Abstract
BACKGROUND:
While uncommon, the incidence of endometrial cancer and atypical hyperplasia among reproductive-aged women is increasing. The fertility outcomes in this population are not well described.
OBJECTIVES
We aim to describe the patterns of care and fertility outcomes of reproductive-aged women with endometrial cancer or atypical hyperplasia.
STUDY DESIGN:
A cohort of women age ≤ 45 with endometrial cancer or atypical hyperplasia diagnosed in 2000 to 2014 were identified in Truven Marketscan, an insurance claims database of commercially-insured patients in the United States. Treatment information, including use of progestin therapy, hysterectomy, and assisted fertility services, was identified and collected using a combination of Common Procedural Terminology codes, International Classification of Diseases codes, and National Drug Codes. Pregnancy events were identified from claims data using a similar technique. Patients were categorized as receiving progestin therapy alone, progestin therapy followed by hysterectomy, or standard surgical management with hysterectomy alone. Multivariable logistic regression was performed to assess factors associated with receiving fertility-sparing treatment.
RESULTS:
A total of 4007 reproductive-aged patients diagnosed with endometrial cancer or atypical hyperplasia were identified. The majority of these patients [n=3189; 79.6%] received standard surgical management. Of the 818 patients treated initially with progestins, 397 [48.5%] subsequently underwent hysterectomy whereas 421 [51.5%] did not. Patients treated with progestin therapy had a lower median age than those who received standard surgical management [median age 36 v. 41 years; p < 0.001]. The proportion of patients receiving progestin therapy increased significantly over the observation period with 24.9% treated at least initially with progestin therapy in 2014 [p < 0.001]. Multivariable analysis shows that younger age, a diagnosis of atypical hyperplasia diagnosis rather than endometrial cancer, and diagnosis later in the study period were all associated with a greater likelihood of receiving progestin therapy [p < 0.0001]. Among the 421 patients who received progestin therapy alone, 92 patients [21.8%; 92/421] had 131 pregnancies including 49 live births for a live birth rate of 11.6%. Among the 397 patients treated with progestin therapy followed by hysterectomy, 25 patients [6.3%; 25/397] had 34 pregnancies with 13 live births. The median age of patients who experienced a live birth following diagnosis during the study period was 36 years [IQR 33–38]. The use of some form of assisted fertility services was observed in 15.5% patients who were treated with progestin therapy. Among patients who experienced any pregnancy event following diagnosis, 54% of patients used some form of fertility treatment. For patients who experienced a live birth following diagnosis, 50% of patients received fertility treatment. Median time to live birth following diagnosis was 756 days [IQR 525–1077]. Patients treated with progestin therapy were more likely to experience a live birth if they had used assisted fertility services [OR 5.9 ; 95% CI 3.4–10.1; p < 0.0001].
CONCLUSIONS:
The number of patients who received fertility-sparing treatment for endometrial cancer or atypical hyperplasia increased over time. However, the proportion of women who experience a live birth following these diagnoses is relatively small.
Keywords: endometrial cancer, endometrial hyperplasia, fertility, fertility conservation, fertility-sparing treatment, oncofertility, health services research
CONDENSATION:
Fertility-conserving treatment for women with endometrial cancer or atypical hyperplasia has increased, although relatively few of these women experience live birth following these diagnoses.
INTRODUCTION:
Endometrial carcinoma is uncommon in reproductive-aged women with only about 7% of new diagnoses occurring in women under age 44.1 For both endometrial cancer and atypical hyperplasia, hysterectomy is part of standard surgical management.2–4 However, uterine-sparing treatment is an option for women diagnosed at a young age who desire fertility preservation. The Society of Gynecologic Oncology describes an ideal candidate for conservative treatment as a woman who strongly desires fertility-sparing management with a well-differentiated (grade 1) tumor, no evidence of myometrial invasion (stage 1A), no contraindications for medical management, and acceptance of non-standard cancer treatment.2,5
Historically, oral progestin therapy has been the primary management strategy for women with endometrial cancer who desire fertility preservation.2 Reported initial response rates to progestins are estimated to be 70–90%, although the optimal dose and progestin formulation is uncertain.6 The use of the levonorgestrel-releasing intrauterine device [LNG-IUD] has been associated with regression rates in women with atypical hyperplasia as high as 90%.7 In a recent case series from the University of Texas MD Anderson Cancer Center, responses to treatment with a LNG-IUD were seen in 67% of patients with grade 1 and 75% of patients with grade 2 endometrial cancer of endometrioid histology.8 While these findings may suggest that progestin therapy is a reasonable alternative treatment strategy in patients who desire fertility preservation, increased disease-specific mortality is seen in young women with endometrial cancer treated with progestin therapy compared with hysterectomy.9,10 Further, approximately 20–40% of patients experience recurrent disease after an initial response.6,11,12
Much of the literature published regarding the fertility outcomes of young women with endometrial cancer or atypical hyperplasia who received fertility-sparing management has been limited to single-institution retrospective reviews at academic institutions or meta-analyses of these reports.6,11–13 Our objective was to describe patterns of care in the treatment of young women diagnosed with endometrial cancer or atypical hyperplasia and to estimate their fertility outcomes from a population-level perspective using a national insurance claims database.
MATERIAL AND METHODS
We performed a retrospective cohort study by analyzing data from the Truven Health MarketScan Research Database [IBM Watson Health, Cambridge, MA], a commercial database of patient-level information derived from health insurance claims. Since 1995, this database has collected enrollment data, hospital admission records, outpatient services, and outpatient prescription drug claims for 250 million health insurance beneficiaries from more than 300 employer-sponsored health plans including fee-for-service coverage, fully capitated and partially-capitated health plans, preferred provider organizations, indemnity plans, health maintenance organizations, and other managed care-type insurance. In the most recent data year, the MarketScan databases contained information on 43.6 million covered individuals.14 Insurance claims from 1998 to 2016 were analyzed for this study.
