Abstract
Purpose
Despite our understanding of diabetes as an established risk factor for progressive kidney disease and cardiac complications, the prognostic significance of prediabetes in patients with chronic kidney disease (CKD) remains largely unknown.
Methods
Participants of the Chronic Renal Insufficiency Cohort (CRIC) were categorized as having normoglycemia, prediabetes, or diabetes according to fasting plasma glucose, glycated hemoglobin A1c (HbA1c), and treatment with antidiabetic drugs at baseline. Unadjusted and adjusted proportional hazards models were fit to estimate the association of prediabetes and diabetes (versus normoglycemia) with: (1) composite renal outcome (end-stage renal disease, 50% decline in estimated glomerular filtration rate to ≤ 15 mL/min/1.73 m2, or doubling of urine protein-to-creatinine ratio to ≥ 0.22 g/g creatinine); (2) composite cardiovascular (CV) outcome (congestive heart failure, myocardial infarction or stroke); and (3) all-cause mortality.
Results
Of the 3701 individuals analyzed, 945 were normoglycemic, 847 had prediabetes and 1909 had diabetes. The median follow-up was 7.5 years. Prediabetes was not associated with the composite renal outcome (adjusted hazard ratio [aHR] 1.13; 95% confidence interval [CI], 0.96–1.32; P = 0.14), but was associated with proteinuria progression (aHR 1.23; 95% CI, 1.03–1.47; P = 0.02). Prediabetes was associated with a higher risk of the composite CV outcome (aHR 1.38; 95% CI, 1.05–1.82; P = 0.02) and a trend towards all-cause mortality (aHR 1.28; 95% CI, 0.99–1.66; P = 0.07). Participants with diabetes had an increased risk of the composite renal outcome, the composite CV outcome, and all-cause mortality.
Conclusions
In individuals with CKD, prediabetes was not associated with composite renal outcome, but was associated with an increased risk of proteinuria progression and adverse CV outcomes.
Keywords: prediabetes, diabetes, chronic kidney disease, cardiovascular outcomes, renal outcomes, all-cause mortality
Prediabetes is a highly prevalent condition, affecting about one-third of adults in the United States (1). Patients with prediabetes have an increased risk for diabetes, with 2% to 10% progressing to diabetes each year (2, 3). Although prediabetes is frequently considered an intermediary stage in the progression between normoglycemia and diabetes, many individuals may have prediabetes for several years, while some may never progress.
Chronic kidney disease (CKD) is a heterogeneous group of disorders characterized by alterations in kidney structure and function (4), and is associated with an increased risk of end-stage renal disease (ESRD) and adverse cardiovascular (CV) outcomes (4). In the United States, the prevalence of CKD is estimated to be approximately 14% (5). In patients with CKD, diabetes has been clearly associated with an increased risk of progression to ESRD and adverse CV outcomes (6); however, the prognostic significance of prediabetes remains uncertain.
The Chronic Renal Insufficiency Cohort (CRIC) includes participants with CKD followed up to 10 years. This cohort includes a high proportion of participants with prediabetes and includes adjudicated renal and CV outcomes, providing a unique opportunity to evaluate the associations of prediabetes with these outcomes among patients with CKD. We hypothesized that prediabetes would be associated with an increased risk of CKD progression and adverse CV outcomes in patients with CKD.
Methods
Study participants
The Chronic Renal Insufficiency Cohort (CRIC) Study is a multicenter prospective cohort that recruited an ethnically and racially diverse group of subjects with prevalent CKD across 7 clinical centers in the United States from 2003 to 2008. CRIC was designed to elucidate risk factors for progression of CKD, development of ESRD, and development of CV disease among patients with varying stages of CKD, half of whom had diabetes. Entry criteria included estimated glomerular filtration rate (eGFR) from 20 to 70 mL/min/1.73 m2 and age of 21 to 74 years. Individuals with polycystic kidney disease, New York Heart Association class (NYHA) III or IV heart failure (HF), known cirrhosis, HIV/AIDS, multiple myeloma or renal cancer were excluded. Further exclusions included active immunosuppression, recent chemotherapy or immunosuppressive therapy; institutionalization; organ transplantation; pregnancy; or dialysis for a month prior to screening. For this secondary analysis, we excluded participants with missing fasting glucose or glycated hemoglobin (HbA1c) measurements at baseline (n = 238) (Supplementary Figure 1) (7). The study design, baseline characteristics and main results of CRIC have been published previously (8). The CRIC study was approved by the institutional review board at each participating study site, and written informed consent was obtained from all participants.
Exposure
The main exposure of the current analysis was prediabetes at baseline, defined as either HbA1c of 5.7% to 6.4% or fasting plasma glucose of 100 to 125 mg/dL and no treatment with antidiabetic drugs. Diabetes was defined as one of the following: HbA1c ≥ 6.5%, fasting plasma glucose ≥ 126 mg/dL, or treatment with antidiabetic drugs at baseline.
In sensitivity analyses, the definitions of prediabetes and diabetes according to HbA1C alone or fasting plasma glucose alone were also evaluated. Previous studies have shown that the association of prediabetes with renal and CV outcomes may differ according to the definition used for classifying prediabetes (9, 10). In this classification, patients treated with antidiabetic drugs were classified as having diabetes and the remaining patients were defined as having normoglycemia, prediabetes, or diabetes, according to fasting plasma glucose or HbA1c cutoffs.
