1. Introduction
a. Lubricin – original discoveries
Lubricin (also known as proteoglycan 4 or PRG4), expressed by the PRG4 gene, is a mucin-like 224 kDa glycoprotein known for its lubricating properties and was originally found within the synovial fluid of diarthrodial joints in bovine and human samples and secreted by synovial fibroblasts 1–7. Lubricin contains many O-linked glycosylations located in a central post-translationally modified mucin domain. The length of the mucin-like repeats in its structure varies across species but is generally proportional to the size of the animal 4,6. However, lubricin can also be expressed with several different isoforms in the N-terminus and each isoform may have different functions 8. Like other mucins and mucin-like substances, lubricin prevents the attachment of cells to surfaces which enables its activity as a boundary lubricant by reducing friction 9.
b. Friction and apoptosis
The ability of lubricin to exert its lubricating effects and decrease friction is dependent upon its concentration, indicated by an increase in joint friction with decreased lubricin concentrations 4. Optimal concentrations of lubricin were found to be between 200–260 μg/mL for proper lubricating activity and reduced friction coefficients in non-cartilage surfaces 7.
Patients born with a rare genetic mutation in the PRG4 gene display camptodactyly-arthropathy-coxa vara-pericarditis (CACP) syndrome which is characterized by increased joint friction, apoptosis and cellular morphological changes that eventually lead to joint failure 10–12. Lubricin null mice (Prg4 −/−) (the orthologous genetic model of CACP syndrome in humans) were bred in order to study joint mechanics and to examine histological abnormalities. Prg4 gene trap (GT/GT) mice have increased friction and activated caspase 3 staining in their chondrocytes, which is reversed after the Prg4 gene is turned back on through Cre-mediated recombination 13. The coefficient of friction and amount of activated caspase-3 staining in lubricin null mice given purified human synoviocyte lubricin was significantly lower than mice that were lubricin null littermates receiving only sham injections 14. Moreover, lubricin null mice had higher levels of ONOO− in femoral head cartilage and synoviocytes when compared to controls 14. Additionally, there was increased gene expression of the cytokines IL-1β and TNF-α, and caspases 3, 6 and 7 in the cartilage of lubricin null mice compared to controls indicating the anti-inflammatory role of PRG4 in preventing apoptosis that is activated through the intrinsic pathway 14.
Other studies reveal that lubricin has a cellular protective effect, as knee joints from lubricin null mice had increased numbers of apoptotic cells which was measured via anti-caspase-3 and terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) 15. In rats with anterior cruciate ligament transection (ACLT), expression levels of lubricin and deposition of lubricin on cartilage surfaces were significantly decreased when assessed at 3 and 5 weeks post transection 16. At 3 weeks, exercised rats with ACLT had expression levels of lubricin that were even lower than rats that had undergone ACLT only, indicating increased damage in the joint 16. Moreover, the rats with ACLT + exercise also showed an increase in activated caspase-3 staining of chondrocytes in comparison to ACLT only rats which was further increased at 5 weeks 16. Interestingly, treatment of ACLT rats with intra-articular purified human lubricin significantly lowered activated caspase-3 staining of chondrocytes compared to those with no lubricin 16.
Osteoarthritis (OA) is a common age-related degenerative disease of the joints that consists of cartilage degeneration, inflammation, and bone remodeling, characterized by pain, joint stiffness, and swelling 17. The causes of OA are still not well understood but are thought to stem from genetic and orthopedic abnormalities, gender, metabolic diseases, environmental factors such as sports injuries, and obesity 17. There is still an unmet need for treatment of patients with osteoarthritis, but several reports point to lubricin not only as a factor in the progression of the disease process and but also as a potential effective therapy for its treatment 11,18,19.
OA in humans presents with downregulated PRG4 in lateral femoral condyle cartilage, which may be location and isoform dependent 20. In a sheep model of osteoarthritis, lubricin immunostaining was decreased in cartilage which also corresponded with downregulated mRNA levels of PRG4 in comparison to sham treated animals 19. Moreover, PRG4 was shown to prevent osteoarthritis in a mouse model using intra-articular injected adenoviral vectors conjugated to antibodies designed to over express PRG421,22. It appears that one of the mechanisms of cellular protection is via PRG4 inhibition of cellular pathways that lead to catabolism of cartilage 21.
Rheumatoid arthritis (RA) is a type of arthritis that has autoimmune and inflammatory components 23. The symptoms of RA are very similar to those of OA however, the causes and co-morbidities are different 23,24. Autoimmunity leads to joint destruction and swelling in addition to inflammation of the synovium 23,24. Interestingly, lubricin concentrations in synovial fluid from both OA and RA patients is significantly lower than that of controls 25. Moreover, synovial fluid from patients with RA failed to lubricate rabbit knee joints after induced injury, indicating that there is a loss of lubricin that occurs within the RA disease process similar to that of OA 26.
These findings indicate the very significant role that PRG4 has in mitigating joint friction and in preventing chondrocyte cell death in normal and disease states. These studies also suggest that PRG4 should be considered as an intra-articular therapeutic and potential biomarker in patients that sustain joint related injuries or in patients with CACP, OA, and RA in order to provide a protective effect on the joint 27.
