Abstract
Purpose:
To evaluate risk and response based multi-agent therapy for patients with rhabdomyosarcoma (RMS) at first relapse.
Patients and Methods:
Patients with RMS and measurable disease at first relapse with unfavorable risk (UR) features were randomized to a six-week Phase 2 window with one of two treatment schedules of irinotecan with vincristine (VI) (previously reported). Those with at least a partial response to VI continued to receive 44 weeks of multi-agent chemotherapy including the assigned VI regimen. UR patients without measurable disease at study entry, without radiographic response after the VI window, or who declined VI window therapy received 31 weeks of multi-agent chemotherapy including tirapazamine (TPZ) at weeks 1, 4, 10, 19 and 28. Favorable risk (FR) patients received 31 weeks of the same multi-agent chemotherapy without VI and TPZ.
Results:
One hundred thirty-six eligible patients were enrolled. For 61 patients not responding to VI, the 3-year failure free survival (FFS) and overall survival (OS) were 17% (95% Confidence Interval 8%, 29%) and 24% (13%, 37%), respectively. For 30 UR patients not treated with VI, the 3-year FFS and OS were 21% (8%, 37%) and 39% (20%, 57%), respectively. FR patients had a 3-year FFS and OS of 79% (47%, 93%) and 84% (50%, 96%), respectively. There were no unexpected toxicities.
Conclusion:
Patients with UR rhabdomyosarcoma at first relapse or disease progression have a poor prognosis when treated with this multi-agent therapy while those with FR have a higher chance of cure with second-line therapy.
Keywords: Rhabdomyosarcoma, relapse, refractory, recurrent, treatment
PRECIS:
This clinical trial evaluated risk and response based multi-agent therapy for patients with rhabdomyosarcoma at first relapse. We found that patients with unfavorable risk rhabdomyosarcoma at first relapse or disease progression have a poor prognosis when treated with this therapy while those with favorable risk have a higher chance of cure with second-line therapy.
INTRODUCTION
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma of childhood and adolescence.1 Multimodality therapy with surgery, radiation therapy and cytotoxic chemotherapy has resulted in approximately 70% of patients achieving long term survival.2 However, patients who are refractory to primary therapy or those who relapse following primary therapy have a poor prognosis.3–5 This is true especially for patients with Stage 2–4, Clinical Group II-IV embryonal RMS (ERMS); those with Stage 1 or Clinical Group I ERMS that were initially treated with vincristine, dactinomycin and cyclophosphamide (VAC); and alveolar RMS (ARMS).3 In aggregate, these unfavorable risk (UR) patients have a 5-year post relapse survival rate of approximately 10%. In contrast, a relatively more favorable risk (FR) group is comprised of those with botryoid histology or Stage 1 or Clinical Group I ERMS treated at diagnosis with vincristine and dactinomycin (VA) where survival is approximately 50%3.
The Soft Tissue Sarcoma (STS) Committee of the Children’s Oncology Group (COG) chose to evaluate the activity of two schedules of irinotecan together with vincristine (VI) in a 6-week phase 2 window in those patients with UR and measurable disease at the time of first relapse followed by multi-agent therapy incorporating VI for patients who achieved at least a partial response at six weeks. We have previously reported the rationale, early response rates for the two VI schedules and outcome for UR patients treated with the VI window.6 The study enrolled two additional categories of patients. The first category included UR patients without measurable disease at study entry, those who declined VI phase 2 window therapy and those that did not achieve a radiographic response following 6 weeks of VI therapy. These patients received the same multi-agent therapy without VI but with the addition of the tirapazamine (TPZ), a hypoxic cell toxin. The second category included FR patients and received multi-agent therapy without VI or TPZ. Here, we report toxicity and outcome for these two categories of patients.
