Abstract
The dynamics of the resting-state activity in brain functional networks are complex, containing meaningful patterns over multiple temporal scales. Such physiologic complexity is often diminished in older adults. Here we aim to examine if the resting-state complexity within functional brain networks is sensitive to functional status in older adults and if repeated exposure to transcranial direct current stimulation (tDCS) would modulate such complexity. Twelve older adults with slow gait and mild-to-moderate executive dysfunction and 12 age- and sex-matched controls completed a baseline resting-state fMRI (rs-fMRI). Ten participants in the functionally-limited group then completed ten 20-minute sessions of real (n=6) or sham (n=4) tDCS targeting the left prefrontal cortex over a two-week period as well as a follow-up rs-fMRI. The resting-state complexity associated with seven functional networks was quantified by averaging the multiscale entropy (MSE) of the blood oxygen level-dependent (BOLD) time-series for all voxels within each network. Compared to controls, functionally-limited group exhibited lower complexity in the motor, ventral attention, limbic, executive and default mode networks (F>6.3, p<0.02). Within this group, those who received tDCS exhibited greater complexity within the ventral, executive and limbic networks (p<0.04) post intervention as compared to baseline, while no significant changes in sham group was observed. This study provides preliminary evidence that older adults with functional limitations had diminished complexity of resting-state brain network activity and repeated exposure to tDCS may increase that resting-state complexity, warranting future studies to establish such complexity as a marker of brain health in older adults.
Keywords: tDCS, resting-state complexity, BOLD, brain networks, functionally-limited older adults
Introduction
Age-related decline in cognitive and motor function diminish the functional independence of older adults [1]. Successful completion of cognitive and motor tasks depends upon the capacity of functional brain networks to process and exchange information with each other over multiple temporal scales [2]. Recent blood oxygen level-dependent (BOLD) functional MRI (fMRI) evidence suggests that even during “resting state,” the dynamics of spontaneous brain activity are not random. Instead, they are “complex”, containing non-random, fractal-like patterns with selfsimilar structures across multiple scales of time [3]. Biological aging has been linked to a loss of such “complexity” in the dynamics of multiple physiologic processes, including resting-state brain activity [4–6], and such diminished physiologic complexity is associated with loss of the system’s function. It seems reasonable, therefore, that individuals with diminished performance on assessments of cognitive and/or motor function would have less complexity of resting-state brain activity.
Transcranial direct current stimulation (tDCS) is a safe and noninvasive technique that selectively modulates the excitability of cortical neurons and their connected neural networks [7]. tDCS works by creating electric fields that polarize populations of neurons, which modulates resting membrane potential and thus the likelihood of firing (Nitsche & Paulus, 2000). Mounting evidence suggests that tDCS targeting the left dorsal lateral prefrontal cortex (dlPFC)—a primary brain region subserving cognitive function—may enhance both cognitive[8,9] and mobility [10,11] in older adults. tDCS-induced facilitation of neural activation and a resulting increase in the interaction between connected neural networks may alter the dynamics of brain activity [12]. In this study, we investigated resting-state brain network complexity in older adults with and without mild-to-moderate functional limitations, and subsequently, explored whether such complexity was sensitive to the effects of a 10-session tDCS intervention targeting prefrontal regions. We hypothesized that as compared to age-matched controls, those with functional limitations would have lower resting-state complexity within brain networks involved in cognitive-motor control. We further hypothesized that within those with functional limitations, a tDCS intervention targeting the dlPFC may increase the resting-state complexity, particularly within the networks connecting to dlPFC (e.g., attention, executive networks).
Experimental Procedures
Participants
We performed a secondary analysis of a completed double-blinded, pilot randomized controlled trial on the effects of tDCS on cognitive-motor function in functionally-limited older adults [9]. Eighteen ambulatory, non-demented older adults with both mild-to-moderate cognitive “executive” dysfunction and slow gait, were randomized to receive a two-week tDCS or sham intervention. Executive dysfunction was defined as performance on the Trail Making Test (TMT) B [13] below the 25th percentile of age- and education-based norms [13]. Slow gait was defined as a preferred 4-meter over-ground walking speed <1.0 m/s [14].
