Abstract
Salisphere-derived adult epithelial cells have been used to improve saliva production of irradiated mouse salivary glands. Importantly, optimization of the cellular composition of salispheres could improve their regenerative capabilities. The Rho Kinase (ROCK) inhibitor, Y27632, has been used to increase the proliferation and reduce apoptosis of progenitor cells grown in vitro. In this study, we investigated whether Y27632 could be used to improve expansion of adult submandibular salivary epithelial progenitor cells or to affect their differentiation potential in different media contexts. Application of Y27632 in medium used previously to grow salispheres promoted expansion of Kit+ and Mist1+ cells, while in simple serum-containing medium Y27632 increased the number of cells that expressed the K5 basal progenitor marker. Salispheres derived from Mist1CreERT2; R26TdTomato mice grown in salisphere media with Y27632 included Mist1-derived cells. When these salispheres were incorporated into 3D organoids, inclusion of Y27632 in the salisphere stage increased the contribution of Mist1-derived cells expressing the proacinar/acinar marker, Aquaporin 5 (AQP5), in response to FGF2-dependent mesenchymal signals. Optimization of the cellular composition of salispheres and organoids can be used to improve the application of adult salivary progenitor cells in regenerative medicine strategies.
Keywords: Salivary gland, Salisphere, Organoid, Proacinar differentiation, Y27632
1. Introduction
The loss of salivary gland function, or salivary hypofunction, is a clinical condition that occurs in patients diagnosed with Sjögren’s Syndrome and in patients that have undergone radiation therapy for head and neck cancer. Currently there are more than 500,000 patients who suffer from this condition (Lombaert et al., 2017). Salivary hypofunction results in the sensation of “dry mouth” or xerostomia and leads to a decline in quality of life as saliva is essential for digestion, mineralization of teeth, lubrication of the oral cavity and immunity against microorganisms, fungi, and viruses. There are commercially available supplements to increase moisture in the oral cavity including synthetic saliva, stimulants such as pilocarpine, and moisturizers. Despite the availability of these substances, they only provide temporary relief and are not a cure. To provide permanent salivary gland functional restoration after xerostomia, progenitor cell therapies hold great promise (Lombaert et al, 2008; Nanduri et al, 2014). To develop future cell-based salivary gland regenerative therapies, increased knowledge of how to expand progenitor cells is needed, and defining the inputs that regulate their growth and differentiation is of primary importance.
Stem and progenitor cells elaborate organs during development and are of interest for application in regenerative therapies. In the salivary gland, multiple epithelial progenitor cell populations have been identified, and the relative contribution of each cell type during development and during injury repair remains a topic of great interest. During the later stages of branching morphogenesis, cells in the end buds that will become secretory acinar cells start to differentiate. mRNA for the water channel protein Aquaporin 5 (AQP5) begins to be expressed in the end buds by embryonic day 14 (E14), with AQP5 protein expression detectable the following day (E15) and membranous localization occurring by E16 (Nelson et al, 2013). In adults, acinar cells replicate through a process of self-duplication rather than through differentiation from stem or progenitor cells, which was shown through lineage tracing of the transcription factor, Mist1 (Aure et al, 2015). Mist1 is not unique to the salivary glands, as it is also expressed by acinar cells in the pancreas (Pin et al, 2001), but it is of interest in the salivary gland as a secretory acinar lineage marker. Multiple progenitor cell populations that replenish the ducts reside in the SMG, including cells which express the intermediate filaments, cytokeratin 5 (K5) and cytokeratin 14 (K14), which are basal progenitor markers, and K5 and K14 are expressed in basal duct cells and in myoepithelial cells in the adult salivary gland (Nelson et al, 2013;Yamammoto et al, 2016). The K5+/K14+ myoepithelial cell population is self-renewing (May et al, 2018). Lineage tracing in embryonic organ explants showed that K5+ cells can yield luminal ductal cells that express Prominin 1 (DeSantis et al, 2017). Kit is a receptor tyrosine kinase that marks progenitor cells in many organs but is not a stem cell marker in adult salivary glands (Kwak et al, 2018; Emmerson and Knox, 2018). Kit+ cells are found distally in end buds in developing glands, but Kit+ cells transition primarily to the intercalated ducts in adult glands (Nelson et al, 2013; Wang et al, 2014). Recent lineage tracing showed Kit+ cells contribute to intercalated ducts in adult gland, but when Kit-Cre was induced at postnatal day 2, Kit+ cells also contributed to acini and other duct cells (May et al, 2018). Kit+ cells remain of interest therapeutically as Kit+ cells isolated from salispheres partially restored saliva production after implantation into irradiated SMGs (Lombaert et al, 2008; Pringle et al, 2016), but through unknown mechanisms. There are multiple progenitor cell populations that show plasticity during development and in response to injury and ductal cells may participate in injury (Aure et al, 2019). However, there remains a lack of understanding of precisely how SMG progenitor cell populations contribute to organ formation and how these cell populations these could be manipulated with cell therapy strategies in hypofunctioning glands.
A potential method to enhance progenitor cell expansion in salisphere cultures is through manipulation of the Rho-associated protein kinase (ROCK) pathway. ROCK is a member of the serine-threonine kinase family that is activated by the small GTPase, RhoA (Amano et al., 2010), and has many functions in the cell, including promoting apoptosis (Coleman et al, 2001). Due to these properties, ROCK inhibition has been used to stimulate proliferation and prevent apoptosis of stem/progenitor cells during expansion in culture. ROCK inhibition by Y27632 has been shown to increase survival of dissociated human embryonic stem cells and to promote expansion of tissue resident stem cells in culture (Watanabe et al, 2007. Zhang et al, 2011). ROCK inhibition has been shown to increase progenitor expansion in many cell types (Kim et al, 2015). ROCK signaling is critical for salivary gland development (Daley et al, 2009, 2011, 2012; Gervais et al, 2016), and ROCK inhibition has been shown to delay senescence and promote proliferation of the epithelial cells from adult SMG (Han et al, 2018; Nanduri et al, 2014; Lee et al, 2015). However, a role for ROCK inhibition to promote expansion of specific progenitor cell populations in adult-derived salispheres has not been investigated.
Organoids are self-assembled 3D collections of cell types that mimic the morphology and functionality of full organs at a smaller scale in-vitro. Single progenitor cells or tissue pieces can be used to generate organoids (Kretzschmar and Clevers, 2016). When specific factors are added in vitro, organoids are able to self-organize into structures that resemble mini organs (Rossi et al, 2018; Lancater and Knoblich, 2014). Significantly, implantation of organoids into damaged or diseased organs has been shown to improve functionality of several adult organs in animal models, and organoids are important models to study cell interactions that control differentiation and tissue organization in vitro (Karthaus et al, 2014; Kretzschmar and Clevers, 2016; Ramachandran et al, 2015; Tan et al, 2017). We previously established methods to form salivary gland organoids from primary embryonic cells (Hosseini et al, 2018, 2019). We used organoids to identify a requirement for FGF2 signaling in the mesenchyme and laminin-111 for development of complex, branched proacinar organoids from embryonic E16 epithelium (Hosseini et al, 2018, 2019). In this study, we investigate the contribution of ROCK signaling in adult salisphere-derived organoid formation. We show that the ROCK inhibitor, Y27632, and different media compositions can be used for improved expansion of defined mouse adult SMG progenitor cell populations in salisphere cultures and that salispheres expanded with Y27632 have an increased capability for generating complex FGF2-induced proacinar salivary organoids.
