Abstract
Rationale
Pulmonary non-tuberculous mycobacterial (PNTM) disease has increased over the past several decades, especially in older women. Abnormal mucociliary clearance and abnormal nasal nitric oxide (nNO) have been associated with PNTM disease in other patient cohorts. Mucociliary clearance can be affected by NO-cyclic guanosine monophosphate signalling and, therefore, modulation of the pathway may be possible with phosphodiesterase inhibitors such as sildenafil as a novel therapeutic approach.
Objective
To define ex vivo characteristics of PNTM disease affected by sildenafil.
Methods
Subjects with PNTM infections were recruited into an open-label dose-escalation trial of sildenafil. Laboratory measurements and mucociliary measurements—ciliary beat frequency, nNO and 24-hour sputum production—were collected throughout the study period. Patients received sildenafil daily during the study period, with escalation from 20 to 40 mg three times per day.
Measurements and main results
Increased ciliary beat frequency occurred after a single dose of 40 mg sildenafil and after extended dosing of 40 mg sildenafil. The increase ciliary beat frequency was not seen with 20 mg sildenafil dosing. There were no changes in sputum production, nNO production, Quality of Life-Bronchiectasis-NTM module (QOL-B-NTM) questionnaire or the St George’s Respiratory Questionnaire during the study period.
Conclusion
Sildenafil, 40 mg, increased ciliary beat frequency acutely as well as with extended administration.
Keywords: airway epithelium, atypical mycobacterial infection, respiratory infection, rare lung diseases, opportunist lung infections, innate immunity
Key messages.
What is the key question?
Is the previously identified ex vivo reduction in ciliary beat frequency in pulmonary non-tuberculous mycobacterial disease capable of being modified in vivo, or is the phenomena limited solely to the ex vivo setting?
What is the bottom line?
This article describes a phase I/II study of sildenafil and ciliary function in patients with pulmonary non-tuberculous mycobacterial infections that demonstrates sildenafil is capable of increasing ciliary beat frequency in vivo.
Why read on?
This study describes a novel therapeutic approach or alternative study direction for research in the pulmonary non-tuberculous mycobacterial disease.
Introduction
Pulmonary non-tuberculous mycobacteria (PNTM) disease in otherwise healthy individuals is increasing in industrialised countries and has been shown to have both environmental and genetic associations.1–5 The clinical syndrome generally occurs in postmenopausal women, with lower body mass indices and without significant immunological abnormalities.6 7 Familial clustering as well as high rates of genetic variants affecting immune, respiratory ciliary, cystic fibrosis transmembrane conductance regulator and connective tissue genes in patients with PNTM disease suggest a genetically complex aspect to the syndrome.8–10 Patients with PNTM infections may have abnormalities in respiratory ciliary function and moderately reduced nasal nitric oxide (nNO) levels.11 Previous work demonstrated that the decreased baseline ciliary beat frequency (CBF) present in ex vivo PNTM patient’s respiratory epithelial cells could be increased through the ex vivo addition of phosphodiesterase V inhibitor, sildenafil.
Mucus clearance rates are associated with linear changes in CBF, and nitric oxide (NO) is known to be involved in regulating CBF through NO synthase and the activation of soluble guanylate cyclase leading to increased concentrations of cyclic guanosine monophosphate (cGMP).12–14 Increases in cGMP concentration can also stimulate numerous other metabolic pathways leading to a myriad of effects on respiratory epithelium, as well as vascular smooth muscles. The ex vivo observation that sildenafil leads to an increase in CBF in primary respiratory epithelial cells obtained from PNTM-infected patients led us to hypothesise that oral administration of sildenafil to patients with PNTM infection may result in increased CBF in vivo secondary to an increase in cGMP signalling. Considering the safety profile of sildenafil, we hypothesise that the augmentation of CBF could have potential benefits on mucociliary clearance and PNTM lung disease course; however, the first step was to determine if the previously demonstrated ex vivo effects could be replicated in vivo.