2. More recent discoveries
Although lubricin was originally found in cartilage and synovial fluid, and secreted by the synovial fibroblasts, it has since been found in other tissues such as lung, heart, liver, eye, uterus, cervix, prostate, bladder, and bone, indicating that it has a multifunctional role that is not limited solely to joints and friction reduction 5,6,28. Therefore, due to lubricin’s ubiquitous nature, there have been numerous reports of lubricin as a potential therapeutic in several non-tribologic disorders which are described below29.
a. Extra articular non-tribologic functions of lubricin
Bowel and bladder permeability
Interstitial cystitis-bladder pain syndrome (IC-BPS) is a relatively common disorder characterized by painful and increased frequency of urination from disruption of epithelial cells in the bladder. Irritable bowel syndrome (IBS) is a common disorder of the large intestine involving pain, cramping, bloating, gas, diarrhea and/or constipation and has a high co-morbidity with IC-BPS, indicating cross-talk of the disorders 30. Currently, patients with either IBS or IC-BPS have very limited treatment options 30,31. In rats, treatment with protamine sulfate (PS) induced bladder and subsequently colon permeability which mimic IC-BPS and IBS in humans 32. When rats that were exposed to PS and later treated with lubricin, there was a reduction of the PS induced permeability in both the bladder and the colon 29. Therefore, lubricin could have potential as a therapeutic for patients with IC-BPS or IBS.
Patients that undergo abdominal surgery have a high chance of developing intra-abdominal lesions, with up to 20% requiring treatment despite newer less invasive surgical techniques 33,34. There are currently no proven methods to completely prevent the formation of intra-abdominal lesions resulting in a clinical need for improved therapies 35. Intra-abdominal adhesions created in rats via both cecal abrasion and cecal enterotomy with only the cecal abrasions leading to significant lesion formation 35. In rats with cecal abrasions, rhPRG4 treatment applied directly after surgery prevented both inflammation and fibrosis of the lesioned areas, indicating a potential role for lubricin as a post-operative treatment in patients that undergo abdominal surgeries 35.
b. Clinical uses for PRG4
Dry eye (xerophthalmia) and dry mouth (xerostomia) diseases
Dry eye (xerophthalmia) is a condition in which there are not enough tears produced in order to lubricate the ocular surface leading to discomfort, light sensitivity, burning sensations, inflammation, and potential eye damage 36,37. There are several other conditions which lead to dry eye including the use of contact lenses, dry eye disease, Sjogren’s syndrome, Stevens-Johnson syndrome, vitamin deficiencies, RA, and refractive surgery 37. The current treatments for dry eye include hyaluronic acid (HA), polyvinyl alcohol, povidone, hydroxypropyl guar, and cellulose derivatives and treatment depends upon the cause of dry eye 36. Dry eye typically has an inflammatory component and if it is severe enough, may need anti-inflammatory or immunosuppressant treatments 36.
Dry mouth (xerostomia) is the feeling of oral dryness and most often occurs in older adult women38. The most common complaints with xerostomia are difficulty talking, chewing and swallowing in addition to altered taste 38. Some of the most common causes of xerostomia include cancer treatment, various medications, and Sjogren syndrome 38.
Sjogren syndrome (SS) is an autoimmune disease that mainly results in both dry eye and dry mouth but can affect other organ systems 39. Women are more effected by SS than men and it most often occurs in later years 39. However, the causes of SS are not well known as it seems there are many contributing factors 39. Due to the lubricating and anti-inflammatory properties of PRG4, several studies have indicated PRG4 as a therapeutic for both dry eye and dry mouth. It has been documented that lubricin deficiency in the eye has a negative impact on corneal functioning 28. In lubricin null mice, there was a significant increase in fluorescein staining and inflammation compared to wild type animals, signifying that substantial corneal damage occurs without lubricin present 28.
HA is commonly used in patients with dry eye and it is commercially available. Recently, a clinical trial indicated that lubricin is an excellent lubricating factor in patients with moderate dry eye disease and is more effective than HA at treating dry eye 40. Moreover, a deficiency of lubricin as seen in lubricin null mice, led to increased corneal fluorescein staining, illustrating the need for lubricin to reduce friction by acting as a natural lubricant in the space between the cornea and eyelid 28. Recently, cathepsin S (CTSS) was found to be increased in the tears of patients with SS 41. Interestingly, CTSS was found to decrease ocular lubrication via degradation of PRG4 in human tears 42. Therefore, it seems that one of the mechanisms of dry eye in SS patients occurs via a reduction of PRG4 at the ocular surface 42.
These results discussed above suggest that PRG4 would be a better treatment for dry eye than current treatments such as HA. Commercialization efforts are currently under way for production of PRG4 intended for lubricating eye and mouth drops by Lubris, LLC and Novartis in the case of the dry eye indication.
c. Anti-inflammatory actions of lubricin
TLR4, CD44, NF-κB, NLRP3
Recently, lubricin has been studied to determine if it serves as a ligand for various receptors that are involved in cellular inflammatory cascades. Several studies indicate that lubricin binds to the integrin CD44 and some members of the toll-like receptor (TLR) family which suggests a role for lubricin in regulating inflammatory cascades 43,44. Moreover, as detailed below, PRG4 reduces nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) nuclear translocation and nod-like receptor pyrin domain containing 3 (NLRP3) inflammasome activation leading to decreases in the production of proinflammatory cytokines.
