Abstract
Background and Objective:
Chemical plaque control acts as an adjunct to mechanical periodontal therapy. Chlorhexidine (CHX) is considered as the gold standard in chemical plaque control, but the main concern is about its fibroblast cytotoxicity. Curcumin, a lipophilic polyphenol, may offer as a promising antiplaque agent. This study was conducted to compare the effect of curcumin (0.003%, 0.03%, 0.06%, 0.1%, and 0.12%) and CHX (0.03%, 0.06%, 0.1%, 0.12%, and 0.2%) on gingival fibroblast cell viability and wound healing at different time periods (1, 2, 4, 6, 8, and 10 min).
Materials and Methods:
The minimum inhibitory concentration (MIC50) was determined before the evaluation of cytotoxicity and wound healing property. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and morphological examination by direct invert microscopy were carried out to determine cytotoxicity. Wound healing was evaluated by scratch wound assay.
Results and Discussion:
The MIC50 of CHX and curcumin was at 0.1% and 0.003%, respectively. The mean percentage of fibroblast viability at different concentrations of CHX and curcumin at each time period showed a significant difference. Curcumin exhibited less cytotoxicity as compared to CHX at all concentrations and at varying time periods. There was a significant difference between mean percentage of fibroblast viability at MIC50 of CHX (0.1%) and curcumin (0.003%) at different time periods. The difference between percentage wound healing at antibacterial concentrations of CHX and curcumin at varying time periods was significant.
Conclusion:
The antibacterial concentration of curcumin (0.003%) exhibits less fibroblast cytotoxicity and excellent wound healing property as compared to CHX. Curcumin may offer as a promising chemical plaque control agent which is less cytotoxic, cost-effective, safe, easily available, and with a possibly beneficial effect on wound healing.
Key words: Chlorhexidine, curcumin, cytotoxicity, fibroblast, wound healing
INTRODUCTION
Periodontal disease is a chronic immunoinflammatory disease, resulting from a complex interplay between periodontopathogens, their byproducts, and host response.[1] It is characterized by the destruction of periodontal ligament and alveolar bone with subsequent clinical attachment loss. In periodontal disease, innate immunity is the first line of defense to bacterial challenge.[2] Pathogen-associated pattern recognition receptors, mainly Toll-like receptors (TLRs), respond in a specific manner to lipopolysaccharides (LPSs) of Gram-negative bacteria.[3] The destruction associated with periodontitis is attributed to the overactivation of TLRs. TLR-4 which is activated by LPS leads to the activation of nuclear factor (NF)κ-β and other inflammatory mediators. Most of the periodontal destruction is caused by these inflammatory mediators and activated free radicals.
The ultimate goal of periodontal therapy is to arrest bacteria/host-induced inflammation and maintain a healthy periodontium. Nonsurgical/surgical therapy has been the mainstay of periodontal disease management, and plaque control is considered as the cornerstone in the prevention of this chronic disease. Various chemical plaque control agents are used as adjuncts along with the mechanical debridement. Chemicals such as chlorhexidine (CHX), essential oils, and triclosan are also used as chemical plaque control agents, and they act as an adjunct to mechanical periodontal therapy.[4]
At present, the most widely used and investigated chemical plaque control agent is CHX.[5] The superior effect of CHX is due to its substantivity.[6] CHX is accepted as a safe and effective antiplaque agent, but several studies showed that CHX is cytotoxic to many cells such as human dermal fibroblasts, gingival and periodontal ligament fibroblasts, and alveolar bone cells.[7] Even low concentrations of CHX inhibited protein and DNA synthesis in fibroblasts and epithelial cells.[8] In vitro studies have shown a time-dependent deleterious effect of CHX on cell viability and a dose-dependent toxic effect on cell proliferation.[9,10] Even though CHX is considered as the gold standard in chemical plaque control, the main concern is about its cytotoxicity to fibroblasts. Hence, an alternative agent with comparable antibacterial and anti-inflammatory property with less fibroblast cytotoxicity needs to be explored as a chemical plaque control agent.
Recently, phytotherapy (use of herbal agents as medicine) is gaining wide attention globally in medicine and dentistry because of its safety.[11] Turmeric one of the widely used home remedies is being given more importance in medical research nowadays. The active ingredient of turmeric is curcumin, and it is a lipophilic polyphenol extracted from the root of curcumin longa.[12] Curcumin possesses anti-inflammatory, antibacterial, antioxidant, and immunomodulatory properties and promotes wound healing.[13] In periodontal disease, curcumin modulates NF-κβ following TLR-4 activation by LPS.[14] Curcumin suppresses an array of cytokines and downregulates several enzymes such as inducible nitric oxide synthase, cyclooxygenase-2 (COX-2), and lipoxygenase.[15] They are potent scavengers of reactive oxygen species that give an added benefit in periodontal disease. Curcumin inhibits the growth of many periodontopathogens in a dose-dependent manner.[16]
To the best of the authors' knowledge, only a few studies[11,14] are available in the literature evaluating the effect of curcumin on fibroblast cytotoxicity and wound healing. This study was conducted to compare the effect of curcumin and CHX digluconate on human fibroblast cell viability and migration.
MATERIALS AND METHODS
Sample size (n) calculation:
The sample size was calculated based on a previous study[10] using the formula,
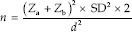
Where Za = 1.96 (constant), Zb = 0.84 (constant), SD = Standard deviation = 11, and d = Effect size = 10.
According to this, the sample size in each category was calculated as 20. For cytotoxic evaluation, the sample size calculated was 200 (twenty samples for each concentration of curcumin [0.003%, 0.03%, 0.06%, 0.1%, and 0.12%] and CHX [0.03%, 0.06%, 0.1%, 0.12%, and 0.2%]). For scratch wound assay, 60 samples were taken. In this in vitro study, the total sample size was calculated as 260.
