Abstract
Background
Vulvar high-grade squamous intraepithelial lesion (vHSIL) is predominantly induced by high-risk human papilloma virus type 16 (HPV16). In two independent trials, therapeutic vaccination against the HPV16 E6 and E7 oncoproteins resulted in objective partial and complete responses (PRs/CRs) in half of the patients with HPV16+ vHSIL at 12-month follow-up. Here, the prevaccination and postvaccination vHSIL immune microenvironment in relation to the vaccine-induced clinical response was investigated.
Methods
Two novel seven-color multiplex immunofluorescence panels to identify T cells (CD3, CD8, Foxp3, Tim3, Tbet, PD-1, DAPI) and myeloid cells (CD14, CD33, CD68, CD163, CD11c, PD-L1, DAPI) were designed and fully optimized for formalin-fixed paraffin-embedded tissue. 29 prevaccination and 24 postvaccination biopsies of patients with vHSIL, and 27 healthy vulva excisions, were stained, scanned with the Vectra multispectral imaging system, and automatically phenotyped and counted using inForm advanced image analysis software.
Results
Healthy vulvar tissue is strongly infiltrated by CD4 and CD8 T cells expressing Tbet and/or PD-1 and CD14+HLA-DR+ inflammatory myeloid cells. The presence of such a coordinated pre-existing proinflammatory microenvironment in HPV16+ vHSIL is associated with CR after vaccination. In partial responders, a disconnection between T cell and CD14+ myeloid cell infiltration was observed, whereas clinical non-responders displayed overall lower immune cell infiltration. Vaccination improved the coordination of local immunity, reflected by increased numbers of CD4+Tbet+ T cells and HLA-DR+CD14+ expressing myeloid cells in patients with a PR or CR, but not in patients with no response. CD8+ T cell infiltration was not increased after vaccination.
Conclusion
A prevaccination inflamed type 1 immune contexture is required for stronger vaccine-induced immune infiltration and is associated with better clinical response. Therapeutic vaccination did not overtly increase immune infiltration of cold lesions.
Keywords: tumor microenvironment, immunotherapy, vulvar HSIL, therapeutic vaccination
Introduction
The immune system is key to the control of tumor outgrowth. A dense infiltration with type 1 CD4+ and CD8+ T cells in the tumor microenvironment (TME) is prognostic for longer survival and associated with a favorable response to therapy in many but not all cancers.1 2 Several effective strategies, including adoptive cell transfer3 4 and immune checkpoint blockade,5 6 demonstrated the power of immunotherapy in cancer, making it one of the pillars of modern cancer therapy in the clinic. With the widespread testing of these immunotherapies, it has become clear that their success builds on the presence of pre-existing tumor-infiltrating T cells, since both their absence and their incapability to infiltrate the tumor cell nests are associated with lack of response after immunotherapy.7 8 Hence, for those patients, there is a need for treatment modalities that can stimulate tumor-reactive T cell immunity and/or promote their infiltration into tumors. Here, cancer vaccines come into play, since they can increase the magnitude and width of the tumor-reactive T cell pool by stimulating both the naive T cell repertoire and the existing tumor-specific T cell population. In a recent review, at least 18 trials were identified to report regression of premalignant lesions or tumors after vaccination with a series of different antigens over a spectrum of different solid tumors. In general, patients with a clinical response showed a robust or more active type 1 T cell response.9 In view of the fact that cancer immunity is influenced by a complex set of host, tumor and environmental factors10 11 it was no surprise that vaccine-induced tumor regression was only observed in a few patients per trial.9
About 20% of human cancers are induced by viruses.12 Human papillomavirus type 16 (HPV16) is a high-risk DNA virus which causes anogenital and oropharyngeal (pre-)cancers. To increase T cell reactivity against HPV16 caused diseases, two DNA vaccines and one synthetic long peptide (SLP) vaccine have been developed to harness the immune system against the HPV16-encoded oncoproteins E6 and E7.13–16 Immunization with these vaccines resulted in the induction of potent CD4+ T helper type 1 (Th1) responses and CD8+ cytotoxic T cell responses in patients with HPV16-induced precancerous cervical lesions15–18 and precancerous vulvar lesions (vulvar high-grade squamous intraepithelial lesion (vHSIL)).19–21 Importantly, these vaccines were able to induce partial and complete regression of the lesions in a substantial number of vaccinated patients.19–22 More recently ISA101, an HPV16 SLP vaccine, showed strong biological signs of clinical activity in patients with either HPV16-positive oropharyngeal or cervical cancer.23 24 The HPV-specific T cell response after vaccination, as measured in the blood of patients, was strongly correlated to clinical outcome.19–22 24 25 Despite these correlations, there are still a number of patients who, based on their vaccine-induced immune response, were expected to show a clinical response but failed to do so. There may be a multitude of reasons for this, but inspired by the studies showing that checkpoint blockade is specifically successful in patients with pre-existing T cell inflamed tumors,10 11 we hypothesized that this may also hold true for therapeutic cancer vaccination.
In order to address this question, we studied a group of patients treated with ISA101, about 50% of whom showed an objective regression of their HPV16-induced vHSIL.21 Based on our previous studies of the TME in vHSIL, demonstrating the impact of different types of immune cells on the recurrence rate of vHSIL,26 27 two antibody panels for studying T cells and myeloid cells were designed and fully optimized for multispectral immunofluorescence imaging, hereby enabling unprecedented in-depth characterization of the immune composition in the TME of vHSIL biopsies before and after vaccination, and in healthy vulvar tissue for comparison. Our data revealed that complete responders (CR) to vaccination had a strong and coordinated pre-existing infiltration with type 1 T helper cells and inflammatory (CD14+) myeloid cells, similar to what was found in healthy vulvar tissue. In contrast, clinical non-responders (NR) displayed an intrinsic incapacity to attract immune cells as they displayed much lower overall infiltration before vaccination and this was not increased after vaccination.
Materials and methods
Patient materials
Prevaccination and postvaccination formalin-fixed paraffin-embedded (FFPE) biopsies of 29 women of ≥18 years old with histologically confirmed HPV16 +vHSIL were taken. These women participated in a phase I/II therapeutic HPV16 SLP vaccination trial with ISA101.21 The ISA101 vaccine was injected subcutaneously and consists of 13 SLPs covering the entire amino acid sequence of HPV16 oncoproteins E6 and E7, dissolved in dimethylsulfoxide in 20 mM of phosphate-buffered saline and emulsified with Montanide adjuvant (ISA-51 Seppic). Clinical efficacy assessments in the trial were performed at 3 and 12 months after the fourth and last vaccination. The patients were grouped into the best clinical response at 12 months after vaccination without additional treatment: CR when the lesion completely disappeared, partial responders (PR) when≥50% of the total lesion area had disappeared, and NR when<50% of the lesion had disappeared. The presence of HPV16 DNA in the lesion was determined by HPV16 PCR analysis. Furthermore, FFPE healthy HPV-negative vulvar tissue from 27 anonymous women who underwent labia reduction surgery was included. All patients gave written informed consent.