Exclusion criteria for the pilot study were the following: unable to stand or ambulate unassisted; severe depression as defined by a Geriatric Depression Scale (GDS) score >12 [15]; self-reported severe arthritis or lower-extremity pain; physician-diagnosed peripheral neuropathy affecting the lower extremities; major neurological disorders (e.g., stroke, Parkinson’s disease); moderate to severe dementia as defined by the score of Mini-Mental State Examination (MMSE) less than 18; contraindications to tDCS or MRI, including use of neuro-active drugs, self-report of seizure within the past two years, open wounds on the scalp, BMI>40, metallic or electrical bio-implants, or claustrophobia.
All participants of the above study completed functional assessments at baseline, post-intervention and again two weeks later. A subset of this cohort (n = 12) completed fMRIs at baseline, and ten of these participants completed an fMRI following intervention (six recevied tDCS; 4 recevied sham).
To test our first hypothesis that resting state complexity would be lower in older adults with functional limitations as compared to controls, we also leveraged the data from a healthy control cohort of 12 age- and sex-matched participants who completed baseline fMRIs within the original pilot study. The inclusion criteria of control group were: a TMT B performance >25th percentile of age and education based norms, a preferred gait speed ≥1ms, and a MMSE score ≥24. Exclusion criteria were similar to the original study as described above.
Ethics Statement
This study was approved by Institutional Review Board of Hebrew SeniorLife, and conducted according to the principles of the Declaration of Helsinki. All participants provided the written informed consent form as approved by the institutional review board.
tDCS
tDCS was delivered with the Starstim® system (Neuroelectrics Inc, Barcelona, Spain) using two saline-soaked 35 cm2 synthetic sponge electrodes placed on the participant’s scalp. The anode (i.e., positive electrode) was placed over the F3 region of the 10/20 EEG electrode placement guide and the cathode (i.e., negative electrode) was placed over the right supraorbital margin (Fp2) [16]. The computer modeling of current flow showed that this tDCS montage induced maximum electric field in the left prefrontal region [9] and the increased excitability of this regions has been linked to improved cognitive [17] and motor performance [10]. Each session of tDCS consisted of 20 minutes of continuous stimulation at a maximum intensity of 2.0 mA. At the beginning of stimulation, current automatically ramped up from 0.1mA in increments of 0.1 mA over 60 seconds in order to minimize discomfort at the onset of stimulation [18]. During the first session, participants were instructed to notify the study personnel if and when they felt any uncomfortable sensations. tDCS was then delivered at an intensity of 0.1mA below the highest level reached at this session and all sessions thereafter. At the end of each session, current was automatically ramped down to 0.0 mA over 60 seconds.
For sham stimulation, the same electrode montage, ramp-up and session duration were used; however, current was automatically ramped down to zero over 60 seconds after ramp-up. This procedure was chosen because cutaneous sensations arising from tDCS diminish considerably within the first minute of stimulation [19].
Participants and research staff administering tDCS were blinded to participant group assignment. Participants were randomly assigned a code linked to their assigned intervention, as developed by the study statistician. Two separate codes were used for each condition to help ensure staff blinding. Personnel uninvolved in any other study procedure preconfigured the tDCS and sham stimulation parameters for each code within the Starstim™ software. At the end of the each tDCS session, participants completed a side effects questionnaire [20]. A blinding efficacy questionnaire was completed after the final tDCS session. Participants were asked to state whether they believed they received the tDCS or sham intervention and their confidence in this belief on a scale of 0–10, with 10 reflecting greatest confidence.