Test materials
The test materials used for this study are commercially available CHX digluconate 0.2% and curcumin powder (99% pure curcumin, Sigma Aldrich, St. Louis, US). CHX concentrations used in this study were 0.03%, 0.06%, 0.1%, 0.12%, and 0.2%, and concentrations of curcumin used in this study were 0.003%, 0.03%, 0.06%, 0.1%, and 0.12%.
In this study, the following procedures were conducted to compare the effect of curcumin and CHX digluconate on human fibroblast cell viability and migration.
Preparation of compound stock
Antibacterial testing
Culturing of fibroblast
Cytotoxicity evaluation
Evaluation of cell proliferation and migration/wound healing (scratch wound assay).
Preparation of compound stock
Curcumin and CHX digluconate stock solutions were diluted to a series of concentrations using Dulbecco's Modified Eagle Medium (DMEM) and dissolved completely by cyclomixer. The solution was filtered through a 0.22-μm Millipore syringe to ensure sterility.
Antibacterial testing (determination of minimum inhibitory concentration (MIC50) of test materials)
The minimum inhibitory concentration (MIC50) was determined before the evaluation of cytotoxicity and wound healing property. MIC50 was determined using a two-fold serial dilution method.[17] A 28-g nutrient broth media (HiMedia) was dissolved in 1000-ml distilled water and was autoclaved at 121°C, 15l bps for 15 min. Mixed culture of bacteria (Streptococcus mutans [MTCC 890] and Enterococcus faecalis [ATCC 29212]) was used as a test inoculum. The growth of test inoculum was adjusted to 1% McFards Standard. The broth dilution assay was done in 96-well microtiter plates (Thermo Scientific). All wells in the plate were added with 100 μl of the diluted (two times) inoculum suspensions (final volume in each well was 200 μl). Samples of both CHX and curcumin were added in increasing concentrations to the respective wells and incubated overnight at 37°C in a microbiological incubator (KEMI). The control well (with bacteria) was also incubated without any test materials. Bacterial growth was observed by visual inspection and by measuring the optical density (OD) at 630 nm using enzyme-linked immunosorbent assay plate reader (ERBA, LisaScan). The OD was measured immediately after the visual reading. The growth inhibition for the test wells at each extract dilution was determined by the formula:
Percentage of inhibition = (OD of control − OD of test)/(OD of control) × 100%.
The results were obtained as percentage inhibition of bacteria corresponding to the concentration of the test solution (curcumin and CHX). MIC50 and lethal dose (LD90) of curcumin and CHX were further calculated using ED50 plus v1 software (AATBioquest, Californi, USA) from the result obtained.
Culturing of fibroblast
Human dermal pulp stem cells were initially procured from HiMedia, India (National Centre for Cell Sciences, Pune, Maharashtra, India). They were cultured in HiMesoXL™ mesenchymal stem cell expansion medium. It is supplemented with 10% mesenchymal stem cell tested fetal bovine serum, L-glutamine, sodium bicarbonate, and antibiotic solution containing penicillin (100U/ml) streptomycin (100 μg/ml) and amphotericin B (2.5 μg/ml). To convert stem cells to gingival fibroblast cells, the cells were trypsinized and cultured in HiFibroXL™ fibroblast expansion medium. Two-day-old confluent monolayer cells are trypsinized, and the cells are suspended in a 10% growth medium. A 100-μl cell suspension (5 × 104 cells/well) was seeded in 96-well tissue culture plates and incubated at 37°C in a humidified 5% CO2 incubator. The cells obtained by this procedure were used for carrying out 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay for cytotoxicity evaluation and scratch wound assay.
Cytotoxicity evaluation
After attaining sufficient growth of fibroblast cells, the growth medium is removed. Freshly prepared solutions of CHX (0.03%, 0.06%, 0.1%, 0.12%, and 0.2%) and curcumin (0.003%, 0.03%, 0.06%, 0.1%, and 0.12%) in 100-μl 5% DMEM are added in triplicates to the respective wells and incubated for 1, 2, 4, 6, 8, and 10 min at 37°C in a humidified 5% CO2 incubator. The viability of cells is evaluated by direct microscopic observation through inverted phase-contrast microscopy and MTT assay.
Cytotoxicity evaluation by direct microscopic observation
After 24 h of incubation of fibroblasts, with varying concentrations of CHX (0.03%, 0.06%, 0.1%, 0.12%, and 0.2%) and curcumin (0.003%, 0.03%, 0.06%, 0.1%, and 0.12%), the entire plate is observed in an inverted phase-contrast tissue culture microscope (Olympus CKX41 with Optika Pro5 CCD Camera). Microscopic observations were recorded as images for each concentration of curcumin and CHX at varying time periods. Each concentration at a varying time period of both CHX and curcumin was imaged. Any detectable changes in the morphology of cells were considered as indications of cytotoxicity.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
A 15-mg MTT (Sigma, M-5655) is reconstituted in 3-ml phosphate buffer saline (PBS) until completely dissolved and sterilized by filter sterilization. The fibroblasts with varying concentrations of CHX (0.03%, 0.06%, 0.1%, 0.12%, and 0.2%) and curcumin (0.003%, 0.03%, 0.06%, 0.1%, and 0.12%) were incubated for 24 h. A 30-μl reconstituted MTT solution was added to all test and control wells. The plate is then shaken well and then incubated at 37°C in a humidified 5% CO2 incubator for 4 h. After the incubation period, the supernatant is removed and 100 μl of MTT solubilization solution diethyl sulfoxide is added and the wells are mixed gently by pipetting up and down in order to solubilize the formazan crystals. The absorbance values are measured using a microplate reader at a wavelength of 540 nm. The same procedure was repeated in twenty samples, provided all other conditions are kept similar.
