Abstract
Pleomorphic Adenoma (PA), a benign neoplasm of glandular origin most commonly involves major salivary glands. It is rare in minor salivary glands such as hard palate, upper lip and buccal mucosa, frequently affecting middle aged females. PA comprises diverse histopathologic features of epithelial, myoepithelial and mesenchymal components. Aberrant histopathologic features in Pleomorhic Adenoma thus calls for judicious discrimination from alike entities which facilitates appropriate surgical management. Here we present a case report of PA in upper lip in a 25 year old female patient showing uncommon findings like clear cells, squamous metaplasia and cribriform pattern.
Keywords: Clear cell, cribriform pattern, pleomorphic adenoma, squamous metaplasia, upper lip
INTRODUCTION
Salivary gland neoplasms are rarely encountered with an annual incidence of 1.0–6.5 cases per 100,000 people worldwide.[1] Although pleomorphic adenoma (PA) is the most common benign salivary gland neoplasm predominantly affecting parotid gland, it rarely involves minor salivary glands.[1] PA of upper lip is an uncommon entity, diagnosis of which needs immense knowledge of variety of cells, tissue architecture and morphology. This benign neoplasm shows that middle-aged female preponderance[2] bears wide spectrum of histopathological diversity simulating an array of benign or malignant lesions often culminating into erroneous diagnosis. Here, we present a case of PA in upper lip in a young female patient having rare findings like clear cells, squamous metaplasia and cribriform pattern which escalate probability of misdiagnosis.
CASE REPORT
A 25-year-old female patient presented with the chief complaint of a slowly enlarging, painless swelling on the upper lip on the right side for 2 years. The onset of the lesion was gradual without any associated injury. Her medical history was noncontributory. Extraoral examination revealed diffuse, mobile, firm, nontender nodule on the anatomical area enclosed between right commissure, right nasolabial fold laterally and philtrum medially [Figure 1]. Overlying skin was free. No regional lymphadenopathy was noted. Intraoral examination revealed a solitary, well-defined, round to ovoid, firm, nontender, nonfluctuant and nonpulsatile nodule (1.5 cm × 2.0 cm) on the upper right labial mucosa in relation to 13, 14 and 15 showing prominent superficial vascular markings [Figure 2]. Based on clinical findings, a provisional diagnosis of benign salivary gland neoplasm was made. Differential diagnosis included canalicular adenoma, PA, fibroma, neurofibroma and lipoma.
Figure 1.
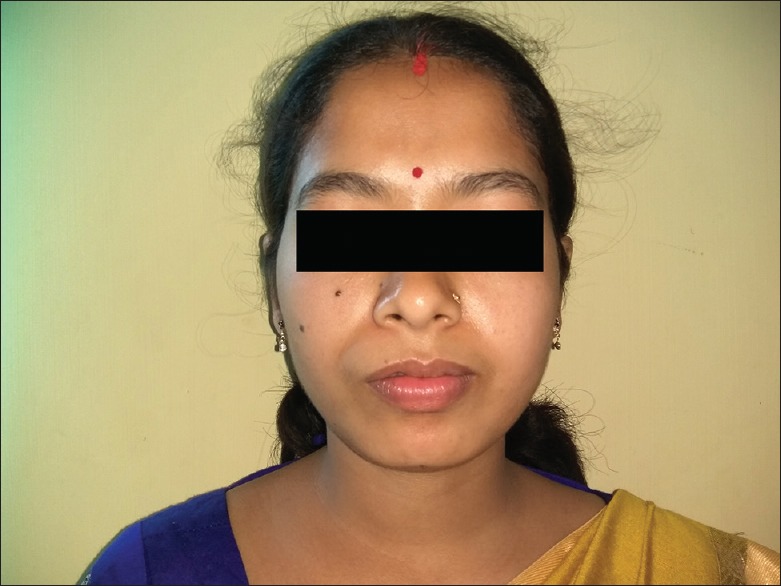
Extraoral photograph showing diffuse swelling above the upper lip region on the right side
Figure 2.