CD44 is a glycoprotein transmembrane cell surface receptor that is well-known for binding to hyaluronic acid (HA) and internalizing it via receptor-mediated endocytosis and is highly involved in inflammatory cascades 45–48. CD44 signaling seems to have function in both pro-inflammatory and anti-inflammatory pathways, as studies show contradictory reports about its function depending upon ligand binding, cell type, and type of infection. For example, in CD44 knockout mice, renal dysfunction and inflammation was delayed after lipopolysaccharide (LPS) treatment 49. On the contrary, CD44 deficient mice with Escherichia coli infections had increased lung inflammation and pro-inflammatory cytokine release 50,51. Therefore, ligand binding to CD44 may elicit both pro and anti-inflammatory cascades depending upon cell type and other factors.
Besides HA, PRG4 was found to be one of CD44’s ligands. Using direct ELISA, recombinant human PRG4 (rhPRG4) was found to bind to recombinant CD44 receptors with a higher affinity than CD44’s known ligand, HA and was concentration dependent 44. However, HA blocked the binding of rhPRG4 to CD44 in a concentration dependent manner 44. Interestingly, removal of the mucin domain’s sialic acid and O-glycosylations of rhPRG4 resulted in an even greater affinity of rhPRG4 to CD44. In the same study, RA fibroblast-like synoviocytes (RA-FLS) proliferation was induced via IL-1β and TNF-α which was suppressed with rhPRG4 44. IM7, an antibody directed against CD44, blocked the suppressive effects of rhPRG4, further verifying that CD44 is one of PRG4’s receptors 44.
The toll-like receptors are a large 11-member family of receptors that are associated with host defense mechanisms for innate immunity 52,53. The TLR family of receptors is responsible for the detection of pathogen-associated molecular patterns (PAMPs) from bacteria, fungi, and viruses and are considered one family of the pathogen-recognition receptors (PRRs) 52. Upon recognition of said pathogens, a cascade of immune events occurs in the host including cytokine release and transcriptional factor activation 52. Therefore, antagonists of the TLRs have been utilized as a means to reverse immune cascades in order to treat various immune related disorders 54,55.
Both rhPRG4 and native human PRG4 (nhPRG4) bind to Toll-like receptors 2 and 4 (TLR2 and TLR4) and binding is concentration dependent which was verified via ELISA and via a TLR2/TLR4 HEK-293 reporter cell line 43. Moreover, nhPRG4 was effectively able to block TLR2 and TLR4 activation when the agonists Pam3CSK4 and LPS were used 43. TLR2 and TLR4 activation can also be stimulated via human synovial fluid (SF) samples from patients with OA and RA which are effectively blocked with both rhPRG4 and nhPRG4 43. Interestingly, OA and RA SF have naturally depleted levels of PRG4 which coincides with increased TLR2 and TLR4 activation 43. Therefore, PRG4 seems to be an effective antagonist of both TLR2 and TLR4, thus blocking downstream inflammatory cellular responses.
Activation of inflammatory pathways including ligand binding of both the TLRs and CD44 stimulates NF-κB translocation into the nucleus which triggers a cascade of inflammatory related events 44,56. NF-κB is a family of transcription factors and are well-known for being activated during innate and acquired immune responses and subsequently stimulate the release of chemokines, cytokines, anti-apoptotic proteins, and stress-response proteins 56,57. Moreover, these same chemokines, cytokines and proteins can also trigger NF-κB translocation in a reciprocal fashion 56. However, more recent findings indicate that the role of NF-κB is tissue specific and is implicated in the production of both inflammatory and anti-inflammatory genes spurring characterization of the canonical and alternative NF-κB signaling pathways 56. These results indicate that the function of NF-κB is difficult to elucidate and that activation of NF-κB stimulates release of both pro and anti-inflammatory factors which would increase or decrease apoptosis depending upon cellular dynamics.
Recent reports indicate that lubricin can prevent NF-κB translocation. For example, treatment of RA-FLS with TNFα induces NF-κB translocation into the nucleus which can be blocked with both rhPRG4 or MG132 (a known inhibitor of NF-κB translocation) 44. Moreover, blockade of NF-κB translocation from rhPRG4 was significantly reduced with the IM7 antibody 44. Similarly, in THP-1 macrophages treated with monosodium urate (MSU) crystals, NF-κB translocation was significantly increased which was reversed by PRG4 treatment 58.
The NLRP3 inflammasome is another facilitator of inflammatory cascades within the cell 58–60. NLRP3 can be stimulated by a variety of factors within the cell such as NF-κB translocation and when “primed”, initiates pro-caspase-1 activity, creating the inflammasome that promotes the cleavage of pro-interleukin 1 beta (IL-1β) and pro-interleukin 18 (IL-18) into their mature forms to further drive the inflammatory response 58–60. PRG4 inhibited both the inflammasome and pro-caspase-1 activation in macrophages that were induced into an inflammatory state via MSU crystals, which are typically deposited in joints in patients with gout 58.