The percentage of cell viability was calculated using the formula:
Percentage of viability = Mean OD samples × 100/Mean OD of the control group (OD = Optical Density)
The result obtained was the mean percentage of viability of fibroblasts corresponding to each concentration of curcumin and CHX at varying time periods.
Scratch wound assay
The scratch wounds were made by a sterile 1-ml pipette tip through a premarked line in the fibroblast culture plates. After removal of the resulting debris from five linear scratches, the cell monolayer was subsequently rinsed three times with PBS (phosphate buffer solution) followed by incubation with samples at antibacterial concentration – CHX – 0.1% and curcumin – 0.003% for 24, 48, and 72 h. The control plate was also incubated without any test materials. Twenty similar culture plates were prepared for both curcumin and CHX. The wound areas were displayed by taking images after staining with acridine orange, just above the interchanges between scratched wound areas and premarked lines. The effect of the sample on wound closure was determined microscopically (Olympus CKX41) after 24, 48, and 72 h of incubation. The effect of the sample on wound closure was measured in terms of the area using MRI-Image J analysis software (Javabased image processing program, (National institute of health and LOCI, University of Wisconsin)). The wound closure was calculated as percentage migration of fibroblast in CHX, curcumin, and control samples at 24, 48, and 72 h.
Statistical analysis
Mean (±SD) was calculated for all quantitative variables. Intergroup comparison of mean percentage of fibroblast viability at the antibacterial concentration of CHX and curcumin at the varying time periods and intergroup comparison of mean percentage of fibroblast migration and proliferation (wound healing) of control, CHX, and curcumin at varying time periods were calculated by one-way ANOVA. Repeated measures ANOVA was used for intragroup comparison of mean percentage of fibroblast viability at different concentrations of control, CHX, and curcumin at each time period and intragroup comparison of mean percentage of fibroblast migration and proliferation of control, CHX, and curcumin at varying time periods. Bonferroni post hoc test was done. The α value was set at 0.05 and the confidence interval set at 95%.
RESULTS
This experimental study focused on five different concentrations of curcumin and CHX at six different time periods. An antibacterial test was carried out to assess the MIC of both curcumin and CHX. A series of concentrations were tested against different strains of bacteria. MIC50 of curcumin was 0.003% [Figure 1] and of CHX was 0.1% [Figure 2]. As the concentration of CHX and curcumin increased, the antibacterial property was also found to be increased. Curcumin showed MIC at a very low concentration (0.003%) as compared to CHX. This study suggested that the antibacterial property of curcumin was higher than that of CHX.
Figure 1.
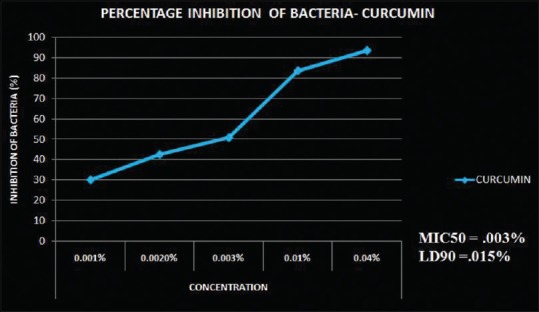
Percentage inhibition of bacteria at different concentrations of CUR on mixed bacterial culture. The MIC50 of CUR was 0.1% and LD90 was 0.01%. Calculated by software – ED50plus V (CUR – Curcumin; MIC – Minimum inhibitory concentration; LD – Lethal dose)
Figure 2.
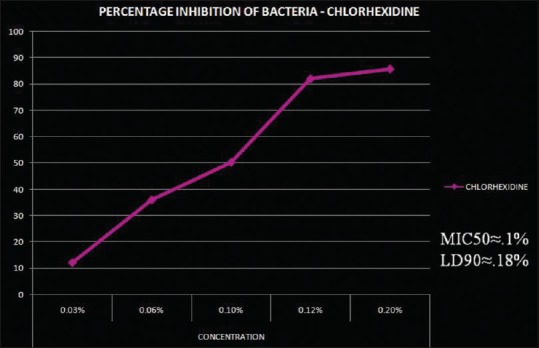
Percentage inhibition of bacteria at different concentrations of CHX on mixed bacterial culture. The MIC50 of CHX was 0.1% and LD90 was 0.18%. Calculated by software – ED50plus v (CHX – Chlorhexidine; MIC – Minimum inhibitory concentration; LD – Lethal dose)
The MTT assay was carried out to evaluate the gingival fibroblast cytotoxicity of curcumin and CHX. It was observed that the mean percentage of fibroblast viability decreases as the concentration of CHX (0.03%, 0.06%, 0.1%, 0.12%, and 0.2%) and curcumin (0.003%, 0.03%, 0.06%, 0.1%, and 0.12%) increases at varying time periods. As the time period increases (1, 2, 4, 6, 8, and 10 min), the mean percentage of fibroblast viability decreases for both agents. At 0.1% concentration of CHX (MIC50), the mean percentage of fibroblast viability ranges from 48.75% to 38.85% for the time period of 1–10 min. At 0.003% concentration of curcumin (MIC50), the mean percentage of fibroblast viability ranges from 99.04% to 68.6% for the time period of 1–10 min. For all other concentrations of curcumin, the fibroblast viability is less than the viability at the concentration of 0.003% for the time period of 1–10 min. The mean percentage of fibroblast viability of curcumin was significant at all concentrations at varying time periods except at a concentration of 0.06% (P < 0.05) [Table 1]. The mean percentage of fibroblast viability was not significant at all concentrations of CHX at varying time periods (P > 0.05) [Table 1].
Table 1.
