Abstract
Background
It is generally assumed that there have been mixed results in the literature regarding the association between ambient particulate matter (PM) and myocardial infarction (MI). The aim of this meta-analysis was to explore the rate of short-term exposure PM with aerodynamic diameters ≤2.5 μm (PM2.5) and examine its potential effect(s) on the risk of MI.
Methods
A systematic search was conducted on databases like PubMed, Scopus, Web of Science, and Embase with components: “air pollution” and “myocardial infarction”. The summary relative risk (RR) and 95% confidence intervals (95%CI) were also calculated to assess the association between the PM2.5 and MI.
Results
Twenty-six published studies were ultimately identified as eligible candidates for the meta-analysis of MI until Jun 1, 2018. The results illustrated that a 10-μg/m 3 increase in PM2.5 was associated with the risk of MI (RR = 1.02; 95% CI 1.01–1.03; P ≤ 0.0001). The heterogeneity of the studies was assessed through a random-effects model with p < 0.0001 and the I2 was 69.52%, indicating a moderate degree of heterogeneity. We also conducted subgroup analyses including study quality, study design, and study period. Accordingly, it was found that subgroups time series study design and high study period could substantially decrease heterogeneity (I2 = 41.61, 41.78).
Conclusions
This meta-analysis indicated that exposure – response between PM2.5 and MI. It is vital decision makers implement effective strategies to help improve air pollution, especially in developing countries or prevent exposure to PM2.5 to protect human health.
Keywords: Fine particulate matter, PM2.5, Air pollution, Myocardial infarction, Exposures
Background
Air pollution (atmospheric pollution) is the release of harmful particles matter into air by one or more harmful gases. It is generally assumed that any exposure to outdoor particulate matter air pollution can pose a big challenge to both public health agencies and physicians in the world, especially in the developing countries [1]. It is also believed that outdoor air pollution is a threat factor contributing to universal mortality and disability-adjusted life-years (DALYs) which rank the fifth and sixth in the world, respectively [2, 3]. Based on the criteria released by the National Ambient Air Quality Standard (NAAQS), there are six major pollutants of ozone (O3), carbon monoxide (CO), lead (PB), sulfur dioxide (SO2), nitrogen dioxide (NO2), particulate matter < 10 μm (PM10), and particulate matter < 2.5 μm (PM2.5). Recent findings suggest that any exposure to PM2.5 can endanger lungs and blood stream more than other pollutants and can lead to adverse cardiovascular, respiratory, and neurological disorders (Stroke, Alzheimer and Parkinson) as well as premature birth [4–8]. In most countries, it is thought that the level of PM2.5 particles is higher than the defined standards, even higher than those set by the World Health Organization (WHO) (WHO) [9, 10]. It is assumed that automobiles and combustion activities are the main sources for the production of PM2.5 [11]. Tehran Province in Iran, has been struggling with the highest air pollution in the last few decades, due to fast-growing industrial activities as well as the large number of automobiles on the road [12]. Cohen et al. (2017), in their study, indicated that 103.1 million years of life lost (YLL) and 4.2 million mortality occurred as a consequence of exposure to PM2.5.
Myocardial infarction (MI) or cardiac infarction is generally defined as detection of an elevated cardiac troponin (cTn) value which is above the 99th percentile upper reference limit [13]. In recent years, the prevalence of MI has been increased in both developed and developing countries [14, 15]. Research evidences indicate that such risk factors as age, sex, and family history cannot be modulated, but some of the risk factors such as ambient air pollution and unhealthy life style are to a great extent preventable [16–18]. Considering the fact that the age for onset of the first MI has been decreasing and that MI is multifactorial in nature, its fundamental function remains unknown [19].
Therefore, incidence of MI with simultaneous concentration of fine particulate matter has been extensively studied all over the world [20, 21] but only two systematic reviews and meta-analyses about the effect of particulate matter on MI were found Mustafic et al. (2012) revealed that all air pollutants, except for ozone, are significantly correlated with the increased risk of MI. In this meta-analysis, 13 research studies on feature of PM2.5 were scrutinized to detect the risk of MI and it was found that the relative risk of overall PM2.5 ranked the second after the relative risk of overall carbon monoxide [22]. Moreover, Luo et al. (2015) conducted a meta-analysis based on thirty-one time-series and case crossover studies in order to investigate the effect of particulate matter on the risk of MI. The results demonstrated that the exposure to PM2.5 can increase the risk of MI much more than the exposure to PM10. The findings also showed that there was a moderate heterogeneity in meta-analysis of the pooled estimates, but the subgroup analyses might not pinpoint the cause of this heterogeneity. Therefore, it is imperative to investigate the source of heterogeneity in a study with more details (Luo et al., 2015) [23]. In this meta-analysis, the rate of PM2.5 and the risk of MI are focused. It should be noted that PM2.5 was a subgroup in other meta-analysis studies. The time scope in this study is broader as the original studies conducted from January 2000 to June 2018 were attempted to be incorporated. This study has been performed in University of Medical Sciences, Iran in 2018.
Methods
This protocol was registered in PROSPERO, the International Prospective Register of Systematic Reviews, on 2 January, 2019 (registration number CRD42019118998). The findings of this study were based on the accommodation guidelines: “Preferred reporting items for a protocol for a meta-analysis (PRISMA-P) 2015” [24]. In this study, two reviewers (ZF and MAD) conducted a research on such electronic bibliographic databases including Scopus, Web of Science, PubMed, and EMBASE. They also searched for components such as “air pollution” and “myocardial infarction” and found synonyms using the Medical Subject Headings (MeSH). In addition, the results were combined using the Emtree term and incorporated all other synonyms, except for those found in PubMed. In this way, it was possible to narrow the syntax down to a specific period from Jan. 1, 2000 to Jun. 1, 2018. It is worth mentioning that any study dealing with the short-term relationship between the pollutants and myocardial infarction was thoroughly reviewed. A thorough search on Google Scholar was also performed using dual combinations of the two main components. In an attempt not to miss any study, the grey literature and conference proceedings were explored, and a list of references was ultimately reviewed [25]. There was no language restriction on the search engines. Having completed the search, one of the reviewers (ST) did the duplications using Endnote software version 8 and started to conduct the initial screening through titles and abstracts. Then, the two reviewers reviewed the full-text of the articles carefully for any potentially-relevant studies according to inclusion and exclusion criteria [26]. The inclusion criteria for this meta-analysis allowed for utilization of original studies with time-series or case-crossover designs dealing with any exposure to particulate matter (PM2.5), including even a short-term exposure such as the same day or 7 days before the occurrence of MI. The excluded studies had the following traits: 1) not being case-crossover or time-series designs, 2) non-original studies, 3) patients with MI, (4) long-term exposure to particulates matter PM10, and 5) no reported relative risk (RR)/odds ratio (OR) and 95% Confidence Interval (CI 95%). Any disagreement in arbitration for the eligibility of the paper was discussed until a consensus was reached by the reviewers.