Eligibility for inclusion into our study cohort was all women aged <45 years diagnosed with endometrial cancer [ICD-9-CM 182.0; ICD-10-CM C54.x] or atypical hyperplasia [ICD-9-CM 621.33; ICD-10-CM N85.02] between 1999 to 2014 [Figure 1]. Outpatient diagnoses were included only if the codes appeared two or more times with at least 30 days between claims. Inpatient diagnoses were included if a single claim was observed. Patients were restricted to those who had continuous coverage for at least 12 months before and 24 months following diagnosis [n=6941 excluded]. An algorithm was applied to remove prevalent cases from the analysis [n=724 excluded]. Patients identified with endometrial cancer or atypical hyperplasia without any corresponding treatment information [e.g. no claims for either hysterectomy or progestin therapy] were also removed [n=1116 excluded].
Figure 1. Cohort identification for reproductive-aged patients with endometrial cancer or atypical hyperplasia.

At least 2 outpatient claims or at least 1 inpatient for endometrial cancer [ICD-9-CM 182.0; ICD-10-CM C54.x] or atypical hyperplasia [ICD-9-CM 621.33; ICD-10-CM N85.02] was required for initial study inclusion.
Description: Figure 1 depicts the process by which the final analyzed sample was selected from the MarketScan Commercial Database, as well as the rationale and number of patients excluded at different points in the cohort selection.
Our primary objective was to estimate the proportion of reproductive-aged patients with endometrial carcinoma or atypical hyperplasia receiving progestin therapy alone, progestin therapy followed by hysterectomy, or definitive surgical management with hysterectomy alone. Secondary objectives included the following: determination of factors associated with progestin therapy, estimation of pregnancy events that occurred after a diagnosis of endometrial carcinoma or atypical hyperplasia, and the utilization of assisted fertility services including use of ovulation induction and in vitro fertilization following diagnosis of endometrial cancer or atypical hyperplasia.
Variables for patient characteristics were collected for analysis, including: age at diagnosis, year of diagnosis, geographic location, and insurance plan type. Comorbidity was estimated using Klabunde-modified Charlson comorbidity score using claims in the 12 months prior to the diagnosis of endometrial cancer/hyperplasia.15,16 Treatment information, including receipt of progestin therapy, hysterectomy, and assisted fertility services was identified in claims data by using a combination of International Statistical Classification of Diseases and Related Health Problems (ICD), revision 9 and 10 diagnosis codes, Common Procedural Terminology (CPT) codes, ICD-9/10 procedure codes, and National Drug Codes (NDC) [Appendix Table 1]. Similarly, pregnancy events, including live birth, spontaneous abortion, and ectopic pregnancy were identified using a combination of ICD-9/10 diagnosis codes, Common Procedural Terminology (CPT) codes, and ICD-9/10 procedure codes [Appendix Table 1]. The cohort of identified patients was subdivided into three treatment categories: patients receiving progestin therapy alone [e.g. fertility-sparing treatment], progestin therapy followed by hysterectomy, or hysterectomy alone. Fertility outcomes were categorized as any pregnancy event and live births. For consistency with other investigations regarding this patient population, live birth rate was defined as number of live births observed divided by the number of patients receiving fertility-sparing treatment or progestin therapy followed by hysterectomy.6,12
Descriptive statistics [means, median and standard deviations of continuous variables and frequencies of discrete variables] of patients’ sociodemographic and clinical characteristics were calculated. The Chi-square test [for discrete variables] or F test [for group means] or Kruskal-Wallis test [for medians] was used to assess differences between patients’ characteristics and therapies. Multivariable logistic regression was performed to assess factors associated with receipt of progestin therapy. A subset of patients was treated with progestin therapy initially but subsequently underwent hysterectomy. As a sensitivity analysis, we repeated multivariate logistic regression after reassigning patients away from the fertility-sparing treatment group if they underwent hysterectomy after less than 2 months [60 days] of progestin therapy. Our rationale was that determining intent of a given treatment is difficult with insurance claims data and that patients who would be excluded by this criterion would more likely represent patients who received progestins for indications other than fertility conservation [e.g. amelioration of bleeding symptoms]. All analyses were conducted with the SAS statistical software program [version 9.3, SAS Institute, Cary, NC]. This project was approved by our Institutional Review Board [PA 14–0796].
RESULTS
With the Marketscan database, we identified 4007 women ≤ 45 years-old who were diagnosed with endometrial cancer [3137; 78.3%] or atypical hyperplasia [870; 21.7%] between 2000 and 2014 [Table 1] [Figure 1]. Median follow-up for patients identified was 4.5 years [IQR 3.2–6.5]. From this cohort, 3189 [79.6%] received definitive surgical management and 818 [20.4%] received progestin therapy either alone or followed by hysterectomy. Of the patients in the sub-cohort initially treated with progestin therapy, 48.5% [397/818] subsequently underwent hysterectomy whereas 51.5% [421/818] did not. The median time from diagnosis to hysterectomy in the patients initially treated with progestins but who subsequently underwent hysterectomy was 168 days [IQR 55–727]. After excluding patients who underwent hysterectomy after less than 2 months of progestin therapy, the median time from diagnosis to hysterectomy in this group was 380 days [IQR 142–959]. Patients who underwent definitive surgical management were older and more likely to have endometrial cancer than atypical hyperplasia [p<.001] [Table 1]. A greater proportion of patients diagnosed later in the study period received fertility-sparing treatment [p <.001] [Figure 2]. In the final year of the study period, 24.9% of patients were treated with progestin therapy, at least initially. Of the patients treated with progestin therapy at least initially, 21.4% were treated with a LNG-IUD [175/818] alone or in combination with other progestins.