Mean percentage of fibroblast viability at different concentrations of chlorhexidine and curcumin at varying time periods
| Samples (%) | Mean percentage of fibroblast viability (%) |
Intragroup comparison |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 min | 2 min | 4 min | 6 min | 8 min | 10 min | F | df | Significance | |
| CHX 0.03 | 77.99 | 71.178 | 65.64 | 63.23 | 57.12 | 53.62 | 7.623 | 1.565 | 0.064 |
| CHX 0.06 | 58.50 | 57.55 | 52.15 | 49.02 | 43.94 | 43.43 | 1.745 | 3.059 | 0.170 |
| CHX 0.1 | 48.75 | 47.54 | 40.55 | 39.73 | 39.34 | 38.85 | 5.423 | 1.483 | 0.103 |
| CHX 0.12 | 42.85 | 40.01 | 37.26 | 35.08 | 34.59 | 34.22 | 2.797 | 1.275 | 0.216 |
| CHX 0.2 | 36.23 | 33.93 | 31.79 | 31.32 | 30.24 | 30.08 | 2.978 | 1.582 | 0.185 |
| CUR 0.003 | 99.04 | 90.80 | 77.031 | 74.04 | 72.41 | 68.66 | 97.24 | 1.460 | 0.000* |
| CUR 0.03 | 90.77 | 85.68 | 75.73 | 72.88 | 70.484 | 65.44 | 56.17 | 1.110 | 0.013* |
| CUR 0.06 | 72.19 | 67.69 | 64.85 | 63.84 | 63.62 | 63.46 | 4.039 | 1.549 | 0.137 |
| CUR 0.1 | 56.43 | 52.60 | 50.950 | 49.74 | 46.66 | 45.89 | 11.69 | 1.860 | 0.025* |
| CUR 0.12 | 39.23 | 36.99 | 35.77 | 34.29 | 34.10 | 32.82 | 7.438 | 1.939 | 0.048* |
*P<0.05 significant. The mean percentage of fibroblast viability of CUR was significant at all concentrations except at concentration of 0.06% at different time periods. (Repeated measures ANOVA, P<0.05). The mean percentage of fibroblast viability was not significant at all concentrations of CHX at varying time periods. (Repeated measures ANOVA, P>0.05). Intragroup comparison of mean percentage of fibroblast viability at different concentrations of CHX and CUR at different time periods was statistically significant. (One-way ANOVA, P<0.05). CHX – Chlorhexidine; CUR – Curcumin;P – Probability value; F – Fstatistic
There was a significant difference in the mean percentage of fibroblast viability between CHX and curcumin at different concentrations (0.03%, 0.06%, 0.1% and 0.12%) at varying time periods (P < 0.05) [Table 1]. Curcumin showed a higher mean percentage of fibroblast viability as compared to CHX at every concentration and time period. There was a significant difference between mean percentage of fibroblast viability at the antibacterial concentration of CHX (0.1%) and curcumin (0.003%) at different time periods (P < 0.05) [Table 2 and Graph 1]. Bonferroni post hoc analysis showed that there was a significant difference in the mean percentage of fibroblast viability between the antibacterial concentration of curcumin and CHX at varying time periods.
Table 2.
Comparison of mean percentage of fibroblast viability at antibacterial concentration of chlorhexidine (0.1%) and curcumin (0.003%) at varying time periods
| Samples (%) | Mean fibroblast viability (%) and SD |
Comparison between antibacterial concentration |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 min | 2 min | 4 min | 6 min | 8 min | 10 min | F | df | Significance | |
| CHX 0.1 | 48.76 (5.64) | 47.55 (5.62) | 40.56 (5.80) | 39.74 (5.58) | 39.34 (4.59) | 38.86 (3.10) | 102.841 | 11.24 | 0.000* |
| CUR 0.003 | 99.05 (.54) | 90.80 (.126) | 77.03 (1.16) | 74.05 (.08) | 72.40 (.43) | 68.66 (1.35) | |||
*P<0.05 significant. The mean percentage fibroblast viability at antibacterial concentration of CHX (0.1%) and CUR (0.003%) at different time periods was statistically significant (One-way ANOVA, P<0.05). Bonferroni post hoc analysis showed that there was a significant difference in the mean percentage of fibroblast viability. CHX – Chlorhexidine; CUR – Curcumin; SD – Standard deviation; P – Probability value; F – Fstatistic
Graph 1.
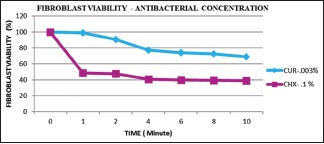
Comparison of mean percentage fibroblast viability at antibacterial concentration of chlorhexidine (0.1%) and curcumin (0.003%) at varying time period. *P < 0.05 significant. The mean percentage fibroblast viability at antibacterial concentration of CHX (0.1%) and CUR (0.003%) at different time periods was statistically significant (One-way ANOVA, P < 0.05). Bonferroni post hoc analysis showed that there was a significant difference in the mean percentage of fibroblast viability. CHX – Chlorhexidine; CUR – Curcumin; SD – Standard deviation; P – Probability value; F – Fstatistic
In this study, morphological change of CHX/curcumin-treated fibroblasts was evaluated through a phase-contrast microscope. As the concentration of CHX increased from 0.03% to 0.2%, changes such as nuclear fragmentation, cytoplasmic blebbing, and echinoid spikes were visible. At the antibacterial concentration of CHX (0.1%), fibroblast exhibited alteration in normal morphology of cells, cell shrinkage and presence of apoptotic cells, but normal morphology of cells was reflected at an antibacterial concentration of curcumin [Figure 3]. As the concentration of curcumin increased minor alteration in the morphology of cells such as loss of spindle shape of cells and cell shrinkage were seen, but was not appreciable.
Figure 3.
