Abstract
Heart failure (HF), a common sequela of cardiovascular diseases, remains a staggering clinical problem, associated with high rates of morbidity and mortality worldwide. Advances in pharmacological, interventional and operative management have improved patient care, but these interventions are insufficient to halt the progression of HF, particularly the end-stage irreversible loss of functional cardiomyocytes. Innovative therapies that could prevent HF progression and improve the function of the failing heart are urgently needed. Following successful preclinical studies, two main strategies have emerged as potential solutions: cardiac gene therapy and cardiac regeneration through stem and precursor cell transplantation. Many potential gene- and cell-based therapies have entered into clinical studies, intending to ameliorate cardiac dysfunction in patients with advanced HF. In this review we focus on the recent advances in cell- and gene-based therapies in the context of cardiovascular disease, emphasizing the most advanced therapies. The principles and mechanisms of action of gene and cell therapies for HF are discussed along with the limitations of current approaches. Finally, we highlight the emerging technologies that hold promise to revolutionize the biological therapies for cardiovascular diseases.
Keywords: Cardiac Regeneration, Cell Therapy, Gene Therapy, Regenerative Medicine, Heart Failure
Introduction
Cardiovascular diseases are debilitating and often deadly, responsible for more deaths worldwide than any other disease.1 Specifically, heart failure (HF) – the common sequela of many cardiovascular diseases – affects over 38 million people, 50% of which are estimated to die within 5 years of diagnosis.2 Given the limited intrinsic regenerative potential of the adult heart3, the endogenous sources of regeneration and repair are insufficient to halt the progression of HF. In end-stage cases, the last available option for treatment is cardiac transplantation, which is limited by the shortage of organs available. Pharmacological and device-based treatments that are currently implemented into HF treatment guidelines have improved patient survival.4,5 Although these therapies are beneficial, they are limited in relieving symptoms and do not address the molecular mechanisms underlying the pathogenesis of HF and hence cannot reverse or slow the adverse remodeling of the heart. This limitation in available remedies has raised the need for therapies that could repair or even regenerate the injured myocardium. Biological therapies, such as gene- and cell-based approaches, have emerged as alternative therapies to treat both acute cardiac events such as myocardial infarction (MI), and chronic cardiovascular diseases, representing a new generation in biological therapeutics for HF.
In this review we discuss the progress in the gene therapy field and the use of stem and precursor cells for stimulating endogenous regeneration and/or as a source for cardiomyocyte renewal, emphasizing the recent human clinical trials. In addition, we highlight the biological processes that underpin the reported therapeutic benefits, and discuss the shortcomings, challenges and future perspectives of gene- and cell-based therapies for HF. Lastly, we examine the most exciting advances in the field, which hold promise as alternative approaches to cardiac regeneration and repair.
Progress and Challenges in Stem Cell Cardiac Regenerative Therapy
Over the past two decades, substantial progress has been made in the field of stem cell therapy for cardiac repair. Despite the absence of an understanding of the mechanism through which donor cells improve cardiac function in patients, research has proceeded rapidly from preclinical models to clinical studies. To date a plethora of relatively small clinical trials have tested the potential benefit of various cell types in patients with HF (Table 1).
Table 1.
Cell Therapy Clinical Trials
| Cell Type | Phase | Name | Patient Number | Condition | Treatment Outcomes | Clinical Trial Identifier | References |
|---|---|---|---|---|---|---|---|
| BMMNC | I | BOOST | 60 | 5–7 days post-MI | improvement of LV systolic function after 6 months; no significant benefit after 18 months | NCT00224536 | 13 |
| II | Leuven-AMI | 67 | 1 day post-MI | no significant effect on recovery of global LV function | NCT00264316 | 12 | |
| II | ASTAMI | 50 | 5–7 days post-MI | no improvement in global LVEF after 6 months | NCT00199823 | 11 | |
| III | REPAIR-AMI | 204 | 3–6 days post-MI | significant increase in LV EF, reduced adverse events after 1 year | NCT00279175 | 9 | |
| II/III | FINCELL | 80 | 2–6 days post-MI | Improvement of global LVEF after 6 months | NCT00363324 | 10 | |
| II | REGENERATE-AMI | 100 | 1 day post-MI | Not significant improvement in LVEF after 1 year | NCT00765453 | 17 | |
| II | TIME | 120 | 3 or 7 days post-MI | No significant effect on recovery of LV function | NCT00684021 | 14 | |
| II | Late-TIME | 87 | 14–21 days post-MI | No significant improvement in LV function after 6 months | NCT00684060 | 15 | |
| II | FOCUS-CCTRN | 153 | CAD | No significant improvement in LV volume, oxygen consumption or defect | NCT00824005 | 16 | |
| III | BAMI* | 3000 | AMI | NCT01569178 | |||
| II | TOPCARE-CHD | 75 | MI | Significant increase in LVEF | NCT00289822 | 8 | |
| CD90+ MSC and CD45+ CD14+ Macrophages (Ixmyelocel-T) | IIa | IMPACT-DCM | 39 | DCM | Improves symptoms in patients with ischemic DCM | NCT00765518 | 23 |
