Abstract
Rationale: Oral treprostinil improves exercise capacity in patients with pulmonary arterial hypertension (PAH), but the effect on clinical outcomes was unknown.
Objectives: To evaluate the effect of oral treprostinil compared with placebo on time to first adjudicated clinical worsening event in participants with PAH who recently began approved oral monotherapy.
Methods: In this event-driven, double-blind study, we randomly allocated 690 participants (1:1 ratio) with PAH to receive placebo or oral treprostinil extended-release tablets three times daily. Eligible participants were using approved oral monotherapy for over 30 days before randomization and had a 6-minute-walk distance 150 m or greater. The primary endpoint was the time to first adjudicated clinical worsening event: death; hospitalization due to worsening PAH; initiation of inhaled or parenteral prostacyclin therapy; disease progression; or unsatisfactory long-term clinical response.
Measurements and Main Results: Clinical worsening occurred in 26% of the oral treprostinil group compared with 36% of placebo participants (hazard ratio, 0.74; 95% confidence interval, 0.56–0.97; P = 0.028). Key measures of disease status, including functional class, Borg dyspnea score, and N-terminal pro–brain natriuretic peptide, all favored oral treprostinil treatment at Week 24 and beyond. A noninvasive risk stratification analysis demonstrated that oral treprostinil–assigned participants had a substantially higher mortality risk at baseline but achieved a lower risk profile from Study Weeks 12–60. The most common adverse events in the oral treprostinil group were headache, diarrhea, flushing, nausea, and vomiting.
Conclusions: In participants with PAH, addition of oral treprostinil to approved oral monotherapy reduced the risk of clinical worsening.
Clinical trial registered with www.clinicaltrials.gov (NCT01560624).
Keywords: pulmonary arterial hypertension, oral treprostinil, clinical study, combination therapy, sequential therapy
At a Glance Commentary
Scientific Knowledge on the Subject
Oral treprostinil improved exercise capacity in treatment-naive patients with pulmonary arterial hypertension (PAH) and substituted for parenteral treprostinil in a carefully selected group of participants, but the effect on clinical outcomes was unknown.
What This Study Adds to the Field
This multicenter, randomized, double-blind placebo-controlled trial demonstrates that initiation of oral treprostinil reduces the risk of clinical worsening events in participants who had recently (median, 5.4 mo) started oral monotherapy for PAH. Secondary endpoints, including N-terminal pro–brain natriuretic peptide and a multifaceted noninvasive risk assessment, improved in oral treprostinil–assigned participants beginning at Week 12 and continuing through Week 60.
Pulmonary arterial hypertension (PAH) is a rare, but progressive and often fatal, pulmonary vascular disease. Treatment options have expanded greatly in the past 20 years (1), and two event-driven studies of sequential combination therapy have established the durable benefit of the endothelin receptor antagonist (ERA) macitentan (2) and the prostacyclin receptor agonist selexipag (3). Epoprostenol, the endogenous agonist for the prostacyclin receptor, is highly effective in PAH, but it is short acting and requires continuous intravenous infusion (4). Selexipag was thus a significant addition to treatment options as a long-acting and orally available, selective prostacyclin IP receptor agonist. Oral extended-release treprostinil diolamine tablets improved exercise capacity when dosed twice daily in treatment-naive patients with PAH (5). Oral treprostinil dosed three times daily had a better pharmacokinetic profile, allowed participants to achieve a higher total daily dose, and substituted for parenteral treprostinil in a cohort of carefully selected participants with PAH (6). Therefore, the FREEDOM-EV study hypothesized that combination therapy with oral treprostinil would reduce the risk of clinical worsening events in patients who had recently started oral monotherapy for PAH. Preliminary results of this study have been previously reported in the form of conference abstracts (7–9).
Methods
Study Design
The FREEDOM-EV trial was a multicenter, randomized, double-blind, placebo-controlled, event-driven study. Investigators from 152 centers across 23 countries conducted the study between June 2012 and June 2018. The steering committee, in collaboration with the sponsor, designed the study protocol (see the online supplement), and the institutional review board at each center approved the protocol. The sponsor collected and analyzed the data according to a prespecified statistical analysis plan. An independent data monitoring committee supervised the study, and all authors had access to the source-verified data and attest to the accuracy and completeness of this report.
Selection of Participants
Participants were 18–75 years of age, met the 2013 consensus definition of World Health Organization (WHO) Group 1 pulmonary hypertension (10), and had a 6-minute-walk distance (6MWD) 150 m or greater at the screening visit. Historical right heart catheterization within 3 years (or during the screening period) must have demonstrated a mean pulmonary artery pressure of 25 mm Hg or greater and a pulmonary artery wedge pressure of 15 mm Hg or less. Based on the AMBITION study (11), protocol amendment 5 excluded participants who had three or more of the following risk factors for heart failure with preserved ejection fraction: 1) body mass index of 30 kg/m2 or greater; 2) essential hypertension; 3) diabetes mellitus; or 4) clinically significant coronary artery disease. The initial protocol sought to enroll participants soon after they began oral monotherapy (between 30 and 90 d of beginning an approved dose and schedule of sildenafil, tadalafil, bosentan, ambrisentan, macitentan, or riociguat). Subsequent amendments expanded the monotherapy treatment window to address slow enrollment. The full set of protocol entry criteria are provided in the online supplement. All the participants provided written informed consent.
Trial Procedures
Randomization (1:1) was stratified by type of background therapy (i.e., phosphodiesterase type 5 [PDE5] inhibitor or soluble guanylate cyclase [SGC] stimulator vs. ERA) and by baseline 6MWD (breakpoint ≤350 m). Participants initially took oral treprostinil or matching placebo 0.125 mg three times daily (spaced carefully every 6–8 h with food). The protocol allowed daily up-titration in 0.125 mg increments for the first 4 weeks and 0.25-mg daily titration thereafter to a maximum dose of 12 mg three times daily. We instructed investigators to increase doses steadily and to assess the need for dose adjustment during weekly telephone calls, attempting to balance the expected adverse drug effects with the apparent clinical benefits (i.e., a reduction in the signs and symptoms of PAH).
Outcome Measures
The primary endpoint was the time to first adjudicated clinical worsening event, which was defined as death from any cause, hospitalization for worsening PAH, disease progression, initiation of inhaled or infused prostacyclin therapy, or unsatisfactory long-term clinical response (definitions provided in the online supplement). Three disease experts (not otherwise participating in the study) formed a blinded, independent clinical event committee, which adjudicated all clinical worsening events using a narrative that was stripped of information about adverse events or dosing that might cue them to treatment assignment. Investigator teams met participants at Weeks 4, 8, and 12, and then at 12-week intervals throughout the study to conduct efficacy assessments, including 6MWD, Borg dyspnea score, plasma N-terminal pro–brain natriuretic peptide (NT-proBNP) levels, and WHO functional class. Before the final statistical analysis plan was submitted to the U.S. Food and Drug Administration and before unblinding, we planned a risk analysis using three noninvasive variables as previously proposed (12) and validated (13) (e.g., 6MWD, NT-proBNP, and WHO functional class). Safety assessments included evaluation of adverse events and clinical laboratory parameters. Beginning in 2015, with protocol amendment 6, we collected vital status by phone every 6 months for those who discontinued the study; survival analysis was prespecified in the statistical plan submitted to the Food and Drug Administration.
Statistical Analysis
The final power calculation estimated that 205 adjudicated events would provide at least 90% power (type I error rate, 0.05; two tailed) to detect a difference in the time to adjudicated clinical worsening event between treatment groups, assuming exponential distributions and an underlying hazard ratio of 0.62. We assumed a placebo event rate of 23% at Month 12 and accrual of subjects over 3 years with 10% attrition. These assumptions indicated a sufficient sample size would be 610–850 participants; we closed enrollment at 690 participants when we approached the required 205 events. The primary efficacy endpoint had been tested at an interim analysis when approximately 75% of the total adjudicated events had occurred with a prespecified decision to stop if the interim type I error was less than 0.02; this required that the final analysis have an α of less than 0.044 for an overall type I error rate at 0.05. The main analyses for the primary and secondary endpoints were performed in the entire population. For the primary efficacy analysis of time to adjudicated clinical worsening event, data were summarized by treatment group using product-limit estimates calculated by the Kaplan-Meier method. The log-rank test adjusted for the type of background PAH therapy (PDE5 inhibitor or SGC stimulator vs. ERA), and the baseline 6MWD (breakpoint ≤350 m) was used to calculate significance for treatment differences in the intention-to-treat population. The risk of clinical worsening was also compared between treatment groups using a Cox proportional hazards regression model to estimate a hazard ratio and its 95% confidence interval (CI), also adjusting for background PAH therapy and baseline 6MWD. All safety analyses were also performed in the entire population.