Data extraction
The two researchers (H SH and M AD) extracted the data independently using a standardized form which was particularly prepared for studies based on the Cochrane guidelines [27]. The study had to be excluded from meta-analysis in the case of receiving no response from the author. In case of disagreement between the two authors, a third person was called upon as an arbitrator to help reach a consensus. The information in the data extraction form was: name of the author(s), publication year, country, city, study design, study period, lag exposure, case of population, adjustment, effect size, level of exposure to pollution, association between MI and lag exposure (0–6 day), and cumulative lags (0–1, 0–2, 0–3, 0–4, 0–5, 0–6) (Table 1).
Table 1.
Features of the studies imported to the meta-analysis
| No | Author/ publication year | Country | City | Design | Study period (month) | Lag exposure | Case population (n) | Adjustment | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | (Peters et al., 2001) [28] | USA | Boston | Case-Crossover | 5 | Lag0 | 772 | Day of the week, season, and meteorological parameters on the same time scales | High |
| 2 | (Peters et al., 2005) [29] | Germany | Augsburg | Case -Crossover | 24 | Lag0, Lag5, Lag0–4 | 851 | Temperature, humidity, days of the week, pressure | High |
| 3 | (Sullivan et al., 2005) [30] | USA | Washington | Case-Crossover | 72 | Lag0 | 5793 | Relative humidity and temperature | High |
| 4 | (Pop et al., 2006) [31] | USA | Utah | Case-Crossover | 120 | Lag0, Lag3 | 3910 | Temperature | Low |
| 5 |
(Zanobetti &Schwartz et al. 2006) [32] |
USA | Boston | Time Series | 48 | Lag0, Lag0–1 | 15,578 | Temperature, days of the week | High |
| 6 | (Barnett et al., 2006) [33] | Australia | Auckland, Brisbane, Canberra, Christchurch, Melbourne, Perth, Sydney | Case-Crossover | 36 | Lag0–1 | 56,036 | Day of week, pressure, holidays, temperature, humidity and others | High |
| 7 | (Ueda et al., 2009) [34] | Japanese | Fukuoka, Kawasaki, Kobe, Nagoya, Osaka, Sapporo, Sakai, Sendai and Tokyo | Time Series | 24 | Lag0, Lag1 | 67,897 | Days of the week, seasonality, relative humidity, ambient, and temperature | Low |
| 8 | (Stieb et al., 2009) [35] | Canada | Edmonton, Halifax, Montreal, Ottawa, Saint John, Vancouver and Toronto | Time Series | 120 | Lag0, Lag1, Lag2 | 63,184 | Seasonal cycles, temperature, and humidity | High |
| 9 | (Belleudi et al., 2010) [36] | Italy | Rome | Case-Crossover | 56 | Lag0, Lag6 | 7520 | Influenza, population reduction, epidemics, pressure, and Temperature | Low |
| 10 | (Zanobetti &Schwartz 2009) [37] | USA | 112 cities (The biggest cities are California, New York City, Los Angeles, Chicago, Illinois and New York) | Time Series | 72 | Lag0–1 | 397,894 | Long-term trend, seasonality, temperature, days of the week | High |
| 11 | (Rich et al., 2010) [38] | USA | New Jersey | Case-Crossover | 24 | Lag0 | 5864 | Weather and days of the week | High |
| 12 | (Berglind et al., 2010)a [39] | Sweden | Boston | Case-Crossover | 24 | Lag0 | 772 | Relative humidity and temperature | Low |
| 13 | (Berglind et al., 2010) b [39] | Sweden | Seattle | Case-Crossover | 24 | Lag0 | 5793 | Relative humidity and temperature | Low |
| 14 | (Berglind et al., 2010) c [39] | Sweden | Augsburg | Case-Crossover | 24 | Lag0 | 691 | Temperature and relative humidity | Low |
| 15 | (Mate et al., 2010) [40] | Spain | Madrid | Time Series | 24 | Lag6 | 1096 | Days of the week, trend, seasonality, influenza and temperature | High |
| 16 | (von Klot et al., 2011) [41] | Germany | Augsburg | Case-Crossover | 48 | Lag0 | 960 | Days of the week and temperature | High |
| 17 | (Chang et al., 2013) [42] | Taiwan | Taipei | Case-Crossover | 48 | Lag0 | 14,353 | Temperature and relative humidity | High |
| 18 | (Rosenthal et al., 2013) [43] | Finland | Helsinki | Case-Crossover | 96 | Lag0, Lag1, Lag2, Lag3,Lag0–3 | 629 | Temperature and humidity | High |
| 19 | (Talbott et al., 2014) [21] | USA | Florida | Case-Crossover | 96 | Lag0, Lag1, Lag2, Lag0–2 | 135,421 | Maximum apparent temperature and ozone | Low |
| 20 | (Gardner et al., 2014) [44] | USA | New York | Case-Crossover | 36 | Lag0–1,Lag0–2, Lag0–3, Lag0–4 | 677 | Relative humidity and temperature | High |
| 21 | (Milojevic et al., 2014) [45] | UK | London | Case-Crossover | 72 | Lag0–4 | 452,343 | Temperature, days of the week | High |
| 22 | (Wichmann et al., 2014) [46] | Sweden | Gothenburg | Case-Crossover | 300 | Lag0, Lag1, Lag0–1 | 28,215 | Relative humidity, temperature and public holiday | High |
| 23 | (Wang et al., 2015) [47] | Canada | Calgary, Edmonton | Case-Crossover | 132 | Lag(0,1.2.3,4) | 22,628 | daily average of temperature, dew point temperature and wind speed | Low |
| 24 | (Zang et al., 2016) [48] | China | Chaoyang | Case-Crossover | 12 | Lag(0,1,2,3,4,5) | 2749 | meteorological conditions and/or other gaseous pollutants | High |