2. Materials and Methods
Materials and methods are included in supplementary information.
3. Results
3.1. Effect of Y27632 treatment on salisphere formation and proliferation
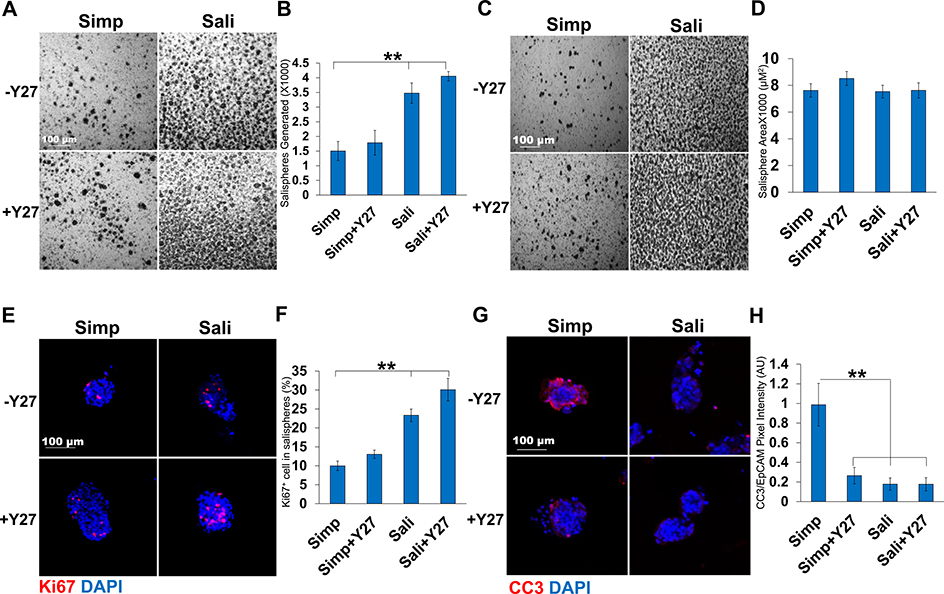
We compared the survival and growth of epithelial cell clusters isolated from adult submandibular salivary glands as salispheres in a simple serum-containing medium (DMEM:F12 +10% (v/v) FBS) that we used previously to culture embryonic epithelial clusters as organoids (Hosseini, et al 2018, 2019) and a complex serum-free medium that was previously used to grow adult salivary gland epithelial cells in 3D clusters known as salispheres (Sali medium) (Nanduri et al., 2013) both with and without the ROCK-selective inhibitor, Y27632. The number of salispheres present after three days of culture in simple medium was 1503 ± 324 while the number was nearly double when Sali medium was used with (3475 ± 345) (Fig. 1a and b). With the addition of Y27632, there was a small increase in the number of salispheres (1783 ± 422 in Simp+Y27632 vs 4052 ± 159 in Sali+Y27632). We also measured the average size of the salispheres grown under different media conditions. Although there was little difference in the size of salispheres with Y27632 treatment in simple medium, salispheres were larger in Sali medium relative to simple media and slightly larger in Sali medum+Y27632 than in Sali medium alone (Fig. 1c and d). We then examined the effect of Y27632 on the proliferation and apoptosis of salispheres. We performed ICC to detect Ki67, a protein that is expressed during all phases of the cell cycle except for G0 (Scholzen and Gerdes, 2000; Nguyen et al, 2018) in salisphere cultures. Comparing salispheres grown in simple medium with salispheres grown in Sali medium for three days, the number of cells expressing Ki67 was twice as high in Sali medium. The inclusion of Y27632 increased the number of Ki67-positive cells grown in both media with Y27632 having a greater impact in Sali medium (Fig. 1e and f). Next, we examined the levels of apoptosis in cultured salispheres using ICC for the apoptosis marker, Cleaved Caspase 3 (CC3). Salispheres cultured in simple medium were highly apoptotic, while treatment with Y27632 significantly reduced CC3 levels after 3 days (0.3 ± 0.08 vs 1 ± 0.2) (Fig. 1g and h). In the Sali medium alone, apoptosis levels were very low and Y27632 did not significantly affect apoptosis levels in this medium. Together, these results indicate that Y27632 treatment primarily promoted cell cycle entry of cells in salispheres cultured in Sali medium but promoted both cell cycle entry and reduction of apoptosis in salispheres cultured in simple medium.
Fig. 1.
Y27632 treatment increases salisphere proliferation and reduces apoptosis. A) Bright field images of salispheres cultured for three days in Simple (Simp) or Salisphere (Sali) medium ± Y27632 (Y27). B) Number of salispheres generated at 3 days. C) Bright field images of salispheres generated per media condition at 3 days. D) Average size of salispheres quantified at 3 days. N = 6. E) ICC of Ki67 in salispheres cultured for 3 days with Ki67 (red, proliferating cells) and DAPI (blue, nuclei). F) Percent of Ki67+ cells per salisphere relative to the total DAPI+ cells present in a salisphere. G) ICC of Cleaved Caspase 3 (CC3, red) and DAPI (blue). H) Quantification of CC3 pixel intensity relative to EpCAM. A-D: N = 6 experiments, E-F: N = 3 experiments. **p < 0.01, One-way ANOVA.
