Abstract
Malignant melanoma in the head and neck area is rare. The incidence of oral malignant melanomas of the head and neck is approximately four per 10 million populations per year. They are derived from epidermal melanocytes and are most frequently seen on the palate and maxillary gingiva. They are asymptomatic initially but become painful with progress and enlargement. Later, they are associated with ulceration, bleeding, mobility of tooth, paresthesia and ill-fitting prosthesis. The diagnosis is often delayed due to silent growth and development of the lesion. Oral malignant melanomas are associated with poor prognosis due to their invasive and metastasizing tendencies. This case report is presented to emphasize the role of a dentist in identifying the pigmented lesions of the oral cavity.
Keywords: Histopathology, immunohistochemistry, oral malignant melanoma, tumor-node-metastasis staging
INTRODUCTION
Melanoma is a malignant tumor that arises from epidermal melanocytes and is most commonly occurs on skin. According to the National Cancer Database Report on cutaneous and noncutaneous melanoma, 91.2% of all melanomas arise on the skin, whereas ocular (5.2%), mucosal (1.3%) and unknown primaries (2.2%) are less frequently seen. Almost 25% of cutaneous melanomas arise in the head and neck area, 40% on the extremities and the rest on the trunk. More than half of the mucosal melanomas occur in head and neck, with the remainder primarily involving the urogenital and anorectal mucosa. Mucosal melanoma is much more aggressive than its cutaneous counterpart. An analysis of various worldwide cancer registries has shown similarly low incidence rates, with primary oral melanoma accounting for only 0.26% of all oral cavity cancers.[1,2]
In contrast to cutaneous melanomas, the etiology and pathogenesis of oral malignant melanoma (OMM) are poorly understood. OMM is believed to arise from pigmented nevi, pre-existing pigmented areas, Hutchinson's premalignant lentigo or de novo from apparently normal mucosa.[3,4] Many genes are implicated in the development of melanoma, including CDKN2A (p16), CDK4 (chromosome 12q15), RB I, CDKN2A (p19), PTEN/MMAC I and ras. They play an important role in both sporadic and hereditary melanomas.[5]
OMM is a lesion of the adulthood, rarely occurring under the age of 20 years. The average age of patients with mucosal melanoma was 56 years and ranges from 22 to 83 years. Males are more often affected than females. Most commonly involved areas are hard palate and maxillary gingiva; other oral sites are mandible, tongue, buccal mucosa and upper and lower lip.[6]
This manuscript reports a rare case of OMM of the maxillary posterior gingiva which has been discussed with histopathology and immunohistochemistry.
CASE REPORT
A 60-year-old male patient reported with a chief complaint of swelling in the upper back region. The swelling was associated with pain since 2 months. The patient observed bleeding in the area of swelling during brushing. The medical history was hypertensive and is on antihypertensive drugs since 1 year. The patient gave a positive history for smoking and other deleterious habits. Extraoral examination revealed no significant findings. On intraoral clinical examination, a solitary, sessile, tender and pigmented (purplish) overgrowth was present on the left maxillary buccal vestibule [Figure 1]. The size of the growth was approximately 3 cm × 5 cm with irregular periphery extending to the marginal gingiva adjacent tooth. Deep proximal caries was detected in 26. Pigmented areas were also seen all over the hard palate.
Figure 1.
