Abstract
Background
Although education, exercise, and weight loss are recommended for management of knee osteoarthritis, the additional benefits of incorporating weight loss strategies into exercise interventions have not been well investigated. The aim of this study is to compare, in a private health insurance setting, the clinical- and cost-effectiveness of a remotely-delivered, evidence- and theory-informed, behaviour change intervention targeting exercise and self-management (Exercise intervention), with the same intervention plus active weight management (Exercise plus weight management intervention), and with an information-only control group for people with knee osteoarthritis who are overweight or obese.
Methods
Three-arm, pragmatic parallel-design randomised controlled trial involving 415 people aged ≥45 and ≤ 80 years, with body mass index ≥28 kg/m2 and < 41 kg/m2 and painful knee osteoarthritis. Recruitment is Australia-wide amongst Medibank private health insurance members. All three groups receive access to a bespoke website containing information about osteoarthritis and self-management. Participants in the Exercise group also receive six consultations with a physiotherapist via videoconferencing over 6 months, including prescription of a strengthening exercise and physical activity program, advice about management, and additional educational resources. The Exercise plus weight management group receive six consultations with a dietitian via videoconferencing over 6 months, which include a very low calorie ketogenic diet with meal replacements and resources to support behaviour change, in addition to the interventions of the Exercise group. Outcomes are measured at baseline, 6 and 12 months. Primary outcomes are self-reported knee pain and physical function at 6 months. Secondary outcomes include weight, physical activity levels, quality of life, global rating of change, satisfaction with care, knee surgery and/or appointments with an orthopaedic surgeon, and willingness to undergo surgery. Additional measures include adherence, adverse events, self-efficacy, and perceived usefulness of intervention components. Cost-effectiveness of each intervention will also be assessed.
Discussion
This pragmatic study will determine whether a scalable remotely-delivered service combining weight management with exercise is more effective than a service with exercise alone, and with both compared to an information-only control group. Findings will inform development and implementation of future remotely-delivered services for people with knee osteoarthritis.
Trial registration
Australian New Zealand Clinical Trials Registry: ACTRN12618000930280 (01/06/2018).
Keywords: Osteoarthritis, Exercise, Telerehabilitation, Weight management, Ketogenic diet, Knee, Pain, Obesity, RCT, Physiotherapy, Dietitian
Background
Knee osteoarthritis (OA) is a major public health problem [1] and incurs enormous indirect and direct healthcare costs [2]. All current clinical guidelines recommend non-surgical non-drug treatments for first-line management of OA, including education/advice, exercise, and, if appropriate, weight loss [3, 4]. However, there is evidence that a substantial proportion of people with OA in Australia are not receiving recommended care [5]. For example, although surgery is only recommended for those with severe symptomatic OA who do not respond to conservative management approaches, data from an Australian hospital found that 33% of people referred for orthopaedic management had not engaged in any non-drug conservative management methods [6]. From 2005 to 06 to 2015–16, Australia has seen a 38% rise in the rate of total knee replacements for OA [7], yet 20% of people undergoing knee replacement surgery report unsatisfactory outcomes [8]. Given that the prevalence of knee OA is projected to rise in the coming decades [2, 9], there is an urgent need to increase uptake of recommended conservative management options through implementation of effective, accessible and scalable models of service delivery.
The benefits of therapeutic exercise for people with knee OA are well-established [10], with similar efficacy to analgesics and non-steroidal anti-inflammatory drugs, but with fewer side-effects [11, 12], and with fewer risks than joint replacement surgery. Given that muscle weakness is common amongst people with OA [13], muscular strengthening exercises are important and can help reduce pain, and improve physical function [14]. In addition, as most people with OA do not meet physical activity recommendations [15], advice to increase physical activity is also important. For example, research has demonstrated that people with knee OA who are less sedentary have better physical function, independent of time spent doing moderate or vigorous physical activity [16, 17], and that a threshold of 6000 steps per day discriminates between people with knee OA who do and do not develop functional limitations 2 years later [18].
Given that people with OA who are overweight or obese tend to experience more severe symptoms [19, 20] and are more likely to undergo joint replacement surgery [21], weight loss is an important component of management. However, although weight loss is recommended by evidence-based guidelines [4, 22, 23] there is only limited evidence to support its efficacy in improving symptoms of pain and dysfunction. In the literature, interventions often combine both exercise and weight loss components, making it difficult to identify the independent effects of weight loss on outcomes. One pivotal trial involving 454 overweight and obese adults with knee OA investigated the effects of an intensive diet program, with and without exercise, conducted in a rigorously monitored environment [24]. The combined diet and exercise group lost approximately 10% of body weight, compared to 2% in the exercise only group, leading to reduced knee loads and inflammation compared to the exercise only group. While statistically significant benefits on pain (1-point difference (95% confidence interval: 0.3 to 1.7) on 20-point scale) and function (4.3-point difference (2.1 to 6.5) on 68-point scale) were also seen, the magnitude of these benefits are small and may be of questionable clinical importance. Furthermore, although the diet and exercise intervention was reported to be more cost-effective than usual care (pharmacological NSAIDs regimen followed by total knee replacement surgery) [25], the cost-effectiveness analyses did not include data from the exercise only group. Thus, it remains unknown whether a combined intervention of diet and exercise is more cost-effective than exercise alone.
Although the findings of Messier and colleagues [24] support the efficacy of combined weight loss and exercise programs, their interventions were time-intensive and potentially costly, with the weight loss component involving 12 individual and 42 group sessions for nutrition education and behavioural support over 18-months, and the exercise component involving three group exercise sessions per week for 6-months. Given that many barriers to self-management of OA relate to inaccessibility and/or costs of healthcare [26, 27], implementation of such an intensive program outside of a research setting is likely to be difficult in most community or hospital settings, either public or private, without considerable cost and time burdens. Identifying effective programs that minimise active involvement by clinicians is therefore important. One way in which to improve the accessibility and scalability of care is to provide it remotely via technology (telerehabilitation), allowing patients to consult from their own home or workplace. There is emerging evidence that telerehabilitation for people with musculoskeletal conditions improves pain and function and is equivalent to outcomes following traditional in-person consultations [28]. More recently, there is evidence that exercise advice and prescription delivered by physiotherapists via videoconferencing leads to improvements in pain and function in people with knee OA [29]. Importantly, such a service is also acceptable to both the people with knee pain receiving care and the physiotherapists delivering it [30]. Similarly, dietary interventions for weight loss can also be effective when delivered remotely. A recent systematic review and meta-analysis found that dietary interventions for people with chronic diseases (i.e. obesity, diabetes, heart disease, hypertension, stroke, or kidney disease) delivered by dietitians or nurses via video or telephone improved diet quality and led to significant weight loss and reduced waist circumference [31]. No previous studies have investigated the effectiveness of remotely-delivered exercise and weight management programs by physiotherapists and dietitians for people with knee OA who are overweight or obese.
For many people with OA, undertaking an exercise and/or weight management program requires considerable changes in lifestyle behaviours over prolonged periods of time. As such, it is important that intervention development is underpinned by behaviour change theory, and informed by best practice in chronic disease management and self-management support programs. Interventions must include provision of high quality information. However, education alone is insufficient to support behavioural change. Healthcare clinicians must also have the communication and psychosocial skills necessary to facilitate long-term changes in behaviour [32, 33]. Incorporating behavioural counselling [34, 35] and specific behaviour change techniques that are effective for supporting change in exercise and eating behaviours in this population [36] into intervention design will facilitate participants to achieve and sustain effective self-management practices in the long-term.
The aim of this study, conducted in a private health insurance setting, is to compare the effectiveness of two remotely-delivered, evidence- and theory-informed, behaviour change interventions to provide information and behaviour change support for i) exercise and; ii) exercise plus weight management, to each other, and to an information-only control for people with knee OA who are overweight or obese. We hypothesise that the exercise plus weight management intervention will lead to greater improvements in pain and function than the exercise intervention and that both interventions will be more effective than information only.
Methods/design
Design
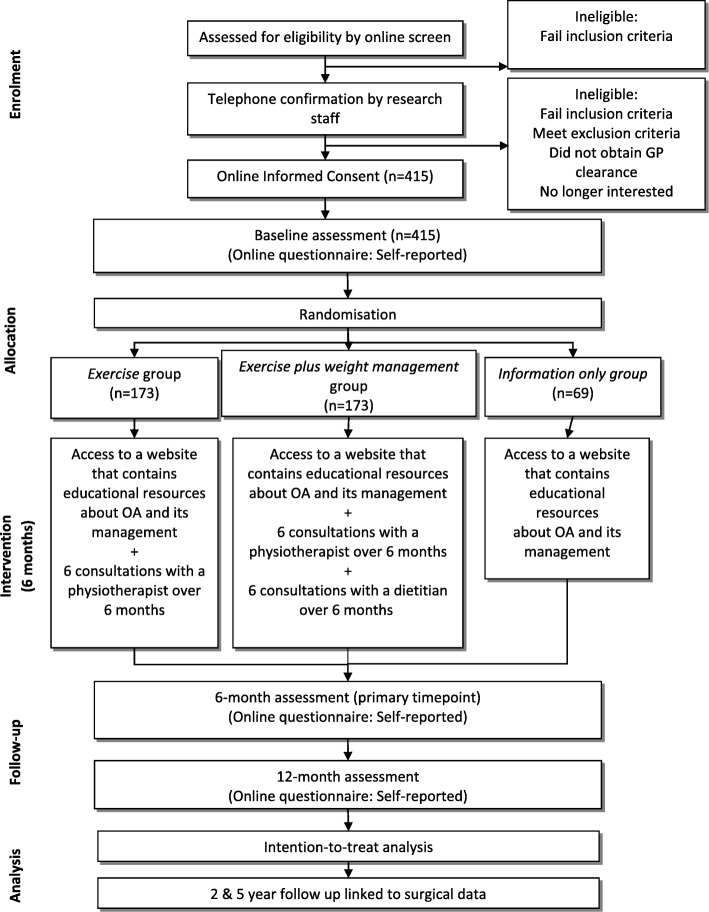
The Better Knee, Better Me™ trial is a three-arm pragmatic superiority parallel-design randomised controlled trial. Figure 1 outlines the RCT phases. This protocol has been developed according to the SPIRIT statement [37]. The trial has been prospectively registered in the Australian New Zealand Clinical Trials Registry (ACTRN12618000930280). The trial is conducted at The University of Melbourne. The effectiveness and cost-effectiveness of interventions will be assessed by analysing a range of outcomes and reported as the main trial results. Nested qualitative studies of participants’ and clinicians’ experiences participating in the trial will be used to explore the acceptability and usefulness of the services, as well as explore the barriers and facilitators to engagement. However, this will be reported separately to the main trial results.
