Abstract
Background
Low-grade fibromyxoid sarcoma (LGFMS) is a rare fibroblastic tumor often involving deep tissue of trunk and lower extremities in young to middle-aged patients. Rarely, LGFMS can occur in other sites including head and neck, chest, abdomen and female reproductive system. Three cases of LGFMS in mesentery of small intestine have been reported and all have conventional histologic features. Herein we reported a unique case of LGFMS in mesentery of small intestine.
Case presentation
A 43 year-old male with chief complaint of lower back pain for 4 years presented to our hospital. Physical exam reveal a firm, non-tender, non-distended, mobile large abdominal mass, which was shown on abdominal CT as a 10 cm retroperitoneal tumor. Biopsy revealed a spindle cell neoplasm in a myxoid background with a delicate vascular network. Tumor resection was performed. Gross examination of the resected specimen showed a 10.8 cm, tan-white, smooth, firm, lobulated mesenteric mass with bulging and gelatinous cut surface and confined within small bowel serosa. Microscopic examination demonstrated foci epithelioid cords and whorls with prominent atypia, in additional of regular, bland-appearing spindle cells in a fibrous and myxoid stroma and osseous metaplasia. The tumor cells stained diffusely positive for DOG1 with moderate staining density, and diffusely and strongly positive for MUC4. Rearrangement involving FUS (16p11.2) gene was identified with break-apart probe and confirmed by Anchored Multiplex PCR. A final diagnosis of low-grade fibromyxoid sarcoma was rendered.
Conclusion
Our case highlights the importance of including LGFMS in the differential diagnosis of mesenteric tumors and the DOG1 positivity which could represent a potential diagnostic pitfall.
Keywords: Low grade fibromyxoid sarcoma, Epithelioid, Small bowel, Mesentery, DOG-1
Introduction
Low-grade fibromyxoid sarcoma (LGFMS), also as known as Evans’ tumor, is a rare fibroblastic tumor. It was first described by Evans in 1987 [1]. The tumor most often involves deep tissue of trunk and lower extremities, especially thigh, in young to middle-aged adults with male predominance in all age groups [2]. Less frequently, LGFMS can occur in abdomen (mesentery, intestine) [3–7] and other sites such as female reproductive system (breast, vagina, vulva, broad ligament) [8–12].
LGFMS is an aggressive low-grade tumor typically composed of deceivingly bland spindle-shaped fibroblast cells residing in variably fibrous/myxoid stroma. The tumor usually grow slowly with infiltration with a propensity for late metastatic potential. The tumor cells have palely eosinophilic cytoplasm and round to ovoid nuclei. Pleomorphic Nuclei, nucleoli and mitotic figures are usually absent [13].
LGFMS can display some variable, focal morphologic features in addition to conventional alternating areas of giant rosettes and hypercellularity. These features include epithelioid morphology, hyalinization, cyst degeneration, calcification/osseous metaplasia, multinucleated giant cells, nuclear palisading, nuclear pleomorphism, and tumor necrosis [14–17]. These histologic variations can be misleading. Especially, the giant rosettes featuring central accumulation of collagen and peripheral palisading epithelioid fibroblastic cells can mimic sclerosing epithelioid fibrosarcoma (SEF). Morphologically, SEF differs from LGFMS in two aspects. First, the tumor cells of SEF are epithelioid cells with clear or eosinophilic cytoplasm, forming nests and cords. Second, the stroma is densely sclerotic. SEF is more aggressive than LGMFS in that local recurrences and distant metastases are seen in more than half of cases [18].
MUC4 is a nearly 100% sensitive and specific marker for LGFMS [19]. Notably, MUC4 expression can also be seen in SEF, synovial sarcomas, ossifying fibromyxoid tumors, epithelioid gastrointestinal stromal tumors and myoepithelial carcinomas [14, 19]. LGFMS can also demonstrate positivity of CD99 and BCL-2 [4]. Interestingly, about 40% of LGFMS cases display patchy expression of DOG1 (discovered on GIST-1) with variable intensity; however, no extensive strong positivity has been ever observed.
Cytogenetically, more than 90% of LGFMS harbor t (7;16)(q34;p11), resulting in FUS-CREB3L2 fusion [20–22]. Alternatively, t (11;16)(p11;p11) translocation resulting in EWSR1-CREB3L1 is seen in less than 10% of cases [23, 24]. Of note, MUC4-negative LGFMS with FUS-CREB3L2 fusion has been described [25].