Multiplex immunofluorescence imaging
The specificity of each primary antibody was first assessed with immunohistochemistry, tonsil slides served as positive and negative control. After selection of the best primary antibodies, the immunodetection conditions for each marker in seven color immunofluorescence were optimized. Dim markers were tyramide signal amplified with Opal to enable their detection by fluorescence microscopy, and primary antibodies with clashing species and isotypes in the panel were directly labeled with fluorochromes or detected with species/isotype-specific antibodies.28 A complete overview of the design of the T cell panel and myeloid cell panel is presented in online supplementary files 1 and 2, respectively. In the seven color immunofluorescent procedure, 4 μm FFPE tissue sections were deparaffinized, endogenous peroxidase was blocked with hydrogen peroxide, and heat-induced epitope retrieval was performed with citrate (10 mM, pH 6.0) in the T cell panel and with tris-EDTA (10 mM/1 mM, pH 9.0) in the myeloid cell panel. Non-specific binding sites were blocked with SuperBlock (ThermoFisher Scientific). First, the antibodies detected by Opal were applied and subjected to the recommended tyramide signal amplification protocol, followed by the unconjugated antibodies which were incubated overnight. On the second day, after detection of the previous with respective fluorescently labeled secondary antibodies, the directly labeled primary antibodies were incubated for 5 hours and, lastly, DAPI was applied as nuclear counterstain.28
jitc-2020-000563supp001.pdf (3.2MB, pdf)
Quantification of immune cells in the TME
Immunofluorescence images were acquired with the Vectra V.3.0.5 multispectral imaging microscope (PerkinElmer) at 20× magnification and exposure times were set to avoid spectral overlap. Immune cells in the TME were automatically phenotyped and counted with inForm V.2.4 image analysis software (PerkinElmer), after manual training and validation of procedures. The software was trained to segment epithelial and stromal fractions, segment DAPI +nucleated cells, and to assign a phenotype to each cell (online supplementary file 3a). All images were visually inspected on accurateness in segmenting tissue and phenotyping cells, and if errors were detected, the training was further optimized. Given the multitude of possible coexpressed markers, each seven color panel was divided into multiple subanalyses (online supplementary file 3b) to preclude the exclusion of relevant phenotypes, after which the phenotypes of the different subanalyses were merged per cell based on X, Y-positions to obtain the full seven marker expression profile of each cell. Immune cell counts were normalized for tissue size (cells/mm2 epithelium and cells/mm2 stroma). After merging all subanalyses, a threshold of a median cell count ≥10 cells/mm2 was applied to enable the analysis of relevant phenotypes.
Statistical analysis
Statistical data analysis was performed with SPSS V.25.0 (IBM Corporation). Immune counts of two groups were compared with the non-parametric Mann-Whitney U test. Pearson correlation was used to study correlations between the T cell infiltrate and the myeloid cell infiltrate in each group (clinical NR, PR, CR and healthy donor vulva). Two sided p values<0.05 were marked as significant. GraphPad Prism V.8.0.1 (GraphPad Software) was used to create graphs.
Results
Healthy vulvar tissue is predominantly infiltrated by type 1 activated T cells and inflammatory macrophages
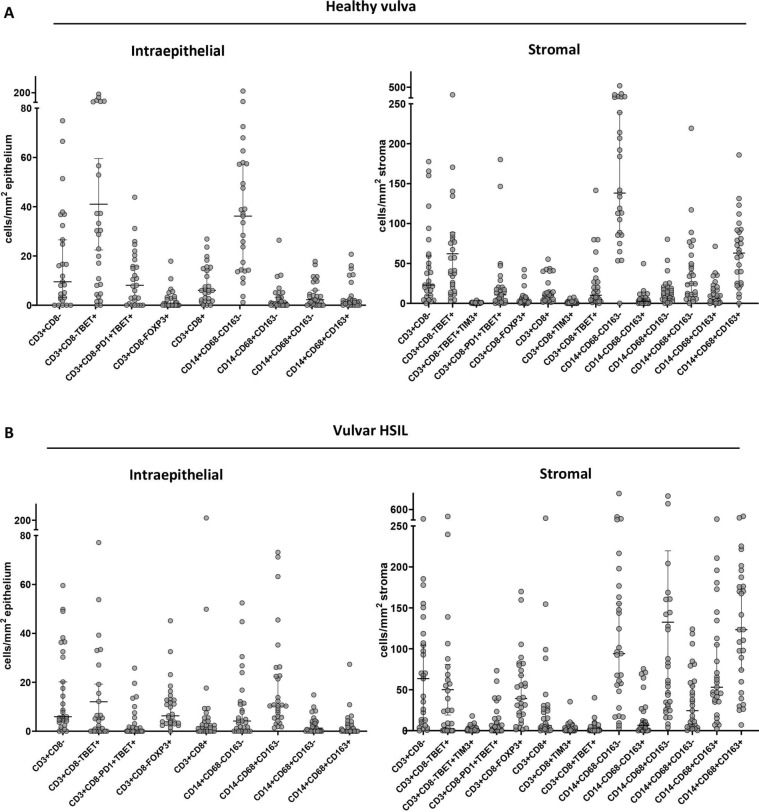
Correct interpretation of the immune infiltration in precancerous vulvar lesions requires a profound understanding of the immune microenvironment in healthy vulvar tissue. In-depth characterization of the immune infiltrate was performed with two panels of antibodies to identify different subsets of T cells and myeloid cells in the epithelium and stroma, using multispectral immunofluorescence imaging (figure 1A, B and online supplementary file 4). Analysis of 27 healthy HPV-negative vulvar tissues revealed that normal vulvar tissue is strongly infiltrated with T cells and myeloid cells, although at variable levels between individuals (figure 2A and online supplementary file 5). The intraepithelial infiltrate was dominated by type 1 Th cells (CD3+CD8−Foxp3−) expressing Tbet and a smaller fraction of which also expressed PD-1 as well as by inflammatory myeloid cells (CD14+ cells). A similar pattern was observed in healthy vulvar stroma; however, here also high numbers of M1 (CD68+CD163−) and M2 (CD68+CD163+) macrophages were found and the number of inflammatory myeloid cells (CD14+) superseded any other immune cell count by far. These data suggest that healthy vulvar tissue is a highly inflammatory microenvironment comprising many type 1 (Tbet+) and activated (PD-1+) T lymphocytes and inflammatory macrophages.