MRI
All MRIs were obtained using a GE Signa HDxt 3 Tesla system with an 8-channel head coil within the Center for Advanced MR Imaging at the Beth Israel Deaconess Medical Center. A T1-weighted MDEFT (Modified Driven Equilibrium Fourier Transform) scan (inversion time=1100ms, TR=6.616ms, TE=2.84ms, flip angle=15°, resolution= 1.000mm × 0.9375 mm × 0.9375 mm) was first acquired for whole-brain high-resolution anatomy. Participants then completed three separate six-minute runs of an eyes-open resting-state fMRI scan (resolution= 3 mm × 3.75 mm × 3.75 mm, TR=3.2 s, TE= 30 ms, flip angel= 90°, axial slices=52), such that each resulting BOLD time series contained 120 sampled points. Immediately prior to each resting-state run, participants were instructed to visually fixate on a cross within the MR bore for the entire duration of the run.
Data analysis
Resting-state fMRI
Resting-state fMRI data were analyzed using a custom combination of software packages, including FSL, SPM, and 4dfp routines, as previously described [21–23]. The following steps were performed: volume registration, alignment to the T1 anatomy, warp into Talairach space, 8mm kernel smoothing, and scaling to a percentage of the mean. A band-pass filter was used to remove fluctuations below 0.01 and above 0.08Hz [24]. Filtered data were entered into a general linear model to remove the effects of 6 degrees of motion, and ventricles, white matter and the global signal nuisance signals were regressed from the time-series. The residual time series in each voxel was then used to calculate multiscale entropy. We also removed the first two points and the last point of each BOLD time series to avoid the artifacts due to the instability of MRI scanning at the beginning and the potential motion artifacts at the end of the scan. BOLD time series consisting of 117 points were thus used in subsequent analyses.
Multiscale entropy
Multiscale entropy (MSE) [25] was used to quantify the complexity of the resting-state BOLD time series associated with each brain voxel. MSE quantifies the degree of re-occurrence of repetitive patterns in a bio-physiological time-series across multiple temporal scales. Less re-occurrence of patterns over multiple scales reflects greater complexity. Here, MSE was quantified using time scales from one to five. To do so, the BOLD time series of each voxel was “coarse-grained” five times by averaging point values using non-overlapping windows of length equaling to the scale factor τ (i.e., τ= 1 to 5). The sample entropy of each coarse-grained time series was then calculated, as defined by the negative natural logarithm of the conditional probability that a time-series, having repeated itself within a tolerance r for m points (defined pattern length), will also repeat itself for m + 1 points without self-matches. Here, we chose m = 1 and r = 0.35 [26,27]. The number of data points in the coarse-grained time series at the maximum scale thus equaled to 23 (i.e., 117 divided by 5), greater than the 10m to 20m points (i.e., 10 to 20 as m=1) required for reliable estimation of sample entropy [25,28].
We then calculated the MSE curves associated with seven known large-scale functional brain cortical networks (i.e., visual, motor, dorsal attention, ventral attention, limbic, executive, and default mode networks) as described by Yeo and colleagues [22]. Network parcellation was Talairach-normalized, resampled to 3×3×3 voxels, and separated into individual networks. Network-level MSE curves were then calculated by averaging entropy values, by time scale, across all voxels contained within each network (Figure 1). We observed that network-level entropy values were consistent across the three fMRI runs and a crossover existed at scale 3, such that the complexity in functionally-limited older adults was smaller on scale 1 and 2, but greater in scales 3 to 5, compared to the healthy controls. We thus averaged entropy values separately across scales 1 and 2 (i.e., short-scale complexity) and 3 to 5 (i.e., large-scale complexity) for each network in each fMRI run. Finally, these complexity metrics derived from each run were averaged and used for statistical analysis.
Figure 1. The multi-scale entropy (MSE) curves (mean ± S.D.) of seven functional brain cortical networks (i.e., visual, motor, dorsal attention (DA), ventral attention (VA), limbic, executive and default mode (DM) networks) in functionally-limited older adults (in black) and age-matched healthy controls (in blue).