Pathology of small intestine mesenteric LGMFS have been described in 3 cases from 3 different case reports in English literature. The results reveal conventional histopathologic features of all these 3 cases [3, 26, 27]. All showed conventional morphology, no DOG1 expression, nor epithelioid cells.
Herein we reported a case of LGMFS arising in small intestine mesentery with unconventional epithelioid cords and whorls and extensive, diffuse positivity for DOG-1. Our case highlight the diagnostic challenges of such occurrence.
Case report
A 43 year-old male who presented with lower back pain for 4 years, loss of 40 pounds in 17 months and urinary urgency for one month. His past medical history was significant for Bell’s palsy, hyperlipidemia, osteoarthritis of cervical spine and vertigo. Physical exam results were normal except a firm, non-tender, mobile large mass palpated in right side of abdomen. The mass was nondistended. Abdominal CT showed a 10 cm retroperitoneal mass (Fig. 1). Laboratory results showed normal hematologic, biochemical and coagulatory results.
Fig. 1.
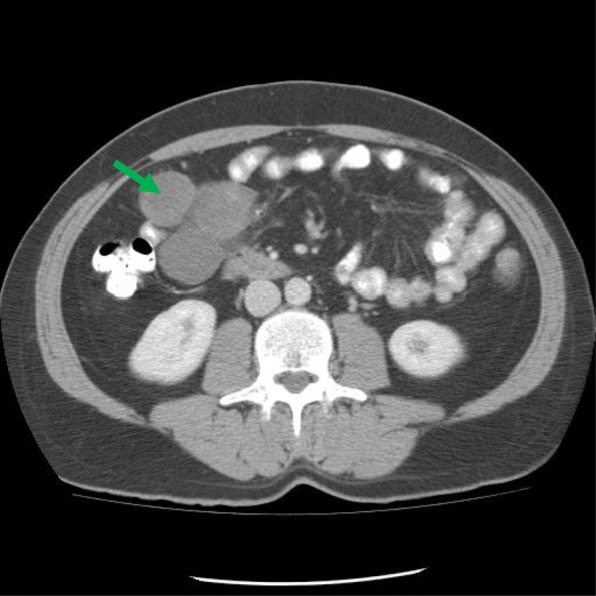
Abdominal CT showed a 10 cm retroperitoneal mesentery mass (put arrows on the mass)
Pathologic findings
Biopsy revealed a spindle cell neoplasm in a myxoid background with a delicate vascular network. The tumor cells showed small oval nuclei with some cytological atypia including hyperchromatism and angulate nuclei. No mitotic figures nor necrosis was noted. Immunohistochemical studies demonstrated that negativity of SMA, desmin, β-catenin, S100, SOX10, AE1/3, EMA, CD117 and CD34 in the tumor cells. DOG1 stain showed rare nonspecific staining in the tumor cells. Proliferative index was low (< 5%) on ki-67 stain (Data not shown). Further molecular classification was attempted but hindered due to the limited specimen.
Tumor resection was performed with the entire tumor removed. At intraoperative examination, retroperitoneal mass was found clearly intimate with the mesentery and adjacent small bowel showed that the. Gross examination of the resected specimen showed an intact 10.8 × 9.0 × 8.3 cm, tan-white, smooth, firm, lobulated mesenteric mass, which was completely confined within small bowel serosa and had bulging and gelatinous cut surface. The mass was predominately solid (90%) and focally cystic (10%) with a 2.0 × 1.2 × 1.0 cm calcified area (Fig. 2).
Fig. 2.