Fig. 1.
Participant flow through the randomized controlled trial
Participants
A total of 415 participants who have private health insurance with Medibank Private at a level that includes cover for arthroplasty surgery are recruited from around Australia. Medibank Private is one of Australia’s largest health funds, with 3.7 million members across Australia [38]. Inclusion and exclusion criteria are outlined in Table 1. Medibank Private send targeted invitations, predominantly via email, to potentially eligible members. They also advertise the study in their member newsletters and on their website. The University of Melbourne manage the clinical screening of volunteers. Screening occurs via a two-step process; i) via an online survey and, if eligible, ii) via additional telephone screening by research personnel at The University of Melbourne. Additional clearance to participate is sought from a general practitioner for anyone who does not pass pre-exercise screening questions [40], reports more than 1 fall in the past 12 months, or is house-bound due to mobility limitations (e.g. concurrent disabling low back pain).
Table 1.
Participant eligibility criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
Fulfil National Institute for Health and Care Excellence [3] clinical criteria for osteoarthritis: • Age > 45 years; • Have activity related joint pain; and • Have morning stiffness ≤30 min. Average knee pain severity ≥4 on 11-point numeric rating scale (NRS, where 0 = no pain, 10 = worst pain possible) in the past week History of knee pain on most days for at least 3 months Aged < 81 years – due to potential safety reasons and additional co-morbidities Body mass index (BMI) ≥ 28 kg/m2 and < 41 kg/m2. The lower BMI limit was chosen according to recommendations [39] that VLCD are effective in supporting weight loss in people with a BMI > 27. The upper limit was chosen as these individuals often require more extensive intervention and for possible safety reasons Member of Medibank with a level of cover that includes arthroplasty surgery Able to give informed consent and to participate fully in the interventions and assessment procedures Willing to follow advice for self-management, participate in exercise/physical activity and/or participate in a weight loss program if part of their treatment program Have the ability to weigh themselves (e.g. have access to the same set of bathroom scales) |
Booked for knee surgery on either knee Have had all eligible knee joints replaced by arthroplasty Knee surgery within the past 6 months Unable to speak or read English Self-reported diagnosis of rheumatoid arthritis or other inflammatory arthritis Other medical condition or upcoming medical procedures that in the opinion of the research staff and/or investigators would preclude participation Private health insurance claims related to cancer treatment or inpatient neurological rehabilitation in the previous 12 months, palliative care, or acquired brain injury Unable to use/access a telephone and internet For those identified as at risk from the pre-exercise and falls screening, doctor does not give clearance Used low-calorie meal replacement products (e.g. Optifast/Optislim) for weight loss in previous 6 months Currently, or in the past 6 months, undertaking regular strengthening exercises for the knee Unable to undertake VLCD for medical reasons including: i. Self-reported diagnosis of Type 1 diabetes ii. Self-reported Type 2 diabetes requiring insulin or other medication apart from metformin iii. Self-reported warfarin use iv. Stroke or cardiac event in previous 6 months v. Unstable heart condition vi. Fluid intake restriction |
BMI Body mass index, VLCD Very low calorie diet
Data collection and management
Data are collected via online questionnaire and stored on a secure password-protected server. The “study knee” is the painful knee or, in people with bilaterally eligible knees, the most painful knee.
Randomisation allocation concealment and blinding
The randomisation schedule is computer generated and prepared by the study biostatistician. Following completion of the baseline questionnaire, participants are randomly allocated to either the Exercise group, the Exercise plus weight management group, or the information only (Control) group. Due to the differences expected to be observed between treatment groups (effect size of 0.3) and between the least-performing treatment group and the control group (effect size of 0.4), to minimise the number of control group participants required, we will recruit fewer participants in the control group than in either treatment group: see details in the Trial sample size section below. This leads to a randomisation ratio of 5:5:2 (for every 5 participants randomised to a treatment group, we will only randomise 2 to the control group). We expect to see a smaller difference between the two treatment groups than between the least-performing treatment group and the control group. Randomisation will be stratified by history of knee surgery (arthroscopy or contralateral arthroplasty). Participants allocated to the Exercise group are randomly allocated to one of three physiotherapists. Participants allocated to the Exercise plus weight management group are randomly allocated to one of the same three physiotherapists as in the Exercise group, and one of five dietitians. If a physiotherapist or dietitian is unavailable (e.g. sick, on holidays), participants are re-randomised to another available clinician. To conceal group allocation, a researcher not involved in participant recruitment accesses the randomisation schedule via a password-protected computer program.
Given that all primary and secondary outcomes are self-reported, participants are also the assessors. Participants are blinded to study hypotheses, however the components of each treatment arm are disclosed during recruitment. This replicates real-world conditions whereby consumers are fully informed about the nature of the treatment components before choosing whether or not to participate/receive an intervention. Physiotherapists and dietitians are not blinded to group allocation.
Information only (control) group
The information only (Control) group receive information, advice, and education about OA and its management via a bespoke website developed by the research team which is only accessible via password login. The website includes: i) educational information including understanding OA, recommended treatment options (including a total knee replacement decision aid), exercise and physical activity, weight loss, understanding and managing pain (including audio files to facilitate mini-relaxations and pleasant imagery), sleep, and “success stories” from other people with knee OA, and; ii) links to recommended external websites for further help and support (e.g. MyJointPain, painHEALTH, Musculoskeletal Australia). Participants have access to the study website from enrolment to completion of the 12-month follow-up questionnaire.
Intervention design
Design of the interventions is underpinned by the Chronic Care model [41], recommendations for design and evaluation of self-management support programs [33], and the information-motivation-behavioural skills theoretical model (IMB) for behaviour change [42, 43]. The Chronic Care model [41] explains the importance of productive interactions between clinicians with the right skills and patients who are informed and activated. An intervention with the aim of facilitating effective self-management by patients must both activate and then support patient behaviour change. The IMB model contends that although information is a pre-requisite for change, on its own information will not activate change [44]. The model explains that having the motivation to change, and the behavioural skills needed in order to change, are independent and essential determinants [43]. Other interventions based on this model have been effective in influencing behavioural change across a variety of clinical applications [45]. This model of change is consistent with the recommendation that in order to be effective, self-management support programs must include motivational coaching interventions as well as educational interventions that link knowledge with skills [33].
Two recent systematic reviews support the effectiveness of coaching interventions, such as Motivational Interviewing, for managing chronic conditions [35] and chronic painful musculoskeletal conditions [34], specifically in regard to increasing physical activity behaviours. Coaching from clinicians must be patient-centred and tailored to the needs and concerns of the individual [33]. Training and protocols for the clinicians are important intervention components, especially since these coaching skills are not often taught during entry to practice training for health professionals and both clinicians and trainees report skills deficits in this area [46]. In addition to being able to effectively deliver high quality information to facilitate desired behaviour change, clinicians also need to incorporate specific behaviour change techniques such as shared decision-making, goal setting, problem solving, confidence building, review and reinforcement. Additional techniques that have been shown to be effective for supporting change in exercise and eating behaviours and that are included in the interventions include providing information about the consequences of the behaviour, encouraging social support, phased action planning, self-monitoring, graded tasks, reinforcing successful behaviour, prompting focus on past success, providing feedback about the participant’s behaviour, barrier identification, and relapse prevention/coping planning [36].
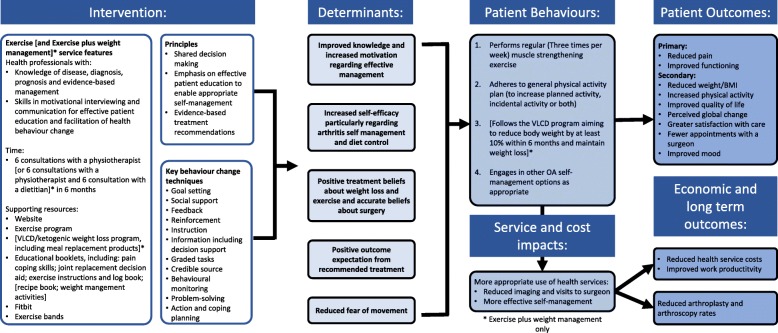
The intervention design is additionally based on best practice recommendations for management of knee OA [3, 4] and obesity [39, 47], plus exercise and weight loss interventions with proven effectiveness. Stakeholder input was sought from our key collaborators: Medibank Private and the Weight Control Clinic at Austin Health as well as our multidisciplinary and cross-sectoral research group. Consumers provided input into various aspects of the trial including the resources and study name. The rationale for the interventions is depicted in a program logic model in Fig. 2.
Fig. 2.
Logic model depicting the rationale underpinning the Exercise and Exercise plus weight management models of service delivery *Exercise plus weight management only VLCD: very low calorie diet
All potential participants are provided with a plain language statement which describes what is involved in each of the study groups and the level of commitment required. Participants are reminded of this during the online and telephone screening process, and are excluded if they feel they are not able to commit fully to either of the three study groups.
Exercise intervention
The Exercise intervention consists of educational information and links to external websites as for the Control group (bespoke website, in addition to exercise modules related only to this intervention) plus six individual consultations with a physiotherapist via videoconferencing over the internet (Zoom Video Communications Inc., California USA) over 6-months (Table 2). The physiotherapist consultations include individualised advice on treatment options, decision support, a structured exercise and physical activity plan, behaviour change support, and facilitation of other self-management strategies including pain coping skills training. Educational resources (Table 2) are provided in hardcopy via post and electronically via the study website. Participants are also posted three exercise resistance bands (green, red, and blue) for strengthening exercises and a Fitbit (Flex 2 model) to help track and monitor physical activity.
Table 2.
