To the Editor:
HIV infection is an independent risk factor for chronic obstructive pulmonary disease (COPD) (odds ratio, 1.14; 95% confidence interval, 1.05–1.25) (1). Mechanisms remain unclear, but some observational studies have suggested that HIV antiretroviral treatment (ART) might lead to worse lung function and subsequent COPD (2, 3).
We addressed the effects of HIV treatment on lung function in the START (Strategic Timing of Antiretroviral Treatment) Trial Pulmonary Substudy. We previously published initial results from this randomized trial in which 1,026 HIV-positive, ART-naive adults (from 80 sites in 20 high- and low- to middle-income countries) with CD4+ T-cell counts >500 cells/mm3 were randomly assigned to begin ART immediately or defer until CD4+ T-cell counts were 350 cells/mm3 or AIDS developed (4). All participants provided informed consent, and all site institutional review boards/ethics committees approved the substudy (ClinicalTrials.gov identifiers: NCT 01797367 and NCT 00867048).
The parent START trial demonstrated clear benefits of immediate ART on reduction of AIDS and non-AIDS events (5), at which time our primary substudy outcome of lung function decline (slope of FEV1) did not differ between immediate and deferred ART. When the primary outcome was analyzed in May 2015, there were two major limitations in our results. First, the initial follow-up time was relatively short, at a median of 2.0 years. Second, the duration of ART exposures was limited by only 55% of participants in the deferred treatment group having started ART, which precluded adequately assessing difference by ART regimen on lung function decline.
After the primary results of START demonstrated the benefits of immediate treatment, ART initiation was recommended to all participants in the deferred group, and all participants in the Pulmonary Substudy continued to be followed through the end of December 2016, at which point median follow-up time was 3.9 years, thereby improving our ability to investigate the effects of differing ART regimens on lung function decline. We now present the final results of the randomized comparisons and an observational analysis comparing rate of longitudinal lung function decline among differing ART regimens.
We collected postbronchodilator spirometry before randomization and annually during follow-up. Blinded central review of all spirometry data was performed by K.M.K. Our primary outcome was rate of lung function decline, expressed as FEV1 slope in milliliters per year. We compared FEV1 slope using repeated measures mixed models (SAS 9.4), using only good-quality spirometry data and strict intention to treat. We additionally compared differing initial ART regimens to assess for potential class-specific effects. This analysis included only those who began ART before study completion and had at least one good-quality FEV1 measurement both before ART initiation and while receiving ART. All ART regimens in START contained two nucleoside/nucleotide reverse-transcriptase inhibitor (NRTI) drugs, and 90% of participants were prescribed NRTI components of tenofovir disoproxil fumarate and emtricitabine (or lamivudine), precluding meaningful comparisons between individual NRTIs. Therefore, we focused on comparisons between the nonnucleoside reverse-transcriptase inhibitor (NNRTI), protease inhibitor, and integrase strand transfer inhibitor (INSTI) classes of antiretroviral drugs.
A total of 1,026 participants were randomly assigned to immediate ART (n = 518) or deferred ART (n = 508). Participants were relatively young (median age, 36 yr), recently diagnosed with HIV (median time since HIV diagnosis, 1.2 yr), with median baseline CD4+ T-cell count of 648 cells/mm3. Of the participants, 29.1% were female, 28.3% were current smokers, and baseline lung function was generally good (median FEV1 of 96.2% of predicted), with COPD prevalence at study entry of 6.8%. By the end of follow-up, ART had been initiated in 98.9% of the immediate ART group and 88.6% of the deferred group. In the immediate ART group, median CD4+ count at the time of ART initiation was 648 cells/mm3, and 95.5% of follow-up time was spent on ART. In the deferred ART group, median CD4+ count at the time of ART initiation was 482 cells/mm3, and 45.1% of follow-up time was spent on ART.