TPZ, a benzotriazine di-N-oxide anti-cancer drug that is activated to a toxic free radical under hypoxic conditions, was the first drug of this class to enter clinical testing.7 In pre-clinical models, additive or greater than additive cytotoxicity was noted when TPZ was combined with cytotoxic chemotherapy presumably by selective killing of hypoxic tumor cells.8, 9 A phase 1 clinical trial in adults defined the maximum tolerated dose (MTD) of TPZ as 330 mg/m2 when administered intravenously once every three weeks as a single agent.10 Reversible deafness and tinnitus were dose limiting toxicities on this trial. In adult studies, TPZ has been safely administered together with cytotoxic chemotherapy11 though patients experienced significantly more nausea and vomiting.12 There were no differences in hematopoietic toxicity when TPZ was investigated in a randomized phase 3 clinical trial with cisplatin in adult patients with small non cell lung cancer.11 A pediatric phase 1 trial of the TPZ/cyclophosphamide combination demonstrated a MTD of 325 mg/m2 for TPZ when combined with 1.5 g/m2 of cyclophosphamide intravenously.13 The dose limiting toxicity (DLT) was reversible ototoxicity. There were three responses including one in a patient with RMS.
The majority of newly diagnosed RMS patients in North America are treated with VAC chemotherapy. Phase 2 clinical trials with doxorubicin, ifosfamide and etoposide in RMS14 formed the basis for risk-based multi-agent treatment regimens for RMS patients with first relapse or disease progression investigated on this clinical trial.
PATIENTS AND METHODS
Patients eligible for COG ARST0121 had biopsy proven RMS, undifferentiated sarcoma or ectomesenchymoma, and were <21 years of age at the time of initial diagnosis; experienced first relapse or disease progression; had an ECOG performance status of 0, 1, or 2 and a life expectancy of at least 2 months. Adequate organ function was required including a hemoglobin ≥ 10 g/dL (transfusion allowed); absolute neutrophil count ≥ 750/mm3; platelet count ≥ 75,000/mm3; serum creatinine < /= 1.5 X normal for age or creatinine clearance/radioisotope glomerular filtration rate ≥70 ml/min/1.73 m2; serum bilirubin </= 1.5 X normal for age; serum alanine transaminase < /= 2.5 X normal for age and left ventricular shortening fraction of ≥ 27% by echocardiogram or ejection fraction of ≥ 50% by gated radionuclide scan. Any prior central nervous system toxicity had to be < grade 2 and adequate control of any seizure disorder with anticonvulsants was required. Patients who had received >1 prior chemotherapy treatment regimen and those with prior exposure to anthracyclines, ischemic heart disease, myeloablative chemotherapy followed by hematopoietic stem cell rescue, disease impinging on or within the brain and spinal cord and those who were pregnant/lactating were excluded. Written informed consent was required from all subjects and/or their parents/legal guardians after institutional, Food and Drug Administration, and National Cancer Institute requirements for human studies were met.
Clinical trial design
The experimental design of the ARST0121 trial is shown in Figure 1. Consenting patients with UR features (Stage 2–4, Clinical Group II-IV ERMS at initial diagnosis; Stage 1 or Clinical Group I ERMS at initial diagnosis with distant recurrence following VA or recurrence following VAC; and ARMS at initial diagnosis); measurable disease (defined as ≥ 1 lesion with a diameter ≥10 mm) at study entry, and no prior exposure to irinotecan were randomized to one of two VI treatment schedules followed by multi-agent chemotherapy that incorporated the assigned VI schedule for responding patients (Table 1- Regimen 1A and 1B). Patients who did not respond to the phase 2 VI window were switched to multi-agent chemotherapy without VI but with TPZ at week 7 (Table 1-Regimen 2). Patients with UR features at the time of first relapse or disease progression without measurable disease at study entry, those that had previous exposure to irinotecan and those that declined randomization to the VI phase 2 window also received multi-agent chemotherapy, without VI but with TPZ (Regimen 2). TPZ was supplied by the NCI (IND# 46525). Patients with FR features (botryoid histology at initial diagnosis or Stage 1 or Clinical Group I ERMS at initial diagnosis, not treated with cyclophosphamide, and who recurred either locally or regionally) at the time of first relapse or disease progression received multi-agent chemotherapy without VI or TPZ starting at week 1(Table 1-Regimen 3). Disease response was assessed using Response Evaluation Criteria in Solid Tumors (RECIST)15 of the NCI at week 6 for patients on Regimens 1 and 2. Patients on Regimen 1 who did not respond to the VI phase 2 window at week 6 had a second disease response assessment at week 12 following two cycles of TPZ administered together with doxorubicin and cyclophosphamide.