| IIa | CATHETER-DCM | 22 | DCM | Improved symptoms in patients with ischemic DCM | NCT01020968 | 23 | |
| II | IxCELL-DCM* | 109 | DCM | reduced cardiac events with treatment | NCT01670981 | 24 | |
| Bone Marrow-derived Mesenchymal Cardiopoietic Cells | II/III | C-CURE | 47 | HF | Improved LVEF and quality of life | NCT00810238 | 18 |
| III | CHART-1* | 240 | HF | NCT01768702 | 32 | ||
| III | CHART-2* | 240 | HF | NCT02317458 | |||
| Allogeneic MPC Stro-1/Stro-3+ | II | 60 | HF | High-dose significantly reduced adverse cardiac events | NCT00721045 | 27 | |
| III | DREAM-HF* | 600 | HF | NCT02032004 | |||
| Autologous and allogeneic MSC | I/II | POSEIDON | 30 | HF | Improved functional capacity, quality of life, ventricular remodeling | NCT01087996 | 26 |
| CSC | I | SCIPIO | 33 | HF | Increased LVEF and decreased infarct size | NCT00474461 | 36, 37 |
| CDC | I | CADUCEUS | 31 | HF | No significant improvement on LVEF or scar reduction | NCT00893360 | 41, 42 |
| I/II | ALLSTAR* | 134 | MI | NCT01458405 | |||
| I | DYNAMIC* | 42 | DCM | NCT02293603 | |||
| CSC + bFGF | I | ALCADIA | 7 | HF | Safe and effective in patients with ischemic cardiomyopathy | NCT00981006 | 44 |
| CSC + MSC | II | CONCERT-HF* | 144 | HF | NCT02501811 | ||
| ESCs CD15+ Isl-1+ | I | ESCORT* | 6 | HF | No major complications after 3 months | NCT02057900 | 47 |
= ongoing
ADRC = adipose-derived regenerative cell; AMI = acute myocardial infarction; bFGF = basic fibroblast growth factor; BMC = bone marrow-derived cell; BMMNC = bone marrow mononuclear cell; CAD = coronary artery disease; CDC = cardiosphere-derived cell; CHD = coronary heart disease; CPC = cardiac progenitor cell; CSC = cardiac stem cell; DCM = dilated cardiomyopathy; EF = ejection fraction; HF = heart failure; LV = left ventricle/ventricular; MI = myocardial infarction; MPC = mesenchymal precursor cell; MSC = mesenchymal stem cell; NICM = non-ischemic cardiomyopathy
Bone Marrow-Derived Cells
Bone marrow consists of hematopoietic stem cells (HSCs) and non-hematopoietic multipotent cells, such as mesenchymal stem cells (MSCs), which can be induced to differentiate into the adipocytic, chondrocytic, or osteocytic lineages.6 Bone marrow mononuclear cells (BMMNCs) can be readily obtained from patients by bone marrow aspiration and density gradient centrifugation without the need for culture in vitro prior to administration.
Since its inception over 15 years ago,7 the use of autologous BMMNCs as cell therapy for HF has been investigated in multiple randomized and non-randomized trials, yielding conflicting and controversial clinical outcomes. Early clinical trials, such as TOPCARE-CHD,8 REPAIR-AMI9 and FINCELL,10 reported improved systolic function in treated acute myocardial infarction (AMI) patients, while others reported either no significant improvements (ASTAMI, Leuven-AMI)11,12 or absence of any long-term benefits (BOOST)13. More recent trials with larger cohorts that were adequately controlled (FOCUS-CCTRN, TIME, Late TIME, REGENERATE-AMI)14–17 found modest or no effect of BMC therapy on ventricular function and pre-specified endpoints. Overall, all trials failed to show any improvements in clinical outcomes in the treated patients.
In an attempt to improve the therapeutic potential of autologous bone marrow cells multiple trials have assessed the safety and efficacy of selected and ex vivo expanded subpopulations, such as MSCs. The C-CURE18 trial was one of the first studies that implemented the concept of delivering cardiac lineage primed bone marrow–derived MSCs (termed ‘cardiopoietic stem cells’) prior to myocardial implantation in patients with ischemic cardiomyopathy. This cardiopoietic cell population was derived by exposure of MSC (CD105, CD166, CD29, and CD44 and negative for CD14, CD34, and CD45) to a growth factor cocktail, including transforming growth factor-β, bone morphogenetic protein, activin A, fibroblast growth factor 2, cardiotrophin, and α-thrombin, which triggers hallmark traits of cardiac development.19 Despite inconsistencies in the reported data20 the study suggested that patients who received cells showed evidence of improved function versus the control arm six months after treatment, suggesting that treatment with cytokine-primed MSCs is safe and feasible with signs of benefit in chronic ischemic HF. Another autologous bone marrow-derived subpopulation, termed ixmyelocel-T, has been tested in clinical trials in HF patients. Ixmyelocel-T is an expanded population of mesenchymal stromal cells and M2-like macrophages, as well as many of the CD45+ cells found in the bone marrow. Although the precise mechanism of action is unknown, it is hypothesized that this expanded multi-cellular product induces tissue remodeling, immunomodulation, angiogenesis, and endothelial protection.21,22 The early phase, open label clinical trials (IMPACT-DCM and CATHETER-DCM) suggested that intramyocardial delivery of ixmyelocel-T might improve clinical, functional, symptomatic, and quality-of-life outcomes in patients with HF due to ischemic dilated cardiomyopathy (DCM).23 More recently, the phase IIB randomized, double blind ixCELL-DCM24 study showed that this multicellular therapy resulted in a significant reduction in adjudicated clinical cardiac events compared with placebo leading to improved patient outcomes, corroborating the findings of early trials.