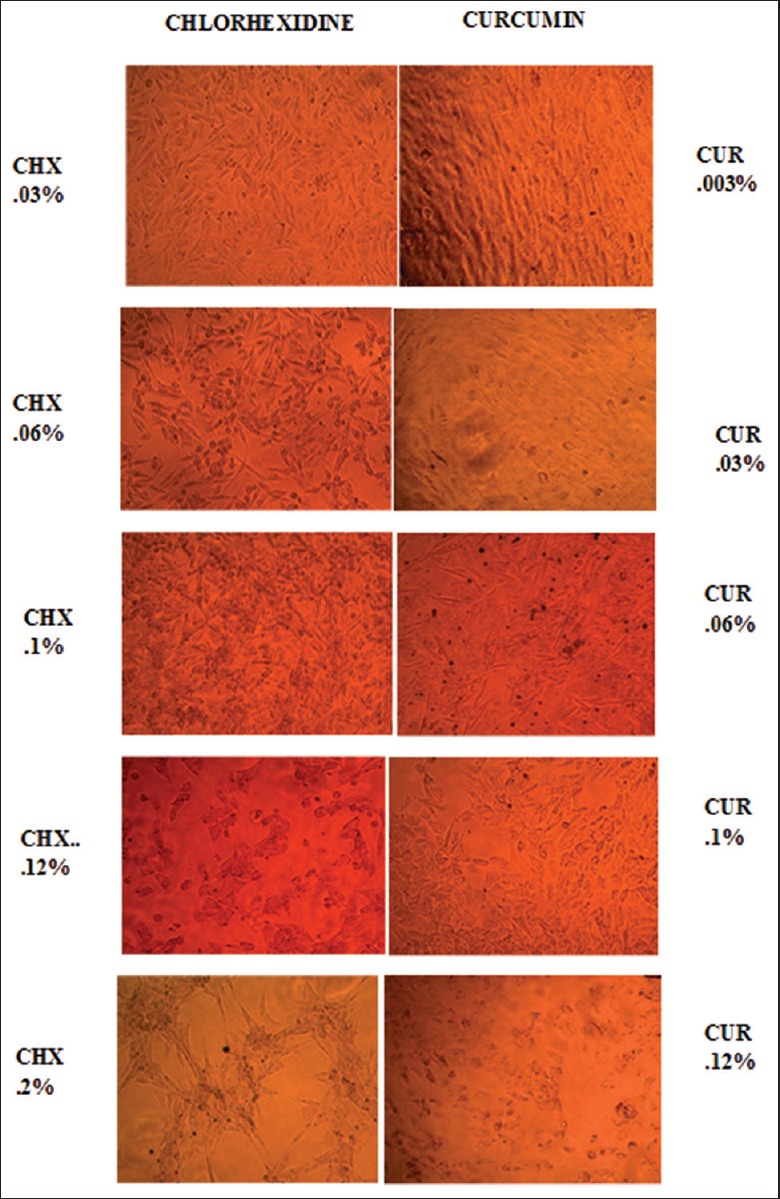
Phase-contrast microscopic images of gingival fibroblasts exposed to varying concentrations of CHX and CUR at 1-min time period. CHX 0.03% – Alteration in spindle-shaped appearance of fibroblasts. 0.06% – Hyperchromatic nuclei/cell shrinkage. 0.1% – Nuclear condensation 0.12% – Cell morphology completely lost, presence of apoptotic bodies. 0.2% – Membrane blebbing/Apoptotic bodies/Echinoid spikes. CUR 0.003% – No changes. 0.03%/0.06% – Cell shrinkage hyperchromatism. 0.1% – Cellular condensation. Membrane blebbing present. 0.12% – Presence of apoptotic bodies. (CHX – Chlorhexidine; CUR – Curcumin)
The wound healing property of CHX and curcumin was compared by carrying out a scratch wound assay for three different time periods (24, 48, and 72 h). The mean percentage of fibroblast migration and proliferation (wound healing) of CHX at the 24th, 48th, and 72nd h was 8.93%, 9.67%, and 10.44%, respectively, whereas the mean percentage of fibroblast migration and proliferation of curcumin at the 24th, 48th, and 72nd h was 17.6%, 26.58%, and 40.92%, respectively. The mean percentage of fibroblast migration and proliferation (wound healing) of control, CHX, and curcumin at different time periods is statistically significant (P < 0.05) [Table 3, Figure 4 and Graph 2]. Intergroup comparison showed that the mean percentage of fibroblast migration and proliferation between control, CHX, and curcumin at the 24th, 48th, and 72nd h was statistically significant (P < 0.05) [Table 3]. The mean percentage of fibroblast migration and proliferation of curcumin at MIC was higher as compared to CHX (0.1%). The mean percentage of fibroblast migration and proliferation of CHX (0.1%) is found to be lower than that of control. Bonferroni post hoc analysis of the mean percentage of fibroblast migration and proliferation between control, CHX (0.1%), and curcumin (0.003%) shows statistical significance at varying time periods.
Table 3.
The mean percentage of fibroblast migration and proliferation (wound healing) of control, chlorhexidine, and curcumin at varying time periods
| Samples | Mean percentage of fibroblast migration and proliferation (wound healing) |
Intragroup comparison |
Intergroup comparison |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | F | df | Significance | F | df | Significance | |
| Control | 2.70 | 14.42 | 22.13 | 4.747E10 | 1.328 | 0.000* | 8.67E10 | 11.228 | 0.000* |
| CHX | 8.93 | 9.66 | 10.44 | 1.569E10 | 2.551 | ||||
| CUR | 17.6 | 26.58 | 40.92 | 3.166E11 | 2.280 | ||||
*P<0.05 significant. Intragroup comparison (repeated measures ANOVA P<0.05) and intergroup comparison (one-way ANOVA P<0.05) of mean percentage of fibroblast migration and proliferation of control, CHX, and CUR at varying time periods was statistically significant. CHX – Chlorhexidine; CUR – Curcumin; P– Probability value; F – Fstatistic
Figure 4.