Study outcomes
The prespecified primary endpoints of the present analysis were: (1) composite renal outcome defined as either the development of ESRD (renal transplantation or dialysis initiation), a 50% decline in baseline eGFR (CKD-EPI equation) to ≤ 15 mL/min/1.73 m2, or doubling of urine protein to creatinine ratio to ≥ 0.22 g/g creatinine (this cutoff corresponds to an urinary protein excretion of 300 mg/day and has been used in previous studies assessing progression of CKD) (11); (2) a composite CV outcome of congestive heart failure (CHF), myocardial infarction (MI), or stroke; and (3) all-cause mortality. Individual components of the composite outcomes were also assessed. As exploratory outcomes we also evaluated (1) the composite endpoint of a 50% decline in baseline eGFR (with or without decrease to ≤ 15 mL/min/1.73 m2) or development of ESRD; and (2) peripheral artery disease events (amputation due to vascular disease, or peripheral surgical or percutaneous revascularization). For the primary renal endpoint, the development of ESRD and eGFR decline were prespecified endpoints in CRIC. For the purpose of the present analyses, we also included proteinuria in order to more comprehensively capture CKD progression. The CV composite of CHF, MI, or stroke was also prespecified in CRIC. The inclusion of CHF is highly relevant, given the body of literature stressing the increased risk of HF among patients with diabetes (12–14), and the growing evidence favoring new therapies (e.g., sodium glucose co-transporter 2 [SGLT2] inhibitors) to prevent HF events in those with and without diabetes (15–17). Clinical endpoints were adjudicated by an independent clinical events committee and outcomes definitions are presented in Supplementary Table 1 (7). Participants were followed until March 2013, withdrawal of consent, loss to follow-up, or death. Outcomes for both prediabetes and diabetes at baseline (compared with normoglycemic individuals), and for alternative definitions of prediabetes and diabetes by HbA1c criteria alone, and fasting plasma glucose alone, are presented.
Statistical analyses
Continuous variables are described as mean ± standard deviation or median (25th-75th percentiles) and categorical variables as proportions (percentages). Baseline characteristics of the study population were compared with ANOVA or the Kruskall-Wallis test for normal and nonnormal continuous variables respectively, and the chi-square test for categorical variables.
The associations of prediabetes and diabetes with CKD progression, composite CV outcome and all-cause mortality were assessed through unadjusted and adjusted Cox proportional hazards models. All adjusted models were stratified by clinic center. Model 1 included age, sex, and race/ethnicity. Model 2 (main model) included those variables in model 1 plus body mass index (BMI), antiplatelet therapy, lipid lowering therapy, systolic blood pressure at baseline, coronary artery disease (CAD, defined as prior MI or prior coronary revascularization), peripheral vascular disease, congestive heart failure, hematocrit, baseline eGFR (CKD-EPI formula), serum albumin, 24-hour urine protein (log-transformed), and inhibition of renin-angiotensin-aldosterone axis (treatment with angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or aldosterone antagonists). The choice of covariates was based on prior knowledge of risk factors/confounders and biological plausibility. The log-rank test was used to compare the survival distribution of outcomes for normoglycemia, prediabetes, and diabetes at baseline, and Kaplan-Meier curves were generated to represent the survival distribution of outcomes by glycemic control at baseline. An additional exploratory model with further adjustment for biomarkers associated with CKD progression and adverse CV outcomes was performed. For this analysis, we included the variables in model 2 plus high-sensitive C reactive protein (hsCRP, log-transformed), brain natriuretic peptide (BNP, log-transformed), and high-sensitivity troponin T (hsTnT, log-transformed), based on prior literature demonstrating the association of these biomarkers with adverse renal and CV outcomes (18–20).
The presence of effect modification (interaction) of the association of glycemic control at baseline with the primary outcomes according to race (white, black, other), sex (women or men), baseline eGFR (as continuous variable), and 24-hour urine protein (as continuous variable, log-transformed) was tested via inclusion of cross-product terms in Cox proportional hazard model, adjusting for the variables in the model 2. Subgroup analyses were performed only if there was evidence for effect modification (P for interaction < 0.1). The proportional hazards assumption was tested for all models. For covariates that violated the proportionality assumption, the corresponding time interaction term was included in the model. An adjusted model (model 2) using a restricted cubic spline with 3 knots was constructed to flexibly display the continuous association between HbA1c or glucose and the hazards of CKD progression, composite CV outcome or all-cause mortality. All analyses were conducted with the statistical software package Stata IC version 14.2 (College Station, TX) using a dataset obtained from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Data Repository. A two-sided P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 3701 individuals were included in the present analysis. The mean HbA1c was 5.3% in normoglycemic subjects (n = 945), 5.9% in participants with prediabetes (n = 847) and 7.6% in participants with diabetes (n = 1909). Relative to those with normoglycemia, participants with diabetes and prediabetes were more likely to be black, and to have a history of CAD, stroke, congestive heart failure, peripheral vascular disease, or hypertension. Participants with diabetes and prediabetes tended to have lower eGFR, higher systolic blood pressure, proteinuria, and BMI at baseline, and were more likely to use antiplatelet and lipid-lowering agents (Table 1).
Table 1.