In addition to the 1,026 baseline tests, we collected 3,484 follow-up spirometry tests over a median follow-up time of 3.9 years for a total of 3,674 patient-years of spirometry follow-up. For the primary outcome of FEV1 slope, we found no difference in slopes between the immediate and deferred ART groups in smokers (difference, +2.2 ml/yr; 95% confidence interval, −17.9 to +22.2 ml/yr; P = 0.83) or nonsmokers (difference, −4.1 ml/yr; 95% confidence interval, −14.5 to +6.3 ml/yr; P = 0.44; Table 1). Analyses pooling baseline smokers and nonsmokers, then adjusting for either baseline smoking status or smoking status at each study visit, similarly showed no difference in FEV1 slopes between the immediate and deferred ART groups.
Table 1.
Primary Outcome of FEV1 Slope Comparisons in Those Randomized to Immediate versus Deferred ART Initiation
| FEV1 Slope (ml/yr) | 95% Confidence Interval (ml/yr) | P Value | |
|---|---|---|---|
| Baseline smokers | |||
| Immediate ART (n = 135) | −36.6 | −22.3 to −51.0 | |
| Deferred ART (n = 156) | −38.8 | −24.8 to −52.8 | |
| Difference | +2.2 | −17.9 to +22.2 | 0.83 |
| Baseline nonsmokers | |||
| Immediate ART (n = 383) | −26.9 | −19.8 to −34.1 | |
| Deferred ART (n = 353) | −22.8 | −15.3 to −30.4 | |
| Difference | −4.1 | −14.5 to +6.3 | 0.44 |
| Pooled analysis, adjusted for baseline smoking status | |||
| Immediate ART (n = 518) | −29.4 | −22.9 to −35.9 | |
| Deferred ART (n = 508) | −27.6 | −20.8 to −34.3 | |
| Difference | −1.8 | −11.2 to +7.5 | 0.70 |
| Pooled analysis, adjusted for smoking status at each study visit | |||
| Immediate ART (n = 518) | −29.3 | −22.8 to −35.8 | |
| Deferred ART (n = 508) | −27.3 | −20.6 to −34.0 | |
| Difference | −2.0 | −11.3 to +7.4 | 0.68 |
Definition of abbreviation: ART = antiretroviral treatment.
Analyses are restricted to only spirometry data from tests passing central review quality control criteria.
Slopes of FVC and FEV1/FVC ratio did not differ between immediate and deferred ART groups. After excluding 67 participants with COPD at study entry, incident COPD occurred in 7.4% of participants randomly assigned to immediate ART and 6.7% of those randomly assigned to deferred ART (odds ratio, 1.13; 95% confidence interval, 0.64 to 1.99; P = 0.67).
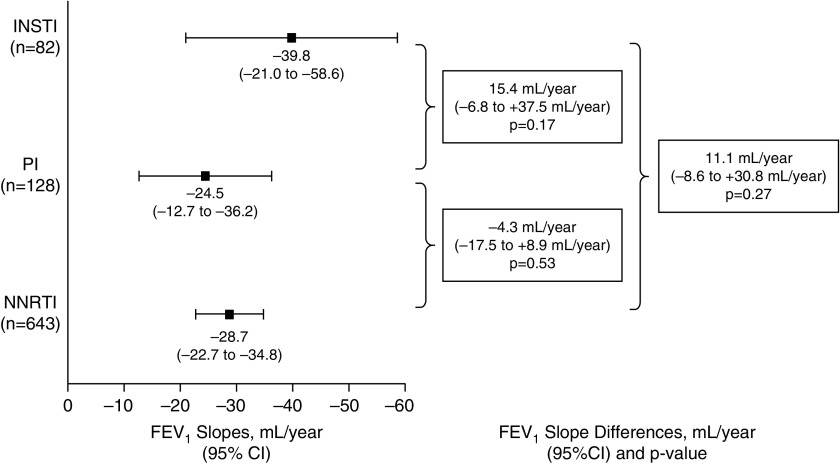
In addition to the dual NRTIs component of ART regimens, NNRTIs (n = 643; 93% efavirenz, 7% rilpivirine) were the most common third drug, followed by protease inhibitors (n = 128; 47% darunavir, 43% atazanavir, and 10% other protease inhibitor) and INSTIs (n = 82; 56% raltegravir, 26% elvitegravir, and 18% dolutegravir). FEV1 slope did not differ by ART class, although the small number of participants on INSTIs resulted in imprecise estimates of FEV1 slope on INSTIs (Figure 1).