Summary of resources provided to participants in the Exercise and Exercise plus weight management groups
| Resource | Description of content/purpose | Exercise group | Exercise plus weight management group |
|---|---|---|---|
| Study website | Information about OA, treatment options, exercise and physical activity, weight loss, managing pain, sleep, and stories from other people with knee OA | √ | √ |
| Consultations with a physiotherapist | 6 video consultations over 6-months. Advice on treatment options, structured exercise and physical activity plan, and behaviour change support | √ | √ |
| Consultations with a dietitian | 6 video consultations over 6-months. Helps participant undertake VLCD with behaviour change support | √ | |
| Exercise bands | 3 exercise resistance bands (green, red, and blue) for strengthening exercises | √ | √ |
| Activity tracker (Fitbit) | To help track and monitor physical activity | √ | √ |
| Plastic portion plate | To help manage portion sizes | √ | |
| Optifast® meal replacements | 6-months’ worth of meal replacements for the VLCD | √ | |
| Educational video about the VLCD | Short video about the VLCD featuring endocrinologists and dietitian experts, and a person with knee OA | √ | |
| Booklets | |||
| Preparing for your consultations | Information about consultations, instructions on how to use Zoom videoconferencing, and Fitbit instructions | √ | √ |
| Osteoarthritis information | Understanding knee OA, common management options, weight loss, pain coping skills, and sleep | √ | √ |
| Exercise booklet | Strengthening exercise instructions and photos | √ | √ |
| Knee care plan and exercise log book | Templates to record details of management plans and completed exercises | √ | √ |
| Knee replacement surgery for osteoarthritis-related pain | Decision aid about joint replacement surgery, including the benefits and harms of surgery | √ | √ |
| Weight management ‘how to’ guide | Describes the VLCD and provides information about healthy food choices and portion sizes | √ | |
| Weight management behavioural support activities | Workbook containing information and templates to track weight, a food diary, tips to find a support person, identifying food triggers, planning for “at risk” situations, overcoming barriers, changing thought patterns, and monitoring hunger levels | √ | |
| Recipe book | Recipes that are suitable for the VLCD | √ | |
| Food list pocket guide | List of suitable low carbohydrate ingredients to consume when on the VLCD | √ | |
OA Osteoarthritis, VLCD Very low calorie diet
Before their first consultation with the physiotherapist, participants complete a pre-consultation survey asking about their main problems and goals, a brief history of their knee symptoms, and other health problems. The initial physiotherapy consultation is approximately 45 min long, with follow-up consultations being approximately 20 min. Consultations are recommended to occur in weeks 1, 3, 7, 11, 16, and 21, but the precise timing is negotiated between each participant and their physiotherapist.
Together with the participant, the physiotherapist develops an individualised management plan aiming to include the following components: i) a structured and progressive muscle strengthening exercise program; ii) a tailored physical activity plan to increase incidental and general physical activity and reduce sedentary behaviour, including a daily step goal if agreed by the participant, and; iii) other practical self-management strategies (e.g. activity pacing, pain coping strategies, sleep hygiene). Physiotherapists use motivational interviewing principles and techniques to develop motivation (readiness to change) and self-efficacy as well as assist the participant to overcome barriers to enacting their agreed management plan. Education about OA is provided from a biopsychosocial perspective to facilitate confidence in ability to manage pain. For participants who have been identified during the baseline questionnaire as having a preference towards total joint replacement surgery in their knee, physiotherapists discuss the benefits and harms of surgery with participants. For those who are not yet considering surgery, information about the likelihood of future need for surgery is discussed. During follow-up consultations, physiotherapists review progress and reassess goals, making modifications as required.
For the strengthening exercise program, physiotherapists choose from the list of exercises shown in Table 3 (based on exercises used in previous trials by the Centre which were found to be effective [29, 48]) and aim to prescribe 5–6 exercises, including at least one from each category (i.e. quadriceps strengthening) and an optional extra. The exact number of exercises, as well as sets/repetitions, is negotiated between the physiotherapist and participant. Intensity is determined using a modified Rating of Perceived Exertion (RPE) [49], where it should feel “hard” to “very hard” to perform a full set of each exercises. Participants are encouraged to complete exercises three times per week. For the physical activity plan, physiotherapists encourage the participant to increase their general and incidental levels of physical/aerobic activity based on their individual needs and goals, as well as their current level of activity. Participants are encouraged to use the provided Fitbit to record and monitor their daily step count. Prescription of exercises/physical activity plans is based on the clinical history and functional ability of the participant. Participants are provided with a study email they can use to contact the clinicians between consultations if they have any questions or encounter any issues.
Table 3.
Home exercise protocol
|
Maximum of 6 exercises (with progression as appropriate) 2 knee extensor strengthening exercises 1 hip abductor strengthening exercise 1 hamstring strengthening exercise 1 calf strengthening exercise 1 other exercise as appropriate | |||
| 1. Quads strengthening (aim to include two exercises) | |||
| Knee extension | Non weight-bearing | Q1. Seated knee extension (with resistance) with 5 s hold | Progression: Increase with theraband resistance – red through to black |
| Non weight-bearing | Q2. Inner range quads over roll (with resistance) with 5 s hold | Instruction: Put a rolled up towel under your arthritis knee. Keep the knee cap and toes pointing toward the roof. Keeping the back of the knee in contact with the towel, push the back of your knee down into the towel and straighten your arthritis leg | |
| Sit-to-stand | Weight-bearing | Q3. Sit to stand without using hands | Progression: lower chair height, hover above the seat without touching down (3 s hold), add resistance band around knees and push outwards keeping knees over toes |
| Weight-bearing | Q4. Sit to stand with more weight on involved knee |
Instruction: Take more weight by either a) placing uninvolved further forward b) shift both feet sideways so study leg is midline |
|
| Steps | Weight-bearing | Q5. Step-ups | Progression: Increase step height |
| Weight-bearing | Q6. Forward touchdowns from a step | Progression: Increase step height, don’t touch floor | |
| Weight-bearing | Q7. Step-ups with weight |
Instruction: Hold 2 kg of weight either a) against chest, b) in each hand, c) in one hand while holding for balance, or d) in backpack Progression: Increase step height, increase weight |
|
| Weight-bearing | Q8. Forward touchdowns from a step with weight |
Instruction: Hold 2 kg of weight either a) against chest, b) in each hand, c) in one hand while holding for balance, or d) in backpack Progression: Increase step height, increase weight |
|
| Wall squats | Weight-bearing | Q9. Partial wall squats for 5 s hold | Progression: more weight on study side |
| Weight-bearing | Q10. Split leg wall squats for 5 s hold | Instruction: Step feet away from wall (about 30 cm) and move non-involved leg a further 15 cm away from the wall. | |
| Controlled squats | Weight-bearing | Q11. Controlled partial squat with 5 s hold | |
| Leg Sliding | Weight-bearing | Q12. Forward and backward sliding of uninvolved leg | Instruction: Keep weight on study leg. Concentrate on keeping knee positioned over the foot |
| Weight-bearing | Q13. Forward and backward sliding of uninvolved leg with resistance band pulling study leg laterally |
Instruction: Place loop of elastic band around involved knee and leg of a table to provide a pull outwards on knee that you must resist by keeping knee in line with foot. Progression: Increase with theraband resistance – red through to black |
|
| Weight-bearing | Q16. Sideways sliding of uninvolved leg | Instruction: Keep weight on study leg. Concentrate on keeping knee positioned over the foot | |
| Weight-bearing | Q17. Sideways sliding of uninvolved leg with resistance band pulling study leg laterally |
Instruction: Place loop of elastic band around study leg and leg of a table to provide a pull outwards on knee that you must resist by keeping knee in line with foot. Progression: Increase with theraband resistance – red through to black |
|
| Step to single leg balance | Weight-bearing | Q14. Step with study leg to about 30° knee flexion for single leg balance | Instruction: Take a step forwards with study leg, keeping knee bent to about 30° knee flexion. Allow uninvolved leg to lift off and practice balancing as long as you can |
| Weight-bearing | Q15. Step with study leg to about 30° knee flexion for single leg balance with arm movements | Instruction: Take a step forwards with involved knee, keeping knee bent to about 30° knee flexion. Allow uninvolved leg to lift off and practice balancing as long as you can, while raising arms out to side and above head in an arc. | |
| 2. Hip abductor strengthening (1 exercise) | |||
| Standing hip abduction | Weight-bearing | HA1. Side leg raises in standing with 5 s hold | Progression: Increase theraband resistance – red through to black, add another 5 s halfway |
| Side stepping | Weight-bearing | HA2. Crab walk with resistance band | Progression: Increase theraband resistance – red through to black |
| Standing hip abduction | Weight bearing | HA3. Wall push standing on study leg for 20 s | Progression: Hold weight in hand, increase the hold time |
| Weight bearing | HA4. Wall push standing on study leg positioned up to 45° knee flexion | ||
| 3. Hamstring strengthening (1 exercise) | |||
| Bridging | Weight-bearing | HG1. Bridge with 5 s hold | |
| Weight-bearing | HG2. Split leg bridge with 5 s hold | Instruction: Place feet hip-width apart, then move study leg slightly closer toward your bottom and slightly in towards centre | |
| Weight-bearing | HG3. Single-leg bridge on study leg with 5 s hold |
Version A: Lift bottom. Keeping hips level, lift uninvolved leg off the floor/bed. Hold for 5 s. Slowly lower uninvolved leg back to the floor. Then slowly lower bottom back to floor. Version B: Lift uninvolved knee off floor/bed. Lift bottom and take all weight through study leg. Hold for 5 s. Slowly lower your bottom back to floor/bed. |
|
| Standing knee flexion | Non weight-bearing | HG4. Hamstring curls -Standing over bench knee flexion with 5 s hold | |
| Non weight-bearing | HG5. Hamstring curls -Standing over bench knee flexion with 5 s hold against resistance band | Instruction: Place one end of an elastic band securely around ankle of study knee. Place the other end of elastic around your opposite foot so you are standing on it. | |
| Seated knee flexion | Non weight-bearing | HG6. Seated knee flexion |
Instruction: Place one end of an elastic band securely around a stable object (e.g. a heavy table leg). Loop the other end around the ankle of your study leg. Keeping your opposite foot on the floor, pull against the elastic band and bend your knee more. Progression: Increase with theraband resistance – red through to black |
| Standing hip extension | Non weight-bearing | HG7. Hip extension with knee bent (90°) - standing over bench with 5 s hold | |
| Non weight-bearing | HG8. Hip extension with knee straight - standing over bench with 5 s hold | ||
| Non weight-bearing | HG9. Hip extension with knee straight with resistance band - standing over bench with 5 s hold | Instruction: Place one end of an elastic band securely around ankle of study knee. Place the other end of elastic around your opposite foot so you are standing on it. | |
| 4. Calf strengthening (1 exercise) | |||
| Standing plantar-flexion | Weight-bearing | C1. Double heel raises with 5 s hold | |
| Weight-bearing | C2. Single heel raises with 5 s hold | ||
| Weight-bearing | C3. Double heel raises with 5 s hold over edge of step | ||
| Weight-bearing | C4. Double heel raises with 5 s hold over edge of step | ||
| 5. Arm strengthening (if appropriate) | |||
| Bicep curls | Non Weight- bearing | 1. Bicep curls | Progression: increase resistance |
| Wall push ups | Weight- bearing | 2. Wall push ups | Progression: increase repetitions |
During each consultation, physiotherapists complete online treatment notes recording the call duration, topics discussed, clinical history, personal motivators, and details of the participant’s management plan.