| 25 | (Argacha et al., 2016) [49] | Belgian | Belgian | Case-Crossover | 48 | Lag0 | 11,428 | Day of the week, temperature | High |
| 26 | (Baneras et al., 2017) [20] | Spain | Barcelona | Time Series | 24 | Lag0 | 4141 | Seasonal, meteorological factors, and time-calendar variables | High |
| 27 | (Akbarzadeh et al., 2018) [50] | Iran | Tehran | Case-Crossover | 24 | Lag0–1 | 208 | Temperature and humidity | Low |
| 28 | (Yu et al., 2018) [51] | China | Changzhou | Time Series | 24 | Lag(0,1,2,3,4,5,6), Lag(0–1,0-2,0-3,0-4,0-5,0–6) | 5545 | Temperature, days of the week, relative humidity, seasonal trends | Low |
Quality score assessment
It is commonly assumed that the quality assessment report for all qualified papers is an indispensable requirement for all case-crossover and time-series studies. Nonetheless, there are currently no valid scales available for assessing the quality of the methodology. To this end, a quality rating scale was adopted and accepted according to the previous meta-analysis (Mustafić et al., 2012). The two reviewers (ST and H AG) managed to evaluate the quality of the study independently based on the following three components [52]. The quality of measurements for ambient concentration PM2.5 (0 and 1) was based on the following criteria. Score 0 was recorded in case that the measurements were done under the condition that more than 25% of the data was missing and not taken daily, or showed that there was no description of pollutant measurements. On the other hand, score 1 was recorded in case that measurements were conducted at least once a day, or under the condition that less than 25% of the data was missing. The arrangement of confounders was based on 0 and 1. It is believed that there is a discrepancy between the time-series and the case-crossover studies in their research methods. As a result, the modalities for the arrangement of confounders would be different. Score 1 was recorded in case that the arrangement for covariates was accomplished for multiple main covariates, containing seasonality, temperature, pressure/moisture/day, and long-term processes of week for case-crossover studies which controlled fixed and stilly varying biases using the scheme itself and also for time-series studies. Score 0, however, was recorded for the original papers without modifying the above-mentioned important variables. Finally, if a research study obtained the highest score for all components, it was defined as a high quality one, whereas a study with a minimum score (0 point) for one of the three components was regarded as a low quality one.
Data synthesis
All studies which examined the relationship between the exposure to PM2.5 and MI with relative risk (RR) or odds ratio (OR) and 95% confidence intervals (95%CI) were included. The studies with the statistical estimation risk of MI, relationship with the exposure to PM2.5 as OR, and with 95% CI were converted to RR, with 95% CI by using the above formula
Since, this meta-analysis aimed to was to explore the rate of short-term exposure on MI. Furthermore, in some original study there was no extended lag patterns of short-term effects of air pollution, thus the exposure lags of 0 or 1 day were selected for calculating the RR. The criteria for heterogeneity were determined through I2, and the I2 values of more than 50% offered a significant heterogeneity [53]. It should be noted that the fixed-effects models were utilized in case of no heterogeneity. Any potential publication bias was detected through the optical audit of the funnel plots and the Egger regression test. All the analyses were conducted using Comprehensive Meta-analysis Software (Version 2.0, Biostat) and SPSS 24. All the tests were two-tailed tests and p < 0.05 was statistically significant.
Results
Study characteristics, the risk of bias, and study selection for the included research studies. The selection procedure for the meta-analysis is shown in Fig. 1.
Fig. 1.
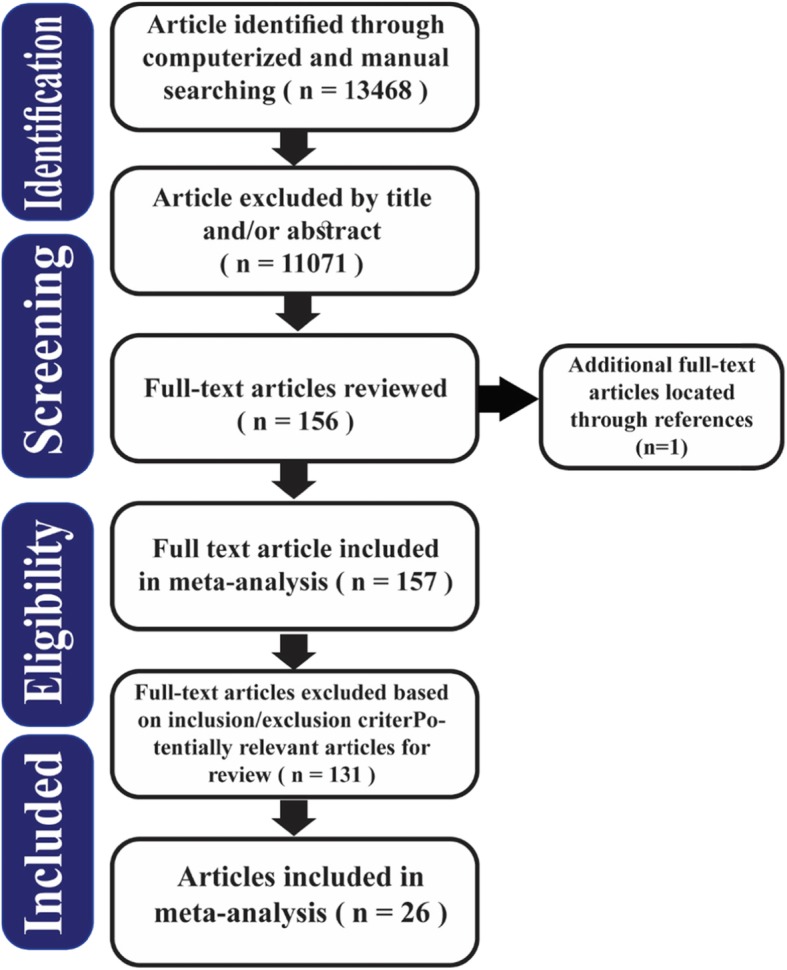
Flow diagram of included /excluded studies
Totally, 13,468 papers were identified. Having excluded 2397 duplicated studies, 11,071 papers were obtained, out of which 10,918 were excluded by title and/or abstract. One hundred fifty-seven full-text papers were opted out and then thoroughly assessed. Finally, 131 unrelated studies were left out and 26 papers were identified eligible for the study. The total number of participants with hospitalization for myocardial infarction was 2,250,473. The largest number of participants was 452,343, which belonged to the study by Milojevic et al., (2014), and the smallest number of participants was 208, which was reported by Akbarzadeh et al., (2018). Considering Berglind et al. (2010), the research was conducted in three cities (Boston, Seattle, and Augsburg), the lag averaging time was 2 h, and the adjusted odds ratio for the PM2.5 pollutant was applied in the analysis [39].