Interestingly, pro-inflammatory cytokines and growth factors also play a role in regulating the production and release of lubricin 61,62. The cytokines tumor necrosis factor alpha (TNF-alpha) and IL-1 decrease both PRG4 mRNA and protein expression from both articular chondrocytes and synoviocytes whereas the growth factor TGF-β increased PRG4 mRNA expression 61,62. PRG4 has also been shown to regulate the release of various cytokines and chemokines 58. For example, MSU treated THP-1 macrophages induced significant increases in the cytokines IL-1β and TNF-alpha, and the chemokines IL8 and MCP-1 all of which were significantly reduced with treatment of rhPRG4 58.
Due to the above findings, a role for PRG4 as an anti-inflammatory biologic has emerged and indicates that a lubricin deficiency may serve as a biomarker of inflammatory processes and inflammation. Lubricin’s antagonism with receptors of the TLR family and interaction with the CD44 receptor indicate that it may be an important treatment option in a variety of disorders with an immunological basis (Figure 1). Therefore, further studies of rhPRG4 and its interaction in immune pathways have more recently been discovered as will be discussed in the next section.
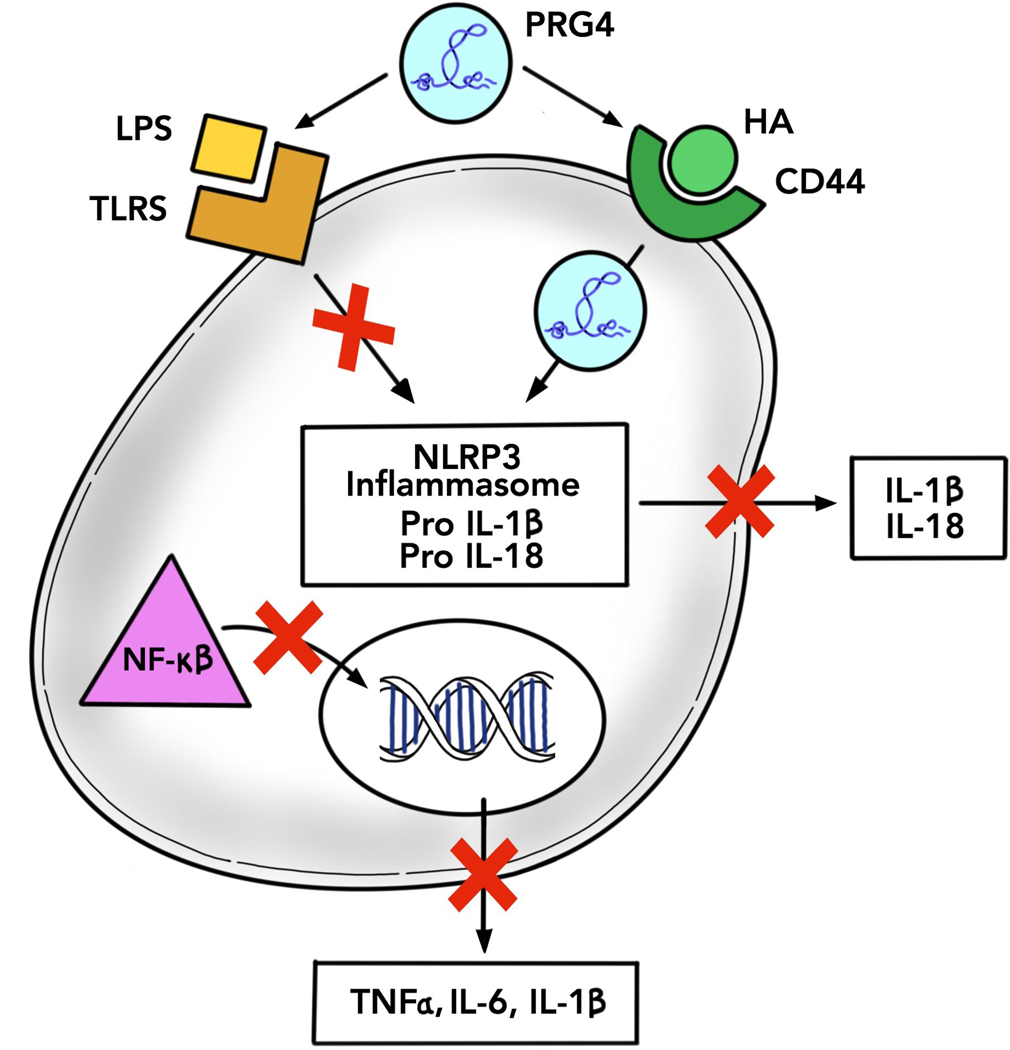
Figure 1: Proposed mechanism of action of lubricin in inflammatory cascades.