Materials and methods
Patient recruitment
PNTM-infected patients were recruited over a 6-month period from March to August (2013) at the Clinical Center, National Institutes of Health (NIH), Bethesda, Maryland, USA. All patients (n=9) provided informed consent under an NIAID IRB-approved protocol (13-I-0075, NCT01853540) (figure 1) and had microbiological and radiographical evidence of longstanding pulmonary NTM infection consistent with the American Thoracic Society (ATS) criteria for PNTM disease.8 15 16 All study participants were concurrently enrolled in a longitudinal observational study of the Natural History, Genetics, Phenotype and Treatment of Mycobacterial Infections (NCT00018044), which allowed verification that none of the current PNTM-infected patients met diagnostic criteria for cystic fibrosis (CF) or primary ciliary dyskinesia (PCD).
Figure 1.
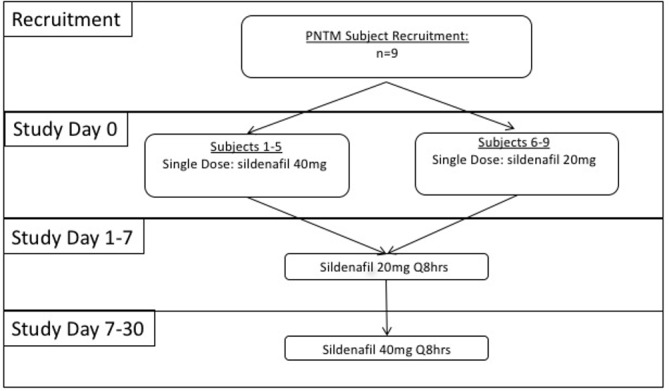
Study design: Nine patients with PNTM disease were enrolled. Sildenafil dosing schedule is graphically displayed for the clinical trial. PNTM, pulmonary non-tuberculous mycobacteria.
Study design
The study was an open-label interventional study that recruited subjects who were concurrently enrolled in a natural history protocol that examined the genetics, phenotype and treatment of mycobacterial infections. Clinical and laboratory disease status measurements were assessed at baseline (day 0) and on days 7 and 30. CBF measurements were done pre-drug and post-drug dosing on days 0, 7 and 30. Subjects 1–5 received a single dose of 40 mg sildenafil on day 0 and subjects 6–9 received a single dose of 20 mg sildenafil on day 0. All nine subjects received 20 mg sildenafil three times per day on days 1–7 and 40 mg sildenafil three times per day on days 8–30 (figure 1).
Collection of respiratory epithelium
Primary human respiratory epithelial cells were collected as previously described.11 Primary patient epithelial samples were visualised at the time of collection (day of collection). Primary human respiratory epithelial cells were collected by scraping the inferior nasal turbinate using a Rhino-Probe (Arlington Scientific). Harvested nasal tissue was suspended in Dulbecco’s modified essential medium, high glucose, without phenol red (DMEM-H; Invitrogen) supplemented with gentamicin (50 µg/mL, Sigma), amphotericin (50 µg/mL, Sigma), ceftazidime (100 µg/mL, Sigma), tobramycin (80 µg/mL, Sigma), vancomycin (100 µg/mL, Sigma), nystatin (100 U/mL, Sigma) and fluconazole (25 µg/mL, Sigma). After collection, the respiratory epithelial cells were resuspended in a hormonally supplemented respiratory epithelial cell medium (Lonza, Walkersvile, Maryland, USA) and keep at 37°C until imaging.
Analysis of CBF
The time interval between the harvest of respiratory epithelial cells and the measurement of CBF was between 60 and 180 min. Cells and cilia were visualised using transmitted light in bright-field mode with a 63× (NA 1.3) glycerol objective on a Leica DM IRBE inverted scope (Leica Microsystems) on a vibration dampened table. Images and videos were captured using a Model A602f-2 Basler area scan high-speed monochromatic digital video camera (Basler AG) at a sampling rate of 100 frames/s with a resolution of 640×480 pixels. The images were analysed using the Sisson-Ammons Video Analysis (SAVA) system V.2.1.15 (Ammons Engineering). All imaging was done at 37°C. Temperature and humidity conditions were maintained through the use of a heated insert, S-2 incubator chamber and objective heater (PeCon). For each respiratory epithelial sample, 20 different field views were continuously recorded for 2.5 s at 100 frames/s. The videos and images were compressed and stored for later analysis. CBF of the de-identified videos was determined by region of interest (ROI) analysis in which CBF was determined by the SAVA software system (Ammons Engineering, Michigan, USA).17 ROI was independently determined by two blinded investigators on the de-identified videos. Multiple videos and ROI were analysed for each time point. The CBF between the two investigators were then averaged for a final CBF per time point.