Exercise plus weight management intervention
Participants who are allocated to the Exercise plus weight management intervention receive all components of the Exercise intervention plus 6 individual videoconferencing consultations with a dietitian (over 6 months) who helps the participant undertake a weight management program involving a ketogenic very low calorie diet (VLCD) [50]. This diet was chosen as it has been shown to lead to greater weight loss in the short-term when compared to low-fat diets [51, 52] and the release of ketones, produced by the liver during fatty acid oxidation while fasting or on a diet that restricts carbohydrates, can help suppress appetite and contribute to further weight loss [50]. Participants are provided with Optifast® meal replacements (Nestlé Health Science, Rhodes, Australia) (or Optislim® [OptiPharm Pty Ltd., Clayton, Australia] if unavailable or the participant is vegetarian) and additional educational resources to support their weight loss (Table 2). Participants are encouraged to lose at least 10% or more of their body weight, as this has been associated with clinically important improvements in pain [53, 54].
In addition to the five educational booklets provided in the Exercise intervention, participants in the Exercise plus weight management intervention are provided with four additional booklets (described in Table 2). Participants are also mailed a plastic “portion plate” (https://www.nestle.com.au/nhw/nestle-portion-plates) to help them manage meal portion sizes. A short educational video about the VLCD featuring endocrinologists and dietitian experts, and a person with knee OA, is also available on the study website.
Participants’ first consultation with the dietitian takes place within the same week as their first physiotherapist consultation and is approximately 45 min in duration, with follow-up consultations approximately 20 min. During the initial consultation, appropriate weight loss goals, including a target weight, and a management plan are developed. Participants are encouraged to commence the VLCD, however they are also able to commence a modified version of the diet (e.g. one meal replacement only) if deemed necessary (e.g. if they do not like the meal replacements or are having difficulty adhering). Participants are asked to weigh themselves weekly and record their progress in their log book. During subsequent consultations, dietitians discuss progress and use motivational interviewing principles and techniques to assist motivation [35], self-efficacy, and overcoming obstacles to completing the agreed self-management plan. Dietitians also guide participants through the resource booklets provided, including activities to help them adhere to their self-management plan such as planning for unforeseen events (e.g. eating out) and choosing a support person. Once the participant has reached their target weight, they can choose whether to transition to a weight maintenance phase or continue with the VLCD program and aim for further weight loss.
The VLCD involves replacing two meals, generally breakfast and lunch, with meal replacements. Very low calorie diet meal replacement products are formulated to provide the adequate micronutrients (vitamins, minerals, and metals), and come as bars, shakes, or soups in a variety of flavours. On the diet, one meal (generally dinner) comprises protein (e.g. white or red meat, fish or seafood, eggs, or tofu) and non-starchy vegetables/salad. A small amount (i.e. 1 tablespoon) of oil/fat is also recommended for this meal to reduce the risk of gallstone formation. In total, the diet contains approximately 800 cal (3280 kJ).
Transitioning off the VLCD (after target weight is reached) is done by reintroducing foods containing carbohydrates and moving to only one meal replacement per day. This transition phase usually lasts at least 2 weeks, after which participants commence a healthy eating diet which is consistent with the principles of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) total wellbeing diet [55] (i.e. high protein, low glycaemic index carbohydrate, low fat). Participants are encouraged to weigh themselves regularly thereafter (e.g. once per week). Participants who regain 2 kg or more are advised to restart the VLCD with meal replacements for 1–2 weeks.
Dietitians complete online consultation notes using the same platform as physiotherapists, where they are able to record details about agreed weight management plans and barriers/motivators that have been discussed, as well as record which resources/activities participants were asked to read/complete. Dietitians and physiotherapists are also encouraged to communicate with each other through their consultation notes (e.g. to flag any important issues that the participant might be having). To reduce the risk of loss of muscle mass during their weight management program, physiotherapists also recommend that participants in this group complete regular arm strengthening exercises (Table 3) in addition to their leg strengthening program. Participants are provided with a study email they can use to contact the clinicians between consultations if they have any questions or encounter any issues.
Physiotherapist and dietitian training
Three musculoskeletal physiotherapists are employed to deliver the intervention. Physiotherapist selection criteria includes having experience managing people with chronic diseases, specifically knee OA, and current registration with the Australian Health Practitioner Regulation Agency. Five accredited dietitians, with at least 2 years of clinical experience and some experience assisting people with weight loss, are employed to deliver the weight management intervention. Prior to the start of the trial, clinicians complete training in:
-
i)
best-practice OA management: dietitians attend a half day workshop in evidence-based management of OA, delivered by members of the research team. Briefly, this includes an introduction to the purpose of the trial, OA definition, diagnosis, risk factors and prognosis, evidence-based practice (including education, exercise therapy, weight loss, and psychological treatments), and principles of shared decision-making and health behaviour change techniques.
-
ii)
motivational interviewing skills: dietitians and physiotherapists attend a 2-day training course from Health & Wellbeing Training Consultants, who specialise in training clinicians in motivational interviewing. Case studies presented during trained are designed to replicate potential scenarios anticipated to arise during the trial. Following these first two training days, clinicians conduct an initial and follow-up video consultation with a practice participant (one per clinician) to practice using their motivational interviewing skills and recording consultation notes. Practice consultations are audio recorded and audited for fidelity by a member of the research team using the Motivational Interviewing Treatment Integrity tool (MITI 4.2.1). Group level feedback is provided to clinicians at a half day workshop approximately 1 month after the initial two training days;
-
iii)
VLCD: dietitians attend a 1-h webinar delivered by the research team on the specifics of the VLCD. Physiotherapists are asked to watch the video recording of the presentation.
-
iv)
study-specific protocols: dietitians and physiotherapists attend a 1–2 h session (supplemented by detailed study treatment manuals) to ensure clinicians were familiar with all study procedures and processes. All clinicians are provided with a hardcopy of the study manual as well as hardcopies of all participant booklets to aid discussions during consultations.
Treatment fidelity
After the trial commences, clinicians are able to discuss with the research team via phone or email any issues with delivering the intervention as planned, including using the motivational interviewing principles. Regular meetings are held with all clinicians and the trial team to discuss any issues or provide feedback. All consultations are audio-recorded and a random subset will be audited for fidelity using the MITI 4.2.1. Fidelity is also evaluated by auditing the electronic treatment notes, which includes details of participant management plans (e.g. the exercises prescribed and dietary plans).
Dealing with adverse events
Expected adverse events include: i) transient increase in knee pain or swelling due to increased exercise or physical activity; ii) feeling hungry, fatigued, fuzzy-headed, and/or having headaches, and either diarrhoea or constipation during the first week after commencing the VLCD. Participants are advised to report any adverse events to the study coordinator as soon as they can, which will be documented in treatments notes and an adverse events log. If necessary, treatment is discontinued and further medical advice is arranged. Adverse events are also collected via self-report in the 6- and 12-month questionnaires.
Clinicians are instructed to report any adverse events to the trial coordinator, record the event in their consultation notes, and refer participants to their general practitioner if necessary. Participants are provided with a “help” email address to contact if they have any issues with their exercise/physical activity or weight management program in between consultations. The email is monitored daily by research staff and, if necessary, staff can seek further advice/guidance from the participant’s physiotherapist and/or dietitian.
Outcomes
Outcome measures and time points are described in Table 4. Our primary and secondary outcome measures are recommended and validated for knee OA trials [56, 57] and are measured at baseline, 6 and 12 months.
Table 4.