Various ligands bind to both the TLRs and CD44, namely, lipopolysaccharide (LPS) and hyaluronic acid (HA). Activation of these receptors activate internal cellular pathways involved in inflammation. The inflammasome becomes activated and initiates conversion of pro IL-18 and pro-IL1β to their mature forms to be released from the cell. NF-κβ translocates to the nucleus which also initiates the production and release of other cytokines. When cells are treated with rhPRG4, these inflammatory cascades are halted. rhPRG4 strongly binds to both TLR2 and TLR4 as an antagonist and competitor with other ligands. rhRPG4 also becomes internalized into the cell via CD44 allowing rhPRG4 to both prevent activation of the inflammasome and translocation of NF-κβ thereby preventing the formation of cytokines.
d. Potential therapeutic role of PRG4 in sepsis
Sepsis background
Sepsis is a major health concern due to its high mortality rate and ineffective current treatments. Sepsis was previously technically defined as a syndrome resulting from systemic inflammatory response syndrome (SIRS) which stems from bacterial and viral infections creating a cascade of inflammatory responses within the host also known as the “cytokine storm”. However, newer reports have proposed that certain terminology be removed in order for clinicians to more accurately diagnosis sepsis and initiate treatment 63. There are also cases in which sepsis can occur from non-infectious conditions such as pancreatitis, burns, and tissue injury 64. In the absence of no adequate intervention or treatment, the inflammatory cascades and cytokine storm usually lead to septic shock, organ failure and death 64.
Because sepsis is a highly multi-factorial syndrome, it is very difficult to treat and most treatments that have been tested in clinical trials were ineffective 64. Another reason that sepsis is challenging to treat is the delay between the time the patient presents with symptoms to the time that white blood cell, blood cultures, and serum lactate test results are received, resulting in a delay of diagnosis 65. Currently, results can take up to 72 hours and the tests are unreliable for sepsis diagnostics 65,66. Presently, broad-spectrum antibiotics, vasopressors, and drugs to treat hypotension are administered to patients with suspected sepsis. Patients treated more quickly are then likely to have a more positive outcome 64,67. Even with current sepsis treatments, there is still a 30% death rate of patients with sepsis, indicating the need for more effective treatments 68.
Therefore, new biomarkers and detection techniques for earlier identification of sepsis are needed 69–72. A new report details an antibody based small microelectrode biosensor to test levels of the cytokine Interleukin-6 (IL-6) within as little as 2.5 minutes. A significant improvement in treatment time for septic patients 70. The biosensor can also be engineered to test multiple biomarkers at the same time which would significantly improve patient wait times and treatment paradigms 70. Other types of biosensors have also been explored, including electrochemical, optical, and microfluidic based sensors 73. However, the issues with biosensors include high cost, low sensitivity, and short shelf-life all of which create a hinderance on use in the clinic 73. However, once the logistical issues are removed, biosensors will become a critical piece of sepsis diagnosis in the future.
e. Cytokine role in sepsis
role of IL-6 in sepsis
The glycoprotein, Interleukin-6 (IL-6) is a cytokine that is released during the body’s inflammatory response resulting from pathogen invasion or tissue damage 74,75. IL-6 is secreted from many different cell types including leukocytes, vascular endothelial (VE) cells, mesenchymal cells, fibroblasts, osteoblasts and tumor cells 76. IL-6 is produced and released via stimulation by other cytokines such as IL-1β and TNF-alpha, via the host cell’s initial recognition of PAMPs through PRR’s, or from damage associated molecular patterns (DAMPs) and acts as a warning signal to other cells 74,77. IL-6 is typically thought of as a pro-inflammatory cytokine but does function in the anti-inflammatory pathway as well 75. When IL-6 expression and secretion becomes dysregulated severe immune related disorders develop, one of them being sepsis 77. Therefore, drugs, biologics and therapeutics that are able to block secretion and/or binding of IL-6 to its receptors have become a major research focus 77.
Sepsis has two phases: the hyper-inflammatory and the immunosuppressive phases both of which contain different cytokine spikes78. There are several cytokines that can be tested as biomarkers of the severity of a sepsis infection although IL-6 is considered one of the best early biomarkers for prognosis of both adult and neonate patients with a severe sepsis infection and/or septic shock 72,79–83.
Therefore, based upon the aforementioned information, IL-6 stood out as an important marker for early cellular inflammatory responses to which lubricin could make a potential therapeutic and impact if it was able to reduce IL-6 protein and gene expression.
Results from our lab
Due to lubricin’s role in the inflammatory pathways involving CD44, TLR4 and NLRP3 as discussed above, our lab has identified lubricin as a possible therapeutic for patients with sepsis due to its ability to lower both protein levels and gene expression of IL-6 in human umbilical vascular endothelial cells (HUVECs) and in human lung microvascular endothelial cells (HLMVECs) 84. HUVECs and HLMVECs were treated with LPS to induce a high inflammatory state, resulting in significantly increased IL-6 protein levels and gene expression, both of which were reversed to control levels with 50, 100 and 150 μg/mL rhPRG4 treatment 84. Moreover, HLMVECs were treated with plasma from septic patients to induce an inflammatory response and rhPRG4 significantly lowered IL-6 protein levels in almost 75% of the patients 84. Because lubricin biosynthesis is downregulated by various cytokines including IL-1β and TNF-alpha, it is possible lubricin could not only serve as a therapeutic but also as a biomarker of inflammation and infections, including sepsis 61.