Figure 1:
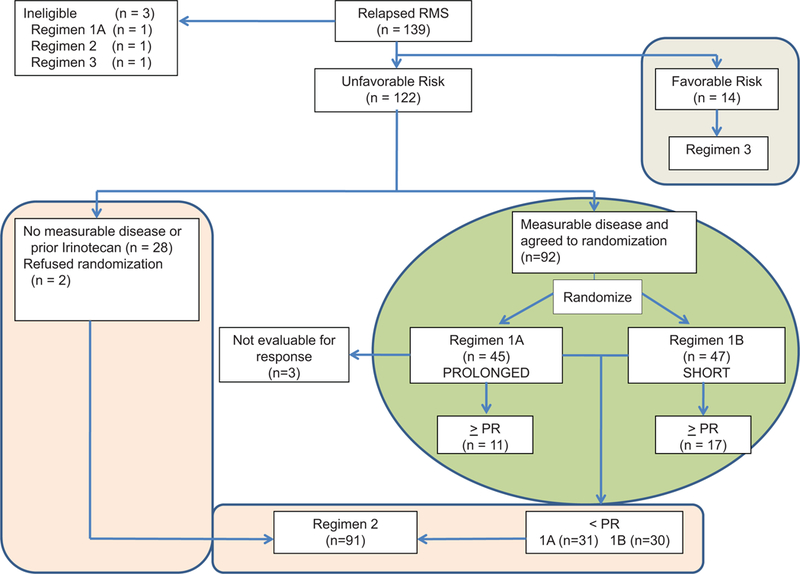
CONSORT diagram for COG ARST0121.
Table 1:
Treatment regimens for patients
| Chemotherapy | Regimen 1A | Regimen 1B | Regimen 2 | Regimen 3 |
|---|---|---|---|---|
| Irinotecan (IV) | 20mg/m2 × 5 days Weeks 1, 2, 4, 5, 13, 14, 25, 26, 34, 35, 46, 47, 49, 50 | 50mg/m2 x 5 daysWeeks 1, 4, 13, 25, 34, 46, 49 | None | None |
| Vincristine (IV)(1.5mg/m2)† | Weeks 1, 2, 4, 5, 13, 14, 25, 26, 34, 35, 46, 47, 49, 50 | Identical to 1A | None | None |
| Doxorubicin (IV)(75mg/m2) | Weeks 7, 16, 28, 37, 40 | Identical to 1A | Weeks 1, 4, 10, 19, 28 | Identical to 2 |
| Cyclophosphamide (IV)(1.2gm/m2) | Weeks 7, 16, 28, 37, 40 | Identical to 1A | Weeks 1, 4, 10, 19, 28 | Identical to 2 |
| Etoposide (IV)(100mg/m2/day x5) | Weeks 10, 19, 22, 31, 43 | Identical to 1A | Weeks 7, 13, 16, 22, 25, 31 | Identical to 2 |
| Ifosfamide (IV)(1.8gm/m2/day x 5) | Weeks 10, 19, 22, 31, 43 | Identical to 1A | Weeks 7, 13, 16, 22, 25, 31 | Identical to 2 |
| Tirapazamine (IV)(330 mg/m2) | None | None | Weeks 1, 4, 10, 19, 28 | None |
Maximum dose 2mg
Surgical resection of disease at previously irradiated sites was strongly encouraged if feasible. Radiation therapy was administered (50.4 Gy for macroscopic disease, 41.4 Gy for microscopic disease) at sites of disease that were not previously irradiated at the discretion of the treating physician either during weeks 16–22 on Regimen 1A and 1B or weeks 10–18 on Regimens 2 and 3. Intraoperative radiation therapy or brachytherapy was recommended following surgical resection for patients with disease at sites that were previously irradiated.