The entropy at each scale was calculated by averaging the entropies at this scale for each voxel within the identified network. Visual inspection revealed that, in general, the entropy values at scales 1 and 2 were most sensitive to group.
Statistical analysis
Analyses were performed with JMP Pro 13 software (SAS Institute, Cary NC). A crossover was observed in the MSE curves (Figure 1) as we described in Methods, thus the complexity metrics (i.e., short-scale and large-scale complexity averaged across three fMRI runs) was used in separate models. Variable normality was examined with the Shapiro-Wilk W test and homogeneity of variance was determined with the Levene test. For those outcomes that were normally distributed and exhibited homogeneity of variance, one-way ANOVAs were used to examine the main effect of group (functionally-limited and controls) on each complexity metric related to each brain network at baseline. Repeated-measures, one-way ANOVAs were also used to examine, within the functionally-limited group, if the complexity associated with each brain network was significantly different from pre- to post-intervention. Given the small sample size and the pilot nature of this work, we separately examined the effects of the tDCS and sham intervention. Non-parametric Wilcoxon Rank Sum tests were utilized when outcomes were not normally distributed and/or did not exhibit homogeneity of variance. Significance level for this pilot study was set to p<0.05.
Results
Useable MRI data was obtained for all participants in the functionally-limited group (Mean ± standard deviation (S.D.) age: 76.2 ± 9.5 years; 8 females; BMI: 30.2 ± 5.8) and in the control group (age: 74.7 ± 8 years; 8 females; BMI: 28.8 ± 5.7). Ten of 12 participants within the functionally-limited group completed the intervention and the follow-up MRI scan. Six received tDCS and the other four received sham stimulation, and all completed all 10 sessions of their assigned intervention. The maximum intensity of tDCS also did not differ between groups [tDCS: 1.9 ± 0.3mA, range=1.7–2.0mA; sham (ramp periods only): 2.0 ± 0.1mA, range=1.8–2.0 mA]. No unexpected side effects of tDCS or adverse events were reported.
At baseline, the resting-state complexity (i.e., both short- and large-scale complexity) of each of the seven brain networks was normally distributed and exhibited homogeneity of variance. The functionally-limited group exhibited lower short-scale resting-state complexity, as compared to controls, within the motor, ventral attention, limbic, executive and default mode networks (Figure 2, F1, 23>6.3, p<0.02). A trend towards lower complexity within the dorsal attention network was also observed in the functionally-limited group as compared to controls (F1, 23=3.4, p=0.08). No significant difference in visual network was observed (F1, 23=1.2, p=0.28). No significant difference was observed in the large-scale complexity associated with any examined brain network (F1, 23<1.34 p>0.26).
Figure 2. The resting-state complexity (mean ± S.D.) associated with seven functional brain networks in functionally-limited older adults and age-matched healthy controls.
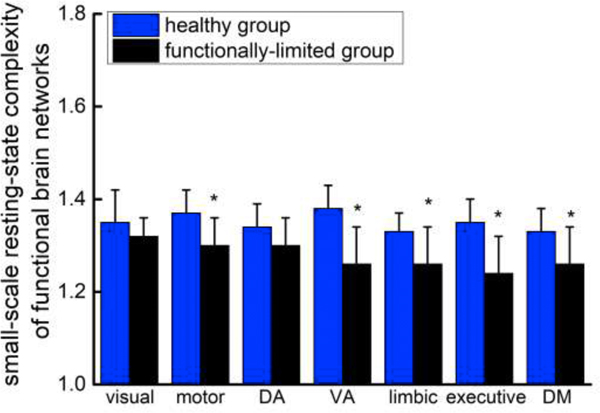
The resting-state complexity of each network was calculated for each participant by averaging the entropy values at scales 1 and 2. Complexity associated with the motor, ventral attention (VA), limbic, executive and default mode (DM) networks were all lower in the functionally-limited group as compared to controls (F1, 23>6.3, p<0.02). A trend towards lower complexity was also observed within the dorsal attention (DA) network (F1, 23=3.4, p=0.08). No group difference in resting-state complexity was observed within the visual network.