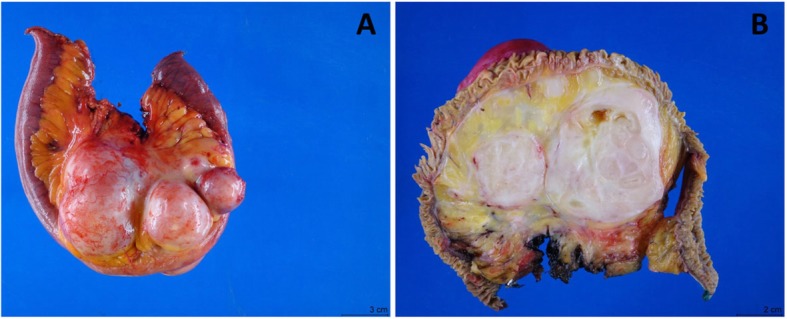
Small intestine mesenteric mass had lobulated outer surface (a) and bulging and gelatinous cut surface with focal cystic degeneration (b)
Microscopic examination confirmed multinodular and infiltrative pattern (Fig. 3A, B). The majority of the tumor was consisted of regular, bland-appearing spindle cells in a fibrous and myxoid stroma (Fig. 3C, D). Thin walled elongated vessels were noted with areas of hyalinization. In certain areas the tumor cells assumed epithelioid morphology and formed a more cord-like and even whorls with more prominent cytological atypia, which is unusual for LGMFS (Fig. 3E-3J). Perivascular hypercellular areas and osseous metaplasia were present (Fig. 3K, L). Yet, no significant atypia, necrosis nor increased mitotic activity was observed.
Fig. 3.
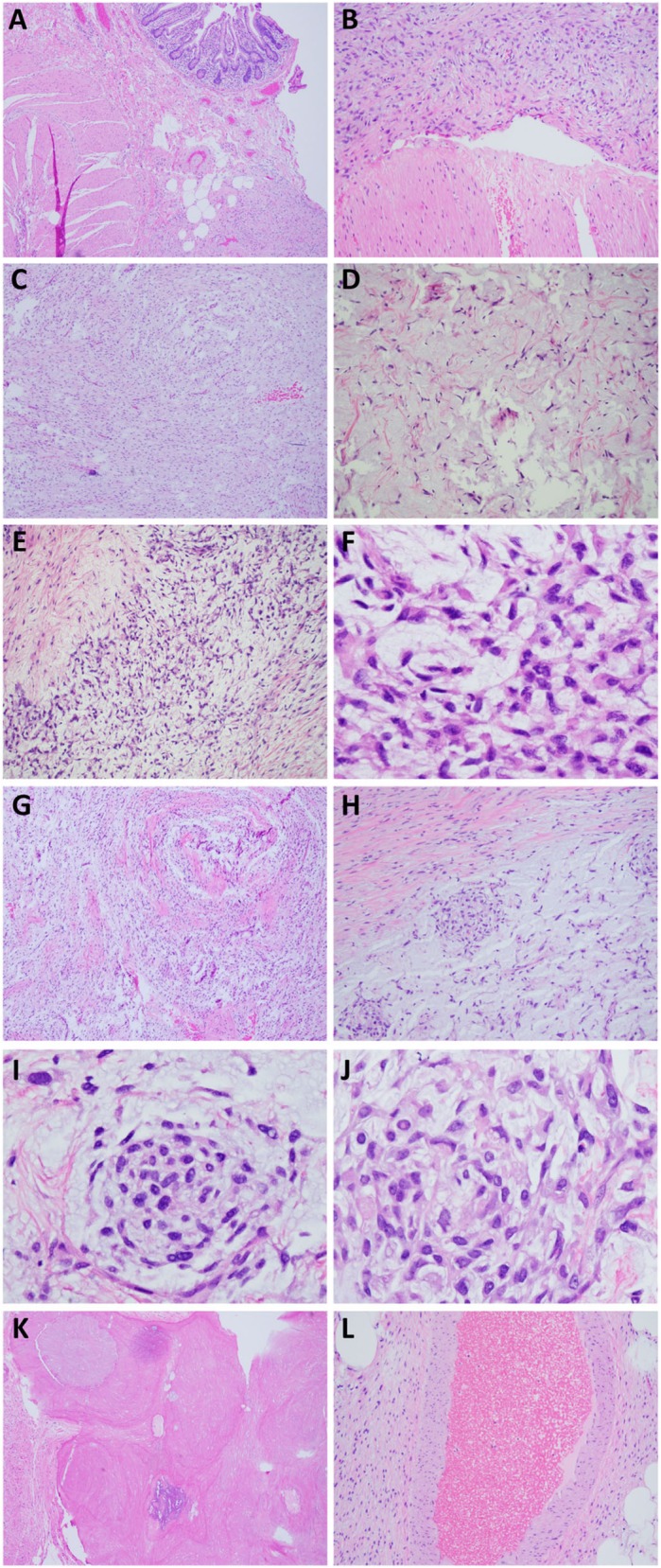
Invasive tumor composed of spindle and epitheloid tumor cells. The tumor invaded muscularis propia (a, 4x; b, 20x). The majority of tumor was comprised of bland spindle cells embedded in myxoid stroma (c, 4x; d, 20x). There were areas comprised of epitheloid tumor cells forming cord-like architecture (e, 10x) and with prominent atypia (f, 40x). There was also focal collagen rosette (g, 4x), and epitheloid cell whorls (h-j) with prominent atypia and rare nuclear inclusion (j). h, 10x; i & j, 40x). Focal osseous metaplasia (k, post decalcification, 4x) and focal perivascular condensation of bland spindle cells (l, 10x) were also present
Immunohistochemically, the tumor cells lacked of reactivity with CD34; CK5/6, EMA, SOX10, S100, desmin, SMA, MDM2 and GFAP. CD117 stain highlighted rare tumor cells. DOG1 stain showed moderate dense immunoreactivity, while MUC4 immunostain demonstrated diffuse and strong positivity in the tumor cell cytoplasm (Fig. 4).