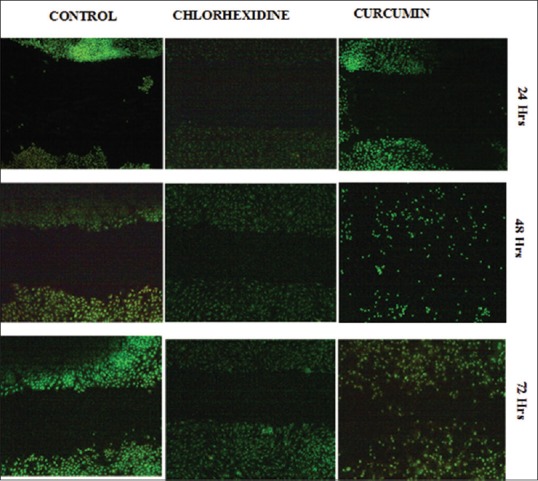
Images of acridine orange stained gingival fibroblasts exposed to CHX and CUR at 24, 48 and72 h of incubation. (×4, Olympus CKX41). The mean percentage of fibroblast migration and proliferation of control 24th, 48th, and 72nd h was 2%, 14%, and 22%, respectively. The mean percentage of fibroblast migration and proliferation of CHX at 24th, 48th, and 72nd h was 8.93%, 9.67%, and 10.44%, respectively. The mean percentage of fibroblast migration and proliferation of CUR at 24th, 48th, and 72nd h was 17.6%, 26.58%, and 40.92%, respectively. (CHX – Chlorhexidine; CUR – Curcumin)
Graph 2.
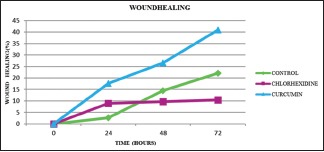
The mean percentage fibroblast migration and proliferation (wound healing) of control, chlorhexidine and curcumin at varying time period. *P < 0.05 significant. Intragroup comparison (repeated measures ANOVA P < 0.05) and intergroup comparison (one-way ANOVA P < 0.05) of mean percentage of fibroblast migration and proliferation of control, CHX, and CUR at varying time periods was statistically significant. CHX – Chlorhexidine; CUR – Curcumin; P – Probability value; F – Fstatistic
DISCUSSION
At present, the most widely used and investigated chemical plaque control agent is CHX digluconate.[5] The most effective commercially available concentration of CHX as an antiplaque agent is 0.12% and 0.2%.[1] Literature gives sufficient evidence regarding the toxic nature of CHX when it is used as an antimicrobial mouthwash adjunct to surgical/nonsurgical periodontal therapy. Recently, many studies have documented the antibacterial, anti-inflammatory, immunomodulatory, and wound healing properties of curcumin.[13] The studies evaluating the effect of curcumin on fibroblast cytotoxicity and wound healing property are scarce in the literature.
The concentrations selected for carrying out cytotoxicity and wound healing studies were based on the antibacterial activity of CHX and curcumin. The MIC50 calculated for CHX was 0.1% and that of curcumin was 0.003% (30 μg/ml). The MIC of CHX exhibited a 33.33-fold increase in concentration as compared to MIC of curcumin for effective bacterial inhibition. In literature, many studies are available to support the antibacterial property of curcumin at the concentration (MIC50) obtained in the present study.[16,18,19]
Curcumin possesses both bacteriostatic and bacteriocidal properties against a wide range of pathogenic microorganisms.[19] The antibacterial property of curcumin is attributed to its chemical structure. Curcumin is a lipophilic polyphenol insert into the lipid bilayer of bacterial cell wall which enhances cell permeability.[20] It inhibits bacterial cell division, biofilm formation, and maturation.[18] Curcumin inhibits Porphyromonas gingivalis proteases (RGP and KGP) in a dose-dependent manner.[16] It possesses a high absorptive affinity to hydroxyapatite and salivary proteases that can be related to its increased local availability in the oral cavity and can be more or less compared to the substantivity of CHX.[18]
Fibroblasts are considered as the main architect-builders of periodontal tissue.[21] The effect of antibacterial mouthwashes on growth, survival, migration, and proliferation of these fibroblasts has to be well evaluated when these agents are used as an adjunct to mechanical periodontal therapy. In this study, a dose-dependent reduction in the mean fibroblast viability was observed after treating with CHX and curcumin. Among the concentrations of CHX and curcumin, the lowest concentration (0.03% and 0.003%, respectively) expressed the highest mean fibroblast viability. The fibroblast viability of curcumin at each concentration was highest compared to all similar concentrations of CHX at varying time intervals. CHX was more cytotoxic to gingival fibroblast at all concentrations that we have studied as compared to curcumin. Cline and Layman in 1992 studied the viability of fibroblasts with increasing concentration of CHX, and they observed a significant reduction of viability of fibroblast in a concentration-dependent manner.[22] CHX even in low concentration was toxic for a variety of human cells including gingival fibroblasts.[8]
Studies regarding fibroblast cytotoxicity of curcumin are scarce in the literature. Liu et al.[23] assayed the effect of curcumin on human fibroblasts and suggested that curcumin did not show any cytotoxic effects. Many comparative studies[10,24,25] were discussed in the literature regarding the fibroblast cytotoxicity of CHX with other chemotherapeutic agents. All of these studies (in vitro) are pointing to the fact that CHX is highly cytotoxic and suggests the cautious use of CHX.
In this study, morphological changes of fibroblasts treated with CHX and curcumin were compared. It was evident that CHX exhibited severe toxic changes in fibroblast morphology in a dose- and time-dependent manner as compared to curcumin. In accordance with these observations, Pucher and Daniel in 1992[9] and Cline and Layman in 1992[22] observed that gingival fibroblast on treatment with 0.2% CHX appeared rounded and retracted from culture dishes.