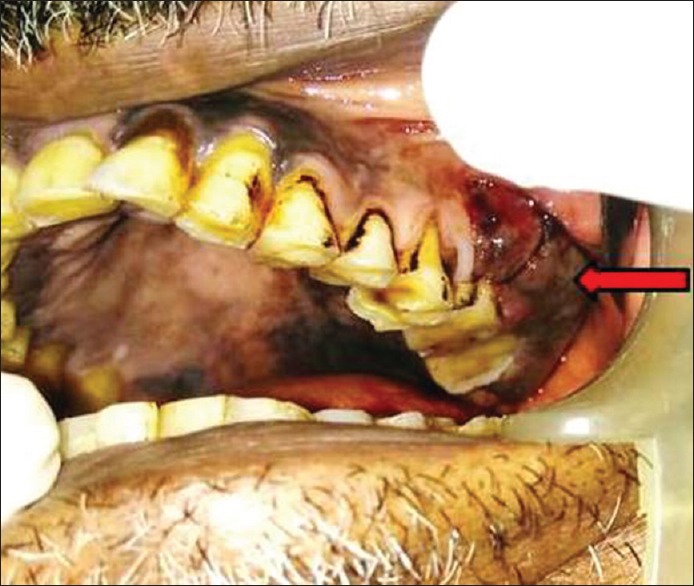
Overgrowth on the left maxillary buccal vestibule and pigmented areas on hard palate
Comparing the patient's complaint, with the history and clinical examination a differential diagnosis of melanosis, drug-induced pigmentation, pyogenic granuloma, mucosal nevus, melanotic macule and melanoacanthoma was attained. A complete blood cell count and biochemical analysis reports were insignificant and under normal limits. An orthopantomogram radiograph revealed a radiolucent area at root apex of tooth in relation to 26, 27 and 28.
An incisional biopsy was taken and sent for histopathological diagnosis. The hematoxylin- and eosin-stained sections showed overlying keratinized stratified squamous epithelium and a very small connective tissue fragment which was not sufficient for a confirmatory diagnosis. Then, an excisional biopsy was done. Gross specimen appeared brownish-black in color, firm in consistency and had irregular borders [Figure 2]. One large bit and many other small bits together measured approximately 3 cm × 3 cm × 1.5 cm. Later, histologic sections showed spindle and plump cells arranged in intersecting fascicles, bundles, clusters and chains. These cells exhibited round, ovoid or elongated nuclei with moderate atypia. Increased mitotic activity was evident. Cells showing intra-cytoplasmic melanin pigmentation [Figure 3a] were very few in number. Therefore, suspecting a malignant melanoma, immunohistochemistry marker homatropine methylbromide (HMB-45) was advised. Immunohistochemical staining with HMB-45 (BioGenex-QD400-60KE), the sections revealed strongly positive tumor cells [Figure 3b]. Malignant melanoma (spindle cell variant) was given as a final diagnosis.
Figure 2.
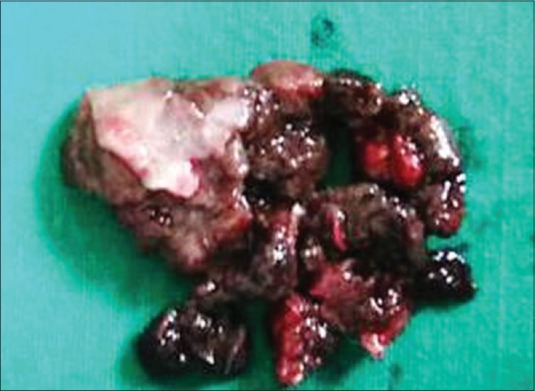
Brownish-black gross specimen
Figure 3.