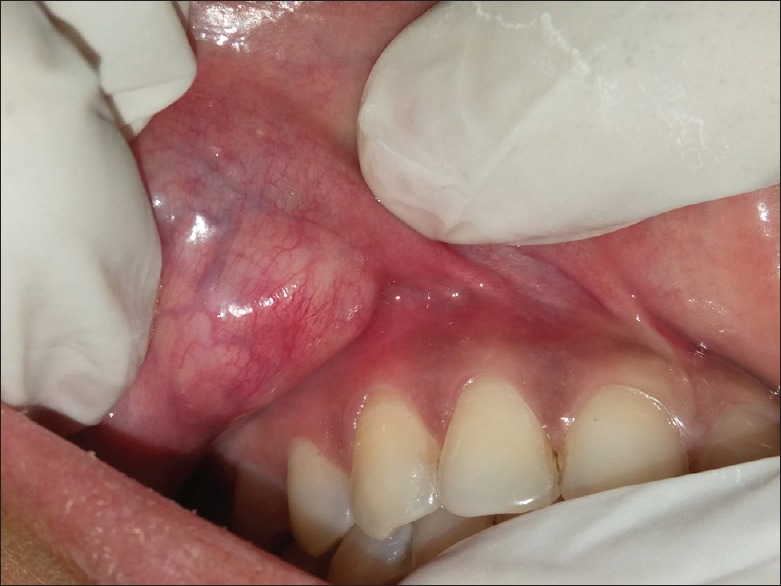
Intraoral photograph showing nodular mass on the upper labial mucosa
Excisional biopsy was performed under local anesthesia after obtaining written informed consent from the patient. The gross specimen was encapsulated, rubbery and resilient in consistency measuring about 1.5 cm × 2.0 cm × 1.0 cm. Cut surface was glistening with a homogeneous tan white color containing multiple microcysts [Figure 3].
Figure 3.
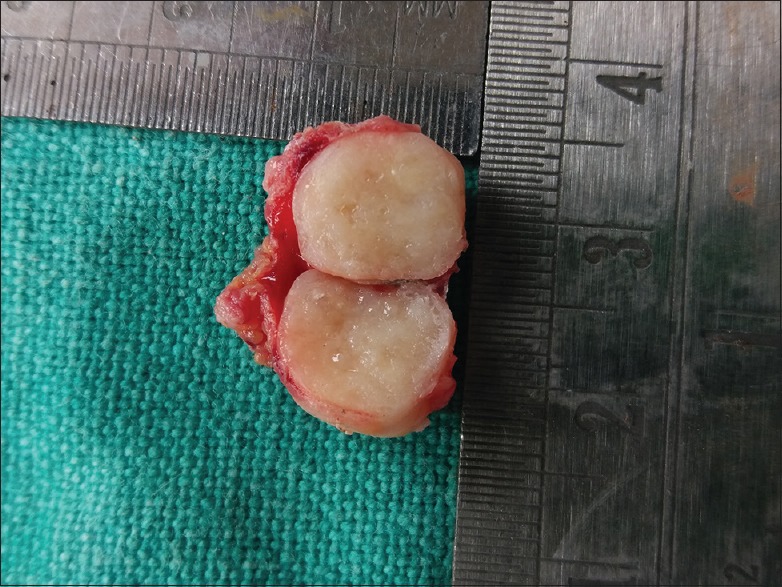
Encapsulated gross-specimen measuring 1.5 cm × 2.0 cm × 1.0 cm with glistening homogeneous tan white cut surfaces
Histopathological examination of the sections stained with hematoxylin and eosin revealed well-circumscribed lesion surrounded by fibrous capsule, composed of neoplastic glandular epithelial cells and myxoid stroma [Figure 4]. High-power magnification showed proliferation of glandular epithelial cells in sheet-like and duct-like pattern. The ducts were peripherally lined by cuboidal luminal cells and abluminal myoepithelial cells having hyperchromatic nuclei [Figure 5]. Lumens of the ducts were filled with periodic acid-Schiff stain-positive mucinous material [Figure 6]. Multiple small to large microcysts resembling cribriform pattern were noted at places [Figure 6]. The stroma predominantly consisted of myxoid zone [Figure 7] interspersed with hyalinized area of dense collagen fibers [Figure 8]. Focal area of keratinization in concentric ring-like fashion was observed suggestive of squamous metaplasia [Figure 9]. Diffuse area of clear cells with inconspicuous nuclei was also noted in close vicinity to the myxoid zone [Figure 10]. Thus, although the overall histopathologic features were suggestive of PA, owing to cribriform pattern, keratinized area and clear cells, adenoid cystic carcinoma (AdCC), mucoepidermoid carcinoma (MEC) and polymorphous low grade adenocarcinoma (PLGA) need to be considered in the differential diagnosis.