3.2. Cellular composition of salispheres ± Y27632
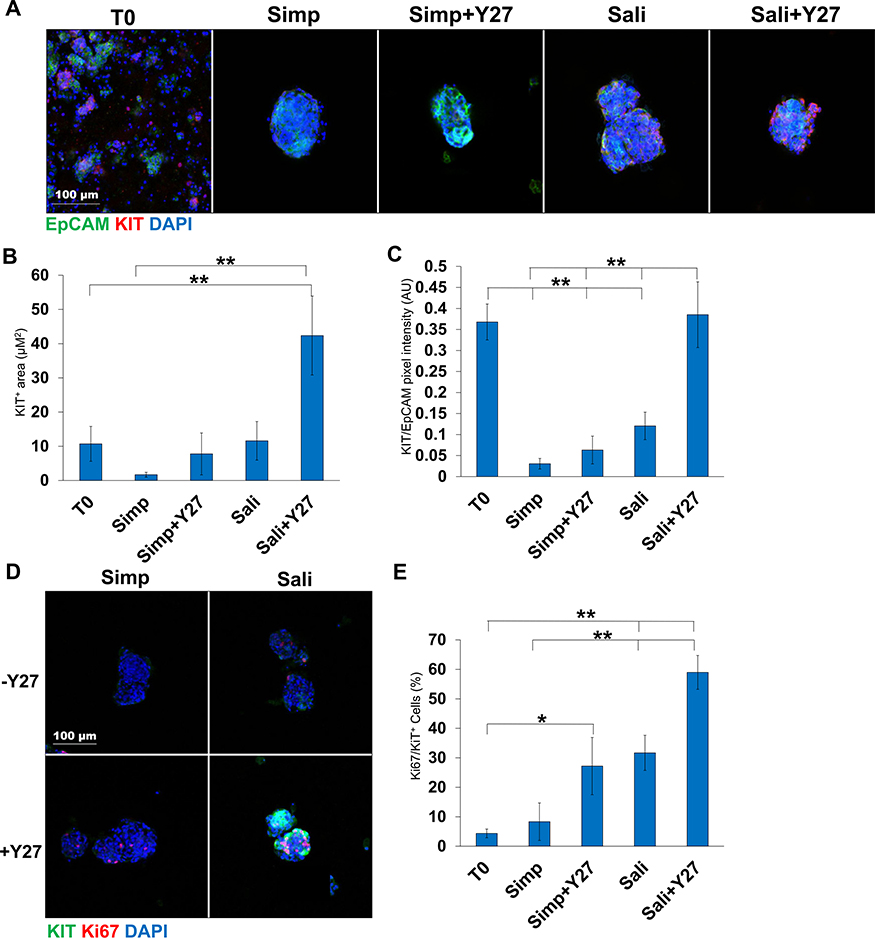
As Kit+ cells are of therapeutic interest in salivary gland regeneration (Lombaert et al, 2008; Nanduri et al, 2014; Pringle et al, 2016) we examined the level of Kit expression in our salispheres. Salispheres were cultured for 3 days and subjected to ICC to detect Kit and the epithelial cell surface marker, EpCAM. Kit was significantly increased in Sali Medium with Y27632 relative to Sali medium alone when the Kit+ area of the salisphere was quantified (11.6 ± 5 vs 42.4 ± 11) and also when the intensity of Kit staining was normalized to EPCAM levels (0.123 ± 0.03 vs 0.399 ± 0.08) (Fig 2a–c). Indeed, compared to samples fixed after preparation of epithelial clusters at the beginning of salisphere culture, Kit levels/intensity were fully restored with Sali+Y27632 treatment together with the total area of cells that were Kit+. By contrast, in salispheres cultured in simple medium, Kit levels were low and did not increase significantly in the presence of Y27632. As the expansion in the epithelial area positive for Kit in the Sali medium implies that Kit+ cells are proliferating, we performed ICC to detect Ki67 together with Kit. Ki67+/Kit+ cells expanded within the EpCAM+ area in salispheres grown in Sali medium+Y27632 relative to Sali medium alone, and much less so in simple medium. These results imply that culture of salispheres in Sali+Y27632 increases the number of cells expressing Kit by stimulating proliferation of Kit+ cells and increasing levels of Kit in individual cells (Fig. 2d). In Sali+Y27632, the percent of Ki67+/Kit+ cells is 59 ± 5.7% (Fig. 2e), demonstrating that Y27632 can be used to in Sali medium to increase the number of proliferative Kit+ cells in salisphere cultures.
Fig. 2.
Y27632 treatment increases Kit levels within salispheres grown in salisphere medium but not serum-containing medium. A) ICC of Kit in salispheres grown in two media formulations ± Y27632: EpCAM (green, epithelium), AQP5 (red, acinar/proacinar) with DAPI (blue, nuclei) in comparison to primary cells at time zero (T0). B) Quantification of Kit+ area in salispheres relative to EpCAM. C) Kit pixel intensity relative to DAPI. D) ICC of Kit (green) and Ki67 (red) in salispheres. E) Quantification of Ki67 in Kit+ cells (%). A-C N = 7 experiments. D-E N = 3 experiments. *p < 0.05, **p < 0.01, One-way ANOVA.
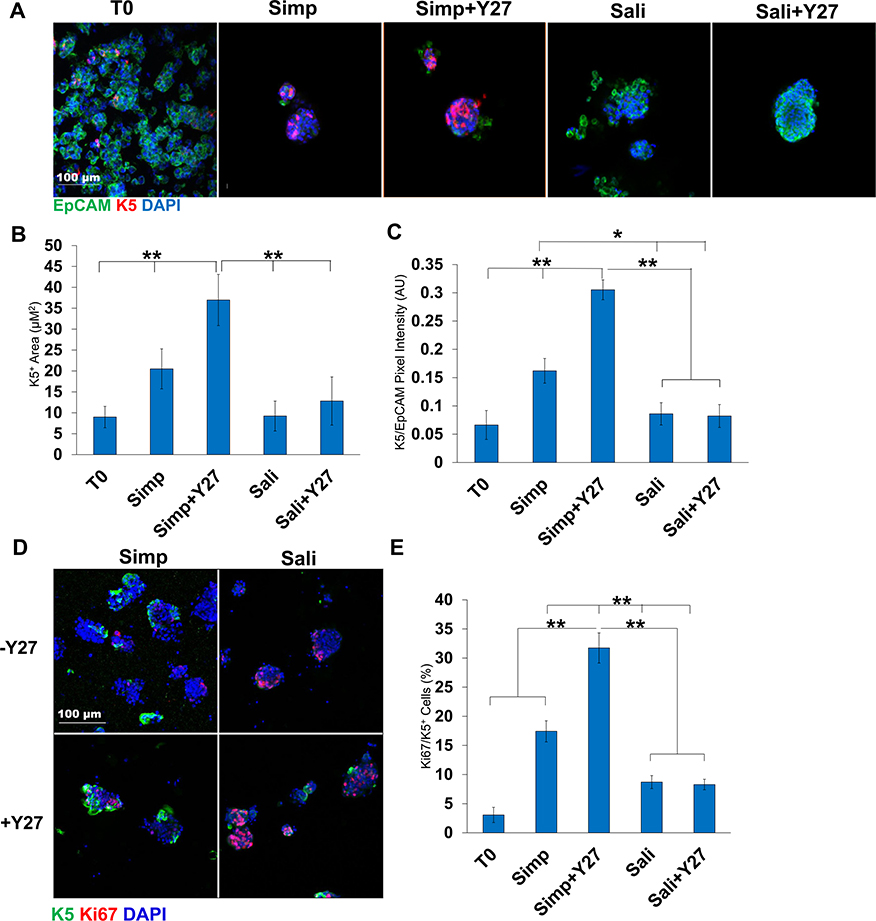
We also examined expression of the basal epithelial cell marker, Keratin 5 (K5), in salisphere culture in the presence or absence of Y27632. In embryonic SMG K5 is expressed in the ducts, while in adult glands K5 is also expressed a subset of the myoepithelial cells. When K5 was examined by ICC in salispheres grown for 3 days either with or without Y27632, K5 expression was robust in simple medium with and without Y27632 (Fig. 3a). The K5+ area was significantly higher with Y27632 (7 ± 3 vs 23 ± 3) and significantly higher than T0 (Fig. 3b). Expression levels of K5 assessed by quantification of pixel intensity were also significantly higher with Y27632 (0.2 ± 0.2 vs 0.3 ± 0.02) and were also significantly higher than T0 (Fig. 3c). Interestingly, salispheres grown in Sali medium and Sali+Y27632 showed little difference in K5 protein levels or salisphere area that is K5+. Keratin 14 (K14), which can dimerize with K5, also showed the highest levels in Simp+Y27632 medium by quantification of pixel intensity (1.0 ± 0.3 vs 0.6 ± 0.1) (Sup 1a). In addition to the overall pixel intensity, the total salisphere area that was positive for K14 in the salispheres was also higher in simple medium+Y27632 salispheres than in simple medium salispheres. The K14+ pixel intensity of salispheres grown in Sali medium was slightly greater than in Sali+Y27632 (1.4 ± 0.09 vs 0.9 ± 0.06) (Sup 1b and c) similarly to what was observed with K5. Interestingly, K5/K14 proliferation in Simp medium was significantly greater than in the Sali medium with or without Y27632, even though salispheres in Sali medium were more proliferative (Fig. 3d and e, Sup 1d and e). These results demonstrate that the simple serum-containing medium supports and can expand the K5+ basal epithelial cell population in salisphere cultures.