The widespread use of BMMNCs can be attributed to immediate availability from the patient. Nonetheless, allogeneic cells could provide an even more readily available “off-the-shelf” therapeutic agent, avoiding the need for bone marrow aspiration and tissue culture delays before treatment. As such, allogeneic BM-derived MSCs have recently emerged as the leading candidate for an “off-the-shelf” product for HF cell-based therapy.25 MSCs are considered immune-privileged and can be expanded in quantities unattainable from an autologous source, undergo cryopreservation, and be available for delivery. The early-stage study (POSEIDON)26 was the first to demonstrate that alloimmune reactions in patients receiving allogeneic MSCs for ischemic LV dysfunction were low, suggesting that allogeneic MSC transplantation might be accomplished without the need for significant host immunosuppression. The trial reported similar safety profiles between the autologous and allogeneic MSCs. Although it was not powered to show efficacy, the MSC treatment favorably affected ventricular remodeling of patients with ischemic cardiomyopathy. Similarly, immunoselected bone marrow–derived mesenchymal precursor cells (MPCs), an enriched Stro-1/Stro-3+ population, were evaluated in a phase II, multicenter, dose escalation study to determine feasibility and safety of three doses in patients with chronic HF. This study concluded that the high-dose allogeneic MPC treatment may reduce adverse cardiovascular events and provide beneficial effects on adverse left ventricular remodeling.27 Taken together these studies suggest that allogeneic immune-selected MSCs are safe and potentially beneficial in treating patients with ischemic cardiomyopathy, offering an off-the-shelf readily available cell product. This beneficial effects have been attributed to multiple mechanisms have been proposed, including transdifferentiation, paracrine factor secretion with antiapoptotic, proangiogenic, and possibly immunomodulatory effects. However, to date the precise mechanisms involved in the positive impact of MSCs remain to be identified.
Despite rapid clinical translation and widespread enthusiasm, the therapeutic benefits of bone marrow-derived cell (BMC) therapy in patients with heart disease remains controversial. Differences in cell types, cell preparation standards, delivery techniques, imaging methods, and patient profiles can lead to incorrect inferences, and the effect of the therapies are difficult to interpret. Systematic review and meta-analysis of data from eligible randomized controlled trials could be informative, but have also yielded conflicting results, highlighting the lack of consistent efficacy in cell-based cardiac regeneration therapies.28–30 Unexplained discrepancies in design, methods, or results of many of the early phase clinical trials have also raised concerns over the validity of the reported benefits of bone marrow stem cell therapy.31 It is apparent that only well-designed and adequately powered trials will establish whether BMC therapy offers a new hope to patients with HF. A series of studies have been designed as phase III confirmative randomized controlled clinical trials, including the BAMI trial (NCT01569178; http://www.bami-fp7.eu) a mortality trial enrolling 3000 patients post-AMI throughout the European Union; the CHART-1 trial,32 which successfully enrolled 240 high-risk patients with advanced congestive HF; and the DREAM-HF study (NCT02032004), with a target enrollment of more than 600 high-risk patients with congestive HF have been designed as phase III confirmative trials. These studies are the most scientifically rigorous human experiments to date in the field of cardiac cell therapy. It is anticipated that the results of these clinical trials will be crucial in establishing whether BMC therapy represents an effective strategy for HF treatment.
Endogenous Cardiac Stem Cells
Recent findings have refuted the long-held belief that the adult mammalian heart is a terminally differentiated organ. There is, in fact, a constant cardiomyocyte turnover within human hearts throughout life, albeit at a very low rate.3 Although the mechanisms of endogenous heart regeneration remain highly debatable, the discovery of putative resident cardiac stem cells (CSCs), such as c-kit+ cells,33 provided the rationale that these cells could be isolated and harnessed to regenerate the failing heart.34 Despite discrepant results, a plethora of preclinical studies demonstrate beneficial effects of c-kit+ cell administration to ischemically damaged hearts despite the observed paucity of cardiomyogenic differentiation of these cells. The phenotype of postnatal c-kit+ cardiac cells resembles traditional MSCs, suggesting their major mechanism of action involves paracrine actions.35
The SCIPIO trial was the first human, randomized, open-label trial of autologous c-kit+ CSCs in patients with ischemic HF undergoing coronary artery bypass grafting.36 The initial results of the study showed a striking improvement of LV function and decreased infarct size at four months and one year after intracoronary infusion.36,37 However, concerns regarding the integrity of the published data have been raised, casting doubts over the validity of the study.38 Another potential source for cardiac-derived stem cell therapy is CDCs, a heterogeneous mixture of many different cell types derived by ex vivo culture of right ventricular endomyocardial biopsies.39 The enhanced potency of cardiospheres for myocardial repair has been attributed to their growth properties that mimic stem cell niche properties with enhanced “stemness” and expression of ECM and adhesion molecules.40 The CADUCEUS trial was a proof-of-concept study that evaluated the safety and efficacy of autologous CDCs in patients with a recent MI. The results showed no significant difference in heart function, end-systolic or end-diastolic volumes with the treatment, but analysis of exploratory efficacy endpoints revealed an increase in viable myocardium after 6 and 12 months, suggestive of therapeutic cardiac regeneration.41,42 In addition, the ALCADIA43,44 study tested a novel approach of combining CDCs with a hydrogel-based delivery method of basic fibroblast growth factor (bFGF) in patients with advanced HF. The interim results of the study demonstrated that the combination of CDCs and bFGF is safe, but given the small size of the study and the absence of a control group, no conclusion be drawn regarding the safety and the efficacy of this approach.
The aforementioned proof-of-concept studies have the potential to revolutionize the treatment of HF. However, the small number of enrolled patients, the short period of follow up and the preliminary nature of the findings preclude any safe conclusions. In addition, although the c-kit+ CSCs and CDCs are cardiac-derived cells that have been named “cardiac progenitor cells’ there is no compelling evidence that they can differentiate into myocardial cells, and therefore the mechanisms involved in the beneficial actions observed remain unknown. Despite these uncertainties, prospective, randomized, placebo-controlled clinical trials are currently investigating the efficacy of cardiac-derived cell therapies in HF. For example, the DYNAMIC (NCT02293603) and ALLSTAR (NCT01458405) trial are currently evaluating the safety and efficacy of allogeneic CDCs in patients with DCM and MI, respectively. Similarly, a hybrid cell therapy composed of autologous c-kit+ CSCs and bone marrow derived MSCs is currently being tested in the CONCERT-HF, a phase II trial (NCT02501811) in patients with ischemic cardiomyopathy. The results of these promising studies are eagerly awaited.