Statistical analyses
The analyses comparing the response rate, toxicities, failure free survival (FFS) and overall survival (OS) of patients treated on regimens 1A and 1B have been previously reported6. The primary endpoint for patients on Regimen 2 was toxicity. Toxicities were reported using NCI Common Terminology Criteria v. 2.0. Based on known toxicities of TPZ, there was monitoring for ototoxicity, cardiotoxicity, stomatitis, hematopoietic toxicity and grade 3 vomiting with stopping rules. DLT was defined as Grade 4 infection, ≥ 40 db hearing loss at 2000 MHz lasting more than 5 days, ≥ Grade 3 vomiting, ≥ Grade 3 cardiac toxicity, and ≥ Grade 3 stomatitis. An ototoxicity rate of 15%, Grade 3+ cardiac toxicity rate 10%, Grade 3+ stomatitis rate of 35% and an incidence of Grade 4 infection or Grade 3 vomiting 25% were set as the acceptable upper bounds for these toxicities above which enrollment would be suspended and modification of the treatment protocol considered. Response rate and OS were the secondary endpoints for patients treated with Regimen 2. Complete response (CR) was defined as disappearance of all target lesions by cross sectional computed tomography or magnetic resonance imaging, partial response (PR) as a > 30% decrease in the dimension used to define the target lesion, progressive disease (PD) as a > 20% increase in the measurement used to define the target lesion and stable disease (SD) as insufficient tumor shrinkage or increase to be considered PR or PD. The estimation of FFS and OS were performed using the Kaplan-Meier method. FFS was defined as the time of enrollment to disease progression or death.16 Confidence interval (CI) for FFS and OS were estimated by the Peto-Peto method.17 OS was defined as the time from enrollment to death from any cause. The primary end-point for Regimen 3 was FFS.
RESULTS
Patient characteristics
COG ARST0121 enrolled 139 patients between June 2002 and October 2006 (Figure 1) and three were deemed ineligible (one each with enrollment error, lack of informed consent and initiation of therapy prior to enrollment). Fourteen patients with FR features were treated on Regimen 3 and the remaining 122 patients were either randomized to one of the two VI schedules (n=92) or assigned to Regimen 2 (n=30, including two patients with measurable disease that declined randomization to the phase 2 VI window). Patient characteristics of those treated on Regimen 1A and 1B have been previously reported6. Characteristics of patients treated on Regimens 2 or 3 are shown in Table 2.
Table 2:
Patient characteristics†
| Patient Characteristics | Regimen 2 (n = 30) |
Regimen 3 (n = 14) |
|---|---|---|
| Age | ||
| < 10 Years | 14 | 8 |
| ≥10 years | 16 | 6 |
| Gender | ||
| Male | 20 | 6 |
| Female | 10 | 8 |
| Histology | ||
| Alveolar | 19 | 0 |
| Embryonal | 6 | 10 |
| Other | 5 | 4 |
| Primary Site‡ | ||
| (at original diagnosis) | ||
| Favorable | 1 | 14 |
| Unfavorable | 29 | 0 |
| Recurrence§ | ||
| Local | 10 | 9 |
| Regional Lymph nodes | 7 | 5 |
| Bone Marrow / Bone | 7 | 0 |
| Other distant sites | 18 | 0 |
Regimen 1 previously reported
Favorable sites- Orbit, Non parameningeal head and neck, Non bladder/ prostate genitourinary and biliary; Unfavorable sites- all others
Numbers indicate distribution of sites of recurrence. Patients may have had
more than one site of recurrence
Toxicity
Toxicity was evaluated for the first six weeks of therapy on regimens 1A and 1B and has been reported previously6. Ninety-one patients were treated with Regimen 2 (including patients not responding to VI phase 2 window) and received TPZ combined with doxorubicin and cyclophosphamide. Grade 3 or greater toxicities experienced during the first six weeks (2 cycles), included vomiting (26.8%), stomatitis (12%), myalgia (8.5%), infection (5%), heart failure (6%) and ototoxicity (2.4%). There was one death due to congestive heart failure that occurred immediately following the first dose of TPZ administered to a patient who was refractory to Regimen 1A. All other toxicities were expected from the known toxicity profile of TPZ and were ≤ the a priori determined boundaries requiring TPZ dose de-escalation or protocol therapy modifications.
Tumor Response
Response rates at week 6 in 89 evaluable patients following treatment on Regimen 1A and 1B have been previously reported and were 26% and 36% respectively (combined response rate of 31.5%)6. Twenty-four of 30 patients assigned to Regimen 2 were evaluable for response. There were 6 CR and 7 PR following six weeks of therapy with TPZ, doxorubicin and cyclophosphamide for an overall objective response rate (CR+PR) of 54%. Sixty-one patients who did not respond to the phase 2 VI window were treated with Regimen 2. Forty-nine of these patients were evaluable for response, with a response rate of 22% following 6 weeks of TPZ, doxorubicin and cyclophosphamide. No patient in this group achieved a CR.