Recently, clinical trials regarding the effectiveness of curcumin in patients with periodontal disease are emerging in literature. Waghmare et al.[26] in 2011, Arunachalam et al.[27] in 2017, and Chatterjee et al. in 2017[28] compared the antiplaque effect of CHX and curcumin mouthrinse, and they reported that curcumin mouthrinse can be considered as an alternative antiplaque agent to CHX. One of the limitations observed in all these studies was that no standardization of concentrations of curcumin in terms of MIC was undertaken. Even though curcumin is a naturally occurring polyphenol, at high concentrations it is found to be highly cytotoxic and leads to apoptosis of cells a property that embarks its use as an anticancer drug.[29]
Normally periodontal tissue cell turnover involves the generation of new cells by proliferation and migration. Fibroblast contributes to the periodontal tissue homeostasis by their ability to remodel tissues and to repopulate wounds. For successful periodontal therapy and predictable regeneration, the presence of viable and healthy cells of the periodontium is essential and these cells should adhere to the root surfaces of the teeth. The antimicrobial mouthwash, when used as an adjunct to mechanical therapy or as a subgingival irrigant, should not produce a deleterious effect on the fibroblasts.
In the scratch wound assay, it was observed that the wound healing property of curcumin was higher as compared to CHX and control. Several investigators have reported a delay in wound healing after exposure to varying concentrations of CHX.[9,30] The delay in wound healing may be either due to the death of fibroblast which impairs its migration and proliferation or due to a marked reduction in protein synthesis.[30] Conflicting reports are also available in the literature regarding wound healing property of CHX. Egelberg et al.[31] suggested that topically applied CHX did not interfere with gingival wound healing in dogs. The varying results that appeared in the literature regarding the effect of CHX on wound healing may be due to diverse study models employed. Hence, the true mechanism underlying the effect of CHX in contact with surgical wounds must be further evaluated both at the molecular and biological levels before reaching a valid conclusion.
The wound healing property of curcumin can be best explained by its antibacterial, anti-inflammatory, and immunomodulating properties. Curcumin is proven to be a potent antimicrobial agent that is effective against a range of organisms. Curcumin suppresses the activation of the transcription factor NF-κB,[11] downregulates the expression of LPS-induced of COX-2, inhibits an array of cytokines,[14] reduces the expression of various vascular endothelial cell surface adhesins, and decreases the formation of prostaglandin E2,[32] which is a potent stimulator of bone resorption and a key player in periodontal disease. Curcumin reduces LPS-induced interferon regulatory factor 3 activation and TLR4 signaling by arresting both myeloid differentiation factor 88 (MyD88) and the transient receptor(TR) domain-containing adaptor-inducing interferon-β-dependent pathways.[23] Curcumin is also considered as a very potent antioxidant.[33] It is a free radical scavenger and hydrogen donor and exhibits both pro- and antioxidant activity.[34] Curcumin-mediated fibroblast migration may be induced by Dkk-1-associated regulation of Wnt signaling.[23] Curcumin is a potent inhibitor of matrix metalloproteinases and an array of cytokines, which reduces the destructive inflammatory process during wound healing enabling more and more cells to migrate and proliferate. It is also possible that curcumin may induce healing by regulating gene expression at posttranscriptional level and is found to be important for cell migration and proliferation.[32]
The design of the present study is in vitro research, and the evidence provided may not be strong. The study was carried out on gingival fibroblast cultured from dental pulp stem cells, and further studies can be carried out using both gingival and periodontal ligament fibroblasts.
CONCLUSIONS
Successful periodontal therapy has been centered on reducing the microbial load by mechanical/antimicrobial therapy, and modulating the host response. Curcumin has a wide biological spectrum that could provide an alternative anti-inflammatory, antimicrobial, and immunomodulatory agent for managing chronic periodontitis which is an immunoinflammatory disease. Within the limitations of the present study, it can be concluded that 0.003% of curcumin exhibits the least fibroblast cytotoxicity, effective inhibition of bacteria, and proper wound healing. Curcumin may offer as a more cost-effective, less cytotoxic, easily available antiplaque agent.
Financial support and sponsorship
This study was supported by the Indian Council of Medical Research, New Delhi, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We are grateful to Dr. Rajesh. Ramachandran, Director, Biogenix Research Centre, Poojappura, Thiruvananthapuram, Dr. John Zacharia, Indian Institute of Spices Research, Kozhikode, and Dr. Biju. George, Associate Professor, Government Medical College, Kozhikode (support for data management) for their support and encouragement; to conduct this study, we acknowledge the financial assistance provided by the Indian Council of Medical Research for carrying out this dissertation.