Conclusions
Lubricin’s original role in the reduction of joint friction has evolved to a multitude of reports on the non-tribologic functions of lubricin, notably within inflammatory pathways. Lubricin is an antagonist of TLR2 and TLR4, which is one mechanism of its role in reducing inflammation. Moreover, lubricin also interacts with CD44 in order to block NF-κB translocation into the nucleus and formation of the NLRP3 inflammasome. Therefore, these results led to further research into lubricin as an anti-inflammatory and possible biomarker of sepsis. Lubricin effectively decreased protein levels and gene expression of IL-6, a prominent sepsis biomarker. We conclude that lubricin has the potential to be an effective therapeutic for many diseases with underlying inflammation, including but not limited to sepsis.
Bullet Points:
Lubricin is a lubricant and has a cellular protective effect as it prevents cellular death and decreases cellular inflammation
Lubricin is an antagonist of TLR2 and TLR4 and gains entry into the cell via CD44 to block inflammatory processes indicating a therapeutic role
Lubricin is downregulated by a number of cytokines, indicating it could serve as an inflammatory and sepsis biomarker
Synopsis.
Proteoglycan 4 (aka PRG4 or Lubricin), a mucin-like glycoprotein, was originally classified as a lubricating substance within diarthrodial joints. More recently, lubricin has been found in other tissues and has been implicated in two inflammatory pathways within the cell, via the toll-like receptors (TLR) and CD44. Lubricin is an antagonist of TLR2 and TLR4 and gains entry into cells via the CD44 receptor. Because of lubricin’s action on these receptors, downstream processes of inflammation are halted, thereby preventing release of cytokines (a hallmark of inflammation and sepsis) from the cell, indicating lubricin’s role as a biomarker and possible therapeutic for sepsis.
Acknowledgments
Funding source: NIH R01AR067748
Footnotes
Gregory Jay has ownership and financial interest in the formation of recombinant human lubricin production (Lubris LLC).
Holly Richendrfer has no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Swann DA, Slayter HS, Silver FH. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J Biol Chem. 1981;256(11):5921–5. [PubMed] [Google Scholar]
- 2.Swann DA, Sotman S, Dixon M, et al. The isolation and partial characterization of the major glycoprotein (LGP-I) from the articular lubricating fraction from bovine synovial fluid. Biochem J. 1977;161(3):473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swann DA, Hendren RB, Radin EL, et al. The lubricating activity of synovial fluid glycoproteins. Arthritis Rheum. 1981;24(1):22–30. [DOI] [PubMed] [Google Scholar]
- 4.Swann DA, Silver FH, Slayter HS, et al. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J. 1985;225(1):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27(3):594–600. [PubMed] [Google Scholar]
- 6.Ikegawa S, Sano M, Koshizuka Y, et al. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet. 2000;90(3–4):291–7. [DOI] [PubMed] [Google Scholar]
- 7.Jay GD, Tantravahi U, Britt DE, et al. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 2001;19(4):677–87. [DOI] [PubMed] [Google Scholar]
- 8.Lord MS, Estrella RP, Chuang CY, et al. Not all lubricin isoforms are substituted with a glycosaminoglycan chain. Connect Tissue Res. 2012;53(2):132–41. [DOI] [PubMed] [Google Scholar]
- 9.Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcelino J, Carpten JD, Suwairi WM, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 1999;23(3):319–22. [DOI] [PubMed] [Google Scholar]
- 11.Bao JP, Chen WP, Wu LD. Lubricin: a novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol Biol Rep. 2011;38(5):2879–85. [DOI] [PubMed] [Google Scholar]
- 12.Bahabri SA, Suwairi WM, Laxer RM, et al. The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum. 1998;41(4):730–5. [DOI] [PubMed] [Google Scholar]
- 13.Hill A, Waller KA, Cui Y, et al. Lubricin restoration in a mouse model of congenital deficiency. Arthritis Rheumatol. 2015;67(11):3070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waller KA, Zhang LX, Jay GD. Friction-Induced Mitochondrial Dysregulation Contributes to Joint Deterioration in Prg4 Knockout Mice. Int J Mol Sci. 2017;18(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waller KA, Zhang LX, Elsaid KA, et al. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci U S A. 2013;110(15):5852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsaid KA, Zhang L, Waller K, et al. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthritis Cartilage. 2012;20(8):940–8. [DOI] [PubMed] [Google Scholar]
- 17.Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016;59(5–6):333–9. [DOI] [PubMed] [Google Scholar]
- 18.Flannery CR, Zollner R, Corcoran C, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60(3):840–7. [DOI] [PubMed] [Google Scholar]
- 19.Young AA, McLennan S, Smith MM, et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8(2):R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neu CP, Reddi AH, Komvopoulos K, et al. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum. 2010;62(9):2680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan MZ, Erez A, Guse K, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5(176):176ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan MZ, Cerullo V, Cela R, et al. Treatment of osteoarthritis using a helper-dependent adenoviral vector retargeted to chondrocytes. Mol Ther Methods Clin Dev. 2016;3:16008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. [DOI] [PubMed] [Google Scholar]