Figure 4.
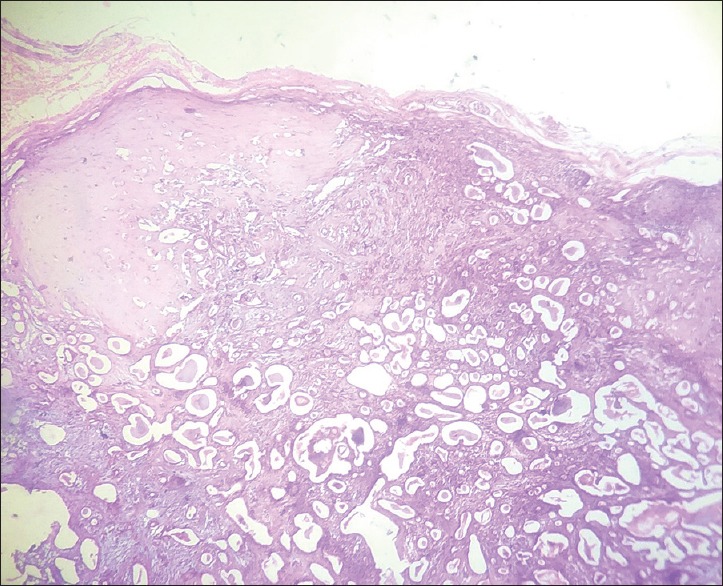
H&E-stained section (×10) showing well-circumscribed mass surrounded by fibrous capsule and glandular epithelial cells with numerous cystic spaces in the stroma
Figure 5.
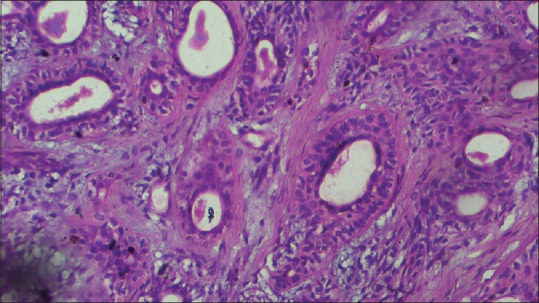
Photomicrograph (H&E stained, ×40) showing ducts lined by luminal cuboidal cells and abluminal myoepithelial cells
Figure 6.
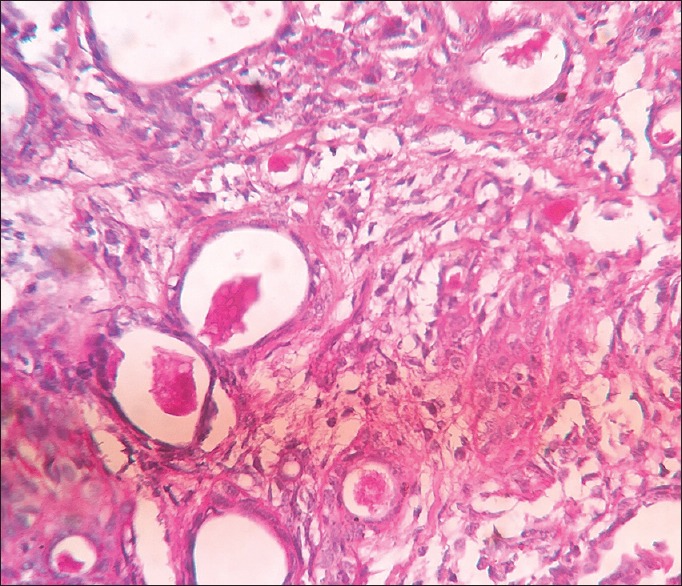
Photomicrograph showing periodic acid-Schiff (×40) positive mucinous material
Figure 7.