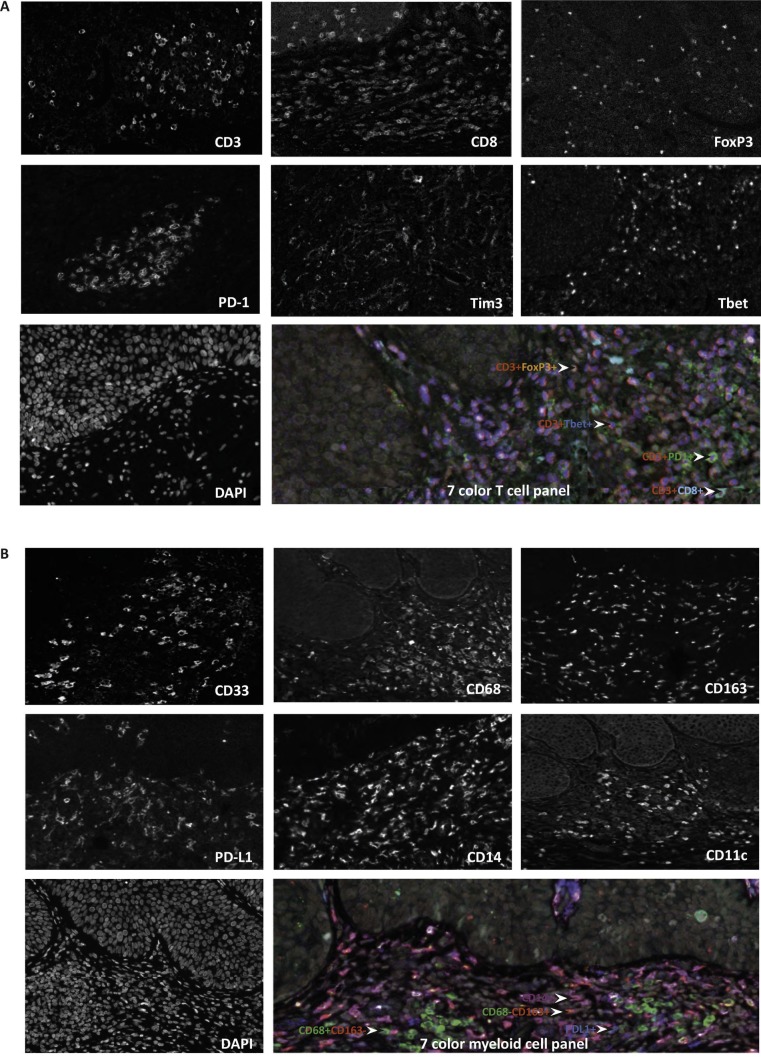
Figure 1.
Multiplex immunofluorescence staining to detect T cells and myeloid cells. Two multiplex panels were developed to detect T cells and myeloid cells in vulvar tissue. (A) The expression of each of the seven markers for T cells showing CD3, CD8, FoxP3, Tbet, Tim3, PD-1 and DAPI as well as a figure showing all seven markers simultaneously. Arrows indicate examples of analyzed phenotypes. (B) The expression of each of the seven markers for myeloid cells showing CD33, CD68, CD163, PD-L1, CD14, CD11c and DAPI as well as a figure showing all seven markers simultaneously. Arrows indicate examples of analyzed phenotypes.
Figure 2.
Healthy vulvar tissue is infiltrated by activated type 1 T cells and several types of myeloid cells while the vulvar high-grade squamous intraepithelial lesion (vHSIL) immune infiltrate is highly heterogenic among different patients. The numbers of intraepithelial and stroma-infiltrating T cell and myeloid cell subtypes, of which the median cell count exceeded the threshold of ≥10 cells/mm2, are presented as cells/mm2 for (A) human papillomavirus-negative healthy vulva (n=27) and (B) vHSIL (n=29) before vaccination. Each dot represents an individual sample, the horizontal bars indicate the median cell counts, and the vertical bars are the 95% CIs.
Strong variations in the lymphoid and myeloid immune infiltration of prevaccination vHSIL
We were able to include 29 of the 34 vaccinated patients with vHSIL21 for the current analysis (table 1). Two patients could not be included due to unavailability of FFPE tissue and three due to unknown response 12 months after vaccination. Analyses of the multispectral immunofluorescence imaging for T cells and myeloid cells revealed that despite the same aetiology, the 29 patients with vHSIL formed a heterogeneous group as the counts of immune cells infiltrating the epithelium and stroma strongly differed per patient (figure 2B and online supplementary file 6). The overall pattern of intraepithelial and stromal immune cell infiltration resembled that found in healthy tissue. However, while 55% of the healthy vulvar tissue-infiltrating CD14+ myeloid cells expressed HLA-DR, this was only 26% in the vHSIL group (online supplementary file 7).
Table 1.