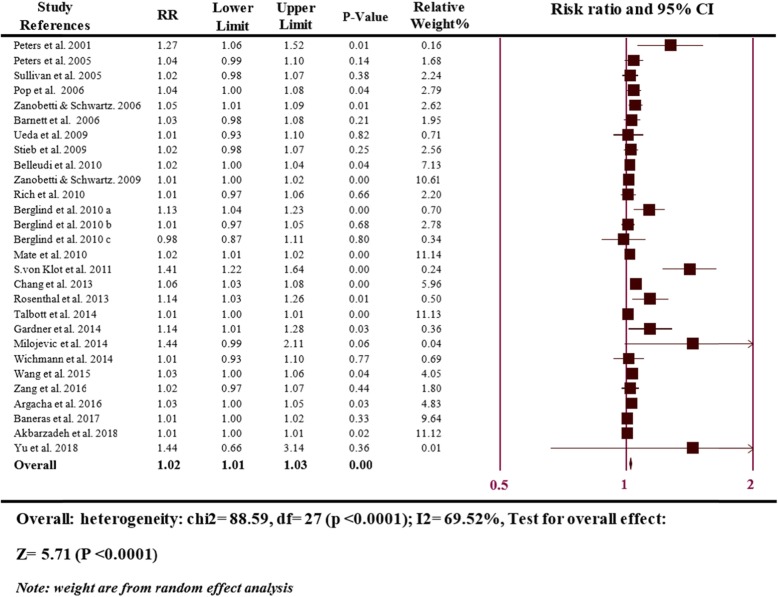
Figure 2 is based on 28 comparisons illustrating the association between a 10 μg/m3 increase in the risk of MI and PM2.5. The heterogeneity of the research studies was evaluated through random-effects with P < 0.0001, and I2 was 69.52%, showing a moderate heterogeneity. The meta-analysis showed a significant positive association between per 10 μg/m3 elevation in PM2.5 and MI risk (RR = 1.02; 95% CI 1.01–1.03; P ≤ 0.0001) at lag exposure of 0 and 0–1 days.
Fig. 2.
Overall analyses of the effect on the risk of MI hospitalizations associated with a 10 μg/m3 increase in PM2.5
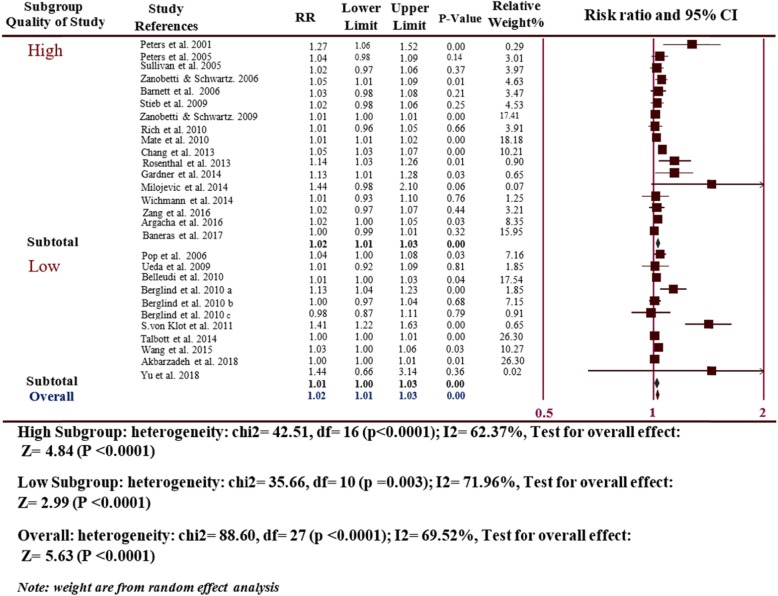
Considering the quality of study subgroup (17 high-quality studies and 11 low-quality studies), a significantly higher rate of MI risk was seen in high quality studies (RR = 1.02, 95% CI: 1.01–1.03, P ≤ 0.0001) with a moderate degree of heterogeneity (I2 = 62.37, P ≤ 0.0001) and in low quality studies (RR = 1.02, 95% CI: 1.01–1.03, p = 0.002) with a moderate to high degree of heterogeneity (I2 = 71.96, P ≤ 0.0001), which was consistent with the results of the overall analyses (Fig. 3).
Fig. 3.