Fig. 4.
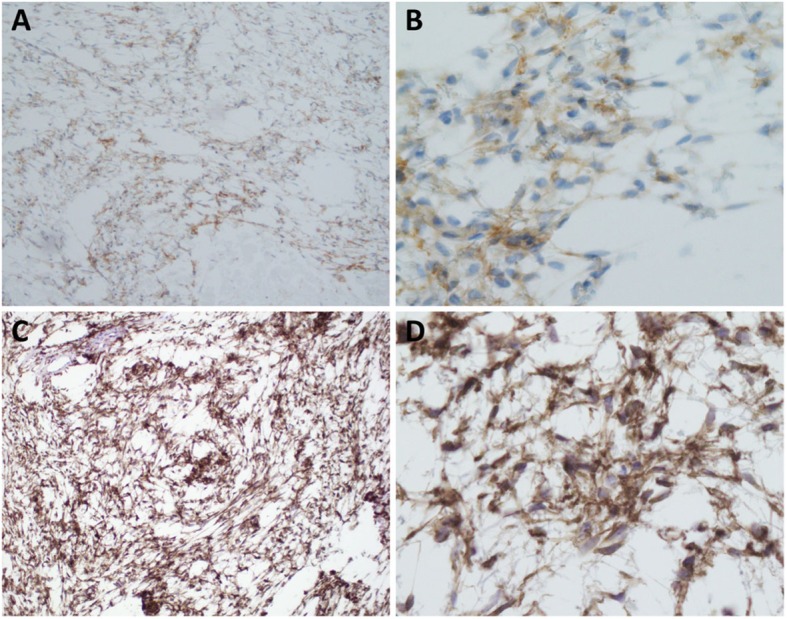
The neoplastic cells were diffusely positive for DOG1 (a, 10x; b, 40x) with moderate dense immunoreactivity, and diffusely and strongly for MUC4 (c, 10x; d, 40x)
Molecular findings
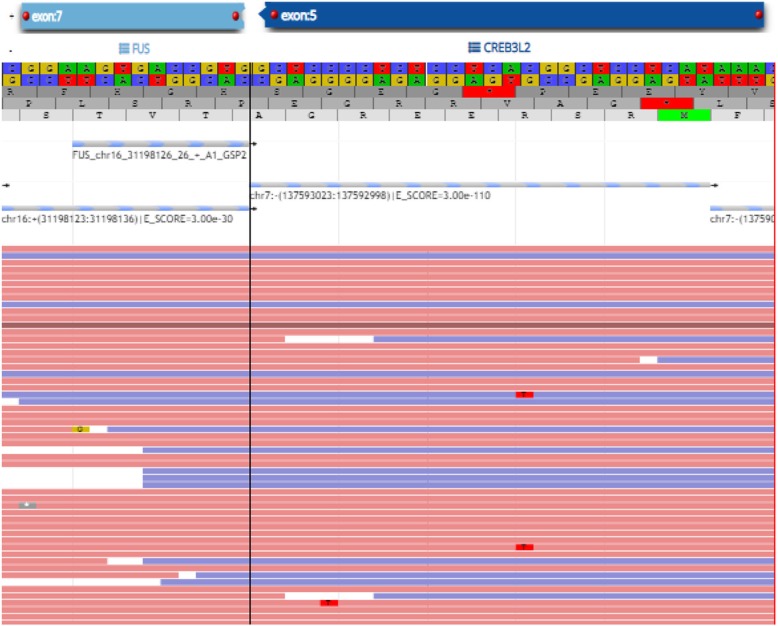
Rearrangement involving FUS (16p11.2) gene was identified with break-apart probe, while rearrangement of the MDM2 and DDIT3 gene regions was not present, excluding dedifferentiated and myxoid liposarcoma. Additional confirmatory RNA from formalin fixed paraffin embedded tissue was extracted, and targeted RNA sequencing using a customized FusionPlex 86 genes panel (Archer, Boulder, CO) [28] was performed to reveal a confirmatory FUS-CREB3L2 fusion (Fig. 5).