Patient characteristics prevaccination and best clinical response during 12-month follow-up after therapeutic human papillomavirus type 16 (HPV16) synthetic long peptide vaccination
| Patient study number | Lesion size (cm2) | Histology | HPV16 | Best response without additional treatment | Prevaccination biopsy analyzed | 3-months postvaccination biopsy analyzed |
| 1 | 15.5 | VIN3 | + | PR | + | + |
| 3 | 3.0 | VIN2 | + | CR | + | -* |
| 6 | 8.0 | VIN3 | + | PR | + | + |
| 9 | 3.5 | VIN3 | + | CR | + | + |
| 10 | 4.0 | VIN2 | + | PR | + | + |
| 11 | 25.3 | VIN3 | + | NR | + | + |
| 14 | 0.3 | VIN3 | + | CR | + | + |
| 15 | 2.0 | VIN2 | + | NR | + | + |
| 18 | 2.0 | VaIN3 | + | NR | + | + |
| 19 | 4.5 | VIN3 | + | NR | + | + |
| 20 | 2.0 | VIN2 | + | PR | + | + |
| 21 | 6.5 | VIN3 | + | NR | + | – |
| 22 | 2.0 | VIN3 | + | NR | + | + |
| 24 | 6.2 | VIN3 | + | NR | + | + |
| 26 | 1.0 | VIN2 | + | NR | + | + |
| 28 | 9.5 | VIN3 | + | NR | + | + |
| 29 | 4.2 | VIN2 | + | PR | + | + |
| 30 | 10.3 | VIN3 | + | PR | + | – |
| 32 | 1.5 | VIN3 | + | CR | + | -* |
| 51 | 19.0 | VIN2 | + | NR | + | + |
| 52 | 120.0 | VIN3 | + | NR | + | + |
| 53 | 42.0 | VIN3 | + | PR | + | + |
| 55 | 104.0 | VIN3 | + | PR | + | + |
| 57 | 3.0 | VIN3 | + | NR | + | + |
| 58 | 44.0 | VIN3 | + | CR | + | -* |
| 59 | 8.0 | VIN3 | + | NR | + | + |
| 61 | 12.0 | VIN3 | + | CR | + | + |
| 62 | 3.0 | VaIN3 | + | PR | + | + |
| 63 | 3.0 | VIN3 | + | PR | + | + |
The data were extracted from our previous report.21
*Vulvar high-grade squamous intraepithelial lesion (vHSIL) already cleared in biopsy.
CR, complete response (100% lesion clearance); NR, no response (<50% lesion size reduction); PR, partial response (≥50% lesion size reduction).
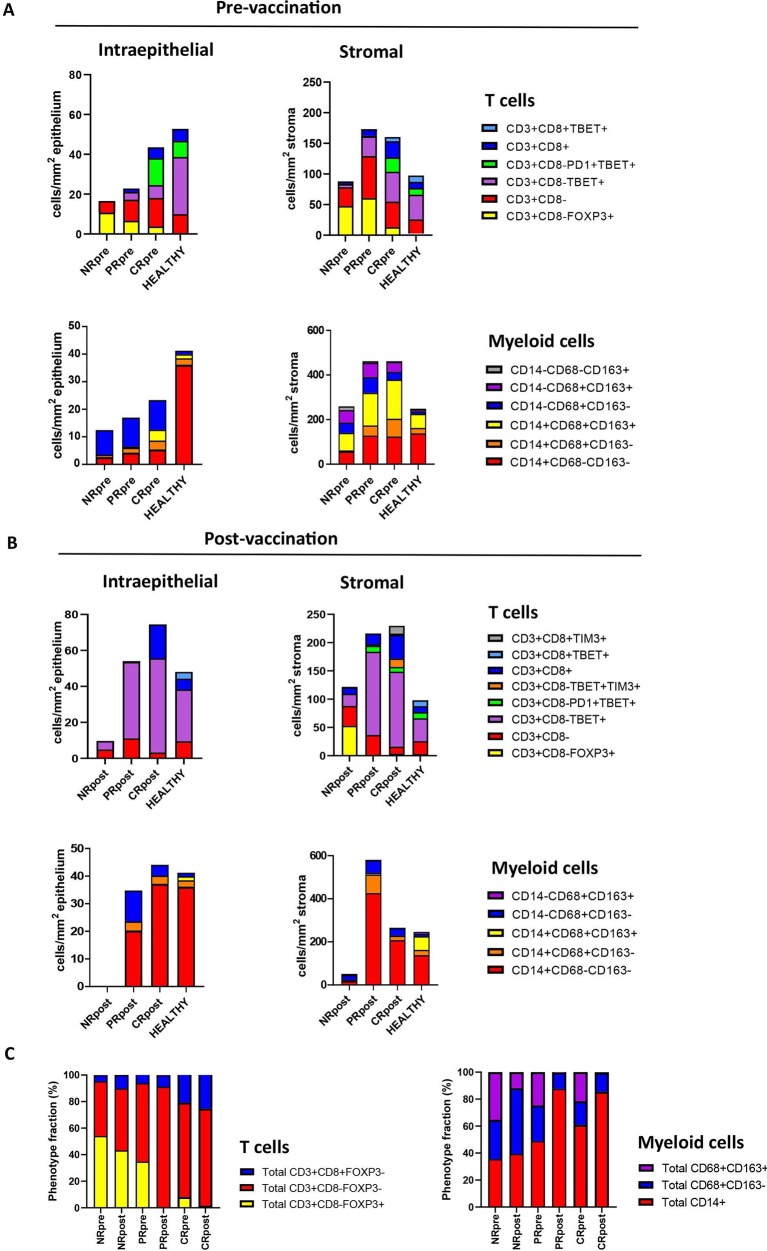
The patients with vHSIL were grouped according to their best clinical response obtained without additional treatment during the 12-month follow-up, into CR (n=7), PR (n=10) or NR (n=12). Plots of the data according to clinical outcome revealed a stepwise increase in the median number of intraepithelial and stromal CD8+ T cells, CD4+ (CD3+CD8−Foxp3−) Tbet+ Th cells and CD14+ inflammatory myeloid cells as clinical outcome improved, while that of Tregs stepwise decreased (figure 3A and online supplementary file 6). In order to ensure that the CD3+CD8− T cells were indeed CD4+ T cells, we analyzed a subset of patients with NR, PR and CR for the expression of CD3+, CD4+ and CD8+, confirming that on average 99% of the CD3+CD8− T cells were CD4+ T cells (online supplementary file 8). Interestingly, the immune infiltration of prevaccination vHSIL in the CR group most closely resembled that of healthy vulvar tissue, whereas this was the opposite for the NR group.
Figure 3.
The tumor microenvironment of complete responders (CR, n=7) displays a similar immune infiltration pattern as found in healthy vulvar tissue. The numbers of intraepithelial and stroma-infiltrating T cell and myeloid cell subtypes, of which the median cell count exceeded the threshold of ≥10 cells/mm2, are presented as cells/mm2 in the (A) prevaccination (n=29) and (B) postvaccination (n=24) vulvar high-grade squamous intraepithelial lesion biopsies of vaccinated patients grouped according to their best clinical response during the 12 months of follow-up, and compared with healthy vulvar tissue (n=27). NR, non-responders (n=12); PR, partial responders (n=10). Statistical differences in the cell types between patient groups are depicted in online supplementary files 6 and 9. (C) Highlighted T cell and myeloid cell subtypes that significantly differed among different response groups, shown as fractional differences in all CD3+ T cells (left) or all CD14+ and CD68+ myeloid cells (right), prevaccination and postvaccination.