- 24.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–108. [DOI] [PubMed] [Google Scholar]
- 25.Kosinska MK, Ludwig TE, Liebisch G, et al. Articular Joint Lubricants during Osteoarthritis and Rheumatoid Arthritis Display Altered Levels and Molecular Species. PLoS One. 2015;10(5):e0125192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsaid KA, Jay GD, Warman ML, et al. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52(6):1746–55. [DOI] [PubMed] [Google Scholar]
- 27.Lord MS, Farrugia BL, Rnjak-Kovacina J, et al. Current serological possibilities for the diagnosis of arthritis with special focus on proteins and proteoglycans from the extracellular matrix. Expert Rev Mol Diagn. 2015;15(1):77–95. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt TA, Sullivan DA, Knop E, et al. Transcription, translation, and function of lubricin, a boundary lubricant, at the ocular surface. JAMA Ophthalmol. 2013;131(6):766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenwood-Van Meerveld B, Mohammadi E, Latorre R, et al. Preclinical Animal Studies of Intravesical Recombinant Human Proteoglycan 4 as a Novel Potential Therapy for Diseases Resulting From Increased Bladder Permeability. Urology. 2018;116:230.e1-.e7. [DOI] [PubMed] [Google Scholar]
- 30.Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1(2):133–46. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa T, Ishizuka O, Ueda T, et al. Current and emerging drugs for interstitial cystitis/bladder pain syndrome (IC/BPS). Expert Opin Emerg Drugs. 2015;20(4):555–70. [DOI] [PubMed] [Google Scholar]
- 32.Greenwood-Van Meerveld B, Mohammadi E, Tyler K, et al. Mechanisms of Visceral Organ Crosstalk: Importance of Alterations in Permeability in Rodent Models. J Urol. 2015;194(3):804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menzies D, Ellis H. Intestinal obstruction from adhesions--how big is the problem? Ann R Coll Surg Engl. 1990;72(1):60–3. [PMC free article] [PubMed] [Google Scholar]
- 34.Gutt CN, Oniu T, Schemmer P, et al. Fewer adhesions induced by laparoscopic surgery? Surg Endosc. 2004;18(6):898–906. [DOI] [PubMed] [Google Scholar]
- 35.Oh J, Kuan KG, Tiong LU, et al. Recombinant human lubricin for prevention of postoperative intra-abdominal adhesions in a rat model. J Surg Res. 2017;208:20–5. [DOI] [PubMed] [Google Scholar]
- 36.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81; quiz 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayo Clinic. Dry eyes. 2019; https://www.mayoclinic.org/diseases-conditions/dryeyes/symptoms-causes/syc-20371863 Accessed February 12, 2019.
- 38.Millsop JW, Wang EA, Fazel N. Etiology, evaluation, and management of xerostomia. Clin Dermatol. 2017;35(5):468–76. [DOI] [PubMed] [Google Scholar]
- 39.Maslinska M, Przygodzka M, Kwiatkowska B, et al. Sjogren’s syndrome: still not fully understood disease. Rheumatol Int. 2015;35(2):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambiase A, Sullivan BD, Schmidt TA, et al. A Two-Week, Randomized, Double-masked Study to Evaluate Safety and Efficacy of Lubricin (150 mug/mL) Eye Drops Versus Sodium Hyaluronate (HA) 0.18% Eye Drops (Vismed(R)) in Patients with Moderate Dry Eye Disease. Ocul Surf. 2017;15(1):77–87. [DOI] [PubMed] [Google Scholar]
- 41.Hamm-Alvarez SF, Janga SR, Edman MC, et al. Tear cathepsin S as a candidate biomarker for Sjogren’s syndrome. Arthritis Rheumatol. 2014;66(7):1872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regmi SC, Samsom ML, Heynen ML, et al. Degradation of proteoglycan 4/lubricin by cathepsin S: Potential mechanism for diminished ocular surface lubrication in Sjogren’s syndrome. Exp Eye Res. 2017;161:1–9. [DOI] [PubMed] [Google Scholar]
- 43.Alquraini A, Garguilo S, D’Souza G, et al. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther. 2015;17:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Sharif A, Jamal M, Zhang LX, et al. Lubricin/Proteoglycan 4 Binding to CD44 Receptor: A Mechanism of the Suppression of Proinflammatory Cytokine-Induced Synoviocyte Proliferation by Lubricin. Arthritis Rheumatol. 2015;67(6):1503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Culty M, Nguyen HA, Underhill CB. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992;116(4):1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hua Q, Knudson CB, Knudson W. Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. J Cell Sci. 1993;106 ( Pt 1):365–75. [DOI] [PubMed] [Google Scholar]
- 47.Underhill CB, Thurn AL, Lacy BE. Characterization and identification of the hyaluronate binding site from membranes of SV-3T3 cells. J Biol Chem. 1985;260(13):8128–33. [PubMed] [Google Scholar]
- 48.Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8(3):208–20. [DOI] [PubMed] [Google Scholar]