Fig. 5.
Screenshot of the Multiplexed PCR using the Archer customize panel. The image showed the fusion between FUS exon 7 and CREB3L2 exon 5. The red and blue lines referred to the different reads supporting the fusion event
A final diagnosis of low-grade fibromyxoid sarcoma was made based on the morphologic, immunohistochemical and cytogenetic features of the tumor.
In summary, we identified a rare case of low-grade myxofibrous sarcoma arising in small intestine mesentery. The tumor cells focally formed unusual epithelioid cords/whorls which contained prominent atypical neoplastic cells.
Discussion
Since LGFMS is a rare tumor that can occur in a wide range of anatomical sites, it should be included in the differential diagnoses of any spindle cell tumor with low cell density. Due to its nature of hypocellularity, fine needle aspiration (FNA) biopsy can yield suboptimal amount of tissue, which may not be adequate for a complete workup to arrive at a definite diagnosis. For the present case, the deep, retroperitoneal location created extra risk of visceral organ injury and additional difficulty in obtaining enough material for further molecular and cytogenetic profiling.
The major differential diagnoses of LGFMS are mesenchymal tumors with fibromyxoid features, such as myxoma, low-grade myxofibrosarcoma, desmoid fibromatosis, nodular fasciitis, perineurioma, neurofibroma, schwannoma, ossifying fibromyxoid tumor, and dermatofibrosarcoma protuberans [13, 29]. These tumors have similar morphologic characteristics include spindle tumor cells and fibromyxoid stroma. The present case had two additional, rare but distinct, morphologic features: (1) focal metaplastic bone, and (2) foci of whorls and cord-like structures of atypical epitheloid cells. The present of bony tissue in the tumor raises the possibility of ossifying fibromyxoid tumor (OFMT), as some OFMTs can be at least focally positive for MUC4 expression. Immunostain with S100, EAAT4, INI1, MUC4 and FISH or PCR for FUS-CREB3L2 or EWSR1-CREB3L1 fusion can make the distinction, as OFMT is immunohistochemically positive for S100 AND EAATA, but negative for INI1, MUC4. It does not harbor t (7;16)(q34;p11) or t (11;16)(p11;p11) either [30].
The epithelioid foci in the present case can easily cause confusion with extraskeletal myxoid chondrosarcoma (EMCS), especially when bony tissue is present. EMCS is a biphasic neoplasm with cartilaginous foci interspersed with spindle mesenchymal cells. The tumor cells are immunostain positive for INSM1, SOX9, CD99 and S100, but negative for MUC4. They carry rearrangement of the NR4A3 but lack translocations of t (7;16)(q34;p11) or t (11;16)(p11;p11) [31, 32]. SEF, a variant of LGFMS with worse prognosis should also be taken into consideration for differential. SEF usually has large areas of hyalinized fibrous stroma and stains positive for EMA and S100 [14, 24, 33]. Synovial sarcoma (SS) can have epitheloid tumor cells as well. The tumor cells in SS are more uniform and retain t(x;18) translocation [34].