Coordinated high infiltration with CD4 and CD8 type 1 T cells and inflammatory myeloid cells identifies the CR to therapeutic vaccination
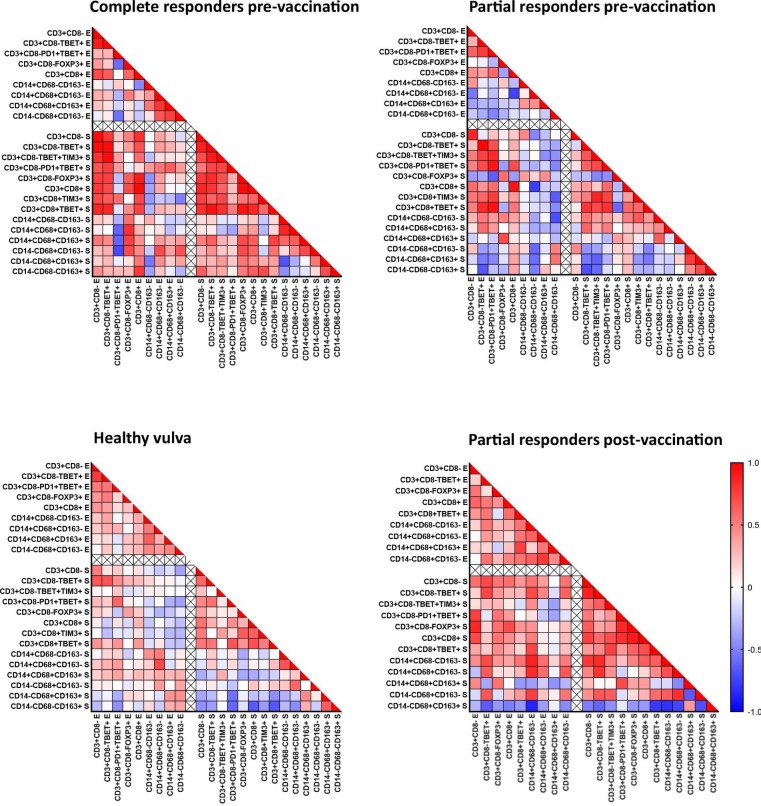
To gain a better insight in the coordination of the local immune response, we plotted the correlation between the absolute intraepithelial and stromal immune cell counts for the PR, CR and healthy donor tissues (figure 4), and not for the NR group as this would be uninformative due to the overall low cell counts. This analysis shows that there are strong correlations between the number of infiltrating CD8+ T cells and Th cells, as well as between the different types of myeloid cells; within a tissue segment (epithelium or stroma) and between both tissue segments in healthy vulva and in CR (figure 4). Importantly, there also is a strong correlation between the number of myeloid cells and CD8+ T cells (healthy, CR) and Th1 cells (CR), suggesting a coordinated immune infiltration of T cells and myeloid cells in both healthy vulvar tissue and in vHSIL of patients who will respond well to therapeutic vaccination. In contrast, there is no or a negative correlation between the vHSIL-infiltrating T cells and myeloid cells in patients with a therapeutic vaccine-induced PR (figure 4), suggesting that either T cells or myeloid cells are under-represented in the prevaccination vHSIL immune microenvironment of patients with PR.
Figure 4.
Correlation between the absolute numbers of tissue-infiltrating T cells and myeloid cells in the pretreatment and post-treatment vulvar high-grade squamous intraepithelial lesion (vHSIL) biopsies and in healthy vulvar tissue. Non-parametric Spearman r correlation analysis (two tailed) was performed to analyze the coinfiltration of the indicated different immune cell subtypes in the epithelium (E) and stroma (S) and is shown in a heatmap for healthy vulvar tissue (n=27), prevaccination complete responder patients with vHSIL (n=7) and partial responder patients with vHSIL (n=10) as well as postvaccination partial responder patients with vHSIL (n=9).
Therapeutic vaccination does not overcome a ‘cold’ low-infiltrated immune microenvironment
Three months post vaccination, all patients with vHSIL were rebiopsied, except for three patients with CR whom already reached complete response at 3-month follow-up, and for one patient with NR and one patient with PR of whom no postvaccination biopsy material was available. Consequently, we were able to analyze the postvaccination biopsies of 11 patients with NR, 9 patients with PR and 4 patients with CR in the same manner as the prevaccination biopsies were analyzed. This revealed a strong increase in the number of CD3+CD8−Tbet+ Th cells and a decrease in Tregs (figure 3B, C, online supplementary file 9), as well as a marked increase in CD14+ inflammatory myeloid cells and decreased numbers of M2 macrophages (figure 3B, C, online supplementary file 9) in the vHSILs of patients with a PR or CR after vaccination. Notably, not only was the number of CD14+ inflammatory myeloid cells increased, but also the proportion of CD14+ cells expressing HLA-DR (online supplementary file 7), suggesting that these cells provide local help to the activation of the Th1 response. While the vHSILs of patients with NR displayed only minor changes in these subsets (figure 3B, C, online supplementary file 9), vaccination improved the coordinated immune infiltration of T cells and CD14+ myeloid cells in patients with PR (figure 4). Last but not least, whereas the vHSIL of patients with CR displayed both intraepithelial and stromal CD8+ T cell infiltration after vaccination, similar to what is seen in healthy vulvar tissue, the vHSIL of patients with PR did not (figure 3B, C). Notably, 2 out of 3 patients with CR who could not be analyzed because their vHSIL disappeared within 3-month follow-up already displayed high numbers of Tbet+ Th cells (79, respectively, 460 cells/mm2 stroma), CD8+ T cells (44, respectively, 107 cells/mm2 stroma), and CD14+ inflammatory myeloid cells (316, respectively, 546 cells/mm2 stroma).