- 49.Rampanelli E, Dessing MC, Claessen N, et al. CD44-deficiency attenuates the immunologic responses to LPS and delays the onset of endotoxic shock-induced renal inflammation and dysfunction. PLoS One. 2013;8(12):e84479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Q, Teder P, Judd NP, et al. CD44 deficiency leads to enhanced neutrophil migration and lung injury in Escherichia coli pneumonia in mice. Am J Pathol. 2002;161(6):2219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Windt GJ, van ‘t Veer C, Florquin S, et al. CD44 deficiency is associated with enhanced Escherichia coli-induced proinflammatory cytokine and chemokine release by peritoneal macrophages. Infect Immun. 2010;78(1):115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen K, Huang J, Gong W, et al. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007;7(10):1271–85. [DOI] [PubMed] [Google Scholar]
- 53.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. [DOI] [PubMed] [Google Scholar]
- 54.Patra MC, Choi S. Recent progress in the development of Toll-like receptor (TLR) antagonists. Expert Opin Ther Pat. 2016;26(6):719–30. [DOI] [PubMed] [Google Scholar]
- 55.Gao W, Xiong Y, Li Q, et al. Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front Physiol. 2017;8:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawrence T The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–34. [DOI] [PubMed] [Google Scholar]
- 58.Qadri M, Jay GD, Zhang LX, et al. Recombinant human proteoglycan-4 reduces phagocytosis of urate crystals and downstream nuclear factor kappa B and inflammasome activation and production of cytokines and chemokines in human and murine macrophages. Arthritis Res Ther. 2018;20(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Afonina IS, Zhong Z, Karin M, et al. Limiting inflammation-the negative regulation of NF-kappaB and the NLRP3 inflammasome. Nat Immunol. 2017;18(8):861–9. [DOI] [PubMed] [Google Scholar]
- 60.He Y, Hara H, Nunez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 2016;41(12):1012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones AR, Flannery CR. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater. 2007;13:40–5; discussion 5. [DOI] [PubMed] [Google Scholar]
- 62.Flannery CR, Hughes CE, Schumacher BL, et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254(3):535–41. [DOI] [PubMed] [Google Scholar]
- 63.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. Bmj. 2016;353:i1585. [DOI] [PubMed] [Google Scholar]
- 65.Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33(7):1538–48. [DOI] [PubMed] [Google Scholar]
- 66.Rhee C, Murphy MV, Li L, et al. Lactate Testing in Suspected Sepsis: Trends and Predictors of Failure to Measure Levels. Crit Care Med. 2015;43(8):1669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.CDC. Sepsis https://www.cdc.gov/sepsis/datareports/index.html 2017. Accessed February 15, 2019.
- 69.Bloos F, Reinhart K. Rapid diagnosis of sepsis. Virulence. 2014;5(1):154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell C, Ward AC, Vezza V, et al. Development of a needle shaped microelectrode for electrochemical detection of the sepsis biomarker interleukin-6 (IL-6) in real time. Biosens Bioelectron. 2019;126:806–14. [DOI] [PubMed] [Google Scholar]
- 71.Ricarte-Bratti JP, Brizuela NY, Jaime-Albarran N, et al. IL-6, MMP 3 and prognosis in previously healthy sepsis patients. Rev Fac Cien Med Univ Nac Cordoba. 2017;74(2):99–106. [PubMed] [Google Scholar]
- 72.Biron BM, Ayala A, Lomas-Neira JL. Biomarkers for Sepsis: What is and What Might Be? Biomarker Insights. 2015;10s4:BMI.S29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar S, Tripathy S, Jyoti A, et al. Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review. Biosens Bioelectron. 2019;124–125:205–15. [DOI] [PubMed] [Google Scholar]
- 74.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. [DOI] [PubMed] [Google Scholar]
- 76.Kobeissi Z, Zanotti-Cavazzoni S. Biomarkers of sepsis Marshall JC, for the International Sepsis Forum (Li Ka Shing Knowledge Inst, Toronto, Ontario, Canada, St. Michael’s Hosp, Toronto, Ontario, Canada, Univ of Toronto, Toronto, Ontario, Canada; Friedrich-Schiller Univ, Jena, Germany) Crit Care Med 37: 2290–2298, 2009. Year Book of Critical Care Medicine. 2010;2010:227–8. [Google Scholar]
- 77.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–70. [DOI] [PubMed] [Google Scholar]
- 78.Tamayo E, Fernandez A, Almansa R, et al. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur Cytokine Netw. 2011;22(2):82–7. [DOI] [PubMed] [Google Scholar]
- 79.Rios-Toro JJ, Marquez-Coello M, Garcia-Alvarez JM, et al. Soluble membrane receptors, interleukin 6, procalcitonin and C reactive protein as prognostic markers in patients with severe sepsis and septic shock. PLoS One. 2017;12(4):e0175254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andaluz-Ojeda D, Bobillo F, Iglesias V, et al. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012;57(3):332–6. [DOI] [PubMed] [Google Scholar]
- 81.Gogos CA, Drosou E, Bassaris HP, et al. Pro-versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181(1):176–80. [DOI] [PubMed] [Google Scholar]
- 82.Franco DM, Arevalo-Rodriguez I, i Figuls MR, et al. Interleukin-6 for diagnosis of sepsis in critically ill adult patients. Cochrane Database of Systematic Reviews. 2015(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun B, Liang LF, Li J, et al. A meta-analysis of interleukin-6 as a valid and accurate index in diagnosing early neonatal sepsis. Int Wound J. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Richendrfer H, Schmidt TM, Levy MM, et al. Recombinant Human Proteoglycan-4 (rhPRG4) Decreases IL-6 in Human Endothelial Cells With a Sepsis Phenotype. Society of Academic Emergency Medicine; May 2019; Las Vegas, Nevada, USA. [Google Scholar]