Survival Outcomes
The 3-year FFS rates for all patients are summarized in Table 3 by regimen. The overall outcomes for patients treated on the VI window have been reported previously.6 Patients randomized to regimen 1A had a 3-year FFS and OS of 14% (95% CI 5%, 27%) and 34% (95% CI 20%, 49%) respectively while those randomized to regimen 1B had a 3- year FFS of 15% (95% CI 7%, 26%) and OS of 22% (95% CI 11%, 35%). Patients ineligible for the phase 2 VI window had a 3-year FFS and OS of 21% (95% CI 8%, 37%) and 39% (95% CI 20%, 57%) respectively, following treatment on Regimen 2 (Figure 2). The 3-year FFS and OS for patients that did not respond to VI were 17% (95% CI 8%, 29%) and 24% (95% CI 13%, 37%) respectively. Figure 3 displays the 3-year FFS and OS for the 14 FR patients treated on Regimen 3. The 3-year FFS and OS for FR patients were 79% (95% CI 47%, 93%) and 84% (95% CI 50%, 96%), respectively.
Table 3:
Three year post-relapse/progression outcome according to regimen
| Regimen | Failure Free Survival (95% Confidence Interval) |
Overall Survival (95% Confidence Interval) |
|---|---|---|
| 1A | 14% (5%, 27%) | 34% (20%, 49%) |
| 1B | 15% (7%, 26%) | 22% (11%, 35%) |
| 2† | 17% (8%, 29%) | 24% (13%, 37%) |
| 2‡ | 21% (8%, 37%) | 39% (20%, 57%) |
| 3 | 79% (47%, 93%) | 84% (50%, 96%) |
Unfavorable risk patients with no radiographic response on phase 2 window
Unfavorable risk patients ineligible for phase 2 window
Figure 2:
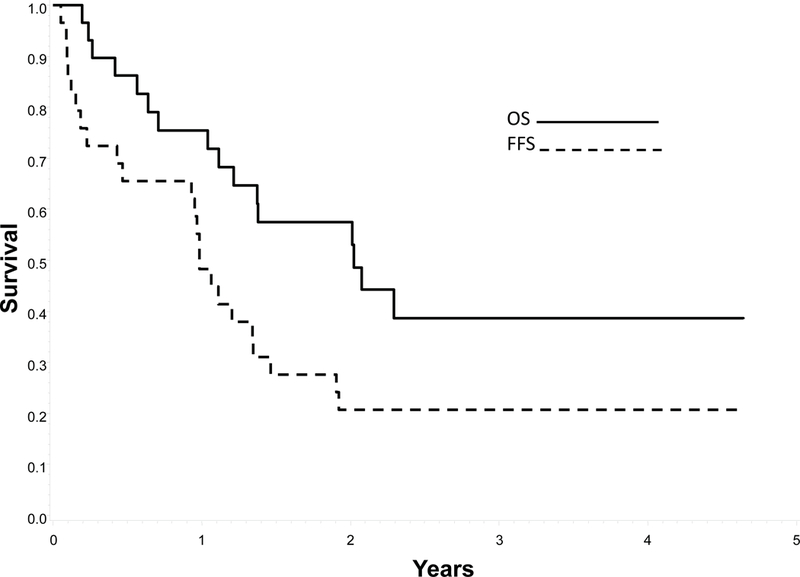
Probability of Failure Free Survival (FFS) and Overall Survival (OS) for patients with unfavorable features not responding to Vincristine/Irinotecan
Figure 3:
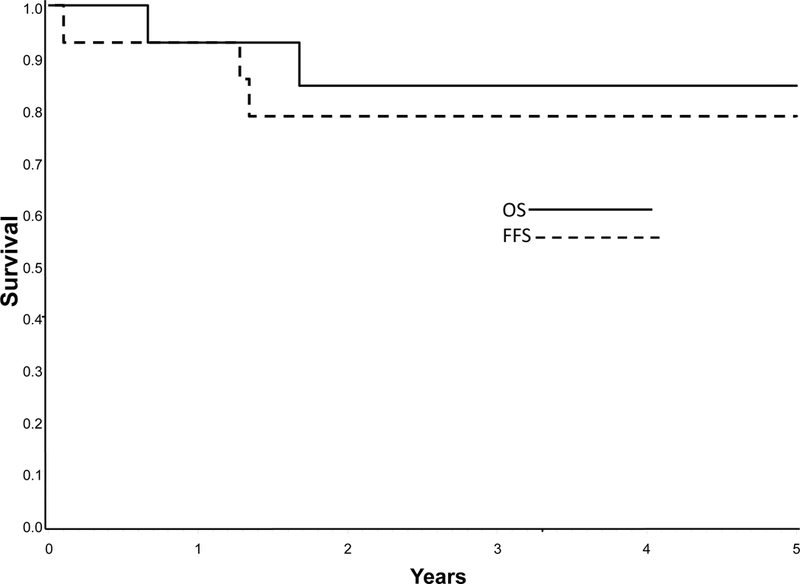
Probability of Failure Free Survival (FFS) and Overall Survival (OS) for patients with favorable features treated on Regimen 3.