Table 1.
Patient characteristics (N = 4,007)
| Characteristics | Treatment Category |
P-valuec | Total = 4,007, n (%) | ||
|---|---|---|---|---|---|
| Progestin Therapy Only |
Progestin Therapy followed by Hysterectomy |
Hysterectomy Only |
|||
| (421/4007; 10.5%) | (397/4007; 9.9%) | (3189/4007; 79.6%) | |||
| Age at diagnosis, y | <.001 | ||||
| 18–29 | 72 (17.1) | 29 (7.3) | 83 (2.6) | 184 (4.5) | |
| 30–34 | 107 (25.4) | 82 (20.7) | 287 (9.0) | 476 (11.9) | |
| 35–39 | 122 (29.0) | 122 (30.7) | 764 (24.0) | 1008 (25.2) | |
| 40–45 | 120 (28.5) | 164 (41.3) | 2055 (64.4) | 2339 (58.4) | |
| Mean age, y (SD) | 35.4 (6) | 37.7 (5.2) | 40.1 (4.5) | <.0001d | 39.4 (5) |
| Median age, y (IQR) | 36 (31–40) | 38 (34–42) | 41 (38–44) | <.0001e | 41 (37–43) |
| Median Duration of Follow-up, y (IQR) | 4.1 (3.1–5.9) | 4.7 (3.4–6.6) | 4.5 (3.2–6.6) | <.001 | 4.5 (3.2–6.5) |
| Diagnosis group | <.001 | ||||
| Endometrial Cancer | 216 (51.3) | 302 (76.1) | 2619 (82.1) | 3137 (78.3) | |
| Atypical Hyperplasia | 205 (48.7) | 95 (23.9) | 570 (17.9) | 870 (21.7) | |
| Year of Diagnosis | <.001 | ||||
| 2000–2004a | 25 (5.9) | 26 (6.5) | 365 (11.4) | 416 (10.4) | |
| 2005 | 16 (3.8) | 19 (4.8) | 163 (5.1) | 198 (4.9) | |
| 2006 | 17 (4.0) | 21 (5.3) | 257 (8.1) | 295 (7.4) | |
| 2007 | 21 (5.0) | 19 (4.8) | 250 (7.8) | 290 (7.2) | |
| 2008 | 49 (11.6) | 50 (12.6) | 373 (11.7) | 472 (11.8) | |
| 2009 | 57 (13.5) | 67 (16.9) | 415 (13.0) | 539 (13.5) | |
| 2010 | 51 (12.1) | 51 (12.8) | 402 (12.6) | 504 (12.6) | |
| 2011 | 49 (11.6) | 42 (10.6) | 336 (10.5) | 427 (10.7) | |
| 2012 | 51 (12.1) | 45 (11.3) | 241 (7.6) | 337 (8.4) | |
| 2013 | 49 (11.6) | 42 (10.6) | 233 (7.3) | 324 (8.1) | |
| 2014 | 36 (8.6) | 15 (3.8) | 154 (4.8) | 205 (5.1) | |
| Regionb | 0.029 | ||||
| Northeast | 92 (22.5) | 77 (19.8) | 542 (17.4) | 711 (18.2) | |
| North Central | 94 (23.0) | 92 (23.7) | 776 (24.9) | 962 (24.6) | |
| South | 150 (36.8) | 138 (35.5) | 1273 (40.9) | 1561 (39.9) | |
| West | 72 (17.6) | 82 (21.1) | 521 (16.7) | 675 (17.3) | |
| Charlson Comorbidity Indexf | 0.045 | ||||
| 0 | 259 (85.8) | 276 (84.1) | 2372 (86.6) | 2907 (86.3) | |
| ≥1 | 43 (14.2) | 52 (15.8) | 368 (13.4) | 463 (13.7) | |
| Patient’s Relationship to the Primary Beneficiaryg | <.001 | ||||
| Employee | 280 (66.5) | 286 (72.0) | 2052 (64.3) | 2618 (65.3) | |
| Spouse | 127 (30.2) | 109 (27.5) | 1115 (35.0) | 1351 (33.7) | |
| Insurance | 0.116 | ||||
| HMO | 62 (14.7) | 80 (20.2) | 498 (15.6) | 640 (16.0) | |
| PPO | 261 (62.0) | 217 (54.7) | 1909 (59.9) | 2387 (59.6) | |
| Other | 98 (23.3) | 100 (25.2) | 782 (24.5) | 980 (24.5) | |
For years 2000–2004, < 11 patients were identified in certain treatment groups per year. These years were grouped together to protect patient privacy.
98 patients are excluded due to unknown region.
P values were derived using the chi-square test for comparing differences between three treatment groups.
P values were derived using the F test for comparing means among three treatment groups.
P values were derived using the Kruskal-Wallis test for comparing medians among three treatment groups.
637 patients are excluded due to missing comorbidity score. For patients in the Progestin Therapy Only and Progestin Therapy followed by Hysterectomy treatment categories, < 11 patients were identified with a Charlson Comorbidity Index score of ≥2. These patients were grouped with patients with patients with score of 1 to protect patient privacy.
To protect patient privacy, the frequency of patients who were the child of or had other relationship to the primary beneficiary (total n=38) is not shown as < 11 patients identified in certain treatment groups.
SD: standard deviation; IQR: interquartile range; HMO: health maintenance organization; PPO: preferred provider organization
Figure 2. Reproductive-aged patients with endometrial cancer or atypical hyperplasia receiving progestin therapy.