Another pitfall in the present case is gastrointestinal stromal tumours (GISTs) for three reasons. First, both of these two entities can occur in abdomen. Second, they display similar, and sometimes identical, histologic features or morphologic spectrum. Lastly, they can both show expression of DOG1 (discovered on GIST1). These similarities in location, morphology and immunophenotype can cause considerable diagnostic confusion among pathologists. Although DOG1 is a sensitive and specific marker for GIST, its expression is reported in up to 94.7% of LGFMS. More specifically, nearly 40% cases of abdomen or retroperitoneum LGFMS have variable staining positivity of DOG1 [35–37]. It should be emphasized that the differential diagnosis between these two entities relies largely on appropriate molecular profiling: LGFMS expresses MUC4 protein and harbors characteristic gene fusions. In contrast, GISTs show expression of CD117 and CD34 and are associated with Kit or PDGFRA mutations [38]. A combination of cytogenetic analysis of gene fusion and immunohistochemical staining of MUC4, CD117 and CD34 will aid in correct diagnosis.
To the best of our knowledge, our present case is the first of its kind given foci of epitheloid core and whorls and diffusely expressing DOG1. Our findings highlight the need for additional immunohistochemical and molecular studies when faced with such tumors. They also underline the importance of including LGFMS in the differential diagnosis of myxoid spindle and epithelioid DOG1+ tumors in the GI tract.
Acknowledgements
Not applicable.
Conflict of interest
The authors disclose no conflicts.
Funding sources
No funding or support is associated with this study.
Abbreviations
- DOG1
Discovered on GIST-1
- EMCS
Extraskeletal myxoid chondrosarcoma
- FNA
Fine needle aspiration
- GIST
Gastrointestinal stromal tumours
- LGFMS
Low-grade fibromyxoid sarcoma
- OFMT
Ossifying fibromyxoid tumor
- SEF
Sclerosing epithelioid fibrosarcoma
Authors contribution
Dr. Jialing Huang put all data together and wrote the manuscript. Dr. Steven Cohen performed the surgery and provided the intraoperative findings. Dr. George Jour did the pathologic examination and gave the pathologic interpretation.
Availability of data and materials
The data and materials are available upon request.
Ethics approval and consent to participate
This publication is approved by the IRB office of NYU Langone Medical Center.
Consent for publication
Consent from the patient is obtained.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Evans HL. Low-grade fibromyxoid sarcoma. A report of two metastasizing neoplasms having a deceptively benign appearance. Am J Clin Pathol. 1987;88(5):615–619. doi: 10.1093/ajcp/88.5.615. [DOI] [PubMed] [Google Scholar]
- 2.Mertens F, Fletcher CD, Antonescu CR, et al. Clinicopathologic and molecular genetic characterization of low-grade fibromyxoid sarcoma, and cloning of a novel FUS/CREB3L1 fusion gene. Lab Investig. 2005;85(3):408–415. doi: 10.1038/labinvest.3700230. [DOI] [PubMed] [Google Scholar]
- 3.Geramizadeh B, Zare Z, Dehghanian AR, Bolandparvaz S, Marzban M. Huge mesenteric low-grade fibromyxoid sarcoma: a case report and review of the literature. Rare Tumors. 2018;10:2036361318777031. doi: 10.1177/2036361318777031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendoza AS, O'Leary MP, Peng SK, Petrie BA, Li AI, French SW. Low-grade fibromyxoid sarcoma of the sigmoid colon. Exp Mol Pathol. 2015;98(2):300–303. doi: 10.1016/j.yexmp.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Kim M, Song TJ, Kang SD, et al. A case of low-grade fibromyxoid sarcoma of the colon. Korean J Gastroenterol. 2014;64(6):375–379. doi: 10.4166/kjg.2014.64.6.375. [DOI] [PubMed] [Google Scholar]