DISCUSSION
There is no defined standard therapy for patients with relapsed or progressive RMS. In general, these patients are treated with intensive multi-agent chemotherapy with or without surgery and/or radiation therapy. Other therapeutic options include enrollment on phase 1 or phase 2 clinical trials with new agents. Prior to ARST0121, no clinical trial had prospectively investigated protocol defined multi-agent chemotherapy exclusively for patients with RMS at first relapse or disease progression. In addition to comparing prolonged and shorter VI schedules in a phase 2 window, ARST0121 investigated risk-based therapy for patients with first relapse RMS based on previously defined UR or FR features. ARST0121 represents the largest clinical trial conducted in relapsed/refractory RMS. This clinical trial confirms the poor outcome for first relapse RMS patients with UR features. In addition, it confirms the favorable outcome among the minority of patients with lower Stage/Clinical Group disease and ERMS or botryoid RMS when treated with multi-agent therapy.
The toxicity on this trial was as expected and similar to other regimens used for the treatment of pediatric solid tumors at diagnosis or after relapse18–20. The response rate of 31.5% to VI was modest,6 particularly compared to the 70% response rate seen in an upfront phase 2 window trial in previously untreated patients with metastatic RMS.21 Following investigation of the VI phase 2 window in the first relapse setting for RMS, the combination was investigated with alternating cycles of VAC chemotherapy in newly diagnosed patients with intermediate risk RMS by COG (ARST0531) but failed to improved outcomes.22 The objective response rate on this trial to TPZ, doxorubicin and cyclophosphamide ranged from 22–54%. The lower response rate of 22% in the group of patients who did not respond to VI suggests that those patients had inherently more resistant disease at the time of first relapse compared with those treated with Regimen 2 immediately at the time of relapse. Treatment including TPZ was tolerable but did not improve FFS or OS over expected. We had hoped to demonstrate safety and evidence of synergistic activity for TPZ combined with doxorubicin and cyclophosphamide in relapsed RMS potentially supporting further investigation of TPZ in newly diagnosed patients with metastatic RMS or with radiation therapy in advanced disease. This study does not support further investigation of TPZ in RMS and is consistent with results of two phase 3 trials in adults with carcinomas, in which TPZ failed to improve outcomes.23, 24 Patients treated on Regimen 2 at initial relapse cannot be compared with patients treated on Regimen 2 following no response to the VI window since the latter group is presumed to be an inherently more resistant and thus a different population.
Local control with radiation therapy and surgery can be challenging in the management of patients with relapsed RMS particularly if there is local tumor progression at previously irradiated sites. These data were not collected on ARST0121 and therefore its impact on outcome could not be analyzed.
Patients with botryoid RMS with first relapse and those with either Stage 1 or Clinical group I ERMS with local/regional recurrence and not treated with cyclophosphamide at original diagnosis had favorable outcomes when treated with multi-agent therapy. Such patients should therefore generally not be considered candidates for investigational therapy with single agent phase 2 or phase 1 clinical trials at the time of first relapse. The recent COG low-risk RMS trial ARST0331 showed excellent outcomes with shorter duration (22 week) therapy with a modest cumulative cyclophosphamide dose (4.8 g/m2), suggesting all low-risk RMS patients might receive some cyclophosphamide in the future to allow shorter therapy.25 It is unknown if the same excellent outcomes seen with Regimen 3 in patients without prior cyclophosphamide exposure will be seen in patients who relapse after modest cyclophosphamide treatment but otherwise FR features.