Subgroup analyses of the risk of MI hospitalizations and PM2.5 for the quality of study
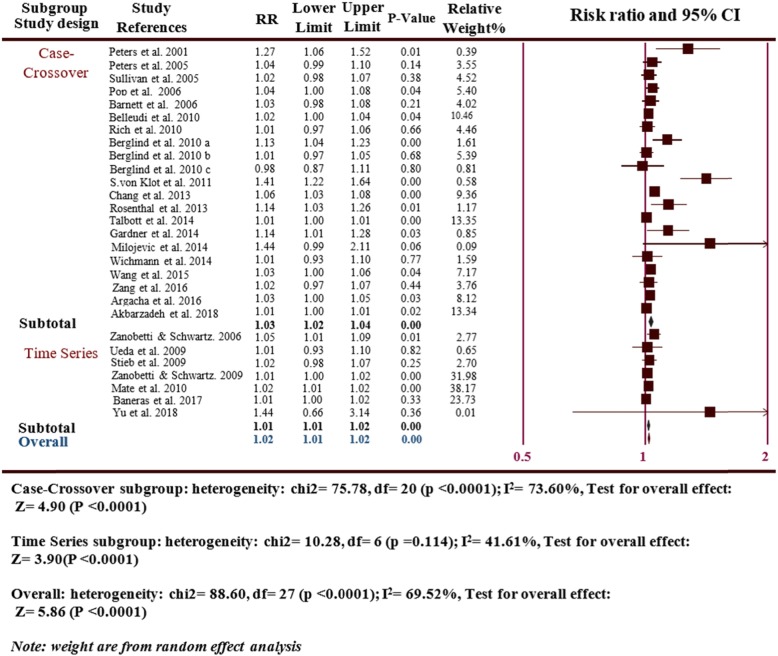
With respect to the study design subgroup, there were a positive association among MI risks in 21 case-crossover studies (RR = 1.03, 95% CI: 1.02–1.04, p ≤ 0.0001; I2 = 75.78, P ≤ 0.0001), which was basically consistent with the overall analyses. There was also statistical significance for 7 time series study subgroup (RR = 1.01, 95% CI: 1.01–1.02, P ≤ 0.0001; I2 = 41.61, P ≤ 0.0001) (Fig. 4).
Fig. 4.
Subgroup analyses the risk of MI hospitalizations and PM2.5 for the design
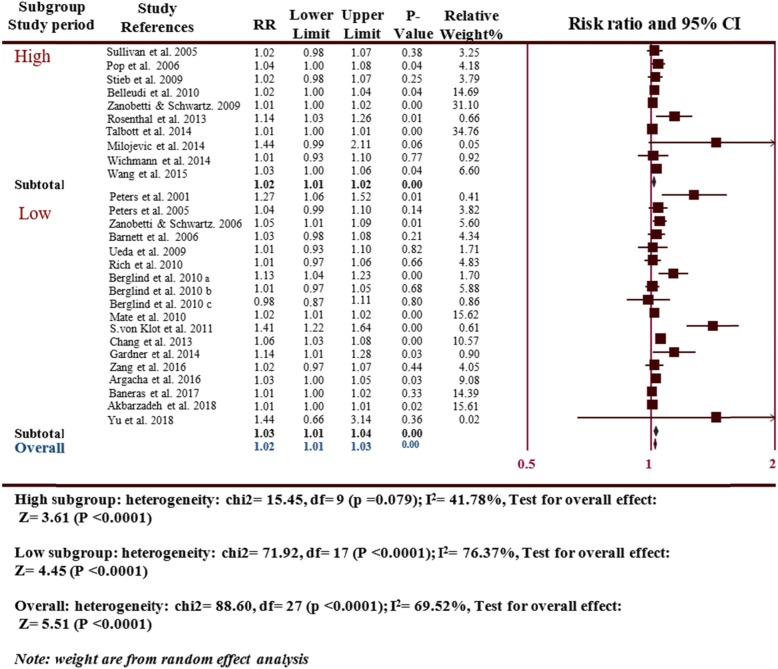
The subgroup analysis of the study period, Accordingly, the original studies were divided into two subgroups based on the follow-up times: the follow-up of less than 4 years as short study period and the follow-up time of more than 4 years as long study period (10 long study period and 19 short study period) revealed a significantly increased MI risk in the long study period (RR = 1.02, 95% CI: 1.01–1.02, P = 0.014) with a moderate degree of heterogeneity (I2 = 41.78 P ≤ 0.0001) and the short study period (RR = 1.03, 95% CI: 1.01–1.04, P ≤ 0.0001) with a moderate degree of heterogeneity (I2 = 76.37, P ≤ 0.0001), which was consistent with the overall analyses (Fig. 5).
Fig. 5.
Subgroup analyses the risk of MI hospitalizations and PM2.5 for the study period
Discussion
This study aimed to assess the association between exposure to PM2.5 and MI hospitalization. The subgroup analyses and the overall analysis were performed based on pooled estimates and relationship between 10 μg/m3 increase in the short-term exposure to PM2.5 and the risk of incident MI was pinpointed. Notably, two previous reviews had also shown this association, and in both of them the heterogeneity was supposedly moderate. In an attempt to identify the sources of heterogeneity, Mustafic et al. (2012), formed two subgroups based on study quality and lag exposure, and Luo et al. (2015), formed four subgroups based on study design, study quality, lag exposure, and geographic locations subgroup analyses. However, neither of them could successfully describe the sources of heterogeneity. In the same vein, three subgroup analyses (study quality, study design, and study period) were performed in the present study. According to the results, there was a relatively little difference among the high quality subgroup of the study, the case-crossover study design, and the short study period, all of which contributed to the overall analyses in terms of statistical significance and evidence of heterogeneity. Except for the low quality subgroup of the study which was not statistically significance, the rest were statistically significant. It is assumed that subgroup time series study design and long study period could substantially decrease heterogeneity (I2 = 41.61, 41.78). Time-series analysis examined both pre-adjustment and co-adjustment. The pre-adjustment method picks up temporal trends from both the health and air pollution, whereas the co-adjustment approach considers air pollution forecasters and temporal trends [54]. It is presumed that the case-crossover design can create bias as a unidirectional control sampling devoid of time trends [55]. Time-series method is more likely to result in more accurate estimates of risk than the case-crossover method [54]. It is reckoned that the reasons behind the observed heterogeneity in the present study could be the varied design of the included original studies, and also the use of case-crossover and time series studies. Future studies are, nonetheless, expected to use time-series studies, which may help clarify the source of heterogeneity. The follow-up accuracy is also a prerequisite for estimating valid consequences and should be acclaimed systematically. The follow-up index is easy to achieve and could be applied as a reporting criterion for indicators [56]. It is thought that the priority put on the long-term follow-up could enhance the capability to prepare more precise estimates [57]. We also found derivation errors in the second meta-analysis which was performed in 2015. The errors were found in three papers authored by Linn et al. (2000) [58], Xie et al. (2014) [59], and Wichmann et al. (2014), [46]. All these studies might be unintentionally entered the forest plot (PM2.5) and did not measure the effect of PM2.5 on the risk of incident MI, which could affect the pooled estimates of the study. All these studies might be unintentionally entered the forest plot (PM2.5) and did not measure the effect of PM2.5 on the risk of Incident MI, which could affect the pooled estimates of the study. In addition, the number of studies identified in the work of Chowdhary et al. (2018), was higher than that of the two previous studies conducted by Mustafic et al. (2012) (13) and Luo et al. (2015) (16). Also, the two reviewers in this study conducted the data extraction phase independently, appraised the papers, investigated all the data, and removed the difference through a third person. Moreover, this study was extensive enough to lower the possibility of publication bias. Even the gray studies were enveloped without any language limitation. This study had some limitations. First, the included original research papers had a great variety and substantially differed from one another in case population, number of people from below 1000 to more than 400,000 people, the city examined, and the study period from under 6 months to over 300 months. Secondly, the assessment of the effect of air pollution on MI could not be well-established as MI is a multifactorial disease associated with diabetes, hypertension, smoking, alcohol, and obesity [60]. Thirdly, the population is chosen in different age group, while the cause of MI in young adults differs from the elderly people. Most of the people with MI are elderly ones with heart problems beforehand. It is estimated that airborne contamination could trigger the undesirable effects to be over-represented for this age group [33, 61].
