Abstract
Benign lymphoepithelial lesion (BLEL) is characterized by extensive lymphocytic infiltration of the major salivary glands and may be associated with Sjogren's syndrome or HIV infection. The involvement of the palatal minor salivary glands is extremely rare. We report an isolated case of BLEL affecting the palatal minor salivary glands, presenting as a palatal swelling in a 37-year-old female patient. Serological tests ruled out potential comorbid conditions. Cone-beam computed tomography showed a palatal soft-tissue mass with thinning of the adjacent cortical plates. A histopathological examination revealed salivary gland tissue with significant acinar destruction, dense lymphocytic infiltration and focal myoepithelial islands. Therefore, BLEL may be considered as a rare differential diagnostic possibility of a palatal soft-tissue mass lesion.
Keywords: Benign lymphoepithelial lesion, minor salivary gland, palate
INTRODUCTION
Benign lymphoepithelial lesions (BLELs) of the salivary glands usually affect the parotid gland (nearly 85%), less commonly the submandibular glands (15%) and may be associated with Sjogren's syndrome.[1] The involvement of the minor salivary glands by BLEL is quite rare as evidenced by very few cases reported in literature.[2] Palatal swellings are frequently encountered in clinical practice and can be infectious, neoplastic or immune mediated. BLEL, however, is an extremely rare cause of a palatal swelling. Here, we report an isolated case of BLEL affecting the palatal minor salivary gland without associated autoimmune diseases or immunodeficiency states.
CASE REPORT
A 37-year-old female patient presented with the chief complaint of swelling in the right side of the palate of 1-month duration associated with dull, intermittent pain. History revealed that the patient had applied rock salt over the swelling to alleviate pain. Medical history revealed that the patient was undergoing infertility treatment for 7 months and had undergone surgery for uterine fibroid 6 years back. There was no history of dry eyes, dry mouth, joint pain or weight loss. The patient was a nonsmoker and nonalcoholic and had no tobacco chewing habits.
On intraoral examination, a single diffuse ovoid swelling measuring approximately 1.5 cm × 1 cm was present on the right side of the posterior hard palate [Figure 1]. The surface of the swelling was predominantly smooth with a focal shallow ulceration in the center. On palpation, the swelling was nontender, soft to firm in consistency and fluctuant. No tooth in the quadrant was tender on percussion. There were no clinical signs of dental caries or periodontal disease.
Figure 1.
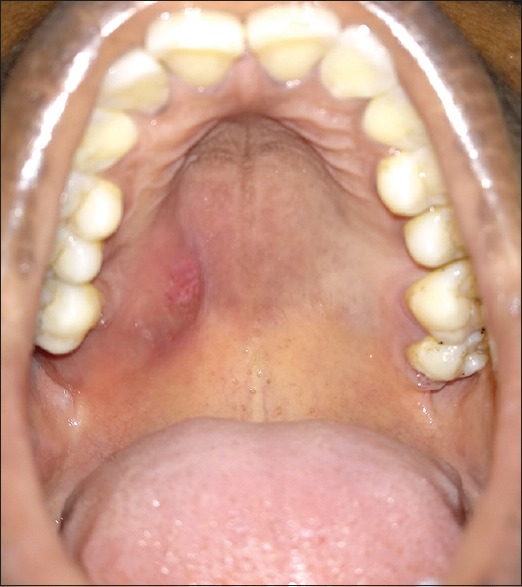
Intraoral photograph showing a single-diffuse swelling on the right side of the posterior hard palate with shallow superficial ulceration
Intraoral periapical radiograph of the right maxillary first molar and maxillary lateral occlusal radiographs revealed no significant changes. Cone-beam computed tomography of maxilla revealed a soft-tissue shadow in the right side of the hard palate in relation to maxillary right first molar with pressure effect on adjacent bone and thinning of cortical bone with no evidence of breach in the continuity of bone [Figures 2 and 3]. There was no periapical lesion in relation to maxillary right premolars and molars. We excluded a palatal abscess or a cyst by clinical and radiographic examination. The ulceration was shallow, associated with a smooth swelling and likely to have been caused by the abrasive effects of rock salt and hence necrotizing sialometaplasia was considered unlikely. Hence, a provisional diagnosis of benign salivary gland tumor or cyst was made with the differentials of malignant salivary gland tumor and nonodontogenic soft-tissue neoplasms.