Embryonic Stem Cells
Pluripotent stem cells (PSCs) – either embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) – have emerged as a renewable cell source for heart regenerative applications.45 Human PSCs are attractive because they can be differentiated with great efficiency into cardiomyocytes (CMs),46 providing an unlimited supply of cardiomyocyte-like cells in vitro, prior to transplantation. After a long period of preclinical and translational work, the first human trial, ESCORT,47 was initiated in 2013 with the first patient receiving purified ESC-derived cardiac progenitors (CD15+ Isl-1+) in an epicardial fibrin gel patch. Although the preliminary results suggested an improvement in the kinetics of the non-bypassed cell-patched area at three months and six months follow up, it would be meaningless to draw any conclusions regarding the efficacy of this treatment based on a single patient. This landmark study demonstrated the feasibility of producing clinical-grade ESC-derived cardiac progenitor cells and represents the first clinical application of this approach in the setting of HF, but additional studies are necessary to evaluate its safety and efficacy. Although the capacity of ESCs to differentiate toward the cardiac lineages is well established, numerous challenges remain for the clinical implementation of ESC-based therapies.48,49 For example, allogeneic ESCs face immunological challenges that might require life-long immunosuppression; theoretically autologous iPSC-derived cardiomyocytes circumvent this issue. PSC derivatives pose the inherent risk of forming teratomas. In addition, cell survival, retention, and engraftment are major obstacles. Even when cells successfully engraft and survive in the injured heart, PSC-derived CMs could potentially trigger malignant arrhythmias, as recently observed in preclinical studies in non-human primates.24 This phenomenon could be attributed to the presence of a heterogeneous population of phenotypically immature cells with spontaneous beating activity.50 Larger preclinical studies investigating cell dose, timing and delivery modalities using consistent and efficient methods are necessary to address the aforementioned concerns, and to conclusively demonstrate that the PSC-derived CMs and/or cardiomyocyte progenitor cells can improve cardiac function.
In summary, stem cell/progenitor cell therapies have been rapidly translated from bench to bedside, and numerous clinical trials have been spurred over the last 15 years. The initial enthusiasm generated by early-stage studies has now been met with skepticism, as the clinical outcomes of most BMC-based trials have yielded inconclusive results. Similarly, putative heart-derived stem cells, such as c-kit+ and CDCs, have been proposed as attractive candidates for heart regeneration, but their therapeutic value remains questionable. Notably, it is not clear whether the modest beneficial effects are cell-type specific and the mechanisms of cardioprotection have not been completely unraveled yet. With the exception of PSC-derived CM progenitor cells, BMCs, MSCs, CDCs, and CSCs do not represent bona fide stem cell populations and are unlikely to regenerate the myocardium. Originally hypothesized to differentiate into new CMs, the aforementioned cell types are now known to engraft poorly, with the majority persisting less than a week after transplantation. Regardless of the cell source, the current consensus is that the transplanted cells do not generate new tissue, and it has been postulated that their beneficial effect is exerted via paracrine mechanisms that stimulate the endogenous repair pathways through the release of various factors. Nevertheless, these paracrine mechanisms have yet to be elucidated, and studies to determine exact mechanisms of action in the diseased human heart are needed in order to develop more targeted and robust cell therapies. Despite these mechanistic uncertainties, it is important to acknowledge that the safety and feasibility of BMCs, MSCs, CDCs, and CSCs has been consistently established. Recent advances with PSCs hold promise for successful clinical translation, but it will take time to develop effective and safe protocols for the use of PSCs in heart failure. The completion of the ongoing Phase II/III studies will certainly contribute knowledge and most likely provide valuable information to the cardiac regenerative medicine conundrum.
Progress and Challenges in Gene Therapy For Cardiovascular Disease
Over the past decade, our understanding of the complex disease mechanisms underlying the pathogenesis of HF has significantly improved,51 and advances in molecular cardiology have identified key targets within the progression of HF. Gene therapy has emerged as a viable therapeutic strategy for specifically modulating underlying disease mechanisms, potentially replacing the symptomatic approach of existing treatments. Rectifying the disease at the gene level could mean a more permanent therapeutic benefit that could slow down or even reverse the detrimental course of HF. Extensive investigation into new treatment modalities has led to the development of gene-based therapeutic interventions, and in recent years there have been rapid advancements in gene therapy for HF (Table 2).
Table 2.