Fig. 3.
Y27632 treatment increases K5 levels in salispheres. A) ICC of K5 in salispheres grown in two media forumulations ± Y27632: K5 (red), EpCAM (green), DAPI (blue). B) K5 pixel area relative to DAPI. C) K5+ pixel area relative to EpCAM. The area and intensity of K5 increases in salispheres grown in Simp medium + Y27632. D) ICC of K5 and Ki67 in salispheres. E) Quantification of Ki67/K5+ cells (%) reveals increased Ki67+ cells in Simp Medium + Y27632. A-C: N = 6 experiments, D-E: N = 3 experiments (Bl6 mice). *p < 0.05, **p < 0.01, One-way ANOVA.
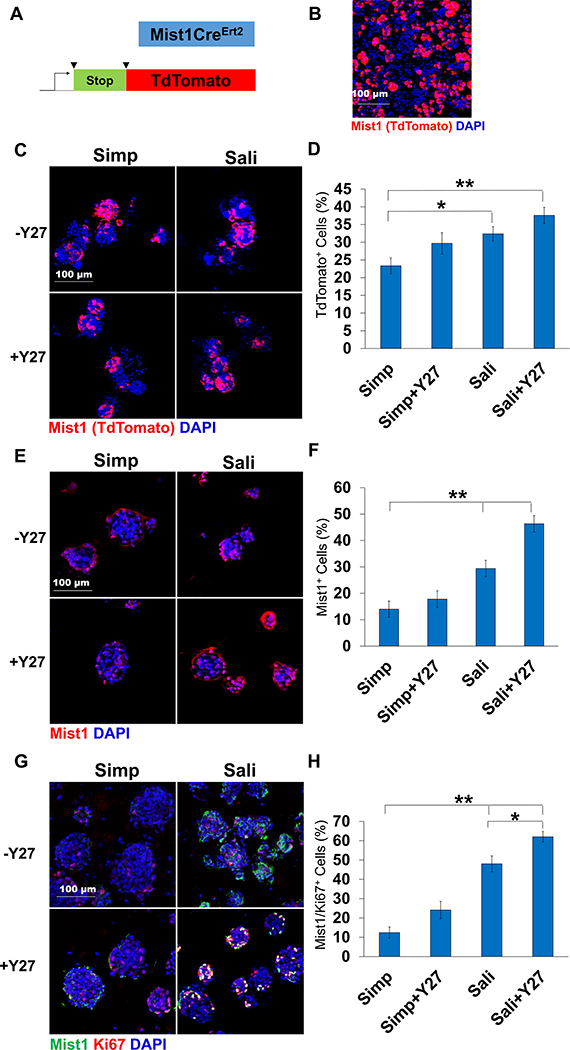
Since salispheres have also been reported to contain cells derived from Mist1+ secretory acinar cells (Varghese et al, 2019), we used Mist1CreERT2;ROSA26TdTomato (R26TdT) reporter mice to isolate salispheres and assayed for expression of reporter cells in both media +/− Y27632 (Fig. 4a and b). Visualization of TdTomato revealed that Mist1+ cells contributed to salispheres formed in each of the four media conditions with no measurable change in TdTomato in salispheres grown under Y27632 treatment in any media condition but increased numbers in Sali medium relative to simple medium (Fig. 4c and d). Mist1+-derived cells thus do not appear to be directly impacted by Y27632 but are enriched by Sali medium relative to simple medium. However, to determine if these salisphere conditions induce Mist1 expression, salisphere ICC was performed to examine the Mist1 epitope. Mist1 expression in Simp salispheres remained low with little increase with Y27632 treatment (14 ± 3 vs 17 ± 8) while in Sali medium, there was an increase in Mist1+ cells with more Mist1+ cells present after Y27632 treatment (29 ± 3 vs 43 ± 3) (Fig. 4e and f). To determine if this increase in Mist1 expression is due to their proliferation in salisphere culture, Mist1 and Ki67 were stained together (Fig. 4g and h). Y27632 treatment of salispheres in simple medium resulted in a minimal increase of proliferating Mist1+ cells (12 ± 3 vs 24 ± 4). In the salisphere medium; however, inclusion of Y27632 resulted in a significant increase in Mist1+ cell proliferation as compared to simple medium, with Sali+Y27 treatment resulting in the greatest number of proliferating Mist1+ cells (48 ± 4 vs 62 ± 3). These results indicate that Sali+Y27632 medium promotes the greatest Mist1+ cell expansion by proliferation, indicating that salispheres grown in Sali+Y27 medium should induce the greatest level of proacinar differentiation in an organoid assay.
Fig. 4.
Mist1-derived cells are present in salispheres and increase in salisphere medium. A) Schematic of TdTomato induction. B) ICC of Mist1CreErt2;R26TdT mouse primary epithelial clusters at T = 0. C) TdT expression in salispheres grown for 3 days. D) Quantification reveals that the average % of salispheres derived from Mist1+ mice increases in Sali relative to Simp medium but is not affected by Y27632 treatment. E) ICC of Mist1 and Ki67. F) % of Mist1- and Ki67-positive cells. N = 3 experiments. *p < 0.05, **p < 0.01, One-way ANOVA.