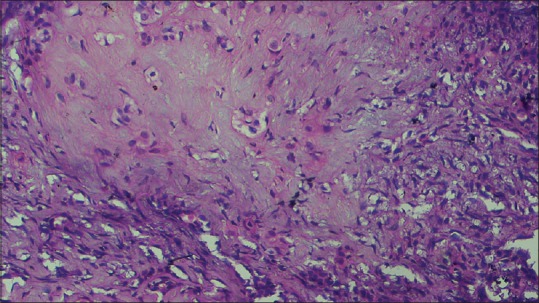
Photomicrograph (H&E stained, ×40) showing myxomatous change of the stromal component
Figure 8.
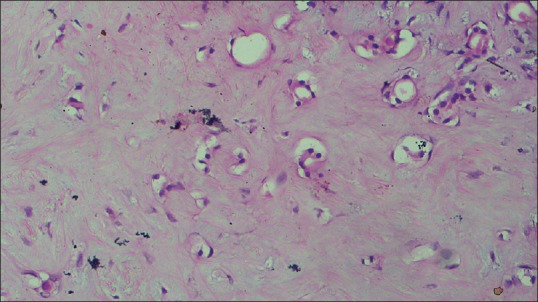
Photomicrograph (×40) showing hyalinized, dense collagen fibers in the connective tissue
Figure 9.
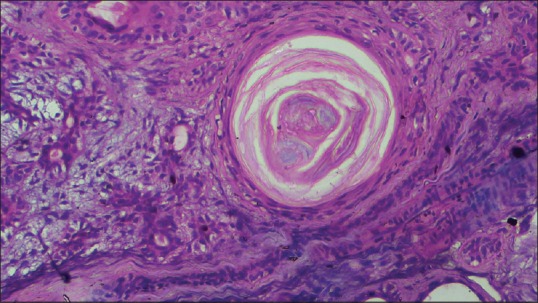
Photomicrograph (H&E, ×40) showing keratin pearl (squamous metaplasia) in the stroma
Figure 10.
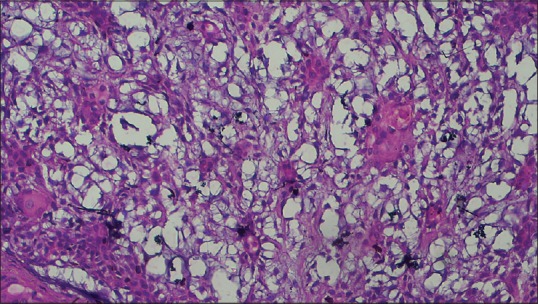
Photomicrograph (×40) showing clear cells along with proliferating myoepithelial cells
The patient was followed up over a period of 12 months and no recurrence was noted.
DISCUSSION
The term “pleomorphic adenoma” was coined by Willis in 1953 due to its distinctive mixed histopathology.[3] It constitutes the largest proportion of all the benign salivary gland neoplasms[1] but rarely involves minor salivary glands accounting for 6.4% of all cases[2] amongst which hard palate is considered as the most common site of occurrence (50%–60%) followed by upper lip (15%–20%) and buccal mucosa (8%–10%).[4] It affects individuals in 3rd to 5th decades with slight female predilection.[5] PA clinically manifests as slow-growing, painless, firm growth without any overlying mucosal ulceration.[6] The surface of the growth may be either smooth or bosselated depending on the size of the lesion.[7] PA on lips are usually mobile, sessile, well-circumscribed, nodular and rubbery mass.[8] Earlier onset is seen in PA affecting minor salivary glands compared to major glands.[9] It is six times more common in the upper lip than the lower lip.[8] In the present case, PA affecting upper lip was found in a 25-year-old female with clinical features corroborative to literature. List of differential diagnoses was excluded based on clinical and histopathological features [Table 1].
Table 1.