Baseline Characteristics of Study Participants
| Normoglycemia | Prediabetes | Diabetes | ||
|---|---|---|---|---|
| (n = 945) | (n = 847) | (n = 1,909) | P-value | |
| Women | 445 (47.1%) | 393 (46.4%) | 845 (44.3%) | 0.30 |
| Age, years | 54.4 ± 12.8 | 59.3 ± 10.4 | 59.5 ± 9.7 | <0.001 |
| Race/ethnicity | <0.001 | |||
| White | 523 (55.3%) | 387 (45.7%) | 653 (34.2%) | |
| Black | 279 (29.5%) | 384 (45.3%) | 861 (45.1%) | |
| Other | 143 (15.1%) | 76 (9.0 %) | 395 (20.7%) | |
| Myocardial infarction or prior revascularization | 111 (11.7%) | 155 (18.3%) | 530 (27.8%) | <0.001 |
| Prior stroke | 64 (6.8 %) | 71 (8.4 %) | 224 (11.7%) | <0.001 |
| Congestive heart failure | 40 (4.2 %) | 54 (6.4 %) | 255 (13.4%) | <0.001 |
| Peripheral vascular disease | 20 (2.1 %) | 30 (3.5 %) | 196 (10.3%) | <0.001 |
| Hypertension | 697 (73.8%) | 718 (84.8%) | 1,766 (92.5%) | <0.001 |
| Systolic blood pressure, mmHg | 121.8 ± 20.1 | 125.0 ± 20.5 | 133.2 ± 22.8 | <0.001 |
| Body mass index, kg/m2 | 29.1 ± 6.8 | 31.5 ± 7.5 | 33.8 ± 7.9 | <0.001 |
| Inhibitors of RAA axis | 511 (54.4%) | 535 (63.7%) | 1520 (80.1%) | <0.001 |
| Antiplatelet agents | 293 (31.2%) | 324 (38.6%) | 1074 (56.6%) | <0.001 |
| Antidyslipidemic agents | 369 (39.3%) | 418 (49.8%) | 1,405 (74.1%) | <0.001 |
| Oral antidiabetic drugs | 0 (0.0 %) | 0 (0.0 %) | 1,024 (54.0%) | <0.001 |
| Insulin | 0 (0.0 %) | 0 (0.0 %) | 885 (46.7%) | <0.001 |
| Hemoglobin A1c, % | 5.3 ± 0.3 | 5.9 ± 0.3 | 7.6 ± 1.6 | <0.001 |
| Fasting glucose, mg/dL | 86.1 ± 7.7 | 95.4 ± 11.5 | 137.1 ± 60.7 | <0.001 |
| eGFR, mL/min/1.73m2 | 47.5 ± 16.4 | 46.1 ± 14.8 | 42.1 ± 14.0 | <0.001 |
| Urine Protein, g/24h | 0.1 (0.1, 0.5) | 0.1 (0.1, 0.4) | 0.3 (0.1, 1.6) | <0.001 |
| Urine Albumin, mg/24h | 30 (7, 271) | 26 (7, 201) | 150 (20, 973) | <0.001 |
| Serum albumin, g/dL | 4.0 ± 0.4 | 4.1 ± 0.4 | 3.8 ± 0.5 | <0.001 |
| Hematocrit, % | 39.0 ± 4.9 | 39.1 ± 4.8 | 36.3 ± 4.9 | <0.001 |
| High-sensitive CRP, mg/L | 2.0 (0.9, 5.0) | 2.7 (1.2, 7.1) | 2.8 (1.1, 6.9) | <0.001 |
| BNP, pg/mL | 30.7 (13.6, 69.4) | 30.7 (14.0, 82.2) | 50.1 (21.5, 114.7) | <0.001 |
| High-sensitivity TnT, pg/mL | 6.9 (1.5, 13.8) | 8.9 (4.2, 15.6) | 17.6 (9.7, 34.6) | <0.001 |
Categorical variables are presented as counts (percentages). Continuous variables are presented as mean ± standard deviation or median (25th, 75th percentile). P-values refer to a test for difference (analysis of variance for normally distributed continuous variables; Kruskal-Wallis test for nonnormally distributed continuous variables; and chi-square test for categorical variables).
Abbreviations: BNP, brain natriuretic peptide; CRP, C-reactive protein; TnT, troponin T; eGFR, estimated glomerular filtration rate (CKD-EPI equation); RAA, renin-angiotensin-aldosterone.
Association with outcomes
Composite renal outcome
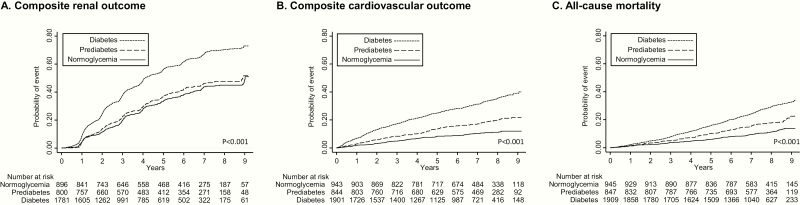
The median follow-up was 7.5 years (25th-75th percentile, 6.2–8.6 years). During this period, 989 had a 50% decline in baseline eGFR to ≤ 15 mL/min/1.73 m2 or developed ESRD (896 developed ESRD), and 1238 had a doubling of urine protein to creatinine ratio to ≥ 0.22 g/g creatinine (Table 2). In unadjusted analyses, prediabetes (versus normoglycemia) was not associated with composite renal outcome, whereas diabetes was associated with an increase of the composite renal outcome (hazard ratio [HR] 1.88; 95% confidence interval [CI], 1.66–2.12; P < 0.001) (Figure 1A). The pattern of association was similar in adjusted models (model 2 adjusted hazard ratio [aHR] 1.13; 95% CI, 0.96–1.32; P = 0.14 for prediabetes; and 1.47; 95% CI, 1.27–1.70; P < 0.001 for diabetes). In relation to the components of the composite, prediabetes was not associated with a decline in eGFR > 50% or ESRD (model 2 aHR 0.88; 95% CI, 0.71–1.10; P = 0.26), or ESRD alone (Model 2 aHR 1.01; 95% CI, 0.79–1.29; P = 0.94), but was associated with increased risk of proteinuria progression (model 2 aHR 1.23; 95% CI, 1.03–1.47; P = 0.02) (Supplementary Table 2) (7). Diabetes was associated with increased risk of ESRD and proteinuria progression (Supplementary Table 2) (7).