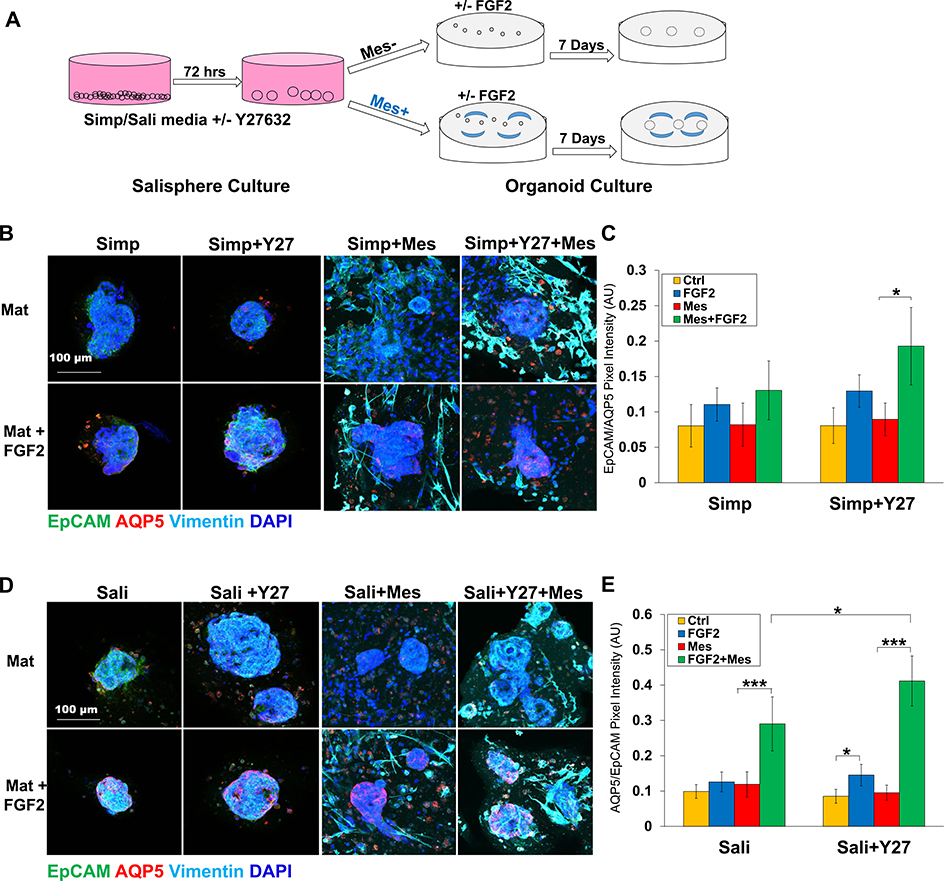
3.3. Proacinar differentiation of salispheres in organoid culture is dependent on FGF2 and primary mesenchyme
Previously, we generated complex salivary organoids with robust, membrane-localized AQP5 derived from E16 salivary epithelial clusters cultured with their own primary mesenchyme cells (Hosseni et al, 2018, 2019). To determine if adult salispheres form AQP5+ budded organoids under organoid culture conditions and if salisphere culture conditions impact organoid formation, salispheres were produced using both medias ± Y27632, and for organoid formation salispheres were transferred to Matrigel® and grown in simple medium containing FGF2, E16 primary mesenchyme, or both (Fig. 5a). Organoids derived from salispheres grown in simple medium exhibited little AQP5 (Fig. 5b and c). Organoids derived from salispheres grown in Sali medium during the salisphere phase exhibited a modest but significant increase in levels of AQP5 when cultured with both FGF2 and E16 mesenchyme, that was further improved by Y27632 (Fig. 5d and e).
Fig. 5.
Salispheres grown in salisphere medium with Y27632 generate proacinar organoids. A) Experimental schematic of organoid experiments. B) ICC of salispheres grown ± Y27632 in Simp medium in the salisphere phase and in the presence or absence of primary E16 salivary mesenchyme cells (Mes) in Matrigel® ± FGF2 in the organoid phase. C) Quantification of salispheres of Ctrl- vs FGF2-treated salispheres. N = 5. *p < 0.05, **p < 0.01, One-Way Anova. D) ICC of salispheres grown ± Y27632 in Sali medium in the salisphere phase and in the presence or absence of primary E16 salivary mesenchyme cells (Mes) in Matrigel® ± FGF2 in the organoid phase. E) Quantification of AQP5/EpCAM intensity in organoids reveals that salispheres grown in Sali medium + Y27632 form proacinar organoids more effectively in the presence of Mes cells than salispheres cultured under other conditions. N = 7 experiments. *p < 0.05, **p < 0.01, ***p < 0.001, One-way ANOVA (C and E).
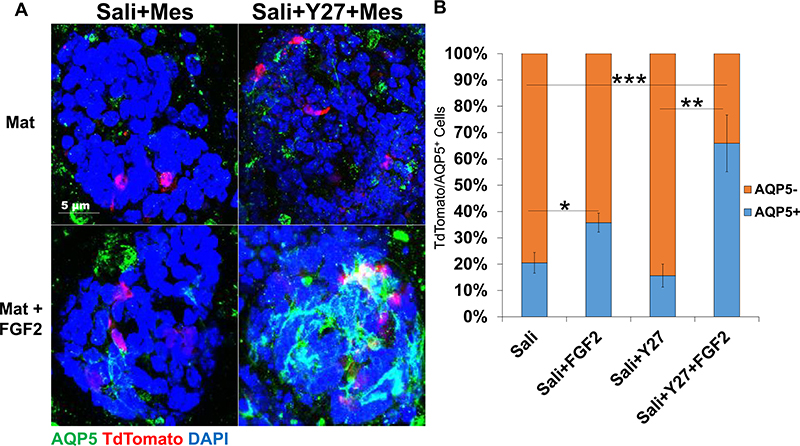
To determine if Mist1-derived cells contribute to cells of a proacinar phenotype in organoids differentially in response to FGF2 influences, organoids were derived from Mist1CreERT2; R26TdT mice Aure et al., 2015 and the co-expression of TdTomato and AQP5 was quantified in all organoid conditions. In organoids derived from salispheres grown in Simp medium, there was not a correlation of TdTomato with AQP5 regardless of whether Y27632 was included in the salisphere culture phase or if FGF2 was included in the organoid phase, with FGF2 showing little to no induction of AQP5 in cells in the organoids (Sup 2a). In organoids derived from salispheres that were cultured in Simp medium, the percent of TdTomato+ cells expressing AQP5 is around 14–20% in the absence or presence of FGF2, respectively, with FGF2 treatment not increasing the correlation of TdTomato with AQP5 in any significant manner (Sup 2b). However, in organoids derived from salispheres cultured in Sali medium, the addition of FGF2 increased the amount of TdTomato+ cells that express AQP5 in organoids derived from salispheres grown both in Sali and in Sali+Y27632 medium (Fig. 6a). In organoids derived from salispheres cultured in Sali medium, FGF2 treatment increased AQP5 expression from 20% to 35%, while in organoids derived from salispheres cultured in Sali+Y27632, FGF2 increased the AQP5+ cells from 16% to 65% (Fig. 6b), respectively. These results indicate that Sali+Y27632-derived organoids treated with mesenchyme and exogenous FGF2 show the highest level of Mist1-derived cells expressing AQP5.
Fig. 6.