Within the functionally-limited group, the resting-state complexity associated with each brain network was similar between those randomized to receive the tDCS and sham intervention. Within the tDCS arm, the short-scale resting-state complexity associated with the limbic and executive function networks did not exhibit homogeneity of variance. For these two networks, Wilcoxon Rank Sum Tests revealed that as compared to baseline, resting-state complexity was greater following tDCS (z>1.9, p<0.04, Figure 3). Resting-state complexity values for the other five networks were normally distributed and demonstrated homogeneity of variance. Of these, short-scale complexity associated with the ventral attention network was greater following tDCS intervention as compared to baseline (F1, 5=5.4, p=0.04, Figure 3). Both short- and long-scale complexity associated with each network was similar between pre- and post-intervention in the group who completed the sham intervention. No effects of current intensity on the complexity metric were observed.
Figure 3. The resting-state complexity (mean ± S.D.) of functional brain networks before and after a two-week tDCS or sham intervention.
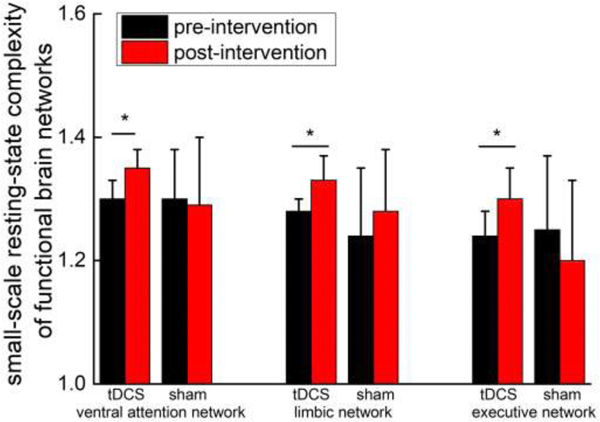
Within the group that received tDCS, resting state was greater following intervention, as compared to baseline, in the limbic, executive and ventral attention networks (p<0.05). In contrast, resting state network complexity did not change in the group that received the sham intervention.
Discussion
This pilot study provides preliminary evidence that older adults with functional limitations exhibit lower complexity in the spontaneous activity within specific functional brain networks. Moreover, this resting-state complexity may be sensitive to repeated exposure to noninvasive tDCS intervention. Those who completed a 10-session tDCS intervention designed to facilitate the excitability of the left prefrontal cortex exhibited an increase of their resting-state complexity, particularly within the executive, ventral attention and limbic networks, which are each structurally connected to the prefrontal regions [29]. Together, these preliminary observations suggest that resting-state complexity may be a modifiable physiologic phenomenon that provides important insight into cognitive-motor function in older adults.
Considerable evidence indicates that the output of a given physiological system is not completely random. Instead, during basal or “free-running” conditions, spontaneous fluctuations in system output are complex, meaning they contain physiologically-meaningful patterns across multiple temporospatial scales [30]. The degree and characteristics of such complexity can be quantified using nonlinear tools derived from chaos theory, such as multiscale entropy or detrended fluctuation analysis. Using these tools, researchers have linked the degree of complexity contained within a given system’s output over time to the functionality of that system as defined by the ability to respond or adapt to stressors (e.g., perturbation applied to the system) [31]. With respect to brain activity, Yang et al. [6] reported that the resting-state complexity, as measured by MSE of BOLD fMRI signals, within the default mode network areas was associated with cognitive performance—those with lower complexity also exhibited poorer performance on short-term memory, orientation and attention tasks. Here, we demonstrated that that resting-state complexity of functional brain networks, particularly over short scales (corresponding to time range of 3 seconds to 6 seconds), may also be sensitive to functional status. Older adults with slowed gait and executive dysfunction had lower complexity in multiple functional brain networks compared to their healthy counterparts. Future longitudinal studies are thus warranted to establish the sensitivity of multi-scale dynamics of resting-state brain network activity to changes in health status, aging into senescence, and disease processes.