Measurement of nasal airway NO
nNO was measured in concordance with ATS guidelines by direct sampling through a NO analyser (model 280i, Sievers Instrument, Boulder, Colorado, USA) and reported as steady-state production of NO in nL/minute.18
Sputum collection and measurement
Twenty-four-hour sputum collections were performed and weights recorded at days 0, 7 and 30 as previously described.19
Questionnaires
Self-administered quality of life and functional questionnaires were administered throughout the study period. The Quality of Life-Bronchiectasis-NTM module (QOL-B-NTM) questionnaire quantifies disease-specific symptom severity and quality of life and was performed as previously described at baseline, day 7 and day 30.20 The St. George’s Respiratory Questionnaire (SGRQ) evaluates the health-related quality of life in subjects with the chronic pulmonary disease with a 30-day recall. The SGRQ was performed at baseline, day 30 and day 45.21
Patient and public involvement
Patient care at the Clinical Centre, NIH, is performed as part of clinical studies. Patients and the public are involved during study design as part of the NIAID IRB approval process. There was no patient involvement in the recruitment and conduct of the study. Patients were not invited to contribute to the writing.
Statistical analysis
Data, including figures, are expressed as mean±SD, statistical testing was done using the two-tailed Student t-test with Welch’s correction and one-way analysis of variance (ANOVA) with Dunnett correction.
Results
Demographics
Nine patients with PNTM disease were enrolled, age 63±8 years (range: 58 to 80 years); all were Caucasian women. The mean body mass index was 20.8±3.2 kg/m2. All patients reported chronic cough and had radiographically demonstrated bronchiectasis prior to enrolment. Sixty-six per cent (n=6) reported haemoptysis and 44% reported sinusitis, but only one had a history of otitis media. Thirty-three per cent (n=3) reported a remote history of smoking. Twenty-two per cent (n=2) had joint hypermobility (Beighton Hypermobility score), 11% (n=1) a positive thumb-wrist sign (self-reported)22 and none had echocardiography-proven mitral valve prolapse. The mean forced expiratory volume in 1 s (% predicted) was 85%±24%. Mutations in cystic fibrosistransmembrane conductance regulator, determined by full gene sequence (Ambry Genetics, Aliso Viejo, California, USA), were absent in 33% (n=3), present on a single allele in 22% (n=2) and unknown in 44% (n=4). At the time of respiratory epithelial collection, 56% (n=5) were on NTM-associated medications (clarithromycin, azithromycin, rifampin, rifabutin, rifapentine, ethambutol, amikacin, isoniazid, imipenem, meropenem, tigecycline, cefoxitin, linezolid, clofazimine or moxifloxacin) and all had positive NTM cultures. NTM was isolated from all the patients at some time prior to enrolment: 45% (n=4) of the patients had mycobacterium avium complex (MAC), 33% (n=3) mycobacterium abscessus (MAB), and 22% (n=2) had both MAC and MAB. Fifty-six per cent had elevated C reactive protein test or beta-2 microglobulin at the time of consent (table 1).
Table 1.
Population characteristics and bacterial organisms
|
Characteristics |
Patients with PNTM disease (N=9) |
| Mean age, mean±SD (years) | 63±8 |
| Female gender, n (%) | 9 (100) |
| Ethnicity | |
| White, n (%) | 9 (100) |
| Never smokers, n (%) | 3 (33) |
| CFTR carriers, n (%) | 2 (22) |
| Scoliosis, n (%) | 3 (33) |
| Joint hypermobility, n (%) | 2 (22) |
| Positive thumb-wrist sign, n (%) | 1 (11) |
| Mitral valve prolapse, n (%) | 0 (0) |
| Cough, n (%) | 9 (100) |
| Bronchiectasis, n (%) | 9 (100) |
| Previous history of NTM-positive sputum, n (%) | 9 (100) |
| On NTM therapy at collection, n (%) | 5 (56) |
| Elevated inflammatory markers at collection, n (%) | 5 (56) |
| Macrolide exposure at collection, n (%) | 4 (44) |
| Immunomodulatory drugs, n % | 0 (0) |
| BMI, mean±SD | 20.8±3.2 |
| Mycobacteria, n (%) | |
| MAC | 4 (45) |
| MAB | 3 (33) |
| MAC & MAB | 2 (22) |
BMI, body mass index; CFTR, cystic fibrosis transmembrane conductance regulator; MAB, mycobacterium abscessus; MAC, mycobacterium avium complex; NTM, non-tuberculous mycobacteria.