Figure 2.
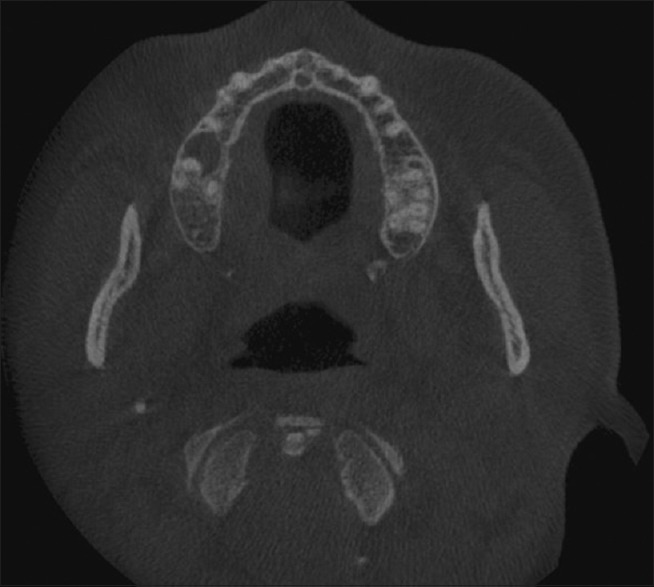
Axial cone-beam computed tomography section showing a soft-tissue shadow in the right side of the hard palate
Figure 3.

Coronal cone-beam computed tomography section showing thinning of the palatal cortical bone associated with a soft-tissue shadow on the right side
Under local anesthesia, an excisional biopsy was done and the tissue was sent for routine histopathological evaluation. Hematoxylin and eosin-stained soft-tissue sections showed salivary gland tissue with significant acinar destruction and replacement with dense lymphocytic infiltration [Figures 4 and 5]. Remnants of ductal structures and focal areas of myoepithelial islands were evident throughout the lesion [Figure 6]. Histopathological findings were consistent with BLEL.
Figure 4.
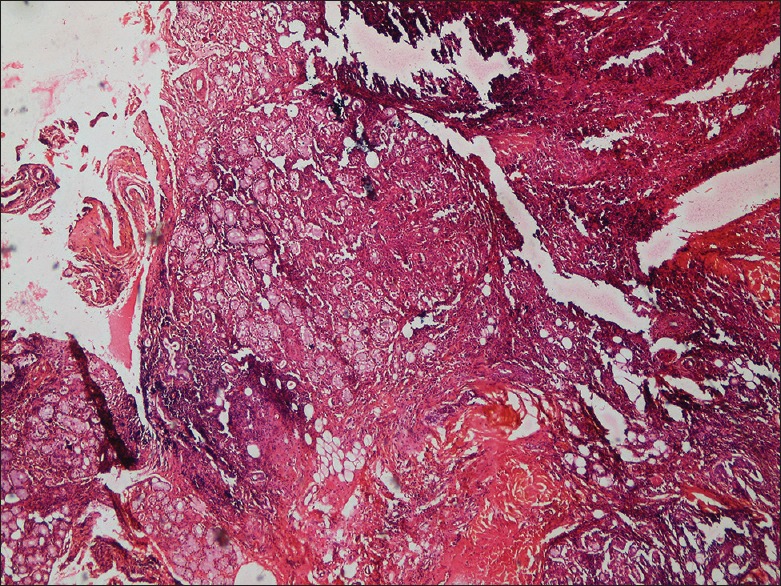
Low power view showing salivary gland acini and ductal structures exhibiting lymphocytic infiltration (H&E stain)
Figure 5.
