To the Editor:
To date, there have been more than 1,000 reports across the United States in which the use of e-cigarettes, or vaping products, have been associated with cases of acute lung injury, a term that has been recently described as “e-cigarette or vaping product use-associated lung injury” (EVALI) (1, 2). In most cases, patients are usually male (70%) and younger than 35 years of age (80%) (2). Clinical manifestations are heterogeneous, with a wide range of symptoms including cough, fever, dyspnea, vomiting, diarrhea, headache, dizziness, and chest pain after the use of vaping devices (1). Imaging findings reveal a range of patterns suggesting different mechanisms of injury. Most cases have basilar-predominant consolidation and ground-glass opacities, usually with areas of lobular or subpleural sparing (3). Histopathologic findings are usually nonspecific, but foamy macrophages and pneumocyte vacuolization appear in most cases and are regarded as useful diagnostic clues (4).
Although the exact pathophysiology of EVALI remains to be established, it appears that there are multiple compounds present in the vaporized material that could potentially cause injury to the lungs, resembling a chemical pneumonitis (1, 2). No single product, or origin, has been linked to all cases in the recent outbreak, but preliminary data suggest the use of tetrahydrocannabinol-containing products to be associated with most cases, particularly from those obtained from street or other informal sources (2). As a consequence, in the United States, there is an active surveillance program recommending that the public stop using vaping products until new safety data emerge (2). The aim of this report is to describe a case of vaping-associated pulmonary illness in a patient from Ecuador. To the best of our knowledge, our case is among the first to be officially reported in South America, highlighting the need for proper implementation of surveillance programs and increasing the awareness of the medical community and general public of the potential risks associated with the use of e-cigarettes and vaping devices.
Case Report
A 20-year old male patient presented to the emergency department after 1 week of worsening symptoms consisting of nasal congestion, rhinorrhea, subjective fever, chest tightness, shortness of breath, wheezing, and persistent tension-like headache in the fronto-orbital region. On admission, his vital signs were blood pressure 130/85 mm Hg, heart rate 95/min, respiratory rate 18/min, temperature 38.3°C, and 94% saturation at room air. On physical examination, the patient was diaphoretic and in visible distress, with scattered wheezes in both pulmonary fields. The rest of the examination was within normal limits. Past medical history was positive for seasonal allergic rhinitis and a 4-year use of e-cigarettes, including various products containing tetrahydrocannabinol compounds. The patient was hospitalized on a presumptive diagnosis of acute rhinosinusitis and possible asthma exacerbation.
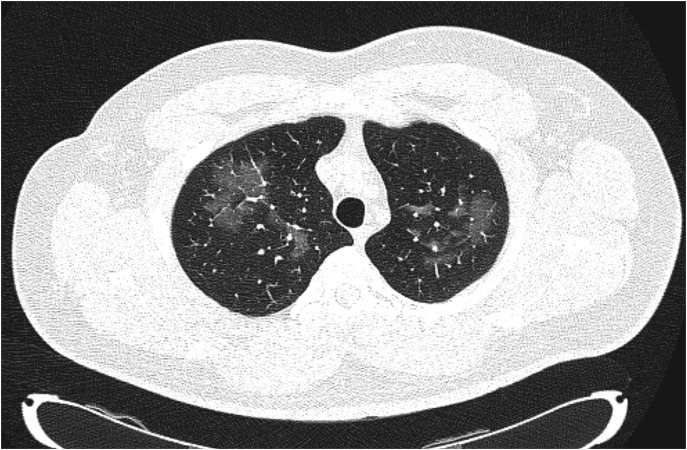
Laboratory results were unremarkable, as were the levels of C-reactive protein (Table 1). Computed tomography (CT) scan of the paranasal sinuses showed changes consistent with a chronic allergic or inflammatory process. An empiric course of antibiotics, intravenous hydration, and analgesia were initiated, as well as respiratory therapy with bronchodilators. Reevaluation at 48 hours since initial hospitalization revealed partial improvement. However, the patient still complained, albeit to a lesser extent, of chest tightness and shortness of breath, which prompted further imaging. CT scan of the chest revealed bilateral ground-glass opacities (Figure 1), raising the suspicion of EVALI. Intravenous steroids were started, and the patient promptly saw improvement in his pulmonary symptoms. One week after initial hospitalization, the patient was discharged home on oral corticosteroids with favorable response and was encouraged to discontinue the use of vaping products. Meanwhile, on a follow-up appointment 1 month later, pulmonary function tests were performed that revealed flow and volume values within normal limits, including the DlCO. The patient also reported complete resolution of his symptoms. Written informed consent was obtained from the patient for the publication of the case report and any accompanying images.
Table 1.