Summary of measurements to be taken
| Domain | Data collection instrument | Time points | ||
|---|---|---|---|---|
| Baseline | 6 M | 12 M | ||
| Descriptive data | ||||
| Age, gender, height, body mass index | ✓ | |||
| Duration of knee OA symptoms | ✓ | |||
| Previous treatments and surgery | ✓ | |||
| Problems in other joints | ✓ | |||
| Medical history | ✓ | |||
| Expectation of treatment outcome | 5-point ordinal scale | ✓ | ||
| Primary outcome measures | ||||
| Average knee pain in past week | 11-point NRS | ✓ | ✓ | ✓ |
| Physical function in past 48 h | WOMAC physical function subscale | ✓ | ✓ | ✓ |
| Secondary outcome measures | ||||
| Weight | Self-reported | ✓ | ✓ | ✓ |
| Physical activity | IPEQ-W questionnaire | ✓ | ✓ | ✓ |
| Health-related quality of life | AQoL-8D questionnaire | ✓ | ✓ | ✓ |
| Perceived change since baseline | Overall change, 7-point ordinal scale | ✓ | ✓ | |
| Change in pain, 7-point ordinal scale | ✓ | ✓ | ||
| Change in function, 7-point ordinal scale | ✓ | ✓ | ||
| Change in physical activity, 7-point ordinal scale | ✓ | ✓ | ||
| Satisfaction with care | 7-point ordinal scale | ✓ | ✓ | |
| Appointment with orthopaedic surgeon | Yes/No | ✓ | ✓ | ✓ |
| Depression, anxiety, and stress | DASS-21 | ✓ | ✓ | ✓ |
| Surgery performed | Self-reported TKJR and arthroscopy | ✓ | ✓ | |
| Willingness to undergo surgery | 5-point ordinal scale | ✓ | ✓ | ✓ |
| Other measures | ||||
| Health economic data | Quality adjusted life years | ✓ | ✓ | ✓ |
| Self-reported medication use | ✓ | ✓ | ✓ | |
| Self-reported use of health services/co-interventions | ✓ | ✓ | ✓ | |
| Cost-effectiveness ratio | ✓ | ✓ | ||
| Work productivity (WHO HPQ Short Form) | ✓ | ✓ | ✓ | |
| Adherence | Number of consultations with physiotherapista | ✓ | ||
| Number of consultations with dietitianb | ✓ | |||
| Duration of consultations with physiotherapista | ✓ | |||
| Duration of consultations with dietitianb | ✓ | |||
| Self-rated adherence to strengthening exercisea/ physical activitya/weight managementb, 11-point NRS | ✓ | |||
| Perceived usefulness | Times accessed website, 5-point ordinal scale | ✓ | ✓ | |
| Usefulness of website, 11-point NRS | ✓ | |||
| Usefulness of physiotherapy consultationsa 11-point NRS | ✓ | |||
| Usefulness of dietitian consultationsb 11-point NRS | ✓ | |||
| Usefulness of educational resourcesa 11-point NRS | ✓ | |||
| Usefulness of Fitbita 11-point NRS | ✓ | |||
| Usefulness of ketogenic dietb 11-point NRS | ✓ | |||
| Usefulness of strengthening exercise programa 11-point NRS | ✓ | |||
| Usefulness of physical activity plana 11-point NRS | ✓ | |||
| Video software ease of usea 11-point NRS | ✓ | |||
| Harms | Adverse events | ✓ | ✓ | |
| Determinants | Self-efficacy for symptom control (ASES) | ✓ | ✓ | ✓ |
| Self-efficacy for eating (WELQ) | ✓ | ✓ | ✓ | |
| Attitudes towards self-management (PAM-13) | ✓ | ✓ | ✓ | |
| Treatment beliefs about arthroplasty (TOA) | ✓ | ✓ | ✓ | |
| Fear of movement (BFMS) | ✓ | ✓ | ✓ | |
| Long-term surgical rates | Number who have had arthroscopy/arthroplasty | 24 months and 60 months | ||
NRS Numeric rating scale, WOMAC Western Ontario and McMaster Universities Osteoarthritis Index, IPEQ-W Incidental and Planned Exercise Questionnaire, AQoL-8D Assessment of Quality of Life Instrument, DASS Depression, Anxiety, and Stress Scale, TKJR Total knee joint replacement, WHO HPQ World Health Organization Health and Work Performance Questionnaire, ASES Arthritis Self-efficacy Scale, WELQ Weight Efficacy Lifestyle Questionnaire, PAM Patient Activation Measure, TOA Treatment beliefs in knee and hip OA – arthroplasty subscale, BFMS Brief Fear of Movement Scale for osteoarthritis
ameasured in Exercise and Exercise plus weight management groups only
bmeasured in Exercise plus weight management group only
Primary outcomes
Primary outcomes are overall average knee pain severity in the last week measured on an 11-point numeric rating scale (NRS), where 0 = ‘no pain’ and 10 = ‘worst pain possible’ [58], and physical function assessed using the physical function subscale of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index [59]. The WOMAC is a disease-specific self-report instrument which has demonstrated validity, reliability, and responsiveness [60]. The physical function subscale contains 17 questions scored from 0 to 4, giving a range of possible scores from 0 (no dysfunction) to 68 (maximum dysfunction).
Secondary outcomes
Secondary outcome measures include:
-
i)
Self-reported weight, measured in kilograms. Participants are asked to use the same set of scales for these measures across the time points;
-
ii)
Physical activity, assessed using the Incidental and Planned Exercise Questionnaire (IPEQ-W) [61];
-
iii)
Health-related quality of life, measured using the Assessment of Quality of Life Instrument (AQoL-8D) [62];
-
iv)
Global rating of change, scored on a 7-point Likert scale for “Overall change in your study knee since you began the study” from “much worse” to “much better” [63];
-
v)
Satisfaction with care, scored on a 7-point Likert scale for “Overall satisfaction with the care you received in this study” from “extremely unsatisfied” to “extremely satisfied”;
-
vi)
Self-reported appointments with an orthopaedic surgeon;
-
vii)
Depression, anxiety, and stress, measures on the Depression, Anxiety, and Stress Scale (DASS-21) [64];
-
viii)
Self-reported total knee joint replacement and knee arthroscopy surgery;
-
ix)
Willingness to undergo surgery, measured on a 5-point Likert scale ranging from “definitely not willing” to “definitely willing”.
Descriptive data
At baseline, descriptive data is collected, including: i) age; ii) gender; iii) height and weight; iv) country of birth; v) postal code; vi) Aboriginal or Torres Strait Islander Heritage; vii) education level; viii) current employment status; ix) duration of symptoms; x) length of time since first visit to a doctor for knee pain; xi) previous history of knee arthroscopy and arthroplasty; xii) pain in other parts of the body; xiii) expectation of treatment outcomes, and; xiv) medication use.
Determinants
-
i)
Self-efficacy for managing arthritis symptoms, measured using the Arthritis Self-Efficacy Scale [65];
-
ii)
Self-efficacy for eating control, measured using the Weight Efficacy Lifestyle Questionnaire [66];
-
iii)
Attitudes towards self-management, measured using the Patient Activation Measure (PAM-13) [67];
-
iv)
Treatment beliefs about arthroplasty, using the Treatment beliefs in knee and hip OsteoArthritis (TOA) questionnaire – Arthroplasty subscale [68];
-
v)
Fear of movement, measured using the Brief Fear of Movement Scale for osteoarthritis [69].
Additional measures
Economic data: assessed by self-reported medication use and healthcare costs (regarding the use of health services), quality-adjusted life years (QALY), cost-effectiveness ratio (calculated using predictions of health care costs and QALYs), and work productivity (using the World Health Organisation Health and Work Performance Questionnaire [70]). Knee surgery costs will be obtained by linking to Medibank data.
Adverse events: any undesirable clinical occurrence, whether considered to be treatment-related or not, that includes a clinical sign, symptom, or condition, are self-reported in questionnaires and recorded by the clinician during the consultation. Participants are asked to provide details on the nature of the event, how long it lasted for, and what action they took.
Adherence: assessed by recording the number of consultations received from the physiotherapist and/or dietitian. Adherence to prescribed exercise and physical activity programs is self-reported by the participant via questionnaire, being scored on an 11-point NRS (0 = not at all and 10 = completely as instructed). Adherence to the weight management plan, if applicable, is also self-reported and scored on a similar 11-point NRS.
Perceived usefulness of treatment components: assessed by self-report with response options ranging from 0 = “not at all” to 10 = “extremely useful”. Usefulness questions relate to the information on the website, consultations with the physiotherapist and/or dietitian, resource booklets, Fitbit, VLCD, strengthening exercise program, and physical activity plan. Participants will also be asked how many times they accessed the website in the last 6-months (5-point scale ranging from “never” to “> 10 times”) and how easy it was for them to use the videoconferencing software (11-point NRS ranging from 0 = “not at all easy” to 10 = “extremely easy”).
Long-term surgical rates: via linkage with the data routinely collected by Medibank Private, and will include the number of individuals who had a knee arthroscopy and/or arthroplasty intervention at 2 and 5 years after baseline. These findings will be reported separately from the main trial outcomes.
Trial sample size
The primary outcomes of this trial include changes in pain (NRS) and physical function (WOMAC) over 6 months and the intervention will be considered beneficial if it changes either one or both of the primary outcomes. A Cochrane Review shows that exercise has a moderate effect size for pain and function in knee OA [10]. Calculations thus assume an effect size between the least-performing treatment group (presumed to be the Exercise group) and the control group of 0.4. Given that the effect size between two treatments will be less than between treatment and control, we assumed an effect size of 0.3 between the Exercise group and the Exercise plus weight management group. We chose 0.3 because any smaller effect is unlikely to be clinically relevant or cost-effective to implement in real-world settings given the considerable expense of the weight management program relative to the exercise components of this combined intervention. Calculations also assume a correlation between baseline and follow-up measurements of 0.4, power 80, 15% loss to follow-up (based on our previous research [71, 72]), and two-sided significance level of 0.05. Based on these calculations we require 173 participants in each treatment group, and 69 participants in the control group arm, giving a total sample size of 415 participants.
There are different levels of clustering for each of the three groups; in the Exercise group, participants are clustered by physiotherapist, in the Exercise plus weight management group, people are clustered by physiotherapist and dietitian, and in the control group, there is no clustering. Given the geographical and demographic spread of participants, we expect that the intra-cluster correlation of outcomes treated by the same dietitian or physiotherapist to be of the order of 0.01. Modifying the formulae [73] to allow for correlations between baseline and follow-up measurements, and assuming that there are five dietitians delivering the Exercise plus weight management treatment, the effect size that can be detected between the Exercise and the Exercise plus weight management groups with 80% power given the originally calculated sample size increases slightly to 0.32.
Analysis
We will use intention-to-treat analyses, using multiple imputation to account for missing data and details on the imputation will be reported [74]. Participants will be included in their randomised treatment groups regardless of their post-randomisation behaviour. P values less than 0.05 will be considered significant. Outcomes (primary, secondary, and determinants) will be compared between groups as follows: i) Exercise vs Exercise plus weight management; ii) Exercise vs control, and; iii) Exercise plus weight management vs control. For continuous data, mean differences in change over time between these groups will be estimated using linear regression models fit to data from both follow-up time points, with random effects for participants, and accounting for clustering by physiotherapist and dietitian in the Exercise and Exercise plus weight management groups. Models will be adjusted for the stratification variable (history of knee surgery) and values of the outcome at baseline. Terms for time and treatment will be included, with an interaction between the two. Regression assumptions will be assessed using standard diagnostic plots. For binary outcomes, logistic regression models will be fit using generalised estimating equations to account for clustering, with risk differences and 95% confidence intervals calculated. This will include a comparison of the proportion of participants in each group whose improvement meets or exceeds the minimal clinically important difference for each of the primary outcomes. For ordinal outcomes, proportional odds models will be fit similarly. Multinominal models will be applied if the assumption of proportional odds is not valid. To assess the sensitivity of the results for the primary outcomes in situations in which the outcomes are missing not at random, we will apply a pattern-mixture approach that involves fitting regression models that adjust for departures from the missing at random assumption [75].