Table 2.
Association of Prediabetes and Diabetes With Outcomes (HR [95% CI])
| Normoglycemia | Prediabetes | Diabetes | |
|---|---|---|---|
| (n = 945) | (n = 847) | (n = 1909) | |
| Composite renal outcome | |||
| No. Events/No. Pts | 351/896 (39.2%) | 340/800 (42.5%) | 1094/1781 (61.4%) |
| Unadjusted HR | (reference) | 1.12 (0.96–1.30) | 1.88 (1.66–2.12) |
| P = 0.15 | P < 0.001 | ||
| Model 1 HR | (reference) | 1.12 (0.96–1.31) | 1.86 (1.64–2.11) |
| P = 0.13 | P < 0.001 | ||
| Model 2 HR | (reference) | 1.13 (0.96–1.32) | 1.47 (1.27–1.70) |
| P = 0.14 | P < 0.001 | ||
| Composite cardiovascular outcome | |||
| No. Events/No. Pts | 93/943 (9.9%) | 151/844 (17.9%) | 579/1901 (30.5%) |
| Unadjusted HR | (reference) | 1.85 (1.43–2.40) | 3.60 (2.89–4.49) |
| P < 0.001 | P < 0.001 | ||
| Model 1 HR | (reference) | 1.59 (1.23–2.07) | 2.97 (2.37–3.71) |
| P < 0.001 | P < 0.001 | ||
| Model 2 HR | (reference) | 1.38 (1.05–1.82) | 1.63 (1.27–2.11) |
| P = 0.021 | P < 0.001 | ||
| All-cause mortality | |||
| No. Events/No. Pts | 104/945 (11.0%) | 151/847 (17.8%) | 520/1909 (27.2%) |
| Unadjusted HR | (reference) | 1.63 (1.27–2.09) | 2.56 (2.07–3.17) |
| P < 0.001 | P < 0.001 | ||
| Model 1 HR | (reference) | 1.36 (1.06–1.76) | 2.07 (1.67–2.57) |
| P = 0.016 | P < 0.001 | ||
| Model 2 HR | (reference) | 1.28 (0.98–1.66) | 1.53 (1.20–1.95) |
| P = 0.071 | P = 0.001 |
Composite renal outcome: Development of ESRD (renal transplantation or dialysis initiation), 50% decline in baseline eGFR (CKD-EPI equation) and eGFR ≤ 15 mL/min/1.73 m2, or doubling of urine protein to creatinine ratio to ≥ 0.22 g/g creatinine.
Composite cardiovascular outcome: Congestive heart failure, myocardial infarction or stroke.
Model 1: age, sex and race/ethnicity.
Model 2: variables in model 1 plus body mass index, antiplatelet therapy, lipid lowering therapy, systolic blood pressure at baseline, coronary artery disease (defined as prior myocardial infarction or prior coronary revascularization), peripheral vascular disease, congestive heart failure, hematocrit, baseline eGFR, serum albumin and 24-hour urine protein.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HR, hazard ratio; Pts, patients
Figure 1.
Kaplan-Meier curves for composite renal outcome (A), composite CV outcome (B), and all-cause mortality (C) in participants with normoglycemia, prediabetes, or diabetes. Composite renal outcome: development of ESRD (renal transplantation or dialysis initiation), a 50% decline in baseline eGFR (CKD-EPI equation) to ≤ 15 mL/min/1.73 m2, or doubling of urine protein to creatinine ratio to ≥ 0.22 g/g creatinine. Composite CV outcome: CHF, MI, or stroke. P values were calculated with the use of log-rank tests. Abbreviations: CHF, congestive heart failure; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; MI, myocardial infarction.
Composite cardiovascular outcome
Compared with normoglycemia, prediabetes was associated with an 85% increased risk of the composite CV outcome (HR 1.85; 95% CI, 1.43–2.40; P < 0.001), while diabetes was associated with a 3.6-fold increased risk of the composite CV outcome (HR 3.60; 95% CI, 2.89–4.49; P < 0.001) in unadjusted models (Figure 1B). In model 2, prediabetes was consistently associated with a 38% increased risk of composite CV outcome (HR 1.38; 95% CI, 1.05–1.82; P = 0.02), and diabetes with a 63% increased risk of composite CV outcome (HR 1.63; 95% CI, 1.27–2.11; P < 0.001) (Table 2). The association of prediabetes and diabetes with individual components of the composite CV endpoint are presented in Supplementary Table 3 (7). Both prediabetes and diabetes were more strongly associated with an increased risk of heart failure.
In an exploratory model evaluating peripheral artery disease as the outcome of interest, prediabetes and diabetes were associated with a 2.33-fold (HR 2.36; 95% CI, 1.05–5.18; P = 0.037) and a 4.34-fold higher risk of peripheral artery disease (HR 4.34; 95% CI, 2.12–8.91; P < 0.001), respectively (Supplementary Table 4) (7).