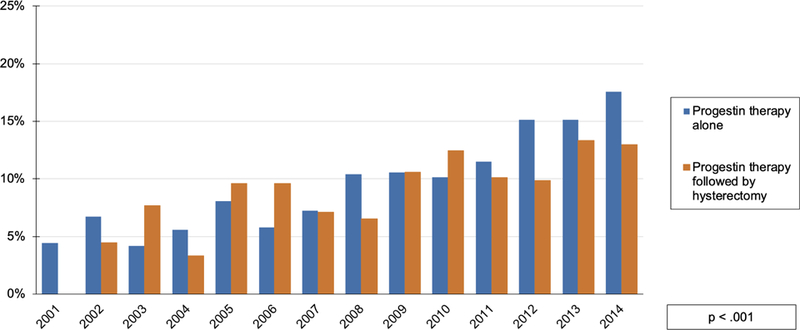
y-axis indicates percentage of patients diagnosed with endometrial cancer or atypical hyperplasia in given year in each treatment group. Patients treated with only hysterectomy are not shown. Fertility-sparing treatment defined as treatment with progestin therapy alone. Patients diagnosed in 1999 not shown due to washout algorithm to exclude prevalent cases. Cases from 2000 not shown as only 22 patients were identified meeting inclusion criteria for analysis; 18% [4/22] received fertility-sparing treatment.
Description: Figure 2 depicts a statistically-significant increasing trend in the proportion of patients who were treated with progestin therapy in each subsequent year of the observation period. The total group of patients treated with progestins is divided into those treated with progestin therapy alone [blue] and progestin therapy followed by hysterectomy [orange].
Of the cohort treated with progestin therapy with or without hysterectomy, 117 patients experienced 165 pregnancies during the observation period [Table 2]. Among the 421 patients receiving fertility-sparing treatment, 92 patients [21.8%; 92/421] had 131 pregnancies. Of these patients who were treated exclusively with progestins, 49 patients had live births for a live birth rate of 11.6% [49/421]. Among the 397 patients treated with progestin therapy followed by hysterectomy, 25 patients [6.3%; 25/397] had 34 pregnancies. Of those, 13 patients had live births for a live birth rate of 3.3% [13/397]. The live birth rate for the entire cohort of patients who received progestin treatment with or without hysterectomy was 7.6% [62/818]. The median age of patients who experienced a live birth during the study period was 36 years [IQR 33–38]. The median time from diagnosis to first pregnancy event was 413 days [IQR 262–758]. The median time from diagnosis to live birth was 756 days [IQR 525–1077].
Table 2.
Fertility events among patients receiving progestin therapy.
| Progestin therapy alone | Progestin therapy followed by hysterectomy | Total cohort initially managed with fertility conservation | |
|---|---|---|---|
| n=421 | n=397 | n=818 | |
| Patients Experiencing Any Pregnancy, n | 92 | 25 | 117 |
| Patients Experiencing Live Birth, n | 49 | 13 | 62 |
| Live Birth Rate, % | 11.6% | 3.3% | 7.6% |
Live birth rate defined as proportion of patients experiencing a live birth (numerator) over patients in treatment category (denominator).
In the group of patients treated with progestin therapy with or without hysterectomy, 15.5% of patients [127/818] utilized assisted fertility services [Table 3]. Of patients who received progestin therapy alone, 20.9% of patients [88/421] received fertility treatments. Of patients who had any pregnancy or specifically a live birth during the study period, 54% [63/117] and 50% [31/62] of patients received fertility treatments, respectively. Of the patients who received fertility treatment and experienced any pregnancy event, 79% [50/63] used in vitro fertilization. Patients treated with progestin therapy were 5.9 times more likely to experience a live birth if they also had received some form of fertility treatment [95% CI 3.4–10.1; p < 0.0001].
Table 3:
Utilization of assisted fertility services.
| Patients Utilizing Assisted Fertility Services | Patients Not Utilizing Assisted Fertility Services | Total, n | |||
|---|---|---|---|---|---|
| Endometrial Cancer | Hyperplasia with Atypia | Endometrial Cancer | Hyperplasia with Atypia | ||
| Entire cohort, n (%) | 151 (3.8) | 69 (1.7) | 2986 (74.5) | 801 (20) | 4007 |
| Patients who received progestin therapya, n (%) | 79 (9.7) | 48 (5.9) | 439 (53.7) | 252 (30.8) | 818 |
| Patients who received fertility-sparing treatmentb, n (%) | 47 (11.2) | 41 (9.7) | 169 (40.1) | 164 (39) | 421 |
| Patients who experienced any pregnancy, n (%) | 35 (29.9) | 28 (23.9) | 27 (23.1) | 27 (23.1) | 117 |
| Patients who experienced a live birth, n (%) | 18 (29) | 13 (21) | 15 (24.2) | 16 (25.8) | 62 |
: Includes patients who receiving progestin therapy exclusively and those who also subsequently underwent hysterectomy.
: Includes patients who were only treated with progestin therapy. All values represent frequencies followed by row percentages in parenthesis, unless otherwise indicated.
Both univariate and multivariable analysis revealed that younger age, diagnosis of atypical hyperplasia, and diagnosis later in the observation period were associated with a greater likelihood of receiving progestin therapy at least initially [Table 4]. Patients aged <25 were 9.5-times more likely to receive progestin therapy compared to those age 40 to 45 [OR 9.5; 95% CI 3.6–25.1; p<0.0001]. Women with atypical hyperplasia were 2.6-times more likely to initially receive progestin therapy compared with those diagnosed with endometrial cancer [OR 2.7; CI 2.2–3.2; p<0.0001]. Analyzing the observation period into 5-year intervals, a diagnosis of endometrial cancer or atypical hyperplasia between 2009 to 2014 was associated with an approximately 47% increased likelihood of receiving progestin therapy at least initially compared to those diagnosed between 1999 to 2004 [OR 1.5; 95% CI 1.1–2.1; p=0.0002]. These factors remained significant predictors of receiving fertility-sparing treatment after the sensitivity analysis of reassigning patients who underwent hysterectomy after less than 2 months of progestin therapy was performed [Table 5].