REFERENCES
- 1.Taubman MA, Valverde P, Han X, Kawai T. Immune response: The key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–41. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 2.Hans M, Hans VM. Toll-like receptors and their dual role in periodontitis: A review. J Oral Sci. 2011;53:263–71. doi: 10.2334/josnusd.53.263. [DOI] [PubMed] [Google Scholar]
- 3.Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontol 2000. 2011;55:36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philip G, Dayakar MM, Divater V, Prasad S. Emerging concepts in oral chemical plaque control – An overview. Int J Dent Clin. 2012;4:49–51. [Google Scholar]
- 5.Walsh TF, Glenwright HD, Hull PS. Clinical effects of pulsed oral irrigation with 0.2% chlorhexidine digluconate in patients with adult periodontitis. J Clin Periodontol. 1992;19:245–8. doi: 10.1111/j.1600-051x.1992.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 6.Jones CG. Chlorhexidine: Is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 7.Westfelt E. Rationale of mechanical plaque control. J Clin Periodontol. 1996;23:263–7. doi: 10.1111/j.1600-051x.1996.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldschmidt P, Cogen R, Taubman S. Cytopathologic effects of chlorhexidine on human cells. J Periodontol. 1977;48:212–5. doi: 10.1902/jop.1977.48.4.212. [DOI] [PubMed] [Google Scholar]
- 9.Pucher JJ, Daniel JC. The effects of chlorhexidine digluconate on human fibroblasts in vitro. J Periodontol. 1992;63:526–32. doi: 10.1902/jop.1992.63.6.526. [DOI] [PubMed] [Google Scholar]
- 10.Tsourounakis I, Palaiologou-Gallis AA, Stoute D, Maney P, Lallier TE. Effect of essential oil and chlorhexidine mouthwashes on gingival fibroblast survival and migration. J Periodontol. 2013;84:1211–20. doi: 10.1902/jop.2012.120312. [DOI] [PubMed] [Google Scholar]
- 11.Nagpal M, Sood S. Role of curcumin in systemic and oral health: An overview. J Nat Sci Biol Med. 2013;4:3–7. doi: 10.4103/0976-9668.107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 13.Esatbeyoglu T, Huebbe P, Insa MA, DawnChin E, Wagner AE, Rimbach G. Curcumin-from molecule to biological function. Chem Int Ed. 2012;51:5308–32. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 14.Guimarães MR, Leite FR, Spolidorio LC, Kirkwood KL, Rossa C., Jr Curcumin abrogates LPS-induced pro-inflammatory cytokines in RAW 264.7 macrophages. Evidence for novel mechanisms involving SOCS-1, -3 and p38 MAPK. Arch Oral Biol. 2013;58:1309–17. doi: 10.1016/j.archoralbio.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–73. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 16.Izui S, Sekine S, Maeda K, Kuboniwa M, Takada A, Amano A, et al. Antibacterial activity of curcumin against periodontopathic bacteria. J Periodontol. 2016;87:83–90. doi: 10.1902/jop.2015.150260. [DOI] [PubMed] [Google Scholar]
- 17.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(Suppl 1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 18.Hu P, Huang P, Chen MW. Curcumin reduces Streptococcus mutans biofilm formation by inhibiting sortase A activity. Arch Oral Biol. 2013;58:1343–8. doi: 10.1016/j.archoralbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Shahzad M, Millhouse E, Culshaw S, Edwards CA, Ramage G, Combet E. Selected dietary (poly) phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015;6:719–29. doi: 10.1039/c4fo01087f. [DOI] [PubMed] [Google Scholar]
- 20.Dani S, Prabhu A, Chaitra KR, Desai NC, Patil SR, Rajeev R. Assessment of Streptococcus mutans in healthy versus gingivitis and chronic periodontitis: A clinico-microbiological study. Contemp Clin Dent. 2016;7:529–34. doi: 10.4103/0976-237X.194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wikesjö UM, Selvig KA. Periodontal wound healing and regeneration. Periodontol 2000. 1999;19:21–39. doi: 10.1111/j.1600-0757.1999.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 22.Cline NV, Layman DL. The effects of chlorhexidine on the attachment and growth of cultured human periodontal cells. J Periodontol. 1992;63:598–602. doi: 10.1902/jop.1992.63.7.598. [DOI] [PubMed] [Google Scholar]
- 23.Dai X, Liu J, Zheng H, Wichmann J, Hopfner U, Sudhop S, et al. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater. 2017;9:368. [Google Scholar]
- 24.Chang YC, Huang FM, Tai KW, Chou MY. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:446–50. doi: 10.1067/moe.2001.116812. [DOI] [PubMed] [Google Scholar]
- 25.Aka B, Özeroglub E, Taspinar M. The use of methylene blue as mouthwash in periodontology. East J Med. 2015;20:215–21. [Google Scholar]
- 26.Waghmare PF, Chaudhari AU, Karhadkar VM, Jamkhande AS. Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: A clinical and microbiological study. J Contemp Dent Pract. 2011;12:221–4. doi: 10.5005/jp-journals-10024-1038. [DOI] [PubMed] [Google Scholar]
- 27.Arunachalam LT, Sudhakar U, Vasanth J, Khumukchum S, Selvam VV. Comparison of anti-plaque and anti-gingivitis effect of curcumin and chlorhexidine mouth rinse in thetreatment of gingivitis: A clinical and biochemical study. J Indian Soc PeriodontoL. 2017;21:478–83. doi: 10.4103/jisp.jisp_116_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee A, Debnath K, Rao NKH. A comparative evaluation of the efficacy of curcumin and chlorhexidine mouthrinses on clinical inflammatory parameters of gingivitis: A double-blinded randomized controlled clinical study. J Indian Soc Periodontol. 2017;21:132–7. doi: 10.4103/jisp.jisp_136_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen AN, Veena MS, Srivatsan ES, Wang MB. Suppression of interleukin 6 and 8 production in head and neck cancer cells with curcumin via inhibition of Ikappa beta kinase. Arch Otolaryngol Head Neck Surg. 2009;135:190–7. doi: 10.1001/archotol.135.2.190. [DOI] [PubMed] [Google Scholar]
- 30.Bassetti C, Kallenberger A. Influence of chlorhexidine rinsing on the healing of oral mucosa and osseous lesions. J Clin Periodontol. 1980;7:443–56. doi: 10.1111/j.1600-051x.1980.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 31.Hirst RC, Egelberg J, Hornbuckle GC, Oliver RC, Rathbun WE. Microscopic evaluation of topically applied chlorhexidine gluconate on gingival wound healing in dogs. J South Calif Dent Assoc. 1973;41:311–7. [PubMed] [Google Scholar]
- 32.Mantovani A, Bussolino F, Introna M. Cytokine regulation of endothelial cell function: From molecular level to the bedside. Immunol Today. 1997;18:231–40. doi: 10.1016/s0167-5699(97)81662-3. [DOI] [PubMed] [Google Scholar]
- 33.Bhaskar Rao A, Prasad E, Deepthi SS, Haritha V, Ramakrishna S, Madhusudan K, et al. Wound healing: A new perspective on glucosylated tetrahydrocurcumin. Drug Des Devel Ther. 2015;9:3579–88. doi: 10.2147/DDDT.S85041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sreejayan, Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–7. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]