Results
Participants
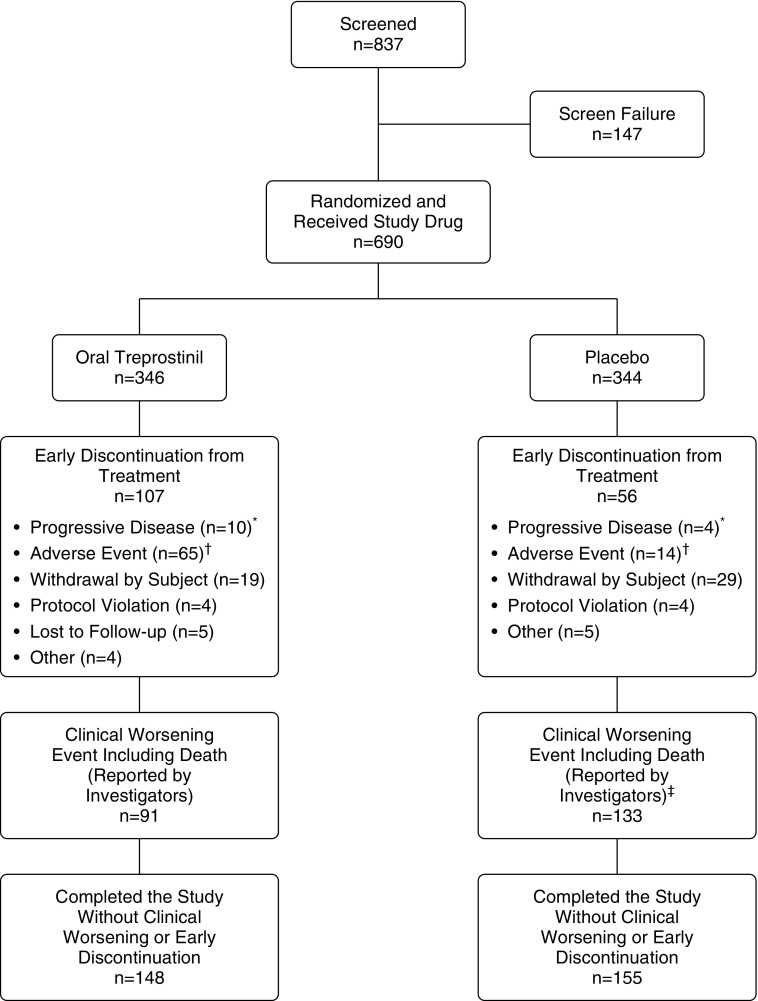
We randomly allocated 690 participants to oral treprostinil (346 participants) or placebo (344 participants) treatment groups (Figure 1). Table 1 shows the demographic and clinical characteristics of participants; individual characteristics were largely balanced at baseline. We enrolled participants after beginning initial monotherapy (median time, 5.4 mo). Participants were predominantly female; 63% had WHO functional class II symptoms, and 72% were taking a PDE5 inhibitor or SGC stimulator. Median dose of oral treprostinil achieved at Week 24 was 3.56 mg three times daily, which corresponds to titration by approximately 0.125 mg once weekly (288 oral treprostinil participants; see Figure E1 in the online supplement). Median dose of placebo at Week 24 was 6 mg three times daily (289 placebo participants).
Figure 1.
Patient disposition. *Includes one subject in the oral treprostinil group and one subject in the placebo group who experienced clinical worsening events due to urgent hospitalization for treatment of worsening pulmonary arterial hypertension. †Includes one subject in the oral treprostinil group and one subject in the placebo group who experienced clinical worsening events due to fatal serious adverse events, and one subject in the oral treprostinil group who discontinued treatment due to an adverse event, but remained in the study until death (which did not qualify as a clinical worsening event). ‡Includes one subject in the placebo group who died after discontinuation of study treatment due to clinical worsening.
Table 1.
Baseline Characteristics*
| Characteristic | Oral Treprostinil (n = 346) | Placebo (n = 344) | Overall (n = 690) |
|---|---|---|---|
| Age, yr | 45.6 ± 15.7 | 44.8 ± 15.4 | 45.2 ± 15.5 |
| Sex, F, n (%) | 275 (79.5) | 269 (78.2) | 544 (78.8) |
| Race, n (%) | |||
| White | 187 (54.0) | 173 (50.3) | 360 (52.2) |
| Black or African American | 8 (2.3) | 13 (3.8) | 21 (3.0) |
| Asian | 150 (43.4) | 156 (45.3) | 306 (44.3) |
| Unknown | 1 (0.3) | 2 (0.6) | 3 (0.4) |
| Region, n (%) | |||
| North America | 39 (11.3) | 54 (15.7) | 93 (13.5) |
| Asia-Pacific | 162 (46.8) | 160 (46.5) | 322 (46.7) |
| Europe | 55 (15.9) | 44 (12.8) | 99 (14.3) |
| Latin America | 90 (26.0) | 86 (25.0) | 176 (25.5) |
| Median time since diagnosis (IQR), mo | 6.2 (2.4–13.3) | 6.5 (2.28–13.2) | 6.4 (2.3–13.3) |
| Etiology of PAH, n (%) | |||
| Idiopathic or heritable PAH | 219 (63.3) | 216 (62.8) | 435 (63.0) |
| Connective tissue disease | 94 (27.2) | 84 (24.4) | 178 (25.8) |
| HIV infection | 2 (0.6) | 7 (2.0) | 9 (1.3) |
| Congenital heart defect | 20 (5.8) | 27 (7.8) | 47 (6.8) |
| Other | 11 (3.2) | 10 (2.9) | 21 (3.0) |
| 6MWD, n (%) | |||
| ≤350 m | 95 (27.5) | 93 (27.0) | 188 (27.2) |
| >350 m | 251 (72.5) | 251 (73.0) | 502 (72.8) |
| 6MWD, m | 392.9 ± 92.5 | 398.5 ± 100.0 | 395.7 ± 96.3 |
| WHO functional class at baseline, n (%) | |||
| I | 9 (2.6) | 13 (3.8) | 22 (3.2) |
| II | 205 (59.2) | 228 (66.3) | 433 (62.8) |
| III | 131 (37.9) | 103 (29.9) | 234 (33.9) |
| IV | 1 (0.3) | 0 | 1 (0.1) |
| Background PAH therapy at baseline, n (%) | |||
| PDE5 inhibitor or SGC stimulator alone | 248 (71.7) | 246 (71.5) | 494 (71.6) |
| ERA alone | 98 (28.3) | 98 (28.5) | 196 (28.4) |
| Median time on background PAH therapy at baseline (IQR), mo | 5.3 (2.3–10.7) | 5.5 (2.4–10.6) | 5.4 (2.4–10.7) |
| Risk stratification by number of low-risk criteria met†‡, n (%) | — | ||
| 0 | 85 (25.2) | 59 (17.7) | |
| 1 | 112 (33.2) | 110 (32.9) | |
| 2 | 102 (30.3) | 94 (28.1) | |
| 3 | 38 (11.3) | 71 (21.3) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; ERA = endothelin receptor antagonist; IQR = interquartile range; PAH = pulmonary arterial hypertension; PDE5 = phosphodiesterase type 5; SGC = soluble guanylate cyclase; WHO = World Health Organization.
Plus/minus values are means ± SD. Testing of baseline characteristics showed that there were no significant between-group differences at baseline, except regarding risk stratification by number of low-risk criteria.
Low-risk criteria defined as WHO functional class I or II, 6MWD greater than 440 m, and/or N-terminal pro–brain natriuretic peptide less than 300 pg/ml. Low-risk criteria met were only counted for subjects with all three measures available; n = 337 oral treprostinil, n = 334 placebo.
P = 0.002; P value was obtained from Fisher’s exact test.