Table 4.
Factors associated with receiving progestin therapy. (n = 4,007)
| Univariate |
p-value | Multivariable |
p-value | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||||
| Age at diagnosis, y | <.0001 | <.0001 | ||||||
| 40–45 | ||||||||
| <25 | 8.0 | 4.2 | 15.4 | 9.5 | 3.6 | 25.1 | ||
| 25–29 | 9.0 | 6.4 | 12.8 | 10.0 | 6.9 | 14.4 | ||
| 30–34 | 4.8 | 3.8 | 5.9 | 4.9 | 3.9 | 6.2 | ||
| 35–39 | 2.3 | 1.9 | 2.8 | 2.3 | 1.9 | 2.8 | ||
| Diagnosis Group | <.0001 | <.0001 | ||||||
| Endometrial | ||||||||
| Cancer | ||||||||
| Atypical | ||||||||
| Hyperplasia | 2.7 | 2.2 | 3.2 | 2.7 | 2.2 | 3.2 | ||
| Year of diagnosis | <.0001 | 0.0002 | ||||||
| 1999–2004 | ||||||||
| 2005–2008 | 1.5 | 1.0 | 2.0 | 1.0 | 0.7 | 1.4 | ||
| 2009–2014 | 2.2 | 1.6 | 3.0 | 1.5 | 1.1 | 2.0 | ||
| Region | 0.0194 | 0.0132 | ||||||
| South | ||||||||
| Northeast | 1.4 | 1.1 | 1.7 | 1.4 | 1.1 | 1.8 | ||
| North Central | 1.1 | 0.9 | 1.3 | 1.2 | 0.9 | 1.5 | ||
| West | 1.3 | 1.0 | 1.6 | 1.4 | 1.1 | 1.8 | ||
| Unknown | 1.2 | 0.7 | 2.0 | 1.4 | 0.8 | 2.4 | ||
| Insurance | 0.4756 | 0.6431 | ||||||
| HMO | ||||||||
| Other | 0.9 | 0.7 | 1.1 | 0.9 | 0.7 | 1.2 | ||
| PPO | 0.9 | 0.7 | 1.1 | 0.9 | 0.7 | 1.1 | ||
| Charlson Comorbidity Score | 0.2799 | 0.4077 | ||||||
| 0 | ||||||||
| 1 | 1.1 | 0.8 | 1.4 | 1.1 | 0.9 | 1.5 | ||
| ≥2 | 0.6 | 0.3 | 1.2 | 0.7 | 0.3 | 1.4 | ||
| Relationship to the Primary Beneficiary | <.0001 | 0.0112 | ||||||
| Employee | ||||||||
| Spouse | 0.8 | 0.6 | 0.9 | 0.8 | 0.6 | 0.9 | ||
| Child/Other | 2.6 | 1.4 | 5.1 | 0.9 | 0.3 | 2.5 | ||
P values are based on Wald test. Abbreviations: CI, confidence interval. OR, Odds ratio. HMO: health maintenance organization; PPO: preferred provider organization.
Table 5.
Factors associated with receiving progestin therapy [sensitivity analysis reassigning patients who underwent hysterectomy after ≤2 months progestin treatment]. (n = 4,007)
| Univariate |
p-value | Multivariable |
p-value | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||||
| Age at diagnosis, y | <.0001 | <.0001 | ||||||
| 40–45 | ||||||||
| <25 | 9.0 | 4.7 | 17.2 | 11.9 | 4.4 | 32.5 | ||
| 25–29 | 12.4 | 8.7 | 17.7 | 14.7 | 10.1 | 21.3 | ||
| 30–34 | 5.6 | 4.5 | 7.1 | 6.0 | 4.7 | 7.7 | ||
| 35–39 | 2.8 | 2.3 | 3.5 | 2.9 | 2.3 | 3.5 | ||
| Diagnosis Group | <.0001 | <.0001 | ||||||
| Endometrial | ||||||||
| Cancer | ||||||||
| Atypical | ||||||||
| Hyperplasia | 3.3 | 2.8 | 4.0 | 3.5 | 2.9 | 4.2 | ||
| Year of diagnosis | <.0001 | 0.0046 | ||||||
| 1999–2004 | ||||||||
| 2005–2008 | 1.8 | 1.2 | 2.5 | 1.1 | 0.8 | 1.7 | ||
| 2009–2014 | 2.6 | 1.8 | 3.6 | 1.5 | 1.0 | 2.2 | ||
| Region | 0.0131 | 0.0029 | ||||||
| South | ||||||||
| Northeast | 1.5 | 1.2 | 1.8 | 1.6 | 1.3 | 2.1 | ||
| North Central | 1.1 | 0.9 | 1.3 | 1.2 | 0.9 | 1.5 | ||
| West | 1.2 | 1.0 | 1.6 | 1.3 | 1.0 | 1.7 | ||
| Unknown | 1.4 | 0.8 | 2.3 | 1.6 | 0.9 | 2.9 | ||
| Insurance | 0.435 | 0.7102 | ||||||
| HMO | ||||||||
| Other | 0.9 | 0.7 | 1.1 | 0.9 | 0.7 | 1.2 | ||
| PPO | 1.0 | 0.8 | 1.2 | 1.0 | 0.8 | 1.3 | ||
| Charlson Comorbidit Score | 0.272 | 0.4253 | ||||||
| 0 | ||||||||
| 1 | 1.0 | 0.8 | 1.4 | 1.1 | 0.8 | 1.5 | ||
| ≥2 | 0.5 | 0.2 | 1.2 | 0.6 | 0.3 | 1.4 | ||
| Relationship to the Primary Beneficiary | 0.0006 | 0.0508 | ||||||
| Employee | ||||||||
| Spouse | 0.8 | 0.7 | 1.0 | 0.8 | 0.6 | 1.0 | ||
| Child/Other | 2.6 | 1.3 | 5.0 | 0.8 | 0.3 | 2.5 | ||
P values are based on Wald test. Abbreviations: CI, confidence interval. OR, Odds ratio. HMO: health maintenance organization; PPO: preferred provider organization.