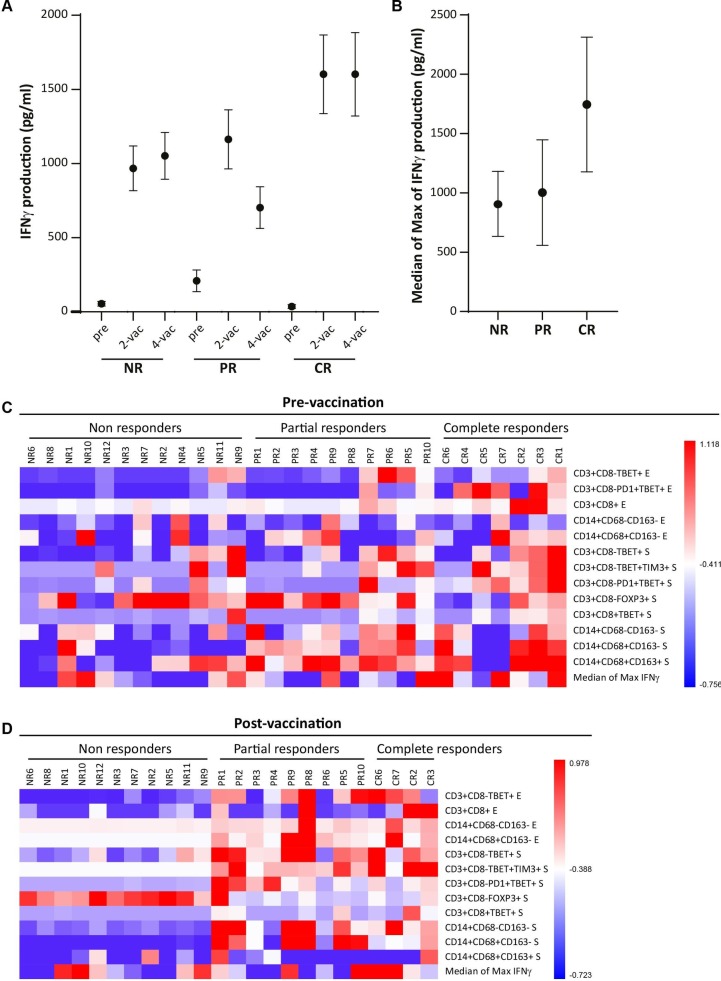
We have previously shown that patients with a CR displayed an overall stronger systemic interferon γ (IFNγ)-associated proliferative response after vaccination,21 and this was also observed after two or four vaccinations in the currently selected cohort when the average production of IFNγ to all peptide pools of all patients in the groups was compared (figure 5A), and when the average IFNγ production was depicted as the median of the maximal IFNγ production after either two or four vaccinations to all six E6 and E7 peptide pools per patient (figure 5B). In order to understand the relative differences in immune infiltration and vaccine-induced T cell response between patients with NR, PR and CR, we z-transformed the data of those immune cell types of which the counts significantly differed between the patient groups either prevaccination or postvaccination, as well as that of the vaccine-specific IFNγ production (figure 5C, D). This revealed a propensity of clinical responders to have pre-existing stromal infiltration with CD4+ T cells (CD3+CD8−) and CD14+ inflammatory myeloid cells. Furthermore, it suggests that in case a CD14+ inflammatory myeloid cell infiltration is already present a strong vaccine-induced immune response is not required (figure 5C). This profile was even more clear after vaccination, where clinical responsiveness was clearly correlated with the intraepithelial and stromal presence of type 1 CD4+ T cells (CD3+CD8−Tbet+) and CD14+ inflammatory myeloid cells lacking the expression of CD163 as well as the relative absence of Tregs (figure 5D). The absence of such a coordinated response before vaccination and the failure to alter this by vaccination explain why some of the patients with a strong vaccine-induced T cell response still displayed an NR. Thus, a generally strong and coordinated immune infiltration present already before or after vaccination in the TME by type 1 CD4+ T cells and CD14+ inflammatory myeloid cells was associated with a better clinical response to vaccination.
Figure 5.
Immune profile of non-responders (NR), partial responders (PR) and complete responders (CR) bases on the relative infiltration of significantly different immune cell subtypes and human papillomavirus (HPV)-specific T cell reactivity. The strength of the vaccine-induced HPV16-specific T cell response present in peripheral blood mononuclear cells (PBMC) as measured by the production of interferon γ (IFNγ) in the supernatant of PBMC stimulated with six different E6 and E7 peptide pools for clinical NR, PR and CR as determined previously by cytokine bead array20 is depicted as (A) the mean±SEM of all patients to all six peptide pools tested, before and after two and four vaccinations; and (B) the median of max calculated by taking the median of the highest responses to each peptide pool after two or four vaccinations. Shown is the mean±SEM of all medians per group. (C) and (D) The cell counts of all immune subsets of which the median cell count exceeded the threshold of ≥10 cells/mm2 and the numbers significantly differed between at least two of the patient groups (NR, PR, CR) either before vaccination (C) or after vaccination (D) as well as the median of max of IFNγ production as determined by cytometric bead array are depicted after the data were z-transformed to enable comparison of relative differences.
Discussion
Our aim was to investigate if the immunological makeup of HPV16-induced vHSIL was of influence on the clinical response of patients to an HPV16 SLP vaccine, shown to be active in two separate trials.19 21 Our study is the first to show that a coordinated immune infiltration by activated type 1 CD8+ and T helper cells as well as HLA-DR+CD14+ myeloid cells is associated with a complete regression of vHSIL after vaccination. In a previous study, no difference was found in the numbers of pretreatment vHSIL-infiltrating CD4+ and CD8+ T cells between patients with or without a complete clinical response after vaccination with an HPV16-E6E7L2 fusion protein vaccine.29 This can be explained by the fact that these patients were pretreated with imiquimod,29 which is known to induce strong local inflammation30 that is reflected by infiltration with CD4+ and CD8+ T cells, activated DCs and macrophages,31 thereby recreating the desired immune microenvironment. But also because no functional assessment of the infiltrating T cells was performed, while our data clearly point at the role of CD4+ Tbet+ (type 1) Th cells. An impact of the pre-existing immune microenvironment on clinical outcome was also not found in patients with cervical HSIL vaccinated with the HPV16/18 vaccine VGX-3100, but here only the number of CD8+ T cells and Tregs was assessed25 whereas it is known that also CD14+ inflammatory myeloid cells are associated with better prognosis in cervical cancer17 and as such are likely to play a similar role in its precursors. A coordinated response of T cells and inflammatory myeloid cells was also found to be required for the regression of tumors in the HPV16-positive tumor mouse models TC-1 and C3.32 Last but not least, we used software that performed automatic tissue segmentation and cell phenotyping, enabling the analysis of the entire tissue slide thereby precluding selection bias of analyzed areas, and also preventing potential interobserver variation.