Gene Therapy Clinical Trials
| Molecular Target | Delivery Mode | Phase | Name | Patient Number | Treatment Outcomes | Clinical Trial Identifier | References |
|---|---|---|---|---|---|---|---|
| VEGF | Adenovirus | II | KAT | 103 | Significant increase in myocardial perfusion | 53 | |
| Plasmid | I | VIVA | 178 | 52 | |||
| Adenovirus | I | KAT301 | 30 | Enhanced myocardial perfusion | NCT01002430 | 57 | |
| Plasmid | III | EUROINJECT-ONE | 80 | No difference in myocardial perfusion | 54 | ||
| Plasmid | II/III | NORTHERN | 93 | NCT00143585 | 55 | ||
| Adenovirus | III | REVASC | 17 | 56 | |||
| FGF4 | Adenovirus | II/III | AGENT-3 | 416 | No beneficial effect | NCT00346437 | 60 |
| II/III | AGENT-4 | 116 | NCT00185263 | ||||
| I | AGENT | 79 | Trend for improved myocardial perfusion | 58 | |||
| I | AGENT-2 | 62 | 59 | ||||
| AC6 | Adenovirus | I/II | AC6 Gene Transfer | 56 | Dose-related improvement of cardiac function | NCT00787059 | 72 |
| SERCA2a | AAV1 | I/II | CUPID | 51 | Decreased HF symptoms remodeling | NCT00454818 | 64–67 |
| II/III | CUPID-2b | 250 | No improvement in the clinical course of HF | NCT01643330 | 68 | ||
| II | AGENT-HF# | 10 | NCT01966887 | ||||
| II | SERCA-LVAD# | 5 | NCT00534703 | ||||
| SDF1 | Plasmid | I | ACRX-100 | 17 | Improvements 6-minute walk | NCT01082094 | 75 |
| II | STOP-HF | 90 | Improvements 6-minute walk | NCT01643590 | 76 | ||
| IIb | STOP-HF2* | 180 |
AAV = adeno-associated virus; AC6 = adenyl cyclase 6; CAD = coronary artery disease; CHF = congestive heart failure; CMV = cytomegalovirus; FGF = fibroblast growth factor; HGF = hepatocyte growth factor; HIF1α = hypoxia-inducible factor; LV = left ventricle/ventricular; MI = myocardial infarction; SDF1 = Stromal-derived factor 1; SERCA2a2 = Sarcoplasmic reticulum Ca2+ ATPase; VEGF = vascular endothelial growth factor.
Terminated.
Ongoing.
Gene therapy was proposed to be particularly valuable in the context of coronary artery disease (CAD), the most common type of cardiovascular disease. Preclinical studies have shown that a number of growth factors including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), and hypoxia-inducible factor (HIF) could promote angiogenesis and induce vascular permeability and cytoprotective effects. Most gene therapy clinical trials for CAD were focused on the administration of genes encoding angiogenic growth factors, such as VEGF and FGF4, aiming to promote the development of collateral blood vessels in ischemia-related conditions. Early trials, such as VIVA52 and KAT,53 suggested a functional improvement in myocardial perfusion and cardiac function in patients with CAD after the administration of either an expression plasmid or an adenoviral vector expressing VEGF-A165, respectively. However, subsequent, double blind, randomized, placebo-controlled studies (EUROINJECT-ONE, NORTHERN)54,55 failed to demonstrate any improvement in myocardial perfusion. Similarly, the phase II randomized, controlled REVASC56 trial that evaluated the efficacy of an adenoviral vector-mediated VEGF delivery (Ad. VEGF-A121) did not show any significant improvement in the primary endpoint of myocardial perfusion and alleviation of symptoms. Recently, another member of the VEGF family, VEGF-D, has also been clinically evaluated in patients with severe CAD. The preliminary data of the KAT301 trial,57 a randomized, placebo-controlled, single-blinded phase I/II study, suggest that adenoviral-mediated VEGF-D gene therapy is safe and could enhance myocardial perfusion. In an alternative approach, early phase I/II trials demonstrated that FGF4 treatment improved exercise capacity and reduced ischemic defect size in CAD patients (AGENT and AGENT-2).58,59 However, the larger phase III trials (AGENT-3 and AGENT-4)60 failed to corroborate these benefits. Consequently, both studies were terminated after an interim analysis of the AGENT-3 trial indicated that there were no significant differences regarding the primary endpoint in the between the treatment and placebo groups.
In advanced HF, cardiac calcium (Ca2+) cycling – the release and reuptake of intracellular Ca2+ that drives muscle contraction and relaxation – is profoundly altered, resulting in impaired contractility and fatal cardiac arrhythmias.61 Key components of the machinery that regulates Ca2+ cycling in the heart have emerged as prominent targets for human HF therapy.62,63 Notably, heart failure is associated with depressed sarcoplasmic reticulum (SR) calcium cycling, reflecting impaired SR Ca2+ -transport and Ca2+ -release, which has been attributed to reductions in gene and protein expression, or activity of the SR Ca2+ uptake pump (SERCA2a). SERCA2a plays a key role in transporting Ca2+ from the cytosol into the lumen of the SR following cardiac contraction, thus regulating cardiac contractility and relaxation. Targeting SERCA2a showed beneficial results in preclinical testing, leading to the first-in-human trial to enhance SR Ca2+ uptake in 2007.64,65 In this phase I/II trial (CUPID), a small number of patients with advanced HF received an intracoronary administration of an adeno-associated viral (AAV1) vector expressing SERCA2a (AAV1.SERCA2a) and later showed improvements in key clinical outcomes.66 Although individual patients did not show improvements across all parameters, improvements in pre-specified primary endpoint criteria were observed in the highest dose cohort67. The promising outcome of the initial trial led to a larger Phase IIB, double-blind, placebo-controlled study, CUPID2, and two smaller auxiliary studies, AGENT-HF and SERCA-LVAD trials. The recently completed CUPID2 study did not meet its primary or secondary endpoints, and overall failed to demonstrate any improvement of clinical outcomes in patients with advanced HF.68 Following the outcome of the CUPID2 trial, patient enrollment in both AGENT-HF and SERCA-LVAD studies was suspended. Importantly, no safety issues emerged - from the procedure of delivering the virus or long-term effects - in the participant patient population at the tested AAV1 therapeutic dose.