COMMENT
Principal Findings
In our study, the use of progestin therapy for reproductive-aged women diagnosed with endometrial cancer or atypical hyperplasia became more common over the study period. Patients diagnosed near the end of the study period were nearly 75% more likely to receive progestin therapy. As would be expected, the youngest patients in our study cohort and those with atypical hyperplasia were much more likely to receive progestin therapy than the oldest. Among patients who received fertility conservation, we observed a live birth rate of <12%. Most of the pregnancy events that occurred following a diagnosis of endometrial cancer or atypical hyperplasia occurred in the context of assisted fertility services.
Meaning
Our findings are consistent with prior population-based analyses of the treatment of young women with endometrial cancer that have shown an increase in the use of progestin therapy over time.9,10 This trend may relate to increased awareness and interest in this treatment strategy by clinicians for women in this age group. Using the National Cancer Database, Ruiz et al found that the proportion endometrial cancer patients that were treated with progestin therapy increased from 2.4% in 2004 to 5.9% in 2014.10 We observed that nearly 25% of our cohort received progestin therapy with or without subsequent hysterectomy during the final year of our study period. This difference in estimates may be accounted for at least partially by the inclusion of patients with atypical hyperplasia in our analysis, who we found were more likely to be treated with progestin therapy compared to those with endometrial cancer. Additionally, Ruiz et al included patients aged 45–49, an age group where the incidence of endometrial cancer and atypical hyperplasia is relatively higher and patients are more likely to be treated surgically than medically.10 Greenwald et al. reported similar findings from an analysis utilizing the Surveillance, Epidemiology, and End Results database.9 Both analyses found that women receiving non-surgical treatment of endometrial cancer were at an increased risk for cancer-specific mortality.9,10 Both of these investigations identified a greater number of reproductive-aged women with endometrial cancer than this report. The discrepancy in number of patients identified in our study from these reports reflects differences in data sources, methodology, and inclusion criteria.
As fertility preservation may be an important factor in pursuing uterine-sparing treatment, the fertility-related outcomes reported here offer context for what may be seen as the benefit for avoiding or deferring hysterectomy. We found that a pregnancy or a live birth was an uncommon event in women following an endometrial cancer or atypical hyperplasia diagnosis. In our cohort, we observed a live birth rate of 11.6% among those women pursuing fertility-conserving therapy. In a systematic review by Gallos et al. the live birth rate in women receiving fertility-sparing treatment for endometrial cancer or atypical hyperplasia was 26% and 28%, respectively.6 In a more recent review, Wei et al. reported a live birth rate of 14–20%.12 Some of the difference in live birth rate estimated by those reviews and this study may be due to patient population differences. Those reports include patients receiving uterine-sparing treatment exclusively for the purpose of fertility preservation. Our methodology does not allow us to discriminate and exclude patients that received uterine-sparing treatment for reasons other fertility preservation. Other common rationale for non-surgical treatment includes medical infirmity, advanced age, or patient preference to avoid surgery. However, as patients in our cohort were by definition young and few had significant medical comorbidities, the live birth rate we report may better reflect the real-world likelihood of live birth for patients following a diagnosis of endometrial cancer or atypical hyperplasia.
Although we are limited in our ability to definitively determine the proportion of patients in this study who sought pregnancy, these data may suggest a more limited reproductive potential for reproductive-aged patients with endometrial cancer or atypical hyperplasia. More than half of the pregnancies achieved in this study occurred in the context of fertility treatment. Given this, individualized assessment of each patient’s likelihood to achieve pregnancy should be carefully incorporated into shared decision-making when fertility-sparing treatment is considered. Advancing age and obesity – well-established risks shared by endometrial cancer and impaired fertility – are clinical factors immediately available to a gynecologic oncologist counseling a patient regarding her reproductive potential and treatment that may affect it.17–19 Collaboration with a fertility specialist may be a highly valuable addition to the evaluation of an individual patient’s reproductive potential, as referral may offer clarity in terms of the potential for pregnancy with and without various forms of assisted reproductive technology. Notably, the American Society of Clinical Oncology recommends physicians “should refer patients who express an interest in fertility preservation to reproductive specialists.”20
With increased utilization of uterine-sparing treatment, questions regarding longer-term management of these patients also arise. As stated above, two population-level analyses of young endometrial cancer patients found an increased risk of cancer-specific mortality among patients who received fertility-sparing treatment.9,10 Durable responses to progestin therapy are suboptimal with up to 40% of patients experiencing disease recurrence.6,11 The true rate may be higher still given that the proportion of patients who will experience a relapse increases with time as women remain at risk. For women with atypical hyperplasia, the Royal College of Obstetricians and Gynaecologists recommends hysterectomy following initial uterine-sparing treatment “once fertility is no longer required” on account of the high recurrence risk.21 Nearly 90% of gynecologic oncologists surveyed would recommend hysterectomy once childbearing was completed for women initially treated with uterine-preservation.22
Strengths & Weaknesses
To our knowledge, this analysis is the first that examines the fertility outcomes of patients with endometrial cancer or atypical hyperplasia using a national-level sample. We identified a large number of reproductive-aged patients with these diagnoses with a median follow-up time of 4.5 years, likely a satisfactory observation period to examine these fertility endpoints. Our study has a several important limitations. First, our analysis does not capture oncologic outcomes or information regarding adjuvant treatment, both of which could affect fertility potential. We cannot estimate the effectiveness of progestin therapy compared with standard therapy. As stated previously, we cannot ascertain what proportion of patients received uterine-sparing treatment for the purpose of fertility conservation as opposed to other clinical indications. We cannot determine what proportion of patients who received fertility conservation subsequently tried to get pregnant. We cannot determine the reason why some patients initially managed with progestin therapy subsequently underwent hysterectomy. Koskas et al. found that the number of patients who will respond to progestin therapy seems to plateau at approximate 80% at 12 months following the start of treatment.23 It is possible that some of the patients initially treated with progestins subsequently underwent hysterectomy either for treatment failure, recurrence, or disease progression. Although our analysis did not limit the observation period, patients changing insurance providers may have been ‘lost to follow-up’ if their new insurance carrier did not report into the MarketScan database. The 24 month requirement of continuous coverage following diagnosis was designed to mitigate this. We may have underestimated pregnancy events in our study population, especially for those diagnosed in the final years of the study who may have experienced pregnancy after the end of the observation period or for those who had miscarriages that did not require medical services. The use of assisted fertility services may also have been underestimated. In many parts of the United States, these services are not a standard health insurance benefit and so a claim for these may have not been submitted for reimbursement. The generalizability of these findings may be limited to persons who are uninsured or underinsured. Lastly, selection and treatment bias are inherent limitations of retrospective investigations and this analysis is not exempt from that.