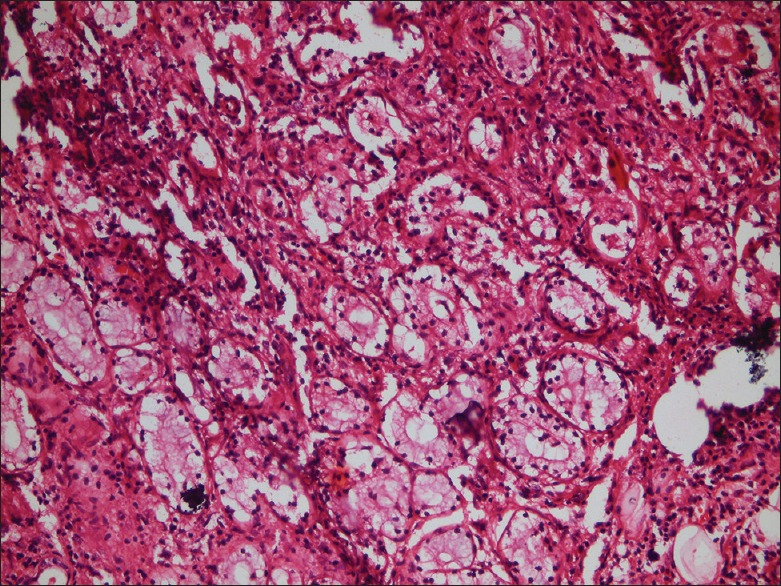
×10 magnification - salivary gland tissue showing significant acinar cell destruction and replacement with dense lymphocytic infiltration (H&E stain)
Figure 6.

×40 magnification - high power view showing acinar cells, lymphocytic infiltrates and myoepithelial islands (H&E stain)
Serological work-up for associated autoimmune diseases were negative. ELISA for HIV was also negative. The patient was followed up for 1 year following the excisional biopsy. The healing has been uneventful, and there are no signs of recurrence.
DISCUSSION
BLEL[3] also referred to as lymphoepithelial sialadenitis, lymphoepithelial lesion,[4] myoepithelial sialadenitis, Sjogren's type sialadenitis[5] or autoimmune sialadenitis of the salivary glands, is characterized histologically by an extensive infiltration of chronic inflammatory cells that replace the salivary parenchymal space, which is composed of acinar and ductal cells.[6] Godwin[7] described BLEL as lymphoepithelial changes in major salivary glands associated with unilateral or bilateral gland enlargement in the absence of the clinical signs of Sjogren's syndrome (dry eyes and mouth). However, Morgan and Castleman were the first to detail the histopathologic hallmarks of BLEL, namely marked lymphoplasmacytic infiltration with residual epimyoepithelial islands (punctuated by hyperplastic and metaplastic ducts) and acinar degeneration.[8]
BLEL is considered an autoimmune disease of salivary glands. Clinical features of Sjögren's syndrome may be manifested by 50%–84% of patients with BLEL, which increases the risk (40:1) of developing malignant lymphoma, thereby underscoring the need for follow-up surveillance for potential malignant transformation.[9] In patients with salivary gland enlargement, the diagnosis of BLEL may be difficult due to the many benign inflammatory conditions that arise and/or the occurrence of salivary cysts or tumors. Isolated involvement of minor salivary glands by BLEL is extremely rare and poses diagnostic challenges due to a plethora of conditions that can present as a palatal swelling. In our case, the clinical presentation mimicked a benign adenoma of the minor salivary gland. The absence of associated syndromes and HIV infection made the diagnosis even more challenging. Careful histopathological examination was required to arrive at a conclusive diagnosis.