Adenylyl-cyclase type 6 (AC6) is an enzyme that serves as the effector molecule for β-adrenergic signaling, playing a key role in contractile responsiveness, cardiac relaxation, and LV diastolic function.69 In preclinical studies, adenoviral-mediated delivery of an AC6 transgene improved LVEF and increased survival rates in animal models of cardiomyopathy in part due to increased SERCA2a activity and improved Ca2+ handling in CMs70–71 A recent randomized, double-blinded, placebo-controlled clinical trial evaluated the safety, tolerability and clinical effectiveness of ascending doses of adenovirus-5 encoding human AC6 (Ad5.hAC6) in patients with stable but severe HF.72 Although the rates of serious adverse events were similar in both groups, the findings of this small clinical study suggests that intracoronary delivery ofAd5.hAC6 in patients with HF appears to be safe with a dose-related improvement of cardiac function at four and twelve weeks after randomization. The size of the study, however, was too small to draw any definitive conclusions regarding the efficacy and long-term benefit of this promising new gene therapy target in patients with advanced HF.
The stromal cell-derived factor-1 (SDF-1), and its receptor, chemokine receptor type 4 (CXCR4) has emerged as a key regulator in endogenous tissue repair. Preclinical studies indicate that a SDF-1 promotes tissue repair through the SDF-1:CXCR4 axis by promoting cell survival, endogenous stem cell recruitment, and vasculogenesis.73,74 The safety and potential efficacy of SDF-1 gene therapy was initially demonstrated in an open-label Phase I study in patients with ischemic cardiomyopathy.75 According to the results of the more recent STOP-HF trial,76 the transient overexpression of SDF-1 has the potential to improve cardiac function in patients with ischemic cardiomyopathy. Although the trial failed to meet its primary efficacy endpoint, a pre-specified sub-analysis demonstrated that the potential benefits were more pronounced in patients with advanced cardiac dysfunction for at least one year post-treatment. These promising findings have led to the design of a larger, prospectively designed clinical study (STOP-HF2) that is expected to enroll up to 180 HF patients.
In short, despite extensive preclinical evaluation and encouraging results from early clinical studies, to date none of the gene therapy approaches have provided compelling evidence of a significant clinical benefit in HF patients. Early studies that focused on neovascularization have shown limited efficacy and consequently the angiogenic gene-therapy approaches with the goal to improve cardiac vascularization have largely been abandoned. More recently, the first human clinical trial of viral vector–based gene transfer for advanced HF was initiated after pilot clinical trials targeting the Ca2+ cycling pathway in HF patients showed favorable clinical outcomes without safety issues. However, the milestone CUPID2 trial failed to meet its pre-specified endpoints, demonstrating that establishing clinical efficacy of novel therapeutic principles is a long and arduous path.
Conclusions and Future Directions
HF, a complex clinical syndrome, represents a major global health problem. Significant progress has been made over the past two decades in cell- and gene-based therapies for HF, promising the development of innovative therapeutic strategies for both treatment and prevention (Figure 1). There are, of course, substantial gaps in knowledge that pose obstacles to the realization of the full potential of such novel biological therapies for clinical benefit. There is still a tremendous amount of work to be done, especially in addressing the need for deeper insights into the underlying disease mechanisms (i.e., which cell types, which genes, and at what levels, which pathways are relevant to any given pathogenic process, and which patients to treat).
Figure 1. Biological therapeutics for the treatment of heart failure.
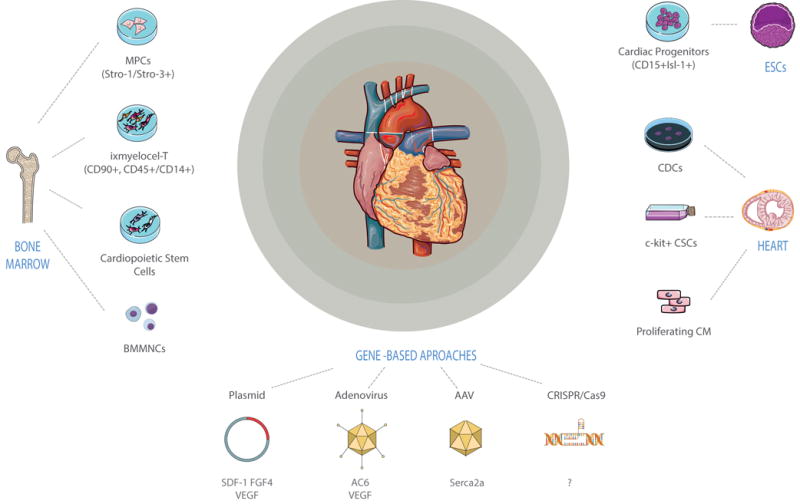
Sources of stem cells used for cardiac repair include a broad range of bone marrow–derived stem cells, resident cardiac stem cells and embryonic stem cell derived progenitors. Current gene therapy approaches utilize DNA plasmids, adenoviruses, adenovirus-associated viruses (AAVs); the application of new genome editing tools, such as the CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats (CRISPR) associated nuclease 9), holds great promise for gene therapy.
Perhaps one of the most promising developments in the field of the regenerative cardiology is the emerging notion of using pre-existing cardiomyocytes as the source for cardiomyocyte replacement to maintain normal myocardial homeostasis as well as after myocardial injury.77–80. The stimulation of proliferation of pre-existing cardiomyocytes could provide new avenues for future therapeutic strategies to regenerate the heart. However, further evidence and characterization for this putative pool of cycling cardiomyocytes as well as development of the means of therapeutic manipulation is a prerequisite to harness the endogenous regenerative properties of the adult heart.