- 6.Kim KJ, Seo JW. Intra-abdominal low-grade fibromyxoid sarcoma of the transverse mesocolon mimicking lymphoma. Jpn J Radiol. 2014;32(6):360–364. doi: 10.1007/s11604-014-0305-1. [DOI] [PubMed] [Google Scholar]
- 7.Park IJ, Kim HC, Yu CS, Kim JS, Jang SJ, Kim JC. Low-grade fibromyxoid sarcoma of the colon. Dig Liver Dis. 2007;39(3):274–277. doi: 10.1016/j.dld.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Kosemehmetoglu K, Ozogul E, Babaoglu B, Tezel GG, Gedikoglu G. Programmed death ligand 1 (PD-L1) expression in malignant Mesenchymal tumors. Turk Patoloji Derg. 2017;1(1):192–197. doi: 10.5146/tjpath.2017.01395. [DOI] [PubMed] [Google Scholar]
- 9.Guducu N, Coban I, Bassullu N, Gonenc G, Aydinli K. Low-grade fibromyxoid sarcoma of the vagina: a tumor, not previously reported at this site. Turk J Obstet Gynecol. 2014;11(3):196–197. doi: 10.4274/tjod.73626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanSandt AM, Bronson J, Leclair C, Mansoor A, Goetsch M. Low-grade fibromyxoid sarcoma of the vulva: a case report. J Low Genit Tract Dis. 2013;17(1):79–81. doi: 10.1097/LGT.0b013e318256da58. [DOI] [PubMed] [Google Scholar]
- 11.Barnhill D, Ismailjee M, Goss N, Ruiz B, Young A. Low-grade fibromyxoid sarcoma of the vulva. J La State Med Soc. 2012;164(2):95–96. [PubMed] [Google Scholar]
- 12.Fras AP, Frkovic-Grazio S. Hyalinizing spindle cell tumor with giant rosettes of the broad ligament. Gynecol Oncol. 2001;83(2):405–408. doi: 10.1006/gyno.2001.6368. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60–67. doi: 10.1016/j.anndiagpath.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Doyle LA, Wang WL, Dal Cin P, et al. MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am J Surg Pathol. 2012;36(10):1444–1451. doi: 10.1097/PAS.0b013e3182562bf8. [DOI] [PubMed] [Google Scholar]
- 15.Hisaoka M, Matsuyama A, Aoki T, Sakamoto A, Yokoyama K. Low-grade fibromyxoid sarcoma with prominent giant rosettes and heterotopic ossification. Pathol Res Pract. 2012;208(9):557–560. doi: 10.1016/j.prp.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Merchant SH. Low grade fibromyxoid sarcoma: report of a case with epithelioid cell morphology, masquerading as a papillary thyroid carcinoma. Acta Cytol. 2009;53(6):689–692. doi: 10.1159/000325411. [DOI] [PubMed] [Google Scholar]
- 17.Folpe AL, Lane KL, Paull G, Weiss SW. Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol. 2000;24(10):1353–1360. doi: 10.1097/00000478-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Meis-Kindblom JM, Kindblom LG, Enzinger FM. Sclerosing epithelioid fibrosarcoma. A variant of fibrosarcoma simulating carcinoma. Am J Surg Pathol. 1995;19(9):979–993. doi: 10.1097/00000478-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Doyle LA, Moller E, Dal Cin P, Fletcher CD, Mertens F, Hornick JL. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am J Surg Pathol. 2011;35(5):733–741. doi: 10.1097/PAS.0b013e318210c268. [DOI] [PubMed] [Google Scholar]
- 20.Panagopoulos I, Storlazzi CT, Fletcher CD, et al. The chimeric FUS/CREB3l2 gene is specific for low-grade fibromyxoid sarcoma. Genes Chromosomes Cancer. 2004;40(3):218–228. doi: 10.1002/gcc.20037. [DOI] [PubMed] [Google Scholar]
- 21.Matsuyama A, Hisaoka M, Shimajiri S, et al. Molecular detection of FUS-CREB3L2 fusion transcripts in low-grade fibromyxoid sarcoma using formalin-fixed, paraffin-embedded tissue specimens. Am J Surg Pathol. 2006;30(9):1077–1084. doi: 10.1097/01.pas.0000209830.24230.1f. [DOI] [PubMed] [Google Scholar]
- 22.Panagopoulos I, Moller E, Dahlen A, et al. Characterization of the native CREB3L2 transcription factor and the FUS/CREB3L2 chimera. Genes Chromosomes Cancer. 2007;46(2):181–191. doi: 10.1002/gcc.20395. [DOI] [PubMed] [Google Scholar]