Adverse events
There were no serious adverse events, adverse events leading to treatment discontinuation or death noted in the study. All adverse events during the study were grade ≤2. Adverse events in patients associated with study drug included: 44% (n=4) headache that spontaneously resolved within 15 min of study drug, and 22% (n=2) nasal congestion. Adverse events occurring in more than one study patient included: 22% (n=2) decreased serum albumin, 22% (n=2) hypomagnesaemia, 22% (n=2) lymphopenia and 22% (n=2) thrombocytopenia (n=2). No other adverse event occurred in more than one patient.
Immediate effects of in vivo addition of sildenafil on CBF
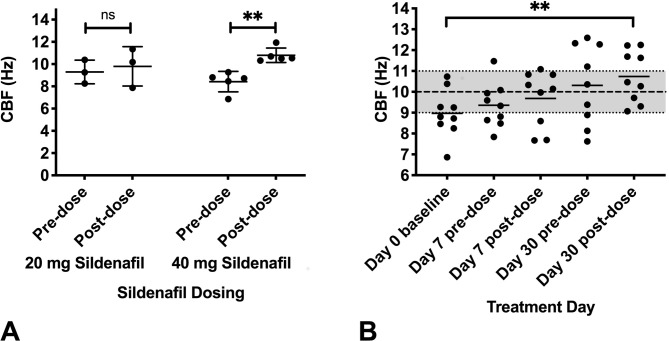
To determine if CBF in PNTM patient respiratory epithelium could be increased by in vivo inhibition of phosphodiesterase V with sildenafil, patients were administered either a single dose of 20 mg sildenafil (n=4) or 40 mg sildenafil (n=5). One patient in the 20 mg sildenafil cohort specimen was unable to have samples successfully processed post sildenafil dosing. CBF was measured before the oral dosing and 2 hours after the oral dosing of sildenafil. Prior to sildenafil dosing, patients with PNTM disease had a mean CBF of 8.96±1.2 Hz, with no significant difference between the two groups, similar to our previous data.11 Two hours after sildenafil dosing, the 40 mg sildenafil group’s CBF was significantly elevated to 10.79±0.6 Hz (figure 2A) (p<0.008 two-tailed t-test). In contrast, the 20 mg sildenafil group’s CBF was not significantly different from baseline.
Figure 2.
CBF response to sildenafil: (A) Absolute CBF on day 0 for patients with PNTM disease prior and 2 hours post a single dose of either 20 mg sildenafil (n=3) or 40 mg sildenafil (n=5). Patients with PNTM disease had a mean CBF of 8.96±1.2 Hz, with no significant difference between the two groups prior to sildenafil dose. The 40 mg sildenafil group’s CBF was significantly elevated to 10.79±0.6 Hz (p<0.008, two-tailed t-test) post sildenafil. (B) Mean absolute CBF at days 0, 7 and 30 (n=9). CBF measurement was done at prior to sildenafil at day 0. CBF was measured prior to and post the 20 mg dose of sildenafil at the end of 1 week (day 7 pre-time and post-time point) and prior to and post the 40 mg dose of sildenafil at 4 weeks (day 30 pre-time and post-time point). CBF at the day 30 post sildenafil dose was significantly elevated to 10.73±1.2 Hz compared with day 0 predose (p<0.004, ANOVA with Dunnett correction). CBF for historical healthy controls11 represented by greyed zone on the y-axis (data are presented as means, error bars show SD, **p<0.01). ANOVA, analysis of variance; CBF, ciliary beat frequency; PNTM, pulmonary non-tuberculous mycobacteria.