All-cause mortality
Compared with normoglycemic individuals, the risk of all-cause mortality was increased in both prediabetes (HR 1.63; 95% CI, 1.27–2.09; P < 0.001) and diabetes (HR 2.56; 95% CI, 2.07–3.17; P < 0.001) in unadjusted models (Figure 1C). In model 1, prediabetes was associated with a 36% increased risk of all-cause mortality, while diabetes was associated with a 2.07-fold increased risk. In model 2, there was a trend for increased all-cause mortality with prediabetes (HR 1.28; 95% CI, 0.98–1.66; P = 0.07), while diabetes was associated with a 53% increased risk of this outcome (HR 1.53; 95% CI, 1.20–1.95; P = 0.001).
Effect modification and subgroup analyses
There was no evidence for effect modification of the association of glycemic control at baseline with the primary outcomes according to race, sex, or 24-hour urine protein (P-interactions > 0.1). We did find evidence for effect modification for the association of prediabetes/diabetes with all-cause mortality according to baseline eGFR (P-interaction < 0.001). In individuals with eGFR > 45 mL/min/1.73 m2, prediabetes at baseline was associated with a 2.2-fold adjusted risk of all-cause mortality (aHR 2.20; 95% CI, 1.31–3.70) and diabetes with a 2.59-fold adjusted risk of all-cause mortality (aHR 2.59; 95% CI, 1.57–4.26); in individuals with eGFR ≤ 45 mL/min/1.73 m2, the aHR for all-cause mortality in prediabetes was 1.04 (95% CI, 0.76–1.42) and 1.26 (95% CI, 0.95–1.67) for diabetes.
Sensitivity analyses: association with outcomes according to the classification of prediabetes and diabetes by HbA1c or fasting plasma glucose
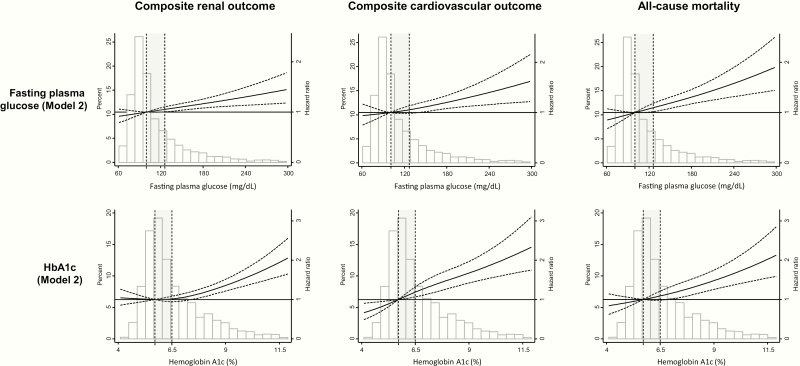
The number of participants diagnosed with prediabetes and diabetes according to HbA1c and fasting plasma glucose is shown in Supplementary Table 5 (7). The states of glucose tolerance at the end of follow-up in each baseline group are shown in Supplementary Table 6 (7). In sensitivity analyses that defined prediabetes as baseline HbA1c 5.7% to 6.4% and diabetes as HbA1c ≥ 6.5%, regardless of baseline fasting plasma glucose, the associations of prediabetes and diabetes with clinical outcomes were consistent with those in the main results. On the other hand, when participants were classified only according to fasting plasma glucose levels, there were no significant associations of prediabetes with composite renal outcome, composite CV outcome, or all-cause mortality (Supplementary Table 7) (7). These associations are more clearly apparent in restricted cubic spline analyses, where the associations of HbA1c and fasting plasma glucose are modeled in a continuous fashion (Figure 2).
Figure 2.
Composite renal outcome and composite CV outcome or all-cause mortality according to baseline fasting plasma glucose and HbA1c levels. Composite renal outcome: development of ESRD (renal transplantation or dialysis initiation), a 50% decline in baseline eGFR (CKD-EPI equation) to ≤ 15 mL/min/1.73 m2, or doubling of urine protein to creatinine ratio to ≥ 0.22 g/g creatinine. Composite CV outcome: CHF, MI, or stroke. Model 2: age, sex, race/ethnicity, body mass index, antiplatelet therapy, lipid lowering therapy, systolic blood pressure at baseline, coronary artery disease (defined as prior MI or prior coronary revascularization), peripheral vascular disease, congestive heart failure, hematocrit, baseline eGFR, serum albumin and 24-hour urine protein. Dashed lines indicate the upper and lower 95% CI for the regression line (solid black line). Vertical dashed lines indicate the cutoffs for transition from normoglycemia to prediabetes range and to diabetes range. Prediabetes range is shaded in gray. Abbreviations: CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; MI, myocardial infarction.
Exploratory model
Similar patterns of association were noted after adjusting for hsCRP, BNP, and hsTnT, such that prediabetes was associated with a 34% higher risk of the composite CV outcome (HR 1.34; 95% CI, 1.01–1.77; P = 0.04), but not with progression of CKD or all-cause mortality (Supplementary Table 8) (7). Diabetes continued to be associated with an increased risk of all outcomes examined.
Discussion
In this cohort of patients with CKD, prediabetes was not associated with increased risk of eGFR decrease or ESRD development, but was associated with an increased risk of proteinuria progression, increased risk of adverse CV outcomes and a trend towards increased all-cause mortality. These patterns of association persisted when prediabetes was defined according to HbA1c, but not when defined according to fasting plasma glucose. In subgroup analyses, the association of prediabetes with all-cause mortality appeared to be restricted to those with higher baseline eGFR.