- 23.Lau PP, Lui PC, Lau GT, Yau DT, Cheung ET, Chan JK. EWSR1-CREB3L1 gene fusion: a novel alternative molecular aberration of low-grade fibromyxoid sarcoma. Am J Surg Pathol. 2013;37(5):734–738. doi: 10.1097/PAS.0b013e31827560f8. [DOI] [PubMed] [Google Scholar]
- 24.Arbajian E, Puls F, Magnusson L, et al. Recurrent EWSR1-CREB3L1 gene fusions in sclerosing epithelioid fibrosarcoma. Am J Surg Pathol. 2014;38(6):801–808. doi: 10.1097/PAS.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 25.Linos K, Bridge JA, Edgar MA. MUC 4-negative FUS-CREB3L2 rearranged low-grade fibromyxoid sarcoma. Histopathology. 2014;65(5):722–724. doi: 10.1111/his.12422. [DOI] [PubMed] [Google Scholar]
- 26.Alatise OI, Oke OA, Olaofe OO, Omoniyi-Esan GO, Adesunkanmi AR. A huge low-grade fibromyxoid sarcoma of small bowel mesentery simulating hyper immune splenomegaly syndrome: a case report and review of literature. Afr Health Sci. 2013;13(3):736–740. doi: 10.4314/ahs.v13i3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans HL. Low-grade fibromyxoid sarcoma. A report of 12 cases. Am J Surg Pathol. 1993;17(6):595–600. doi: 10.1097/00000478-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20(12):1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 29.Alfaro-Cervello C, Benavent Casanova O, Nieto G, Mares Diago FJ, Navarro S. Low-grade fibromyxoid sarcoma, an essential differential diagnosis in myxoid tumors with benign appearance. Rev Esp Patol. 2018;51(3):178–182. doi: 10.1016/j.patol.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Schneider N, Fisher C, Thway K. Ossifying fibromyxoid tumor: morphology, genetics, and differential diagnosis. Ann Diagn Pathol. 2016;20:52–58. doi: 10.1016/j.anndiagpath.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida A, Makise N, Wakai S, Kawai A, Hiraoka N. INSM1 expression and its diagnostic significance in extraskeletal myxoid chondrosarcoma. Mod Pathol. 2018;31(5):744–752. doi: 10.1038/modpathol.2017.189. [DOI] [PubMed] [Google Scholar]
- 32.Broehm CJ, Wu J, Gullapalli RR, Bocklage T. Extraskeletal myxoid chondrosarcoma with a t (9;16)(q22;p11.2) resulting in a NR4A3-FUS fusion. Cancer Genet. 2014;207(6):276–280. doi: 10.1016/j.cancergen.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Patterson JW, Tchernev G, Chokoeva AA, Wick MR. Sclerosing epithelioid fibrosarcoma. Wien Med Wochenschr. 2017;167(5–6):120–123. doi: 10.1007/s10354-016-0509-3. [DOI] [PubMed] [Google Scholar]
- 34.Turc-Carel C, Dal Cin P, Limon J, et al. Involvement of chromosome X in primary cytogenetic change in human neoplasia: nonrandom translocation in synovial sarcoma. Proc Natl Acad Sci U S A. 1987;84(7):1981–1985. doi: 10.1073/pnas.84.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ud Din N, Ahmad Z, Zreik R, Horvai A, Folpe AL, Fritchie K. Abdominopelvic and retroperitoneal low-grade Fibromyxoid sarcoma: a Clinicopathologic study of 13 cases. Am J Clin Pathol. 2018;149(2):128–134. doi: 10.1093/ajcp/aqx137. [DOI] [PubMed] [Google Scholar]
- 36.Vallejo-Benitez A, Rodriguez-Zarco E, Carrasco SP, et al. Expression of dog1 in low-grade fibromyxoid sarcoma: a study of 19 cases and review of the literature. Ann Diagn Pathol. 2017;30:8–11. doi: 10.1016/j.anndiagpath.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Thway K, Ng W, Benson C, Chapman J, Fisher C. DOG1 expression in low-grade Fibromyxoid sarcoma: a study of 11 cases, with molecular characterization. Int J Surg Pathol. 2015;23(6):454–460. doi: 10.1177/1066896915593801. [DOI] [PubMed] [Google Scholar]
- 38.Mei L, Smith SC, Faber AC, et al. Gastrointestinal stromal tumors: the GIST of precision medicine. Trends Cancer. 2018;4(1):74–91. doi: 10.1016/j.trecan.2017.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials are available upon request.