We observed that as compared to an age- and sex-matched control group, functionally-limited older adults exhibited lower overall resting-state complexity within functional brain networks. These between-group differences were observed specifically when complexity was computed from relatively short time scales. These results may indicate that cognitive and motor dysfunction may be related to the change of dynamics in resting-state brain activity within these temporal scales. Through future work is needed to confirm observed trends, these results may indicate that physiologic or pathologic stressors and/or states (e.g., vigilance) may affect resting-state brain activity uniquely by time scale [5,24,32]. In support of our findings, Yang and his colleagues [24] observed that patients with schizophrenia had varied characteristics in resting-state brain complexity. Compared to healthy control, the complexity across all scales (scale 1 to 5) were lower in some brain regions (e.g., left middle frontal) in schizophrenia, but were relatively greater short-scale entropy (scale 1~2) but lower large-scale entropy (scale 3 to 5) within some other regions (e.g., inferior frontal).Future studies are warranted to delineate the physiologic mechanisms in health, aging and disease that give rise to time-scale-specific differences in resting-state brain activity.
The observed decreased complexity in the temporal dynamics of resting-state activity in motor, ventral attention, limbic, executive and default mode networks in the functionally-limited group are supported by traditional resting-state fMRI analyses linking the strength of functional connectivity within and between several of these large-scale networks to both cognitive and/or motor functions in various populations. For example, Yuan and colleagues [33] reported that in older adults, those with greater strength of functional connectivity within the motor, attention and executive networks tended to walk faster. In separate studies, those with greater functional connectivity within frontoparietal control network had faster walking speed [34]; Nestor et al. [35] demonstrated that those suffering from mild Alzheimer’s disease had relatively weak functional connectivity within the limbic network; and Greicius et al. [36] demonstrated that in healthy younger adults, those with greater functional connectivity within the default mode network performed better in working memory task.. It is thus highly desirable to combine the functional connectivity and complexity metric together to more fully characterize the temporospatial communication within and between brain networks.
Despite the small sample size, our results suggest that within the functionally-limited group, a two-week, ten-session tDCS intervention targeting the prefrontal cortices increased resting-state complexity within the ventral attention, limbic and executive networks. At least four of the six participants demonstrated an increase in the complexity associated with each of these large-scale networks. Numerous studies have demonstrated that tDCS induces improvements in functional performance via modulating the excitability of brain functional regions. The general conclusion is that tDCS enables more efficient recruitment of brain resources for the execution of a given cognitive-motor task [37]. Here, our piloting results indicate that the low-level current delivered by tDCS may alter the dynamics of resting-state brain network activity across multiple scales of time, and potentially, augment the capacity of such networks to effectively respond to a given cognitive-motor task. Results of the current study thus warrant larger, more definitive studies to determine if tDCS intervention can increase and/or restore age- and disease-related loss of resting-state complexity, along with the clinical meaningfulness of such changes.
Due to small sample size (n=6 in tDCS and 4 in sham group), we did not investigate the association between tDCS-induced changes in resting-state complexity and functional outcomes. Future research is thus warranted to not only confirm the results of this pilot study, but also establish associations between tDCS-induced changes of resting-state complexity and functional performance. A relatively long MRI TR time was used in the initial study and as such, the temporal resolution of resting-state activity was relatively low. To this end, we were only able to investigated entropy of the BOLD signal at five scales of time. Studies with longer scans and/or faster TR times are thus needed to better understand the neurophysiological basis of “complexity” and its relationship to brain health and functional outcomes. It is also of note that the current study focused only on the dynamics of resting-state cortical activity and does not provide insight into subcortical function. Additionally, other complexity metrics, such as approximate entropy, should also be implemented in future work to more fully explore the complex structural and functional characteristics of the entire brain. This pilot study nevertheless demonstrated that the resting-state complexity of spontaneous brain activity may be a sensitive marker of functional status in older adults and may be responsive to noninvasive brain stimulation interventions.
