risk of progression of early COPD.
In conclusion, among individuals under 50 years of age and 10 pack-years or greater of tobacco consumption from the general population, 15% fulfill criteria of early COPD. Individuals with early COPD more often have chronic respiratory symptoms and severe lung function impairment, and an increased risk of acute respiratory hospitalizations and early death.
Supplementary Material
Footnotes
Supported by the Lundbeck Foundation and by the National Institute for Health Research Manchester Biomedical Research Centre (J.V.).
Author Contributions: Y.Ç. and P.L. had full access to all data in the study and had final responsibility for the decision to submit for publication. Y.Ç., S.A., B.G.N., J.V., and P.L. contributed to the study concept and design. Y.Ç., S.A., B.G.N., J.V., and P.L. collected, analyzed, and interpreted the data. Y.Ç. wrote the draft manuscript and did the statistical analyses. Y.Ç., S.A., B.G.N., J.V., and P.L. revised the manuscript for important intellectual content. B.G.N. obtained funding and provided administrative, technical, and material support. J.V. and P.L. supervised the study.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201908-1644OC on November 26, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375:871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 3.Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet. 2015;385:1778–1788. doi: 10.1016/S0140-6736(15)60647-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soriano JB, Polverino F, Cosio BG. What is early COPD and why is it important? Eur Respir J. 2018;52:1801448. doi: 10.1183/13993003.01448-2018. [DOI] [PubMed] [Google Scholar]
- 5.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 6.Martinez FJ, Han MK, Allinson JP, Barr RG, Boucher RC, Calverley PMA, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:1540–1551. doi: 10.1164/rccm.201710-2028PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5:426–434. doi: 10.1016/S2213-2600(17)30119-4. [DOI] [PubMed] [Google Scholar]
- 8.Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Young and middle-aged adults with airflow limitation according to lower limit of normal but not fixed ratio have high morbidity and poor survival: a population-based prospective cohort study. Eur Respir J. 2018;51:1702681. doi: 10.1183/13993003.02681-2017. [DOI] [PubMed] [Google Scholar]
- 9.Løkke A, Marott JL, Mortensen J, Nordestgaard BG, Dahl M, Lange P. New Danish reference values for spirometry. Clin Respir J. 2013;7:153–167. doi: 10.1111/j.1752-699X.2012.00297.x. [DOI] [PubMed] [Google Scholar]
- 10.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4:103–112. [Google Scholar]
- 11.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12:737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 12.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 13.Ingebrigtsen TS, Marott JL, Lange P, Hallas J, Nordestgaard BG, Vestbo J. Medically treated exacerbations in COPD by GOLD 1–4: a valid, robust, and seemingly low-biased definition. Respir Med. 2015;109:1562–1568. doi: 10.1016/j.rmed.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 15.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aaron SD, Donaldson GC, Whitmore GA, Hurst JR, Ramsay T, Wedzicha JA. Time course and pattern of COPD exacerbation onset. Thorax. 2012;67:238–243. doi: 10.1136/thoraxjnl-2011-200768. [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Çolak Y, Nordestgaard BG, Vestbo J, Lange P, Afzal S. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur Respir J. 2019;54:1900734. doi: 10.1183/13993003.00734-2019. [DOI] [PubMed] [Google Scholar]
- 19.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 20.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med. 2016;193:662–672. doi: 10.1164/rccm.201511-2210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax. 2009;64:894–900. doi: 10.1136/thx.2008.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalhan R, Dransfield MT, Colangelo LA, Cuttica MJ, Jacobs DR, Jr, Thyagarajan B, et al. Respiratory symptoms in young adults and future lung disease: the CARDIA Lung Study. Am J Respir Crit Care Med. 2018;197:1616–1624. doi: 10.1164/rccm.201710-2108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vestbo J, Lange P. Can GOLD stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2002;166:329–332. doi: 10.1164/rccm.2112048. [DOI] [PubMed] [Google Scholar]
- 24.Vestbo J, Prescott E, Lange P Copenhagen City Heart Study Group. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153:1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 25.Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, et al. Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Salcedo P, Divo M, Casanova C, Pinto-Plata V, de-Torres JP, Cote C, et al. Disease progression in young patients with COPD: rethinking the Fletcher and Peto model. Eur Respir J. 2014;44:324–331. doi: 10.1183/09031936.00208613. [DOI] [PubMed] [Google Scholar]
- 28.Morice AH, Celli B, Kesten S, Lystig T, Tashkin D, Decramer M. COPD in young patients: a pre-specified analysis of the four-year trial of tiotropium (UPLIFT) Respir Med. 2010;104:1659–1667. doi: 10.1016/j.rmed.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Bakke PS, Rönmark E, Eagan T, Pistelli F, Annesi-Maesano I, Maly M, et al. European Respiratory Society Task Force. Recommendations for epidemiological studies on COPD. Eur Respir J. 2011;38:1261–1277. doi: 10.1183/09031936.00193809. [DOI] [PubMed] [Google Scholar]
- 30.Hansen JG, Pedersen L, Overvad K, Omland Ø, Jensen HK, Sørensen HT. The prevalence of chronic obstructive pulmonary disease among Danes aged 45–84 years: population-based study. COPD. 2008;5:347–352. doi: 10.1080/15412550802522635. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed Hoesein FA, Zanen P, Sachs AP, Verheij TJ, Lammers JW, Broekhuizen BD. Spirometric thresholds for diagnosing COPD: 0.70 or LLN, pre- or post-dilator values? COPD. 2012;9:338–343. doi: 10.3109/15412555.2012.667851. [DOI] [PubMed] [Google Scholar]
- 32.Lange P, Çolak Y, Ingebrigtsen TS, Vestbo J, Marott JL. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma–chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: a prospective population-based analysis. Lancet Respir Med. 2016;4:454–462. doi: 10.1016/S2213-2600(16)00098-9. [DOI] [PubMed] [Google Scholar]
- 33.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 34.Tagiyeva N, Devereux G, Fielding S, Turner S, Douglas G. Outcomes of childhood asthma and wheezy bronchitis: a 50-year cohort study. Am J Respir Crit Care Med. 2016;193:23–30. doi: 10.1164/rccm.201505-0870OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.