Economic evaluations will assess and compare the cost-effectiveness of the interventions. This will involve analysis of: i) cost per extra person with a clinically significant improvement in pain (measured as 1.8 point reduction on the pain score) and function (6 unadjusted WOMAC units), and; ii) per quality-adjusted life years (QALYs) gained for the intervention compared to control at 12 months. QALYs will be calculated based on utility scores using the AQoL-8D at baseline and 12 months. The difference in health care usage and productivity lost between baseline and 12 months will be compared for intervention and control groups. The association between utility gains on the AQoL-8D and productivity will also be compared between the intervention and control groups.
To evaluate the longer-term effects of the Exercise and Exercise plus weight management interventions on total knee joint replacement and knee arthroscopy surgical rates, logistic regression models for surgery will be fit using generalised estimating equations to account for clustering by physiotherapist and dietitian, with treatment effects summarised using risk differences and 95% confidence intervals. The stratifying variable of history of knee surgery will be adjusted for.
To evaluate whether there is a relationship between amount of weight loss and changes in pain and/or function, linear regression models for changes in pain and outcome will be fit and will include a term for weight lost at 6 and 12 months (as percentage of baseline weight), adjusting for baseline level of the outcome, assigned treatment group, and stratifying variables. Demographic variables thought to affect both weight loss and outcomes levels, including age and sex, will also be included in the model. Fractional polynomial terms for weight lost will be assessed to investigate if the relationship between outcomes and weight loss is non-linear. Standard diagnostic plots will be used to assess regression assumptions. We will also evaluate whether there is a relationship between adherence to exercise and changes in pain and/or function by estimating a complier-average causal effect using a two-stage least squares approach [76].
Finally, we will conduct exploratory analyses to investigate whether the relative effects of each intervention on change in each of the primary outcomes is moderated by: i) expectation of treatment effects; ii) sex; iii) pain self-efficacy; iv) BMI; v) employment situation; vi) history of knee surgery (contralateral total knee joint replacement surgery and/or knee arthroscopic surgery); vii) self-efficacy for eating control, and; viii) depression. These moderators were selected based on previous research and/or theoretical plausibility (Table 5). Models will include terms for treatment, the moderator variables, and an interaction between the two. Fractional polynomials will be included for the continuous moderators [88]. The estimated effects of treatment and 95% confidence intervals will be presented for each moderator, with visual representations included for continuous moderators.
Table 5.
Overview of selected demographic and clinical moderators
| Selected moderator variables | Justification | ||
|---|---|---|---|
| Expectation of treatment effects |
Based on evidence that greater treatment expectations are associated with more favourable outcomes in people with osteoarthritis [77–79]. Hypothesis: Participants in the intervention groups who have greater treatment expectations will report greater improvement in primary outcomes than those who have lower treatment expectations (relative to control group). |
||
| Sex |
Based on evidence that being male is associated with better outcomes in pain and physical function after supervised strengthening exercises [80] and evidence from a review that being female is associated with greater weight loss intentions [81]. Hypothesis: Participants in the intervention groups who are male will report greater improvement in primary outcomes than those who are female (relative to control). Participants in the Exercise plus weight management group who are female will report greater improvement in primary outcomes than those who are male (relative to Exercise group). |
||
| Pain self-efficacy |
Based on evidence that higher self-efficacy associated with better outcomes in pain and quality of life after supervised neuromuscular exercise [82], and greater improvements in pain after and internet-delivered exercise and education program [83]. Hypothesis: Participants in the intervention groups who have higher self-efficacy at baseline will report greater improvement in primary outcomes than those who have lower self-efficacy (relative to control group). |
||
| Body mass index |
Based on evidence that obesity is associated with better outcomes in quality of life after supervised aquatic exercise [84] and evidence from a review that higher initial BMI is associated with greater weight loss [85]. Hypothesis: Participants in the intervention groups who have a higher BMI will report greater improvement in primary outcomes (relative to control) than those who have a lower BMI. Participants in the Exercise plus weight management group who have a higher BMI will report greater improvement in primary outcomes than those with a lower BMI (relative to Exercise group). |
||
| Employment situation |
Based on evidence that being employed associated with greater improvements in pain after an internet-delivered exercise and education program [83]. Hypothesis: Participants in the intervention groups who are employed will report greater improvement in primary outcomes than those who are not employed (relative to control group). |
||
| History of knee surgery |
Chosen based on theoretical plausibility that knee surgical experience could affect expectations of outcomes and motivation Hypothesis: Participants in the intervention groups who have a history of knee surgery will report less improvement in primary outcomes than those without (relative to control group). |
||
| Self-efficacy for eating control |
Based on evidence from a review that better control of over-eating and dietary restraint is associated with weight loss and maintenance [86, 87]. Hypothesis: Participants in the Exercise plus weight management group who have higher self-efficacy for eating control will report greater improvement in primary outcomes than those with lower self-efficacy for eating control (relative to Exercise group and to control group). |
||
| Depression |
Based on evidence that fewer depressive symptoms is associated with better outcomes in pain and physical function after supervised strengthening exercises [80]. Hypothesis: Participants in the intervention groups who have more depressive symptoms at baseline will report less improvement in primary outcomes than those who have less depressive symptoms (relative to control group). |
||
Ethics
Ethical approval has been obtained from the University of Melbourne Human Research Ethics Committee (HREC No. 1750443). All participants will provide written informed consent. The funding agency (Medibank) will be responsible for marketing the study to support recruitment, by making the study known (via email, letters, articles, newsletters and social media) to potentially eligible members. All marketing material viewed by member will include a statement that their involvement in the study will not affect their relationship or policy with Medibank. Medibank will not be involved in statistical analysis.
Discussion
With the rapidly rising burden of knee OA, there is an urgent need for interventions that improve outcomes and that are also widely accessible to people in rural, regional, and metropolitan communities [89]. Although the effectiveness of exercise therapy on pain and function is well-established, there is relatively limited robust evidence about the additional benefits of weight loss in people with knee OA [4].
This trial will be the first to investigate the clinical- and cost-effectiveness of a remotely delivered service combining dietary weight management and exercise, compared to a service involving exercise only and to an online information-only control group. Such a service has the potential to be a low-cost and accessible way in which people with OA can receive care, thus potentially increasing uptake of recommended management methods, like weight loss and exercise. Findings from this study will inform healthcare providers about the effects of incorporating weight loss components into exercise interventions. Moderator analyses using data from this study will also allow us to determine whether specific sub-groups of participants are more likely to respond to these remotely delivered services, which will allow health service providers to better target interventions to those that would benefit most. Qualitative explorations will provide insight into the feasibility and viability of implementing similar weight management programs into private and public healthcare settings from the perspective of both the participants receiving care, as well as the clinicians involved in delivering it.
A potential limitation of this trial is the targeted sample of participants, including only those who are Medibank Private members with a level of membership that includes arthroplasty surgery. This may potentially limit the generalisability of our findings, however, Medibank Private is one is one of the largest providers of private health insurance in Australia, and participants will be recruited Australia-wide to attempt to maximise the generalisability of the cohort.
Acknowledgements
We thank Justin Braver, Medibank, for his contribution to the production of the total joint replacement decision aid.
Abbreviations
- BMI
Body mass index
- CSIRO
Commonwealth Scientific and Industrial Research Organisation
- MITI
Motivational Interviewing Treatment Integrity tool
- NRS
Numeric rating scale
- NSAIDS
Non-steroidal anti-inflammatory drugs
- OA
Osteoarthritis
- RCT
Randomised controlled trial
- RPE
Rating of perceived exertion
- VLCD
Very low calorie diet
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
Authors’ contributions
KLB, RSH, CK and TE conceived the idea for the study. KLB and RSH are leading the trial. KLB, RSH, TE, CK, JK, JQ, JP, AH, AMB, CP, PC, MD, FK and CR obtained funding for the study. All designed the trial protocol. JP, PS, CK and CB produced the VLCD video. CK, CB, PC, MD, KLB, RSH, and TE produced the total joint replacement decision aid. KLB, RSH, CK, CB, AK, LS, BJL, JQ, TE, JP, PS, FK and CR designed the participant resources. CK and CB managed the marketing to Medibank members, development of the cookbook and data linkage to support evaluation. AK developed the website. JK performed the sample size calculation, randomisation procedures and devised the statistical plan. AH designed the health economic evaluation. KLB and BJL drafted the manuscript with input from other authors. All authors read and approved the final manuscript.
Funding
This study is funded by Medibank and the Medibank Better Health Foundation Research Fund as well as a National Health and Medical Research Council (NHMRC) Centre of Research Excellence (APP1079078). Medibank staff (CK, CB) were involved in the design of the study protocol, development of participant resources, and are responsible for inviting members to participate in the trial. Medibank staff are not otherwise involved in the collection or analysis of data.
RSH is supported by a National Health & Medical Research Council Fellowship (#1154217) and KLB by a NHMRC Principal Research Fellowship (APP1058440). JQ is supported by a NIHR Clinical Research Network West Midlands Research Scholars Fellowship. MD is supported by a National Health & Medical Research Council Career Development Fellowship (APP1122526). PC is supported by a National Health & Medical Research Council Practitioner Fellowship (APP1154203). PS is supported by a David Bickart Clinical Research Fellowship from the University of Melbourne.
Availability of data and materials
The datasets used and/or analysed during the current study will be available from the corresponding author (k.bennell@unimelb.edu.au) on reasonable request once the study has been completed.
Ethics approval and consent to participate
Ethical approval has been obtained from the University of Melbourne Human Research Ethics Committee (HREC No. 1750443). All participants will provide written informed consent.
Consent for publication
Not applicable.