Primary Efficacy Endpoint
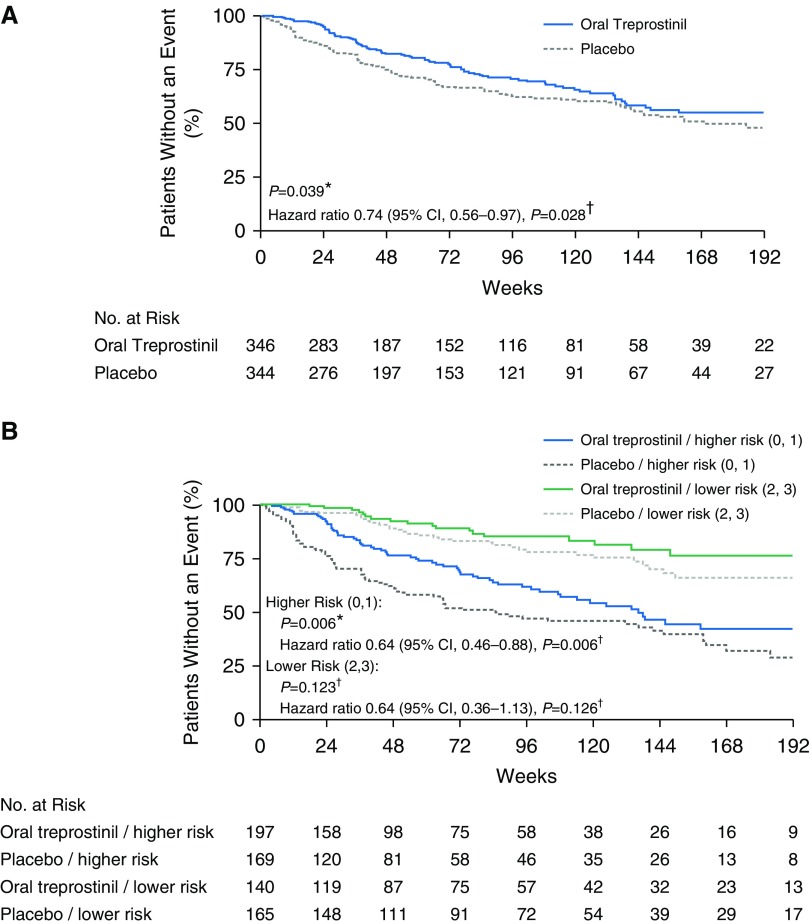
Overall, 90 (26%) participants in the oral treprostinil group experienced an adjudicated clinical worsening event compared with 124 (36%) placebo participants. Kaplan-Meier estimates of the time to adjudicated clinical worsening event suggested group separation before Week 24 (Figure 2A, log-rank test, P = 0.039); the hazard ratio adjusted for background therapy and baseline 6MWD as a continuous variable was 0.74 (95% CI, 0.56–0.97; P = 0.028). When adjusted for baseline 6MWD as a categorical variable (breakpoint ≤350 m), the hazard ratio was 0.75 (95% CI, 0.57–0.99; P = 0.040). The median time to clinical worsening was 46 weeks with oral treprostinil and 37 weeks with placebo. The treatment-attributable difference in clinical worsening was driven by a reduced incidence of disease progression in the oral treprostinil group (hazard ratio, 0.39; 95% CI, 0.23–0.66; P < 0.001). Deaths and hospitalizations were balanced. Subgroup analyses of the primary endpoint, based on age, sex, baseline 6MWD, WHO functional class, PAH etiology, geographic region, and background oral PAH therapy did not show any significant interactions between subgroup and treatment (see Figure E2).
Figure 2.
Kaplan-Meier plots of primary endpoint and primary endpoint by baseline risk stratification. (A) Time to adjudicated clinical worsening events. (B) Time to adjudicated clinical worsening events by baseline risk stratification. “Lower risk” is defined as subjects with two or three low-risk criteria met; “higher risk” is defined as subjects with zero or one low-risk criterion met. *P values were calculated with log-rank test stratified by background pulmonary arterial hypertension (PAH) therapy and baseline 6-minute-walk distance (6MWD) category. †Hazard ratios, 95% confidence intervals (CIs), and P values were calculated with proportional hazard model with explanatory variables of treatment, background PAH therapy, and baseline 6MWD as a continuous variable.
Individual components of the demographics suggested balanced participant characteristics at baseline; however, a prespecified (before unblinding), noninvasive risk stratification (12) indicated that the oral treprostinil–assigned group had a higher mortality risk at baseline. Placebo-assigned participants had more low-risk criteria (e.g., WHO functional class I or II symptoms, 6MWD >440 m, and NT-proBNP <300 pg/ml) compared with the oral treprostinil group (Fisher’s exact test, P = 0.002, Table 1). We thus conducted a post hoc analysis accounting for baseline risk profile (number of low-risk factors, 0–3); the hazard ratio for a clinical worsening event dropped further to 0.61 (95% CI, 0.46–0.81; P < 0.001). Arbitrarily classifying those with two to three low-risk factors as “lower risk” and those with zero to one low-risk factors as “higher risk” resulted in two groups of similar size (n = 366 vs. 305). We re-estimated Kaplan-Meier time-to-event curves for these two groups, finding that oral treprostinil protected higher-risk participants from clinical worsening events (log-rank, P = 0.006; Figure 2B).
Secondary and Exploratory Efficacy Endpoints
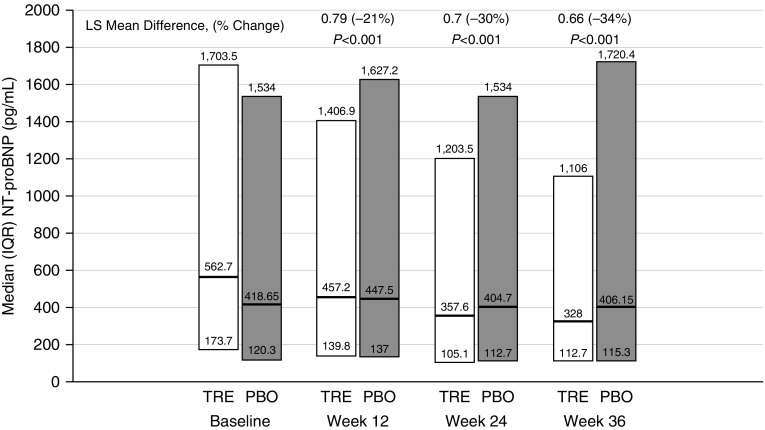
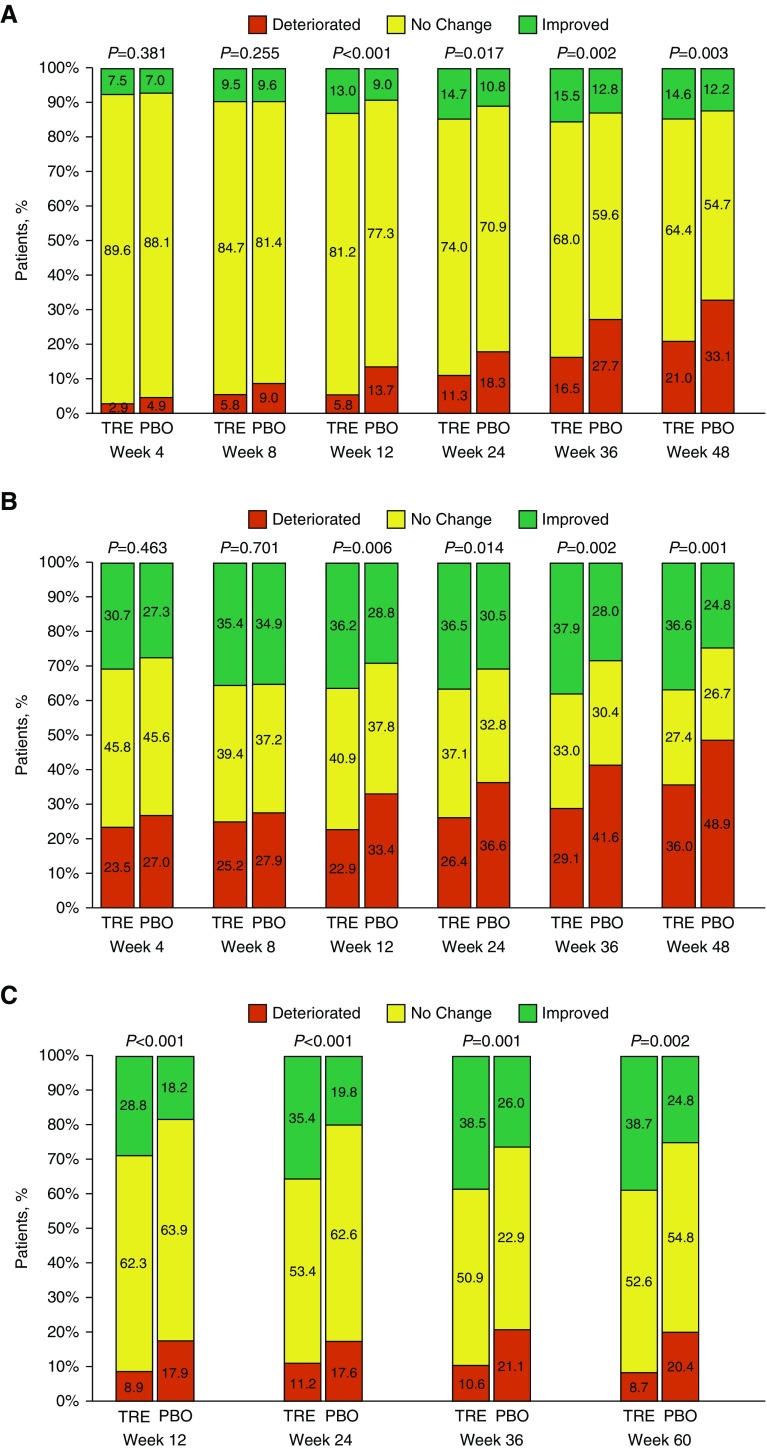
Plasma NT-proBNP levels decreased in the oral treprostinil group beginning at Week 12 (Figure 3). WHO functional class improved significantly for participants in the oral treprostinil group at all visits from Week 12 to Week 48 when compared with the placebo group (Figure 4A). Improvement was observed as both a higher proportion of favorable (“improved”) and a lower proportion of worsening (“deteriorated”) categorical change from baseline. At Week 24, oral treprostinil participants increased their 6MWD 16 m (least squares mean) compared with 8 m in the placebo group (mixed-model repeated measurement [MMRM] estimate of treatment effect, 8 m [95% CI, −2 to 18; P = 0.12], Table 2). By Week 36, treatment difference in 6MWD was clear (MMRM = 13 m [95% CI, 1–25]; P = 0.04), and this increased further at Week 48 (MMRM = 22 m [95% CI, 8–35; P = 0.002]; see Figure E3). Hodges-Lehmann estimate of treatment effect yielded similar results (see Figure E4). Although the change in 6MWD was not statistically different between the treatment groups at Weeks 12 or 24, categorical changes in Borg dyspnea score measured at the end of each walk test favored oral treprostinil at assessments from Week 12 to Week 48 (Figure 4B). Similarly, the combined 6MWD/Borg dyspnea score ranking, a statistical method for analyzing changes in walk distance and associated changes in Borg dyspnea score, favored the oral treprostinil treatment group at Week 24 (see Figure E5).
Figure 3.
Plasma N-terminal pro–brain natriuretic peptide (NT-proBNP) results by study visit. Per protocol, NT-proBNP values were not measured at Week 48. P value was obtained from the analysis of covariance with change from baseline in log-transformed data in NT-proBNP as the dependent variable, treatment as fixed effect, and log-transformed baseline NT-proBNP as a covariate. NT-proBNP assay centrally performed by Covance via the Immulite 2000 on a Seimens platform. The normal range for both sexes less than 75 years of age is less than 125 pg/ml. The normal range for both sexes over 75 years of age is less than 450 pg/ml. IQR = interquartile range; LS = least squares; PBO = placebo; TRE = oral treprostinil.