Mist1-derived cells show greatest percentage of acinar differentiation in organoids if first expanded in Sali+Y27632 in salisphere culture. A) ICC of organoids derived from salispheres grown ± Y27632 in Sali medium in the salisphere phase and in the presence or absence of primary E16 salivary mesenchyme cells (Mes) in Matrigel ± FGF2 in the organoid phase. Images are Maximum projection confocal images. AQP5 is Green, TdTomato is Red, and DAPI is blue. B) Quantification of the ratio of TdTomato-positive cells that are AQP5+ or AQP5−. N = 3 experiments. *p < 0.05, **p < 0.01, ***p < 0.001, One-way ANOVA.
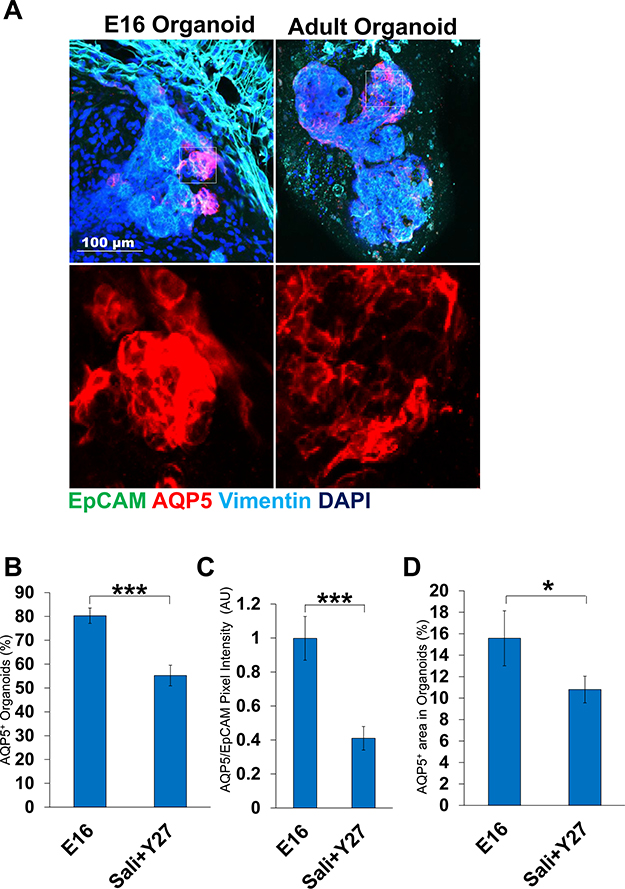
We compared the optimal adult salisphere-derived organoids with proacinar organoids that we previously generated from E16 embryonic tissue (Hosseini et al, 2018). As salisphere culture was previously used to enrich for Kit+ progenitor cells, we examined the levels of Kit+ cells and found that the levels of Kit+ cells were similar in both types of organoids (Sup 3a and b). When compared to complex E16 epithelium-derived organoids that are budded and show robust, membrane-localized AQP5 in response to FGF2 at the tips of these buds, organoids derived from adult salispheres grown in Sali medium with Y27632 exhibited considerably fewer buds, lower AQP5 intensity levels, and less AQP5/EpCAM+ area (Fig. 7a–c). There was also lower percentage of adult organoids that express AQP5 when compared to E16 organoids (Fig. 7d). Our results indicate that while the adult salisphere-derived organoids contain Kit+ cells and proacinar differentiation is similarly FGF2- and mesenchyme-dependent, morphogenesis and proacinar differentiation is not as robust as in organoids derived from embryonic salivary gland cells.
Fig. 7.
Adult cell-derived salivary organoids are less robust and less complex than E16 embryonic-derived organoids. A) ICC of E16 derived organoids vs Adult Salisphere derived organoids grown in optimal conditions. White boxes indicate AQP5+ area. B) Quantification of AQP5 intensity of organoids relative to EpCAM. C) AQP5/EpCAM+ area. D) AQP5+ % organoids. Adult cells are less effective than embryonic cells at forming budded AQP5+ proacinar organoids. *p < 0.05, ***p < 0.001, Student’s t-test.
4. Discussion
Expansion of specific adult epithelial progenitor cells is a goal for current applications in regenerative medicine. Organoid assays have been used to grow a multitude of mini-organs in culture and are useful for exploring the contribution of specific cell types in forming organs. In this report, we demonstrate Y27632-mediated expansion of adult salisphere cultures facilitated salivary organoid formation. We demonstrate that treatment of adult SMG-derived salisphere cultures with the ROCK inhibitor, Y27632, can be used in combination with specific media formulations to reduce apoptosis and expand specific epithelial cell populations in salisphere culture. In serum-containing medium, Y27632 generally reduced cell death, whereas in a more defined media previously used to culture salispheres, Y27632 significantly increased cell proliferation. Examination of cells expressing progenitor markers, showed that inclusion of Y27632 in serum-containing medium promoted proliferation of K5+ cells but not Kit+ cells or Mist1+ cells. In Sali medium, inclusion of Y27632 increased proliferation of Kit+ cells but not K5+ cells or Mist1+ cells. However, Mist1+ cells proliferated more in Sali medium than in the simple serum-containing medium. Organoids grown in culture that mimic specific aspects of whole organs can be used to examine cell potential. We used assays that we previously developed for embryonic SMG epithelial to examine the potential of adult salispheres to form complex salivary organoids with secretory proacinar differentiation. We previously reported that FGF2 in the presence of primary mesenchyme can stimulate a proacinar phenotype in organoids derived from E16 epithelium (Hosseini et al, 2018, 2019). We report here that the adult-derived salivary gland organoids are not identical to the embryonic organoids. Interestingly, while the organoids derived from salispheres grown in Sali+Y27632 showed the highest levels of AQP5 amongst all other salisphere conditions, the overall levels of AQP5 expression in those organoids was lower than that achieved by E16-derived organoids that we previously described (Hosseini et al, 2018, 2019). We demonstrate that Y27632 can enhance the ability of adult salispheres to generate salivary organoids with secretory proacinar differentiation in organoid culture with FGF2-stimulated E16 salivary mesenchyme (Hosseini et al, 2018, 2019). As with E16 embryonic epithelial cells, we identified a requirement for FGF2 and the presence of mesenchyme cells for efficient organoid formation from adult salivary gland epithelial cells, suggesting that cells grown within salispheres may retain the ability to further differentiate in vitro and in vivo in response to other signals.