Laboratory Work-up at Initial Hospitalization
| Laboratory Test | Result | Reference Values |
|---|---|---|
| Platelets, × 109/L | 334 | 140–400 |
| WBC, × 109/L | 9,970 | 4.1–11 |
| Neutrophils, % | 74 | 55–75 |
| Lymphocytes, % | 14* | 17–45 |
| Hb, g/dl | 16.3 | 12–16.0 |
| CRP, mg/L | 3.1 | 0–5 |
| BUN, mg/dl | 27 | 19–43 |
| Creatinine, mg/dl | 1.2 | 0.8–1.5 |
| Sodium, mg/dl | 138 | 135–145 |
| Potassium, mg/dl | 4.3 | 3.5–5.1 |
| Chloride, mg/dl | 95 | 98–106 |
| PT, s | 11.8 | 9.1–12.1 |
| PTT, s | 29.7 | 24.3–35 |
| INR | 1.06* | 1.0 |
Definition of abbreviations: BUN = blood urea nitrogen; CRP = C-reactive protein; Hb = hemoglobin; INR = international normalized ratio; PT = prothrombin time; PTT = partial thromboplastin time; WBC = white blood cells.
Denotes an out-of-range value.
Figure 1.
High-resolution computed tomography scan of the chest demonstrating bilateral areas of ground-glass opacities in the apical regions of the lungs (B70f protocol).
Discussion
According to the CDC, a confirmed case of pulmonary disease associated with e-cigarette use requires a history of vaping or dabbing 90 days before the onset of symptoms, pulmonary infiltrates on the CT scan, absence of pulmonary infection, and no evidence of an alternative viable diagnosis (5). In the case presented, the patient reported a 4-year history of various e-cigarette and vaping products. Moreover, bilateral ground-glass opacities in the lung fields were observed in the CT scan, accompanied with unremarkable laboratory findings. We were unable to find evidence in our patient that might have explained the lower respiratory symptoms. These diagnostic criteria help to define a confirmed case of EVALI from an epidemiologic standpoint; however, we believe that they also represent a useful tool for the clinical practice, as other studies such as pulmonary function tests, BAL, and histopathology result in heterogeneous data that involve a more technical and time-consuming approach, especially in the acute setting (2).
In Latin America, only 7 countries have regulations that include either product prohibitions or restrictions related to e-cigarettes (6). Even though these policies are focused on restricting advertisement and promotion, as well as sales numbers, none of them enforces the inclusion of a health warning labeling or a description of the ingredients/flavors included (6). This is of utmost importance, as some chemicals that are used to flavor e-liquids, such as diacetyl, are associated with lung disease (7). Moreover, in another publication assessing the level of awareness of e-cigarette smoking and its adverse effects, roughly one third of participants realized that e-cigarettes may have negative respiratory health effects, whereas only 16% acknowledge its toxic nature (8). When we asked our patient about specific brands and components, he reported that at first, he consumed a trademarked product, but he switched to flavors with no official brand or regulation, as they were easier and cheaper to obtain.
Vaping-associated lung injury is not only a medical problem but also a social issue. Although its diagnostic criteria have not been defined with a high level of evidence, the situation is also challenged by low awareness of the potential risks by consumers, wide availability of these products, and loose regulations involving these devices. Finally, we want to stress the need for the implementation of surveillance programs in the region, as well as for increasing the awareness of both the general public and medical community. Further research is needed, particularly in South America, where to the best of our knowledge, no cases of EVALI have been published prior to this one.
Supplementary Material
Acknowledgments
Acknowledgment
Special thanks to Clínica Guayaquil and Universidad Espíritu Santo for their continuous support in the field of medical research.
Footnotes
Author Contributions: E.B. and I.C.-O. developed the research question for the case report; C.V.P. participated in the data recollection process; E.B., M.F., E.V., C.V.P., and I.C.-O. wrote the final manuscript; and all authors read and approved the final version.
Originally Published in Press as DOI: 10.1164/rccm.201910-2002LE on December 6, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Carlos WG, Crotty Alexander LE, Gross JE, Dela Cruz CS, Keller JM, Pasnick S, et al. Vaping-associated pulmonary illness (VAPI) Am J Respir Crit Care Med. 2019;200:P13–P14. doi: 10.1164/rccm.2007P13. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Outbreak of lung injury associated with E-cigarette use, or vaping. 2019 [accessed 2019 Oct 12] Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html.
- 3.Henry TS, Kanne JP, Kligerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381:1486–1487. doi: 10.1056/NEJMc1911995. [DOI] [PubMed] [Google Scholar]
- 4.Butt YM, Smith ML, Tazelaar HD, Vaszar LT, Swanson KL, Cecchini MJ, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381:1780–1781. doi: 10.1056/NEJMc1913069. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Severe pulmonary disease associated with electronic-cigarette–product use: interim guidance. 2019 [accessed 2019 Oct 15] Available from: https://www.cdc.gov/mmwr/volumes/68/wr/mm6836e2.htm.
- 6.Kennedy RD, Awopegba A, De León E, Cohen JE. Global approaches to regulating electronic cigarettes. Tob Control. 2017;26:440–445. doi: 10.1136/tobaccocontrol-2016-053179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, et al. Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect. 2016;124:733–739. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohde JA, Noar SM, Mendel JR, Hall MG, Baig SA, Ribisl KM, et al. E-cigarette health harm awareness and discouragement: implications for health communication Nicotine Tob Res[online ahead of print] 9 Oct 2019 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.