Finally, genome-editing tools such as programmable engineered nucleases81 are becoming more accessible,82 and are being utilized to increase our understanding of disease mechanisms as well as to develop novel therapeutic approaches. Gene correction by genome editing has shown great promise for clinical translation, as highlighted by recent studies for the treatment of Duchenne Muscular Dystrophy in vivo83–85 and DCM in vitro.86 Nevertheless, these novel approaches will likely have to address the problem of delivery that has been a key issue in gene therapeutic strategies targeting the heart. Although the genome editing field is in its infancy, these studies represent an important step in the treatment of hereditary forms of cardiovascular diseases.
Acknowledgments
This study was funded by the NIH 4R00 HL104002-03, AHA 15BGIA22730027, and Stanford CVI Seed Grant (I.K.); Prince Mahidol Award Foundation, Thailand (V.T.); and the Sarnoff Cardiovascular Research Foundation (C.C.). The authors gratefully acknowledge Alessandra Briganti for preparing the illustration.
Footnotes
Author contribution:
Caressa Chen: Conception and design, manuscript writing, collection and/or assembly of data and final approval of manuscript.
Vittavat Termglinchan: Conception and design, manuscript writing, collection and/or assembly of data and final approval of manuscript.
Ioannis Karakikes: Conception and design, manuscript writing, collection and/or assembly of data, financial support and final approval of manuscript
References
- 1.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMurray JJ, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. 2013. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Hamano K, et al. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: clinical trial and preliminary results. Japanese circulation journal. 2001;65:845–847. doi: 10.1253/jcj.65.845. [DOI] [PubMed] [Google Scholar]
- 8.Assmus B, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. The New England journal of medicine. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 9.Schachinger V, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. The New England journal of medicine. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 10.Huikuri HV, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. European heart journal. 2008;29:2723–2732. doi: 10.1093/eurheartj/ehn436. [DOI] [PubMed] [Google Scholar]
- 11.Lunde K, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. The New England journal of medicine. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 12.Janssens S, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 13.Wollert KC, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 14.Traverse JH, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. Jama. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traverse JH, et al. LateTIME: a phase-II, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Texas Heart Institute journal. 2010;37:412–420. [PMC free article] [PubMed] [Google Scholar]
- 16.Perin EC, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. Jama. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudry F, et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trialdagger. European heart journal. 2016;37:256–263. doi: 10.1093/eurheartj/ehv493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartunek J, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. Journal of the American College of Cardiology. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 19.Behfar A, Terzic A. Derivation of a cardiopoietic population from human mesenchymal stem cells yields cardiac progeny. Nature clinical practice Cardiovascular medicine. 2006;3(Suppl 1):S78–82. doi: 10.1038/ncpcardio0429. [DOI] [PubMed] [Google Scholar]
- 20.Mielewczik M, et al. The C-CURE Randomized Clinical Trial (Cardiopoietic stem Cell therapy in heart failURE) Journal of the American College of Cardiology. 2013;62:2453. doi: 10.1016/j.jacc.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Ledford KJ, Murphy N, Zeigler F, Bartel RL, Tubo R. Therapeutic potential of ixmyelocel-T, an expanded autologous multicellular therapy for treatment of ischemic cardiovascular diseases. Stem cell research & therapy. 2015;6:25. doi: 10.1186/s13287-015-0007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ledford KJ, Zeigler F, Bartel RL. Ixmyelocel-T, an expanded multicellular therapy, contains a unique population of M2-like macrophages. Stem cell research & therapy. 2013;4:134. doi: 10.1186/scrt345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry TD, et al. Safety and efficacy of ixmyelocel-T: an expanded, autologous multi-cellular therapy, in dilated cardiomyopathy. Circulation research. 2014;115:730–737. doi: 10.1161/CIRCRESAHA.115.304554. [DOI] [PubMed] [Google Scholar]
- 24.Patel AN, et al. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet. 2016;387:2412–2421. doi: 10.1016/S0140-6736(16)30137-4. [DOI] [PubMed] [Google Scholar]
- 25.Karantalis V, Schulman IH, Balkan W, Hare JM. Allogeneic cell therapy: a new paradigm in therapeutics. Circulation research. 2015;116:12–15. doi: 10.1161/CIRCRESAHA.114.305495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hare JM, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. Jama. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perin EC, et al. A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circulation research. 2015;117:576–584. doi: 10.1161/CIRCRESAHA.115.306332. [DOI] [PubMed] [Google Scholar]
- 28.Fisher SA, Doree C, Mathur A, Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circulation research. 2015;116:1361–1377. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- 29.Afzal MR, et al. Adult Bone Marrow Cell Therapy for Ischemic Heart Disease: Evidence and Insights From Randomized Controlled Trials. Circulation research. 2015;117:558–575. doi: 10.1161/CIRCRESAHA.114.304792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gyongyosi M, Wojakowski W, Navarese EP, Moye LA, Investigators*, A. Meta-Analyses of Human Cell-Based Cardiac Regeneration Therapies: Controversies in Meta-Analyses Results on Cardiac Cell-Based Regenerative Studies. Circulation research. 2016;118:1254–1263. doi: 10.1161/CIRCRESAHA.115.307347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowbar AN, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. Bmj. 2014;348:g2688. doi: 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartunek J, et al. Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial design. European journal of heart failure. 2016;18:160–168. doi: 10.1002/ejhf.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 34.Koudstaal S, et al. Concise review: heart regeneration and the role of cardiac stem cells. Stem cells translational medicine. 2013;2:434–443. doi: 10.5966/sctm.2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keith MC, Bolli R. “String theory” of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circulation research. 2015;116:1216–1230. doi: 10.1161/CIRCRESAHA.116.305557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolli R, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Chugh AR, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Lancet E. Expression of concern: the SCIPIO trial. Lancet. 2014;383:1279. doi: 10.1016/S0140-6736(14)60608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith RR, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 40.Li TS, et al. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makkar RR, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malliaras K, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) Journal of the American College of Cardiology. 2014;63:110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takehara N, et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. Journal of the American College of Cardiology. 2008;52:1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 44.Matsubara H. First-in-man Cell Therapy Clinical Trial for Heart Failure -AutoLogous human Cardic-Derived Stem Cell to Treat Ischemic cardiomyopathy (ALCADIA) Journal of Cardiac Failure. 17:S130. doi: 10.1016/j.cardfail.2011.06.408. [DOI] [Google Scholar]