Competing interests
JP was Chair of the Medical advisory Board for Liraglutide 3 mg (Saxenda) for Novo Nordisk Australia and Has given lectures on the management of obesity for I Nova marketers of Duromine (Phentermine) and Contrave (Bupropion and Naltrexone).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pereira D, Peleteiro B, Araujo J, Branco J, Santos R, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthr Cartil. 2011;19(11):1270–1285. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Ackerman IN, Bohenseky MA, Pratt C, Gorelik A, Liew D. Counting the cost: the current and future burden of arthritis. 2016. [Google Scholar]
- 3.National Institute for Health and Care Excellence. Osteoarthritis - Care and management, clinical guideline CG177. National Institute for Health and Care Excellence, editor. London: National Institute for Health and Care Excellence; 2014.
- 4.The Royal Australian College of General Practitioners . Guideline for the management of knee and hip osteoarthritis. 2. East Melbourne: RACGP; 2018. [Google Scholar]
- 5.Runciman WB, Hunt TD, Hannaford NA, Hibbert PD, Westbrook JI, Coiera EW, et al. CareTrack: assessing the appropriateness of health care delivery in Australia. Med J Aust. 2012;197(2):100–105. doi: 10.5694/mja12.10510. [DOI] [PubMed] [Google Scholar]
- 6.Haskins R, Henderson JM, Bogduk N. Health professional consultation and use of conservative management strategies in patients with knee or hip osteoarthritis awaiting orthopaedic consultation. Aus J Primary Health. 2014;20(3):305–310. doi: 10.1071/PY13064. [DOI] [PubMed] [Google Scholar]
- 7.Australian Institute of Health and Welfare. Osteoarthritis snapshot: AIHW; 2018. Cat. no: PHE 232. Available from: https://www.aihw.gov.au/reports/chronic-musculoskeletal-conditions/osteoarthritis/contents/what-is-osteoarthritis.
- 8.Baker P, Van der Meulen J, Lewsey J, Gregg P. The role of pain and function in determining patient satisfaction after total knee replacement: data from the National Joint Registry for England and Wales. J Bone Joint Surg. 2007;89(7):893–900. doi: 10.1302/0301-620X.89B7.19091. [DOI] [PubMed] [Google Scholar]
- 9.Arthritis and Osteoporosis Victoria . A problem worth solving. The rising cost of musculoskeletal conditions in Australia. Melbourne: Arthritis and Osteoporosis Victoria; 2015. [Google Scholar]
- 10.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376. doi: 10.1002/14651858.CD004376.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46–54. doi: 10.7326/M14-1231. [DOI] [PubMed] [Google Scholar]
- 12.Quicke J, Foster N, Thomas M, Holden M. Is long-term physical activity safe for older adults with knee pain?: a systematic review. Osteoarthr Cartil. 2015;23(9):1445–1456. doi: 10.1016/j.joca.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Hinman RS, Hunt MA, Creaby MW, Wrigley TV, McManus FJ, Bennell KL. Hip muscle weakness in individuals with medial knee osteoarthritis. Arthritis Care Res (Hoboken). 2010;62(8):1190–1193. doi: 10.1002/acr.20199. [DOI] [PubMed] [Google Scholar]
- 14.Runhaar J, Luijsterburg P, Dekker J, Bierma-Zeinstra S. Identifying potential working mechanisms behind the positive effects of exercise therapy on pain and function in osteoarthritis; a systematic review. Osteoarthr Cartil. 2015;23(7):1071–1082. doi: 10.1016/j.joca.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Wallis J, Webster K, Levinger P, Taylor N. What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthr Cartil. 2013;21(11):1648–1659. doi: 10.1016/j.joca.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Dunlop DD, Song J, Arntson EK, Semanik PA, Lee J, Chang RW, et al. Sedentary time in US older adults associated with disability in activities of daily living independent of physical activity. J Phys Act Health. 2015;12(1):93–101. doi: 10.1123/jpah.2013-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Chang RW, Ehrlich-Jones L, Kwoh CK, Nevitt M, Semanik PA, et al. Sedentary behavior and physical function: objective evidence from the osteoarthritis initiative. Arthritis Care Res (Hoboken). 2015;67(3):366–373. doi: 10.1002/acr.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White DK, Tudor-Locke C, Zhang Y, Fielding R, LaValley M, Felson DT, et al. Daily walking and the risk of incident functional limitation in knee osteoarthritis: an observational study. Arthritis Care Res (Hoboken). 2014;66(9):1328–1336. doi: 10.1002/acr.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackerman IN, Osborne RH. Obesity and increased burden of hip and knee joint disease in Australia: results from a national survey. BMC Musculoskelet Disord. 2012;13(1):1. doi: 10.1186/1471-2474-13-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasilic-Brasnjevic S, Marinkovic J, Vlajinac H, Vasiljevic N, Jakovljevic B, Nikic M, et al. Association of body mass index and waist circumference with severity of knee osteoarthritis. Acta Reumatologica Portuguesa. 2016;41(3):226–231. [PubMed] [Google Scholar]
- 21.Smith WA, Zucker-Levin A, Mihalko WM, Williams M, Loftin M, Gurney JG. Physical function and physical activity in obese adults after Total knee Arthroplasty. Orthop Clin N Am. 2017;48(2):117–125. doi: 10.1016/j.ocl.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Brosseau L, Wells GA, Tugwell P, Egan M, Dubouloz C-J, Casimiro L, et al. Ottawa Panel evidence-based clinical practice guidelines for the management of osteoarthritis in adults who are obese or overweight. Phys Ther. 2011;91(6):843–861. doi: 10.2522/ptj.20100104. [DOI] [PubMed] [Google Scholar]
- 23.Alrushud AS, Rushton AB, Kanavaki AM, Greig CA. Effect of physical activity and dietary restriction interventions on weight loss and the musculoskeletal function of overweight and obese older adults with knee osteoarthritis: a systematic review and mixed method data synthesis. BMJ Open. 2017;7(6):e014537. doi: 10.1136/bmjopen-2016-014537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Losina E, Smith KC, Paltiel AD, Collins JE, Suter LG, Hunter DJ, et al. Cost-Effectiveness of Diet and Exercise for Overweight and Obese Knee Osteoarthritis Patients. Arthritis Care Res (Hoboken). 2018. 10.1002/acr.23716. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 26.Papandony MC, Chou L, Seneviwickrama M, Cicuttini FM, Lasserre K, Teichtahl AJ, et al. Patients’ perceived health service needs for osteoarthritis care: a scoping systematic review. Osteoarthr Cartil. 2017;25(7):1010–1025. doi: 10.1016/j.joca.2017.02.799. [DOI] [PubMed] [Google Scholar]
- 27.Dobson F, Bennell KL, French SD, Nicolson PJ, Klaasman RN, Holden MA, et al. Barriers and facilitators to exercise participation in people with hip and/or knee osteoarthritis: synthesis of the literature using behavior change theory. Am J Phys Med Rehabil. 2016;95(5):372–389. doi: 10.1097/PHM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 28.Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2016;31(5):625–638. doi: 10.1177/0269215516645148. [DOI] [PubMed] [Google Scholar]
- 29.Bennell KL, Nelligan R, Dobson F, Rini C, Keefe F, Kasza J, et al. Effectiveness of an internet-delivered exercise and pain-coping skills training intervention for persons with chronic knee pain: a randomised trial. Ann Intern Med. 2017;166(7):453–462. doi: 10.7326/M16-1714. [DOI] [PubMed] [Google Scholar]
- 30.Hinman R, Nelligan R, Bennell K, Delany C. “Sounds a bit crazy, but it was almost more personal”: a qualitative study of patient and clinician experiences of physical therapist-prescribed exercise for knee osteoarthritis via Skype™. Arthritis Care Res (Hoboken) 2017;69(12):1834–1844. doi: 10.1002/acr.23218. [DOI] [PubMed] [Google Scholar]
- 31.Kelly JT, Reidlinger DP, Hoffmann TC, Campbell KL. Telehealth methods to deliver dietary interventions in adults with chronic disease: a systematic review and meta-analysis, 2. Am J Clin Nutr. 2016;104(6):1693–1702. doi: 10.3945/ajcn.116.136333. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes L, Hagen KB, Bijlsma JW, Andreassen O, Christensen P, Conaghan PG, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125–1135. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 33.Pearson ML, Mattke S, Shaw R, Ridgely MS, Wiseman SH. In: Patient self-management support programs. Services USDoHaH, editor. 2007. [Google Scholar]
- 34.Martin E, Dobson F, Hall M, Marshall C, Egerton T. The effects of behavioural counselling on the determinants of health behaviour change in adults with chronic musculoskeletal conditions making lifestyle changes: a systematic review and meta-analysis. Musculoskelet. 2019;17(3):170–197. doi: 10.1002/msc.1410. [DOI] [PubMed] [Google Scholar]
- 35.O’Halloran PD, Blackstock F, Shields N, Holland A, Iles R, Kingsley M, et al. Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clin Rehabil. 2014;28(12):1159–1171. doi: 10.1177/0269215514536210. [DOI] [PubMed] [Google Scholar]
- 36.Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011;26(11):1479–1498. doi: 10.1080/08870446.2010.540664. [DOI] [PubMed] [Google Scholar]
- 37.Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medibank Private Ltd . Annual Report 2018. Melbourne: Medibank Private Ltd; 2018. [Google Scholar]
- 39.National Health and Medical Research Council. In: Council NHaMR, editor. Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. Melbourne: National Health and Medical Research Council; 2013.