Hyperglycemia is known to increase the production of reactive oxygen species, promote the accumulation of advanced glycation end products, activate intracellular signaling molecules such as protein kinase C, and increase the effects of the renin-angiotensin system (21, 22). In patients with diabetes these effects lead to glomerular hyperfiltration, mesangial expansion, glomerular basement membrane thickening, podocyte injury, and glomerular sclerosis, thereby promoting the development of albuminuria and the progression of CKD (22). Whether the milder hyperglycemia of prediabetes results in similar adverse renal effects is uncertain.
The association of prediabetes with the risk of kidney disease has not been consistent across studies. Some studies have suggested an increased risk of kidney disease among participants with prediabetes, although most of those were cross-sectional (23–26). In a prospective cohort study of Korean adults, impaired glucose tolerance and HbA1C 5.7% to 6.4%, but not impaired fasting glucose, were independent predictors of incident CKD (10). In a post hoc analysis of the Systolic Blood Pressure Intervention Trial (SPRINT) impaired fasting glucose was also not associated with a higher incidence of CKD, incident albuminuria, or worsening kidney function (27). A meta-analysis of 9 cohort studies reported a modest increased risk of CKD development in participants with prediabetes, but there was a significant heterogeneity across the included studies and a limited adjustment for potential confounders (28).
Few studies have assessed the effects of prediabetes in participants with CKD at baseline. A cohort study of 1165 adults with nondialysis CKD stages 1 to 5 and without diabetes (29) reported that HbA1c in the prediabetes range was not associated with increased progression to ESRD. In our study, although prediabetes was not associated with the composite renal outcome, it was associated with a higher risk of proteinuria progression. The use of change in albuminuria or proteinuria as a surrogate endpoint for progression of CKD and increased risk of ESRD in clinical trials has recently gained support (30). In diabetic kidney disease, hyperfiltration and proteinuria are proposed to be the early clinical manifestations of kidney damage (31). We hypothesize that, in patients with nondiabetic CKD, prediabetes might also contribute to hyperfiltration, glomerular dysfunction, and proteinuria. Although prediabetes was not associated with eGFR decrease or ESRD, with a median follow-up of 7.5 years, it might have contributed to these outcomes if the follow-up had been longer. Furthermore, proteinuria has been associated with increased CV risk (32), suggesting the possibility that the increased risk of adverse CV outcomes in our study may have been partly mediated by the proteinuria progression.
Many mechanistic theories have been postulated to explain the association of prediabetes with higher CV risk. These include associations with endothelial dysfunction (33), changes in myocardial substrate utilization (34), microvascular dysfunction (35), increased in proinflammatory cytokines (36), impaired fibrinolysis and hypercoagulability (37). However, in the general population, the association of prediabetes with adverse CV events is not consistent. Some studies have suggested an increased CV risk (38, 39), while other have not found significant associations (40, 41), suggesting that the CV risk may be dependent on the population studied. Perhaps the most robust observational evidence comes from a meta-analysis including 53 prospective cohort studies, which reported that prediabetes was associated with an increased risk of composite CV events, coronary heart disease, stroke, and all-cause mortality (42). Importantly, most studies evaluated in this meta-analysis included only a small portion of patients with CKD. Regarding patients with CKD, in a study by Huang et al. including non-dialysis CKD participants without diabetes, HbA1c values in the prediabetes range were associated with increased all-cause mortality (adverse CV events were not assessed in this study) (29). In this study, HbA1c was associated with increased mortality even after adjustment for fasting glucose levels (29). The observation in our study, that prediabetes defined by hemoglobin HbA1c, but not defined by fasting plasma glucose, was associated with increased risk of adverse CV outcomes, suggests that HbA1c may be a better predictor of CV events in patients with CKD. Interestingly, in our study, there was evidence for effect modification of the association of prediabetes with all-cause mortality according to baseline eGFR, such that the association appeared to be restricted to those with higher baseline eGFR. This suggests that while prediabetes may be an important risk factor for all-cause mortality in early phases of CKD, in patients with more advanced CKD (and higher CV risk), prediabetes may not further increase the risk of mortality.
In our study, prediabetes was also associated with a higher risk of peripheral artery disease events, which is in agreement with previous reports in the general population (38). However, the component of the composite CV outcome that was more strongly associated with prediabetes was heart failure. Our results are consistent with the higher risk of heart failure in prediabetes reported in the general population (43). Furthermore, in the Atherosclerosis Risk in the Community Study (ARIC) including participants without prevalent CV disease, prediabetes was associated with increased left ventricular mass, diastolic dysfunction, and subtle reduction in left ventricular systolic function (44). The identification of prediabetes as a risk factor for heart failure in CKD is important given the high incidence of heart failure in this group (45).
From a clinical perspective, our study reports a high prevalence of prediabetes in a representative cohort of participants with CKD, and highlights the risk of CV events in such individuals. Recently, SGLT2 inhibitors have been shown to decrease the risk of adverse CV and renal outcomes in diabetes (46, 47). The mechanism of benefit of SGLT2 inhibitors is likely to be independent of glucose levels and may involve a reduction in intraglomerular pressure. Whether treatment with SGLT2 can also reduce adverse CV and renal outcomes in patients with CKD and prediabetes is unknown. Our finding that diabetes is associated with higher risk for adverse CV and CKD progression is concordant with most previous studies (48–51).