Figure 4.
Categorical changes from baseline in World Health Organization (WHO) functional class, Borg dyspnea score, and risk stratification criteria. (A) WHO functional class categorical change from baseline by study visit; participants who had a missing assessment at Week 24 and had deteriorated were assigned functional class IV; P value was obtained from Fisher’s exact test. (B) Borg dyspnea score categorical change from baseline by study visit; participants who had a missing assessment at Week 24 and had deteriorated were assigned worst case of 10; P value was obtained from Fisher’s exact test. (C) Risk categorical change from baseline through Week 60. Percentages are calculated based on the number of participants at each visit within each treatment group. Low-risk criteria are defined as WHO functional class I or II, 6-minute-walk distance >440 m, or N-terminal pro–brain natriuretic peptide <300 pg/ml. Low-risk criteria met were only counted for subjects with all three measures. “Improved” indicates any increase in the number of low-risk criteria met; “no change” indicates the same number of low-risk criteria met; and “deteriorated” indicates any decrease in the number of low-risk criteria met. P values were obtained from Fisher’s exact test. PBO = placebo; TRE = oral treprostinil.
Table 2.
Primary and Secondary Efficacy Endpoints
| Endpoint | Oral Treprostinil (n = 346) | Placebo (n = 344) | Treatment Effect (95% CI) |
|---|---|---|---|
| Primary endpoint: adjudicated clinical worsening event, n (%) | |||
| All events | 90 (26.0) | 124 (36.0) | HR, 0.74 (0.56 to 0.97); P = 0.028*; P = 0.039† |
| Death (all causes) | 15 (4.3) | 14 (4.1) | |
| Hospitalization due to PAH and/or right heart failure | 35 (10.1) | 35 (10.2) | |
| Initiation of inhaled or infused prostacyclin | 2 (0.6) | 5 (1.5) | |
| Disease progression | 19 (5.5) | 50 (14.5) | |
| Unsatisfactory long-term clinical response | 19 (5.5) | 20 (5.8) | |
| Secondary endpoints (at Week 24) | |||
| 6MWD, LS mean change, m | 16 | 8.03 | 7.96 (−2 to 17.92); P = 0.117‡ |
| NT-proBNP, concentration ratio to baseline, LS mean change | 0.82 | 1.16 | 0.71 (0.61 to 0.82); P < 0.001§ |
| Borg dyspnea score, shift from baseline, n (%) | P = 0.014‖ | ||
| Improved | 126 (36.5) | 105 (30.5) | |
| No change | 128 (37.1) | 113 (32.8) | |
| Deteriorated | 91 (26.4) | 126 (36.6) | |
| Combined ranking of 6MWD and Borg dyspnea score | — | — | P = 0.006¶ |
| WHO functional class, shift from baseline, n (%) | P = 0.017‖ | ||
| Improved | 51 (14.7) | 37 (10.8) | |
| No change | 256 (74) | 244 (70.9) | |
| Deteriorated | 39 (11.3) | 63 (18.3) | |
| Deaths (all causes), n (%) | |||
| Deaths during study | 17 (4.9) | 18 (5.2) | HR, 1.00 (0.52 to 1.95); P = 0.992*; P = 0.978† |
| Deaths at closure of study** | 38 (11.0) | 60 (17.4) | HR, 0.63 (0.42 to 0.95); P = 0.026*; P = 0.032† |
Definition of abbreviations: 6MWD = 6-minute-walk distance; CI = confidence interval; HR = hazard ratio; LS = least squares; MMRM = mixed-model repeated measurement; NT-proBNP = N-terminal pro–brain natriuretic peptide; PAH = pulmonary arterial hypertension; WHO = World Health Organization.
Hazard ratio, 95% CI, and P value were calculated with proportional hazard model with explanatory variables of treatment, background PAH therapy, and baseline 6MWD as a continuous variable.
P value was obtained from log-rank test stratified by background PAH therapy and baseline 6MWD category.
LS mean, P value, estimated difference, and its 95% CI were from the MMRM with the change from baseline in 6MWD as the dependent variable, treatment, week, treatment-by-week interaction, and background PAH therapy as the fixed effects, and baseline 6MWD as the covariate. An unstructured variance/covariance structure shared across treatment groups was used to model the within-subject errors.
LS mean, P value, estimated difference, and its 95% CI were from the MMRM with the change from baseline in log-transformed data in NT-proBNP as the dependent variable, treatment, week, and treatment-by-week interaction as the fixed effects, and log-transformed baseline NT-proBNP as the covariate. An unstructured variance/covariance structure shared across treatment groups was used to model the within-subject errors.
P value was obtained from Fisher’s exact test.
P value obtained from nonparametric analysis of covariance.
Vital status was collected at the study closure for all subjects including subjects who rolled over to extension study and who discontinued early from the study. For subjects whose vital status was not available at the study closure, their time to death was censored at the subjects’ last known date to be alive. Subjects who were alive at the study closure have their time to death censored at the last contact date.