Highlights.
The dynamics of the resting-state brain activity is complex.
The resting-state brain complexity is sensitive to cognitive and motor function.
Multi-session tDCS targeting dlPFC increases the resting-state brain complexity.
Acknowledgments
Funding: This study was supported by NIA training grant (T32-AG023480), an NIA career development grant (K01-AG044543-01A1), an NIA research project grant (R01 AG041785), the Dr. Ralph and Marian Falk Medical Research Trust, the Boston Claude D. Pepper Older Americans Independence Center (P30-AG013679), and the Hebrew SeniorLife Applebaum grant. L.L. holds the Irving and Edyth S. Usen Chair in Geriatric Medicine at Hebrew SeniorLife.
Footnotes
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM, Gait and cognition: a complementary approach to understanding brain function and the risk of falling, J. Am. Geriatr. Soc. 60 (2012) 2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jor’dan AJ, Poole VN, Iloputaife I, et al. , Executive network activation is linked to walking speed in older adults: functional MRI and TCD ultrasound evidence from the MOBILIZE Boston study, J. Gerontol. A-Biol. 72 (2017) 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fox MD, Snyder AZ, Vincent JL, Raichle ME, Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior, Neuron. 56 (2007) 171–184. [DOI] [PubMed] [Google Scholar]
- [4].Liu Z, Ma H, Poole VN, et al. , Effects of multi-session repetitive transcranial magnetic stimulation on motor control and spontaneous brain activity in multiple system atrophy: a pilot study, Front. Behav. Neurosci. 12 (2018) 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smith RX, Yan L, Wang DJ, Multiple time scale complexity analysis of resting state FMRI, Brain. Imaging. Behav. 8 (2014) 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang AC, Huang CC, Yeh HL, et al. , Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: a multiscale entropy analysis, Neurobiol. Aging. 34 (2013) 428–438. [DOI] [PubMed] [Google Scholar]
- [7].Nitsche MA, Paulus W, Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation, J. Physiol. 527 (2000) 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hsu WY, Ku Y, Zanto TP, Gazzaley A, Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: a systematic review and metaanalysis, Neurobiol. Aging. 36 (2015) 2348–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Manor B, Zhou J, Harrison R, Transcranial direct current stimulation may improve cognitive-motor function in functionally limited older adults, Neurorehab. Neural. Re. 32 (2018) 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Manor B, Zhou J, Jor’dan A, Zhang J, Fang J, Pascual-Leone A, Reduction of dual-task costs by noninvasive modulation of prefrontal activity in healthy elders, J. Cog. Neurosci. 28 (2016) 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhou J, Hao Y, Wang Y, Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control, Eur. J. Neurosci. 39 (2014) 1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Keeser D, Meindl T, Bor J, et al. , Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI, J. Neurosci. 31 (2011) 15284–15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arbuthnott K, Frank J, Trail making test, part B as a measure of executive control: validation using a set-switching paradigm, J. Clin. Exp. Neuropsychol. 22 (2000) 518–528. [DOI] [PubMed] [Google Scholar]
- [14].Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB, Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability, New. Engl. J. Med. 332 (1995) 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van Marwijk HW, Wallace P, de Bock GH, Hermans JO, Kaptein AA, Mulder JD, Evaluation of the feasibility, reliability and diagnostic value of shortened versions of the geriatric depression scale, Brit. J. Gen. Pract. 45 (1995) 195–199. [PMC free article] [PubMed] [Google Scholar]
- [16].Boggio PS, Rigonatti SP, Ribeiro RB, et al. , A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression, Int. J. Neuropsychoph. 11 (2008) 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fregni F, Boggio PS, Nitsche M, et a., Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory, Exp. Brain. Res. 166 (2005) 23–30. [DOI] [PubMed] [Google Scholar]