Temporal effects of in vivo addition of sildenafil on CBF
To determine whether the increase in CBF in PNTM patient respiratory epithelium was maintained with prolonged dosing of sildenafil, all nine patients with PNTM disease were dosed with 20 mg sildenafil three times per day for 1 week followed by 40 mg sildenafil three times per day for 3 weeks. Measurement of CBF was done before and after the 20 mg dose of sildenafil at day 7 and before and after the 40 mg dose of sildenafil at day 30. The CBF in the patient with PNTM disease respiratory epithelium was significantly elevated to 10.73±1.2 Hz after 40 mg sildenafil on day 30 (figure 2B) (p<0.004, ANOVA).
Effects of in vivo addition of sildenafil on nNO
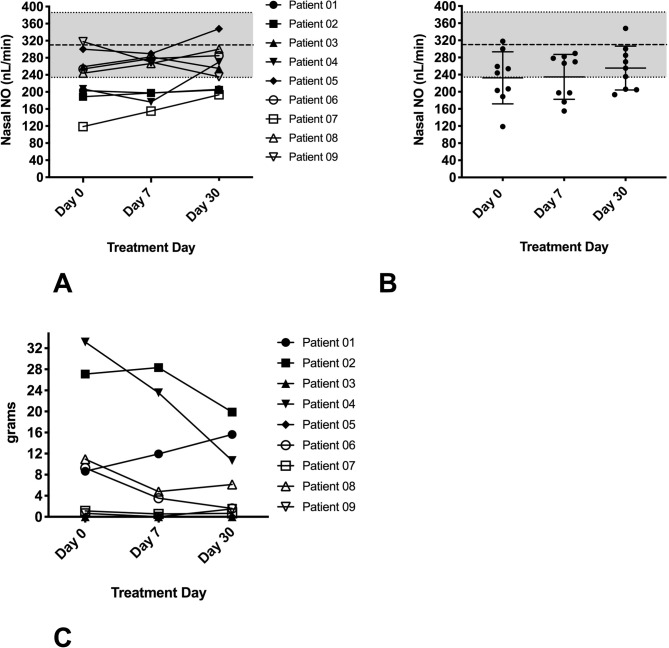
Patients with PNTM disease have been previously shown to have moderately reduced nNO levels in vivo. The patients with PNTM disease enrolled in the clinical trial had a mean nNO level of 232±61 nL/min at the time of consent. There was no significant change in the average nNO throughout the trial (figure 3A, B).
Figure 3.
nNO and sputum production in study patients with PNTM disease. (A) Individual nNO in patients with PNTM disease (n=9), done predosing at days 0, 7 and 30. there was no significant trend in nNO throughout the study. (B) Mean nNO for patients with PNTM disease. at baseline, nNO was 232±61 nL/min, similar to previous results. nNO for historical PNTM controls11 represented by greyed zone on the y-axis (data are presented as means, error bars show SD). (C) Individual 24-hour sputum weights in patients with PNTM disease (n=9) at days 0, 7 and 30 of the study. No significant trends were noted in the amount of sputum produced over the course of the study. nNO, nasal nitric oxide; PNTM, pulmonary non-tuberculous mycobacteria.
Effects of in vivo addition of sildenafil on sputum production
We measured sputum weight over time in patients with PNTM disease, as enhanced ciliary clearance might be expected to alter sputum production. Previous research has demonstrated that patients with PNTM disease have variable sputum production.4 The 24-hour sputum weights before and during exposure to study the drug showed no significant trend during the study period (figure 3C).