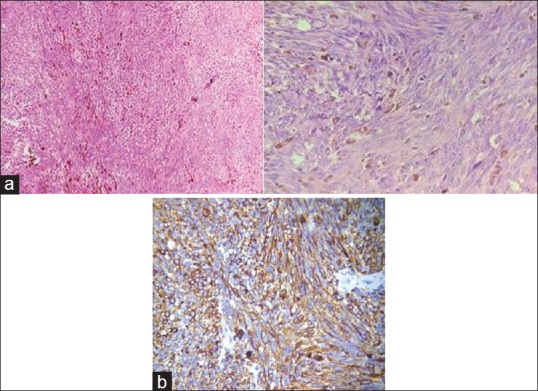
(a) Histologic sections showing spindle and plump cells with round, ovoid, or elongated nuclei and intracytoplasmic melanin pigmentation (4× and 40×). (b) Immunohistochemical staining for homatropine methylbromide-45, sections showed strongly positive tumor cells (40×)
DISCUSSION
In oral mucosa, melanocytes are located along the basal layer of the epithelium. Melanocytes and keratinocytes are in a ratio of 1:15 in single tissue sections of gingiva. Melanocytes, nevus cells and melanoma cells differ markedly in their appearance, organization and biologic properties. Nevus cells lack cellular atypia, nuclear pleomorphism and rarely have mitotic activity. Melanoma cells retain some features of nevus cells, such as lack of dendritic processes, round to ovoid or spindle shape and loss of contact inhibition. These malignant cells show significant cellular and nuclear pleomorphism, hyperchromatic nuclei, prominent nucleoli and detectable mitotic activities.[6,7]
The diagnosis of OMM often remains difficult. The differential diagnoses should include benign, malignant and exogenous pigmented lesions. Amelanotic malignant melanoma can also affect the mouth and can be challenging for diagnosis.[7,8] Pigmented lesions can be seen in some of the systemic diseases such as Cushing's syndrome, Laugier–Hunziker syndrome, patients with pulmonary diseases, especially in lung cancer, hemosiderosis, Addison's disease, and Peutz–Jeghers syndrome.[4,9,10] To aid in diagnosis clinically, the tumors are classified into five types as pigmented nodular, nonpigmented nodular, pigmented macular, pigmented mixed and nonpigmented mixed type.[6] Further for pigmented OMM a simple and practical method for the clinical diagnosis was proposed by Delgado Azañero and Mosqueda Taylor through a study consisting of 13 cases. This method suggests rubbing the pigmented lesion with a gauze piece and observing for dark brown or black staining which attributes to the presence of melanin-containing cells in the epithelium.[11,12] They also concluded that negative results for the above test do not exclude the diagnosis of malignant melanoma since there are cases in which tumor cells would not have invaded the superficial epithelial cells.
The lesion has a marked tendency to regional and distant metastasis which is supported by the intralesional blood vessels or lymphatics. It also seen that there is a lack of peripheral lymphoid reaction and the absence of giant tumor cells. Few authors have described that mucosal and submucosal lymphatic vessels are relatively large, readily permeate and richly intercommunicate with the lesion. There are no obstructions to the lymphatic flow till the tubules reach parapharyngeal or lateral neck nodal systems.[13]
As we know that OMM is an aggressive lesion, they are large at presentation and have a poorer prognosis than cutaneous melanoma. Hence, clinical tumor-node-metastasis staging in association with histopathological microstaging is an advantageous factor in the prognosis of OMM.[4,5]
-
Stage I: The presence of primary tumor (TanyN0M0)
- Level I: Pure in situ melanoma with either absence of invasion or in situ melanoma with “microinvasion”
- Level II: Involvement of the lamina propria
- Level III: Invasion into the deep skeletal tissue (skeletal muscle, bone or cartilage).
Stage II: Metastasis of tumor to regional lymph nodes (TanyN1M0)
Stage III: Metastasis of tumor to distant sites (TanyNanyM1).
The treatment of choice is surgical excision with safety margins. According to Zitelli et al., the margins should be at least 1.5 cm for the lesion in head and neck melanoma or 2.5 cm for melanomas larger than 3 cm in diameter. Many patients with early-stage lesions are cured by surgery alone. However, adjuvant radiation therapy and immunotherapy (with interferon-alpha) can be considered. Even though melanoma is not very radiosensitive, patients in early melanomas have had good response to radiation therapy. For patients with distant metastasis vemurafenib and ipilimumab are two novel treatments but agents such as high-dose interleukin-2, dacarbazine, imatinib and paclitaxel have also been tried. These recent developments in genotype directed and immunotherapy have led to prolonged survival of patients.[5,14]
Poor prognosis of melanoma is known fact. It is reported that 79% of the patients die within 5 years from the point of diagnosis. In situ lesions of melanoma are curable completely by excision. Five years' survival rate holds good for 95% of the patients with lesions <1 mm thickness and without ulcerations. The survival rates for lesions with metastasis is very poor.[15,16]
CONCLUSION
The unusual morphological features, extremely rare occurrence and aggressive behavior make it mandatory to report such cases, thus facilitating the early diagnosis and treatment for better prognosis.