Regarding the strengths of our study, we performed an analysis of a large prospective cohort with rigorous data collection. Furthermore, we evaluated an ethnically and racially diverse population of participants with varying stages of CKD with adjudicated renal and CV outcomes. There are limitations to our analysis. First, despite the adjustment for several biologically plausible confounders, there may still be residual confounding due to the observational design. We cannot exclude that some associations of prediabetes or diabetes with CV or renal outcomes could have been different if we were able to further reduce residual confounding. Second, the classification of prediabetes and diabetes was performed based on a single baseline analysis. Although this approach is common in similar studies, we cannot exclude the possibility of misclassification of some participants. Third, our classification of glucose metabolism status was based only on the fasting plasma glucose and HbA1c levels. Some participants might have been classified differently if the oral glucose tolerance test had also been evaluated. Finally, our study might have been not powered enough to detect small differences between prediabetes and normoglycemia regarding renal outcomes.
In summary, in participants of CRIC, prediabetes is common, is not associated with an increased risk of eGFR decrease or ESRD development, but is associated with proteinuria progression, and an increased risk of adverse CV outcomes. Given the high CV risk profile of patients with CKD, future studies targeting risk reduction for individuals with CKD and prediabetes are warranted.
Acknowledgments
CRIC was conducted by the CRIC Investigators and supported by the NIDDK. The funders of this study had no role in the analysis, interpretation of data, writing of the report, or the decision to submit the report for publication. Dr Mc Causland is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK102511 and R03DK122240.
Glossary
Abbreviations
- aHR
adjusted hazard ratio
- BMI
body mass index
- BNP
brain natriuretic peptide
- CHF
congestive heart failure
- CI
confidence interval
- CKD
chronic kidney disease
- CRIC
Chronic Renal Insufficiency Cohort
- CV
cardiovascular
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- HbA1c
glycated hemoglobin A1c
- HF
heart failure
- hsCRP
high-sensitivity C-reactive protein
- hsTnT
high-sensitivity troponin T
- MI
myocardial infarction
- SGLT2
sodium glucose co-transporter 2
Additional Information
Disclosure Summary: The authors declare that there are no conflicts of interest relevant to this article.
Data Availability: The dataset analyzed during the current study is available in the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository: https://repository.niddk.nih.gov/studies/cric/.
References
- 1. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 2. Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10:CD012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007;78(3):305–312. [DOI] [PubMed] [Google Scholar]
- 4. Eknoyan G, Lameire N, Eckardt K, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(1):5–14. [DOI] [PubMed] [Google Scholar]
- 5. Saran R, Robinson B, Abbott KC, et al. US renal data system 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3S1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perkovic V, Agarwal R, Fioretto P, et al. ; Conference Participants Management of patients with diabetes and CKD: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016;90(6):1175–1183. [DOI] [PubMed] [Google Scholar]
- 7. Neves JS, Correa S, Baeta Baptista R, Bigotte Vieira M, Waikar SS, Mc Causland FR. Supplementary Material of “Association of prediabetes with CKD progression and adverse cardiovascular outcomes: an analysis of the CRIC (Chronic Renal Insufficiency Cohort Study).” Harvard Dataverse Digital Repository Deposited 10 December 2019. 10.7910/DVN/7KSPDT [DOI]
- 8. Lash JP, Go AS, Appel LJ, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Group Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warren B, Pankow JS, Matsushita K, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2017;5(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim GS, Oh HH, Kim SH, Kim BO, Byun YS. Association between prediabetes (defined by HbA1C, fasting plasma glucose, and impaired glucose tolerance) and the development of chronic kidney disease: a 9-year prospective cohort study. BMC Nephrol. 2019;20(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen TK, Tin A, Peralta CA, et al. APOL1 risk variants, incident proteinuria, and subsequent eGFR decline in blacks with hypertension-attributed CKD. Clin J Am Soc Nephrol. 2017;12(11):1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3(2):136–145. [DOI] [PubMed] [Google Scholar]
- 13. Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. Jama. 1979;241(19):2035–2038. [DOI] [PubMed] [Google Scholar]
- 14. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34(1):29–34. [DOI] [PubMed] [Google Scholar]
- 15. McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. [DOI] [PubMed] [Google Scholar]
- 16. Wiviott SD, Raz I, Bonaca MP, et al. ; DECLARE–TIMI 58 Investigators Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. [DOI] [PubMed] [Google Scholar]
- 17. Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 18. Hellemons ME, Lambers Heerspink HJ, Gansevoort RT, de Zeeuw D, Bakker SJ. High-sensitivity troponin T predicts worsening of albuminuria in hypertension; results of a nested case-control study with confirmation in diabetes. J Hypertens. 2013;31(4):805–812. [DOI] [PubMed] [Google Scholar]
- 19. Welsh P, Woodward M, Hillis GS, et al. Do cardiac biomarkers NT-proBNP and hsTnT predict microvascular events in patients with type 2 diabetes? Results from the ADVANCE trial. Diabetes Care. 2014;37(8):2202–2210. [DOI] [PubMed] [Google Scholar]