We hypothesized a priori (before unblinding) that oral treprostinil applied as sequential combination therapy would improve risk assessments at follow-up. We obtained the necessary variables to define the recently proposed, noninvasive risk assessment at Weeks 12, 24, 36, and 60. At each assessment, the Fisher’s exact analysis of those having categorical change was highly significant (P < 0.002), with more oral treprostinil participants having an improved risk profile and fewer having a deteriorated risk profile (Figure 4C). A post hoc analysis using the Reveal 2.0 risk score (14) yielded a similar result beginning at Week 12 (see Table E1).
The initial data collection plan stopped following participants 30 days after discontinuing randomized treatment unless they consented to participate in an open-label follow-up study. An amended protocol issued in 2015 collected vital status every 6 months for consenting participants until final study closure in October 2018. As of October 2018, 38 (11%) participants initially assigned to oral treprostinil were confirmed dead compared with 60 (17.4%) in the placebo group (hazard ratio, 0.63; 95% CI, 0.42–0.95; P = 0.026; Table 2). Because we did not begin collecting vital status until some participants had exited the study (and some investigative sites had closed), survival could not be confirmed for 74 (11%) participants. A sensitivity analysis assuming the observed mortality rate in those with unknown vital status still favored oral treprostinil (see Tables E2 and E3).
Safety
A total of 334 (96.5%) participants in the oral treprostinil group and 219 (63.7%) participants in the placebo group reported at least one adverse event attributable to study drug (Table 3). Headache, diarrhea, flushing, nausea, and vomiting were more commonly attributed to oral treprostinil and were more often severe compared with events attributed to placebo. Study drug discontinuation because of adverse events was substantially more common in oral treprostinil–assigned participants (18.8%) than in placebo participants (4.1%). Discontinuation because of an adverse event was more common before Week 24 (see Figure E6) and occurred at a median (interquartile range) oral treprostinil dose of 1.4 (0.4–3.0) mg three times daily.
Table 3.
Most Frequent Adverse Events
| Variable | Oral Treprostinil (n = 346) [n (%)] | Placebo (n = 344) [n (%)] |
|---|---|---|
| Any event reported | 342 (98.8) | 328 (95.3) |
| Any event probably or possibly related to study drug | 334 (96.5) | 219 (63.7) |
| Study drug–related serious adverse event | 27 (7.8) | 18 (5.2) |
| Study drug–related severe adverse event | 78 (22.5) | 27 (7.8) |
| Adverse events* | ||
| Headache | 242 (69.9) | 102 (29.7) |
| Diarrhea | 227 (65.6) | 68 (19.8) |
| Flushing | 151 (43.6) | 26 (7.6) |
| Nausea | 128 (37.0) | 58 (16.9) |
| Vomiting | 111 (32.1) | 26 (7.6) |
| Pain in jaw | 60 (17.3) | 8 (2.3) |
| Dizziness | 52 (15.0) | 45 (13.1) |
| Pain in extremity | 48 (13.9) | 11 (3.2) |
| Myalgia | 44 (12.7) | 23 (6.7) |
Adverse events listed are those probably or possibly related to study drug that occurred in more than 10% of participants in either study group.
Discussion
In the present study, oral treprostinil dosed three times daily delayed a composite clinical endpoint of time to first adjudicated clinical worsening event by reducing the likelihood of disease progression in participants, all of whom were taking an approved oral monotherapy for PAH. Hospitalizations and deaths were balanced between groups as components of the primary endpoint. This study differs from previous sequential combination studies (2, 3) in that participants had a younger age, more recent diagnosis, less severe symptoms, and better baseline exercise capacity. Although prostacyclin-class adverse events were common and 18.8% of oral treprostinil participants discontinued therapy because of adverse events, active treatment facilitated steady reductions in plasma NT-proBNP, improved WHO functional class, and reduced Borg dyspnea score after 6MWT, all beginning at Week 12. Actual 6MWD was improved at Weeks 36 and 48 in an analysis that does not require imputation. Total daily dose among actively treated participants at Week 24 was 50% higher than that at Week 16 in the previous combination studies of oral treprostinil (15, 16), and we postulate that higher doses were possible, because three times daily dosing reduced peak–trough excursions in plasma concentrations of treprostinil (6, 17).
Functional improvements, measured as part of a multifaceted risk assessment, have been repeatedly associated with improved outcomes (12, 13, 18, 19). We prespecified (before submission of the final statistical analysis plan) use of the French risk assessment, because we had collected the three required noninvasive variables at nearly all of the quarterly follow-up visits. For those who remained on therapy, participants taking oral treprostinil had a favorable shift in this noninvasive risk at Week 12, and the measured treatment benefit in risk reduction persisted at Week 60. This shift reflected an improvement in risk profile for 39% of oral treprostinil participants at Week 60 and was independent of the differences in the primary event of clinical worsening (because those participants had been censored). Our data support the recently suggested “net clinical benefit” strategy for clinical studies of novel therapies in PAH (20).
The balanced appearance of baseline characteristics in our study was deceiving and revealed the need for a multifaceted assessment of prognosis to ensure a reliable assessment of subsequent treatment efficacy. The unexpected imbalance in risk profiles at baseline indicates that our randomization strategy failed to create comparable baseline groups. This may be because the 350 m or lower breakpoint for 6MWD included less than 30% of the baseline walks and/or because this 350-m value is not a recognized transition point for prognosis (21). The post hoc, risk-adjusted analysis of the primary endpoint demonstrating a greater treatment effect indicates that future studies should consider stratifying randomization based upon background therapy and a validated risk score to create cohorts that have a similar prognosis at baseline (22). A failure in this regard could lead to under- or overestimation of the treatment effect.
Participants initially assigned placebo had a similar rate of death at the end of randomized treatment. A total of 108 of the 117 participants with an investigator-reported, nonfatal clinical worsening event in the primary study began therapy with oral treprostinil in the extension study. An apparent increase in survival for those initially assigned oral treprostinil emerged late in the study, but this observation must be treated cautiously, because vital status was unknown for 74 participants (11%). The results still favored oral treprostinil, assuming a proportional mortality among those with unknown vital status (Tables E2 and E3). We know very little about participants who discontinued the study. Only vital status was collected via phone call; we do not know the causes of death. Deaths were distributed relatively uniformly throughout the world with the exception of India, which had a death rate of approximately 20%. However, other countries with less access to expensive, approved PAH therapies (e.g., Mexico and China) did not have excess deaths relative to countries with more ready access. We know nothing about treatment status for those who died after discontinuing the study, and treatment differences between the groups might be an important confounder. Thus, this apparent difference in mortality (using the strategy prescribed in the final statistical analysis plan) is intriguing, but must be interpreted with appropriate context due to the amount of missing data. In a recent meta-analysis of 17 randomized, controlled therapeutic trials in PAH, sequential therapy was associated with a significant risk reduction for clinical worsening (−35%; P < 0.001), but not mortality (−14%; P = 0.09) (23). Macitentan was associated with a trend toward improved survival (2) in a prespecified analysis, and initial combination therapy improved survival (as compared with initial monotherapy) in a post hoc analysis (24). Although selexipag prevented clinical worsening events overall (3), death was numerically greater at the end of randomized treatment and similar to placebo through the end of the study.
Our study has important limitations. Adverse effects typical for prostacyclin-class medications were common, and the 18.8% discontinuation rate was higher than for a previous study of selexipag (14.3%). It is conceivable that some of the oral treprostinil–assigned participants who stopped taking the drug because of adverse events might have later had clinical worsening, but we used standard censoring methodology in generating the primary outcome analysis. The protocol was launched in 2013 when sequential combination therapy was standard, but initial combination therapy is becoming increasingly common. It is unknown whether the present results are generalizable to patients in clinical practice who are already taking two approved therapies.
In conclusion, oral treprostinil administered three times daily to a relatively homogenous group of participants with PAH who were taking oral monotherapy reduced the likelihood of clinical worsening due to disease progression. Plasma levels of NT-proBNP dropped markedly with oral treprostinil, and we also observed improvements in investigator-assessed WHO functional class and participant-reported Borg dyspnea score after hallway walking. Serial, noninvasive risk score measurements appeared useful to document treatment-related benefits, and a prognostic score should be considered for future outcome studies to balance baseline risk profiles between the randomized treatment groups.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Aliou Ousmanou, Pharm.D. (United Therapeutics), for successfully leading the global operation throughout the project. Andrew Nelsen, Pharm.D., and Derek Solum, Ph.D. (United Therapeutics), provided critical support to the data analysis and the development of figures and tables. Erick Borg, Pharm.D. (United Therapeutics), and Tina Lin, Pharm.D. (Write Science), provided excellent editorial support; the latter was funded by United Therapeutics.