Conclusion
In summary, this study offers a real-world perspective on the utilization of fertility-conserving treatment in patients diagnosed with endometrial cancer and atypical hyperplasia. We observed more wide-spread use of this treatment strategy across the study period. The live birth rate in these women was lower than some estimates currently available in the literature and most pregnancies in this cohort occurred in the context of assisted fertility services. This observation underscores the need to evaluate each patient’s reproductive potential when exploring fertility conservation as uterine-sparing treatment may be associated with greater cancer-specific mortality. An individualized assessment of a patient’s chance to achieve pregnancy should facilitate shared decision-making and ensure the choice to pursue fertility-preserving treatment is well-informed.
AJOG AT A GLANCE:
-
Why was the study conducted?
This study was performed to examine national patterns of care for reproductive-aged women with endometrial cancer or atypical hyperplasia. Desire for fertility is an important clinical indication for pursuing non-surgical management. However, the fertility outcomes in this group of patients is not well described apart from retrospective single institution reviews.
-
What are the key findings?
From 1999 to 2014, the proportion of reproductive-aged women with endometrial cancer or atypical hyperplasia who received fertility-conserving treatment increased significantly. Younger age, diagnosis later in the study period, and a diagnosis of atypical hyperplasia as opposed to cancer were all associated with an increased likelihood of receiving fertility-sparing treatment, at least initially. However, less than 12% of patients experienced a live birth following fertility-sparing treatment.
-
What does this study add to what is already known?
This analysis provides a broader perspective on the utilization over time of fertility-sparing treatment for reproductive-aged women with endometrial cancer or atypical hyperplasia. With certain limitations considered, it offers a population-based estimate of the proportion of women who may experience a live birth following fertility-conserving treatment.
Acknowledgments
Disclosures:
Outside the scope of the current work, CCS reports research support from AstraZeneca. Outside the scope of the current work, SNW reports research support from ArQule, AstraZeneca, Bayer, Clovis Oncology, Cotinga Pharmaceuticals, Novartis, Roche/Genentech, and Tesaro. Outside of the scope of the current work, SNW reports consulting fees from AstraZeneca, Clovis Oncology, MediVation, Merck, Ovation, Pfizer, Roche/Genentech, Takeda, and Tesaro. Outside the scope of the current work, LAM reports research support from AstraZeneca. The remaining authors report no conflicts of interest, financial or otherwise, related to the subject matter of the article submitted.
Financial support for this research investigation was provided in part by grant funding to the following individuals or organizations:
Ross Harrison: National Institutes of Health T32 grant (#5T32 CA101642).
Larissa Meyer: National Cancer Institute K award (#K07 CA201013)
Shannon Westin: National Cancer Institute SPORE for Uterine Cancer (2P50 CA098258–13) and Andrew Sabin Family Fellowship
Sharon Giordano: CPRIT RP160674; Susan Komen SAC150061
MD Anderson Cancer Center: National Cancer Institute Cancer Center Support Grant (P30 CA016672)
Appendix
Table 1.