- 20. Zelniker TA, Morrow DA, Mosenzon O, et al. Cardiac and inflammatory biomarkers are associated with worsening renal outcomes in patients with type 2 diabetes mellitus: observations from SAVOR-TIMI 53. Clin Chem. 2019;65(6):781–790. [DOI] [PubMed] [Google Scholar]
- 21. Gallagher H, Suckling RJ. Diabetic nephropathy: where are we on the journey from pathophysiology to treatment? Diabetes Obes Metab. 2016;18(7):641–647. [DOI] [PubMed] [Google Scholar]
- 22. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Metcalf PA, Baker JR, Scragg RK, Dryson E, Scott AJ, Wild CJ. Microalbuminuria in a middle-aged workforce. Effect of hyperglycemia and ethnicity. Diabetes Care. 1993;16(11):1485–1493. [DOI] [PubMed] [Google Scholar]
- 24. Hoehner CM, Greenlund KJ, Rith-Najarian S, Casper ML, McClellan WM. Association of the insulin resistance syndrome and microalbuminuria among nondiabetic native Americans. The Inter-Tribal Heart Project. J Am Soc Nephrol. 2002;13(6):1626–1634. [DOI] [PubMed] [Google Scholar]
- 25. Plantinga LC, Crews DC, Coresh J, et al. ; CDC CKD Surveillance Team Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5(4):673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang XL, Lu JM, Pan CY, Tian H, Li CL. A comparison of urinary albumin excretion rate and microalbuminuria in various glucose tolerance subjects. Diabet Med. 2005;22(3):332–335. [DOI] [PubMed] [Google Scholar]
- 27. Vieira MB, Neves JS, Leitao L, et al. Impaired fasting glucose and chronic kidney disease, albuminuria, or worsening kidney function: a secondary analysis of the SPRINT. J Clin Endocrinol Metab. 2019;104(9):4024-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Echouffo-Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG. Association between prediabetes and risk of chronic kidney disease: a systematic review and meta-analysis. Diabet Med. 2016;33(12):1615–1624. [DOI] [PubMed] [Google Scholar]
- 29. Trivin C, Metzger M, Haymann JP, et al. ; NephroTest Study Group Glycated hemoglobin level and mortality in a nondiabetic population with CKD. Clin J Am Soc Nephrol. 2015;10(6):957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coresh J, Heerspink HJL, Sang Y, et al. ; Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28(4):1023–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agrawal V, Marinescu V, Agarwal M, McCullough PA. Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat Rev Cardiol. 2009;6(4):301–311. [DOI] [PubMed] [Google Scholar]
- 33. Eringa EC, Serne EH, Meijer RI, et al. Endothelial dysfunction in (pre)diabetes: characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Rev Endocr Metab Disord. 2013;14(1):39–48. [DOI] [PubMed] [Google Scholar]
- 34. Nielsen R, Jorsal A, Iversen P, et al. Heart failure patients with prediabetes and newly diagnosed diabetes display abnormalities in myocardial metabolism. J Nucl Cardiol. 2018;25(1):169–176. [DOI] [PubMed] [Google Scholar]
- 35. Sörensen BM, Houben AJ, Berendschot TT, et al. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: the maastricht study. Circulation. 2016;134(18):1339–1352. [DOI] [PubMed] [Google Scholar]
- 36. Grossmann V, Schmitt VH, Zeller T, et al. Profile of the immune and inflammatory response in individuals with prediabetes and type 2 diabetes. Diabetes Care. 2015;38(7):1356–1364. [DOI] [PubMed] [Google Scholar]
- 37. Meigs JB, Mittleman MA, Nathan DM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. Jama. 2000;283(2):221–228. [DOI] [PubMed] [Google Scholar]
- 38. Warren B, Pankow JS, Matsushita K, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2017;5(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eastwood SV, Tillin T, Sattar N, Forouhi NG, Hughes AD, Chaturvedi N. Associations between prediabetes, by three different diagnostic criteria, and incident CVD differ in South Asians and Europeans. Diabetes Care. 2015;38(12):2325–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schöttker B, Müller H, Rothenbacher D, Brenner H. Fasting plasma glucose and HbA1c in cardiovascular risk prediction: a sex-specific comparison in individuals without diabetes mellitus. Diabetologia. 2013;56(1):92–100. [DOI] [PubMed] [Google Scholar]
- 41. Deedwania P, Patel K, Fonarow GC, et al. Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population-based cohort study. Int J Cardiol. 2013;168(4):3616–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. Bmj. 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nielson C, Lange T. Blood glucose and heart failure in nondiabetic patients. Diabetes Care. 2005;28(3):607–611. [DOI] [PubMed] [Google Scholar]
- 44. Skali H, Shah A, Gupta DK, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk In the Community study. Circ Heart Fail. 2015;8(3):448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18(4):1307–1315. [DOI] [PubMed] [Google Scholar]
- 46. Wanner C, Inzucchi SE, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. [DOI] [PubMed] [Google Scholar]
- 47. Perkovic V, Jardine MJ, Neal B, et al. ; CREDENCE Trial Investigators Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. [DOI] [PubMed] [Google Scholar]
- 48. Tsai WC, Wu HY, Peng YS, et al. Risk factors for development and progression of chronic kidney disease: a systematic review and exploratory meta-analysis. Medicine. 2016;95(11):e3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Evans M, Fryzek JP, Elinder CG, et al. The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis. 2005;46(5):863–870. [DOI] [PubMed] [Google Scholar]
- 50. Chang YT, Wu JL, Hsu CC, Wang JD, Sung JM. Diabetes and end-stage renal disease synergistically contribute to increased incidence of cardiovascular events: a nationwide follow-up study during 1998-2009. Diabetes Care. 2014;37(1):277–285. [DOI] [PubMed] [Google Scholar]
- 51. Tonelli M, Muntner P, Lloyd A, et al. ; Alberta Kidney Disease Network Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380(9844):807–814. [DOI] [PubMed] [Google Scholar]