Importantly, this clinical trial showed prospectively a median FFS of 50% at six months in RMS patients with UR features at first relapse/progression. This establishes a survival benchmark that can be used in future investigation in RMS patients with UR features at first relapse or progression. Given our increased understanding of the molecular basis of cancer and the availability of targeted therapy, COG investigated the combination of molecularly targeted therapy and a cytotoxic chemotherapy backbone in a randomized phase 2 clinical trial in the UR group of RMS patients at the time of first relapse or disease progression. The mammalian target of rapamycin inhibitor temsirolimus improved progression free survival when compared to the vascular endothelial growth factor inhibitor bevacizumab (Mascarenhas L, ASCO 2014). In order to improve survival in RMS, novel strategies are required either to improve the outcome for patients with recurrent disease or to decrease the relapse rate in newly diagnosed patients. Optimizing treatment to improve FFS for newly diagnosed patients with RMS is thus of paramount importance given the poor survival outcome following relapse and the increased burden of therapy to cure those who survive. Genomic studies may provide further insight to inform further investigation in relapsed RMS and other strategies such as immune check point blockade and epigenetic modification provide further avenues to explore.26–28 This clinical trial prospectively confirms risk features for RMS at the time of first relapse/progression and provides a unique model for clinical investigation in patients with relapsed RMS.
Acknowledgments
Supported by:
National Cancer Institute, Bethesda, MD – Grant Numbers U10CA180886, U10CA180899, U10CA98543, and U10CA98413.
Footnotes
Conflict of Interest Statement:
LM: grants to institution from AstraZeneca Pharmaceuticals LP, grants, non-financial support and other from Eli Lilly and Company, personal fees from Bayer Pharma AG, grants to institution from Pfizer Inc., grants to institution from Novartis Pharmaceuticals Corporation, grants to institution from Merck Sharpe and Dome Corporation, grants to institution from E.R. Squibb & Sons L.L.C., outside the submitted work;
ERL: none
PPB: employment, stock ownership, travel, personal fees, accommodation and expenses from Quintiles; Board member and stock ownership- Champions Oncology; personal fees from Grid Therapeutics.
DOW: none
SSD: none
DAR: travel, accommodation and expenses from Navidea
DMP: none
JRA: employment, stock from Merck, consulting for Merck, Amgen and SFS Pharma
WHM: none
DSH: travel, accommodation and expenses from Loxo Oncology, Bristol Meyers Squibb, Celgene and Bayer; grants to institution from Loxo Oncology, Eisai, Merck Sharp and Dome, Novartis, Glaxo Smith Kline and Sanofi.
REFERENCES
- 1.Li J, Thompson TD, Miller JW, Pollack LA, Stewart SL. Cancer incidence among children and adolescents in the United States, 2001–2003. Pediatrics. 2008;121: e1470–1477. [DOI] [PubMed] [Google Scholar]
- 2.Malempati S, Hawkins DS. Rhabdomyosarcoma: review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer. 2012;59: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappo AS, Anderson JR, Crist WM, et al. Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 1999;17: 3487–3493. [DOI] [PubMed] [Google Scholar]
- 4.Raney RB, Crist WM, Maurer HM, Foulkes MA. Prognosis of children with soft tissue sarcoma who relapse after achieving a complete response. A report from the Intergroup Rhabdomyosarcoma Study I. Cancer. 1983;52: 44–50. [DOI] [PubMed] [Google Scholar]
- 5.Chisholm JC, Marandet J, Rey A, et al. Prognostic Factors After Relapse in Nonmetastatic Rhabdomyosarcoma: A Nomogram to Better Define Patients Who Can Be Salvaged With Further Therapy. Journal of Clinical Oncology. 2011;29: 1319–1325. [DOI] [PubMed] [Google Scholar]
- 6.Mascarenhas L, Lyden ER, Breitfeld PP, et al. Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2010;28: 4658–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Research. 1998;58: 1408–1416. [PubMed] [Google Scholar]
- 8.Dorie MJ, Brown JM. Tumor-specific, schedule-dependent interaction between tirapazamine (SR 4233) and cisplatin. Cancer Research. 1993;53: 4633–4636. [PubMed] [Google Scholar]
- 9.Langmuir VK, Rooker JA, Osen M, Mendonca HL, Laderoute KR. Synergistic interaction between tirapazamine and cyclophosphamide in human breast cancer xenografts. Cancer Research. 1994;54: 2845–2847. [PubMed] [Google Scholar]