Figure 1.
Slopes of FEV1 by initial antiretroviral treatment (ART) drug class. Differences were adjusted for age, sex, race, region of world, treatment assignment (immediate vs. deferred ART strategy), smoking status, baseline chronic obstructive pulmonary disease, and log10 HIV-RNA before initiating ART. CI = confidence interval; INSTI = integrase strand transfer inhibitor; PI = protease inhibitor; NNRTI = nonnucleoside reverse transcriptase inhibitor.
These final results of the randomized START Pulmonary Substudy further support that immediate ART initiation does not significantly worsen the rate of lung function decline in early HIV infection. We caution that our participants were relatively young, with recent HIV diagnoses, so generalizability to older persons and those with longer duration of HIV infection is not clear. We also had limited power for sex-specific analysis, which is relevant, given the general population data that women have unique susceptibility factors for cigarette smoke–related COPD (6, 7). Our data also suggest that the class of initial ART selected does not affect subsequent lung function decline, although we had limited ability to accurately estimate FEV1 slope in the increasingly prescribed INSTI class of first-line ART (8).
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all START Pulmonary Substudy participants for their contributions to our scientific understanding of lung disease in HIV infection.
Footnotes
The START (Strategic Timing of Antiretroviral Treatment) Pulmonary Substudy was supported by the NHLBI (R01 HL096453); the parent START trial was primarily supported by the National Institute of Allergy and Infectious Diseases Division of AIDS (UM1 AI068641 and UM AI120197), with additional support from the German Ministry of Education and Research, the European AIDS Treatment Network (NEAT), the Australian National Health and Medical Research Council, and the UK Medical Research Council and National Institute for Health Research. The Veterans Health Administration Office of Research and Development also provided protected research time in support of this study. The University of Minnesota served as sponsor of the study. None of the funders nor the sponsor had any input regarding the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not reflect the views of the U.S. Government, the NIH, the Department of Veterans Affairs, the funders, the sponsors, or any of the authors’ affiliated academic institutions.
Author Contributions: K.M.K. conceived the study, obtained funding, and drafted the manuscript; K.M.K., J.V.B., and G.C. designed the study; E.B., K.M.E.F., E.N., and A.L.R. acquired the data; G.C. and J.E.C. performed the primary statistical analyses; all authors critically revised the manuscript for important intellectual content and approved the final manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis.
Originally Published in Press as DOI: 10.1164/rccm.201911-2266LE on December 16, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Bigna JJ, Kenne AM, Asangbeh SL, Sibetcheu AT. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob Health. 2018;6:e193–e202. doi: 10.1016/S2214-109X(17)30451-5. [DOI] [PubMed] [Google Scholar]
- 2.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4:e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, Weinman R, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunisaki KM, Niewoehner DE, Collins G, Aagaard B, Atako NB, Bakowska E, et al. INSIGHT START Pulmonary Substudy Group. Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial. Lancet Respir Med. 2016;4:980–989. doi: 10.1016/S2213-2600(16)30319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tashkin DP, Altose MD, Connett JE, Kanner RE, Lee WW, Wise RA The Lung Health Study Research Group. Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:1802–1811. doi: 10.1164/ajrccm.153.6.8665038. [DOI] [PubMed] [Google Scholar]
- 7.Prescott E, Bjerg AM, Andersen PK, Lange P, Vestbo J. Gender difference in smoking effects on lung function and risk of hospitalization for COPD: results from a Danish longitudinal population study. Eur Respir J. 1997;10:822–827. [PubMed] [Google Scholar]
- 8.Saag MS, Benson CA, Gandhi RT, Hoy JF, Landovitz RJ, Mugavero MJ, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379–396. doi: 10.1001/jama.2018.8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.