Self-administered questionnaires
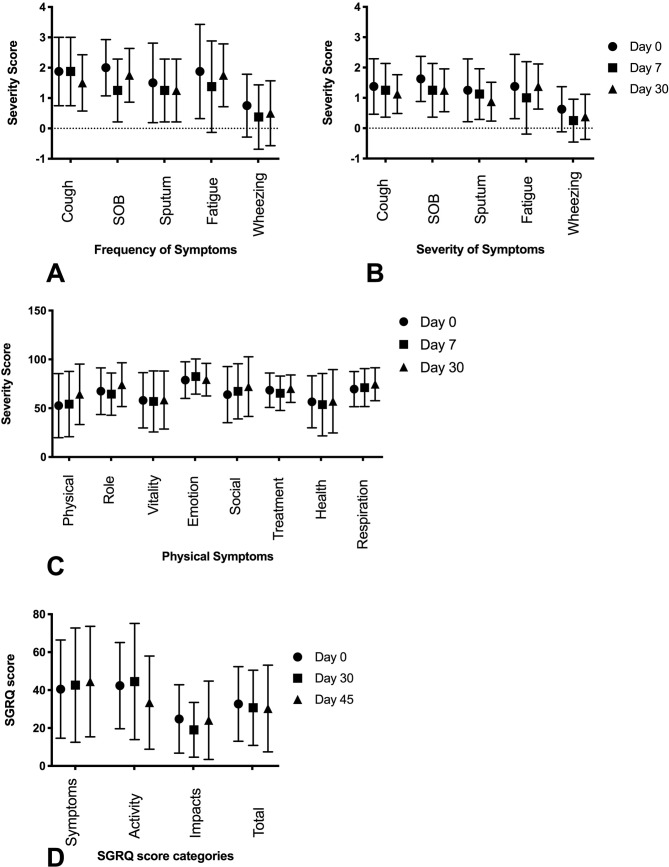
Self-administered quality of life and functional questionnaires were completed throughout the study period. We found no significant changes in the reports of symptoms on the QOL-B-NTM during the course of the clinical trial (figure 4A–C). Similarly, we found no significant trends in the SGRQ over the course of the study (figure 4D).
Figure 4.
Self-administered questionnaires (QOL-B-NTM and SGRQ) in patients with PNTM disease. (A) The QOL-B-NTM questionnaire (n=9) was performed at baseline, day 7 and day 30. We found no significant trends in frequency symptoms. (B) The QOL-B-NTM questionnaire (n=9) was performed at baseline, day 7, day 30. we found no significant trends in severity of symptoms. (C) The QOL-B-NTM questionnaire (n=9) was performed at baseline, day 7 and day 30. We found no significant changes in physical symptoms. (D) The SGRQ was performed at baseline, day 30 and day 45. We found no significant trends in the SGRQ over the course of the study (data are presented as means, error bars show SD). PNTM, pulmonary non-tuberculous mycobacteria; QOL-B-NTM, Quality of Life-Bronchiectasis-Non-Tuberculous Mycobacterial; SGRQ, St. George’s Respiratory Questionnaire.
Discussion
NO donors have been previously shown to rapidly increase mucociliary activity ex vivo.23 We have previously shown that patients with PNTM infection have decreased nNO and their respiratory epithelial cells’ CBF is reduced when compared with healthy controls or other respiratory disease states.11 We have also recently shown that mycobacterial infection of respiratory epithelial cells reduces the expression of ciliary-related genes.24 Therefore, we hypothesised that modulation of the NO-cGMP pathway might be attractive in this patient population, even though the precise role of mucociliary clearance in PNTM infection is undefined.
Sildenafil increases intracellular concentrations of cGMP through the inhibition of phosphodiesterase V, which affects the NO-cGMP pathway. The modulation of cGMP by sildenafil has been extensively studied in the cardiovascular system,25 as well as in the treatment of pulmonary arterial hypertension.26 It has also been studied as a therapy for patients with chronic obstructive pulmonary disease.26
We found a significant increase in ex vivo CBF with a single oral dose of 40 mg of sildenafil (figure 2A), which was not seen with the 20 mg dose. Prolonged dosing of sildenafil noted a significant increase in CBF only after the final dose of sildenafil 40 mg (figure 2B), suggesting that continued dosing of sildenafil did not lead to tachyphylaxis or downregulation of the effect of sildenafil on the NO-cGMP pathway. The absence of sustained response and the non-significance of the predose sample collection at day 30 could be due to the timing of the last dose of sildenafil. The terminal half-life of sildenafil is approximately 4 hours.27 The day 29 previous dose of sildenafil would have been >10 hours prior to sample collection.
No significant increase in nNO in PNTM-infected patients treated with sildenafil was noted (figure 3A, B). This was consistent with our previous demonstration of the absence of a linear correlation between CBF and nNO levels. It is interesting to note the trend toward increased nNO at treatment day 30, which corresponds to the increased CBF seen with higher sildenafil dosing (figure 3B). Sputum production was quite variable throughout the study period (figure 3C), possibly secondary to the difficulty of adequately measuring sputum production while accounting for saliva production and the small sample size of the study.