Our analyses revealed that the pre-existing vHSIL-immune infiltrate of CR resembled that found in healthy vulvar tissue, whereas the immune infiltrate in vHSIL of patients with PR and NR clearly differed. The vHSIL of patients with NR showed an overall low immune cell infiltration except for Tregs which were most abundant in this patient group, indicating that these lesions lacked inflammation and were immune suppressed; hence, they can be branded as relatively immune deserted/immunosuppressed.10 Since healthy vulvar tissue comprises an actively inflamed immune microenvironment, the absence of it in the TME of NR must be labeled as aberrant. The underlying mechanism for this is currently unknown but potentially the answer can be found in the genomic instability of the lesions, which is often found on top of the expression of the HPV16 oncoproteins,33–35 and has been related to immune evasion and reduced response to immunotherapy.36 Analyses of the postvaccination samples at 3 months of follow-up made it clear that therapeutic vaccination was incapable of overcoming the ‘cold’ local microenvironment that is present in NR. The intrinsic or extrinsic escape mechanism underlying this incapacity to attract immune effector cells is part of future studies. While the vHSIL of PR generally was infiltrated more densely with immune cells, a correlation analysis of the pre-existing immune cell infiltrate revealed that there was either no or a negative correlation between the numbers of vHSIL-infiltrating T cells and myeloid cells in this PR group. Some of them were relatively strongly infiltrated with T cells, a phenotype previously found to be correlated with partial responses of vHSIL after vaccination,37 whereas others displayed relatively high levels of myeloid cells only. After vaccination, strong increases in both type 1 Th cells and HLA class II-positive inflammatory macrophages were observed in the vHSIL of patients with a PR or CR at 12 months of follow-up. This coincided with lower numbers of Tregs and M2 macrophages. Hence, therapeutic vaccination can incite and modulate inflamed lesions so that the immune infiltration becomes more coordinated and the effector cells increase to similar or higher numbers when compared with healthy vulvar tissue. In this aspect, it does not differ from other T cell-based approaches, including adoptive T cell transfer38 and checkpoint blockade10 39 all of which have the best possible clinical impact when a tumor is inflamed or hot before start of therapy. The HPV16 SLP vaccine did not increase the numbers of vHSIL-infiltrating CD8+ T cells, which were present already in the vHSIL that became a CR but not in vHSIL ending up as PR.
Finally, several trials with therapeutic vaccines against HPV-induced HSIL lesions have reported overall high immunogenicity of the vaccine but complete clinical responses in only part of the treated subjects.19–22 29 37 40 The data of this study suggest that the HSIL microenvironment has an impact on the vaccine-induced clinical outcome and as such calls for the prospective validation of the combination of CD4+ cells, Tbet+ cells and CD14+ myeloid cells as predictive biomarkers for clinical response of HSIL to therapeutic vaccination. These biomarkers can easily be applied in routine diagnostics, as they only require routinely taken FFPE tissue and regular single immunohistochemical stainings. The combination of multiple biomarkers is likely to increase the sensitivity of the prediction tool.41 Furthermore, our data strongly suggest that the efficacy of therapeutic vaccines in this patient group can be increased when it is combined with other drugs that induce local acute inflammation, to support the infiltration of vaccine-activated T cells and inflammatory myeloid cells. Hence, our findings show that the current paradigm of personalized cancer immunotherapy also applies to therapeutic vaccines for the treatment of patients with virally induced lesions as well as provides a foundation for future combination therapy studies.
Acknowledgments
The authors thank the patients for their participation in this study.
Footnotes
Contributors: Conception and design: ZA, NdM, EMGvE, PJdVvS, HWN, MIEvP, SHvdB. Development of methodology: ZA, NdM, MJPW. Collection and assembly of clinical data and data analysis: MIEvP, EMGvE, PJdVvS and HWN. Immunological analyses: ZA and MJPW. Analysis and interpretation of data: ZA, NdM, MJPW, MIEvP and SHvdB. Writing, review, and/or revision of the manuscript: ZA, MIEvP and SHvdB. Study supervision: MIEvP and SHvdB. All authors read, commented and approved the final manuscript.
Funding: ZA received an MD/PhD-track grant from Leiden University Medical Center, the Netherlands.
Competing interests: SHvdB is being named as an inventor on the patent for the use of synthetic long peptides as vaccine for the treatment of HPV-induced diseases, which is exploited by ISA Pharmaceuticals. SHvdB serves as a paid member of the strategy board of ISA Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Patient consent for publication: Not required.
Ethics approval: This study was conducted in accordance with the Declaration of Helsinki and approved by the National Central Committee on Research Involving Human Subjects (CCMO, NL21215.000.08) and the local institutional ethical committee (P08.197). All patients gave written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All data generated or analyzed during this study are included in this published article (and its additional files) and are available from the corresponding author on reasonable request.
References
- 1. Galon J, Angell HK, Bedognetti D, et al. . The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013;39:11–26. 10.1016/j.immuni.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 2. Fridman WH, Pagès F, Sautès-Fridman C, et al. . The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298–306. 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 3. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62–8. 10.1126/science.aaa4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 2016;16:566–81. 10.1038/nrc.2016.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56–61. 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- 6. Whiteside TL, Demaria S, Rodriguez-Ruiz ME, et al. . Emerging opportunities and challenges in cancer immunotherapy. Clin Cancer Res 2016;22:1845–55. 10.1158/1078-0432.CCR-16-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res 2016;22:1865–74. 10.1158/1078-0432.CCR-15-1507 [DOI] [PubMed] [Google Scholar]
- 8. Tumeh PC, Harview CL, Yearley JH, et al. . Pd-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Burg SH. Correlates of immune and clinical activity of novel cancer vaccines. Semin Immunol 2018;39:119–36. 10.1016/j.smim.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 10. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019;18:197–218. 10.1038/s41573-018-0007-y [DOI] [PubMed] [Google Scholar]