- 10.Senan S, Rampling R, Graham MA, et al. Phase I and pharmacokinetic study of tirapazamine (SR 4233) administered every three weeks. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 1997;3: 31–38. [PubMed] [Google Scholar]
- 11.von Pawel J, von Roemeling R, Gatzemeier U, et al. Tirapazamine plus cisplatin versus cisplatin in advanced non-small-cell lung cancer: A report of the international CATAPULT I study group. Cisplatin and Tirapazamine in Subjects with Advanced Previously Untreated Non-Small-Cell Lung Tumors. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2000;18: 1351–1359. [DOI] [PubMed] [Google Scholar]
- 12.Aghajanian C, Brown C, O’Flaherty C, et al. Phase I study of tirapazamine and cisplatin in patients with recurrent cervical cancer. Gynecologic Oncology. 1997;67: 127–130. [DOI] [PubMed] [Google Scholar]
- 13.Aquino VM, Weitman SD, Winick NJ, et al. Phase I trial of tirapazamine and cyclophosphamide in children with refractory solid tumors: a pediatric oncology group study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2004;22: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 14.G, Lager JJ, Lyden ER, et al. Pooled analysis of phase II window studies in children with contemporary high-risk metastatic rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2006;24: 3415–3422. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92: 205–216. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53: 457–481. [Google Scholar]
- 17.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. Journal of the Royal Statistical Society: Series A. 1972;135: 185–198. [Google Scholar]
- 18.Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children’s Oncology Group Study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2009;27: 2536–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felgenhauer J, Hawkins D, Pendergrass T, Lindsley K, Conrad EU, Miser JS. Very intensive, short-term chemotherapy for children and adolescents with metastatic sarcomas. Medical and Pediatric Oncology. 2000;34: 29–38. [DOI] [PubMed] [Google Scholar]
- 20.Loss JF, Santos PPA, Leone LD, Brunetto AL. Outcome of pediatric recurrent and refractory malignant solid tumors following ifosfamide/carboplatin/etoposide (ICE): A phase II study in a pediatric oncology centre in Brazil. Pediatric Blood & Cancer. 2004;42: 139–144. [DOI] [PubMed] [Google Scholar]
- 21.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children’s Oncology Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2007;25: 362–369. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins DS, Chi YY, Anderson JR, et al. Addition of Vincristine and Irinotecan to Vincristine, Dactinomycin, and Cyclophosphamide Does Not Improve Outcome for Intermediate-Risk Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J Clin Oncol. 2018;36: 2770–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiSilvestro PA, Ali S, Craighead PS, et al. Phase III randomized trial of weekly cisplatin and irradiation versus cisplatin and tirapazamine and irradiation in stages IB2, IIA, IIB, IIIB, and IVA cervical carcinoma limited to the pelvis: a Gynecologic Oncology Group study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2014;32: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rischin D, Peters LJ, O’Sullivan B, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2010;28: 2989–2995. [DOI] [PubMed] [Google Scholar]
- 25.Walterhouse DO, Pappo AS, Meza JL, et al. Shorter-duration therapy using vincristine, dactinomycin, and lower-dose cyclophosphamide with or without radiotherapy for patients with newly diagnosed low-risk rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2014;32: 3547–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shern JF, Chen L, Chmielecki J, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discovery. 2014;4: 216–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Highfill SL, Cui Y, Giles AJ, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Science Translational Medicine. 2014;6: 237ra267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seki M, Nishimura R, Yoshida K, et al. Integrated genetic and epigenetic analysis defines novel molecular subgroups in rhabdomyosarcoma. Nature Communications. 2015;6: 7557. [DOI] [PMC free article] [PubMed] [Google Scholar]