Medical classification codes used for identification of outcomes of interest
| Endometrial cancer |
| ICD-9-CM 182.0; ICD-10-CM C54.x |
| Atypical hyperplasia |
| ICD-9-CM 621.33; ICD-10-CM N85.02 |
| Miscarriage |
| CPT 59812–59866; ICD-9-CM 634.xx; ICD-10-CM O03.xx; ICD-10-PCS 10A00ZZ, 10A03ZZ, 10A04ZZ |
| Ectopic pregnancy |
| ICD-9-CM 633.xx; ICD-10-CM O00.xx |
| Pregnancy |
| CPT 76801–76817; ICD-9-CM V22.x, V23.x, 650, 651xx; ICD-10-CM Z33.x, Z34.x, Z36.2, O09.x, O30.x, O31.x, O35.x, O36.x |
| Delivery |
| CPT 59400–59410, 59510–59525, 59610–59614; ICD-9-CM 74.x, 72.x, ICD-10-PCS 10D00Z1, 10D00Z0, 10D00Z2, 0W8NXZZ, 10D07Z5, 10D07Z4, 10D07Z3 |
| Assisted fertility services & in-vitro fertilization |
| CPT 58322, 58970–58976, 89250–89291, 89335–89356; ICD-9-CM 65.91, 256.1, 628.0, V26.81; ICD-10-CM N97.x, Z31.83; ICD-10-PCS 0U90xx, 0U91xx, 0UDNxxx; HCPCS S4011-S4042, S4989, J0725, J1620, J1675, S0132, S0122, S0126, S0128, J3355, J9202, J9217, J9218, J9219, J1950 |
| Hysterectomy |
| CPT 58150, 58180, 58200, 58210, 58541–58548, 58550–58554, 58570–58573, 58953–58956, 59100; ICD-9-CM 68.x; ICD-10-PCS 0UT9xxx, 0UTC0ZZ, 0UTC4ZZ, 0UTC7ZZ, 0UTC8ZZ, 0UT40ZZ, 0UT44ZZ, 0UT47ZZ, 0UT48ZZ; HCPCS S2900 |
| Use of ovulation induction agents |
| letrozole, clomiphene citrate, tamoxifen citrate, follitropin, menotropin, urofollitropin, chorionic gonadotropin, choriogonadotropin alfa, cetrorelix acetate, triptorelin, ganirelix acetate |
| Use of progestin therapy |
| megestrol acetate, medroxyprogesterone acetate, levonorgestrel intrauterine device |
ICD: International Classification of Diseases; CPT: Current Procedural Terminology; HCPCS: Healthcare Common Procedure Coding System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The investigation described in this manuscript was presented in part by during the International Gynecologic Cancer Society Global Meeting in Lisbon, Portugal [October 29–31, 2016], and the 50th Society of Gynecologic Oncology Annual Meeting in Honolulu, Hawai’i, USA [March 15–19, 2019].
BIBLIOGRAPHY
- 1.Noone AMHN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2015 Bethesda, MD: National Cancer Institute; April 2018 2018. [Google Scholar]
- 2.Group SGOCPECW, Burke WM, Orr J, et al. Endometrial cancer: a review and current management strategies: part II. Gynecol Oncol 2014;134(2):393–402. [DOI] [PubMed] [Google Scholar]
- 3.Group SGOCPECW, Burke WM, Orr J, et al. Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol 2014;134(2):385–392. [DOI] [PubMed] [Google Scholar]
- 4.Trimble CL, Method M, Leitao M, et al. Management of endometrial precancers. Obstet Gynecol 2012;120(5):1160–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodolakis A, Biliatis I, Morice P, et al. European Society of Gynecological Oncology Task Force for Fertility Preservation: Clinical Recommendations for Fertility-Sparing Management in Young Endometrial Cancer Patients. Int J Gynecol Cancer 2015;25(7):1258–1265. [DOI] [PubMed] [Google Scholar]
- 6.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol 2012;207(4):266 e261–212. [DOI] [PubMed] [Google Scholar]
- 7.Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol 2010;203(6):547 e541–510. [DOI] [PubMed] [Google Scholar]
- 8.Pal N, Broaddus RR, Urbauer DL, et al. Treatment of Low-Risk Endometrial Cancer and Complex Atypical Hyperplasia With the Levonorgestrel-Releasing Intrauterine Device. Obstet Gynecol 2018;131(1):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwald ZR, Huang LN, Wissing MD, Franco EL, Gotlieb WH. Does hormonal therapy for fertility preservation affect the survival of young women with early-stage endometrial cancer? Cancer 2017;123(9):1545–1554. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz MP, Huang Y, Hou JY, et al. All-cause mortality in young women with endometrial cancer receiving progesterone therapy. Am J Obstet Gynecol 2017;217(6):669 e661–669 e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 2012;125(2):477–482. [DOI] [PubMed] [Google Scholar]
- 12.Wei J, Zhang W, Feng L, Gao W. Comparison of fertility-sparing treatments in patients with early endometrial cancer and atypical complex hyperplasia: A meta-analysis and systematic review. Medicine (Baltimore) 2017;96(37):e8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chae SH, Shim SH, Lee SJ, Lee JY, Kim SN, Kang SB. Pregnancy and oncologic outcomes after fertility-sparing management for early stage endometrioid endometrial cancer. Int J Gynecol Cancer 2019;29(1):77–85. [DOI] [PubMed] [Google Scholar]
- 14.IBM Corporation. IBM MarketScan Research Databases for Health Services Researchers https://www.ibm.com/downloads/cas/6KNYVVQ2. Published 2018. Accessed May 7, 2019.
- 15.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care 2006;44(10):921–928. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 17.Gambadauro P. The reproductive prognosis of women considering fertility preservation for early stage endometrial cancer. Arch Gynecol Obstet 2019. [DOI] [PubMed]
- 18.Practice Committee of the American Society for Reproductive M. Obesity and reproduction: a committee opinion. Fertil Steril 2015;104(5):1116–1126. [DOI] [PubMed] [Google Scholar]
- 19.American College of O, Gynecologists Committee on Gynecologic P, Practice C. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014;101(3):633–634. [DOI] [PubMed] [Google Scholar]
- 20.Oktay K, Harvey BE, Partridge AH, et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36(19):1994–2001. [DOI] [PubMed] [Google Scholar]
- 21.Gynaecologists RCoOa. Green-top Guideline No. 67: Management of Endometrial Hyperplasia 2016:1–30.
- 22.La Russa M, Zapardiel I, Halaska MJ, et al. Conservative management of endometrial cancer: a survey amongst European clinicians. Arch Gynecol Obstet 2018;298(2):373–380. [DOI] [PubMed] [Google Scholar]
- 23.Koskas M, Uzan J, Luton D, Rouzier R, Darai E. Prognostic factors of oncologic and reproductive outcomes in fertility-sparing management of endometrial atypical hyperplasia and adenocarcinoma: systematic review and meta-analysis. Fertil Steril 2014;101(3):785–794. [DOI] [PubMed] [Google Scholar]