Finally, as the people who are exposed to a mix combination of air pollutants for longest periods are those at elevated risk of adverse health, outcomes related to individual exposure to a single pollutant cannot be obtained with a high degree of certainty [61].
Conclusions
The results of this Meta-analysis demonstrated the severity of the relationship between PM2.5 and MI with more accurate estimates than analysis presented by 26 studies alone, substantiating the notion that PM2.5 levels are key factors in the development of MI hospitalizations. It is highly imperative to conduct further investigations to determine all possible causal relationships and explore potential mechanisms affecting MI. The economic burden of air pollution health-related outcomes is very significant, especially for healthcare providers. Fiscal implications attributed to air pollution are calculated as 253 million to 2.9 billion USD in Asia. Policy makers adopt more effective strategies to help improve air pollution, especially in developing countries, or prevent exposure to PM2.5 so as to protect public health.
Acknowledgements
The study was supported by Iran University of Medical Sciences [IR.IUMS.REC.1398.392]. Authors would like to thank Iran University of Medical Sciences in supporting of this work.
Abbreviations
- CI
Confidence intervals
- Cm2
Comprehensive meta-analysis software version 2
- CO
Carbon monoxide
- cTn
Cardiac troponin
- DALYs
Disability-adjusted Life-years Myocardial infarction
- MeSH
Medical Subject Headings
- MI
Myocardial infarction
- NAAQS
National Ambient Air Quality Standard
- NO2
Nitrogen dioxide
- O3
Ozone
- OR
Odds ratio
- PB
Lead
- PM
Particulate matter
- PM10
Particulate matter < 10 μm aerodynamic diameter
- PM2.5
Particulate matter < 2.5 μm aerodynamic diameter
- PRISMA-P
-
Preferred reporting items for Systematic review and meta-analysis protocols; RR
Relative risk
- SO2
Sulfur dioxide
- SPSS
Statistical Packages for the Social Science
- WHO
World Health Organization
- YLL
Years of healthy life loss
Authors’ contributions
ZF, HAG, and HS conceived the research idea. ZF, MAD, and ST designed the search strategy. ZF, HAG, and MAD assessed quality score. ZF and MAD analyzed the data. ZF, MAD, HAG, and HS wrote the manuscript. All authors have seen and approved the final version of the manuscript.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included from preliminary studies are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by Iran University of Medical Sciences pharse[IUMS/SHMIS_98-2-37-15312] in return[IR.IUMS.REC.1398.392].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet. 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brauer M, Freedman G, Frostad J, Van Donkelaar A, Martin RV, Dentener F, Dingenen RV, Estep K, Amini H, Apte JS. Ambient air pollution exposure estimation for the global burden of disease 2013. Environ Sci Technol. 2015;50(1):79–88. doi: 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- 4.Berger K, Malig BJ, Hasheminassab S, Pearson DL, Sioutas C, Ostro B, Basu R. Associations of source-apportioned fine particles with cause-specific mortality in California. Epidemiology. 2018;29(5):639–648. doi: 10.1097/EDE.0000000000000873. [DOI] [PubMed] [Google Scholar]
- 5.Fu P, Guo X, Cheung FMH, Yung KKL. The association between PM2.5 exposure and neurological disorders: a systematic review and meta-analysis. Sci Total Environ. 2019;655:1240–1248. doi: 10.1016/j.scitotenv.2018.11.218. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Liu Y, Liu F, Wang Y, Yang X, Yu J, Xue X, Jiao A, Lu Y, Tian L, et al. Analysis of short-term and sub-chronic effects of ambient air pollution on preterm birth in Central China. Environ Sci Pollut Res Int. 2018;25(19):19028–19039. doi: 10.1007/s11356-018-2061-8. [DOI] [PubMed] [Google Scholar]
- 7.Sahu SK, Zhang H, Guo H, Hu J, Ying Q, Kota SH. Health risk associated with potential source regions of pm 2.5 in indian cities. Air Qual Atmosphere Health. 2019;12(3):327–340. doi: 10.1007/s11869-019-00661-4. [DOI] [Google Scholar]
- 8.Van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, Lyapustin A, Sayer AM, Winker DM. Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environ Sci Technol. 2016;50(7):3762–72. [DOI] [PubMed]
- 9.Han L, Zhou W, Li W, Qian Y. Global population exposed to fine particulate pollution by population increase and pollution expansion. Air Qual Atmosphere Health. 2017;10(10):1221–1226. doi: 10.1007/s11869-017-0506-8. [DOI] [Google Scholar]
- 10.Lin Y, Zou J, Yang W, Li C-Q. A review of recent advances in research on PM2. 5 in China. Int J Environ Res Public Health. 2018;15(3):438. doi: 10.3390/ijerph15030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee A, Agrawal M. A global perspective of fine particulate matter pollution and its health effects. In: Reviews of environmental contamination and toxicology. edn.: Springer. 2017;244:5–51. [DOI] [PubMed]
- 12.Arhami M, Shahne MZ, Hosseini V, Haghighat NR, Lai AM, Schauer JJ. Seasonal trends in the composition and sources of PM2. 5 and carbonaceous aerosol in Tehran, Iran. Environ Pollut. 2018;239:69–81. doi: 10.1016/j.envpol.2018.03.111. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 14.Mohseni J, Kazemi T, Maleki MH, Beydokhti H. A systematic review on the prevalence of acute myocardial infarction in Iran. Heart Views. 2017;18(4):125–132. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_71_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullasari AS, Balaji P, Khando T. Managing complications in acute myocardial infarction. J Assoc Physicians India. 2011;59(12):43–48. [PubMed] [Google Scholar]