A well-known function of Y27632 in progenitor cells cultured ex vivo is to interfere with apoptosis in dissociated cells (Zhang et al, 2011). Although Y27632 has been included in salivary adherent cultures and in salisphere cultures in previous studies (Nanduri et al, 2014; Han et al, 2018), its direct role in salisphere formation was not previously documented. While we show that treatment of salispheres with Y27632 decreases apoptosis in serum-containing medium, there appears to be no difference in Y27632 effects on apoptosis in cells grown in salisphere medium, suggesting the presence of a survival factor in the salisphere medium that is lacking in the serum-containing medium. Our data indicates that in salisphere medium, Y27632 has a more significant role in maintaining cell proliferation and in serum-containing medium has a more significant contribution to apoptosis, indicating that crosstalk with other pathways stimulated by the media components modifies the effects of Y27632. While ROCK proteins are activated by caspases during apoptosis, and ROCK function stimulates multiple components of both intrinsic and extrinsic apoptosis pathways in a multifactorial manner, including activation of death receptors, apoptotic blebbing, nuclear and organelle fragmentation, and DNA fragmentation (Street and Bryan, 2011), ROCK’s involvement in apoptosis is complex. ROCK signaling also impacts survival signaling through regulation of the pro-survival PI3K/Akt signaling through the phosphatase and tensin homolog (PTEN) in some cell types. In the context of organoids derived from cells grown ex vivo in which in vivo ECM and basement membrane-mediated and cell-cell-mediated signaling was disrupted, interruption of ROCK signaling differentially affects specific cell types. ROCK inhibition by Y27632 has been also shown to delay senescence in the salivary gland epithelium (Lee et al, 2015). There are thus many possible mechanisms through which Y27632 could affect salivary progenitor cells.
ROCK signaling is known to regulate embryonic stem cell and progenitor cell proliferation (Watanabe et al, 2007; Chapman et al, 2014; Sun et al, 2015; Croze et al, 2016; Miyashita et al, 2013; Kim et al, 2015). We here demonstrate that Y27632 can be used to differentially expand progenitor cell populations within salispheres in specific media formulations. We report that treatment of salispheres with Y27632 increases proliferation of the K5/K14+ basal population in Simple medium. In embryonic cells grown in the absence of serum, K5+ cell proliferation is controlled by parasympathetic innervation (Knox et al, 2010) and retinoic acid receptor (RAR) signaling (Abashev et al., 2017; Amano et al., 2010; Aure et al., 2015; DeSantis et al, 2017), but other factors likely impact proliferation of K5+ adult cells in the context of salispheres. Proliferation of Kit+ cells was increased with salisphere culture in in Sali medium. Although little is known regarding the proliferation of adult Kit+ salivary gland cells, miRNA-regulation of p53 is involved in proliferation of Kit+ cardiac cells (Liu et al, 2017). Defining the regulation of proliferation of adult salivary gland Kit+ cells as well as defining how transplanted Kit+ cells can contribute to regeneration in vivo remains of interest.
Our results demonstrate that Mist1+ cells contribute to formation of salispheres and that their behavior in the context of organoids can be manipulated by culture conditions in the salisphere stage. Our results are consistent with a prior report that salispheres are not exclusively ductally derived (Varghese et al, 2019). As there are cells that are AQP5+ that are not TdTomato+ in lineage traced organoids, it is possible that other progenitor cells expressing p63, Sox9, and/or Sox2 undergo acinar differentiation in this context (Chatzeli et al, 2017; Emmerson et al, 2017; Song et al, 2018). Interestingly, the expansion of Mist1+ cells was media-dependent and Y27632-dependent in Sali medium. These results highlight the potential to manipulate salisphere and organoid cellular composition with culture conditions for future application in regenerative medicine strategies. Investigation into the utility of transplanted Mist1+ cells for cell therapy applications in the context of salispheres, organoids, and cell-scaffold constructs is of interest. Defining the molecular mechanisms driving proliferation of specific salivary gland progenitor cell populations ex vivo requires further investigation both for in vitro expansion and for potential manipulation of these cells in vivo in future therapeutic applications. Future investigation into niche requirements for adult salivary gland progenitor and proacinar/acinar cells may lead to improved ex vivo growth conditions for adult cells for application in regenerative strategies.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Catherine Ovitt for generously providing us with the Mist1CreERT2 mice with permission of Dr. Stephen Konieczny.
Funding
This work was funded by NIH grants: R56DE02246706, R01DE02795301, and R21DE027571 and by the University at Albany, SUNY.
Footnotes
Declaration of Competing Interest
None.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.scr.2019.101608.
References
- Abashev TM, Metzler MA, Wright DM, Sandell LL, 2017. Retinoic acid signaling regulates Krt5 and Krt14 independently of stem cell markers in submandibular salivary gland epithelium. Dev. Dyn 246, 135–147. 10.1002/dvdy.24476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Nakayama M, Kaibuchi K, 2010. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67 (9), 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aure MH, Konieczny SF, Ovitt CE, 2015. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev. Cell 33, 231–237. 10.1016/j.devcel.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aure MH, Symonds JM, Mays JW, Hoffman MP, 2019. Epithelial cell lineage and signaling in murine salivary glands. J. Dental Res, 0022034519864592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S, McDermott DH, Shen K, Jang MK, McBride AA, 2014. The effect of Rho kinase inhibition on long-term keratinocyte proliferation is rapid and conditional. Stem Cell Res. Ther 5. 10.1186/scrt449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzeli L, Gaete M, Tucker AS, 2017. Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development 144 (12), 2294–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF, 2001. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol 3 (4), 339. [DOI] [PubMed] [Google Scholar]
- Croze RH, Thi WJ, Clegg DO, 2016. ROCK inhibition promotes attachment, proliferation, and wound closure in human embryonic stem cell-derived retinal pigmented epithelium. Transl. Vis. Sci. Technol 5, 7. 10.1167/tvst.5.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Gervais EM, Centanni SW, Gulfo KM, Nelson DA, Larsen M, 2012. ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development 139 (2), 411–422. 10.1242/dev.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Gulfo KM, Sequeira SJ, Larsen M, 2009. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev. Biol 336, 169–182. 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Kohn JM, Larsen M, 2011. A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Dev. Dyn. 240, 2069–2083. 10.1002/dvdy.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis KA, Stabell AR, Spitzer DC, O’Keefe KJ, Nelson DA, Larsen M, 2017. RARα and RARγ reciprocally control K5 + progenitor cell expansion in developing salivary glands. Organogenesis 13, 125–140. 10.1080/15476278.2017.1358336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson E, Knox SM, 2018. Salivary gland stem cells: A review of development, regeneration and cancer. Genesis 56 (5), e23211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson E, May AJ, Nathan S, Cruz-Pacheco N, Lizama CO, Maliskova L, Zovein AC, Shen Y, Muench MO, Knox SM, 2017. SOX2 regulates acinar cell development in the salivary gland. Elife 6, 1–22. 