Patient-reported outcomes of physical function, emotional state and social interactions are important in a chronic disease such as PNTM infection and are likely to be important markers in the development of new therapies. Patients with PNTM infection have decreased health-related quality of life compared with healthy controls.28 29 The QOL-B-NTM questionnaire20 showed no significant difference during the study period in frequency, severity of disease-specific symptoms or physical impact of the disease (figure 4). SGRQ detected a clinical reduction in symptoms, but it was not significant (figure 4D). The dosing and/or the time course of the study could be too short to note changes in patient-reported clinical outcomes. Enrollment of patients with well-established PNTM disease can provide a cohort with a well-defined disease state, but the chronic infection stage may not be an optimal period for intervention with this type of therapy. The patient-reported measures were included in the study as secondary outcomes to assess feasibility, as well as to assist with future study directions. Importantly, sildenafil did not appear to worsen any patient’s self-reported outcomes.
Conclusions
The abnormality in CBF in PNTM infection that we have identified is modest, aligning with the fact that most aspects of life are relatively normal in patients with PNTM infection until the fifth or sixth decades. The abnormalities in the NO-cGMP pathway seen previously ex vivo in patients with PNTM disease are measurable and modifiable in vivo with sildenafil. This novel therapy has a strong safety record and appears to have minimal adverse events. Although further studies will be necessary to assess the potential effect of modulating the NO-cGMP pathway in the disease course of patients with PNTM infection, this class of medication may offer a novel therapeutic approach or alternative study direction for research in patients with PNTM infection.
Descriptors
Nontuberculous Mycobacterial Disease; Mycobacterial Disease: Host Defenses; Mucosal Immunity of the Respiratory Tract
Acknowledgments
The authors would like to acknowledge the NIAID imaging core for providing invaluable imaging assistance.
Footnotes
Contributors: CF: designed experiments, analysed data and composed manuscript. U-IW: assisted with data analysis. RS and CS: extracted clinical values and coordinated patient recruitment. LB: ensured IRB compliance. CB: provided valuable input throughout the process. KO and SMH: treated patients and were responsible for overall design of the project as well as revision of the manuscript.
Funding: This work supported by the Divisions of Intramural Research, NIAID and NHLBI, NIH.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the ‘Materials and Methods’ section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data is publicly available. Due to the nature of the phase I/II clinical trial, data would be available for collaboration upon reasonable request.
References
- 1.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 2015;36:13–34. 10.1016/j.ccm.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epson E, Cassidy M, Marshall-Olson A, et al. Patients with nontuberculous mycobacteria: comparison of updated and previous diagnostic criteria for lung disease. Diagn Microbiol Infect Dis 2012;74:98–100. 10.1016/j.diagmicrobio.2012.05.035 [DOI] [PubMed] [Google Scholar]
- 3.Henkle E, Aksamit T, Barker A, et al. Patient-centered research priorities for pulmonary nontuberculous mycobacteria (NTM) infection. An NTM research Consortium workshop report. Ann Am Thorac Soc 2016;13:S379–84. 10.1513/AnnalsATS.201605-387WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss CH, Glassroth J. Pulmonary disease caused by nontuberculous mycobacteria. Expert Rev Respir Med 2012;6:597–613. 10.1586/ers.12.58 [DOI] [PubMed] [Google Scholar]
- 5.Marras TK. Host susceptibility or environmental exposure in Mycobacterium avium complex lung disease: it takes two to tango. Am J Respir Crit Care Med 2012;186:585–6. 10.1164/rccm.201208-1432ED [DOI] [PubMed] [Google Scholar]
- 6.Chan ED, Iseman MD. Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin Respir Crit Care Med 2013;34:110–23. 10.1055/s-0033-1333573 [DOI] [PubMed] [Google Scholar]