- 16.Rathore V, Singh N, Mahat RK, Kocak MZ, Fidan K, Ayazoglu TA, Aydin Karahan YG, Onk D, Akar E, Yolcu A. 1. Risk factors for acute myocardial infarction: a review. Eurasian J Med Investig. 2018;2(1):1–7. [Google Scholar]
- 17.Bhandari M, Singh V, Venkatraman D. A study of risk factors for acute myocardial infarction in patients below 35 years in eastern India. Niger J Cardiol. 2017;14(2):84. doi: 10.4103/njc.njc_10_17. [DOI] [Google Scholar]
- 18.Kiani F, Hesabi N, Arbabisarjou A. Assessment of risk factors in patients with myocardial infarction. Glob J Health Sci. 2015;8(1):255–262. doi: 10.5539/gjhs.v8n1p255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nia HS, Sivarajan-Froelicher E, Haghdoost AA, Moosazadeh M, Huak-Chan Y, Farsavian AA, Nazari R, Yaghoobzadeh A, Goudarzian AH. The estimate of average age at the onset of acute myocardial infarction in Iran: a systematic review and meta-analysis study. ARYA Atheroscler. 2018;14(5):225. doi: 10.22122/arya.v14i5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baneras J, Ferreira-Gonzalez I, Marsal JR, Barrabes JA, Ribera A, Lidon RM, Domingo E, Marti G, Garcia-Dorado D. Short-term exposure to air pollutants increases the risk of ST elevation myocardial infarction and of infarct-related ventricular arrhythmias and mortality. Int J Cardiol. 2018;250:35–42. doi: 10.1016/j.ijcard.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Talbott EO, Rager JR, Benson S, Brink LA, Bilonick RA, Wu C. A case-crossover analysis of the impact of PM (2.5) on cardiovascular disease hospitalizations for selected CDC tracking states. Environ Res. 2014;134:455–465. doi: 10.1016/j.envres.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Mustafić H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Périer MC, Marijon E, Vernerey D, Empana JP. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307(7):713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 23.Luo C, Zhu X, Yao C, Hou L, Zhang J, Cao J, Wang A. Short-term exposure to particulate air pollution and risk of myocardial infarction: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2015;22(19):14651–14662. doi: 10.1007/s11356-015-5188-x. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopewell S, McDonald S, Clarke MJ, Egger M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. 2007;(2). [DOI] [PMC free article] [PubMed]
- 26.Atkinson LZ, Cipriani A. How to carry out a literature search for a systematic review: a practical guide. BJPsych Adv. 2018;24(2):74–82. doi: 10.1192/bja.2017.3. [DOI] [Google Scholar]
- 27.Higgins L, Greenwood S, Wareing M, Sibley C, Mills T. Obesity and the placenta: a consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta. 2011;32(1):1–7. doi: 10.1016/j.placenta.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–2815. doi: 10.1161/01.CIR.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 29.Peters A, Heier M, Trentinaglia I, Cyrys J, Hörmann A, Hauptmann M, Wichmann H, Löwel H. Particulate air pollution and nonfatal cardiac events. Part I. Air pollution, personal activities, and onset of myocardial infarction in a case-crossover study. Research report (Health Effects Institute). 2005;(124):1–66. [PubMed]
- 30.Sullivan J, Sheppard L, Schreuder A, Ishikawa N, Siscovick D, Kaufman J. Relation between short-term fine-particulate matter exposure and onset of myocardial infarction. Epidemiology. 2005;(1):41–8. [DOI] [PubMed]
- 31.Pope C, III, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114(23):2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 32.Zanobetti A, Schwartz J. Air pollution and emergency admissions in Boston, MA. J Epidemiol Community Health. 2006;60(10):890–895. doi: 10.1136/jech.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnett AG, Williams GM, Schwartz J, Best TL, Neller AH, Petroeschevsky AL, Simpson RW. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ Health Perspect. 2006;114(7):1018–1023. doi: 10.1289/ehp.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueda K, Nitta H, Ono M. Effects of fine particulate matter on daily mortality for specific heart diseases in Japan. Circ J. 2009;73(7):1248–1254. doi: 10.1253/circj.CJ-08-1149. [DOI] [PubMed] [Google Scholar]
- 35.Stieb DM, Szyszkowicz M, Rowe BH, Leech JA. Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis. Environ Health. 2009;8(1):25. doi: 10.1186/1476-069X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belleudi V, Faustini A, Stafoggia M, Cattani G, Marconi A, Perucci CA, Forastiere F. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology. 2010;(1):414–23. [DOI] [PubMed]
- 37.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117(6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rich DQ, Kipen HM, Zhang J, Kamat L, Wilson AC, Kostis JB, Group MIDASS Triggering of transmural infarctions, but not nontransmural infarctions, by ambient fine particles. Environ Health Perspect. 2010;118(9):1229–1234. doi: 10.1289/ehp.0901624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berglind N, Ljungman P, Möller J, Hallqvist J, Nyberg F, Rosenqvist M, Pershagen G, Bellander T. Air pollution exposure—a trigger for myocardial infarction? Int J Environ Res Public Health. 2010;7(4):1486–99. [DOI] [PMC free article] [PubMed]
- 40.Maté T, Guaita R, Pichiule M, Linares C, Díaz J. Short-term effect of fine particulate matter (PM2. 5) on daily mortality due to diseases of the circulatory system in Madrid (Spain) Sci Total Environ. 2010;408(23):5750–5757. doi: 10.1016/j.scitotenv.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 41.Von Klot S, Cyrys J, Hoek G, Kühnel B, Pitz M, Kuhn U, Kuch B, Meisinger C, Hörmann A, Wichmann H-E. Estimated personal soot exposure is associated with acute myocardial infarction onset in a case-crossover study. Prog Cardiovasc Dis. 2011;53(5):361–368. doi: 10.1016/j.pcad.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Chang C-C, Kuo C-C, Liou S-H, Yang C-Y. Fine particulate air pollution and hospital admissions for myocardial infarction in a subtropical city: Taipei, Taiwan. J Toxic Environ Health A. 2013;76(7):440–448. doi: 10.1080/15287394.2013.771559. [DOI] [PubMed] [Google Scholar]