- 40.Norton K, Norton L. Pre-exercise screening: guide to the Australian adult pre-exercise screening system. Australia: F.A.a.S.M.A. Exercise and Sports Science Australia; 2012. [Google Scholar]
- 41.Wagner EH, Austin BT, Von Korff MJTMQ. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. doi: 10.2307/3350391. [DOI] [PubMed] [Google Scholar]
- 42.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Clin Rehabil. 1992;111(3):455. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 43.Fisher JD, Fisher WA, Misovich SJ, Kimble DL, Malloy TE. Changing AIDS risk behavior: effects of an intervention emphasizing AIDS risk reduction information, motivation, and behavioral skills in a college student population. Health Psychol. 1996;15(2):114. doi: 10.1037/0278-6133.15.2.114. [DOI] [PubMed] [Google Scholar]
- 44.Mazzuca SA. Does patient education in chronic disease have therapeutic value? J Chronic Dis. 1982;35(7):521–529. doi: 10.1016/0021-9681(82)90071-6. [DOI] [PubMed] [Google Scholar]
- 45.Sabaté E. In: Adherence to long-term therapies: evidence for action. World Health Organization, editor. 2003. [Google Scholar]
- 46.Briggs AM, Houlding E, Hinman RS, Desmond LA, Bennell KL, Darlow B, et al. Health professionals and students encounter multi-level barriers to implementing high-value osteoarthritis care: a multi-national study. Osteoarthr Cartil. 2019;27(5):788–804. doi: 10.1016/j.joca.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 47.The Australian & New Zealand Obesity Society . In: Australian Obesity Management Algorithm. ANZOS, editor. 2016. [Google Scholar]
- 48.Bennell KL, Kyriakides M, Metcalf B, Egerton T, Wrigley TV, Hodges PW, et al. Neuromuscular versus quadriceps strengthening exercise in patients with medial knee osteoarthritis and Varus Malalignment: a randomized controlled trial. Arthritis Rheumatol. 2014;66(4):950–959. doi: 10.1002/art.38317. [DOI] [PubMed] [Google Scholar]
- 49.Borg G. Borg’s perceived exertion and pain scales: human kinetics. 1998. [Google Scholar]
- 50.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur J Clin Nutr. 2013;67(7):759–764. doi: 10.1038/ejcn.2013.90. [DOI] [PubMed] [Google Scholar]
- 51.Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr, Brehm BJ, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(3):285–293. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 52.Purcell K, Sumithran P, Prendergast LA, Bouniu CJ, Delbridge E, Proietto J. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2(12):954–962. doi: 10.1016/S2213-8587(14)70200-1. [DOI] [PubMed] [Google Scholar]
- 53.Messier SP, Resnik AE, Beavers DP, Mihalko SL, Miller GD, Nicklas BJ, et al. Intentional weight loss in overweight and obese patients with knee osteoarthritis: is more better? Arthritis Care Res (Hoboken). 2018;70(11):1569–1575. doi: 10.1002/acr.23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons - a scoping review. Obes Rev. 2014;15(7):578–586. doi: 10.1111/obr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noakes M, Clifton P. The CSIRO total wellbeing diet. Camberwell, Vic. Australia: Penguin; 2005. [Google Scholar]
- 56.Messier SP, Callahan LF, Golightly YM, Keefe FJ. OARSI clinical trials recommendations: design and conduct of clinical trials of lifestyle diet and exercise interventions for osteoarthritis. Osteoarthr Cartil. 2015;23(5):787–797. doi: 10.1016/j.joca.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Fitzgerald GK, Hinman RS, Zeni J, Jr, Risberg MA, Snyder-Mackler L, Bennell KL. OARSI clinical trials recommendations: design and conduct of clinical trials of rehabilitation interventions for osteoarthritis. Osteoarthr Cartil. 2015;23(5):803–814. doi: 10.1016/j.joca.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 59.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 60.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster universities osteoarthritis index (WOMAC): a review of its utility and measurement properties. Arthritis Care Res (Hoboken). 2001;45(5):453–461. doi: 10.1002/1529-0131(200110)45:5<453::AID-ART365>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 61.Delbaere K, Hauer K, Lord SR. Evaluation of the incidental and planned activity questionnaire (IPEQ) for older people. Br J Sports Med. 2010;44(14):1029–1034. doi: 10.1136/bjsm.2009.060350. [DOI] [PubMed] [Google Scholar]
- 62.Richardson J, Iezzi A, Khan MA, Maxwell A. Validity and reliability of the assessment of quality of life (AQoL)-8D multi-attribute utility instrument. The patient. 2014;7(1):85–96. doi: 10.1007/s40271-013-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ten Klooster PM, Drossaers-Bakker KW, Taal E, van de Laar MA. Patient-perceived satisfactory improvement (PPSI): interpreting meaningful change in pain from the patient's perspective. Pain. 2006;121(1–2):151–157. doi: 10.1016/j.pain.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 64.Lovibond SH, Lovibond PF. Manual for the depression anxiety stress scales. 2. Psychology Foundation: Sydney; 1991. [Google Scholar]
- 65.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32(1):37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 66.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. J Consult Clin Psychol. 1991;59(5):739–744. doi: 10.1037/0022-006X.59.5.739. [DOI] [PubMed] [Google Scholar]
- 67.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40(6 Pt 1):1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Selten EMH, Vriezekolk JE, Schers HJ, Nijhof MW, van der Laan WH, van der Meulen-Dilling RG, et al. Development of the “treatment beliefs in knee and hip OsteoArthritis (TOA)” questionnaire. BMC Musculoskelet Disord. 2017;18(1):402. doi: 10.1186/s12891-017-1762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shelby RA, Somers TJ, Keefe FJ, DeVellis BM, Patterson C, Renner JB, et al. Brief fear of movement scale for osteoarthritis. Arthritis Care Res (Hoboken). 2012;64(6):862–871. doi: 10.1002/acr.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kessler RC, Barber C, Beck A, Berglund P, Cleary PD, McKenas D, et al. The world health organization health and work performance questionnaire (HPQ) J Occup Environ Med. 2003;45(2):156–174. doi: 10.1097/01.jom.0000052967.43131.51. [DOI] [PubMed] [Google Scholar]
- 71.Bennell KL, Ahamed Y, Jull G, Bryant C, Hunt MA, Forbes AB, et al. Physical therapist–delivered pain coping skills training and exercise for knee osteoarthritis: randomized controlled trial. Arthritis Care Res (Hoboken). 2016;68(5):590–602. doi: 10.1002/acr.22744. [DOI] [PubMed] [Google Scholar]
- 72.Bennell KL, Nelligan RK, Rini C, Keefe FJ, Kasza J, French S, et al. Effects of internet-based pain coping skills training before home exercise for individuals with hip osteoarthritis (HOPE trial): a randomised controlled trial. Pain. 2018;159(9):1833–1842. doi: 10.1097/j.pain.0000000000001281. [DOI] [PubMed] [Google Scholar]
- 73.Moerbeek M, Wong WK. Sample size formulae for trials comparing group and individual treatments in a multilevel model. Stat Med. 2008;27(15):2850–2864. doi: 10.1002/sim.3115. [DOI] [PubMed] [Google Scholar]
- 74.Carpenter J, Kenward M. Multiple imputation and its application. West Sussex: Wiley; 2013. [Google Scholar]
- 75.White IR, Kalaitzaki E, Thompson SG. Allowing for missing outcome data and incomplete uptake of randomised interventions, with application to an internet-based alcohol trial. Stat Med. 2011;30(27):3192–3207. doi: 10.1002/sim.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stuart EA, Perry DF, Le H-N, Ialongo NS. Estimating intervention effects of prevention programs: accounting for noncompliance. Prev Sci. 2008;9(4):288–298. doi: 10.1007/s11121-008-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang W, Robertson J, Jones A, Dieppe P, Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67(12):1716–1723. doi: 10.1136/ard.2008.092015. [DOI] [PubMed] [Google Scholar]
- 78.Foster NE, Thomas E, Hill JC, Hay EM. The relationship between patient and practitioner expectations and preferences and clinical outcomes in a trial of exercise and acupuncture for knee osteoarthritis. Eur J Pain. 2010;14(4):402–409. doi: 10.1016/j.ejpain.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Foster NE. Beliefs and preferences: do they help determine the outcome of musculoskeletal problems? Phys Ther Rev. 2007;12(3):199–206. doi: 10.1179/108331907X222976. [DOI] [Google Scholar]
- 80.French HP, Galvin R, Cusack T, McCarthy GM. Predictors of short-term outcome to exercise and manual therapy for people with hip osteoarthritis. Phys Ther. 2014;94(1):31. doi: 10.2522/ptj.20130173. [DOI] [PubMed] [Google Scholar]
- 81.Assari S, Lankarani MM. The association between obesity and weight loss intention weaker among blacks and men than whites and women. J Racial Ethn Health Disparities. 2015;2(3):414–420. doi: 10.1007/s40615-015-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Skou ST, Simonsen ME, Odgaard A, Roos EM. Predictors of long-term effect from education and exercise in patients with knee and hip pain. Danish Medical Journal. 2014;61(7):A4867. [PubMed] [Google Scholar]
- 83.Lawford BJ, Hinman RS, Kasza J, Nelligan R, Keefe F, Rini C, et al. Moderators of effects of internet-delivered exercise and pain coping skills training for people with knee osteoarthritis: exploratory analysis of the IMPACT randomized controlled trial. J Med Internet Res. 2018;20(5):e10021. doi: 10.2196/10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cadmus L, Patrick MB, Maciejewski ML, Topolski T, Belza B, Patrick DL. Community-based aquatic exercise and quality of life in persons with osteoarthritis. Med Sci Sports Exerc. 2010;42(1):8–15. doi: 10.1249/MSS.0b013e3181ae96a9. [DOI] [PubMed] [Google Scholar]
- 85.Teixeira P, Going SB, Sardinha L, Lohman T. A review of psychosocial pre-treatment predictors of weight control. Obes Rev. 2005;6(1):43–65. doi: 10.1111/j.1467-789X.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- 86.Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6(1):67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 87.Dalle Grave R, Calugi S, Corica F, Di Domizio S, Marchesini G. Psychological variables associated with weight loss in obese patients seeking treatment at medical centers. J Am Diet Assoc. 2009;109(12):2010–2016. doi: 10.1016/j.jada.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Royston P, Sauerbrei W. Two techniques for investigating interactions between treatment and continuous covariates in clinical trials. Stata J. 2009;9(2):230–251. doi: 10.1177/1536867X0900900204. [DOI] [Google Scholar]
- 89.Briggs A, Page C, Shaw B, Bendrups A, Philip K, Cary B, et al. A Model of Care for Osteoarthritis of the Hip and Knee: Development of a System-Wide Plan for the Health Sector in Victoria, Australia. Healthc Policy. 2018;14(2):47–58. doi: 10.12927/hcpol.2018.25686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study will be available from the corresponding author (k.bennell@unimelb.edu.au) on reasonable request once the study has been completed.