10.7554/eLife.26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais EM, Sequeira SJ, Wang W, Abraham S, Kim JH, Leonard D, Desantis KA, Larsen M, 2016. Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development. Organogenesis 12 (4), 194–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, An GH, Woo DH, Kim JH, Park HK, 2018. Rho-associated kinase inhibitor enhances the culture condition of isolated mouse salivary gland cells in vitro. Tissue Cell 54, 20–25. 10.1016/j.tice.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Hosseini ZF, Nelson DA, Moskwa N, Larsen M, 2019. Generating embryonic salivary gland organoids. Curr. Protoc. Cell Biol e76. 10.1002/cpcb.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini ZF, Nelson DA, Moskwa N, Sfakis LM, Castracane J, Larsen M, 2018. FGF2-dependent mesenchyme and laminin-111 are niche factors in salivary gland organoids. J. Cell Sci 10.1242/jcs.208728.jcs.208728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, Van Boxtel R, Wongvipat J, Dowling CM, Gao D, Begthel H, Sachs N, Vries RGJ, Cuppen E, Chen Y, Sawyers CL, Clevers HC, 2014. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159, 163–175. 10.1016/j.cell.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Ossipova O, Sokol SY, 2015. Neural crest specification by inhibition of the ROCK/Myosin II pathway. Stem Cells 33, 674–685. 10.1002/stem.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SM, Lombaert IMA, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP, 2010. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329 (80), 1645–1647. 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar K, Clevers H, 2016. Organoids: modeling development and the stem cell niche in a dish. Dev. Cell 38, 590–600. 10.1016/j.devcel.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Kwak M, Ninche N, Klein S, Saur D, Ghazizadeh S, 2018. c-Kit+ cells in adult salivary glands do not function as tissue stem cells. Sci. Rep 8, 14193. 10.1038/s41598-018-32557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA, 2014. Organogenesisin a dish: modeling development and disease using organoid technologies. Science 80, 345. 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S, Roh S, 2015. Y-27632, a ROCK inhibitor, delays senescence of putative murine salivary gland stem cells in culture. Arch. Oral Biol 60, 875–882. 10.1016/j.archoralbio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Y, Cui J, Sun M, Pu Z, Wang C, Du W, Hou J, Zhang S, Yu B, 2017. miR199a-3p regulates P53 by targeting CABLES1 in mouse cardiac c-kit+ cells to promote proliferation and inhibit apoptosis through a negative feedback loop. Stem Cell Res. Therapy 8 (1), 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IMA, Brunsting JF, Weirenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP, 2008. Rescue of Salivary gland function after stem cell transplantation in irradiated glands. PLoS One 3, 1–13. 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I, Movahednia MM, Adine C, Ferreira JN, 2017. Concise review: salivary gland regeneration: therapeutic approaches from stem cells to tissue organoids. Stem Cells 35, 97–105. 10.1002/stem.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AJ, Cruz-Pacheco N, Emmerson E, Gaylord EA, Seidel K, Nathan S, Muench MO, Klein OD, Knox SM, 2018. Diverse progenitor cells preserve salivary gland ductal architecture after radiation-induced damage. Development 145 (21) dev166363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita H, Yokoo S, Yoshida S, Kawakita T, Yamagami S, Tsubota K, Shimmura S, 2013. Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor. Stem Cells Transl. Med. 758–765. 10.5966/sctm.2013-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri LSY, Baanstra M, Faber H, Rocchi C, Zwart E, De Haan G, Van Os R, Coppes RP, 2014. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep. 3, 957–964. 10.1016/j.stemcr.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri LS, Lombaert IM, van der Zwaag M, Faber H, Brunsting JF, van Os RP, Coppes RP, 2013. Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother. Oncol 108, 458–463. 10.1016/j.radonc.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Manhardt C, Kamath V, Sui Y, Santamaria-Pang A, Can A, Bello M, Corwin A, Dinn SR, Lazare M, Gervais EM, Sequeira SJ, Peters SB, Ginty F, Gerdes MJ, Larsen M, 2013. Quantitative single cell analysis of cell population dynamics during submandibular salivary gland development and differentiation. Biol. Open 2, 439–447. 10.1242/bio.20134309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Dawson P, Zhang Q, Harris Z, Limesand KH, 2018. Administration of growth factors promotes salisphere formation from irradiated parotid salivary glands. PLoS One 13, 1–17. 10.1371/journal.pone.0193942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin CL, Michael Rukstalis J, Johnson C, Konieczny SF, 2001. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J. Cell Biol 155, 519–530. 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle S, Maimets M, van der Zwaag M, Stokman MA, van Gosliga D, Zwart E, Witjes M, Haan G, Os R, Coppes RP, 2016. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 34 (3), 640–652. 10.1002/stem.2278. [DOI] [PubMed] [Google Scholar]
- Ramachandran SD, Schirmer K, Münst B, Heinz S, Ghafoory S, Wölfl S, Simon-Keller K, Marx A, Oie CI, Ebert MP, Walles H, Braspenning J, Breitkopf-Heinlein K, 2015. In vitro generation of functional liver organoid-like structures using adult human cells. PLoS One 10, 1–14. 10.1371/journal.pone.0139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Manfrin A, Lutolf MP, 2018. Progress and potential in organoid research.Nat. Rev. Genet 19, 671–687. 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J, 2000. The Ki-67 protein: from the known and the unknown. J. Cell Physiol 182, 11–322 10.1002. [DOI] [PubMed] [Google Scholar]
- Song EAC, Min S, Oyelakin A, Smalley K, Bard JE, Liao L, Xu J, Romano RA, 2018. Genetic and scrna-seq analysis reveals distinct cell populations that contribute to salivary gland development and maintenance. Sci. Rep 8 (1), 14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street CA, Bryan BA, 2011. Rho kinase proteins—pleiotropic modulators of cell survival and apoptosis. Anticancer Res. 31 (11), 3645–3657. [PMC free article] [PubMed] [Google Scholar]
- Sun CC, Chiu HT, Lin YF, Lee KY, Pang JHS, 2015. Y-27632, a ROCK inhibitor, promoted limbal epithelial cell proliferation and corneal wound healing. PLoS One 10, 1–18. 10.1371/journal.pone.0144571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q, Choi KM, Sicard D, Tschumperlin DJ, 2017. Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials 113, 118–132. 10.1016/j.biomaterials.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese JJ, Hansen ME, Sharipol A, Ingalls MH, Ormanoski MA, Newlands SD, Benoit DS, 2019. Salivary gland cell aggregates are derived from self-organization of acinar lineage cells. Arch. Oral Biol 97, 122–130. 10.1016/j.archoralbio.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Qi S, Wang J, Xia D, Qin L, Zheng Z, Wang L, Zhang C, Luyuan J, Ding G, Wang S, Fan Z, 2014. Spatial and temporal expression of c-Kit in the development of the murine submandibular gland. J. Mol. Histol 45, 381–389. 10.1007/s10735-014-9570-7. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa SI, Muguruma K, Sasai Y, 2007. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol 25, 681–686. 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Nakata H, Kumchantuek T, Sakulsak N, Iseki S, 2016. Immunohistochemical localization of keratin 5 in the submandibular gland in adult and postnatal developing mice. Histochem. Cell Biol 145 (3), 327–339. [DOI] [PubMed] [Google Scholar]
- Zhang L, Valdez JM, Zhang B, Wei L, Chang J, Xin L, 2011. ROCK inhibitor Y-27632 suppresses dissociation-induced apoptosis of murine prostate stem/progenitor cells and increases their cloning efficiency. PLoS One. 10.1371/journal.pone.0018271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.