- 45.Freund C, Mummery CL. Prospects for pluripotent stem cell-derived cardiomyocytes in cardiac cell therapy and as disease models. Journal of cellular biochemistry. 2009;107:592–599. doi: 10.1002/jcb.22164. [DOI] [PubMed] [Google Scholar]
- 46.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell stem cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menasche P, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. European heart journal. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 48.Gerbin KA, Murry CE. The winding road to regenerating the human heart. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2015;24:133–140. doi: 10.1016/j.carpath.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simonson OE, Domogatskaya A, Volchkov P, Rodin S. The safety of human pluripotent stem cells in clinical treatment. Annals of medicine. 2015;47:370–380. doi: 10.3109/07853890.2015.1051579. [DOI] [PubMed] [Google Scholar]
- 50.Karakikes I, Ameen M, Termglinchan V, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circulation research. 2015;117:80–88. doi: 10.1161/CIRCRESAHA.117.305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braunwald E. Research advances in heart failure: a compendium. Circulation research. 2013;113:633–645. doi: 10.1161/CIRCRESAHA.113.302254. [DOI] [PubMed] [Google Scholar]
- 52.Henry TD, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 53.Hedman M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 54.Kastrup J, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. Journal of the American College of Cardiology. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 55.Stewart DJ, et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17:1109–1115. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart DJ, et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment. Gene therapy. 2006;13:1503–1511. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 57.Hassinen I, et al. Abstract 11987: Adenoviral Intramyocardial VEGF-D Gene Transfer Increases Myocardial Perfusion in Refractory Angina Patients. Circulation. 2015;132:A11987–A11987. [Google Scholar]
- 58.Grines CL, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 59.Grines CL, et al. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. Journal of the American College of Cardiology. 2003;42:1339–1347. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 60.Henry TD, et al. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. Journal of the American College of Cardiology. 2007;50:1038–1046. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 61.Lompre AM, et al. Ca2+ cycling and new therapeutic approaches for heart failure. Circulation. 2010;121:822–830. doi: 10.1161/CIRCULATIONAHA.109.890954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circulation research. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritterhoff J, Most P. Targeting S100A1 in heart failure. Gene therapy. 2012;19:613–621. doi: 10.1038/gt.2012.8. [DOI] [PubMed] [Google Scholar]
- 64.Hajjar RJ, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. Journal of cardiac failure. 2008;14:355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Jaski BE, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. Journal of cardiac failure. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zsebo K, et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circulation research. 2014;114:101–108. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]
- 67.Jessup M, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016 doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 69.Feldman AM. Adenylyl cyclase: a new target for heart failure therapeutics. Circulation. 2002;105:1876–1878. doi: 10.1161/01.cir.0000016965.24080.12. [DOI] [PubMed] [Google Scholar]
- 70.Tang T, Gao MH, Roth DM, Guo T, Hammond HK. Adenylyl cyclase type VI corrects cardiac sarcoplasmic reticulum calcium uptake defects in cardiomyopathy. American journal of physiology Heart and circulatory physiology. 2004;287:H1906–1912. doi: 10.1152/ajpheart.00356.2004. [DOI] [PubMed] [Google Scholar]
- 71.Rebolledo B, et al. Adenylylcyclase gene transfer increases function of the failing heart. Human gene therapy. 2006;17:1043–1048. doi: 10.1089/hum.2006.17.1043. [DOI] [PubMed] [Google Scholar]
- 72.Hammond H, Penny WF, Traverse JH, et al. Intracoronary gene transfer of adenylyl cyclase 6 in patients with heart failure: A randomized clinical trial. JAMA Cardiology. 2016;1:163–171. doi: 10.1001/jamacardio.2016.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Askari AT, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi J, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 75.Penn MS, et al. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circulation research. 2013;112:816–825. doi: 10.1161/CIRCRESAHA.111.300440. [DOI] [PubMed] [Google Scholar]
- 76.Chung ES, et al. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized Phase II trial. European heart journal. 2015;36:2228–2238. doi: 10.1093/eurheartj/ehv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ali SR, et al. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8850–8855. doi: 10.1073/pnas.1408233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Senyo SE, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kimura W, et al. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature. 2015;523:226–230. doi: 10.1038/nature14582. [DOI] [PubMed] [Google Scholar]
- 80.Nakada Y, et al. Hypoxia induces heart regeneration in adult mice. Nature. 2016 doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
- 81.Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nature reviews Genetics. 2014;15:321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 82.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 83.Nelson CE, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351:403–407. doi: 10.1126/science.aad5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tabebordbar M, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351:407–411. doi: 10.1126/science.aad5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Long C, et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351:400–403. doi: 10.1126/science.aad5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karakikes I, et al. Correction of human phospholamban R14del mutation associated with cardiomyopathy using targeted nucleases and combination therapy. Nature communications. 2015;6:6955. doi: 10.1038/ncomms7955. [DOI] [PMC free article] [PubMed] [Google Scholar]