For differential diagnostic purposes, imaging modalities such as computed tomography, ultrasound, technetium scan and sialography may be helpful, although such technical advancements are not routinely utilized in the management of salivary gland tumors. Fine-needle aspiration (FNA) biopsy is controversial in this setting because for many surgeons, the presence of tumor alone is sufficient indication to operate. With sensitivity near 90% and a specificity of 75%, FNA biopsy may have a role in documenting the benign disease of poor-risk patients, thus avoiding inappropriate surgery.[10] FNA may reveal epimyoepithelial islands, numerous small lymphocytes and a granular proteinaceous background that could suggest BLEL.[10]
To summarize, the diagnosis of BLEL requires careful history taking to look for associated Sjogren's syndrome and HIV infection, careful clinical examination of the regions of major and minor salivary glands, appropriate imaging and serological investigations, a detailed histopathological evaluation and a multidisciplinary approach including dental surgeons, rheumatologists and ophthalmologists. Although rare, BLEL should be considered in the differential diagnosis of palatal swellings with marked lymphocytic infiltration.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schneider M, Rizzardi C. Lymphoepithelial carcinoma of the parotid glands and its relationship with benign lymphoepithelial lesions. Arch Pathol Lab Med. 2008;132:278–82. doi: 10.5858/2008-132-278-LCOTPG. [DOI] [PubMed] [Google Scholar]
- 2.Metwaly H, Cheng J, Suzuki M, Takata Y, Saitoh C, Hayashi T, et al. Benign lymphoepithelial lesion of minor salivary gland: Report of a case involving the palatal mucosa. Oral Oncol Extra. 2004;40:113–6. [Google Scholar]
- 3.Yoshihara T, Morita M, Ishii T. Ultrastructure and three-dimensional imaging of epimyoepithelial islands in benign lymphoepithelial lesions. Eur Arch Otorhinolaryngol. 1995;252:106–11. doi: 10.1007/BF00168030. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhry AP, Cutler LS, Yamane GM, Satchidanand S, Labay G, Sunderraj M. Light and ultrastructural features of lymphoepithelial lesions of the salivary glands in Mikulicz's disease. J Pathol. 1986;148:239–50. doi: 10.1002/path.1711480308. [DOI] [PubMed] [Google Scholar]
- 5.Ihrler S, Zietz C, Sendelhofert A, Riederer A, Löhrs U. Lymphoepithelial duct lesions in Sjögren-type sialadenitis. Virchows Arch. 1999;434:315–23. doi: 10.1007/s004280050347. [DOI] [PubMed] [Google Scholar]
- 6.Metwaly H, Cheng J, Ida-Yonemochi H, Ohshiro K, Jen KY, Liu AR, et al. Vascular endothelial cell participation in formation of lymphoepithelial lesions (epi-myoepithelial islands) in lymphoepithelial sialadenitis (benign lymphoepithelial lesion) Virchows Arch. 2003;443:17–27. doi: 10.1007/s00428-003-0824-0. [DOI] [PubMed] [Google Scholar]
- 7.Godwin JT. Benign lymphoepithelial lesion of the parotid gland adenolymphoma, chronic inflammation, lymphoepithelioma, lymphocytic tumor, Mikulicz disease. Cancer. 1952;5:1089–103. doi: 10.1002/1097-0142(195211)5:6<1089::aid-cncr2820050604>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Ma Q, Song H. Diagnosis and management of lymphoepithelial lesion of the parotid gland. Rheumatol Int. 2011;31:959–62. doi: 10.1007/s00296-010-1617-9. [DOI] [PubMed] [Google Scholar]
- 9.Goto TK, Shimizu M, Kobayashi I, Chikui T, Kanda S, Toshitani K, et al. Lymphoepithelial lesion of the parotid gland. Dentomaxillofac Radiol. 2002;31:198–203. doi: 10.1038/sj/dmfr/4600690. [DOI] [PubMed] [Google Scholar]
- 10.Chhieng DC, Cangiarella JF, Cohen JM. Fine-needle aspiration cytology of lymphoproliferative lesions involving the major salivary glands. Am J Clin Pathol. 2000;113:563–71. doi: 10.1309/2AR0-RFGW-GTTD-G65E. [DOI] [PubMed] [Google Scholar]