Dentists who treat problems in the oral cavity should be aware of silent killer pigmented lesions and the significance of biopsy as an aid in the prompt diagnosis of such lesions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Neville BW, Damm D, Allenc R, Bouquot JE. Oral and Maxillofacial Pathology. 2nd ed. Philadelphia, PA: WB Saunders; 2002. pp. 376–80. [Google Scholar]
- 2.Prabhu SR, Wilson DF, Daftary DK. Oral Diseases in the Tropics. New York: Oxford University Press; 1992. pp. 460–1. [Google Scholar]
- 3.Warszawik-Hendzel O, Słowińska M, Olszewska M, Rudnicka L. Melanoma of the oral cavity: Pathogenesis, dermoscopy, clinical features, staging and management. J Dermatol Case Rep. 2014;8:60–6. doi: 10.3315/jdcr.2014.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma N. Primary oral malignant melanoma: Two case reports and review of literature. Case Rep Dent. 2012;2012:975358. doi: 10.1155/2012/975358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivapathasundharam B. Shafer's Text Book of Oral Pathology. 8th ed. Chennai: Elsevier; 2016. pp. 170–6. [Google Scholar]
- 6.Hashemi Pour MS. Malignant melanoma of the oral cavity: A review of literature. Indian J Dent Res. 2008;19:47–51. doi: 10.4103/0970-9290.38932. [DOI] [PubMed] [Google Scholar]
- 7.Powell JP, Cummings CW. Melanoma and the differential diagnosis of oral pigmented lesions. Laryngoscope. 1978;88:1252–65. doi: 10.1288/00005537-197808000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhry AP, Hampel A, Gorlin RJ. Primary malignant melanoma of the oral cavity: A review of 105 cases. Cancer. 1958;11:923–8. doi: 10.1002/1097-0142(195809/10)11:5<923::aid-cncr2820110507>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Babburi S, Subramanyam RV, Aparna V, Sowjanya P. Intraoral malignant melanoma. Niger Med J. 2013;54:278–81. doi: 10.4103/0300-1652.119667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Femiano F, Lanza A, Buonaiuto C, Gombos F, Di Spirito F, Cirillo N. Oral malignant melanoma: A review of the literature. J Oral Pathol Med. 2008;37:383–8. doi: 10.1111/j.1600-0714.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- 11.Hasan S, Jamdar SF, Jangra J, Al Beaiji SM. Oral malignant melanoma: An aggressive clinical entity report of a rare case with review of literature. J Int Soc Prev Community Dent. 2016;6:176–81. doi: 10.4103/2231-0762.175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado Azañero WA, Mosqueda Taylor A. A practical method for clinical diagnosis of oral mucosal melanomas. Med Oral. 2003;8:348–52. [PubMed] [Google Scholar]
- 13.Shah JP, Hubos AG, Strong E. Mucosal melanomas of head and neck. Am J Surg. 1977;134:212–9. doi: 10.1016/0002-9610(77)90393-2. [DOI] [PubMed] [Google Scholar]
- 14.Zitelli JA, Brown CD, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422–9. doi: 10.1016/s0190-9622(97)70144-0. [DOI] [PubMed] [Google Scholar]
- 15.Shah H, Vyas Z. Malignant melanoma: A case report. J Adv Dent Res. 2010;1:59–62. [Google Scholar]
- 16.Symvoulakis EK, Kyrmizakis DE, Drivas EI, Koutsopoulos AV, Malandrakis SG, Skoulakis CE, et al. Oral mucosal melanoma: A malignant trap. Head Face Med. 2006;2:7. doi: 10.1186/1746-160X-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