- 7.Kartalija M, Ovrutsky AR, Bryan CL, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med 2013;187:197–205. 10.1164/rccm.201206-1035OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo RE, Hill SC, Claypool RJ, et al. Familial clustering of pulmonary nontuberculous mycobacterial disease. Chest 2010;137:629–34. 10.1378/chest.09-1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung JM, Fowler C, Smith C, et al. A familial syndrome of pulmonary nontuberculous mycobacteria infections. Am J Respir Crit Care Med 2013;188:1373–6. 10.1164/rccm.201306-1059LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szymanski EP, Leung JM, Fowler CJ, et al. Pulmonary nontuberculous mycobacterial infection. A multisystem, multigenic disease. Am J Respir Crit Care Med 2015;192:618–28. 10.1164/rccm.201502-0387OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler CJ, Olivier KN, Leung JM, et al. Abnormal nasal nitric oxide production, ciliary beat frequency, and Toll-like receptor response in pulmonary nontuberculous mycobacterial disease epithelium. Am J Respir Crit Care Med 2013;187:1374–81. 10.1164/rccm.201212-2197OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao J, Wang H, Lou W, et al. Regulation of ciliary beat frequency by the nitric oxide signaling pathway in mouse nasal and tracheal epithelial cells. Exp Cell Res 2011;317:2548–53. 10.1016/j.yexcr.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 13.Teff Z, Priel Z, Gheber LA. The forces applied by cilia depend linearly on their frequency due to constant geometry of the effective stroke. Biophys J 2008;94:298–305. 10.1529/biophysj.107.111724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braiman A, Priel Z. Efficient mucociliary transport relies on efficient regulation of ciliary beating. Respir Physiol Neurobiol 2008;163:202–7. 10.1016/j.resp.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 15.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 16.Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066–74. 10.1164/rccm.200805-686OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sisson JH, Stoner JA, Ammons BA, et al. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 2003;211:103–11. 10.1046/j.1365-2818.2003.01209.x [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society, European Respiratory Society . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30 10.1164/rccm.200406-710ST [DOI] [PubMed] [Google Scholar]
- 19.Teper A, Jaques A, Charlton B. Inhaled mannitol in patients with cystic fibrosis: a randomised open-label dose response trial. J Cyst Fibros 2011;10:1–8. 10.1016/j.jcf.2010.08.020 [DOI] [PubMed] [Google Scholar]
- 20.Quittner AL, O'Donnell AE, Salathe MA, et al. Quality of life Questionnaire-Bronchiectasis: final psychometric analyses and determination of minimal important difference scores. Thorax 2015;70:1–9. 10.1136/thoraxjnl-2014-205918 [DOI] [PubMed] [Google Scholar]
- 21.Wilson CB, Jones PW, O'Leary CJ, et al. Validation of the St. George's respiratory questionnaire in bronchiectasis. Am J Respir Crit Care Med 1997;156:536–41. 10.1164/ajrccm.156.2.9607083 [DOI] [PubMed] [Google Scholar]
- 22.De Paepe A, Devereux RB, Dietz HC, et al. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 1996;62:417–26. [DOI] [PubMed] [Google Scholar]
- 23.Runer T, Lindberg S. Ciliostimulatory effects mediated by nitric oxide. Acta Otolaryngol 1999;119:821–5. 10.1080/00016489950180487 [DOI] [PubMed] [Google Scholar]
- 24.Matsuyama M, Martins AJ, Shallom S, et al. Transcriptional response of respiratory epithelium to nontuberculous mycobacteria. Am J Respir Cell Mol Biol 2018;58:241–52. 10.1165/rcmb.2017-0218OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai EJ, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther 2009;122:216–38. 10.1016/j.pharmthera.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butrous G. The role of phosphodiesterase inhibitors in the management of pulmonary vascular diseases. Glob Cardiol Sci Pract 2014;2014:42–290. 10.5339/gcsp.2014.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation 2010;122:88–95. 10.1161/CIRCULATIONAHA.110.944603 [DOI] [PubMed] [Google Scholar]
- 28.Mehta M, Marras TK. Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med 2011;105:1718–25. 10.1016/j.rmed.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 29.Hong JY, Lee SA, Kim SY, et al. Factors associated with quality of life measured by EQ-5D in patients with nontuberculous mycobacterial pulmonary disease. Qual Life Res 2014;23:2735–41. 10.1007/s11136-014-0727-3 [DOI] [PubMed] [Google Scholar]