- [18].Antal A, Alekseichuk I, Bikson M, Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines, Clin. Neurophysiol. 128 (2017) 1774–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gandiga PC, Hummel FC, Cohen LG, Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation, Clinic. Neurophysiol. (2006) 117, 845–850. [DOI] [PubMed] [Google Scholar]
- [20].Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F, A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation, Int. J. Neuropsychoph. 14 (2011) 1133–1145. [DOI] [PubMed] [Google Scholar]
- [21].Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A, Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner, P. Natl. Acad. Sci. USA. 108 (2011) 21229–21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yeo BT, Krienen FM, Sepulcre J, et al. , The organization of the human cerebral cortex estimated by intrinsic functional connectivity, J. Neurophysiol. 106 (2011) 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A, Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network, J. Neurosci. 34 (2014) 12049–12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang AC, Hong CJ, Liou YJ, et al. , Decreased resting- state brain activity complexity in schizophrenia characterized by both increased regularity and randomness, Hum. Brain. Mapp. 36 (2015) 2174–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Costa M, Goldberger AL, Peng CK, Multiscale entropy analysis of complex physiologic time series, Phys. Rev. Lett, 89 (2002) 068102. [DOI] [PubMed] [Google Scholar]
- [26].Yang AC, Tsai SJ, Lin CP, Peng CK, A strategy to reduce bias of entropy estimates in resting-state fMRI signals, Front. Neurosci. 12 (2018) 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou J, Poole VN, Wooten T, et al. , Multi-scale dynamics of spontaneous brain activity is associated with walking speed in older adults, J. Gerontol. A-Biol. (2019) doi: 10.1093/gerona/glz231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Costa M, Goldberger AL, Peng CK, Multiscale entropy analysis of biological signals, Phys. Rev. E. 71 (2005) 021906. [DOI] [PubMed] [Google Scholar]
- [29].Perlstein WM, Elbert T, Stenger VA, Dissociation in human prefrontal cortex of affective influences on working memory-related activity, P. Natl. Acad. Sci. USA, 99 (2002) 1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lipsitz LA, Physiological complexity, aging, and the path to frailty, Sci. SAGE. KE. (2004) pe16. [DOI] [PubMed] [Google Scholar]
- [31].Manor B, Costa MD, Hu K, et al. , Physiological complexity and system adaptability: evidence from postural control dynamics of older adults. J. Appl. Physiol. 109 (2010) 17861791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yousefi B, Shin J, Schumacher EH, Keilholz SD, Quasi-periodic patterns of intrinsic brain activity in individuals and their relationship to global signal, Neuroimage. 167 (2018) 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yuan J, Blumen HM, Verghese J, Holtzer P, Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: A resting-state fMRI study, Hum. Brain. Mapp. 36 (2015) 1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lo OY, Halko MA, Zhou J, Harrison R, Lipsitz LA, Manor B, Gait Speed and Gait Variability Are Associated with Different Functional Brain Networks, Front. Aging. Neurosci. 9 (2017) 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nestor PJ, Fryer TD, Smielewski P, Hodges JR, Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment, Ann. Neurol. 54 (2003) 343–351. [DOI] [PubMed] [Google Scholar]
- [36].Greicius MD, Krasnow B, Reiss AL, Menon V, Functional connectivity in the resting brain: a network analysis of the default mode hypothesis, P. Natl. Acad. Sci. USA., 100 (2003) 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Filmer HL, Mattingley JB, Dux PE, Improved multitasking following prefrontal tDCS, Cortex. 49 (2013) 2845–2852. [DOI] [PubMed] [Google Scholar]