FREEDOM EV Investigators: Argentina—Hospital Italiano de Buenos Aires, Buenos Aires: Graciela Noemi Svetliza; Sanatorio San Jose, Buenos Aires: Adrian Jose Lescano; Sanatorio de la Trinidad Mitre, Santa Fe: Guillermo Roberto Bortman; Hospital Italiano Garibaldi, Rosario: Fabian Antonio Diez; Hospital Provincial Dr. Jose Maria Cullen, Santa Fe: Christian Edgardo Botta. Australia—The Prince Charles Hospital, Brisbane, Queensland: John Fitzgerald, Eelke Feenstra, and Fiona Dawn Kermeen; St. Vincent’s Hospital, Sydney, New South Wales: Anne Margaret Keogh; The Alfred Hospital, Melbourne, Victoria: Trevor John Williams; Royal Prince Alfred Hospital, Sydney, New South Wales: Peter Paul Yousseff; Nepean Hospital, Kingswood, New South Wales: Benjamin Joh-Han Ng; Royal Melbourne Hospital, Parkville, Victoria: David McNaughton Smallwood; Royal Hobart Hospital, Sandy Bay, Tasmania: Nathan Brent Dwyer; Macquarie University, Macquarie Park, New South Wales: Martin Russell Brown. Austria—Medical University of Vienna, Vienna: Irene M Lang; Krankenhaus der Elisabethined Linz GmbH, Linz, Oberosterreich: Regina Steringer-Mascherbauer. Brazil—Universidade Federal de Sao Paulo–Unifesp/EPM, San Paulo: Jaquelina Ota Arakaki; Instituto das Pequenas Missionarias de Maria Imaculada-Hospital Madre Teresa, Belo Horizonte: Frederico Campos; Hospital das Clinicas da Universidade Federal de Minas Gerais, Belo Horizonte: Ricardo de Amorim Correa; Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo Instituto do Coração, San Paulo: Rogerio de Souza; Complexo Hospitalar Santa Casa de Porto Alegre, Porto Alegre: Gisela Martina Bohns Meyer; Hospital das Clinicas da Universidade Federal de Goiás, Goiania: Maria Carmo Moreira; Universidade Estadual Paulista Julio de Mesquita Filho–Campus de Botucatu–Faculdade de Medicina, Botucatu: Hugo Yoo; Hospital Alemao Oswaldo Cruz, Sao Paulo: Monica Silveira Lapa. Canada—Vancouver General Hospital, the Lung Centre, Vancouver, British Columbia: John Swiston; Peter Lougheed Centre, Calgary, Alberta: Naushad Hirani; London Health Sciences Centre–Victoria Hospital, London, Ontario: Sanjay Mehta. Mazankowski Heart Institute, University of Alberta Hospital, Edmonton, Alberta: Evangelos Michelakis. Chile—Pontificia Universidad Católica de Chile, Santiago: Pablo Sepulveda; Asesorias E Investigaciones Medicas Tasol Limitada, Santiago: Monica Zagolin. China—Shanghai Pulmonary Hospital, Shanghai: Jimming Liu; Peking Union Medical College Hospital, Beijing: Zhang Shuyang; Beijing Shijitan Hospital, Beijing: Lei Pan; Renji Hospital Shanghai Jiaotong University School of Medicine, Shanghai: Bao Chunde; West China Hospital of Sichuan University, Chengdu: Yi Qun; the Second Affiliated Hospital of Nanchang Medical University, Nanchang: Cheng Xiaoshu; Xiangya Hospital, Central South University, Changsha: Yu Zaixin; the First Affiliated Hospital of Nanjing Medical University, Nanjing: Xinli Li; Guangdong General Hospital, Guangzhou: Yao Hua; Wuhan Asia Heart Hospital, Wuhan: Zhang Gangcheng; the General Hospital of Shenyang Military Region, Shenyang: Xianyang Zhu; Chinese People’s Liberation Army Hospital, Beijing: Yundai Chen; the Affiliated Hospital of Qindao University, Qingdao: Cheng Zhaozhong; Beijing Chao-Yang Hospital, Capital Medical University, Beijing: Yuanhua Yang; Zongshan Hospital Fudan University, Shanghai: Zhou Daxin; Renji Hospital Shanghai Jiaotong University School of Medicine, Shanghai: Shen Jieyan. Denmark—Aarhus University Hospital, Skejby Department of Cardiology, Hillerod: Jens Erik Nielsen-Kudsk; Rigshospitalet, Copenhagen University Hospital, Hillerod: Jorn Carlsen. France—Centre Hospitalier Universitaire de Montpellier, Hopital Arnaud de Villeneuve, Montpellier: Arnaud Bourdin; Centre Hospitalier Régional Universitaire de Lille–Hopital Claude Huriez, Lille: Eric Hachulla; Hopital Haut Leveque, Centre Francois Magendie, Pessac: Claire Dromer; Hopital Brabois, Haute-Normandie: Ari Chaouat; Centre Hospitalier Universitaire Nord, Marseilles: Martine Reynaud-Gauber; Centre Hospitalier Regional Universitaire de Besancon–Hopital Jean Minjoz, Besancon: Marie-France Seronde. Germany—Universitätsklinikum Hamburg-Eppendorf, Hamburg: Hans Klose; Universitätsklinikum Carl Gustav Carus, Dresden: Michael Halank and Gert Hoffken; Universitätsmedizin Greifswald Klinik und Poliklinik fur Innere Medizin B, Greifswald: Ralf Ewert; Universitätsklinikum Köln, Cologne: Stephan Rosenkranz; Thorax Clinic at University Hospital Heidelberg, Heidelberg: Ekkehard Grunig; Herzzentrum Duisberg, Dusseldorf: Ulrich Kruger; Berufsgenossenschaftliches Universitätsklinikum Bergmannsheil GmbH, Bochum: Juliane Kronsbein, Barbara Monika Hauptmeier, and Andrea Koch; Klinikum Wurzburg Mitte gGmbH, Munich: Matthias Held; Universitätsklinikum Regensburg, Regensburg: Tobias Johannes Lange; Universität München–Klinikum Großhadern, Munich: Claus Neurohr; Universitätsklinikum des Saarlandes, Saarbrucken: Heinrike Wilkens; Universitätsklinikum Leipzig AöR, Leipzig: Hubert Wirtz; Universitätsmedizin der Johannes Gutenberg–Universität Mainz, Mainz: Stavros Konstantinides. Greece—General Hospital of Thesaloniki “G. Papanikolaou,” Exochi: Paraskevi Argyropoulou-Pataka; University General Hospital “Attikon,” Athens: Stylianos Orfanos. India—Ruby Hall Clinic, Grant Medical Foundation, Pune: Shirish Hiremath; King Edward Memorial VII Hospital & Seth Gordhandas Sunderdas Medical College, Mumbai: Prafulla Gopinath Kerkar; Narayana Health–Narayana Institute of Cardiac Sciences, Bangalore: Pujar Venkateshacharya Suresh; Care Institute of Medical Sciences, Ahmedabad: Hemang Ashwinkumar Baxi; Apollo Hospitals, Chennai: Abraham Oomman; G. Kupppuswamy Naidu Memorial Hospital, Coimbatore: Rajpal Kanaklal Abhaichand; Maxcure Mediciti Hospitals, Hyderabad: P. K. Edla Kumar; Medanta, the Medicity, Gurgaon: Vijay Chopra and Rahul Mehrotra; Indraprastha Apollo Hospitals, New Delhi: Rajeev Kumar Rajput; Sir Ganga Ram Hospital, New Delhi: Jitendra Singh Sawhney; Apollo Hospitals International Ltd, Gandhinagar: Subir Bimalendu and Kamal Harishchandra Sharma; Care Hospitals, Hyderabad: B. K. Srinivasa Sastry. Israel—Rabin Medical Center, Beilinson Campus, Petah Tikva: Mordechai Reuben Kramer; the Chaim Sheba Medical Center, Ramat Gan: Michael Jonathan Segel and Issahar Ben-Dov; Hadassah–Hebrew University Hospital, Hadassah Medical Organization, Jerusalem: Neville Berkman; Rambam Health Care Campus, Haifa: Mordechai Yigla; the Lady Davis Carmel Medical Center, Haifa: Yochai Adir. Italy—Azienda Ospedaliera Specialistica dei Colli-Ospedale Monaldi, Napoli: Michael D’Alto; Universita di Roma “Sapienza”–Policlinico Umberto I, Rome: Carmine Dario Vizza; Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo di Pavia, Pavia: Laura Scelsi; Instituto Mediterraneo Trapianti Terapie Alta Specializzazione di Palmero, Palermo: Patrizio Vitulo. Mexico—Instituto Nacional de Cardiología Ignacio Chávez, Mexico City: Tomas Rene Pulido; Unidad De Inv Clinica En Medicina, Monterrey: Carlos Jerjes-Sanchez. The Netherlands—Vrije Universiteit Medisch Centrum, Amsterdam: Anko Boonstra; Radbound University Nijmegan Medical Center, Amsterdam: Madelon Clementina Vonk. Poland—Uniwersytecki Szpital Kliniczny w Bialymstoku, Warsaw: Bozena Sobkowicz; Szpital Kliniczny Przemienienia Panskiego, Warsaw: Tatiana Mularek-Kubzdela; Europejskie Centrum Zdrowia Otwock, Warsaw: Adam Torbicki; Krakowski Szpital Specjalistyczny im Jana Pawla II, Warsaw: Piotr Podolec. Singapore—National Heart Centre Singapore: Lim Soo Teik; National University Heart Centre, Singapore: Wei Luen James Yip. South Korea—Severance Hospital, Yonsei University Health System, Seoul: Hyuk-Jae Chang; Seoul National University Hospital, Seoul: Hyung-Kwan Kim and Jun-Bean Park; Samsung Medical Center, Seoul: Sung-A Chang, and Duk-Kyung Kim; Gachon University Gil Medical Center, Incheon: Wook-Jin Chung; Assan Medical Center, Seoul: Jong-Min Song. Sweden—Karolinska University Hospital, Solna: Magnus Nissell; Sahlgrenska University Hospital, Stockholm: Clara Hjalmarsson and Bengt Rundqvist. Taiwan—Kaohsiung Veterans General Hospital, Kaohsiung: Wei-Chun Huang and Chin-Chang Cheng; National Cheng Kung University Hospital, Tainan: Chih-Hsin Hsu; National Taiwan University Hospital, Taipei: Hsao-Hsun Hsu; China Medical University Hospital, Taichung: Kuo-Yang Wang. United Kingdom—Royal Free London National Health Service Foundation Trust, London: John Gerard Coghlan; Sheffield Teaching Hospitals National Health Service Foundation Trust, Royal Hallamshire Hospital, Sheffield: David Gerard Kiely; Royal Papworth Hospital, Cambridgeshire: Joanna Wanda Pepke-Zaba; Freeman Hospital, Newcastle-upon-Tyne: James Lawrence Lordan and Paul Anthony Corris. United States—University of Iowa Hospitals and Clinics, Iowa City, Iowa: Linda Cadaret and Sif Hansdottir; Harbor University of California Los Angeles Medical Center, Los Angeles, California: Ronald Jack Oudiz; University of Colorado Denver, Denver, Colorado: David B. Badesch; University of Pittsburgh Medical Center Presbyterian Hospital, Pittsburgh, Pennsylvania: Michael Mathier; University Hospitals Case Medical Center, Cleveland, Ohio: Robert Schilz; Tufts Medical Center, Boston, Massachusetts: Nicholas Hill; Brigham and Women’s Hospital, Boston, Massachusetts: Aaron Waxman; Legacy Medical Group–Pulmonary and Sleep Clinic, Portland, Oregon: Catherine J. Markin; Aurora St. Luke’s Medical Center, Milwaukee, Wisconsin: Diane Lynn Zwicke; Emory University School of Medicine, the Emory Clinic, Atlanta, Georgia: Micah Fisher; the Ohio State University Wexner Medical Center–Martha Morehouse Medical Plaza, Columbus, Ohio: Veronica Franco and Namita Sood; University of Maryland Hospital Division of Cardiology, Baltimore, Maryland: Myung H. Park; University of California Davis Medical Center, Sacramento, California: Roblee Allen; Arizona Pulmonary Specialists, Ltd, Phoenix, Arizona: Jeremy P. Feldman; Community Regional Medical Centers/University of California San Francisco Fresno, Fresno, California: Vijay Balasubramanian; University of Florida College of Medicine, Gainesville, Florida: Vandana Kavita Seeram and Abubakr Bajwa; University of Nebraska Medical Center, Omaha, Nebraska: Austin B. Thompson III; Barnabas Health Newark Beth Israel Medical Center, Newark, New Jersey: Christina Migliore; University of Cincinnati/UC Health, Cincinnati, Ohio: Jean Elwing; Kentuckiana Pulmonary Research Center, Louisville, Kentucky: John W. McConnell; Cleveland Clinic Florida, Weston, Florida: Jinesh P. Mehta and Franck Farzad Rahaghi; Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania: J. Eduardo Rame; Oregon Health and Science University, Portland, Oregon: Akram Khan; University of Texas Medical School, Houston, Texas: Bela Patel; HeartCare Midwest, Peoria, Illinois: Ron M. Oren; Rhode Island Hospital, Providence, Rhode Island: James R. Klinger; University of Florida Clinical Research Center, Gainesville, Florida: Hassan Alnuaimat; Beaumont Health Pulmonary Hypertension Center, Royal Oak, Michigan: Samuel Allen; Indiana University Health North Hospital, Indianapolis, Indiana: William Harvey; Sentara Pulmonary & Critical Care Specialists, Sentara Heart Hospital, Cincinnati, Ohio: Michael S. Eggert; Cedars-Sinai Medical Center, Beverly Hills, California: Antoine Hage; Piedmont Healthcare Pulmonary & Critical Care Research, Atlanta, Georgia: Chad E. Miller; Henry Ford Health System, Detroit, Michigan: Rana Awdish and Hector Cajigas; Virginia Commonwealth University Medical Center, Richmond, Virginia: Daniel Grinnan; Asheville Cardiology Associates, Asheville, North Carolina: Benjamin Howard Trichon; Augusta University, Augusta, Georgia: Clark McDonough; Mary M. Parkes Center, University of Rochester Medical Center, Rochester, New York: R. James White; University of Arizona Clinical and Translational Science Research Center, Tucson, Arizona: Franz Rischard.
Footnotes
Supported by United Therapeutics Corporation.
A complete list of the FREEDOM-EV Investigators may be found before the beginning of the References.