- 11. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321–30. 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- 12. McLaughlin-Drubin ME, Munger K. Viruses associated with human cancer. Biochim Biophys Acta 2008;1782:127–50. 10.1016/j.bbadis.2007.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welters MJP, Kenter GG, Piersma SJ, et al. . Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res 2008;14:178–87. 10.1158/1078-0432.CCR-07-1880 [DOI] [PubMed] [Google Scholar]
- 14. Kenter GG, Welters MJP, Valentijn ARPM, et al. . Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 2008;14:169–77. 10.1158/1078-0432.CCR-07-1881 [DOI] [PubMed] [Google Scholar]
- 15. Bagarazzi ML, Yan J, Morrow MP, et al. . Immunotherapy against HPV16/18 generates potent Th1 and cytotoxic cellular immune responses. Sci Transl Med 2012;4:155ra138–38. 10.1126/scitranslmed.3004414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim TJ, Jin H-T, Hur S-Y, et al. . Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun 2014;5:5317 10.1038/ncomms6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Vos van Steenwijk PJ, Ramwadhdoebe TH, Goedemans R, et al. . Tumor-Infiltrating CD14-positive myeloid cells and CD8-positive T-cells prolong survival in patients with cervical carcinoma. Int J Cancer 2013;133:n/a–94. 10.1002/ijc.28309 [DOI] [PubMed] [Google Scholar]
- 18. de Vos van Steenwijk PJ, van Poelgeest MIE, Ramwadhdoebe TH, et al. . The long-term immune response after HPV16 peptide vaccination in women with low-grade pre-malignant disorders of the uterine cervix: a placebo-controlled phase II study. Cancer Immunol Immunother 2014;63:147–60. 10.1007/s00262-013-1499-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kenter GG, Welters MJP, Valentijn ARPM, et al. . Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009;361:1838–47. 10.1056/NEJMoa0810097 [DOI] [PubMed] [Google Scholar]
- 20. Welters MJP, Kenter GG, de Vos van Steenwijk PJ, et al. . Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci U S A 2010;107:11895–9. 10.1073/pnas.1006500107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Poelgeest MIE, Welters MJP, Vermeij R, et al. . Vaccination against oncoproteins of HPV16 for noninvasive Vulvar/Vaginal lesions: lesion clearance is related to the strength of the T-cell response. Clin Cancer Res 2016;22:2342–50. 10.1158/1078-0432.CCR-15-2594 [DOI] [PubMed] [Google Scholar]
- 22. Trimble CL, Morrow MP, Kraynyak KA, et al. . Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2B trial. Lancet 2015;386:2078–88. 10.1016/S0140-6736(15)00239-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Massarelli E, William W, Johnson F, et al. . Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melief CJM, Welters MJP, Vergote I, et al. . Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Nature 2019. [DOI] [PubMed] [Google Scholar]
- 25. Morrow MP, Kraynyak KA, Sylvester AJ, et al. . Clinical and immunologic biomarkers for histologic regression of high-grade cervical dysplasia and clearance of HPV16 and HPV18 after immunotherapy. Clin Cancer Res 2018;24:276–94. 10.1158/1078-0432.CCR-17-2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Esch EMG, van Poelgeest MIE, Kouwenberg S, et al. . Expression of coinhibitory receptors on T cells in the microenvironment of usual vulvar intraepithelial neoplasia is related to proinflammatory effector T cells and an increased recurrence-free survival. Int J Cancer 2015;136:E95–106. 10.1002/ijc.29174 [DOI] [PubMed] [Google Scholar]
- 27. van Esch EMG, van Poelgeest MIE, Trimbos JBMZ, et al. . Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV-induced vulvar neoplasia. Int J Cancer 2015;136:E85–94. 10.1002/ijc.29173 [DOI] [PubMed] [Google Scholar]
- 28. Ijsselsteijn ME, Brouwer TP, Abdulrahman Z, et al. . Cancer immunophenotyping by seven-colour multispectral imaging without tyramide signal amplification. J Pathol Clin Res 2019;5:3–11. 10.1002/cjp2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daayana S, Elkord E, Winters U, et al. . Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer 2010;102:1129–36. 10.1038/sj.bjc.6605611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mauldin IS, Wages NA, Stowman AM, et al. . Topical treatment of melanoma metastases with imiquimod, plus administration of a cancer vaccine, promotes immune signatures in the metastases. Cancer Immunol Immunother 2016;65:1201–12. 10.1007/s00262-016-1880-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barnetson RSC, Satchell A, Zhuang L, et al. . Imiquimod induced regression of clinically diagnosed superficial basal cell carcinoma is associated with early infiltration by CD4 T cells and dendritic cells. Clin Exp Dermatol 2004;29:639–43. 10.1111/j.1365-2230.2004.01614.x [DOI] [PubMed] [Google Scholar]
- 32. van der Sluis TC, Sluijter M, van Duikeren S, et al. . Therapeutic peptide vaccine-induced CD8 T cells strongly modulate intratumoral macrophages required for tumor regression. Cancer Immunol Res 2015;3:1042–51. 10.1158/2326-6066.CIR-15-0052 [DOI] [PubMed] [Google Scholar]
- 33. Aulmann S, Schleibaum J, Penzel R, et al. . Gains of chromosome region 3q26 in intraepithelial neoplasia and invasive squamous cell carcinoma of the vulva are frequent and independent of HPV status. J Clin Pathol 2008;61:1034–7. 10.1136/jcp.2008.056275 [DOI] [PubMed] [Google Scholar]
- 34. Bryndorf T, Kirchhoff M, Larsen J, et al. . The most common chromosome aberration detected by high-resolution comparative genomic hybridization in vulvar intraepithelial neoplasia is not seen in vulvar squamous cell carcinoma. Cytogenet Genome Res 2004;106:43–8. 10.1159/000078559 [DOI] [PubMed] [Google Scholar]
- 35. Rosenthal AN, Ryan A, Hopster D, et al. . High frequency of loss of heterozygosity in vulval intraepithelial neoplasia (VIN) is associated with invasive vulval squamous cell carcinoma (VSCC). Int J Cancer 2001;94:896–900. 10.1002/ijc.1549 [DOI] [PubMed] [Google Scholar]
- 36. Davoli T, Uno H, Wooten EC, et al. . Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 2017;355:eaaf8399 10.1126/science.aaf8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davidson EJ, Boswell CM, Sehr P, et al. . Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res 2003;63:6032–41. [PubMed] [Google Scholar]
- 38. Melief SM, Visconti VV, Visser M, et al. . Long-Term survival and clinical benefit from adoptive T-cell transfer in stage IV melanoma patients is determined by a Four-parameter tumor immune signature. Cancer Immunol Res 2017;5:170–9. 10.1158/2326-6066.CIR-16-0288 [DOI] [PubMed] [Google Scholar]
- 39. Gajewski TF. The next hurdle in cancer immunotherapy: overcoming the Non-T-Cell-Inflamed tumor microenvironment. Semin Oncol 2015;42:663–71. 10.1053/j.seminoncol.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baldwin PJ, van der Burg SH, Boswell CM, et al. . Vaccinia-Expressed human papillomavirus 16 and 18 E6 and E7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin Cancer Res 2003;9:5205–13. [PubMed] [Google Scholar]
- 41. Signorelli D, Giannatempo P, Grazia G, et al. . Patients selection for immunotherapy in solid tumors: overcome the naïve vision of a single biomarker. Biomed Res Int 2019;9056417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-000563supp001.pdf (3.2MB, pdf)