- 43.Rosenthal FS, Kuisma M, Lanki T, Hussein T, Boyd J, Halonen JI, Pekkanen J. Association of ozone and particulate air pollution with out-of-hospital cardiac arrest in Helsinki, Finland: evidence for two different etiologies. J Expo Sci Environ Epidemiol. 2013;23(3):281. doi: 10.1038/jes.2012.121. [DOI] [PubMed] [Google Scholar]
- 44.Gardner B, Ling F, Hopke PK, Frampton MW, Utell MJ, Zareba W, Cameron SJ, Chalupa D, Kane C, Kulandhaisamy S. Ambient fine particulate air pollution triggers ST-elevation myocardial infarction, but not non-ST elevation myocardial infarction: a case-crossover study. Part Fibre Toxicol. 2014;11(1):1. doi: 10.1186/1743-8977-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milojevic A, Wilkinson P, Armstrong B, Bhaskaran K, Smeeth L, Hajat S. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart. 2014;100(14):1093–1098. doi: 10.1136/heartjnl-2013-304963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wichmann J, Sjoberg K, Tang L, Haeger-Eugensson M, Rosengren A, Andersson EM, Barregard L, Sallsten G. The effect of secondary inorganic aerosols, soot and the geographical origin of air mass on acute myocardial infarction hospitalisations in Gothenburg, Sweden during 1985-2010: a case-crossover study. Environ Health. 2014;13:61. doi: 10.1186/1476-069X-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Dickinson RE, Su L, Zhou C, Wang K. PM2. 5 pollution in China and how it has been exacerbated by terrain and meteorological conditions. Bull Am Meteorol Soc. 2018;99(1):105–119. doi: 10.1175/BAMS-D-16-0301.1. [DOI] [Google Scholar]
- 48.Zhang Q, Qi W, Yao W, Wang M, Chen Y, Zhou Y. Ambient particulate matter (PM2. 5/PM10) exposure and emergency department visits for acute myocardial infarction in Chaoyang District, Beijing, China during 2014: A case-crossover study. J Epidemiol. 2016;26(10):538–545. doi: 10.2188/jea.JE20150209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Argacha J-F, Collart P, Wauters A, Kayaert P, Lochy S, Schoors D, Sonck J, de Vos T, Forton M, Brasseur O. Air pollution and ST-elevation myocardial infarction: a case-crossover study of the Belgian STEMI registry 2009–2013. Int J Cardiol. 2016;223:300–305. doi: 10.1016/j.ijcard.2016.07.191. [DOI] [PubMed] [Google Scholar]
- 50.Akbarzadeh MA, Khaheshi I, Sharifi A, Yousefi N, Naderian M, Namazi MH, Safi M, Vakili H, Saadat H, Parsa SA. The association between exposure to air pollutants including PM10, PM2. 5, ozone, carbon monoxide, sulfur dioxide, and nitrogen dioxide concentration and the relative risk of developing STEMI: a case-crossover design. Environ Res. 2018;161:299–303. doi: 10.1016/j.envres.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 51.Yu Y, Yao S, Dong H, Ji M, Chen Z, Li G, Yao X, Wang S-L, Zhang Z. Short-term effects of ambient air pollutants and myocardial infarction in Changzhou, China. Environ Sci Pollut Res. 2018;25(22):22285–22293. doi: 10.1007/s11356-018-2250-5. [DOI] [PubMed] [Google Scholar]
- 52.Chowdhary P, Raj A, Bharagava RN. Environmental pollution and health hazards from distillery wastewater and treatment approaches to combat the environmental threats: a review. Chemosphere. 2018;194:229–246. doi: 10.1016/j.chemosphere.2017.11.163. [DOI] [PubMed] [Google Scholar]
- 53.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fung KY, Krewski D, Chen Y, Burnett R, Cakmak S. Comparison of time series and case-crossover analyses of air pollution and hospital admission data. Int J Epidemiol. 2003;32(6):1064–1070. doi: 10.1093/ije/dyg246. [DOI] [PubMed] [Google Scholar]
- 55.Navidi W. Bidirectional case-crossover designs for exposures with time trends. Biometrics. 1998;(1):569–605. [PubMed]
- 56.von Allmen RS, Weiss S, Tevaearai HT, Kuemmerli C, Tinner C, Carrel TP, Schmidli J, Dick F. Completeness of follow-up determines validity of study findings: results of a prospective repeated measures cohort study. PLoS One. 2015;10(10):e0140817. doi: 10.1371/journal.pone.0140817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hill KG, Woodward D, Woelfel T, Hawkins JD, Green S. Planning for long-term follow-up: strategies learned from longitudinal studies. Prev Sci. 2016;17(7):806–818. doi: 10.1007/s11121-015-0610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linn WS, Szlachcic Y, Gong H, Jr, Kinney PL, Berhane KT. Air pollution and daily hospital admissions in metropolitan Los Angeles. Environ Health Perspect. 2000;108(5):427–434. doi: 10.1289/ehp.00108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie J, He M, Zhu W. Acute effects of outdoor air pollution on emergency department visits due to five clinical subtypes of coronary heart diseases in shanghai, China. J Epidemiol. 2014;24(6):452–459. doi: 10.2188/jea.JE20140044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhaskaran K, Hajat S, Armstrong B, Haines A, Herrett E, Wilkinson P, Smeeth L. The effects of hourly differences in air pollution on the risk of myocardial infarction: case crossover analysis of the MINAP database. BMJ. 2011;343:d5531. doi: 10.1136/bmj.d5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: shifting from a single-pollutant to a multi-pollutant approach. Epidemiology (Cambridge, Mass) 2010;21(2):187. doi: 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included from preliminary studies are available from the corresponding author on reasonable request.