Author Contributions: R.J.W. is the Principal Investigator of the study and contributed substantially to the protocol, beginning with amendment 2; actively recruited and treated participants in the study; served as the academic lead for analysis and interpretation of the data; and wrote the initial manuscript draft. C.J.-S., G.M.B.M., T.P., P.S., K.Y.W., E.G., S.H., Z.Y., Z.G., W.L.J.Y., S.Z., and A.K. are investigators in the study and actively recruited and treated participants in the study and made revisions to the manuscript. V.F.T. shared leadership responsibility with R.J.W. in the analysis and interpretation of data and early revisions of the manuscript. R.G. served as medical monitor for the study and provided critical input to data analysis and manuscript revision. C.Q.D. managed the study database, performed and directed all the statistical analyses per the statistical analysis plan, helped with interpretation of the statistics and their inclusion in the manuscript, supported additional analyses requested by the other authors, and revised the manuscript throughout its development. All authors approved the decision to submit the manuscript for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201908-1640OC on November 25, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the FREEDOM-EV Investigators, Graciela Noemi Svetliza, Adrian Jose Lescano, Guillermo Roberto Bortman, Fabian Antonio Diez, Christian Edgardo Botta, John Fitzgerald, Eelke Feenstra, Fiona Dawn Kermeen, Anne Margaret Keogh, Trevor John Williams, Peter Paul Yousseff, Benjamin Joh-Han Ng, David McNaughton Smallwood, Nathan Brent Dwyer, Martin Russell Brown, Irene M Lang, Regina Steringer-Mascherbauer, Jaquelina Sonoe Ota Arakaki, Frederico Thadeu Assis Figueiredo Campos, Ricardo de Amorim Correa, Rogerio de Souza, Gisela M. Bohns Meyer, Maria Auxiliadora Carmo Moreira, Hugo Hyung Bok Yoo, Monica Silveira Lapa, John Swiston, Naushad Hirani, Sanjay Mehta, Evangelos Michelakis, Pablo Andres Sepulveda, Monica Maria Zagolin Blancaire, Jimming Liu, Zhang Shuyang, Lei Pan, Bao Chunde, Yi Qun, Cheng Xiaoshu, Yu Zaixin, Xinli Li, Yao Hua, Zhang Gangcheng, Xianyang Zhu, Yundai Chen, Cheng Zhaozhong, Yuanhua Yang, Zhou Daxin, Shen Jieyan, Jens Erik Nielsen-Kudsk, Jorn Carlsen, Arnaud Bourdin, Eric Hachulla, Claire Dromer, Ari Chaouat, Martine Reynaud-Gauber, Marie-France Seronde, Hans Klose, Michael Halank, Gert Hoffken, Ralf Ewert, Stephan Rosenkranz, Ekkehard Grunig, Ulrich Kruger, Juliane Kronsbein, Barbara Monika Hauptmeier, Andrea Koch, Matthias Held, Tobias Johannes Lange, Claus Neurohr, Heinrike Wilkens, Hubert Rolf Wilhelm Wirtz, Stavros Konstantinides, Paraskevi Argyropoulou-Pataka, Stylianos Orfanos, Shirish Hiremath, Prafulla Gopinath Kerkar, Pujar Venkateshacharya Suresh, Hemang Ashwinkumar Baxi, Abraham Oomman, Rajpal Kanaklal Abhaichand, Padma Kumar Edla Arjun, Vijay Chopra, Rahul Mehrotra, Rajeev Kumar Rajput, Jitendra Pal Singh Sawhney, Subir Bimalendu, Kamal Harishchandra Sharma, Bhagavathula Kutumba Srinivasa Sastry, Mordechai Reuben Kramer, Michael Jonathan Segel, Issahar Ben-Dov, Neville Berkman, Mordechai Yigla, Yochai Adir, Michael D’Alto, Carmine Dario Vizza, Laura Scelsi, Patrizio Vitulo, Tomas Rene Pulido, Carlos Jerjes-Sanchez, Anko Boonstra, Madelon Clementina Vonk, Bozena Sobkowicz, Tatiana Mularek-Kubzdela, Adam Torbicki, Piotr Podolec, Lim Soo Teik, Wei Luen James Yip, Hyuk-Jae Chang, Hyung-Kwan Kim, Jun-Bean Park, Sung-A Chang, Duk-Kyung Kim, Sung-A Chang, Wook-Jin Chung, Jong-Min Song, Magnus Nissell, Clara Hjalmarsson, Bengt Rundqvist, Wei-Chun Huang, Chin-Chang Cheng, Chih-Hsin Hsu, Hsao-Hsun Hsu, Kuo-Yang Wang, John Gerard Coghlan, David Gerard Kiely, Joanna Wanda Pepke-Zaba, James Lawrence Lordan, Paul Anthony Corris, Linda Cadaret, Sif Hansdottir, Ronald Jack Oudiz, David B. Badesch, Michael Mathier, Robert Schilz, Nicholas Hill, Aaron Waxman, Catherine J. Markin, Diane Lynn Zwicke, Micah Fisher, Veronica Franco, Namita Sood, Myung H. Park, Roblee Allen, Jeremy P. Feldman, Vijay Balasubramanian, Vandana Kavita Seeram, Abubakr Bajwa, Austin B. Thompson, III, Christina Migliore, Jean Elwing, John W. McConnell, Jinesh P. Mehta, Franck Farzad Rahaghi, J. Eduardo Rame, Akram Khan, Bela Patel, Ron M. Oren, James R. Klinger, Hassan Alnuaimat, Samuel Allen, William Harvey, Michael S. Eggert, Antoine Hage, Chad E. Miller, Rana Lee Adawi Awdish, Hector Cajigas, Daniel Grinnan, Benjamin Howard Trichon, Clark McDonough, R. James White, and Franz Rischard
References
- 1.Humbert M, Lau EM, Montani D, Jaïs X, Sitbon O, Simonneau G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation. 2014;130:2189–2208. doi: 10.1161/CIRCULATIONAHA.114.006974. [DOI] [PubMed] [Google Scholar]
- 2.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani HA, et al. SERAPHIN Investigators. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 3.Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, et al. GRIPHON Investigators. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522–2533. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 4.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 5.Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127:624–633. doi: 10.1161/CIRCULATIONAHA.112.124388. [DOI] [PubMed] [Google Scholar]
- 6.Chakinala MM, Feldman JP, Rischard F, Mathier M, Broderick M, Leedom N, et al. Transition from parenteral to oral treprostinil in pulmonary arterial hypertension. J Heart Lung Transplant. 2017;36:193–201. doi: 10.1016/j.healun.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 7.White RJ, Sanchez Diaz CJ, Bohns Meyer GM, Pulido T, Sepulveda P, Wang KY, et al. Treatment with oral treprostinil is associated with improved survival in pulmonary arterial hypertension participants from the FREEDOM-EV Study [abstract] Presented at the 13th PVRI Annual World Congress on PVD; January 31–February 3, 2019, Barcelona, Spain. [Google Scholar]
- 8.Tapson VF, Sanchez Diaz CJ, Bohns Meyer GM, Pulido T, Sepulveda P, Wang KY, et al. Treatment with oral treprostinil delays time to clinical worsening in patients with pulmonary arterial hypertension: results from FREEDOM-EV [abstract] J Heart Lung Transplant. 2019;38:S94–S95. [Google Scholar]
- 9.White RJ, Sanchez Diaz CJ, Bohns Meyer GM, Pulido T, Sepulveda P, Wang KY, et al. Risk scores and risk-based stratification of clinical worsening events in pulmonary arterial hypertension participants treated with oral treprostinil: FREEDOM-EV [abstract] Am J Respir Crit Care Med. 2019;199:A5587. [Google Scholar]
- 10.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(Suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al. AMBITION Investigators. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373:834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 12.Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50:1700889–1700899. doi: 10.1183/13993003.00889-2017. [DOI] [PubMed] [Google Scholar]
- 13.Hoeper MM, Pittrow D, Opitz C, Gibbs JSR, Rosenkranz S, Grünig E, et al. Risk assessment in pulmonary arterial hypertension. Eur Respir J. 2018;51:1702606. doi: 10.1183/13993003.02606-2017. [DOI] [PubMed] [Google Scholar]
- 14.Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156:323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Tapson VF, Jing ZC, Xu KF, Pan L, Feldman J, Kiely DG, et al. FREEDOM-C2 Study Team. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. 2013;144:952–958. doi: 10.1378/chest.12-2875. [DOI] [PubMed] [Google Scholar]
- 16.Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142:1383–1390. doi: 10.1378/chest.11-2212. [DOI] [PubMed] [Google Scholar]
- 17.White RJ, Torres F, Allen R, Jerjes C, Pulido T, Yehle D, et al. Pharmacokinetics of oral treprostinil sustained release tablets during chronic administration to patients with pulmonary arterial hypertension. J Cardiovasc Pharmacol. 2013;61:474–481. doi: 10.1097/FJC.0b013e31828685da. [DOI] [PubMed] [Google Scholar]
- 18.Benza RL, Farber HW, Frost A, Ghofrani HA, Gómez-Sánchez MA, Langleben D, et al. REVEAL risk scores applied to riociguat-treated patients in PATENT-2: impact of changes in risk score on survival. J Heart Lung Transplant. 2018;37:513–519. doi: 10.1016/j.healun.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Benza RL, Miller DP, Foreman AJ, Frost AE, Badesch DB, Benton WW, et al. Prognostic implications of serial risk score assessments in patients with pulmonary arterial hypertension: a Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) analysis. J Heart Lung Transplant. 2015;34:356–361. doi: 10.1016/j.healun.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Weatherald J, Boucly A, Sahay S, Humbert M, Sitbon O. The low-risk profile in pulmonary arterial hypertension: time for a paradigm shift to goal-oriented clinical trial endpoints? Am J Respir Crit Care Med. 2018;197:860–868. doi: 10.1164/rccm.201709-1840PP. [DOI] [PubMed] [Google Scholar]
- 21.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 22.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 23.Lajoie AC, Lauzière G, Lega JC, Lacasse Y, Martin S, Simard S, et al. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med. 2016;4:291–305. doi: 10.1016/S2213-2600(16)00027-8. [DOI] [PubMed] [Google Scholar]
- 24.Hoeper MM, McLaughlin VV, Barberá JA, Frost AE, Ghofrani HA, Peacock AJ, et al. Initial combination therapy with ambrisentan and tadalafil and mortality in patients with pulmonary arterial hypertension: a secondary analysis of the results from the randomised, controlled AMBITION study. Lancet Respir Med. 2016;4:894–901. doi: 10.1016/S2213-2600(16)30307-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.