List of differential diagnosis
| Differential diagnosis | Clinical features | Histopathology |
|---|---|---|
| Canalicular adenoma | Age: Older age group (peak prevalence in 7th decade) Sex: Slight female predominance (1.2:1) Site: Upper lip (most common)[1] Clinical presentation: slow-growing, nontender, firm (sometimes fluctuant) mass |
Encapsulated benign salivary gland neoplasm characterized by cuboidal epithelial cells enclosing ductal structures in the form of long canals (monomorphic). Unlike PA it lacks stromal variation[1] |
| PA | Age: 3rd to 6th decades Sex: Female > male Site: Parotid gland > submandibular gland (major) Hard palate > upper lip > buccal mucosa (minor gland) Clinical presentation: Slow-growing, nontender, firm, mobile mass. Size varies with location |
Completely or partially encapsulated benign salivary gland neoplasm composed of mixture of glandular epithelium and myoepithelial cells in varied stromal background. Epithelium shows ductal or cystic patterns while stromal component may reveal osseous, chondroid, myxoid, hyalinized or lipomatous differentiation[1,2] |
| Fibroma | Age: 4th to 8th decades Sex: Male:female=2:1 Site: Buccal mucosa[1] Clinical presentation: Smooth-surfaced pale pink, nontender, soft to firm sessile (sometimes pedunculated) nodule |
Un-encapsulated where atrophied surface epithelium is backed by dense and collagenized fibrous connective tissue[1] |
| Neurofibroma | Age: Young adults Sex: Male > female Site: Tongue and buccal mucosa (most common)[1] Clinical presentation: Slow-growing, soft, painless mass. Size may vary | It is a well-circumscribed peripheral nerve neoplasm, composed of interlacing bundles of spindle shaped cells consisting of wavy nuclei[1] |
| Lipoma | Age: 4th to 6th[1] Sex: No such predilection Site: Buccal mucosa and buccal vestibules are more frequently involved intraoral sites Clinical presentation: Smooth-surfaced with subtle yellowish hue, soft, nontender mass | It is well-circumscribed mass composed of thin fibrous capsule and mature adipocytes. Adipocytes are vacuolated cells with eccentrically placed flattened nucleus |
| Adenoid cystic carcinoma | Age: Mean age is 30-40 years Sex: 3:2 (female:male) Site: Submandibular gland > parotid gland (major) > hard palate > upper lip > buccal mucosa (minor gland) Clinical features: Slow-growing, soft, slightly painful mass which often ulcerates |
Unencapsulated lesion composed of uniform basaloid cells arranged in solid, tubular and cribriform pattern. Perineural invasion is characteristic |
| Mucoepidermoid carcinoma | Age: Mean age is 60-65 years Sex: 3:2 (female:male) Site: Parotid gland > submandibular gland (major) Hard palate > upper lip > buccal mucosa (minor gland) Clinical features: Slow-growing, soft, slightly painful mass which often ulcerates. Nodal involvement is seen in regional lymphnodes |
Unencapsulated mass predominantly composed of epidermoid, mucous and intermediate cells. Keratin pearls and clear cells are found often along with varying degrees of cystic space, neural spread, mitosis and anaplasia |
| Polymorphous low-grade adenocarcinoma | Age: Mean age is 50-70 years Sex: Female:male=3:2 Site: Hard palate Clinical features: Slow-growing, painless mass which may be associated with surface ulceration or telangiectasia |
Usually, unencapsulated lesion composed of small to cuboidal luminal cells having bland, minimally hyperchromatic nuclei arranged in cribriform, lobular, papillary and papillary-cystic pattern. Clear cells and perineural invasion in targetoid pattern are often seen |
PA: Pleomorphic adenoma
Histopathologically, PA exhibits variable cellular and stromal characteristics. Epithelial and myoepithelial cells in duct like fashion are arranged in a background of osseous, chondroid, myxoid, mucoid or hyalinized stroma.[8] The ducts are peripherally lined by luminal cuboidal cells and abluminal myoepithelial cells.[8] Abundance of myoepithelial cells were evident in this case along with myxoid stroma, that is in accordance with previous observations.[1,10] These cystic spaces occasionally contain eosinophilic deposits that may sometimes push apart epithelial elements giving rise to cribriform appearance mimicking AdCC.[10] The present case showed cystic areas resembling cribriform pattern in a background of myxoid stroma at places. Chondroid and osseous areas, that are prominent features in PA,[1,10] were absent in this case. Hyalinized area in the stroma has been frequently reported;[1,10] this was present in our case. Evidence of squamous metaplasia and conspicuous clear cell changes in PA[4,10] raises diagnostic perplexity due to resemblance with MEC and PLGA, although hallmarks of malignancy such as cellular and nuclear pleomorphism, anaplasia, mitotic figures, necrosis and intraneural spread are not found in PA [Table 1]. Hypoxic injury and repair or necrosis of the salivary gland are presumed to be responsible for focal squamous metaplasia.[4] This case exhibited these rare findings of squamous metaplasia and clear cells changes.
The most accepted treatment for PA in major salivary glands is surgical excision of the tumor with the removal of the affected lobe or the entire gland, as the case may be.[1] PA in the minor salivary glands mandate enucleation or wide local excision with complete removal of the capsule.[1,4,10] Adequate removal ensures cure rate of more than 95%[1] with rare chance of recurrence.[10] Recurrence of PA is mostly attributed to inadequate excision or spillage of the tumor and/or capsule while surgery.[3] In the present case, the tumor was excised en mass. Postoperative follow-ups were continued for 12 months without any evidence of recurrence.
CONCLUSION
True to its name, PA shows wide spectrum of histopathological features with variation in both epithelial and stromal components. Meticulous histopathological distinction of PA is of utmost importance in the light of rare findings to avoid misdiagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Neville BW, Damm DD, Allen CM, Chi A. Salivary Gland Pathology Oral and Maxillofacial Pathology. First South Asia Edition. India: Elsevier; 2015. pp. 444–8. [Google Scholar]
- 2.Verma P, Sachdeva SK, Verma KG, Sachdeva K. Pleomorphic adenoma of cheek: A rare case report and review of literature. Indian J Dent Res. 2014;25:122–4. doi: 10.4103/0970-9290.131166. [DOI] [PubMed] [Google Scholar]
- 3.Sood A, Chung S, Datiashvili RO. An incidental finding of pleomorphic adenoma of the minor salivary glands in the skin area of the lower lip. Eplasty. 2014;14:e39. [PMC free article] [PubMed] [Google Scholar]
- 4.Tandon A, Jaiswal R, Siddiqui S, Bordoloi B. Keratinizing pleomorphic adenoma: An unusual case report. J Oral Maxillofac Pathol. 2018;22:S69–S72. doi: 10.4103/jomfp.JOMFP_200_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao PK, Shetty SR, Hegde D. Ectopic pleomorphic adenoma. N Am J Med Sci. 2012;4:190–2. doi: 10.4103/1947-2714.94947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalati T, Hussein MR. Juvenile pleomorphic adenoma of the cheek: A case report and review of literature. Diagn Pathol. 2009;4:32. doi: 10.1186/1746-1596-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh AK, Kumar N, Sharma P, Singh S. Pleomorphic adenoma involving minor salivary glands of upper lip: A rare phenomenon. J Cancer Res Ther. 2015;11:1025. doi: 10.4103/0973-1482.148682. [DOI] [PubMed] [Google Scholar]
- 8.Taiwo AO, Akinshipo A, Braimah RO, Ibikunle AA. Pleomorphic adenoma of the upper lip: A case report. Saudi J Med Med Sci. 2018;6:32–5. doi: 10.4103/sjmms.sjmms_109_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrestha A, Reddy NS, Ganguly SN. Pleomorphic adenoma of the upper lip: A case report? Nepal Med Coll J. 2010;6:51–3. DOI: https://doi.org/10.3126/jcmsn.v6i1.3603. [Google Scholar]
- 10.Eveson JW, Kusafuka K, Stenman G, Nagao T. Pleomorphic adenoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumors; Pathology & Genetics; Head and Neck Tumours. Geneva, Lyon, (Switzerland): WHO Press, IARC Press; 2005. pp. 254–8. [Google Scholar]


